Abstract
The role and metabolism of indole-3-acetic acid in gram-negative bacteria is well documented, but little is known about indole-3-acetic acid biosynthesis and regulation in gram-positive bacteria. The phytopathogen Rhodococcus fascians, a gram-positive organism, incites diverse developmental alterations, such as leafy galls, on a wide range of plants. Phenotypic analysis of a leafy gall suggests that auxin may play an important role in the development of the symptoms. We show here for the first time that R. fascians produces and secretes the auxin indole-3-acetic acid. Interestingly, whereas noninfected-tobacco extracts have no effect, indole-3-acetic acid synthesis is highly induced in the presence of infected-tobacco extracts when tryptophan is not limiting. Indole-3-acetic acid production by a plasmid-free strain shows that the biosynthetic genes are located on the bacterial chromosome, although plasmid-encoded genes contribute to the kinetics and regulation of indole-3-acetic acid biosynthesis. The indole-3-acetic acid intermediates present in bacterial cells and secreted into the growth media show that the main biosynthetic route used by R. fascians is the indole-3-pyruvic acid pathway with a possible rate-limiting role for indole-3-ethanol. The relationship between indole-3-acetic acid production and the symptoms induced by R. fascians is discussed.
The phytopathogenic actinomycete Rhodococcus fascians induces the development of a wide range of malformations on its numerous monocotyledonous and dicotyledonous host plants: leaf deformation, growth inhibition, witches' brooms, fasciations, and leafy galls (reference 23 and references therein). The leafy-gall structure, considered an extreme form of apical dominance (59), is the most severe outcome of the R. fascians-plant interaction. Consisting of small misshapen leaves and numerous buds that are inhibited for further outgrowth, the leafy gall is believed to result from the alteration of the endogenous hormone balance of the host plant (23). Leafy-gall features, such as leaf wrinkling, shoot multiplication, and delayed senescence, are typical cytokinin effects. In this respect, R. fascians pathogenicity has been linked to a linear plasmid (pFiD188) that carries different virulence genes. On pFiD188 an isopentenyl transferase (ipt) homologue is located that is essential for symptom development and has been found in all virulent strains examined thus far (14, 15, 53). Moreover, it is known that R. fascians can produce at least 11 different cytokinins in vitro (2, 18, 24, 28, 48, 51). In R. fascians, cytokinins probably originate from two sources, either release from tRNA (36, 38) and/or condensation of isopentenyl pyrophosphate and 5′-AMP (14). Nevertheless, hitherto no clear correlation between known cytokinins and symptom development could be established (16, 18), suggesting that new cytokinin-like molecules could be involved in the formation of the leafy gall (23).
Formation of a leafy gall cannot be attributed solely to the action of cytokinin. Some leafy-gall characteristics, such as vascular tissue differentiation, cell enlargement, and inhibition of bud outgrowth, are known as typical auxin effects. Moreover, other phenotypes induced by R. fascians, such as cell swelling and lateral root initiation (59), can also be attributed to auxin. Although auxin production is reported for several plant-associated bacteria (42), only few data are available on the possible involvement of auxin during R. fascians-plant interaction. Although it has been suggested that the production of cytokinins and the simultaneous degradation of auxins by R. fascians could be at the origin of symptom development (3), elevated levels of the auxin indole-3-acetic acid (IAA) were detected in infected plant tissues compared to noninfected tissues (16, 59), suggesting that R. fascians synthesizes IAA and/or induces its production by the plant.
The production of IAA and its main intermediates by R. fascians is described here for the first time. The production of IAA by the highly virulent strain D188 and the nonvirulent strain D188-5 was compared to assess the importance of IAA secretion in symptom development. Furthermore, the effect of the natural environment of the bacteria on the production of IAA and IAA intermediates was explored. We show that R. fascians produces IAA mainly through the indole-3-pyruvic acid (IPyA) pathway, and we discuss the importance of IAA production in relation to the capacity of R. fascians to colonize plant tissues and/or to induce symptoms.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
R. fascians strains D188 and D188-5 are, respectively, a highly virulent strain and a nonvirulent plasmid-free strain (17). Both strains were grown in liquid YEB medium (37) and cultures were incubated at 28°C with agitation at 130 rpm (shaker Innova 2100; New Brunswick) until an optical density at 600 nm of 2 (typically, 48 h). Cells were harvested by centrifugation (5,000 × g, 5 min), washed in minimal medium (JM medium; 3.875 g of Gamborg's B5 salts/liter, 500 mg of morpholineethanesulfonic acid/liter, 250 mg of NH4NO3/liter, 0.001% thiamine-HCl; pH 5.5), and diluted 4.25-fold in JM medium. When required, tryptophan (TRP), indole-3-ethanol (IEt), indole-3-acetamide (IAM), or tryptamine (TRA) was supplied at a concentration of 20 mg/liter. When appropriate, 23.5 μl of an aqueous extract of either leafy galls (LG extract) or noninfected plant tissues (NIP extract) was added per milliliter of JM medium. All proper controls, including medium plus LG extract, medium plus NIP extract, medium plus LG extract plus TRP, medium plus NIP extract plus TRP, medium plus TRP, medium plus TRA, or medium plus IEt, were included in the experiments. In each control, the IAA levels were significantly lower than in the presence of the bacteria (data not shown). For time course experiments the samples were harvested for extraction of IAA and precursors, and for CFU measurements. The numbers of CFU were calculated by plating serial dilutions (10−8, 10−9, and 10−10) of the samples on solid YEB medium. Plates were incubated at 28°C until single colonies could be counted to determine the number of CFU per milliliter for each bacterial culture. The cultures were centrifuged (5,000 × g for 5 min); bacterial cells and supernatants were separately stored at −20°C until indole analysis.
Preparation of LG and NIP extracts.
Nicotiana tabacum cv. Havana seeds were aseptically germinated and grown (24°C, 16/8-h photoperiod at 70 μmol photons/m2/s) on solid half-strength MS medium (39). After 4 weeks, the tobacco plants were decapitated and infected with a drop (∼5 μl, optical density at 600 nm of 2) of exponentially growing R. fascians D188 cells (growth in YEB for 24 h) or mock inoculated with an equivalent volume of YEB medium. Eight-week-old leafy galls or mock-inoculated plants were harvested and extensively crushed in water (ca. 2 g of plant tissues for 10 ml of sterile water) and filtered to remove tissue debris. The capacity of the LG extract to induce R. fascians virulence gene expression was evaluated by assessing the expression of the att locus as described previously (32). The levels of attH expression in JM medium, with or without extracts of noninfected plant, were low as evaluated by measuring the β-glucuronidase activity (data not shown). However, when LG extract was added to the medium, attH expression was strongly induced (data not shown), confirming that the LG extract contains compounds that trigger virulence-associated gene expression as described previously (14, 32).
Extraction of IAA and precursors from liquid culture medium.
The supernatants were mixed with an equal volume of 0.1 N HCl and the internal standards [13C6]IAA (150 ng, [phenyl-13C6]indole-3-acetic acid; Cambridge Isotope Laboratories, Andover, Mass.), [13C6]IAM (10 ng, synthesis from 13C6-IAA), [15N1]IEt (100 ng, synthesis from indole[15N1]TRP; Cambridge Isotope Laboratories), [13C1]indole-3-acetonitrile ([13C1]IAN; 100 ng, a gift from N. Ilic), d5-TRP (100 ng of l-TRP-2′,4′,5′,6′,7′-d5; CDN Isotopes, Pointe-Claire, Quebec, Canada), and [15N]AA (100 ng; Cambridge Isotope Laboratories) were added. Acidified samples were applied to a C18 cartridge (Bond-Elut 500 mg; Varian, Inc., Harbor City, Calif.) equilibrated with 50 mM HCl. Samples were eluted with 5 ml of acetonitrile. After vacuum evaporation (RVC2-25; Christ, Osterode am Harz, Germany), the residue was dissolved in 100% acidified methanol and methylated with diazomethane prior to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis performed as described previously (45). Although methylation is not an absolute prerequisite for analysis of indole compounds, the samples containing all indole intermediates were methylated before analysis to improve the detection limit of the carboxylic indoles and to prevent degradation of IPyA (45).
LC ES(+)-MS/MS analysis.
Micro LC was performed on a Prodigi 5 μm OD83 100 to 150 mm by 1 mm (Phenomenex, Torrance, Calif.) column. The total sample volume injected was 25 μl. The mobile phase consisted of the following gradient system: 1 min, sample loading at 10/90 (vol/vol) methanol-ammonium oxaloacetate (MeOH-NH4OAc; 0.01 M; pH 7); from 1 to 5 min, a linear gradient from 10/90 to 95/5 (vol/vol) MeOH-NH4OAc (0.01 M; pH 7); from 5 to 7.5 min, isocratic at 95/5 MeOH-NH4OAc (0.01 M); and from 7.5 to 11 min, isocratic equilibration at start conditions at 10/90 (vol/vol) MeOH-NH4OAc (0.01 M; pH 7). A constant flow rate of 60 μl/min was achieved by using a Kontron BIO-TEK P522 binary gradient pump (Kontron Instruments, Milan, Italy) with mixing at high pressure. The effluent was directly introduced at a flow rate of 60 μl/min into the MS source. The LC system was linked to a Quattro II mass spectrometer (Micromass, Ltd., Manchester, United Kingdom) equipped with an electrospray (ES) interface and Z-spray (Micromass). The source temperature was 80°C, the nebulizing gas flow was 20 liters/h, the drying gas flow was 400 liters/h, and the capillary voltage was +3.5 kV. The cone voltage depended on the appropriate compound (45, 46). Collision activated dissociation of the protonated molecular ion ([MH]+) was obtained by using argon as a collision gas at the appropriate compound-specific collision energy, which ranged between 10 and 20 eV (46), and a PAR of 4.10 to 3 mbar. Quantification was done by multiple reactant monitoring of the [MH]+ ion (dwell time, 0.05 s; interchannel delay, 0.01 s; span, 0 atomic mass unit) and the appropriate product ion. All indole compounds present in one sample were analyzed simultaneously during a single LC-MS/MS run. All mass spectra were background subtracted and smoothed once. All data were processed by using Masslynx 3.5 software.
RESULTS
The virulent R. fascians strain D188 produces IAA independently from the TRP supply.
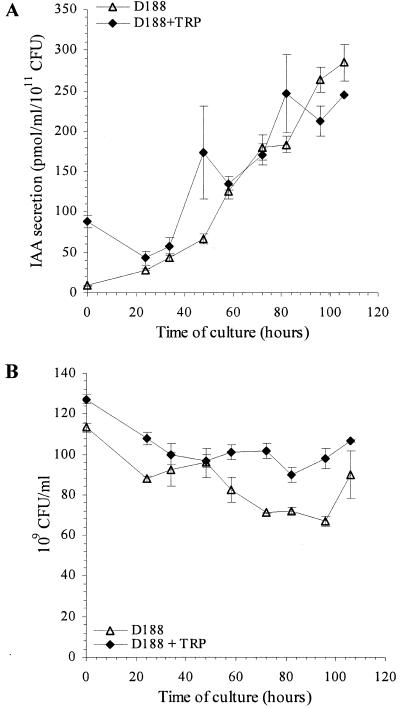
The production of IAA by the highly virulent R. fascians strain D188 was assessed during growth in a minimal medium. At regular time intervals over a period of 106 h, IAA levels present in bacterial cells and secreted into the culture media were measured. Because of the selectivity of multiple reactant monitoring MS/MS, using a unique diagnostic transition for each compound analyzed based on tandem mass spectrometric analysis, the MS/MS data are not only used for quantification but are also a reliable identification criterion for the compounds analyzed (45). We report only on the IAA levels secreted by R. fascians in the culture media, since in the bacterial cells 200-fold-lower levels were detected (data not shown), although the kinetic profiles for both samples were comparable. IAA concentrations increased linearly in time, reaching 285 ± 23 pmol/ml/1011 CFU at the end of the experiment (Fig. 1A). Only background traces of IAA were detected in the bacterium-free media (between 1 and 5 pmol/ml), indicating that R. fascians strain D188 produces IAA under the growth conditions tested. Because l-TRP, considered to be the main IAA biosynthetic precursor (42), could be limiting for IAA biosynthesis, R. fascians D188 was also grown in a minimal medium supplemented with TRP (20 mg/liter). Under these conditions IAA concentrations also increased linearly with time, and the level of secreted IAA was comparable to that measured upon growth in TRP-free medium (Fig. 1A). As shown in Fig. 1B, the growth of strain D188 in the minimal medium is stable during the time course of the experiment. Addition of TRP to the medium had no major effect on the growth of R. fascians D188.
FIG. 1.
Kinetics of IAA secretion by (A) and growth of (B) R. fascians D188. IAA levels and growth were measured in three independent experiments. Error bars represent the standard errors.
An LG extract induces IAA production by R. fascians strain D188 upon TRP supply.
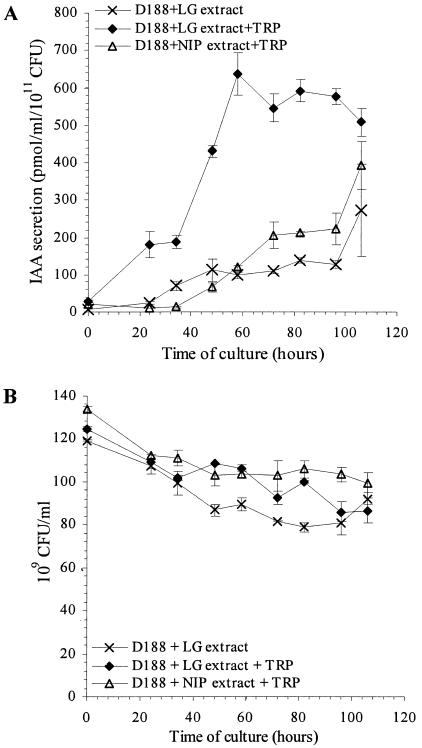
It has been shown that LG extracts contain specific compounds that are not found in noninfected plant tissues and that trigger specific responses in R. fascians, such as induction of the expression of virulence genes (14, 32, 54). Therefore, the level and kinetics of IAA production by R. fascians were investigated when the bacteria were grown in a medium supplemented with LG extract. Under these conditions, IAA concentrations increased linearly and reached levels comparable to those detected in LG extract-free cultures (Fig. 2A). However, when TRP was added to the minimal medium supplemented with LG extract, IAA secretion by strain D188 significantly increased two- to sixfold (Fig. 2A). IAA levels reached a maximum of 640 ± 60 pmol/ml/1011 CFU after 58 h of incubation and then slightly decreased until the end of the experiment, reaching a level of 508 ± 36 pmol/ml/1011 CFU. In the presence of an NIP extract and TRP, IAA secretion by strain D188 was comparable to that of bacteria grown in LG extract-supplemented medium in the absence of exogenous TRP (Fig. 2A). The induction of IAA synthesis in vitro was not the result of a supply of IAA or its precursors, nor of a stimulation of the bacterial growth by the LG extracts itself (Fig. 2B). This indicates that leafy-gall-specific compounds have a major effect on IAA secretion by R. fascians.
FIG. 2.
Kinetics of IAA secretion by (A) and growth of (B) R. fascians D188 upon addition of plant extracts. Bacteria were grown in the minimal medium supplemented with LG extract (×) and in the TRP-supplemented medium after the addition of either LG extract (♦) or NIP tissue extract (▵). IAA levels and growth were measured in three independent experiments. Error bars represent the standard errors.
The nonvirulent, plasmid-free R. fascians strain D188-5 has a delayed IAA production.
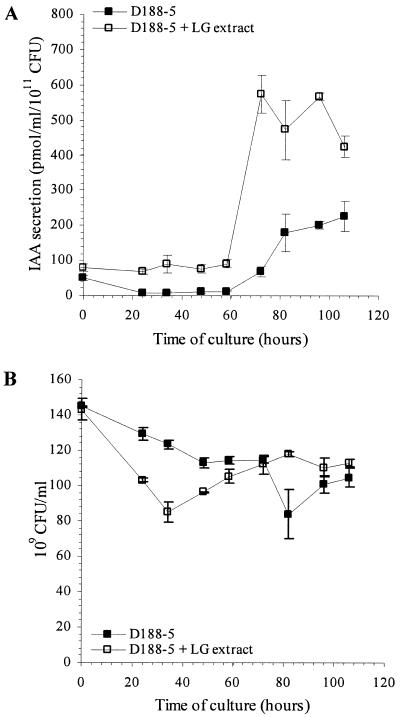
IAA production by the plasmid-free strain D188-5 was also evaluated. This nonvirulent strain was incubated in TRP-supplemented minimal medium, and IAA levels were measured at regular time intervals over a period of 106 h. IAA levels remained low and constant during the first 58 h of culture and then increased linearly, reaching a maximum level after 84 h (Fig. 3A) that was comparable to the level produced by strain D188. In the presence of both TRP and LG extract, IAA levels remained constant (between 70 and 90 pmol/ml/1011 CFU) during the first 58 h of culture and then increased rapidly to reach levels comparable to those measured for strain D188 cultured under similar conditions. These data indicate that the IAA biosynthetic genes are located on the bacterial chromosome. The observed delay in IAA production by strain D188-5 suggests that a plasmid-encoded locus contributes to the regulation of IAA biosynthesis in R. fascians. As shown in Fig. 3B, addition of LG extract had no major effect on the growth of strain D188-5.
FIG. 3.
Kinetics of IAA production by (A) and growth of (B) R. fascians D188-5. Bacteria were grown in TRP-supplemented medium with (□) or without (▪) LG-extract. IAA levels and growth were measured in three independent experiments. Error bars represent the standard errors.
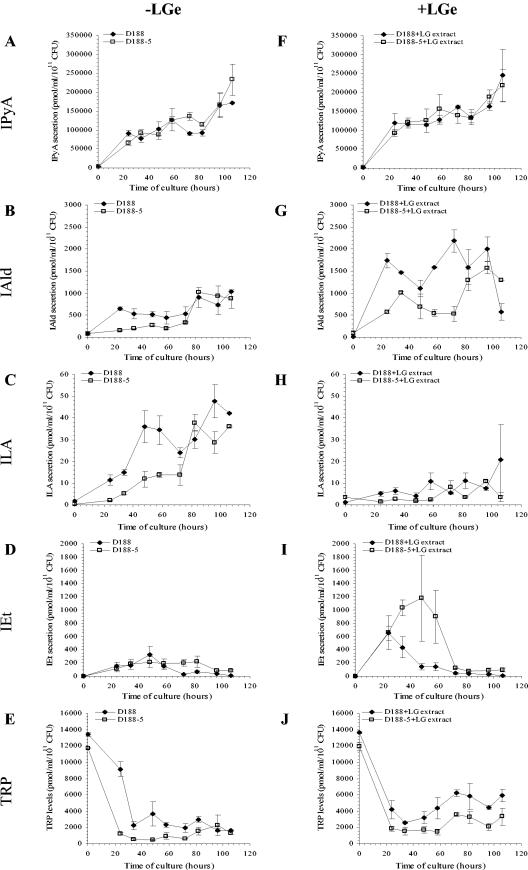
Kinetics of IAA intermediates during culture growth of virulent and nonvirulent R. fascians strains.
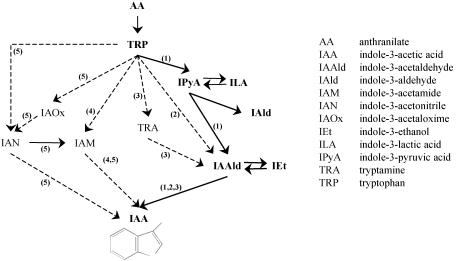
The secretions of known IAA intermediates by the two R. fascians strains were compared to pinpoint an eventual limiting step in IAA biosynthesis and to investigate the biosynthetic pathways involved. Because the levels of several IAA-intermediates were extremely low or under the detection limit upon growth in TRP-free medium (data not shown), analyses were performed on TRP-supplemented bacterial cultures. IAA conjugates were below the detection limit under all conditions tested (i.e., both strains grown in TRP-supplemented medium with or without addition of the LG extract) both in supernatants and bacterial cells (data not shown), indicating that there is no significant production or breakdown of IAA conjugates. Similarly, the IAA intermediates (see Fig. 6) TRA, IAN, IAM, and indole-3-acetaldehyde were present in very low concentrations or were under the detection limit (data not shown).
FIG. 6.
Schematic representation of IAA biosynthetic pathways in R. fascians and other plant-associated bacteria. Numbered arrows: 1, the IPyA pathway; 2, the tryptophan side chain oxidation pathway; 3, the TRA pathway; 4, the IAM pathway; 5, the IAN pathway. The IAA pathway in R. fascians is indicated by continuous lines. (The diagram follows the example set in references 11 and 42).
The main indolic compounds secreted to the medium upon TRP supplementation were the same for both strains, and all were intermediates of the IPyA pathway: IPyA, indole-3-aldehyde (IAld), indole-3-lactic acid (ILA), and IEt. IPyA, IAld, and ILA concentrations increased with time concomitantly with the increase in IAA levels (Fig. 4A to C). In contrast, IEt levels transiently increased during the first 48 h of culture and then decreased until the end of the experiment (Fig. 4D). Finally, the levels of TRP dropped rapidly within the first 40 h of culture (Fig. 4E), along with the rapid increase in IPyA concentrations (Fig. 4A).
FIG.4.
Kinetics of IAA intermediate secretion by R. fascians strains D188 (♦) and D188-5 (□). Bacteria were grown in TRP-supplemented medium in the absence (A to E) or in the presence (F to J) of LG extract (LGe). (A and F) IPyA levels; (B and G) IAld levels; (C and H) ILA levels; (D and I) IEt levels; (E and J) TRP levels. IAA levels were measured in three independent experiments. Error bars represent the standard errors. The corresponding IAA data of these samples are presented in Fig. 2 and 3.
The levels of IAA intermediates were also investigated in both strains upon addition of TRP and LG extract (Fig. 4F to J). IPyA levels increased concomitantly with the decrease in TRP level (Fig. 4F and J, respectively). When compared to the LG extract-free cultures, slightly higher IAld levels were detected in the media of both strains (Fig. 4G), whereas ILA production decreased in both strains (Fig. 4H). Particularly in the presence of LG extract, significantly higher amounts of IEt were measured for both strains (Fig. 4I). In the culture medium of strain D188, the IEt levels decreased rapidly after 24 h. For strain D188-5 the IEt level remained high until 58 h of culture, after which it dropped rapidly. The observed rapid decrease of IEt in both strains coincided with an increase in IAA levels (see Fig. 3A), suggesting that IEt is converted into IAA. A similar kinetic profile of IEt production was observed in the absence of LG extract, although the maximal production level was significantly lower (Fig. 4D). The increased concentrations of indolic compounds did not result from the addition of the LG extract, since only low levels of IAA (2.1 ± 0.5 pmol/ml), IAld (145 ± 13 pmol/ml), anthranilic acid (110 ± 10 pmol/ml), and TRP (4,100 ± 100 pmol/ml) could be detected in bacterium-free, LG extract-supplemented media. The concentrations of all of the other IAA intermediates in this medium were under detection limits (data not shown).
The overall data from the kinetic studies of the IAA intermediates indicate that in R. fascians strain D188 the IAA is mainly synthesized via the IPyA pathway under the conditions tested. Nevertheless, the capacity of R. fascians to use other IAA biosynthetic pathways was assessed by feeding experiments with two IAA precursors that were detected in low concentrations in R. fascians cultures: IAM and TRA. IAM was clearly taken up by the bacterial cells (data not shown); however, this feeding did not result in an increase of the IAA levels (data not shown), indicating that the IAM pathway is probably not used by R. fascians in the applied growth conditions. When R. fascians D188 was incubated in a minimal medium supplemented with LG extract and TRA (20 mg/ml), IAA was no longer secreted although TRA had no effect on bacterial growth (data not shown).
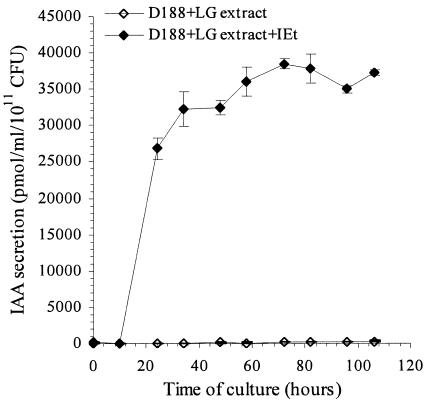
R. fascians converts IEt into IAA.
Comparison of IAA and IEt kinetic studies (see Fig. 2A, 3A, and 4D and I) strongly suggested that IEt was converted into IAA. To test this hypothesis, we conducted feeding experiments in which both strains were incubated in IEt-supplemented TRP-free medium. Very high levels of IAA were reached (26,800 ± 1,500 pmol/ml/1011 CFU) within 24 h of incubation (Fig. 5), suggesting that IEt is rapidly converted into IAA and that the production of IEt could constitute a limiting step in IAA biosynthesis by R. fascians. Both strains were able to convert IEt into IAA irrespective of the presence of LG extract. In all cases, the IAA levels detected in culture medium were ca. 100 times higher than without IEt (data not shown). The conversion of IEt into IAA could not be attributed to a nonspecific degradation of IEt since only very low levels of IAA were detected in the bacterium-free medium containing both IEt and the LG extract (data not shown).
FIG. 5.
Conversion of IEt into IAA by R. fascians strain D188. The kinetics of IAA production by R. fascians in medium supplemented with LG extract (⋄) and with both LG extract and IEt (20 mg/liter) (♦) are indicated. IAA levels were measured over a 106-h growth period in three independent experiments. Error bars represent the standard errors.
DISCUSSION
The shooty phenotype induced by R. fascians on its host plants directed earlier studies toward the synthesis of cytokinins by this bacterium (2, 18, 24, 28, 48, 51). Nevertheless, careful examination of the various symptoms resulting from R. fascians infection revealed that auxin could also play a role during the interaction with the plant (16, 59). The involvement of auxin had only been considered at the level of degradation (3, 27, 50), and possible synthesis of IAA by R. fascians had not been evaluated.
We show here for the first time that R. fascians is able to produce and secrete significant amounts of IAA. The amount of IAA secreted by these bacteria is higher than or comparable to that secreted by the plant pathogens Agrobacterium rhizogenes and Xanthomonas campestris, respectively, and comparable to that secreted by the plant-growth-promoting bacterium Azospirillum irakense (E. Prinsen, unpublished results). IAA synthesis is a common feature of plant-pathogenic and plant-growth-promoting bacteria (see, for example, reference 42) and is not restricted to gram-negative bacteria (21). Generally, IAA production increases after the addition of TRP to the growth medium (20, 26, 29). In the case of R. fascians, the addition of a minimal amount of TRP to the growth medium had no significant effect on IAA production. Interestingly, the amount of IAA secreted by R. fascians in the culture medium was highly increased upon the addition of infected-plant extracts only when TRP was supplied. These data suggest that a TRP threshold concentration and infected-plant-tissue compound(s) are required simultaneously. Although TRP synthesis is a high energy-consuming process (1, 13) and the TRP level in plant tissues is low (47), these data support the idea that TRP-dependent IAA synthesis rises only when R. fascians grows in appropriate symptomatic-tissue-specific conditions that are mimicked in the in vitro growth condition used in the present study. Possibly, one or more IAA biosynthetic genes are regulated by compound(s) synthesized during the establishment of the leafy gall. In R. fascians, it is known that the expression of virulence-associated genes, such as fas and att, is induced in vitro by an aqueous LG extract but not by NIP extracts (14, 32, 54). On the other hand, expression of the chromosomal vic locus is induced by both extracts (60). Upon addition of NIP extracts, IAA secretion did not increase, suggesting that leafy-gall-specific compounds are responsible for the observed enhancement of IAA production. Previous studies have indeed shown that the composition of amino acids and phenolic compounds differs between R. fascians-infected plant tissues and uninfected plants (19, 58). Our data and previous reports also indicate that bacterial gene expression and physiology are modified in the presence of LG extracts. Nevertheless, the structure of the activating compounds in the LG extract is currently unknown. Furthermore, whether the compounds inducing virulence-associated gene expression in R. fascians are those involved in the stimulation of IAA production remains to be determined. In other bacteria that synthesize IAA, environmental signals, such as particular host specific flavonoids (44, 56), compounds present in leaf extracts (12), the plant surface environment (8), osmotic stress (7), or auxins (57), are known to affect IAA production or IAA biosynthetic gene expression (8).
Based on the IAA precursor analyses, the IPyA pathway is likely the only IAA biosynthetic route used by R. fascians in the growth conditions tested. Indeed, the main IAA intermediates detected belong to the IPyA pathway (Fig. 6), and precursors of all other IAA biosynthetic pathways were detected in concentration below or near the detection limits. The eventual contribution of other pathways, such as the TRA and IAM pathways frequently found in bacteria (42), to the IAA biosynthesis in R. fascians was tested by feeding experiments, but none resulted in a clear production of IAA. The TRP side chain oxidation pathway shares IAA precursors with the IPyA pathway, but its role in IAA biosynthesis in R. fascians, as is the case in Pseudomonas fluorescens (40, 41), is doubtful based on the kinetics of ILA and IPyA. Comparison of IAA production by strain D188 and strain D188-5, a plasmid-free strain, indicates that the IAA biosynthetic genes are located on the bacterial chromosome. This is consistent with the chromosomal location of the genes encoding the IPyA pathway in other bacteria (42).
Irrespective of the presence of LG extract, strain D188 started to produce IAA earlier than did strain D188-5. Interestingly, whatever the case, the progressive increase in IAA levels coincided with the decrease in IEt levels in both strains. The capacity of R. fascians to use IEt as a precursor to synthesize IAA was further confirmed by feeding experiments. The regulatory role of IEt in IAA biosynthesis is strengthened by the fact that addition of TRP does not result in accumulation of IAA, as was shown for all IAA intermediates in the IPyA pathway. Conversion of IEt into IAA was also observed in Streptomyces spp. (35) and in Rhizobium lupini (21). This step in the IPyA pathway is indeed considered a regulatory reaction for IAA synthesis in bacteria (5, 21) and also in plants (55). Since strain D188-5 lacks the virulence-associated linear plasmid pFiD188 and the cadmium resistance-associated plasmid pD188 (17), plasmid-encoded proteins are likely contributing to the production of IAA through the conversion of IEt. Indeed, it has already been shown that different virulence-associated loci require plasmid-encoded factors to be optimally expressed (54, 60). Another possibility is that one of the plasmids carries a gene involved in the conversion of IEt into IAA, explaining the rapid production of IAA in strain D188. Nevertheless, since IAA production from IEt also occurs in strain D188-5, other signals must be involved in the regulation of the IAA biosynthetic genes. In this context, it has recently been shown that stress-specific “σ factors” are involved in the synthesis of IAA by Pseudomonas putida GR12-2 (43), Rhizobium sp. strain NGR234 (56), and Azospirillum brasilense (30).
Based on all currently available data, a working hypothesis is proposed in which the basal IAA production contributes to the colonization efficiency and to the growth and survival of R. fascians on its host plants. Once the development of the symptoms induced by R. fascians is initiated, bacterium-derived IAA synthesis is stimulated by compounds produced in infected plant tissues. This boost of auxin, which is comparable to 10 times the concentration per gram (fresh weight) present in noninfected plant tissues, participates in the disruption of the hormonal balance of the host. The latter is indicated by the high IAA levels detected in infected plants (16, 59), and its physiological relevance is illustrated by some of the phenotypic features of leafy galls, such as the inhibition of bud outgrowth. This working hypothesis is supported by several reports on different plant-associated bacteria that show the importance of bacterial IAA release for full development of the induced symptoms or for epiphytic fitness. Indeed, in Pantoea agglomerans (Erwinia herbicola pv. gypsophilae), the IPyA pathway has been associated with epiphytic fitness of the bacteria on the host plants, while the IAM pathway has been linked to the development of galls induced by this bacterium (33, 34). Likewise, the importance of IPyA-derived IAA in the epiphytic fitness of nonpathogenic Erwinia herbicola strains was exemplified by the less efficient plant colonization of IAA mutants compared to parental strains (6). Also, Pseudomonas syringae pv. savastanoi requires IAA for colonization and survival on its host and for symptom development (9, 10, 25, 52). Proposed roles for bacterial IAA synthesis include the detoxification of TRP analogues present on host plant surfaces (4, 31), the stimulation of the release of plant metabolites (5, 22, 30), the downregulation of plant defense (61), and/or the inhibition of the hypersensitive response of infected plants (49).
In conclusion, the work presented here demonstrates that R. fascians, a teratogenic phytopathogen generally associated merely with cytokinins, is also able to produce the auxin IAA through the IPyA pathway. Identification of this biosynthetic route is a prerequisite for the isolation of the genes involved and for the molecular analysis of this pathway in gram-positive bacteria. It is the first important step toward understanding the role of auxin in the interaction of R. fascians with plants.
Acknowledgments
This study was supported by grants from the Interuniversity Poles of Attraction Program (Belgian Prime Minister's Office [P4/15]), by Région Wallonne grant 14547, and by Fonds National de la Recherche Scientifique grant FRFC 2.4565.00F. O.V. is indebted to the Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture for a doctoral fellowship.
REFERENCES
- 1.Akashi, H., and T. Gojobori. 2002. Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc. Natl. Acad. Sci. USA 99:3695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, D. J., E. Scarbrough, F. Skoog, D. L. Cole, and N. J. Leonard. 1976. Cytokinins in Corynebacterium fascians cultures: isolation and identification of 6-(4-hydroxy-3-methyl-cis-2-butenylamino)-2-methylthiopurine. Plant Physiol. 58:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balázs, E., and I. Sziráki. 1974. Altered levels of indoleacetic acid and cytokinin in geranium stems infected with Corynebacterium fascians. Acta Phytopathol. Acad. Sci. Hungaricae 9:287-292. [Google Scholar]
- 4.Bar, T., and Y. Okon. 1992. Induction of indole-3-acetic acid synthesis and possible toxicity of tryptophan in Azospirillum brasilense Sp 7. Symbiosis 13:191-198. [Google Scholar]
- 5.Brandl, M. T., and S. E. Lindow. 1996. Cloning and characterization of a locus encoding an indolepyruvate decarboxylase involved in indole-3-acetic acid synthesis in Erwinia herbicola. Appl. Environ. Microbiol. 62:4121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandl, M. T., and S. E. Lindow. 1998. Contribution of indole-3-acetic acid production to the epiphytic fitness of Erwinia herbicola. Appl. Environ. Microbiol. 64:3256-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandl, M. T., and S. E. Lindow. 1997. Environmental signals modulate the expression of an indole-3-acetic biosynthetic gene in Erwinia herbicola. Mol. Plant-Microbe Interact. 10:499-505. [Google Scholar]
- 8.Brandl, M. T., B. Quiñones, and S. E. Lindow. 2001. Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc. Natl. Acad. Sci. USA 98:3454-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, E., S. Manulis, Y. Ophir, I. Barash, and Y. Gafni. 1993. Cloning and characterization of iaaM and iaaH from Erwinia herbicola pathovar gypsophilae. Phytopathology 83:234-240. [Google Scholar]
- 10.Comai, L., G. Surico, and T. Kosuge. 1982. Relation of plasmid DNA to indoleacetic acid production in different strains of Pseudomonas syringae pv. savastanoi. J. Gen. Microbiol. 128:2157-2163. [Google Scholar]
- 11.Costacurta, A., and J. Vanderleyden. 1995. Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 21:1-18. [DOI] [PubMed] [Google Scholar]
- 12.Costacurta, A., P. Mazzafera, and Y. B. Rosato. 1998. Indole-3-acetic acid biosynthesis by Xanthomonas axonopodis pv. citri is increased in the presence of plant leaf extracts. FEMS Microbiol. Lett. 159:215-220. [Google Scholar]
- 13.Craig, C. L., and R. S. Weber. 1998. Selected costs of amino acid substitutions in ColE1 and ColIa gene clusters harboured by Escherichia coli. Mol. Biol. Evol. 15:774-776. [DOI] [PubMed] [Google Scholar]
- 14.Crespi, M., E. Messens, A. B. Caplan, M. Van Montagu, and J. Desomer. 1992. Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J. 11:795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crespi, M., D. Vereecke, W. Temmerman, M. Van Montagu, and J. Desomer. 1994. The fas operon of Rhodococcus fascians encodes new genes required for efficient fasciation of host plants. J. Bacteriol. 176:2492-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de O'Manes, C. L., M. Van Montagu, E. Prinsen, K. Goethals, and M. Holsters. 2001. De novo cortical cell division triggered by the phytopathogen Rhodococcus fascians in tobacco. Mol. Plant-Microbe Interact. 14:189-195. [DOI] [PubMed] [Google Scholar]
- 17.Desomer, J., P. Dhaese, and M. Van Montagu. 1988. Conjugative transfer of cadmium resistance plasmids in Rhodococcus fascians strains. J. Bacteriol. 170:2401-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eason, J. R., R. O. Morris, and P. E. Jameson. 1996. The relationship between virulence and cytokinin production by Rhodococcus fascians (Tilford 1936) Goodfellow 1984. Plant Pathol. 45:323-331. [Google Scholar]
- 19.El-Wakil, M., and E. Blakeny. 1980. Quantitative analysis of free amino acids in either leafy galls induced by Corynebacterium fascians or its tissue culture. Egyptian J. Phytopathol. 12:145-148. [Google Scholar]
- 20.Ernsten, A., G. Sandberg, A. Crozier, and C. T. Wheeler. 1987. Endogenous indoles and the biosynthesis and metabolism of indole-3-acetic acid in cultures of Rhizobium phaseoli. Planta 171:422-428. [DOI] [PubMed] [Google Scholar]
- 21.Frankenberger, W. T., and M. Arshad. 1995. Auxins, p. 17-136. In W. T. Frankenberger and M. Arshad (ed.), Phytohormones in soils: microbial production and function. Marcel Dekker, Inc., New York, N.Y.
- 22.Gaudin, V., T. Vrain, and L. Jouanin. 1994. Bacterial genes modifying hormonal balances in plants. Plant Physiol. Biochem. 32:11-29. [Google Scholar]
- 23.Goethals, K., D. Vereecke, M. Jaziri, M. Van Montagu, and M. Holsters. 2001. Leafy gall formation by Rhodococcus fascians. Annu. Rev. Phytopathol. 39:27-52. [DOI] [PubMed] [Google Scholar]
- 24.Helgeson, J. P., and N. J. Leonard. 1966. Cytokinins: identification of compounds isolated from Corynebacterium fascians. Proc. Natl. Acad. Sci. USA 56:60-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iacobellis, N. S., A. Sisto, G. Surico, A. Evidente, and E. Dimaio. 1994. Pathogenicity of Pseudomonas syringae subsp. savastanoi mutants defective in phytohormone production. J. Phytopathol. 140:238-248. [Google Scholar]
- 26.Kaneshiro, T., M. E. Slodki, and R. D. Plattner. 1983. Tryptophan catabolism to indolepyruvic and indoleacetic acids by Rhizobium japonicum L-259 mutants. Curr. Microbiol. 8:301-306. [Google Scholar]
- 27.Kemp, D. R. 1978. Indole-3-ylacetic acid metabolism of Corynebacterium fascians, p. 341-345. In M. W. Loutit and J. A. R. Miles (ed.), Microbial ecology. Springer-Verlag, Berlin, Germany.
- 28.Klämbt, D., G. Thies, and F. Skoog. 1966. Isolation of cytokinins from Corynebacterium fascians. Proc. Natl. Acad. Sci. USA 56:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga, J., T. Adachi, and H. Hidaka. 1991. IAA biosynthesis pathway from tryptophan via indole-3-pyruvic acid in Enterobacter cloacae. Agric. Biol. Chem. 55:701-706. [Google Scholar]
- 30.Lambrecht, M., Y. Okon, A. Vande Broek, and J. Vanderleyden. 2000. Indole-3-acetic acid: a reciprocal signalling molecule in bacteria-plant interactions. Trends Microbiol. 8:298-300. [DOI] [PubMed] [Google Scholar]
- 31.Lebuhn, M., T. Heulin, and A. Hartmann. 1997. Production of auxin and other indolic and phenolic compounds by Paenobacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiol. Ecol. 22:325-334. [Google Scholar]
- 32.Maes, T., D. Vereecke, T. Ritsema, K. Cornelis, H. Ngo Thi Thu, M. Van Montagu, M. Holsters, and K. Goethals. 2001. The att locus of Rhodococcus fascians strain D188 is essential for full virulence on tobacco through the production of an autoregulatory compound. Mol. Microbiol. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 33.Manulis, S., A. Haviv-Chesner, M. T. Brandl, S. E. Lindow, and I. Barash. 1998. Differential involvement of indole-3-acetic acid biosynthetic pathways in pathogenicity and epiphytic fitness of Erwinia herbicola pv. gypsophilae. Mol. Plant-Microbe Interact. 11:634-642. [DOI] [PubMed] [Google Scholar]
- 34.Manulis, S., L. Valinski, Y. Gafni, and J. Hershenhorn. 1991. Indole-3-acetic acid biosynthetic pathways in Erwinia herbicola in relation to pathogenicity on Gypsophila paniculata. Physiol. Mol. Plant Pathol. 39:161-171. [Google Scholar]
- 35.Manulis, S., H. Shafrir, E. Epstein, A. Lichter, and I. Barash. 1994. Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology 140:1045-1050. [DOI] [PubMed] [Google Scholar]
- 36.Matsubara, S., D. J. Armstrong, and F. Skoog. 1968. Cytokinins in tRNA of Corynebacterium fascians. Plant Physiol. 43:451-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Murai, N., F. Skoog, M. E. Doyle, and R. S. Hanson. 1980. Relationships between cytokinin production, presence of plasmids, and fasciation caused by strains of Corynebacterium fascians. Proc. Natl. Acad. Sci. USA 77:619-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bio essay with tobacco tissue cultures. Physiol. Plant 15:473-497. [Google Scholar]
- 40.Narumiya, S., K. Takai, T. Tokuyama, Y. Noda, H. Ushiro, and O. Hayaishi. 1979. A new metabolic pathway of tryptophan initiated by tryptophan side chain oxidase. J. Biol. Chem. 254:7007-7015. [PubMed] [Google Scholar]
- 41.Oberhänsli, T., G. Défago, and D. Haas. 1991. Indole-3-acetic acid (IAA) synthesis in the biocontrol strain CHAO of Pseudomonas fluorescens: role of tryptophan side chain oxidase. J. Gen. Microbiol. 137:2273-2279. [DOI] [PubMed] [Google Scholar]
- 42.Patten, C. L., and B. R. Glick. 1996. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 42:207-220. [DOI] [PubMed] [Google Scholar]
- 43.Patten, C. L., and B. R. Glick. 2002. Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can. J. Microbiol. 48:635-642. [DOI] [PubMed] [Google Scholar]
- 44.Prinsen, E., N. Chauvaux, J. Schmidt, M. John, U. Wieneke, J. De Greef, J. Schell, and H. Van Onckelen. 1991. Stimulation of indole-3-acetic acid production in Rhizobium by flavonoids. FEBS Lett. 282:53-55. [DOI] [PubMed] [Google Scholar]
- 45.Prinsen, E., W. Van Dongen, E. Esmans, and H. Van Onckelen. 1997. HPLC linked electrospray tandem mass spectrometry: a rapid and reliable method to analyze indole-3-acetic acid metabolism in bacteria. J. Mass Spectrom. 32:12-22. [Google Scholar]
- 46.Prinsen, E., W. Van Dongen, E. Esmans, and H. Van Onckelen. 1998. Micro and capillary LC-MS/MS: a new dimension in phytohormone research. J. Chromatgr. A 826:25-37. [Google Scholar]
- 47.Radwanski, E. R., and R. L. Last. 1995. Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7:921-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathbone, M. P., and R. H. Hall. 1972. Concerning the presence of the cytokinin, N 6-(Δ2-isopentenyl)adenine in cultures of Corynebacterium fascians. Planta 108:93-102. [DOI] [PubMed] [Google Scholar]
- 49.Robinette, D., and A. G. Matthysse. 1990. Inhibition by Agrobacterium tumefaciens and Pseudomonas savastanoi of development of the hypersensitive response elicited by Pseudomonas syringae pv. phaseolicola. J. Bacteriol. 172:5742-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roussaux, J. 1965. Etude préliminaire des modifications induites chez le pois express Alaska par Corynebacterium fascians (Tilford) Dowson. Rev. Gen. Botan. 72:21-53. [Google Scholar]
- 51.Scarbrough, E., D. J. Armstrong, F. Skoog, C. R. Frihart, and N. J. Leonard. 1973. Isolation of cis-zeatin from Corynebacterium fascians cultures. Proc. Natl. Acad. Sci. USA 70:825-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverstone, S., D. G. Gilchrist, R. M. Bostock, and T. Kosuge. 1993. The 73-kb pIAA plasmid increases competitive fitness of Pseudomonas syringae subspecies savastanoi in oleander. Can. J. Microbiol. 39:659-664. [DOI] [PubMed] [Google Scholar]
- 53.Stange, R. R., Jr., D. Jeffares, C. Young, D. Scott, J. Eason, and P. Jameson. 1996. PCR-amplification of the fas-1 gene for the detection of virulent strains of Rhodococcus fascians. Plant Pathol. 45:407-417. [Google Scholar]
- 54.Temmerman, W., D. Vereecke, R. Dreesen, M. Van Montagu, M. Holsters, and K. Goethals. 2000. Leafy gall formation is controlled by fasR, and AraC-type regulatory gene, in Rhodococcus fascians. J. Bacteriol. 182:5832-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terziivanova-Dimova, S. D., and M. Kutacek. 1991. Enzymes of auxin biosynthesis and their regulation. I. Tryptophan and phenylalanine aminotransferase in pea plant. Biol. Plant. 33:227-286. [Google Scholar]
- 56.Vande Broek, A., M. Lambrecht, K. Eggermont, and J. Vanderleyden. 1999. Auxins upregulate expression of the indole-3-pyruvate decarboxylase gene in Azospirillum brasilense. J. Bacteriol. 181:1338-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theunis, M., H. Kobayashi, W. J. Broughton, and E. Prinsen. 2004. Flavonoids, NodD1, NodD2, and Nod-Box NB15 modulate expression of the y4wEFG locus that is required for indole-3-acetic acid synthesis in Rhizobium sp. strain NGR234. Mol. Plant-Microbe Interact. 17:1153-1161. [DOI] [PubMed] [Google Scholar]
- 58.Vereecke, D., E. Messens, K. Klarskov, A. De Bruyn, M. Van Montagu, and K. Goethals. 1997. Patterns of phenolic compounds in leafy gall of tobacco. Planta 201:342-348. [DOI] [PubMed] [Google Scholar]
- 59.Vereecke, D., S. Burssens, C. Simón-Mateo, D. Inzé, M. Van Montagu, K. Goethals, and M. Jaziri. 2000. The Rhodococcus fascians-plant interaction: morphological traits and biotechnological applications. Planta 210:241-251. [DOI] [PubMed] [Google Scholar]
- 60.Vereecke, D., K. Cornelis, W. Temmerman, M. Jaziri, M. Van Montagu, M. Holsters, and K. Goethals. 2002. Chromosomal locus that affects pathogenicity of Rhodococcus fascians. J. Bacteriol. 184:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada, T. 1993. Role of auxin in plant-disease development. Annu. Rev. Phytopathol. 31:253-273. [DOI] [PubMed] [Google Scholar]