Abstract
Introduction
Increasing incidence of Enterococcus faecium resistant to key antimicrobials used in therapy of hospitalized patients is a worrisome phenomenon observed worldwide. Our aim was to characterize a tigecycline-, linezolid- and vancomycin-resistant E. faecium isolate with the vanA and vanB genes, originating from a hematoma of a patient hospitalized in an intensive care unit in Poland.
Methods
Antimicrobial susceptibility (a broad panel) was tested using gradient tests with predefined antibiotic concentrations. The complete genome sequence was obtained from a mixed assembly of Illumina MiSeq and Oxford Nanopore’s MinION reads. The genome was analyzed with appropriate tools available at the Center for Genomic Epidemiology, PubMLST and GenBank. Transferability of oxazolidinone, tigecycline and vancomycin resistance genes was investigated by conjugation, followed by PCR screen of transconjugants for antimicrobial resistance genes and plasmid rep genes characteristic for the donor and genomic sequencing of selected transconjugants.
Results
The isolate was resistant to most antimicrobials tested; susceptibility to daptomycin, erythromycin and chloramphenicol was significantly reduced, and only oritavancin retained the full activity. The isolate represented sequence type 18 (ST18) and carried vanA, vanB, poxtA, fexB, tet(L), tet(M), aac(6')-aph(2''), ant(6)-Ia and ant(6')-Ii. The vanA, poxtA and tet(M) genes located on ~ 40-kb plasmids were transferable by conjugation yielding transconjugants resistant to vancomycin, linezolid and tigecycline. The substitutions in LiaS, putative histidine kinase, SulP, putative sulfate transporter, RpoB and RpoC were potential determinants of an elevated daptomycin MIC. Comparative analyses of the studied isolate with E. faecium isolates from other countries revealed its similarity to ST18 isolates from Ireland and Uganda from human infections.
Conclusions
We provide the detailed characteristics of the genomic determinants of antimicrobial resistance of a clinical E. faecium demonstrating the concomitant presence of both vanA and vanB and resistance to vancomycin, linezolid, tigecycline and several other compounds and decreased daptomycin susceptibility. This isolate is a striking example of an accumulation of resistance determinants involving various mechanisms by a single hospital strain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00881-3.
Keywords: Enterococcus faecium, HAI, LRE, Plasmid, VRE
Key Summary Points
| Why carry out this study? |
| Enterococcus faecium is one of the two clinically important enterococcal species, causing an increasing number of hospital-acquired infections (HAIs). Although isolates with both vanA and vanB vancomycin-resistance determinants as well as isolates resistant to “last resort” drugs linezolid and tigecycline have been reported, they remain rare or even very rare, and this combination of resistance phenotypes is very unusual. |
| The study was performed on a clinical E. faecium isolate demonstrating a concomitant resistance to vancomycin, linezolid and tigecycline and reduced susceptibility to daptomycin. We aimed at answering the question of what the character and possible origins of resistance determinants to antimicrobials in this isolate were and what the possibility of their further horizontal dissemination. |
| What was learned from the study? |
| We established the complete genome of the isolate, and on this basis we identified it as a representative of sequence type 18, typical for hospital meroclone of E. faecium. The vanA determinant was located in Tn1546 in a 39.5-kb transferable plasmid while vanB resided in Tn1549 on a chromosome. Linezolid and tigecycline resistance was due to the acquisition of a 42.4-kb transferable plasmid, carrying poxtA and tet(M)/tet(L) genes. |
| Although E. faecium is well known for accumulation of various antimicrobial resistance determinants, the studied isolate was a particularly striking example of this phenomenon. Genomic analyses revealed different mechanisms beyond acquisition of a plethora of antimicrobial resistance determinants, in several cases facilitated by mobile genetic elements. |
| Appearance of a strain with extremely limited treatment options and ability to further disseminate genes conferring resistance to glycopeptides, linezolid and tigecycline are particularly worrisome. |
Introduction
Enterococci, usually harmless human and animal commensals, are also causative agents of serious hospital-associated infections (HAIs). Their intrinsic and acquired resistance to several antimicrobials often limits treatment options. This is particularly observed for Enterococcus faecium, a species currently demonstrating an increasing prevalence in HAIs [1]. Strains of E. faecium infecting hospitalized patients almost exclusively belong to a large meroclone, initially described as clonal complex 17 (CC17) [2], based on the multilocus sequence typing (MLST) approach [3]. CC17 was later split into two major lineages, 17/18 and 78, named from their main sequence types (STs) [4]. One of important early adaptations of CC17 to the hospital environment was development of resistance to aminopenicillins and ciprofloxacin [2], followed by acquisition of high-level resistance to aminoglycosides (HLAR) and glycopeptides [5]. The spread of vancomycin-resistant E. faecium (VREfm), which is typically resistant to many other antimicrobial agents, is considered a significant epidemiological threat, and such pathogens belong to the so-called ESKAPE group, responsible for most HAIs worldwide [6]. The World Health Organization (WHO) recently listed VREfm as the highest priority Gram-positive pathogen for which new antibiotics are urgently needed [7]. Some antimicrobials, such as linezolid, daptomycin and tigecycline, are used as last-line drugs against VREfm; however, resistance to these compounds has been increasingly reported [8].
The most common vancomycin-resistance phenotypes include VanA (vancomycin and teicoplanin resistance) and VanB (vancomycin resistance and teicoplanin susceptibility), determined by the vanA and vanB operons, respectively [9]. The Tn1546-type transposons, usually carried on Inc18 and RepA_N family conjugative plasmids, constitute basic genetic elements responsible for dissemination of vanA operons among clinical enterococci [10, 11], while the vanB gene clusters are usually present on the bacterial chromosome, most commonly within the Tn1549-Tn5382 integrative conjugative elements [12, 13]. Linezolid resistance mechanisms observed in enterococci include mutational changes in 23S rRNA and ribosomal proteins L3 and L4, as well as acquisition of resistance genes, including cfr, cfr(B), cfr(D), optrA and poxtA [14–17]. Sporadically described tigecycline resistance is associated in enterococci with ribosomal genes mutations and an overexpression of tet(M) and tet(L) genes resulting from an increased copy number of plasmids carrying these genes [18–20]. Daptomycin resistance is also still rare in enterococcal HAIs and may appear because of mutations in cell envelope stress response regulatory pathways and genes involved in phospholipid metabolism [8]. Here, we report a clinical vancomycin-, linezolid- and tigecycline-resistant E. faecium isolate carrying both vanA and vanB determinants. Since, to our knowledge, no such multiresistant vanA-vanB E. faecium have been identified, we further analyzed this isolate by phenotypic tests and whole-genome sequencing (WGS).
Methods
Bacterial Isolate and Patient Data
The 4995/20 isolate of E. faecium was obtained by the National Reference Centre for Susceptibility Testing (NRCST) in 2020 for verification of the resistance mechanisms. The isolate used in the current study was obtained during a routine national surveillance activity of the NRCST under the mandate of the Ministry of Health according to the national legislation relevant to human infections and infectious diseases. The study was performed in a retrospective manner with anonymization of patient data; thus, ethical approval and informed consent were not required. The strain was cultured from an infected hematoma and intra-abdominal abscess from a patient (age range, 30–39 years) who, prior to the isolation of 4995/20, had been hospitalized for > 2 months, first in cardiology, then in the intensive care unit (ICU), underwent surgery and again, in the time of strain isolation, was in the ICU. During hospitalization the patient received treatment with linezolid and tigecycline as well as piperacillin/tazobactam, metronidazol, meropenem, imipenem, cefuroxime, ciprofloxacin, levofloxacin, amikacin, colistin and trimethoprim/sulfamethoxazole but not vancomycin or daptomycin. The patient had no history of travel, hospitalization or long-term care facility stay for 6 months prior to the admission. No other E. faecium isolates with the concomitant resistance to vancomycin, tigecycline and linezolid were observed in the hospital.
Antibiotic Susceptibility Testing
Antimicrobial susceptibility was tested using the gradient tests with predefined antibiotic concentrations (Liofilchem, Roseto degli Abruzzi, Italy; BioMérieux, Marcy-l’Etoile, France), ComASP™ Oritavancin Test (Liofilchem, Roseto degli Abruzzi, Italy) and broth microdilution method for streptomycin (ISO 20776–1 standard). The results were interpreted according to the EUCAST clinical breakpoints [21] and the ecological cut-off (ECOFF) values (http://mic.eucast.org/Eucast2/, last accessed 12th January 2022). Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213 were used as controls.
PCR-Based Detection of Antimicrobial Resistance and Plasmid-Specific Genes
Bacterial DNA was isolated using the Genomic DNA Prep Plus kit (A&A Biotechnology, Gdansk, Poland), and the detection of vanA, vanB, aac(6')-aph(2″), poxtA, tet(M), tet(L) and plasmid rep genes repUS15DO3, rep2pRE25, repUS1pVEF1, rep18ap200B, rep29AUS0085p4, repp4995_5, repUS12pUB110, repp4995_6 and rep14aAUS0085p5 was performed by PCR [22–27] in 4995/20 and/or its transconjugants with appropriate controls from the laboratory collection. Primers specific for repp4995_5 and repp4995_6 were designed in the current study (sequences available upon request).
Genome Sequencing and Analysis
The total DNA of 4995/20 was obtained using the Genomic Mini AX Bacteria Kit (A&A Biotechnology, Gdynia, Poland), and WGS was carried out as an external service (Genomed S.A., Warsaw, Poland) on the Illumina MiSeq Platform with the PE300 mode (Illumina Inc., San Diego, CA) and Oxford Nanopore’s MinION (Oxford Nanopore Technologies, UK). Reads were trimmed with Cutadapt v 3.1 [28] and used in a mixed assembly with Unicycler v 0.4.7., which also provided a relative approximate sequencing depth for chromosomes and plasmids [29]. Complete genomic sequences were annotated with PROKKA 1.11 [30] and supplementary manual BLASTx analyses (https://blast.ncbi.nlm.nih.gov/; last accessed on the 17th of May 2023). The assembled genome was screened using services available at the Center for Genomic Epidemiology (CGE; http://www.genomicepidemiology.org; last accessed on 17 May 2023) for genes encoding antibiotic resistance (ResFinder 4.1, LRE-Finder 1.0), virulence factors (VirulenceFinder 2.0) and plasmid Rep (PlasmidFinder 2.1). For the in silico MLST, the https://pubmlst.org/organisms/enterococcus-faecium database was used (last accessed on 9 August 2023) [31]. Conjugation transfer-associated regions were analyzed by oriTFinder and MOBScan [32, 33]. The Geneious Prime v.2022.1.1 software (Biomatters, Auckland, New Zealand) was used for sequence alignments and comparisons and for reference-guided assembly of sequencing reads with the complete genome of 4995/20 as a reference. Plasmid and transposon sequences were visualized using the BLAST Ring Image Generator (BRIG, http://brig.sourceforge.net; 7 November 2022 date last accessed) [34]. Visualization of sequence comparisons was done with Artemis Comparison Tool (ACT) [35]. Searches for E. faecium isolates related to 4995/20 were done using the Pathogen Detection website of GenBank (https://www.ncbi.nlm.nih.gov/pathogens/; accessed on the 9th August 2023). The core-genome MLST (cgMLST) profiles were obtained using the https://pubmlst.org/organisms/enterococcus-faecium database and analyzed with GrapeTree v 1.5.0 [36]. For all the software, default parameters were used.
Conjugation
Mating experiments were performed with the E. faecalis OG1RF and E. faecium 64/3 recipients according to the procedure developed for strains with a low transfer efficiency [37]. Transconjugants were selected on BHI agar with fusidic acid (25.0 mg/l) supplemented with vancomycin (VAN, 32.0 mg/l), linezolid (LZD, 4.0 and 6.0 mg/l), tigecycline (TGC, 2.0 mg/l) or tetracycline (TET, 16.0 mg/l). Selected transconjugants were confirmed by the PFGE analysis [38] and tested for the presence of vanA, vanB, aac(6′)-aph(2″), poxtA, tet(M), tet(L) and all nine rep genes present in 4995/20 using PCR. Representative transconjugants were then analyzed by establishing their susceptibility to vancomycin, teicoplanin, linezolid, chloramphenicol, tigecycline, tetracycline, streptomycin and gentamicin, and by WGS.
Accession Numbers
The assembled sequences of 4995/20 and its transconjugants have been deposited at DDBJ/ENA/GenBank in the BioProjects PRJNA766534 and PRJNA1002867, respectively.
Results
Antimicrobial Susceptibility of 4995/20, General Features of its Genome and Relationships with Other Isolates of E. faecium
The 4995/20 E. faecium isolate showed resistance or reduced susceptibility to almost all compounds tested (Table 1). The PCR analysis, a standard procedure for all VRE in the NRCST, demonstrated the presence of both vanA and vanB. Mixed assembly of Illumina and MinION reads yielded seven circular replicons, including a chromosome and six plasmids (Table 2); in addition, two putative free phages (40.0 kb and 36.2 kb) were observed (data not shown). Altogether, the genome contained 3145 genes with 2928 protein-coding sequences, 6 complete rRNAs operons, 69 tRNAs genes, 4 noncoding RNA (ncRNA) genes and 126 pseudogenes. In MLST, this isolate represented ST18 of the 17/18 lineage. ResFinder revealed the presence of the aac(6′)-aph(2″), ant(6)-Ia, aac(6′)-Ii, msr(C); tet(L), tet(M), poxtA, fexB; vanA and vanB resistance genes, in concordance with observed antimicrobial resistance phenotypes and the previous PCR results for vanA and vanB. The isolate also contained genes of virulence-associated factors such as collagen adhesin (acm), endocarditis antigen A (efaAEfm), enterococcal surface protein (espEfm) and hyaluronidase (hylEfm), as established by the VirulenceFinder analysis, and six plasmid replication genes, including rep2pRE25 (in two plasmids), rep14aAUS0085p5-like, rep29AUS0085p4, repUS1pVEF1, repUS12pUB110 and repUS15DO3, found using PlasmidFinder. Analyses with the Pathogen Detection system revealed that 4995/20 belonged to the PDS000100071 SNP group together with six isolates from Ireland, three isolates from Uganda and three isolates of an unreported origin (Supplementary Table 1). The isolates from Ireland and Uganda were obtained from human infections. All 12 isolates carried dfrG, absent from 4995/20, and they all had tet(M). In addition, the isolates from Uganda were also positive for tet(L). The isolates from Ireland and the isolates of an unknown origin had vanA; none of the PDS000100071.5 group except 4995/20 showed the presence of vanB, poxtA or fexB. The cgMLST revealed the closest linkage of 4995/20 with the EFM0469 isolate of unknown origin while isolates from Ireland and Uganda formed two separate, more distant clusters (Supplementary Fig. 1).
Table 1.
Antimicrobial susceptibility profile of the 4995/20 isolate of E. faecium
| Compound | MIC (mg/l) | EUCAST breakpoint (R) | ECOFFa/TECOFFb/PK/PDc | Interpretationd | Determinant |
|---|---|---|---|---|---|
| Vancomycin | > 256 | > 4 | R | vanA, vanB | |
| Teicoplanin | 256 | > 2 | R | vanA | |
| Dalbavancin | > 256 | n/a | > 0.25c | R | vanA |
| Oritavancin | 0.06 | n/a | > 0.06a,f | S | n/p |
| Telavancin | 2 | n/a | > 0.5a | R | vanA |
| Linezolid | 8 | > 4 | R | poxtA | |
| Tedizolid | 1.5 | n/a | > 1a | R | poxtA |
| Tigecycline | 2 | > 0.25 | R | [tet(M), tet(L)]e | |
| Tetracycline | 48 | n/a | > 4a | R | [tet(M), tet(L)]e |
| Doxycycline | 32 | n/a | > 0.5a | R | [tet(M), tet(L)]e |
| Minocycline | 2 | n/a | > 0.5b | R | [tet(M), tet(L)]e |
| Omadacycline | 0.5 | n/a | > 0.25a | R | [tet(M), tet(L)]e |
| Eravacycline | 1.5 | > 0.125 | R | [tet(M), tet(L)]e | |
| Penicillin | > 256 | n/a | > 16a | R | n/d |
| Ampicillin | > 256 | > 8 | R | n/d | |
| Imipenem | > 64 | > 4 | R | n/d | |
| Gentamicin | > 1024 | > 128 | HLGR | aac(6')-aph(2'') | |
| Streptomycin | 2048 | > 512 | HLSR | ant(6)-Ia, ant(6')-Ii | |
| Ciprofloxacin | > 256 | > 4 | R | S80I in ParC, S83I in GyrA | |
| Levofloxacin | > 256 | > 4 | R | S80I in ParC, S83I in GyrA | |
| Moxifloxacin | > 256 | n/a | > 1a | R | S80I in ParC, S83I in GyrA |
| Rifampicin | > 256 | n/a | > 8a,f | R | S491F in RpoB |
| Quinupristin/dalfopristin | 2 | > 4 | S | n/p | |
| Daptomycin | 4 | n/a | > 8a | S | See text |
| Chloramphenicol | 64 | n/a | > 32a | R | fexB |
| Erythromycin | 1.5 | n/a | > 4a | S | n/p |
| Fosfomycin | > 256 | n/a | > 128a,f | R | Unknown |
| Nitrofurantoin (100 μg disc) | 10 mm inhibition zone | < 15 mmf | < 15 mma,f | R | See text |
aECOFF, ecological cut-off (used when the EUCAST breakpoint was not available)
bTECOFF, tentative ECOFF
cPK/PD, pharmacokinetic/pharmacodynamic breakpoint; n/p, not applicable; n/a, not available; n/d, not determined; R, resistant; S, susceptible
dHLGR, high-level gentamicin resistance; HLSR, high-level streptomycin resistance
eIncreased copy number
fAvailable for E. faecalis only
Table 2.
Genome of 4995/20 isolate of E. faecium
| Contig | Size (bp) | Copy numbera | %GC | CDSsb | AMR gene(s)c | Virulence-associated genesd | Plasmid rep typee | ISsf |
|---|---|---|---|---|---|---|---|---|
| Chromosome | 2 726 121 | 1.0 | 38.3 | 2522 | aac(6′)-Ia, vanB operon, msr(C) |
acm efaAEfm espEfm |
n/a | n/d |
| Plasmids | ||||||||
| p4995_1 | 282 637 | 3.4 | 35.8 | 298 | ant(6’)-Ia, aac(6')-aph(2″) | hylEfm | repUS15DO3 | IS16 IS256 (3) IS1062 (2) IS1216 (2) IS1251 IS1252 (5) IS1297 IS1476 (3) IS1485 (2) ISEf1 (3) ISEfa4 (2) ISEfa5 (2) ISEfa7 ISEfa8 ISEfa11 (2) ISEfa13 ISfm1 ISRob1 (4) ISSpn10 (2) |
| p4995_2 | 42 382 | 10.5 | 34.7 | 52 | poxtA, fexB, tet(M), tet(L) | None |
rep2pRE25 rep29AUS0085p4 rep14aAUS0085p5 |
IS1062 IS1216 (7) |
| p4995_3 | 39 491 | 1.9 | 35.9 | 52 | vanA operon | None | rep2pRE25 repUS1pVEF1 repUS12pUB110 | IS1062 IS1216 (7) IS1251 IS1485 |
| p4995_4 | 11 625 | 11.9 | 32.6 | 13 | None | None | rep18ap200B | ISEfa4 |
| p4995_5 | 4 461 | 12.1 | 31.9 | 4 | None | None | Unknown (Rep_3) | None |
| p4995_6 | 2 056 | 13.4 | 37.7 | 4 | None | None | Unknown (Rep_2) | None |
n/a not applicable, n/d not determined
aBased on the sequencing depth
bCDSs, coding DNA sequences
cEstablished using ResFinder
dEstablished using VirulenceFinder
eEstablished using PlasmidFinder
fNumber of copies, if different from one, given within the brackets
Vancomycin Resistance, Tn1546 and Tn1549 Transposons and Their Localization
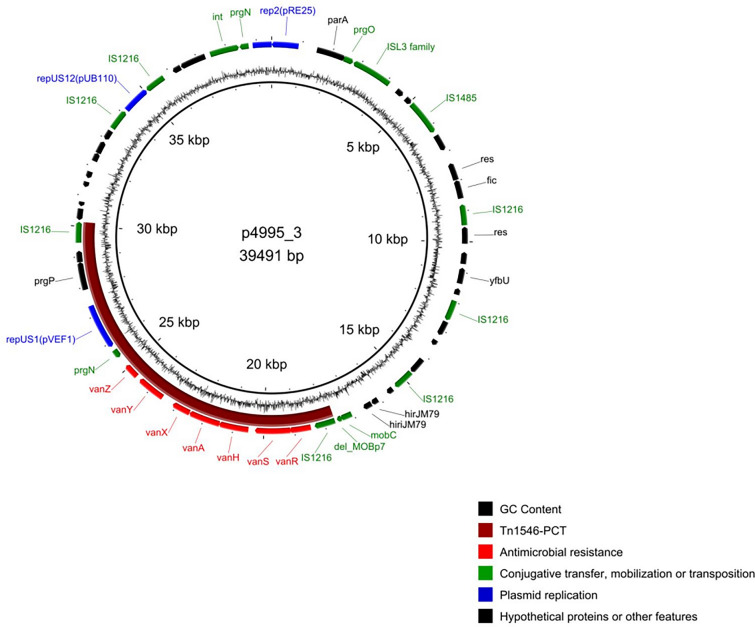
The vanA gene was located within the truncated Tn1546-type transposon of the B5-type [39] on the p4995_3 plasmid (Fig. 1). The 12.6-kb segment of p4995_3 plasmid, flanked by two direct IS1216 copies and encompassing the B5-Tn1546 with the downstream prgN, repUS1pVEF1 and parA genes, differed only by a single nucleotide substitution from the region present on the p1207_4 plasmid from the Polish E. faecalis clinical isolate 1207/04 [40]. This segment likely represents a composite transposon or even more likely a pseudo-compound transposon, PCT, typically associated with IS1216 in enterococci [41]. This 12.6-kb putative PCT remains unique for Polish enterococci (GenBank query on the 10th of August 2023). The carrier of this presumable PCT, the p4995_3 plasmid, was 39.5 kb in size and harbored 52 probable protein-coding genes in total, including three rep genes, rep2pRE25 (Inc18), repUS1pVEF1 (Inc18) and repUS12pUB110 (Rep1). The 5.2-kb region of p4995_3 encompassing prgP, rep2pRE25, prgN, IS1062 and a part of an ORF of unknown function was almost identical to its counterpart in the prototype plasmid pRE25 [42] (Supplementary Fig. 2), differing by the presence of five SNPs. No plasmids with a structure corresponding to p4995_3 were reported as yet.
Fig. 1.
The p4995_3 plasmid map. Inner circle, backbone of plasmid; outer circle, CDS with predicted functions marked with colors as in the legend; middle circle, CG skew. The putative PCT containing Tn1546 marked in purple
The vanB operon was located on the chromosome in the Tn1549-type transposon, 33,813 bp in size (Supplementary Fig. 3). Tn1549 in 4995/20 shared the structure with the original Tn1549 transposon [43] but demonstrated the presence of several substitutions and 1–2 bp indels in the vanB operon, resulting in 98.7% identity in this region. The transposon integration site was located in a counterpart of the M23 family-peptidase gene EFAU004_02748 present in the E. faecium Aus0004 genome [44]. The 6-bp coupling sequence 5’-ATTATG was present at the right transposon terminus. The same Tn1549 integration site was observed in two other E. faecium isolates, UW13781 and UW13763, from Germany (GCA_015365525.1 and GCA_015363845.1, respectively). These isolates represented ST564, a triple-locus variant of ST18, and belonged to a different SNP group (PDS000063135.1), thus being only distantly related to 4995/20.
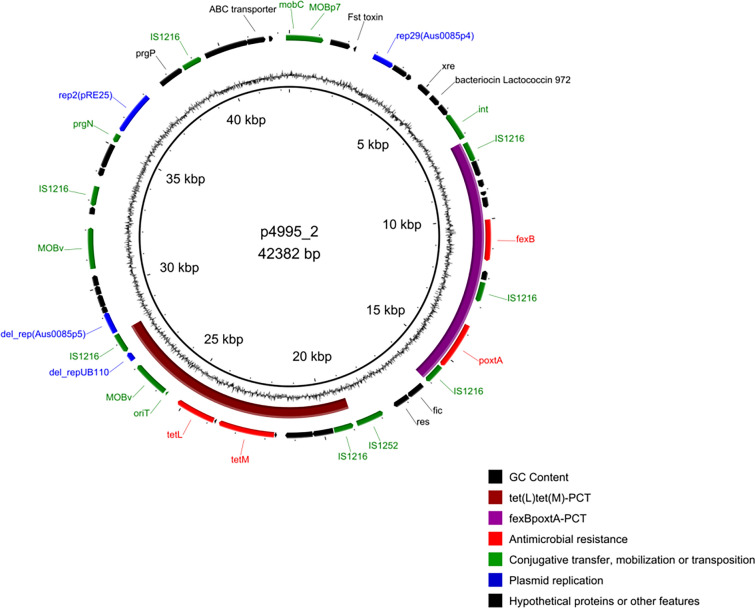
Linezolid and Tigecycline Resistance and the poxtA-fexB-tet(M)-tet(L) Plasmid
The LRE Finder analysis of 4995/20 sequencing reads yielded the wild-type genes of 23S rRNA and revealed the presence of poxtA_1 [45] on the p4995_2 plasmid. The comparison of ribosomal protein genes rplC, rplD and rplV with their counterparts in the DO strain of E. faecium (CP003583) revealed no mutations. The only detected linezolid resistance determinant, poxtA, was located between two direct copies of IS1216 (Fig. 2 and Supplementary Fig. 4). The fexB gene located upstream from the poxtA-PCT was responsible for the observed chloramphenicol resistance [46]. The whole 8.9-kb segment, consisting of poxtA, fexB and three copies of IS1216, formed a potential PCT. Very similar structures occurred also in plasmids of E. faecium (21.4–52.5 kb) and Enterococcus hirae (24.8–53.0 kb) and in chromosomes of E. faecalis and S. aureus from various sources and countries (Supplementary Table 2). These PCTs differed from 4995/20 and, among each other, by the length of sequences immediately adjacent to terminal inverted repeats of two of IS1216 elements as well as by the presence/absence of a 904-nt deletion affecting an ORF of unknown function, located in the fexB-IS1216 region (Supplementary Fig. 4).
Fig. 2.
The p4995_2 plasmid map. Inner circle, backbone of plasmid; outer circle, CDS with predicted functions marked with colors as in the legend; middle circle, CG skew. The putative PCTs containing the tet(L) and tet(M) and the fexB and poxtA genes marked in purple and brown, respectively
No mutations were observed in the rpsJ gene and 5′ untranslated region (UTR) of tet(M), described as determinants of tigecycline resistance in enterococci [18–20, 47]. The tet(M) and tet(L) tetracycline-resistance genes, together with the MOBV gene, were included in a 9.5-kb structure flanked by direct copies of IS1216, also a potential PCT (Fig. 2 and Supplementary Fig. 4), commonly found on enterococcal plasmids, 18.5–149.5 kb in size, reported to GenBank (Supplementary Table 3). The most similar PCT in the pAT456-d plasmid of E. faecium differed from its counterpart in 4995/20 by four nucleotide substitutions and a 3-nt indel in tet(M); PCTs in other enterococcal plasmids demonstrated differences in length of the sequences adjacent to terminal repeats of one of IS1216 (Supplementary Fig. 4), similarly to the situation described above for the fexB-poxtA-PCTs. Based on sequencing depth, the p4995_2 plasmid had approximately 10 copies/cell (Table 2), resulting in tigecycline resistance [20].
The p4995_2 plasmid (42.4 kb; Fig. 2), carrying poxtA, fexB, tet(M) and tet(L), had 52 probable protein-coding genes, including three rep genes, rep2pRE25 (Inc18), rep29Aus0085p4 (Rep_3) and a 5’-truncated rep14aAUS0085p5 (Rep_trans). p4995_2 also carried two mobilization genes belonging to MOBv family, with oriT sequence adjacent to one of these genes, and a single gene of MOBP7 family. Similarly to p4995_3, p4995_2 represented a unique mosaic structure, composed of segments separated by IS1216. The 5.8-kb part of p4995_2 surrounding the rep2pRE25 gene shared an extensive similarity with the 6.9-kb region in p4995_3, differing by the presence of IS1062 in p4995_3 (Supplementary Fig. 2). As described above, most of this region in p4995_3 was almost identical to the region prgP-rep2pRE25-prgN-IS1062 in pRE25.
Conjugative Transfer of Vancomycin, Linezolid and Tigecycline Resistance
Transconjugants were obtained only with the E. faecium 64/3 as a recipient and selection for TGC, TET or VAN; selection on LIN regardless of its concentration repeatedly yielded no transconjugants. Obtained transconjugants were characterized by PCR detection of vanA, vanB, poxtA, tet(M), tet(L) and aac(6′)-aph(2″) and all nine plasmid rep genes found in 4995/20 as well as by WGS and antimicrobial susceptibility testing for selected representatives (Table 3). Selection on VAN yielded transconjugants TC64_3 × 4995_20_VAN with an efficiency of 2.5 × 10–4, harboring genes characteristic for the p4995_3 plasmid, namely vanA, repUS1pVEF1, repUS12pUB110 and, with an exception of a single transconjugant, rep2pRE25. All transconjugants selected on TGC (TC64_3 × 4995_20_TGC; efficiency of 1.6 × 10–7) and on TET (TC64_3 × 4995_20_TET; efficiency of 1.5 × 10–4) carried the tet(M), tet(L) and vanA genes. Moreover, most of them (19 of 28) contained poxtA, and five showed the presence of aac(6′)-aph(2″). All TGC/TET transconjugants were positive for rep2pRE25 and repUS1pVEF1. All poxtA-positive transconjugants had rep29AUS0085p4, and 16 of them harbored repUS12pUB110. In addition, repUS15DO3 was detected in 15 transconjugants, all of which were positive for aac(6′)-aph(2″) in the original p4995_1 located 86.1 kb apart. All 42 analyzed VAN and TGC/TET transconjugants, except for two selected on VAN, harbored repp4995_5, and five transconjugants selected on TIG/TET contained rep18ap200B characteristic for p4995_4. The repp4995_6 gene was not detected in any transconjugant.
Table 3.
Antimicrobial resistance genes, plasmid rep genes, STs and antimicrobial resistance profiles of the transconjugants of the 4995/20 isolate
| Strain/isolate/transconjugants | aac(6')-aph(2'') | ant(6)-Ia | aac(6')-Ii | msr(C) | fexB | poxtA | tet(M) | tet(L) | vanA | vanB |
|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial resistance genesA | ||||||||||
| 4995/20 (localization)^ | + (p1) | + (p1) | + (ch) | + (ch) | + (p2) | + (p2) | + (p2) | + (p2) | + (p3) | + (ch) |
| 64/3 (CP012522.1)^ | − | − | + | + | − | − | − | − | − | − |
| TC64_3 × 4995_20_VAN (14) | − | ND | ND | ND | ND | − | − | − | 14 | − |
| TC64_3 × 4995_20_V3 | − | − | + | + | − | − | − | − | + | − |
| TC64_3 × 4995_20_TIG (4) | − | ND | ND | ND | ND | 4 | 4 | 4 | 4 | − |
| TC64_3 × 4995_20_LTV6 | − | − | + | + | + | + | + | + | + | − |
| TC64_3 × 4995_20_LTV7 | − | − | + | + | + | + | + | + | + | − |
| TC64_3 × 4995_20_TET (24) | 5 | ND | ND | ND | ND | 15 | 24 | 24 | 24 | − |
| TC64_3 × 4995_20_TV8 (localization)^ | − | − |
+ (ch) |
+ (ch) |
− | − |
+ (p1) |
+ (p1) |
+ (p1) |
− |
| Strain/isolate/transconjugants | RepA_N | Inc18 | Rep_3 | Rep1 | Rep_2 | Rep_trans | |||
|---|---|---|---|---|---|---|---|---|---|
| repUS15DO3 | rep2pRE25 | repUS1pVEF1 | rep18ap200B | rep29AUS0085p4 | repp4995_5 | repUS12pUB110 | repp4995_6 | rep14aAUS0085p5 | |
| Plasmid rep genesB | |||||||||
| 4995/20 (localization)^ | + (p1) | + (p2, p3) | + (p3) | + (p4) | + (p2) | + (p5) | + (p3) | + (p6) | + (p2) |
| 64/3 (CP012522.1)^ | − | − | − | − | − | − | − | − | − |
| TC64_3 × 4995_20_VAN (14) | − | 13 | 14 | − | − | 12 | 14 | − | 1 |
| TC64_3 × 4995_20_V3 | − | + | + | − | − | + | + | − | + |
| TC64_3 × 4995_20_TIG (4) | − | 4 | 4 | 3 | 4 | 4 | 1 | − | 2 |
| TC64_3 × 4995_20_LTV6 | − | + | + | − | + | + | − | − | − |
| TC64_3 × 4995_20_LTV7 | − | + | + | + | + | + | − | − | − |
| TC64_3 × 4995_20_TET (24) | 15 | 24 | 24 | 2 | 15 | 24 | 15 | − | 22 |
| TC64_3 × 4995_20_TV8 (localization)^ | − |
+ (p1) |
+ (p1) |
− | − |
+ (p2) |
− | − |
+ (p1) |
| Strain/isolate | ST | Streptomycin | Gentamicin | Chloramphenicol | Linezolid | Tigecycline | Tetracycline | Vancomycin | Teicoplanin |
|---|---|---|---|---|---|---|---|---|---|
| STs and MIC values (mg/l) for selected transconjugants submitted for WGSC | |||||||||
| 4995/20 | 18 | 2048 (HLSR) | > 1024 (HLGR) | 64 (R) | 8 (R) | 2 (R) | 48 (R) | > 256 (R) | 256 (R) |
| 64/3 | 21 | 96 (S) | ≤ 8 (S) | 8 (S) | 4 (S) | 0.023 (S) | ≤ 1 (S) | ≤ 1 (S) | 0,5 (S) |
| TC64_3 × 4995_20_V3 | 21 | 32 (S) | 4 (S) | 8 (S) | 4 (S) | 0.03 (S) | 0.25 (S) | 256 (R) | 32 (R) |
| TC64_3 × 4995_20_LTV6 | 21 | 32 (S) | 2 (S) | 32 (S) | 8 (R) | 8 (R) | 64 (R) | 32 (R) | 16 (R) |
| TC64_3 × 4995_20_LTV7 | 21 | 32 (S) | 2 (S) | 32 (S) | 8 (R) | 8 (R) | 64 (R) | 32 (R) | 32 (R) |
| TC64_3 × 4995_20_TV8^ | 21 | 32 (S) | 8 (S) | 8 (S) | 4 (S) | 2 (R) | 64 (R) | 256 (R) | 16 (R) |
A+ positive; −, negative; ch, chromosome; p1, p4995_1; p2, p4995_2; p3, p4995_3; ^, the complete genome sequence available; TC_TIG, TC_TET and TC_VAN, transconjugants obtained with TIG, TET and VAN selection, respectively (the number of representatives within brackets); ND, not determined
B+, positive; −, negative; p1, p4995_1; p2, p4995_2; p3, p4995_3; p4, p4995_4; p5, p4995_5; p6, p4995_6; ^, the complete genome sequence available; TC_TIG, TC_TET and TC_VAN, transconjugants obtained with TIG, TET and VAN selection, respectively (the number of representatives within brackets)
CInterpretation provided within the brackets. HLSR, high-level streptomycin resistance; HLGR, high-level gentamicin resistance; R, resistant; S, susceptible; ^, complete genome sequence available
The results of WGS analysis of a representative transconjugant TC64_3 × 4995_20_V3 selected on VAN were consistent with an acquisition of p4995_3 and p4995_5. This transconjugant demonstrated vancomycin and teicoplanin resistance but remained susceptible to tetracycline, tigecycline and linezolid. The WGS and antimicrobial susceptibility testing of two representative TGC transconjugants (TC64_3 × 4995_20_LTV6 and TC64_3 × 4995_20_LTV7) indicated an acquisition of both p4995_2 and p4995_3. A complete genomic sequence of the TC64_3 × 4995_20_TV8 transconjugant, which was selected on TET and carried tet(M), tet(L) and vanA but lacked poxtA, revealed a 50.2-kb recombinant plasmid, harboring all three resistance genes as well as rep2pRE25, repUS1pVEF1 and rep14aAUS0085p5. This plasmid was composed of parts of p4995_2 and p4995_3 (Supplementary Fig. 6). This isolate also contained the intact p4995_5.
Decreased Susceptibility to Daptomycin
Susceptibility testing of 4995/20 resulted in MIC of 4 mg/l for daptomycin, which represented a relatively high value, considering that the EUCAST epidemiological cut-off value for E. faecium equals 8 mg/l. The genome of 4995/20 was searched for known and potentially novel resistance mutations in genes of 43 proteins associated with daptomycin resistance in E. faecium [48]. Two amino acid substitutions were detected, including the T120A substitution in LiaS, a putative histidine kinase of the LiaFSR regulatory system, and the S340L substitution in SulP, a putative sulfate transporter. Moreover, the isolate had the S491F mutation in RpoB (see also below) and the T641K mutation in RpoC, also proposed to be involved in daptomycin resistance [49].
Resistance to Other Antimicrobials
The isolate demonstrated resistance to penicillin, ampicillin and fluoroquinolones (Table 1), a phenotype typical for hospital E. faecium, and carried aac(6')-aph(2''), ant(6)-Ia and ant(6')-Ii, responsible for high-level resistance to aminoglycosides. Analysis of rpoB in the search for rifampicin resistance mutations revealed the S491F change in the deduced amino acid RpoB sequence. The same mutation was seen in rifampicin-resistant isolates of E. faecium in Australia and New Zealand [49]. The studied isolate was also resistant to fosfomycin but lacked any fos genes, including fosB3 and fosX, described as fosfomycin resistance determinants in enterococci [50, 51], and had the wild-type murA gene, whose mutation resulted in fosfomycin resistance in E. faecium [52]. Analysis of two nitroreductase genes present in the 4995/20 genome, the counterparts of the RS06170 and RS12585 loci in the DO genome, revealed mutations Q48K and T207M in the deduced amino acid sequence of the RS12585 counterpart as potential determinants of nitrofurantoin resistance [53], specific for 4995/20.
Other Plasmids
Besides two resistance plasmids p4995_2 and p4995_3 described above, four other plasmids were detected in 4995/20 (Table 2). The p4995_1 plasmid was a 282.6-kb megaplasmid carrying repUS15DO3 (RepA_N) typical for pDO3 and pLG1 [54, 55]. p4995_1 also had mobilization gene mobL [56] belonging to the MOBP2 family and genes of type IV coupling proteins (T4CP). The p4995_1 plasmid also harbored hylEfm, aminoglycoside resistance determinants aac(6′)-aph(2″) and ant(6)-Ia, regions encoding heavy-metal resistance and carbohydrate metabolism enzymes, as well as genes of four plasmid addiction systems (Phd/Doc, MazE/F, PemK/PemI and RelB/RelE). The structure of p4995_1 was unique but this plasmid shared the 149.6-kb part, located from 14.3 to 163.9 kb, with the 215.9-kb plasmid unnamed1 from the E. faecium VRE3382 strain isolated in Australia (CP065529.1). The 11.6-kb plasmid p4995_4 represented the rep18ap200B (Rep3_theta) replicon. Nearly identical plasmids (99.6–99.9% identity) were recently detected in E. faecium (Supplementary Table 4). Two remaining small plasmids, p4995_5 and p4995_6, had rep genes unclassified so far, and both of these genes exhibited high similarity (> 99%) to rep characteristic for several plasmids from E. faecium in GenBank (Supplementary Tables 5 and 6, respectively). Moreover, repp4995_6 was also observed in Poland in E. faecalis [27].
Discussion
The isolate of E. faecium analyzed in the current study demonstrated resistance to several drugs used in anti-enterococcal therapy, including (amino)penicillins, imipenem, aminoglycosides (high level), almost all glycopeptides, fluoroquinolones and other compounds as well as “last-resort” drugs linezolid and tigecycline. This isolate also had reduced susceptibility to daptomycin and erythromycin. Thus, therapeutic options were limited to oritavancin, a glycopeptide not affected by the presence of vanA and vanB [57], but available on the market in Poland since May 2022, and quinupristin/dalfopristin, which, because of severe side effects, is not used for treatment. The patient, hospitalized for an extensive period of time, received several various antimicrobials, including linezolid and tigecycline, prior to isolation of 4995/20. Isolations of linezolid- and tigecycline-resistant enterococci in several cases followed the therapy with these drugs [58–61]. Moreover, the use of cephalosporins and carbapenems, which were also received by the patient, represents a significant risk factor for colonization and infection of hospital patients by VRE [62–66]. The genome size of 4995/20 isolate, high load of various antimicrobial resistance genes and presence of several plasmids, including a megaplasmid, are typical features of hospital E. faecium distributed worldwide [67]. The isolate, as deduced from genomic data, represented ST18. Enterococci of this ST showed a wide distribution and were observed in 22 countries over a 30-year period (https://pubmlst.org/organisms/enterococcus-faecium; date last accessed 26 September 2022).
The vanA and vanB gene clusters are the most common vancomycin resistance determinants among clinical isolates of E. faecium. Both genotypes were reported for Polish VRE [39, 67]. Plasmid localization of vanA and chromosomal localization of vanB are typical for these determinants [10, 68]. Although E. faecium carrying concomitantly vanA and vanB was observed already in 1993 in the UK [69], such strains are still reported very rarely, either as single isolates or in small clusters in various countries, including Finland, France, Greece, Saudi Arabia, Vietnam and Australia, and in different STs, such as 17, 117 and 796 [70–76], all belonging to the hospital meroclone of E. faecium. The vanA gene in 4995/20 was located in B5-type Tn1546, commonly observed in both E. faecium and E. faecalis VanA isolates in Poland. This variant of transposon lacks ORF1 and ORF2, which are replaced by IS1216. Moreover, the B5-type harbors a characteristic single-nucleotide deletion at the 9064-bp position, resulting in a frameshift in vanY and truncated VanY [27, 39, 40]. Tn1546 in 4995/20 was located within a potential 12.6-kb PCT, almost identical to the one present in previously characterized E. faecalis [40], in agreement with proposed genetic exchange of vanA among hospital strains of these two species [27]. The acquisition of vanA by E. faecium results in resistance to all glycopeptides except oritavancin [57] as indeed observed for 4995/20. The vanB gene was associated with Tn1549, the most typical carrier of this determinant [68]. The localization site of Tn1549 constitutes an important feature of a particular disseminating vanB clone [77, 78]. The observed Tn1549 integration site in 4995/20 indicated that this isolate did not belong to any previously characterized Polish clones [77]. Instead, the identical localization of Tn1549 was observed in two unrelated isolates from Germany, consistent with an acquisition of the vanB determinant by a chromosomal recombination event [79].
Linezolid-resistant enterococci, mostly E. faecium, were observed in Poland previously, demonstrating the presence of such determinants as mutations in the 23S rRNA genes and plasmid-borne optrA [80]. The poxtA gene was observed for the first time in clinical settings in Poland in the current study; however, it represented the most frequent linezolid resistance determinant in enterococci from food of animal origin in our country, constituting a potential source of such genes for clinical strains [81]. The poxtA gene was first described in E. faecalis and S. aureus [45], and this gene was reported then in clinical isolates of E. faecium in Portugal, Germany, France, Switzerland and Pakistan [59, 82–85].
Tigecycline resistance remains sporadic among E. faecium, and so far invasive enterococci in Polish hospitals have showed full susceptibility to this drug [86]. The 4995/20 isolate lacked typical tigecycline resistance determinants in enterococci such as mutations in the rpsJ gene or deletions in the 5′UTR of tet(M), resulting in higher expression of tet(M) and tet(L), located downstream [18–20, 47]. Observed resistance was most likely caused by an increased copy number of plasmid harboring these two genes, reaching approximately 10 copies per cell. Such a mechanism was proposed previously [20]. Moreover, our study demonstrated that the conjugative transfer of tet(M)/tet(L)-plasmid yielded transconjugants resistant to tigecycline.
The vanA, fexB-poxtA and tet(M)-tet(L) resistance genes were located in structures corresponding to potential PCTs [41]. Although structures very similar to both fexB-poxtA and tet(M)-tet(L) PCTs of the 4995/20 isolate were observed in several other isolates of enterococci and S. aureus, the differences in sequence length immediately adjacent to the IS1216 termini (Supplementary Figs. 3 and 4) would be consistent with an independent formation of such PCTs rather than with a single origin of these structures.
Vancomycin, linezolid and tigecycline resistance was transferable from 4995/20 to a susceptible E. faecium by conjugation. Both plasmids carrying the respective resistance determinants were devoid of their own conjugation genes, and only p4995_3 carried mobilization genes and oriT. Transfer of such plasmids requires the presence of a helper plasmid and is frequently accompanied by such events as plasmid recombination, plasmid fusion and transfer of PCT [87, 88]. In the case of 4995/20, the p4995_1 megaplasmid might provide transfer functions for other co-resident plasmids; genes specific for p4995_1, such as aac(6′)-aph(2'') and repUS15DO3 were indeed detected in some transconjugants but only in these selected with TET. All tested transconjugants selected with VAN lacked poxtA and the tet genes; however, all transconjugants selected on TET or TGC also harbored vanA despite the lack of VAN selection. In addition, only some transconjugants selected on TET or TGC had poxtA, residing on the same plasmid as the tet genes. Obtaining a complete sequence of one of the tranconjugants positive for vanA, tet(M) and tet(L) and negative for poxtA revealed that a 50.2-kb plasmid in one of transconjugants was composed of parts of p4995_2 and p4995_3. Regions shared by these two plasmids, namely the approximately 3.5-kb part with rep2 and one of IS1216, might have facilitated formation of a fusion plasmid via recombination. However, further investigations, involving establishing the complete sequences of plasmids in other transconjugants would be required to follow events during the transfer of resistance plasmids from 4995/20.
The isolate showed a borderline MIC value for daptomycin (4 mg/l), and it might be in fact clinically non-susceptible to daptomycin. A failure of high-dose daptomycin patient treatment against E. faecium with the MIC equal to 3 mg/l was described [89]. Recently, an association of daptomycin resistance with the vanA genotype was noticed among E. faecium isolates from Australia and New Zealand [49]. Mutations in LiaFSR, a regulatory system involved in cell envelope homeostasis, are the most common changes in daptomycin-resistant E. faecium [48, 90]; however, the T120A substitution in LiaS present in the 4995/20 isolate was also commonly found in unrelated E. faecium isolates with low daptomycin MIC values [48] and may probably occur even without daptomycin selective pressure [91]. Several substitutions in SulP were observed in E. faecium isolates with high daptomycin MICs [48, 90]. The S340L substitution in SulP in 4995/20, located three amino acids before the beginning of the eighth predicted transmembrane domain, is a novel mutation as yet with no obvious association with daptomycin resistance. Mutations in RpoB, RpoC and DltC were proposed as novel determinants of daptomycin resistance among vanA-E. faecium from Australia and New Zealand [49], and an independent appearance of the same changes in RpoB and RpoC in 4995/20 strongly supports the potential role of these determinants. Moreover, a replacement of the wild-type rpoB by its mutated variant in S. aureus leads to heteroresistance to daptomycin and vancomycin mediated by an increased expression of the dlt operon and thickening of the cell wall [92]. Thus, selection of resistant rpoB mutants by rifampicin might contribute to reducing susceptibility to daptomycin in E. faecium.
Our study had certain limitations. No other E. faecium isolates from the same center and the same period were obtained; thus, it was not possible to find a potential direct ancestor, retaining susceptibility to some of the antimicrobial compounds, which by an acquisition of resistance yielded the 4995/20 strain. This study also raises further questions about the events occurring during the conjugal transfer of enterococcal plasmids. This issue was, however, beyond the scope of the current work.
Conclusions
The 4995/20 isolate of E. faecium is, to our knowledge, the first reported clinical representative of this species combining resistance or decreased susceptibility to such wide range antimicrobial compounds, including drugs crucial for successful therapy of infections caused by E. faecium. Our genomic analyses demonstrated the presence of several resistance determinants responsible for this phenotype in the genetic background of a strain belonging to a hospital-adapted meroclone of E. faecium. The acquisition of resistance was associated with mutations of chromosomal genes, plasmids and transposons harboring resistance genes, increased copy number of resistance genes and yet unknown determinants. Moreover, further concomitant dissemination of vancomycin-, tigecycline- and linezolid resistance is possible because of a localization of the respective determinants on mobilizable plasmids of mosaic structure within potential PCTs. The appearance of such a strain with extremely limited treatment options in the hospital settings is a particularly worrisome phenomenon.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplementary Fig. 1. The cgMLST analysis of relationships of the 4995/20 isolate and other isolates reported to GenBank belonging to the PDS000100071 SNP group. Black circles, isolates (names provided within a circle); lines, links among isolates with the length of a line proportional to a number of differences in allelic profiles; the clusters of isolates from Uganda and Ireland marked with dashed-line blue circles; the 4995/20 isolate marked with a dashed-line red circle. The AUS0004 genome (CP003351.1) was used as an outgroup in analysis. The scale bar 50 corresponds to 50 differences between two allelic profiles. Supplementary Fig. 2. Comparison of the rep2 region in the p4995_2 and p4995_3 plasmids using ACT. The extent of homology between p4995_3 and pRE25 (X92945) indicated with a double-headed arrow below the . p4995_3 sequence; black arrow, the rep2 gene; red arrow, IS1216; empty red arrow, IS1062; gray arrows, other genes. Supplementary Fig. 3. The structure of Tn1549 and its integration site in the 4995/20. Green arrows, the vanB operon genes; red arrow, the int integrase gene; blue arrows, two parts of a counterpart of the EFAU004_02748 gene; gray arrows, other genes of Tn1549; black triangle, the coupling sequence (drawn not to scale). Supplementary Fig. 4. The structure of poxtA-PCT in 4995/20 and its variants reported to GenBank. Green arrows, the poxtA and fexB genes; red arrows, IS1216; gray arrows, other genes; insertions/deletions differing the poxtA-PCT in 4995/20 from other PCTs indicated by empty triangles. Supplementary Fig. 5. The structure of tet(M)-tet(L)-PCT in 4995/20 and its variants reported to GenBank. Blue arrows, the tet(M) and tet(L) genes; red arrows, IS1216; gray arrows, other genes; insertions/deletions differing the tet(M)-tet(L)-PCT in 4995/20 from other PCTs indicated by empty triangles. Supplementary Fig. 6. The structure of the p4995TC_TV8_1 recombinant plasmid. The extent of tet(M)-tet(L)-PCT, vanA-PCT and p4995_2 and p4995_3 plasmids indicated by yellow arrows; regions of presumable recombination indicated by empty double-headed arrows; empty gray arrows, rep genes; red arrows, IS1216; gray arrows, other genes (PDF 345 KB)
Author Contribution
Ewa Wardal was involved in the conception of the study, designed the experiments, performed the experiments, analyzed and interpreted the data, and drafted the manuscript; Dorota Żabicka was involved in the conception of the study, designed the experiments, analyzed and interpreted the data; Tomasz Skalski obtained the studied isolate, performed its initial characterization, collected the relevant epidemiological data and analyzed and interpreted the data; Joanna Kubiak-Pulkowska obtained the studied isolate, performed its initial characterization, collected the relevant epidemiological data, and analyzed and interpreted the data; Waleria Hryniewicz was involved in the conception of the study and analyzed and interpreted the data; Ewa Sadowy was involved in the conception of the study, designed the experiments, performed the experiments, analyzed and interpreted the data, and drafted the manuscript. All authors critically revised and approved the final manuscript.
Funding
This study was supported by the statutory funding from the Ministry of Education and Science, Warsaw, Poland. Isolate collection and maintenance were supported by the grant Narodowy Program Ochrony Antybiotyków (NPOA) from the Ministry of Health, Warsaw, Poland, and by the grant SPUB MIKROBANK from the Ministry of Education and Science. The funders had no role in study design, data collection and interpretation or the decision to submit the work for publication. No funding or sponsorship was received for the publication of this article.
Data Availability
The datasets generated during the current study are available in the GenBank repository in the BioProjects PRJNA1002867 and PRJNA1002867.
Declarations
Conflict of interest
Ewa Wardal, Dorota Żabicka, Tomasz Skalski, Joanna Kubiak-Pulkowska, Waleria Hryniewicz and Ewa Sadowy have no relevant financial or non-financial interests to disclose.
Ethical approval
The isolate used in the current study was obtained during a routine national surveillance activity of the National Reference Centre for Susceptibility Testing, under the mandate of the Ministry of Health. The study was performed in a retrospective manner with an anonymization of the patient’s data; thus, ethical approval and informed consent were not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leavis HL, Bonten MJM, Willems RJL. Identification of high-risk enterococcal clonal complexes: global dispersion and antibiotic resistance. Curr Opin Microbiol. 2006;9:454–460. doi: 10.1016/j.mib.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Garbajosa P, Bonten MJM, Robinson DA, Top J, Nallapareddy SR, Torres C, et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol. 2006;44:2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willems RJL, Top J, van Schaik W, Leavis H, Bonten M, Sirén J, et al. Restricted gene flow among hospital subpopulations of Enterococcus faecium. mBio. 2012;3:e00151–00112. [DOI] [PMC free article] [PubMed]
- 5.Top J, Willems R, Bonten M. Emergence of CC17 Enterococcus faecium: from commensal to hospital-adapted pathogen. FEMS Immunol Med Microbiol. 2008;52:297–308. doi: 10.1111/j.1574-695X.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 6.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 7.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 8.Bender JK, Cattoir V, Hegstad K, Sadowy E, Coque TM, Westh H, et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: towards a common nomenclature. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother. 2018;40:25–39. doi: 10.1016/j.drup.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Kristich CJ, Rice LB, Arias CA. Enterococcal infection—treatment and antibiotic resistance. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci commensals lead causes drug resist infect [Internet]. Boston: Massachusetts Eye and Ear Infirmary; 2014 [cited 2022 Dec 2]. http://www.ncbi.nlm.nih.gov/books/NBK190420/.
- 10.Freitas AR, Novais C, Tedim AP, Francia MV, Baquero F, Peixe L, et al. Microevolutionary events involving narrow host plasmids influences local fixation of vancomycin-resistance in Enterococcus populations. PLoS ONE. 2013;8:e60589. doi: 10.1371/journal.pone.0060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sletvold H, Johnsen PJ, Wikmark O-G, Simonsen GS, Sundsfjord A, Nielsen KM. Tn1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. J Antimicrob Chemother. 2010;65:1894–1906. doi: 10.1093/jac/dkq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjord A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin Microbiol Infect. 2010;16:541–554. doi: 10.1111/j.1469-0691.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- 13.Werner G, Coque TM, Franz CMAP, Grohmann E, Hegstad K, Jensen L, et al. Antibiotic resistant enterococci—tales of a drug resistance gene trafficker. Int J Med Microbiol. 2013;303:360–379. doi: 10.1016/j.ijmm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Sadowy E. Linezolid resistance genes and genetic elements enhancing their dissemination in enterococci and streptococci. Plasmid. 2018;99:89–98. doi: 10.1016/j.plasmid.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Turner AM, Lee JYH, Gorrie CL, Howden BP, Carter GP. Genomic insights into last-line antimicrobial resistance in multidrug-resistant Staphylococcus and vancomycin-resistant Enterococcus. Front Microbiol. 2021;12:637656. doi: 10.3389/fmicb.2021.637656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenciani A, Morroni G, Schwarz S, Giovanetti E. Oxazolidinones: mechanisms of resistance and mobile genetic elements involved. J Antimicrob Chemother. 2022;77:2596–2621. doi: 10.1093/jac/dkac263. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz S, Zhang W, Du X-D, Krüger H, Feßler AT, Ma S, et al. Mobile oxazolidinone resistance genes in Gram-positive and Gram-negative bacteria. Clin Microbiol Rev. 2021;34:e0018820. doi: 10.1128/CMR.00188-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattoir V, Isnard C, Cosquer T, Odhiambo A, Bucquet F, Guérin F, et al. Genomic analysis of reduced susceptibility to tigecycline in Enterococcus faecium. Antimicrob Agents Chemother. 2015;59:239–244. doi: 10.1128/AAC.04174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beabout K, Hammerstrom TG, Wang TT, Bhatty M, Christie PJ, Saxer G, et al. Rampant parasexuality evolves in a hospital pathogen during antibiotic selection. Mol Biol Evol. 2015;32:2585–2597. doi: 10.1093/molbev/msv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiedler S, Bender JK, Klare I, Halbedel S, Grohmann E, Szewzyk U, et al. Tigecycline resistance in clinical isolates of Enterococcus faecium is mediated by an upregulation of plasmid-encoded tetracycline determinants tet(L) and tet(M) J Antimicrob Chemother. 2016;71:871–881. doi: 10.1093/jac/dkv420. [DOI] [PubMed] [Google Scholar]
- 21.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0 [Internet]. The European Committee on Antimicrobial Susceptibility Testing.; 2022. http://www.eucast.org.
- 22.Clark NC, Cooksey RC, Hill BC, Swenson JM, Tenover FC. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993;37:2311–7. [DOI] [PMC free article] [PubMed]
- 23.Dahl KH, Simonsen GS, Olsvik O, Sundsfjord A. Heterogeneity in the vanB gene cluster of genomically diverse clinical strains of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1999;43:1105–1110. doi: 10.1128/AAC.43.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosecka-Strojek M, Sadowy E, Gawryszewska I, Klepacka J, Tomasik T, Michalik M, et al. Emergence of linezolid-resistant Staphylococcus epidermidis in the tertiary children’s hospital in Cracow, Poland. Eur J Clin Microbiol Infect Dis. 2020;39:1717–1725. doi: 10.1007/s10096-020-03893-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty N, Trzcinski K, Pickerill P, Zawadzki P, Dowson CG. Genetic diversity of the tet (M) gene in tetracycline-resistant clonal lineages of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:2979–2984. doi: 10.1128/AAC.44.11.2979-2984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen LB, Garcia-Migura L, Valenzuela AJS, Løhr M, Hasman H, Aarestrup FM. A classification system for plasmids from enterococci and other Gram-positive bacteria. J Microbiol Methods. 2010;80:25–43. doi: 10.1016/j.mimet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Wardal E, Żabicka D, Hryniewicz W, Sadowy E. VanA- Enterococcus faecalis in Poland: hospital population clonal structure and vanA mobilome. Eur J Clin Microbiol Infect Dis. 2022;41:1245–1261. doi: 10.1007/s10096-022-04479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17:10–2.
- 29.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinforma Oxf Engl. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 31.Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:124. [DOI] [PMC free article] [PubMed]
- 32.Li X, Xie Y, Liu M, Tai C, Sun J, Deng Z, et al. oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 2018;46:W229–W234. doi: 10.1093/nar/gky352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcillán-Barcia MP, Redondo-Salvo S, Vielva L, de la Cruz F. MOBscan: automated annotation of MOB relaxases. Methods Mol Biol Clifton NJ. 2020;2075:295–308. doi: 10.1007/978-1-4939-9877-7_21. [DOI] [PubMed] [Google Scholar]
- 34.Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genom. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J. ACT: the Artemis Comparison Tool. Bioinformatics. 2005;21:3422–3423. doi: 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z, Alikhan NF, Sergeant MJ, Luhmann N, Vaz C, Francisco AP, Carriço JA, Achtman M. GrapeTree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018;28:1395–1404. doi: 10.1101/gr.232397.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manson JM, Hancock LE, Gilmore MS. Mechanism of chromosomal transfer of Enterococcus faecalis pathogenicity island, capsule, antimicrobial resistance, and other traits. Proc Natl Acad Sci USA. 2010;107:12269–12274. doi: 10.1073/pnas.1000139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lencastre H, Severina EP, Roberts RB, Kreiswirth BN, Tomasz A. Testing the efficacy of a molecular surveillance network: methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) genotypes in six hospitals in the metropolitan New York City area. The BARG Initiative Pilot Study Group. Bacterial Antibiotic Resistance Group. Microb Drug Resist Larchmt N. 1996;2:343–51. [DOI] [PubMed]
- 39.Wardal E, Kuch A, Gawryszewska I, Żabicka D, Hryniewicz W, Sadowy E. Diversity of plasmids and Tn1546-type transposons among VanA Enterococcus faecium in Poland. Eur J Clin Microbiol Infect Dis. 2017;36:313–328. doi: 10.1007/s10096-016-2804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wardal E, Sadowy E. Complete genome sequence of a Polish Enterococcus faecalis vanA-positive hospital isolate. Microbiol Resour Announc. 2021;10:e0066821. doi: 10.1128/MRA.00668-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harmer CJ, Pong CH, Hall RM. Structures bounded by directly-oriented members of the IS26 family are pseudo-compound transposons. Plasmid. 2020;111:102530. doi: 10.1016/j.plasmid.2020.102530. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz FV, Perreten V, Teuber M. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid. 2001;46:170–187. doi: 10.1006/plas.2001.1544. [DOI] [PubMed] [Google Scholar]
- 43.Garnier F, Taourit S, Glaser P, Courvalin P, Galimand M. Characterization of transposon Tn1549, conferring VanB-type resistance in Enterococcus spp. Microbiol Read Engl. 2000;146(Pt 6):1481–1489. doi: 10.1099/00221287-146-6-1481. [DOI] [PubMed] [Google Scholar]
- 44.Lam MMC, Seemann T, Bulach DM, Gladman SL, Chen H, Haring V, et al. Comparative analysis of the first complete Enterococcus faecium genome. J Bacteriol. 2012;194:2334–2341. doi: 10.1128/JB.00259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonelli A, D’Andrea MM, Brenciani A, Galeotti CL, Morroni G, Pollini S, et al. Characterization of poxtA, a novel phenicol-oxazolidinone-tetracycline resistance gene from an MRSA of clinical origin. J Antimicrob Chemother. 2018;73:1763–1769. doi: 10.1093/jac/dky088. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Wang Y, Wu C, Schwarz S, Shen Z, Jeon B, et al. A novel phenicol exporter gene, fexB , found in enterococci of animal origin. J Antimicrob Chemother. 2012;67:322–325. doi: 10.1093/jac/dkr481. [DOI] [PubMed] [Google Scholar]
- 47.Niebel M, Quick J, Prieto AMG, Hill RLR, Pike R, Huber D, et al. Deletions in a ribosomal protein-coding gene are associated with tigecycline resistance in Enterococcus faecium. Int J Antimicrob Agents. 2015;46:572–575. doi: 10.1016/j.ijantimicag.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Diaz L, Tran TT, Munita JM, Miller WR, Rincon S, Carvajal LP, et al. Whole-genome analyses of Enterococcus faecium isolates with diverse daptomycin MICs. Antimicrob Agents Chemother. 2014;58:4527–4534. doi: 10.1128/AAC.02686-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Higgs C, Turner AM, Nong Y, Gorrie CL, Sherry NL, et al. Daptomycin resistance occurs predominantly in vanA-type vancomycin-resistant Enterococcus faecium in Australasia and is associated with heterogeneous and novel mutations. Front Microbiol. 2021;12:749935. doi: 10.3389/fmicb.2021.749935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X, Chen C, Lin D, Guo Q, Hu F, Zhu D, et al. The fosfomycin resistance gene fosB3 is located on a transferable, extrachromosomal circular intermediate in clinical Enterococcus faecium isolates. PLoS ONE. 2013;8:e78106. doi: 10.1371/journal.pone.0078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xin L, Xu X, Shi Q, Han R, Wang J, Guo Y, et al. High prevalence and overexpression of fosfomycin-resistant gene fosX in Enterococcus faecium from China. Front Microbiol. 2022;13:900185. doi: 10.3389/fmicb.2022.900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xin L, Hu Z, Han R, Xu X, Wang C, Li D, et al. Asp50Glu mutation in MurA results in fosfomycin resistance in Enterococcus faecium. J Glob Antimicrob Resist. 2022;30:50–55. doi: 10.1016/j.jgar.2022.05.026. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Wang L, Zhou C, Lin Y, Liu S, Zeng W, et al. Unraveling mechanisms and epidemic characteristics of nitrofurantoin resistance in uropathogenic Enterococcus faecium clinical isolates. Infect Drug Resist. 2021;14:1601–1611. doi: 10.2147/IDR.S301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin X, Galloway-Peña JR, Sillanpaa J, Roh JH, Nallapareddy SR, Chowdhury S, et al. Complete genome sequence of Enterococcus faecium strain TX16 and comparative genomic analysis of Enterococcus faecium genomes. BMC Microbiol. 2012;12:135. doi: 10.1186/1471-2180-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laverde Gomez JA, van Schaik W, Freitas AR, Coque TM, Weaver KE, Francia MV, et al. A multiresistance megaplasmid pLG1 bearing a hylEfm genomic island in hospital Enterococcus faecium isolates. Int J Med Microbiol IJMM. 2011;301:165–175. doi: 10.1016/j.ijmm.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 56.Ramachandran G, Miguel-Arribas A, Abia D, Singh PK, Crespo I, Gago-Córdoba C, et al. Discovery of a new family of relaxases in Firmicutes bacteria. PLoS Genet. 2017;13:e1006586. doi: 10.1371/journal.pgen.1006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhanel GG, Calic D, Schweizer F, Zelenitsky S, Adam H, Lagacé-Wiens PRS, et al. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs. 2010;70:859–886. doi: 10.2165/11534440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Smith TT, Tamma PD, Do TB, Dzintars KE, Zhao Y, Cosgrove SE, et al. Prolonged linezolid use is associated with the development of linezolid-resistant Enterococcus faecium. Diagn Microbiol Infect Dis. 2018;91:161–163. doi: 10.1016/j.diagmicrobio.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 59.Olearo F, Both A, Belmar Campos C, Hilgarth H, Klupp E-M, Hansen JL, et al. Emergence of linezolid-resistance in vancomycin-resistant Enterococcus faecium ST117 associated with increased linezolid-consumption. Int J Med Microbiol IJMM. 2021;311:151477. doi: 10.1016/j.ijmm.2021.151477. [DOI] [PubMed] [Google Scholar]
- 60.Kessel J, Bender J, Werner G, Griskaitis M, Herrmann E, Lehn A, et al. Risk factors and outcomes associated with the carriage of tigecycline- and vancomycin-resistant Enterococcus faecium. J Infect. 2021;82:227–234. doi: 10.1016/j.jinf.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Papadimitriou-Olivgeris M, Drougka E, Fligou F, Kolonitsiou F, Liakopoulos A, Dodou V, et al. Risk factors for enterococcal infection and colonization by vancomycin-resistant enterococci in critically ill patients. Infection. 2014;42:1013–1022. doi: 10.1007/s15010-014-0678-1. [DOI] [PubMed] [Google Scholar]
- 62.Batistão DW da F, Gontijo-Filho PP, Conceição N, Oliveira AG de, Ribas RM. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz. 2012;107:57–63. [DOI] [PubMed]
- 63.Drews SJ, Richardson SE, Wray R, Freeman R, Goldman C, Streitenberger L, et al. An outbreak of vancomycin-resistant Enterococcus faecium in an acute care pediatric hospital: lessons from environmental screening and a case-control study. Can J Infect Dis Med Microbiol. 2008;19:233–236. doi: 10.1155/2008/727062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pochhammer J, Kramer A, Orth M, Schäffer M, Beckmann JH. Treatment with ceftriaxone in complicated diverticulitis increases the incidence of intra-abdominal Enterococcus faecium detection. Surg Infect. 2021;22:543–550. doi: 10.1089/sur.2020.057. [DOI] [PubMed] [Google Scholar]
- 65.McKinnell JA, Kunz DF, Chamot E, Patel M, Shirley RM, Moser SA, et al. Association between vancomycin-resistant Enterococci bacteremia and ceftriaxone usage. Infect Control Hosp Epidemiol. 2012;33:718–724. doi: 10.1086/666331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Hal SJ, Willems RJL, Gouliouris T, Ballard SA, Coque TM, Hammerum AM, et al. The global dissemination of hospital clones of Enterococcus faecium. Genome Med. 2021;13:52. doi: 10.1186/s13073-021-00868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadowy E, Sieńko A, Gawryszewska I, Bojarska A, Malinowska K, Hryniewicz W. High abundance and diversity of antimicrobial resistance determinants among early vancomycin-resistant Enterococcus faecium in Poland. Eur J Clin Microbiol Infect Dis. 2013;32:1193–1203. doi: 10.1007/s10096-013-1868-y. [DOI] [PubMed] [Google Scholar]
- 68.Sadowy E. Mobile genetic elements beyond the VanB-resistance dissemination among hospital-associated enterococci and other Gram-positive bacteria. Plasmid. 2021;114:102558. doi: 10.1016/j.plasmid.2021.102558. [DOI] [PubMed] [Google Scholar]
- 69.Woodford N, Chadwick PR, Morrison D, Cookson BD. Strains of glycopeptide-resistant Enterococcus faecium can alter their van genotypes during an outbreak. J Clin Microbiol. 1997;35:2966–2968. doi: 10.1128/jcm.35.11.2966-2968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suppola JP, Kolho E, Salmenlinna S, Tarkka E, Vuopio-Varkila J, Vaara M. vanA and vanB incorporate into an endemic ampicillin-resistant vancomycin-sensitive Enterococcus faecium strain: effect on interpretation of clonality. J Clin Microbiol. 1999;37:3934–3939. doi: 10.1128/JCM.37.12.3934-3939.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marcadé G, Micol JB, Jacquier H, Raskine L, Donay JL, Nicolas-Viaud S, Rouveau M, Ribaud P, Dombret H, Leclercq R, Cambau E. Outbreak in a haematology unit involving an unusual strain of glycopeptide-resistant Enterococcus faecium carrying both vanA and vanB genes. J Antimicrob Chemother. 2014;69:500–505. doi: 10.1093/jac/dkt376. [DOI] [PubMed] [Google Scholar]
- 72.Papagiannitsis CC, Malli E, Florou Z, Medvecky M, Sarrou S, Hrabak J, Petinaki E. First description in Europe of the emergence of Enterococcus faecium ST117 carrying both vanA and vanB genes, isolated in Greece. J Glob Antimicrob Resist. 2017;11:68–70. doi: 10.1016/j.jgar.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Khan MA, Shorman M, Al-Tawfiq JA, Hays JP. New type F lineage-related Tn 1546 and a vanA / vanB type vancomycin-resistant Enterococcus faecium isolated from patients in Dammam, Saudi Arabia during 2006–2007. Epidemiol Infect. 2013;141:1109–1114. doi: 10.1017/S0950268812001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santona A, Taviani E, Hoang HM, Fiamma M, Deligios M, Ngo TVQ, Van Le A, Cappuccinelli P, Rubino S, Paglietti B. Emergence of unusual vanA / vanB2 genotype in a highly mutated vanB2 -vancomycin-resistant hospital-associated E. faecium background in Vietnam. Int J Antimicrob Agents. 2018;52:586–592. doi: 10.1016/j.ijantimicag.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Dendle C, Ballard SA, Grabsch EA, Gao W, Grayson ML. Outbreak of vancomycin-resistant Enterococcus faecium containing both vanA and vanB gene clusters. J Hosp Infect. 2009;71:379–381. doi: 10.1016/j.jhin.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 76.Coombs GW, Daley DA, Yee NWT, Shoby P, Mowlaboccus S. Australian Group on Antimicrobial Resistance (AGAR) Australian Enterococcal Sepsis Outcome Programme (AESOP) Annual Report 2020. Commun Dis Intell (2018). 2022:46. [DOI] [PubMed]
- 77.Sadowy E, Gawryszewska I, Kuch A, Żabicka D, Hryniewicz W. The changing epidemiology of VanB Enterococcus faecium in Poland. Eur J Clin Microbiol Infect Dis. 2018;37:927–936. doi: 10.1007/s10096-018-3209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinholt M, Mollerup S, Boye K, Worning P, Holzknecht BJ, Nygaard S, et al. Investigation of the introduction and dissemination of vanB Enterococcus faecium in the Capital Region of Denmark and development of a rapid and accurate clone-specific vanBE. faecium PCR. J Antimicrob Chemother. 2021;76:2260–7. [DOI] [PubMed]
- 79.García-Solache M, Lebreton F, McLaughlin RE, Whiteaker JD, Gilmore MS, Rice LB. Homologous recombination within large chromosomal regions facilitates acquisition of β-lactam and vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 2016;60:5777–5786. doi: 10.1128/AAC.00488-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gawryszewska I, Żabicka D, Hryniewicz W, Sadowy E. Linezolid-resistant enterococci in Polish hospitals: species, clonality and determinants of linezolid resistance. Eur J Clin Microbiol Infect Dis. 2017;36:1279–1286. doi: 10.1007/s10096-017-2934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zarzecka U, Zakrzewski AJ, Chajęcka-Wierzchowska W, Zadernowska A. Linezolid-resistant Enterococcus spp. isolates from foods of animal origin-the genetic basis of acquired resistance. Foods Basel Switz. 2022;11:975. [DOI] [PMC free article] [PubMed]
- 82.Freitas AR, Tedim AP, Duarte B, Elghaieb H, Abbassi MS, Hassen A, et al. Linezolid-resistant (Tn 6246 :: fexB-poxtA ) Enterococcus faecium strains colonizing humans and bovines on different continents: similarity without epidemiological link. J Antimicrob Chemother. 2020;75:2416–2423. doi: 10.1093/jac/dkaa227. [DOI] [PubMed] [Google Scholar]
- 83.Dejoies L, Sassi M, Schutz S, Moreaux J, Zouari A, Potrel S, et al. Genetic features of the poxtA linezolid resistance gene in human enterococci from France. J Antimicrob Chemother. 2021;76:1978–1985. doi: 10.1093/jac/dkab116. [DOI] [PubMed] [Google Scholar]
- 84.Nüesch-Inderbinen M, Biggel M, Zurfluh K, Treier A, Stephan R. Faecal carriage of enterococci harbouring oxazolidinone resistance genes among healthy humans in the community in Switzerland. J Antimicrob Chemother. 2022;77:2779–2783. doi: 10.1093/jac/dkac260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wardenburg KE, Potter RF, D’Souza AW, Hussain T, Wallace MA, Andleeb S, et al. Phenotypic and genotypic characterization of linezolid-resistant Enterococcus faecium from the USA and Pakistan. J Antimicrob Chemother. 2019;74:3445–3452. doi: 10.1093/jac/dkz367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gawryszewska I, Żabicka D, Bojarska K, Malinowska K, Hryniewicz W, Sadowy E. Invasive enterococcal infections in Poland: the current epidemiological situation. Eur J Clin Microbiol Infect Dis. 2016;35:847–856. doi: 10.1007/s10096-016-2607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shan X, Li X-S, Wang N, Schwarz S, Zhang S-M, Li D, et al. Studies on the role of IS1216E in the formation and dissemination of poxtA -carrying plasmids in an Enterococcus faecium clade A1 isolate. J Antimicrob Chemother. 2020;75:3126–3130. doi: 10.1093/jac/dkaa325. [DOI] [PubMed] [Google Scholar]
- 88.Shan X, Yang M, Wang N, Schwarz S, Li D, Du X-D. Plasmid fusion and recombination events that occurred during conjugation of poxtA-carrying plasmids in enterococci. Microbiol Spectr. 2022;10:e0150521. doi: 10.1128/spectrum.01505-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Munita JM, Mishra NN, Alvarez D, Tran TT, Diaz L, Panesso D, et al. Failure of high-dose daptomycin for bacteremia caused by daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Clin Infect Dis. 2014;59:1277–1280. doi: 10.1093/cid/ciu642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tran TT, Panesso D, Gao H, Roh JH, Munita JM, Reyes J, et al. Whole-genome analysis of a daptomycin-susceptible Enterococcus faecium strain and its daptomycin-resistant variant arising during therapy. Antimicrob Agents Chemother. 2013;57:261–268. doi: 10.1128/AAC.01454-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mishra NN, Bayer AS, Moise PA, Yeaman MR, Sakoulas G. Reduced susceptibility to host-defence cationic peptides and daptomycin coemerge in methicillin-resistant Staphylococcus aureus from daptomycin-naive bacteremic patients. J Infect Dis. 2012;206:1160–1167. doi: 10.1093/infdis/jis482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cui L, Isii T, Fukuda M, Ochiai T, Neoh H-M, Camargo ILBC, et al. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:5222–5233. doi: 10.1128/AAC.00437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplementary Fig. 1. The cgMLST analysis of relationships of the 4995/20 isolate and other isolates reported to GenBank belonging to the PDS000100071 SNP group. Black circles, isolates (names provided within a circle); lines, links among isolates with the length of a line proportional to a number of differences in allelic profiles; the clusters of isolates from Uganda and Ireland marked with dashed-line blue circles; the 4995/20 isolate marked with a dashed-line red circle. The AUS0004 genome (CP003351.1) was used as an outgroup in analysis. The scale bar 50 corresponds to 50 differences between two allelic profiles. Supplementary Fig. 2. Comparison of the rep2 region in the p4995_2 and p4995_3 plasmids using ACT. The extent of homology between p4995_3 and pRE25 (X92945) indicated with a double-headed arrow below the . p4995_3 sequence; black arrow, the rep2 gene; red arrow, IS1216; empty red arrow, IS1062; gray arrows, other genes. Supplementary Fig. 3. The structure of Tn1549 and its integration site in the 4995/20. Green arrows, the vanB operon genes; red arrow, the int integrase gene; blue arrows, two parts of a counterpart of the EFAU004_02748 gene; gray arrows, other genes of Tn1549; black triangle, the coupling sequence (drawn not to scale). Supplementary Fig. 4. The structure of poxtA-PCT in 4995/20 and its variants reported to GenBank. Green arrows, the poxtA and fexB genes; red arrows, IS1216; gray arrows, other genes; insertions/deletions differing the poxtA-PCT in 4995/20 from other PCTs indicated by empty triangles. Supplementary Fig. 5. The structure of tet(M)-tet(L)-PCT in 4995/20 and its variants reported to GenBank. Blue arrows, the tet(M) and tet(L) genes; red arrows, IS1216; gray arrows, other genes; insertions/deletions differing the tet(M)-tet(L)-PCT in 4995/20 from other PCTs indicated by empty triangles. Supplementary Fig. 6. The structure of the p4995TC_TV8_1 recombinant plasmid. The extent of tet(M)-tet(L)-PCT, vanA-PCT and p4995_2 and p4995_3 plasmids indicated by yellow arrows; regions of presumable recombination indicated by empty double-headed arrows; empty gray arrows, rep genes; red arrows, IS1216; gray arrows, other genes (PDF 345 KB)
Data Availability Statement
The datasets generated during the current study are available in the GenBank repository in the BioProjects PRJNA1002867 and PRJNA1002867.