Summary
Pathogen testing is effective to prevent food poisoning. Here, an electrochemical biosensor was explored for Salmonella detection by combining magnetic grid based bacterial separation with enzymatic catalysis based signal amplification on a PCB interdigitated electrode in a microfluidic chip. First, immune magnetic nanobeads, target bacteria, and immune polystyrene microspheres decorated with glucose oxidase were sufficiently mixed to form nanobead-bacteria-microsphere sandwich conjugates. Then, these conjugates were injected into the chip to form conjugate chains right over the electrode under an iron grid enhanced magnetic field. After non-conductive glucose was injected and catalyzed by glucose oxidase on the conjugate chains, conductive glucose acid and non-conductive hydrogen peroxide were continuously produced and rapidly diffused from the conjugate chains to the electrode. Finally, the impedance change was real-timely monitored and used to determine the bacterial amount. This sensor enabled detection of 50 CFU/mL Salmonella typhimurium in 1 h.
Subject areas: Health technology, Materials science
Graphical abstract

Highlights
-
•
The sandwich conjugates were formed into chains over electrode using magnetic grid
-
•
The impedance was rapidly measured using PCB electrode due to fast ionic diffusion
-
•
The biosensor was able to detect Salmonella as low as 50 CFU/mL in 1 h
Health technology; Materials science
Introduction
Over the decade, food poisoning incidents frequently occurred and seriously threatened public health. World Health Organization reported 600 million illnesses and approximate 420,000 deaths each year due to pathogen infections.1 The Center for Disease Control estimated that Salmonella annually caused around 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the US,2 because Salmonella appeared in any link of food supply chains.3 Testing of pathogenic bacteria is effective to prevent food poisoning.4 Among traditional bacterial detection measures, culture plating is accurate but time-consuming, while PCR and ELISA are rapid but either complex or not sensitive.5 Therefore, there is an urgent demand for simple, fast, and sensitive detection measures.6
Various biosensors were reported with low cost, short time, and high selectivity for pathogen testing, including electrochemical,7 optical,8 surface-enhanced Raman spectroscopy,9 surface plasmon resonance,10 surface acoustic wave,11 and quartz crystal microbalance.12 As an important kind of electrochemical biosensors, impedance biosensors, which generally rely upon the analysis of impedance change resulting from target binding onto the surface of electrochemical transducer, have received wide attentions due to batch production, easy operation, and small size.13 Interdigitated array electrodes (IAEs) are featured with high signal-to-noise ratio, low resistance and fast equilibration, and frequently used as impedance transducer.14 For conventional impedance biosensors, biological recognition elements were often modified onto the surface of IAEs through protein A, protein G, streptavidin, covalent binding, etc.15,16,17,18 However, the sensitivity of these impedance biosensors is generally not high because of inefficient solid-liquid reaction between recognition elements and targets. To address this issue, various enzymes or nanozymes, such as glucose oxidase (GOx),19 urease,20 alkaline phosphatase,21 horseradish peroxidase,22 and manganese dioxide,23 were employed for impedance signal amplification by catalyzing non-conductive substrates into conductive products. In addition, some materials with high specific surface area, such as polystyrene microspheres (PSs),24 mesoporous silica nanospheres,25 flower-like porous nanocomposites,26 and metal-organic frameworks,27 were reported for effective loading of more signal probes to further improve the sensitivity. After the products were obtained, they were often dropped onto the surface of an IAE for impedance measurement. Generally, tens microliters of products were needed to cover effective surface of the IAE, and thus same or larger volume of substrates were used for catalysis. However, only those substrates around the enzymes or nanozymes were catalyzed, i.e., most substrates did not participate in enzymatic catalysis but they did dilute the products, which greatly reduced the sensitivity. More importantly, the impedance of the IAE was dominated by the solution ranging from its surface to the sum of finger width and spacing.28 The typical finger width and spacing were only several to tens microns, i.e., the effective zone of the solution for impedance measurement was only at microliter level. Besides, microfluidics has shown its great potential on precise manipulation of solutions and been widely applied for detection of foodborne pathogens.29 Therefore, it might be effective to improve impedance biosensors by performing enzymatic catalysis at effective zone of the IAE.
Due to low concentration of pathogenic bacteria and complex background of real foods, various immunomagnetic separation methods using magnetic nanobeads have been reported for separating pathogenic bacteria from sample, such as conventional static magnetic separation (SMS) at centrifuge tube, injection magnetic separation (IMS) at high-gradient column, continuous-flow magnetic separation (CMS) at narrow tubing/channel, etc.30,31,32,33 Among them, CMS has attracted more attention because it could isolate targets from large volume by constructing a gradient magnetic field to form magnetic nanobeads into chains in the tubing/channel for capturing the flowthrough bacteria.34 The gradient magnetic field played an important role in CMS, which was often generated through soft magnetic materials (like iron gears, sawteeth, and grids) to gather the magnetic field lines at the designated zones and result in the change of magnetic field distribution, which might have the potential to precisely manipulate the conjugates with magnetic nanobeads at their designated place.
In this work, a novel electrochemical impedance biosensor was proposed for Salmonella detection through combining magnetic grid separation and biological signal amplification on a printed circuit board (PCB) interdigitated electrode in a microfluidic chip. From Figure 1, immune magnetic nanobeads, target bacteria, and immune polystyrene microspheres with glucose oxidase were first mixed and incubated to form nanobead-bacteria-microsphere sandwich conjugates. Then, these conjugates were injected into this microfluidic chip for forming conjugate chains right over the electrode under the magnetic field with iron grid. After non-conductive glucose was injected to immerse the conjugate chains, conductive gluconic acid and non-conductive hydrogen peroxide were continuously produced and rapidly diffused to the effective zone of the electrode. Finally, the impedance change was real-timely monitored to calculate the target bacteria. The major innovation of this work was elaborative forming of conjugate chains over the electrode for real-timely monitoring the impedance change due to enzymatic catalysis of non-conductive substrates into conductive products. The products were not diluted and led to attenuation of impedance signal.
Figure 1.
Principle of the electrochemical biosensor
Results and discussion
Simulation of magnetic field distribution in microfluidic channel
The distribution of this magnetic field was crucial to this biosensor because it determined where the sandwich conjugates were captured and whether these conjugates were formed into chains in the microfluidic channel. Thus, the magnetic field was simulated by finite element method magnetics (FEMM). The results were shown in Figures 2A and 2B. Because magnetic field lines aggregated at these tips of iron grid, where magnetic strength and gradient were both the strongest, the conjugates were captured against the tips when they flowed through the channel. Besides, because the magnetic nanobeads tended to flow from the low-gradient zone to the high-gradient one, the nanobead-bacteria-microsphere conjugates were aggregated one by one to form the conjugate chains. From Figure 2A, the magnetic field lines basically passed through the channel vertically, and the magnetic field strength changed from 0.42 T at the top to 0.38 T at the bottom (mean gradient: 207 T/m).
Figure 2.
Simulation and evaluation of this magnetic field
(A) The FEMM simulation on this magnetic field.
(B) The magnetic strength in the channel.
(C) The capture efficiency of magnetic bacteria at different flow rates.
(D) The front and side views of magnetic nanobead chains in this microfluidic channel. Data were presented as mean ± SD. One-way ANOVA with LSD: p < 0.05.
The flow rate was important for stable forming of conjugate chains in the channel. The capture efficiency was defined as the ratio of captured nanobeads/conjugates to initial ones and used to select the optimal flow rate. The immune magnetic nanobeads and target bacteria (1.8× 103 CFU/mL) were first mixed to form the magnetic bacteria. Then, they were pumped into the channel at different flow rates (25–100 μL/min). Finally, the waste was collected for culture plating to calculate the amount of captured magnetic bacteria. From Figure 2C, the capture efficiency of magnetic bacteria was more than 95% at the flow rate between 25 μL/min and 75 μL/min, while the capture efficiency was less than 95% at the flow rate of 100 μL/min. This was because the faster flow rate resulted in partial loss of the magnetic bacteria. Thus, the optimal flow rate was selected as 75 μL/min. To further verify the forming of conjugate chains at this flow rate under this magnetic field, a photo was taken using a microscope (Zhiqi ZQ-603, Shanghai, China). From Figure 2D, it was observed that the magnetic nanobeads were collected over the electrode and formed into chains with the largest height of ∼75 μm, which were right above the electrode with a space of ∼75 μm.
Diffusion of the conductive ions in the microfluidic channel
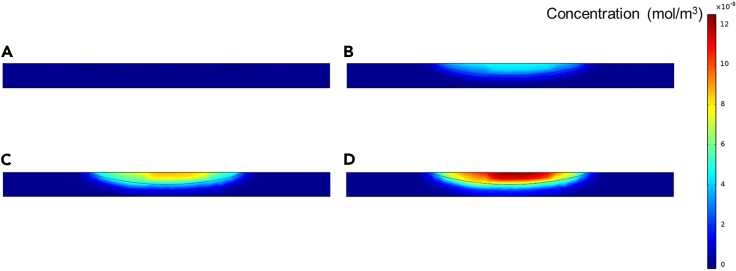
The ionic diffusion from conjugate chains to electrode surface is the basis of this biosensor. To understand the ionic diffusion of conductive catalytic products, COMSOL Multiphysics software was used for simulation by selecting 2D space dimension, ionic diffusion, and time dependence. The diffusion coefficient was established as 1 × 10−9 m2/s. The initial concentration for all domains was set as 0 mol/m3. The reaction was set at the top central arc region and the reaction rate was set as 9 × 10−8 mol/(m3·s). The free triangle mesh and time range of 0–1.5 s were used. The simulation results at 0, 0.5, 1.0, and 1.5 s were shown in Figure 3. When the products were continuously produced, they rapidly diffused toward all directions and the products were observed to reach the bottom (the electrode) in ∼1 s. In theory, the diffusion time (t) could be obtained as t = L2/4D, where L was diffusion distance and D was diffusion coefficient. In general, it need take the ions ∼5.6 s to diffuse from the top of the channel to the bottom (the height of the channel was 150 μm). However, because the conjugates with GOx were formed into chains and this shortened the distance between the products and the electrode, the ionic diffusion from the conjugate chains to the electrode surface only took ∼1.4 s, which was basically consistent with the simulation results. Moreover, according to Fick’s first law, the diffusion velocity could be expressed by diffusion flux (J), which was related to concentration gradient and diffusion coefficient. When the rate of the enzymatic reaction increased, the concentration gradient of products increased and this could lead to faster diffusion.
Figure 3.
The COMSOL simulation of ionic diffusion in the microfluidic channel at different time
(A) 0 s, (B) 0.5 s, (C) 1.0 s, and (D) 1.5 s.
The impedance biosensing mechanism
To demonstrate this impedance biosensoring mechanism, the electrochemical impedance responses to gluconic acid at different concentrations were analyzed. Thus, 100 μL gluconic acid from low to high concentration was injected sequentially into this microfluidic channel at 25 μL/min. From Figure 4A, the Bode plots of gluconic acid with different concentrations were first measured, and 10 kHz was selected as the characteristic frequency for impedance monitoring. Figure S1A showed the results for impedance monitoring of gluconic acid at different concentrations (0.3125–80 μM), and the impedance for each concentration of gluconic acid basically did not change with time after stopping the injection. When gluconic acid concentration increased, its impedance decreased, indicating that the impedance change depended on the change of the ion concentration. In addition, to investigate the impact of immune magnetic nanobeads on the impedance monitoring, 20 μg immune magnetic nanobeads were formed into chains in the channel and different concentrations of gluconic acid were measured in the same way. From Figure S1B, the impedance for each concentration with the magnetic nanobeads was consistent with that without the magnetic nanobeads, indicating the magnetic nanobeads did not have obvious influence on impedance change. From Figure 4B, the relative standard deviation (RSD) of the impedance for different concentrations ranged from 1.31% to 4.92% without nanobeads and from 1.41% to 4.59% with nanobeads, indicating that the magnetic nanobeads basically did not affect the precision of this proposed assay. The impedance (Z) of gluconic acid was linear with its concentration (C), which was described as Z = −12764 ∗C + 143154 (R2 = 0.99).
Figure 4.
Mechanism of this electrochemical biosensor
(A) The electrochemical impedance spectra of gluconic acid at 0.3125 μM–80 μM.
(B) The impedance of gluconic acid at 0.3125 μM–80 μM. Data were presented as mean ± SD.
Performance of impedance biosensor
To assess the performance of the biosensor, triple tests on pure Salmonella at 1.8 × 102 to 1.8 × 106 CFU/mL were performed. From Figure 5A, the impedance decreased with the catalytic time, i.e., the impedance change increased with the catalytic time, because gluconic acids were continuously produced due to the catalysis of glucose by the glucose oxidase. Besides, the impedance monitoring curve for higher concentration had a larger slope. This was because more bacteria resulted in the forming of more conjugate chains, which led to the higher production rate of gluconic acids and thus the faster diffusion to the electrode surface. From Figure 5B, the impedance change for different amounts of bacteria had shown a significant difference in a much shorter catalytic time (∼200 s), which often required 30 min or longer time. A good linear model between impedance change (ΔZ) and bacterial concentration (C) of 1.8 × 102-1.8 × 106 CFU/mL was acquired as ΔZ = 15796 ∗ log (C) - 15075 (R2 = 0.98). Furthermore, the detection limit was calculated as 50 CFU/mL using triple signal-to-noise ratio. Compared with some reported methods in Table S1, this biosensor showed the superiority in either sensitivity or time. Its good performance was mostly owing to: (1) efficient separation of target bacteria by the immune magnetic nanobeads; (2) stable forming of conjugate chains over the PCB electrode; (3) effective amplification of impedance signal by the immune polystyrene microspheres; (4) fast diffusion of catalytic products to the PCB electrode; (5) sensitive monitoring of catalytic products by the PCB electrode.
Figure 5.
Performance of this impedance biosensor
(A) Real-time monitoring of impedance changes for Salmonella at 102-106 CFU/mL.
(B) Impedance changes for different amounts of bacteria with 200 s catalytic time and linear model of the biosensor.
(C) Specificity of the biosensor.
(D) TEM picture for nanobead-bacteria conjugates.
(E) TEM pictures at different magnifications for nanobead-bacteria-microsphere conjugates. Data were presented as mean ± SD. One-way ANOVA with LSD: p < 0.05.
To assess the specificity of this biosensor, Salmonella typhimurium (St) at 1.8 × 105 CFU/mL as target bacteria, Listeria monocytogenes (Lm), Vibrio parahaemolyticus (Vp), and E. coli O157:H7 (Ec) at 1.8 × 107 CFU/mL as non-target bacteria and their mixture were detected. From Figure 5C, the impedance changes of target bacteria and their mixture were significantly higher than those of non-target bacteria, indicating the sensor exhibited a satisfied specificity. Besides, from Figures 5D and 5E, transmission electron microscopy (TEM) validated the forming of magnetic bacteria and sandwich conjugates.
Application of impedance biosensor
The practical application of the impedance biosensor for detecting target bacteria in real samples was verified by triplicate detection of spiked chicken samples. The recovery was determined as the ratio of biosensor result to culture plating one. From Table 1, for bacterial concentration of 1.8 × 102 - 1.8 × 105 CFU/mL, the recoveries were 95.55%, 91.80%, 102.26%, and 97.01%, respectively. The difference between the biosensor and culture plating may be owing to the non-specific adsorption caused by the complex food matrix. The relative standard deviation ranged from 2.51% to 9.06%, showing that this impedance biosensor had good practicability in detecting bacteria.
Table 1.
Detection of Salmonella in spiked chicken supernatants
| Spiked (CFU/mL) | Impedance change (kΩ) | Detected (CFU/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 180 | 20.24d | 172 | 95.55 | 9.06 |
| 1800 | 35.76c | 1652 | 91.80 | 3.42 |
| 18000 | 52.29b | 18406 | 102.26 | 3.06 |
| 180000 | 67.73a | 174616 | 97.01 | 2.51 |
One-way ANOVA with LSD: p < 0.05.
Conclusion
A new impedance biosensor using a PCB interdigitated electrode to real-timely monitor the impedance change resulting from enzymatic catalysis of non-conductive glucose into conductive gluconic acids by glucose oxide on the conjugate chains was successfully developed. This biosensor was demonstrated with a linear detection range of 1.8 × 102-1.8 × 106 CFU/mL, a low detection limit of 50 CFU/mL, and a total analysis time of 60 min for Salmonella typhimurium. Compared to our previous developed impedance biosensors using the same glucose-glucose oxidase system or the different urea-urease and H2O2-MnO2 sytems,35,36,37,38,39 the forming of conjugate chains over the PCB electrode was verified with efficient measurement of impedance change in a much shorter time (from previous ∼30 min to current ∼3 min). In addition, the in situ impedance measurement using this PCB electrode to monitor the iron diffusion was demonstrated to prevent the catalytic products from being diluted and thus resulting in attenuation of impedance signal.
Limitations of the study
This biosensor might be further improved using other impedance signal amplification strategies, such as the urea-urease or H2O2-peroxidase systems.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-Salmonella | Meridian | Cat#C86309M; RRID: AB_152809 |
| Rabbit polyclonal anti-Salmonella | Fitzgerald | Cat#20C-CR7100RP; RRID: AB_1288673 |
| Bacterial and virus strains | ||
| Salmonella typhimurium | ATCC | ATCC 14028 |
| Listeria monocytogenes | ATCC | ATCC 13932 |
| Vibrio parahaemolyticus | ATCC | ATCC 17802 |
| Escherichia coli O157:H7 | ATCC | ATCC 43888 |
| Software and algorithms | ||
| FEMM 4.2 | David Meeker | https://www.femm.info/wiki/Download |
| COMSOL Multiphysics 6.0 | COMSOL AB | https://cn.comsol.com/product-download/6.0 |
| IBM SPSS Statistics 26 | IBM | https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-26 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jianhan Lin (jianhan@cau.edu.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
All these bacterial strains were stored in a broth medium containing 20% glycerol at -20°C and revived by shaking in sterile Luria-Bertani (LB) medium (Aoboxing Biotech, Beijing, China) at 37°C for 16-24 h. The bacterial cultures were serial diluted 10 times with sterile PBS for bacterial counting.
Method details
Materials
Polystyrene microspheres (300 nm, 100 mg/mL) from Bangs Laboratories (Fishers, IN), anti-Salmonella monoclonal antibodies (3.35 mg/mL) from Meridian (Memphis, TN) and GOx (155,000 units/g solid) from Sigma Aldrich (St. Louis, MO) were used to prepare immune polystyrene microspheres. Magnetic nanobeads (180 nm, 10 mg/mL) from Allrun (Shanghai) and anti-Salmonella polyclonal antibodies (2 mg/mL) from Fitzgerald (Acton, MA) were used to prepare immune magnetic nanobeads. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-Hydroxysulfosuccinimide sodium (NHSS) were bought from Aladdin (Shanghai). Glucose, sucrose, bovine serum albumin (BSA), skim milk powder, proclin were bought from Sigma Aldrich. D-gluconic acid solution (2.5 mM) and phosphate buffer (PBS) were from Solarbio (Beijing). NaOH, HCl, anhydrous ethanol, Na2HPO4·12H2O and NaH2PO4·2H2O were bought from Sinopharm (Shanghai). Deionized water was produced by Millipore Advantage A10 (Billerica, MA).
Methodology
Construction of impedance biosensing platform
As shown in Figure 1, the impedance biosensing platform was consisted of three components: (1) a microfluidic chip for performing the enzymatic catalysis, (2) a gradient magnetic field for forming the conjugate chains, and (3) a PCB interdigitated electrode for monitoring the impedance change. The microfluidic chip (size: 35 mm × 20 mm × 4 mm) mainly contained a narrow straight channel (28 mm long, 1-2 mm wide, 150 μm high) and a rectangular groove (size: 13 mm × 14 mm × 2.85 mm) for housing the magnetic field. More information of this microfluidic chip could be found in Figure S2. The magnetic field was generated using two repulsive rectangular NdFeB magnets (size: 10 mm × 10 mm × 5 mm, grade: N52) and an iron grid (10 mm long, 10 mm wide, 1 mm thick) in a 3D printed holder (size: 10 mm × 11 mm × 10 mm, thickness: 1.5 mm). The PCB electrode (size: 40 mm × 20 mm × 1 mm) with 10 pairs of fingers (length: 6 mm, width: 200 μm, spacing: 200 μm),20 which was shown in Figure S3, was stuck to the microfluidic chip through an ultra-thin 3M double-sided adhesive, while the fingers (working area) of the electrode crossed this channel of the chip. The iron grid was parallel with the channel and located at the center of the working area, allowing the forming of conjugate chains right on the top of the fingers.
Synthesis of immune magnetic nanobeads and polystyrene microspheres
The preparation of immune magnetic nanobeads and polystyrene microspheres was on the basis of our reported work with some modifications.35 The EDC/NHSS method was used to cross-link magnetic nanobeads and polyclonal antibodies. After 1 mg magnetic nanobeads were washed 3 times and re-dissolved in 3 mL PB (10 mM, pH 6.0), 1 mg EDC and 1 mg NHSS were first added to react at 37°C for 60 min, followed by washing. Then, 100 μg antibodies in PB (10 mM, pH 8.4) were added to incubate 2 h, and blocked with skim milk powder (10%, w/v) in PB (10 mM, pH 7.4) for 2 h. Finally, immunomagnetic nanobeads were re-dissolved in 1 mL storage solution (containing 10 mg skim milk powder, 250 mg sucrose and 7 μL proclin) after washing with PBS and stored at 4°C. To further characterize successful conjugation of the polyclonal antibodies onto the magnetic nanobeads, the HRP-labeled colorimetric assay and the BCA-based colorimetric assay were conducted and their results were shown in Figures S4 and S5 to verify the antibody conjugation.
The EDC method was used to prepare immune polystyrene microspheres. Prior to use, 10 mL glass bottle and 1 cm rotor were washed overnight with 5 mL PB (10 mM, pH 6.0) through a magnetic stirrer. First, 200 μg microspheres were prepared by centrifugation (12,000 rpm, 20 min) for removing background, followed by electrostatic adsorption with 100 μg monoclonal antibodies in 2.5 mL PB on the magnetic stirrer for 1 h. Then, 100 μg GOx and 30 μg EDC were added to incubate 1 h, and 30 μg EDC was added twice hourly. Subsequently, 320 μL skim milk powder (10%, w/v) and 50 μg EDC were used for blocking (1 h). Finally, immune microspheres were obtained by centrifugation (10,000 rpm, 20 min) and stored in 200 μL storage solution. To confirm the elements in the polystyrene microspheres and immune polystyrene microspheres, the EDS mapping was conducted and the results were shown in Figures S6 and S7.
Detection of pure bacterial culture
Prior to use, the PCB electrode was washed by NaOH (1 M, 5 min) and HCl (1 M, 2 min) respectively, and gently wiped by soft tissue paper with anhydrous ethanol. Besides, the microfluidic chip was blocked with BSA for 1 h and washed with deionized water. To relate impedance change with bacterial amount, 20 μg immune magnetic nanobeads, 500 μL bacterial sample (1.8 × 102-1.8 × 106 CFU/mL) were first incubated with 10 μg immune polystyrene microspheres for 45 min to form the sandwich conjugates. Then, magnetic separation was conducted to obtain these conjugates, followed by washing two times with 500 μL deionized water to remove residual conductive ions. After they were re-suspended in 100 μL deionized water and injected into the microfluidic chip at 75 μL/min through a precision syringe pump (Harvard Apparatus, Holliston, MA), the conjugate chains were formed in the channel and hanged over the electrode. Finally, 100 μL non-conductive glucose (50 mM) was injected at 25 μL/min, and conductive gluconic acid was continuously produced and rapidly diffused towards the electrode, followed by real-time monitoring of impedance change using the PCB electrode.
The PCB electrode was applied with a sinusoidal AC voltage (amplitude: 5 mV, DC bias: 0 V, frequency: 10 kHz) on an electrochemical workstation (Zahner IM6, Kronach, Germany). The impedance data was collected every 2 seconds. The impedance change (ΔZ) was defined as ΔZ = Z0 – Zt, where Z0 and Zt were the impedances at initial moment and t moment respectively, and used for analyzing the impedance data to determine the bacterial amount.
Detection of spiked chicken samples
The spiked chicken supernatant containing different amounts of target bacteria were used for simulating real samples to evaluate the applicability of this biosensor. According to the national food safety standard for Salmonella detection in China (GB 4789.4-2016), 25 g chicken meat was blended with 225 mL PBS at a clean homogenizing bag to obtain the supernatant after keeping static for 10 min. The spiked chicken samples at 1.8 × 102-1.8 × 105 CFU/mL were obtained by adding target bacteria into the supernatant, and tested by this biosensor and culture plating in parallel. The detailed procedure was shown in Figure S8.
Quantification and statistical analysis
Data were plotted by Microsoft excel and presented as mean ± SD. One-way ANOVA (p<0.05) was used to assess the significance between multiple groups by the SPSS software.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (32302962) and Walmart Foundation (UA2020-154).
Author contributions
F. J.: Conceptualization, methodology, formal analysis, data curation, writing – original draft. L. W.: Methodology, formal analysis. N. J.: Formal analysis, data curation. J. Y.: Methodology. Y. L.: Supervision. J. L.: Conceptualization, formal analysis, supervision, project administration, funding acquisition, writing – reviewing and editing.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: October 18, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108245.
Contributor Information
Lei Wang, Email: wanglei123@cau.edu.cn.
Jianhan Lin, Email: jianhan@cau.edu.cn.
Supplemental information
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Van Puyvelde L., Aissa A., Panda S.K., De Borggraeve W.M., Mukazayire M.J., Luyten W. Bioassay-guided isolation of antibacterial compounds from the leaves of Tetradenia riparia with potential bactericidal effects on food-borne pathogens. J. Ethnopharmacol. 2021;273:113956. doi: 10.1016/j.jep.2021.113956. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Salmonella. https://www.cdc.gov/Salmonella/
- 3.Shen Y., Xu L., Li Y. Biosensors for rapid detection of Salmonella in food: A review. Compr. Rev. Food Sci. Food Saf. 2021;20:149–197. doi: 10.1111/1541-4337.12662. [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Tan L., Wu J., Li T., Xie H., Wu D., Gan N. A universal signal-on electrochemical assay for rapid on-site quantitation of vibrio parahaemolyticus using aptamer modified magnetic metal–organic framework and phenylboronic acid-ferrocene co-immobilized nanolabel. Anal. Chim. Acta. 2020;1133:128–136. doi: 10.1016/j.aca.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y., Hassan M.M., Sharma A.S., Li H., Chen Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2023;63:486–504. doi: 10.1080/10408398.2021.1950117. [DOI] [PubMed] [Google Scholar]
- 6.Liu S., Zhao K., Huang M., Zeng M., Deng Y., Li S., Chen H., Li W., Chen Z. Research progress on detection techniques for point-of-care testing of foodborne pathogens. Front. Bioeng. Biotechnol. 2022;10:958134. doi: 10.3389/fbioe.2022.958134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesewski E., Johnson B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020;159:112214. doi: 10.1016/j.bios.2020.112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pebdeni A.B., Roshani A., Mirsadoughi E., Behzadifar S., Hosseini M. Recent advances in optical biosensors for specific detection of E. coli bacteria in food and water. Food Control. 2022;135 doi: 10.1016/j.foodcont.2022.108822. [DOI] [Google Scholar]
- 9.Chen H., Das A., Bi L., Choi N., Moon J.I., Wu Y., Park S., Choo J. Recent advances in surface-enhanced Raman scattering-based microdevices for point-of-care diagnosis of viruses and bacteria. Nanoscale. 2020;12:21560–21570. doi: 10.1039/d0nr06340a. [DOI] [PubMed] [Google Scholar]
- 10.Bhardwaj H., Sumana G., Marquette C.A. A label-free ultrasensitive microfluidic surface Plasmon resonance biosensor for Aflatoxin B1 detection using nanoparticles integrated gold chip. Food Chem. 2020;307:125530. doi: 10.1016/j.foodchem.2019.125530. [DOI] [PubMed] [Google Scholar]
- 11.Tsougeni K., Kaprou G., Loukas C.M., Papadakis G., Hamiot A., Eck M., Rabus D., Kokkoris G., Chatzandroulis S., Papadopoulos V., et al. Lab-on-Chip platform and protocol for rapid foodborne pathogen detection comprising on-chip cell capture, lysis, DNA amplification and surface-acoustic-wave detection. Sens. Actuators, B. 2020;320 doi: 10.1016/j.snb.2020.128345. [DOI] [Google Scholar]
- 12.Wachiralurpan S., Chansiri K., Lieberzeit P.A. Direct detection of Listeria monocytogenes DNA amplification products with quartz crystal microbalances at elevated temperatures. Sens. Actuators, B. 2020;308 doi: 10.1016/j.snb.2020.127678. [DOI] [Google Scholar]
- 13.Riu J., Giussani B. Electrochemical biosensors for the detection of pathogenic bacteria in food. TrAC Trends Anal. Chem. (Reference Ed.) 2020;126 doi: 10.1016/j.trac.2020.115863. [DOI] [Google Scholar]
- 14.Furst A.L., Francis M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2019;119:700–726. doi: 10.1021/acs.chemrev.8b00381. [DOI] [PubMed] [Google Scholar]
- 15.Chiriacò M., Parlangeli I., Sirsi F., Poltronieri P., Primiceri E. Impedance Sensing Platform for Detection of the Food Pathogen Listeria monocytogenes. Electronics-Switz. 2018;7:347. doi: 10.3390/electronics7120347. [DOI] [Google Scholar]
- 16.Cimafonte M., Fulgione A., Gaglione R., Papaianni M., Capparelli R., Arciello A., Bolletti Censi S., Borriello G., Velotta R., Della Ventura B. Screen Printed Based Impedimetric Immunosensor for Rapid Detection of Escherichia coli in Drinking Water. Sensors-Basel. 2020;20 doi: 10.3390/s20010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelrasoul G.N., Anwar A., MacKay S., Tamura M., Shah M.A., Khasa D.P., Montgomery R.R., Ko A.I., Chen J. DNA aptamer-based non-faradaic impedance biosensor for detecting E. coli. Anal. Chim. Acta. 2020;1107:135–144. doi: 10.1016/j.aca.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Jasim I., Shen Z., Mlaji Z., Yuksek N.S., Abdullah A., Liu J., Dastider S.G., El-Dweik M., Zhang S., Almasri M. An impedance biosensor for simultaneous detection of low concentration of Salmonella serogroups in poultry and fresh produce samples. Biosens. Bioelectron. 2019;126:292–300. doi: 10.1016/j.bios.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 19.Xu M., Wang R., Li Y. Rapid detection of Escherichia coli O157:H7 and Salmonella Typhimurium in foods using an electrochemical immunosensor based on screen-printed interdigitated microelectrode and immunomagnetic separation. Talanta. 2016;148:200–208. doi: 10.1016/j.talanta.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Huang F., Cai G., Yao L., Zhang H., Lin J. An Electrochemical Aptasensor Using Coaxial Capillary with Magnetic Nanoparticle, Urease Catalysis and PCB Electrode for Rapid and Sensitive Detection of Escherichia coli O157:H7. Nanotheranostics. 2017;1:403–414. doi: 10.7150/ntno.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D.b., Li Y., Sun M.q., Huang J.p., Shao H.b., Xin Q.l., Wang M. Rapid detection of Salmonella using a redox cycling-based electrochemical method. Food Control. 2016;72:81–87. doi: 10.1016/j.foodcont.2015.10.021. [DOI] [Google Scholar]
- 22.Wang S., Sun C., Hu Q., Li S., Wang C., Wang P., Zhou L. A homogeneous magnetic bead-based impedance immunosensor for highly sensitive detection of Escherichia coli O157:H7. Biochem. Eng. J. 2020;156 doi: 10.1016/j.bej.2020.107513. [DOI] [Google Scholar]
- 23.Wang S., Peng T., Meng Q., Zhu X., Guo L., Yao K., Wang Z., Zheng P., Ren Z., He Z., et al. Rapid and ultrasensitive detection of Salmonella typhimurium using a novel impedance biosensor based on SiO2@MnO2 nanocomposites and interdigitated array microelectrodes. Sens. Actuators, B. 2020;324 doi: 10.1016/j.snb.2020.128654. [DOI] [Google Scholar]
- 24.Somvanshi S.B., Ulloa A.M., Zhao M., Liang Q., Barui A.K., Lucas A., Jadhav K.M., Allebach J.P., Stanciu L.A. Microfluidic paper-based aptasensor devices for multiplexed detection of pathogenic bacteria. Biosens. Bioelectron. 2022;207:114214. doi: 10.1016/j.bios.2022.114214. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Du X., Wang X., Yan T., Yuan M., Yang Y., Jurado-Sánchez B., Escarpa A., Xu L.P. Patterned Liquid-Infused Nanocoating Integrating a Sensitive Bacterial Sensing Ability to an Antibacterial Surface. ACS Appl. Mater. Interfaces. 2022;14:23129–23138. doi: 10.1021/acsami.1c24821. [DOI] [PubMed] [Google Scholar]
- 26.Bu S.J., Wang K.Y., Liu X., Ma L., Wei H.G., Zhang W.G., Liu W.S., Wan J.Y. Ferrocene-functionalized nanocomposites as signal amplification probes for electrochemical immunoassay of Salmonella typhimurium. Mikrochim. Acta. 2020;187:600. doi: 10.1007/s00604-020-04579-y. [DOI] [PubMed] [Google Scholar]
- 27.Wang S., Zhu X., Meng Q., Zheng P., Zhang J., He Z., Jiang H. Gold interdigitated micro-immunosensor based on Mn-MOF-74 for the detection of Listeria monocytogens. Biosens. Bioelectron. 2021;183:113186. doi: 10.1016/j.bios.2021.113186. [DOI] [PubMed] [Google Scholar]
- 28.Van Gerwen P., Laureyn W., Laureys W., Huyberechts G., Op De Beeck M., Baert K., Suls J., Sansen W., Jacobs P., Hermans L., Mertens R. Nanoscaled interdigitated electrode arrays for biochemical sensors. Sens. Actuators, B. 1998;49:73–80. doi: 10.1016/S0925-4005(98)00128-2. [DOI] [Google Scholar]
- 29.Weng X., Zhang C., Jiang H. Advances in microfluidic nanobiosensors for the detection of foodborne pathogens. LWT--Food Sci. Technol. 2021;151 doi: 10.1016/j.lwt.2021.112172. [DOI] [Google Scholar]
- 30.Bai X., Chen G., Wang Z., Xie G., Deng M., Xu H. Simultaneous detection of Bacillus cereus and Staphylococcus aureus by teicoplanin functionalized magnetic beads combined with triplex PCR. Food Control. 2022;132 doi: 10.1016/j.foodcont.2021.108531. [DOI] [Google Scholar]
- 31.Guo R., Huang F., Cai G., Zheng L., Xue L., Li Y., Liao M., Wang M., Lin J. A colorimetric immunosensor for determination of foodborne bacteria using rotating immunomagnetic separation, gold nanorod indication, and click chemistry amplification. Mikrochim. Acta. 2020;187:197. doi: 10.1007/s00604-020-4169-z. [DOI] [PubMed] [Google Scholar]
- 32.Lee H., Han H., Jeon S. Baleen-Mimicking Virtual Filters for Rapid Detection of Pathogenic Bacteria in Water Using Magnetic Nanoparticle Chains and a Halbach Ring. ACS Sens. 2020;5:3432–3437. doi: 10.1021/acssensors.0c01334. [DOI] [PubMed] [Google Scholar]
- 33.Lee H., Han H., Jeon S. Autonomous Internal Reflux of Magnetic Nanoparticle Chains in a Flow Channel for Efficient Detection of Waterborne Bacteria. Anal. Chem. 2021;93:12237–12242. doi: 10.1021/acs.analchem.1c01469. [DOI] [PubMed] [Google Scholar]
- 34.Lee H., Kim G., Park E., Jeon S. Lenz’s Law-Based Virtual Net for Detection of Pathogenic Bacteria from Water. Anal. Chem. 2019;91:15585–15590. doi: 10.1021/acs.analchem.9b03636. [DOI] [PubMed] [Google Scholar]
- 35.Xue L., Guo R., Jin N., Wang S., Duan H., Qi W., Wang L., Zheng Y., Li Y., Lin J. Rapid and automatic Salmonella typhimurium detection integrating continuous-flow magnetic separation and dynamic impedance measurement. Food Control. 2023;143 doi: 10.1016/j.foodcont.2022.109316. [DOI] [Google Scholar]
- 36.Liu Y., Jiang D., Wang S., Cai G., Xue L., Li Y., Liao M., Lin J. A microfluidic biosensor for rapid detection of Salmonella typhimurium based on magnetic separation, enzymatic catalysis and electrochemical impedance analysis. Chin. Chem. Lett. 2022;33:3156–3160. doi: 10.1016/j.cclet.2021.10.064. [DOI] [Google Scholar]
- 37.Wang D., Chen Q., Huo H., Bai S., Cai G., Lai W., Lin J. Efficient separation and quantitative detection of Listeria monocytogenes based on screen-printed interdigitated electrode, urease and magnetic nanoparticles. Food Control. 2017;73:555–561. doi: 10.1016/j.foodcont.2016.09.003. [DOI] [Google Scholar]
- 38.Wang L., Xue L., Guo R., Zheng L., Wang S., Yao L., Huo X., Liu N., Liao M., Li Y., Lin J. Combining impedance biosensor with immunomagnetic separation for rapid screening of Salmonella in poultry supply chains. Poultry Sci. 2020;99:1606–1614. doi: 10.1016/j.psj.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue L., Guo R., Huang F., Qi W., Liu Y., Cai G., Lin J. An impedance biosensor based on magnetic nanobead net and MnO2 nanoflowers for rapid and sensitive detection of foodborne bacteria. Biosens. Bioelectron. 2020;173:112800. doi: 10.1016/j.bios.2020.112800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.