Abstract
Nondestructive ingestion by soilborne protozoa may enhance the environmental resiliency of important bacterial pathogens and may model how such bacteria evade destruction in human macrophages. Here, the interaction of Salmonella enterica serovar Thompson with a soilborne Tetrahymena sp. isolate was examined using serovar Thompson cells labeled with the green fluorescent protein. The bacteria were mixed in solution with cells of Tetrahymena at several ratios. During incubation with serovar Thompson, Tetrahymena cells released a large number of vesicles containing green fluorescent serovar Thompson cells. In comparison, grazing on Listeria monocytogenes cells resulted in their digestion and thus the infrequent release of this pathogen in vesicles. The number of serovar Thompson cells per vesicle increased significantly as the initial ratio of serovar Thompson to Tetrahymena cells increased from 500:1 to 5,000:1. The density of serovar Thompson was as high as 50 cells per vesicle. Staining with propidium iodide revealed that a significantly higher proportion of serovar Thompson cells remained viable when enclosed in vesicles than when free in solution. Enhanced survival rates were observed in vesicles that were secreted by both starved (F = 28.3, P < 0.001) and unstarved (F = 14.09, P < 0.005) Tetrahymena cells. Sequestration in vesicles also provided greater protection from low concentrations of calcium hypochlorite. Thus, the release of this human pathogen from Tetrahymena cells in high-density clusters enclosed in a membrane may have important implications for public health.
Protozoa are an integral part of most microbial consortia and are ubiquitous in nature, particularly in environments where water is present. Many species of protozoa feed on bacteria, which they ingest by phagocytosis and sequester within food vacuoles. Bacterivorous protozoa have been termed the “Trojan horses” of the microbial world as they may shield pathogenic bacteria that they internalize from the early defense responses of human hosts (2). Recent reviews report over 23 species and groups of pathogens found to associate with amoebae (16, 27). Passage of intracellular pathogens such as Legionella pneumophila and Mycobacterium avium through Acanthamoeba castellanii results in these pathogens' greater infectivity (11, 12). Barker et al. (3) have shown that L. pneumophila cells passaged through amoebae are more resistant to certain biocides and disinfectants. Increased resistance to antimicrobial agents has been demonstrated for M. avium inside amoebae compared with those bacteria inside human macrophages (23). The production of vesicles containing viable L. pneumophila has been observed with Acanthamoeba spp. (5) and Tetrahymena spp. (20). Recently, Chen et al. (10) reported the identification of effectors in L. pneumophila that may promote its exocytic release from A. castellanii.
The role of protozoa in the virulence of food-borne pathogens and their persistence in the environment has been relatively unexplored. Several pathogenic coliform species were found to be more resistant to chlorination while internalized within A. castellanii and Tetrahymena pyriformis (17). In addition, multiplication of Escherichia coli O157:H7 within Acanthamoeba polyphaga has been reported (4). However, the significance of bacterial entrapment within protozoa cysts or vesicles in the contamination cycle of food-borne pathogenic bacteria has not been addressed. The objective of our study was to determine whether Salmonella enterica could be released in a viable form in vesicles of Tetrahymena and whether this internalization affords a survival advantage over those bacteria that remain free in an aquatic environment.
MATERIALS AND METHODS
Strains and culture.
S. enterica serovar Thompson strain RM1989 is a clinical isolate associated with an outbreak linked to cilantro in California (6, 9). Serovar Thompson strain STN pGT-Kan is a spontaneous nalidixic-acid-resistant mutant of strain RM1989 that is intrinsically labeled with the green fluorescent protein (GFP) by transformation with plasmid pGT-Kan. Plasmid pGT-Kan was constructed by fusion of the nptII promoter fragment from Tn5 to gfp on pPROBE-GT (22) and is stably maintained in serovar Thompson. Strain STN pGT-Kan was used throughout this study and cultured with agitation at 28°C in Luria-Bertani broth (without NaCl) containing gentamicin at 15 μg ml−1. Listeria monocytogenes strain RM2194 pNF8 is a clinical isolate labeled with GFP and was described previously (15). L. monocytogenes pNF8 was cultured at 28°C in Luria-Bertani broth (without NaCl) containing erythromycin and lincomycin at 1 and 25 μg ml−1, respectively.
Tetrahymena was isolated from soil in California, and the isolate was named SSU. Enrichment for Tetrahymena SSU was performed by the addition of E. coli strain DH5-α cells to aqueous soil suspensions. An axenic culture of this isolate was then obtained by incubation of the suspension in Neff's culture medium supplemented with penicillin G and streptomycin sulfate at 250 μg ml−1 each and in amphotericin B at 1.25 μg ml−1, followed by incubation in Tris-NaCl buffer (10 mM Tris, 7 mM NaCl, pH 7.4; TSB) containing tetracycline at 20 μg ml−1. Stock cultures were prepared as described by Bruns et al. (8) and stored in liquid nitrogen. For all experiments, Tetrahymena was grown in two-thirds-strength modified plate count broth (Becton Dickinson, Sparks, Md.) in a stationary culture flask for 4 days at 25°C.
Identification of the protozoan isolate.
Total genomic DNA was extracted from cultures of the unidentified protozoan and used as template in PCRs by using primers complementary to strongly conserved motifs which amplify bases 97 through 1731 of the nuclear small-subunit rRNA gene (5′-GAA ACT GCG AAT GGC TC-3′ and 5′-TGA TCC TTC TGC AGG TTC ACC TAC-3′) and Platinum Taq high-fidelity DNA polymerase (Invitrogen Corp., Carlsbad, Calif.). An initial 3-min denaturation at 94°C preceded 35 cycles consisting of 30-s denaturation at 94°C, 45-s annealing, and 90-s extension at 72°C. A single, bright band corresponding to approximately 1,800 bp was obtained in each of eight reactions whose annealing temperatures ranged from 50 to 55°C. No amplification resulted from any of eight similarly extracted negative controls. After enzymatic removal of unincorporated primers and deoxynucleoside triphosphates by using ExoSAP-IT (USB Corp., Cleveland, Ohio), PCR products were directly sequenced using Big Dye terminator 3.1 chemistries and an ABI 3100 automated fluorescent sequencer (Applied Biosystems, Foster City, Calif.) with the original primers as well as a series of internal sequencing primers (21). Sequence chromatograms were aligned and edited using the ContigExpress module of vector NTI 9 (Invitrogen Corp.).
The public nucleotide database was searched using BLAST for those sequences most similar to that obtained from our unidentified protozoan isolate. 18S rRNA gene sequences of Tetrahymena species, as well as all sequences especially similar to the query sequence, were retrieved and used to construct a multiple-sequence alignment that also included our new sequence by using CLUSTALW. The resulting alignment was used to reconstruct gene trees employing the neighbor-joining algorithm and using the minimum-evolution and maximum-parsimony objective criteria as implemented in molecular evolutionary genetics analysis 2 software (18). Kimura 2-parameter distances were employed when using the minimum-evolution and neighbor-joining tree methods of phylogenetic analysis. All analyses were applied to 500 bootstrap resamplings of the data matrix as a means to evaluate the robustness of the resulting phylogenetic hypothesis to sampling variance across the molecule.
Grazing and vesicle production.
Suspensions of early-stationary-phase bacteria cultured as described above were washed twice in TSB and resuspended in fresh TSB. Tetrahymena cells were washed three times by repeated centrifugation in TSB, gradually diluting to full-strength TSB to prevent osmotic shock of the cells, and then washed twice in full-strength TSB. Tetrahymena cells were enumerated with a hemacytometer by direct microscopy. Mixed suspensions of washed Tetrahymena and S. enterica or L. monocytogenes cells were prepared in microcentrifuge tubes at several ratios of bacteria to ciliate ranging from 500:1 to 10,000:1, with a constant ciliate concentration of 5 × 104 cells ml−1. Other tests used Tetrahymena cells starved in TSB for 16 h at 25°C after culturing and washing as described above. All mixed suspensions were incubated without agitation at 25°C. In accord with the terminology used by Berk et al. (5), the vacuole containing bacterial cells that was expelled at the protozoan cytoproct will be heretofore termed a vesicle.
Measurement of bacterial-cell number per vesicle.
The frequency distribution of bacterial-cell number per vesicle was determined with vesicle samples obtained from starved and nonstarved Tetrahymena cells as described above. The vesicles were produced in mixed suspensions with STN pGT-Kan-to-Tetrahymena ratios ranging from 500:1 to 10,000:1, keeping the ciliate concentration at 104 cells ml−1. After a 6-h feeding period, the number of STN pGT-Kan cells per vesicle was counted for each of 50 vesicles under the epifluorescence microscope with a fluorescein filter. Vesicles were examined from three replicate tubes.
Assessment of bacterial-cell viability.
Mixed suspensions of Tetrahymena and STN pGT-Kan were incubated in microcentrifuge tubes at concentrations of 5 × 104 and 107 cells ml−1, respectively, for ca. 6 h until most bacterial cells had been ingested. The mixed suspensions were then placed on ice for 16 h to kill all the Tetrahymena cells without lysis. This enabled the assessment of bacterial-cell survival without the interference of live ciliates. The suspensions were then incubated without agitation for 5 days at 25°C for assessment of bacterial-cell viability at regular time intervals.
In order to measure the proportion of viable bacterial cells among the population of cells contained in expelled Tetrahymena vesicles versus that of cells that remained free in suspension, propidium iodide (PI; Molecular Probes, Eugene, Oreg.) was added at a final concentration of 37 μM to each of two replicate tubes containing the mixed suspension described above. The tubes were incubated for 15 min, and 12-μl aliquots of each replicate suspension were placed on Polysine slides (Erie Scientific Company, Portsmouth, N.H.). At least 100 Tetrahymena vesicles were examined at random per slide under an epifluorescence microscope equipped with a fluorescein filter (Leica Microsystems, Wetzlar, Germany), and the numbers of green fluorescent bacterial cells (viable) and of red fluorescent bacterial cells (nonviable) per vesicle were estimated. For each vesicle examined, the fluorescence of 15 free cells in the vicinity of the vesicle was also recorded. The proportions of viable cells per vesicle and per area around the vesicle were estimated at regular time intervals from two replicate tubes and compared statistically. Cells that had yellow fluorescence because of simultaneous red and green fluorescence, which were rare in our study, were not included in the counts. Assessment of viability of GFP-labeled bacterial cells with PI was confirmed with staining of non-GFP bacterial cells by using the Live/Dead BacLight viability system (Molecular Probes).
Survival to calcium hypochlorite treatment.
The suspension containing the vesicles, the ciliates, and the few free bacteria that remained uningested was filtered by gravity flow through a 0.2-μm-pore-size Isopore black membrane (Millipore, Billerica, Mass.). The membrane was transferred and submerged twice in TSB in the well of a six-well tissue culture plate to remove most of the ciliates. The membrane with the vesicles and free bacteria bound to it was then immersed into a solution of calcium hypochlorite in deionized water for 10 min at 0, 1, 2, and 3 ppm, corresponding to free chlorine concentrations of 0.016, 0.13, 0.42, and 0.63, respectively. Free chlorine in the suspension in the presence of the membrane was estimated with a free chlorine kit (Chemetrics, Calverton, Va.). Tap water was estimated to contain 0.036 ppm of free chlorine. Following exposure to the chlorine solution, the membrane was washed twice by immersion in deionized water for 10 min and viability of the bacteria was assessed by PI staining as described above. Two replicate membranes with vesicles and bacteria from two separate grazing suspensions were used per chlorine concentration, and a total of 100 vesicles or areas around the vesicles were examined for cell viability.
Statistical methods.
The Kilmogorov-Smirnov test was carried out to determine if the data or transformed data followed a normal distribution. The runs test was used to determine that the proportion of viable cells varied linearly with time of incubation. Slopes of all regressions were determined to be significantly different from zero at P < 0.01. Statistical tests were performed with the program Prism version 3.0 (GraphPad Software, Inc., San Diego, Calif.). All experiments were performed in replicate with different cultures of each organism and at different times.
Nucleotide sequence accession number.
The DNA sequence coding for the small-subunit rRNA of Tetrahymena SSU was deposited in the GenBank database under the accession number AY755629.
RESULTS
Identification of ciliate isolate.
The ciliate was identified using a 1,635-bp portion of small-subunit rRNA. Variation in the full-length small-subunit rRNA provided sufficient information to confidently place the ciliate within a subclade of Tetrahymena that also contained Tetrahymena farleyi, Tetrahymena mobilis, Tetrahymena tropicalis, and an unnamed species of Tetrahymena corresponding to GenBank accession number TSP511862 (Fig. 1). Although its rRNA contains unique features, its membership in this subclade is supported by 98 and 94% of the bootstrap replicate analyses performed using the minimum-evolution and neighbor-joining methods, respectively. In 85% of trees reconstructed under the criterion of maximum parsimony, T. farleyi was inferred as the ciliate with which our isolate shared the most recent common ancestry (data not shown). Limiting phylogenetic analyses to only the most closely related species of Tetrahymena did not afford us additional precision in estimating the phylogenetic position of our isolated ciliate.
FIG. 1.
Phylogenetic tree showing the relationship of Tetrahymena SSU to other organisms based on the sequence of their 18S rRNA gene. The tree was reconstructed from Kimura 2-parameter distances under the criterion of minimum evolution. Nodes are labeled by their percentage of support in 500 bootstrap replicate analyses.
Grazing and vesicle production.
Tetrahymena fed readily on S. enterica and L. monocytogenes cells. Food vacuoles were apparent almost immediately after the addition of bacteria to the suspension, and nearly all Tetrahymena cells had internal food vacuoles within 60 min. This was observed for starved and unstarved cells of the ciliate. During the course of the grazing period, numbers of Tetrahymena cells remained constant and no Tetrahymena cell lysis was detected. Tetrahymena packaged STN pGT-Kan cells at high density in as many as 40 vacuoles per ciliate. As shown in Fig. 2A, most STN pGT-Kan cells in the internal food vacuoles of Tetrahymena retained their integrity and cell profile. In contrast, L. monocytogenes pNF8 cells were digested soon after phagocytosis and few vacuoles containing intact L. monocytogenes cells were detected in the ciliate cells (Fig. 2B). The digestion of L. monocytogenes by Tetrahymena imparted a homogeneous green fluorescence on the food vacuoles, presumably caused by the release of GFP from the lysed bacterial cells (Fig. 2B, inset). SYTO 9 (Molecular Probes), a general nucleic acid stain that imparts green fluorescence to bacterial cells, was used to confirm our observation that most L. monocytogenes cells were digested in the vacuoles. Similar to our results obtained with GFP-labeled L. monocytogenes, SYTO 9 staining of Tetrahymena cells that fed on L. monocytogenes cells not transformed with GFP revealed very few fluorescent bacterial cells in the food vacuoles.
FIG. 2.
Confocal micrographs of the interaction of Tetrahymena SSU with S. enterica serovar Thompson and L. monocytogenes. Staining with the green fluorescent DNA stain SYTO 9 revealed that (A) serovar Thompson was maintained at high density within the food vacu-oles of Tetrahymena while (B) L. monocytogenes cells were mostly digested (white arrows). The inset in panel B shows that grazing on L. monocytogenes pNF8 resulted in the presence of vacuoles that appeared fluorescent but with few bacterial cells (blue arrows) due to the release of GFP from digested cells. (C) Single optical slice through a large vesicle secreted by Tetrahymena and containing GFP-labeled serovar Thompson cells, only a few of which appeared nonviable by PI staining (yellow arrow), is shown. The micrographs were acquired by using a confocal laser scanning microscope (Leica Microsystems). Bars, 10 μm.
In addition to surviving in the Tetrahymena food vacuoles, S. enterica cells in the vacuoles were expelled by the nonlytic elimination of ingested material at the posterior end (cytoproct) of Tetrahymena cells. By this process, clusters of S. enterica cells presumably surrounded by a membrane were released by Tetrahymena (Fig. 3) (see http://www.tntech.edu/wrc/119.htm and http://www.tntech.edu/wrc/7293.htm for supplementary material showing video clips of the active release by Tetrahymena of vesicles containing S. enterica cells and the laboratory-induced burst of a vesicle discharging many S. enterica cells, respectively). During the grazing experiments, Tetrahymena cells began expelling vesicles within 1 h after initiation of feeding. Tetrahymena cells that were starved for 18 h and then fed with STN pGT-Kan cells at a ratio of 1:300 (ciliate to bacteria) produced ca. 40 vesicles per ciliate and 2 × 106 vesicles per ml of suspension. In comparison, Tetrahymena produced very few vesicles while grazing on L. monocytogenes pNF8 and most vesicles harbored fewer than five cells. Figure 2B shows a vesicle (white arrow) being released by Tetrahymena and containing one L. monocytogenes cell.
FIG. 3.
(A through F) Time sequence of the active release by Tetrahymena SSU of a vesicle (black arrows) containing S. enterica serovar Thompson cells after the starved cells of the protozoan grazed on this human pathogen. Note the membrane protruding from the Tetrahymena cell at the time of vesicle release (panels E and F, white arrows). The differential interference contrast images were acquired through a Nikon Microphot microscope with a VHS video, and the frames were converted to digital images. Bar, 10 μm.
The STN pGT-Kan cells in the vesicles appeared viable since they had a distinct cell profile and showed green fluorescence. Cell viability within vesicles was confirmed by PI staining. This DNA stain permeates only bacterial cells that have lost membrane integrity and may be nonviable, imparting red fluorescence to such cells. PI staining revealed that a small percentage of the vesicles contained a few STN pGT-Kan cells that had a compromised membrane or were dead, while most cells displayed green fluorescence due to GFP (Fig. 2C). Similar observations were made after Live/Dead BacLight staining of vesicles containing S. enterica cells that were not transformed with GFP, showing that most cells in the vesicles were green (viable), while very few or none were red (nonviable). The release of viable STN pGT-Kan cells in vesicles by Tetrahymena was observed in tap water and noncarbonated mineral water purchased at the supermarket.
Frequency distribution of bacterial-cell number per vesicle.
The number of STN pGT-Kan cells per vesicle increased significantly as the ratio of bacteria to ciliate increased, with means of 8.2, 10.2, 12.8, and 12.6 at ratios of 500:1, 1,000:1, 5,000:1, and 10,000:1, respectively (Fig. 4A through C for the three lowest ratios). The Tukey-Kramer multiple-comparison test on the mean of the log-normal value of the number of cells per vesicle showed that the number of bacteria per vesicle was significantly greater at the higher ratio in all pairs (P < 0.01), with the exception of the two highest ratios, for which the means were not significantly different (P > 0.05). The box plot shown in Fig. 4D demonstrates that the distribution of the number of cells per vesicle is increasingly skewed toward high values and has an increasing number of outliers as the ratio increases to 5,000:1.
FIG. 4.
Frequency distribution of the number of S. enterica serovar Thompson pGT-Kan cells in vesicles released by Tetrahymena SSU when both were incubated in TSB buffer at initial ratios of bacteria to protozoa of 500:1 (A), 1,000:1 (B), and 5,000:1 (C). Box plot of the frequency distribution data showing the increase in the median (divider line in the box) and mean (✖) values of the number of cells per vesicle and the increased skewed state of the distribution as the ratio of bacteria to protozoa increases (D).
Although prior starving of Tetrahymena resulted in fewer expelled vesicles, these vesicles contained a similar frequency distribution of bacterial cells (data not shown). The diameter of the vesicles under the light microscope ranged from 3.1 to 8.0 μm, with a mean of 5.09 and standard deviation of 0.99, at an initial ratio of bacteria to ciliate of 500:1. Thus, the vesicles are small enough to potentially be aerosolized and spread in the environment.
Bacterial survival in Tetrahymena vesicles.
The survival of STN pGT-Kan cells in vesicles was compared with that of cells that remained free in the suspension by using GFP fluorescence in combination with PI viability staining. Cells that emitted GFP fluorescence and lacked red fluorescence were considered viable. This method provided a reliable assessment of bacterial-cell viability and integrity since most STN pGT-Kan cells stained with PI after being exposed to various lethal stresses (high temperature, desiccation, or exposure to calcium hypochlorite) fluoresce red exclusively. A few such cells fluoresce only green, and only 1% of the cells fluoresce both red and green simultaneously (M. Brandl, unpublished data). Lowder et al. (19) reported similar observations with Pseudomonas fluorescens. Thus, GFP and PI yield a good segregation of fluorescent labels in a mixed population of viable and nonviable cells. Viability of the bacterial cells within the vesicles was confirmed qualitatively by their culturability on Luria-Bertani agar plates. However, plating was not deemed an adequate method in this study for quantitative measurement. Because of the difficulty in breaking open the vesicles, it was expected that single colonies would arise from clusters of bacterial cells within the vesicles and thus CFU counts would not reflect single-bacterial-cell populations accurately.
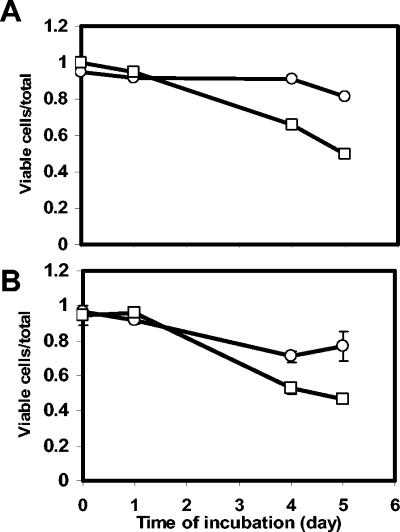
Most STN pGT-Kan cells in the vesicles were viable at the time of their release by Tetrahymena (Fig. 5). Cells of STN pGT-Kan that were not ingested by Tetrahymena and remained in suspension lost viability over a period of 5 days at a significantly higher rate than STN pGT-Kan cells located in the vesicles expelled by Tetrahymena into the same suspension. This difference in survival rate was observed for Tetrahymena cells that were both starved (Fig. 5A) and unstarved (Fig. 5B). Regression analysis of the proportion of viable cells to the total number of bacterial cells over time demonstrated that the survival rate of STN pGT-Kan cells was significantly higher in vesicles than when remaining free in the suspension (for starved Tetrahymena, F = 28.3, P < 0.001; for unstarved Tetrahymena, F = 14.09, P < 0.005).
FIG. 5.
Comparative survival of S. enterica serovar Thompson pGT-Kan in vesicles released by Tetrahymena SSU (○) and as free cells remaining uningested in the same solution (□). Cell viability was evaluated by PI staining. The vesicles were produced by Tetrahymena cells that were starved (A) or not starved (B) prior to grazing. Each value represents the mean and standard error of the mean of the proportion of viable serovar Thompson cells over the total number of cells per vesicle or per area around the vesicle for 100 observations under the epifluorescence microscope. Due to the scale of the y axis, the error bars in panel A are too small to be visualized.
Resistance to calcium chloride treatment.
STN pGT-Kan cells had similar viability in deionized water without calcium hypochlorite over a short period of time (10 min), whether they were free or entrapped in Tetrahymena vesicles. However, when exposed to calcium hypochlorite at concentrations of 1 and 2 ppm for 10 min, a significantly higher proportion of STN pGT-Kan cells survived when within vesicles than when free (Fig. 6) (for 1 ppm, t = 7.0, P < 0.01; for 2 ppm, t = 3.25, P < 0.05). At a concentration of 2 ppm of calcium hypochlorite, the average proportion of cells that were viable was 4.6-fold higher for vesicle-enclosed cells than for free cells. When treated with calcium hypochlorite at a concentration of 3 ppm, all STN pGT-Kan cells appeared to be dead, whether the cells were present in vesicles or free.
FIG. 6.
Comparative survival of S. enterica serovar Thompson pGT-Kan in vesicles released by Tetrahymena SSU (gray bars) and as free cells remaining uningested in the same solution (black bars). Cell viability was evaluated by PI staining. Each value represents the mean and standard error of the mean of the proportion of viable serovar Thompson cells over the total number of cells per vesicle or per area around the vesicle for 100 observations under the epifluorescence microscope. For each treatment, bars with different letters depict significant differences between the means at P < 0.05.
DISCUSSION
The interaction of protozoa with bacteria has been examined mostly from the traditional perspective of predator-prey relationships. Insightful investigations into the ecology of L. pneumophila in association with Acanthamoeba spp. (1) and increasing concerns over the survival and dissemination of food-borne pathogens in the environment have sparked a new interest in the interaction of human pathogens with protists. Although the protective effect of the internalization of enteric bacteria within protozoa has been reported (17), few studies have investigated the active release of enteric pathogens from protozoa. Here, we examined the interaction of S. enterica serovar Thompson, a prevalent food-borne pathogen, with a soilborne ciliated protozoan that we identified as a Tetrahymena of unknown species based on its 18S rRNA sequence.
Temporal observations of the behavior of serovar Thompson pGT-Kan after phagocytosis by Tetrahymena were made with the use of GFP as an intrinsic label to track the fate of the bacterial cells following ingestion by the ciliate. These observations revealed that serovar Thompson has the ability to evade digestion in the food vacuoles of this protozoan. This was further established by the staining with Live/Dead BacLight of serovar Thompson cells that were not transformed with GFP, which showed that the Tetrahymena vacuoles contained a high number of viable bacterial cells. In contrast to serovar Thompson, L. monocytogenes pNF8 was digested at a high rate by Tetrahymena. SYTO 9 staining of the protozoa during grazing on L. monocytogenes cells that were not labeled with GFP failed to detect a higher density of bacterial cells within the vacuoles. Thus, the rare detection of L. monocytogenes pNF8 cells in Tetrahymena vacuoles was not due to the loss of GFP signal from the bacterial cells or to lower survival of the cells in the vacuoles because of GFP. S. enterica serovar Typhimurium strain SL1344 and various L. monocytogenes isolates behaved similarly to serovar Thompson and L. monocytogenes pNF8 (data not shown), respectively, indicating that these behaviors are not restricted to the Salmonella serovar and L. monocytogenes strain used in this study. Other studies have suggested that various serovars of S. enterica (14, 25), as well as E. coli O157:H7 (4), have the ability to replicate within the vacuole of Acanthamoeba species.
The difference in resistance of S. enterica serovar Thompson and L. monocytogenes to digestion by Tetrahymena indicates that the two pathogens interact with this protozoan in different fashions. It is unlikely to be due simply to the different nature of the cell wall of the two pathogens, since we observed that the human pathogen Enterococcus avium, a gram-positive bacterium like L. monocytogenes, resists digestion by Tetrahymena. Rather, it is possible that serovar Thompson has the ability to alter the normal events related to digestion of bacterial cells within the Tetrahymena food vacuole. It is known that serovar Typhimurium avoids killing in host epithelial and phagocytic cells by redirecting the maturation of the invasion vacuole or phagosome, respectively, thus preventing its fusion to the lysosome (7). Likewise, serovar Thompson may sense conditions in the food vacuoles of Tetrahymena that are similar to those in the vacuoles or phagosomes of host cells and upregulate various genes that allow it to escape digestion by Tetrahymena. Gao et al. (13) have demonstrated that various aspects of the infection of protozoa and macrophages by L. pneumophila are similar at the molecular level. The molecular basis of the interaction of S. enterica with ciliates such as Tetrahymena has not been explored and is the subject of current studies in our laboratory.
The persistence of serovar Thompson through the digestive cycle of Tetrahymena led to its release as viable cells in defecation vacuoles at the cytoproct of the ciliate cells. The egestion of viable bacterial cells in vesicles was reported also for L. pneumophila during grazing by Acanthamoeba spp. (5) and for E. coli K12 during grazing by T. pyriformis (24). Release of vesicles containing viable serovar Thompson cells was observed with a ratio of bacteria to starved ciliate as low as 50 to 1, with bacterial and ciliate concentrations of 5 × 105 and 104, respectively. These concentrations are relevant to the biology of Tetrahymena since it has been reported that T. pyriformis stops feeding below the bacterial concentration of 105 cells per ml and then seeks other food sources by increasing its swimming speed (26). The higher ratios of serovar Thompson to Tetrahymena cells used in the remainder of our study ensured that sufficient vesicles were produced to enable statistical analysis of the data. Frequency distribution analysis indicated that the bacterial-cell density within the vesicles released by Tetrahymena increases as the initial ratio of bacterial to ciliate cells increases. Thus, it is probable that Tetrahymena releases vesicles containing high densities of S. enterica in natural aqueous environments where S. enterica is present at high concentration, such as sites that are contaminated with manure. Because the bacterial cells are difficult to physically release from the vesicles, the presence of S. enterica within vesicles likely leads to an underestimation of actual population sizes of the pathogen during predation studies or surveys due to the packaging of the bacterial cells as high-density clusters that act as a single CFU.
Our results suggest that serovar Thompson survives at a higher rate over time within the vesicles released by Tetrahymena than when free in the same suspension. This protective effect may be due to the presence of a membrane around the vesicle, such as that demonstrated for L. pneumophila-containing vesicles released by A. polyphaga (5) and by Tetrahymena sp. (R. Garduòo, personal communication). The membrane and/or the presence of the bacteria as aggregates may have shielded the bacteria from exposure to inhibiting compounds present in the suspension or other types of stresses. Survival of L. pneumophila sequestered in Tetrahymena vesicles was enhanced compared with free cells during desiccation (S. G. Berk, J. S. Thomas, and R. S. Ting, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. Q195, 2003) and exposure to germicidal UV light (S. G. Berk, K. S. Redding, J. S. Thomas, and E. L. Williams, Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. Q057, 2004). In the present study, reduced biocidal activity of calcium hypochlorite against serovar Thompson cells that are sequestered in vesicles, compared to that for free cells, further supports this hypothesis. The concentrations of calcium hypochlorite at which this survival difference was observed are relevant to conditions where free chlorine is dilute, as in tap water, or when it has dissipated, become sequestered in the organic biomass, or been diffused to microsites where microbes colonize food or food-processing equipment.
Our observation that S. enterica cells can be released from Tetrahymena as high-density aggregates protected by a membrane and in which they have enhanced survival has implications for the ecology of this human pathogen that reach beyond the trophic relationship characteristic of predator-prey interactions. The role of this sequestration in the ecology of S. enterica is worthy of exploring in relation to public health issues.
Acknowledgments
We thank E. Williams and S. Sasser for technical assistance and D. Dunams for sequencing the Tetrahymena rRNA gene. We are grateful to L. Gorski for the gift of L. monocytogenes pNF8 and S. Schneider for providing the soil filtrate sample from which we isolated Tetrahymena SSU.
This work was funded by the U.S. Department of Agriculture, Agriculture Research Service CRIS projects 201-5325-210-40 and 401-1265-140-10, and by the Center for the Management, Utilization and Protection of Water Resources, Tennessee Technological University, Cookeville, TN.
REFERENCES
- 1.Abu Kwaik, Y., L.-Y. Gao, B. J. Stone, C. Venkataraman, and O. S. Harb. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, J., and M. R. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. [DOI] [PubMed] [Google Scholar]
- 3.Barker, J., M. R. Brown, P. J. Collier, I. Farrell, and P. Gilbert. 1992. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 58:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker, J., T. J. Humphrey, and M. W. Brown. 1999. Survival of Escherichia coli O157 in a soil protozoan: implications for disease. FEMS Microbiol. Lett. 173:291-295. [DOI] [PubMed] [Google Scholar]
- 5.Berk, S. G., R. S. Ting, G. W. Turner, and R. J. Ashburn. 1998. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 64:279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandl, M. T., and R. E. Mandrell. 2002. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl. Environ. Microbiol. 68:3614-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brumell, J. H., and S. Grinstein. 2004. Salmonella redirects phagosomal maturation. Curr. Opin. Microbiol. 7:78-84. [DOI] [PubMed] [Google Scholar]
- 8.Bruns, P. J., H. R. Smith, and D. Cassidy-Hanley. 2000. Long-term storage. .In J. D. Forney (ed.), Methods in cell biology, vol. 62: Tetrahymena thermophila. Academic Press, San Diego, Calif. [PubMed]
- 9.Campbell, J. V., J. Mohle-Boetani, R. Reporter, S. Abbott, J. Farrar, M. T. Brandl, R. E. Mandrell, and S. B. Werner. 2001. An outbreak of Salmonella serotype Thompson associated with fresh cilantro. J. Infect. Dis. 183:984-987. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., K. S. de Felipe, M. Clarke, H. Lu, O. R. Anderson, G. Segal, and H. A. Shuman. 2004. Legionella effectors that promote nonlytic release from protozoa. Science 303:1358-1361. [DOI] [PubMed] [Google Scholar]
- 11.Cirillo, J. D., S. Falkow, and L. S. Tompkins. 1994. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 62:3254-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirillo, J. D., S. Falkow, L. S. Tompkins, and L. E. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, L. Y., O. S. Harb, and Y. Abu Kwaik. 1997. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect. Immun. 65:4738-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaze, W. H., N. Burroughs, M. P. Gallagher, and E. M. H. Wellington. 2003. Interactions between Salmonella typhimurium and Acanthamoeba polyphaga, and observation of a new mode of intracellular growth within contractile vacuoles. Microb. Ecol. 46:358-369. [DOI] [PubMed] [Google Scholar]
- 15.Gorski, L., J. D. Palumbo, and K. D. Nguyen. 2004. Strain-specific differences in the attachment of Listeria monocytogenes to alfalfa sprouts. J. Food Prot. 67:2488-2495. [DOI] [PubMed] [Google Scholar]
- 16.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King, C. H., E. B. Shotts, Jr., R. E. Wooley, and K. G. Porter. 1988. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 54:3023-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 19.Lowder, M., A. Unge, N. Maraha, J. K. Jansson, J. Swiggett, and J. D. Oliver. 2000. Effect of starvation and the viable-but-nonculturable state on green fluorescent protein (GFP) fluorescence in GFP-tagged Pseudomonas fluorescens A506. Appl. Environ. Microbiol. 66:3160-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNealy, T., A. L. Newsome, R. A. Johnson, and S. G. Berk. 2001. Impact of amoebae, bacteria, and Tetrahymena on Legionella pneumophila multiplication and distribution in an aquatic environment. .In R. Marre et al. (ed.), Legionella. ASM Press, Washington, D.C.
- 21.Medlin, L., H. J. Elwood, S. Stickel, and M. L. Sogin. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491-499. [DOI] [PubMed] [Google Scholar]
- 22.Miller, W. G., J. H. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 23.Miltner, E. C., and L. E. Bermudez. 2000. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 44:1990-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlimme, W., B. Baur, K. Hanselmann, and B. Jenni. 1995. An agarose slide method to follow the fate of bacteria within digestive vacuoles of protozoa. FEMS Microbiol. Lett. 133:169-173. [DOI] [PubMed] [Google Scholar]
- 25.Tezcan-Merdol, D., M. Ljungström, J. Winiecka-Krusnell, E. Linder, L. Engstrand, and M. Rhen. 2004. Uptake and replication of Salmonella enterica in Acanthamoeba rhysodes. Appl. Environ. Microbiol. 70:3706-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson, P. J., K. Ohtaguchi, and A. G. Frederickson. 1981. Kinetics of growth of the ciliate Tetrahymena pyriformis on Escherichia coli. J. Gen. Microbiol. 122:323-333. [DOI] [PubMed] [Google Scholar]
- 27.Winiecka-Krusnell, J., and E. Linder. 2001. Bacterial infections of free-living amoebae. Res. Microbiol. 152:613-619. [DOI] [PubMed] [Google Scholar]