Abstract
Contemporary agriculture heavily relies on pesticides for pest eradication and disease management. Consequently, current study was carried out to assess the acaricidal/antifungal efficacy of emulsifiable concentrate (10 % EC) derived from Boswellia carterii (B. carterii) against adult females of Tetranychus urticae (T. urticae), and five fungal pathogens. The meticulous examination of the chemical constitution of the crude extracts derived from the resin of B. carterii was conducted through the employment of the venerable technique known as Gas-Liquid Chromatography (GLC). The formulated petroleum-ether extract (FPEE) and formulated ethyl-acetate extract (FEAE) of B. carterii at a concentration of 10 mg ml−1 exhibited notable antioxidant activity with rates of 62.0 % and 90.8 %, respectively. In vitro, the FEAE exhibited potent inhibition against all the tested phytopathogenic fungi at different concentrations, whereas FPEE showed comparatively less efficacy. Interestingly, at 4000 ppm concentration, FEAE completely ceased the mycelial growth compared with the control. Moreover, following a span of 72 h of intervention, FPEE exhibited a greater degree of toxicity towards mature females of the T. urticae. This was evidenced by the LC50 value of 422.52 parts per million (ppm) for FPEE, which surpassed the LC50 value of 539.50 ppm observed for FEAE. In summary, the present study indicates that B. carterii resin formulated as an emulsifiable concentrate (10 % EC) can offer a natural and effective alternative for integrated pest management, thereby reducing reliance on synthetic pesticides and offering a more environmentally sustainable strategy for pest control.
Keywords: Frankincense resin, Tetranychus urticae, Antioxidant, Antifungal, Unsaponifiable matters, Fatty acids
1. Introduction
Frankincense, a splendidly organic resin extracted from the majestic Boswellia trees of the esteemed Burseraceae family (Han et al., 2017), emanates from none other than the illustrious Boswellia carterii (B. carterii), which thrives in the arid and lofty realms of East Africa, China, and India (Frank et al., 2009). Boswellia trees offer abundant reservoirs of natural resins and various bioactive compounds. The essential oil found in resin comprises 5–9 % of its composition. Additionally, it contains alcohol-soluble resin comprising 65–85 % and water-soluble gum accounting for approximately 20 %, which is a blend of heteropolysaccharides, polysaccharides, arabinogalactans, glycoproteins and polymeric substances (Al-Yasiry and Kiczorowska, 2016).
Furthermore, the resin possesses a significant amount of non-volatile triterpenoic constituents, including ursane (β-boswellic acid), oleonane (α-boswellic acid), and lupine, which have been associated with a range of biological actions (Karlina et al., 2007, Al-Yasiry and Kiczorowska, 2016). Studies by Ahmed et al. (2015) and Sultan (2020) have verified the presence of fatty acids in the resin, such as oleic, myristic, palmitic, linoleic, arachidic, arachidonic, and lignoceric acids, which contribute to its antibacterial properties. Furthermore, Ayub et al. (2018) have elucidated the presence of phenolic compounds, such as thujene, camphene, β-pinene, myrcene, limonene, M−cymene, and cis-verbenol, which manifest antimicrobial properties.
The resin has a long history of use in traditional medicine for diverse purposes, including rheumatoid arthritis (Banno et al., 2006), and acknowledged properties such as antifungal, antibacterial, and anti-inflammatory effects (Huang et al., 2000). Recently, European countries have witnessed an upsurge in the popularity of frankincense resin due to its potential to manage persistent inflammatory problems (Mishra et al., 2020), promote skin health (Han et al., 2017), and exhibit anticancer activity (Frank et al., 2009, Swallah et al., 2020). Additionally, it's worth noting that the US Food and Drug Administration (FDA) has granted approval for its safe usage as a food additive (Raja et al., 2011).
The two-spotted spider mite (TSSM), Tetranychus urticae Koch (Tetranychidae), can infest a wide range of vegetable crops globally. It is found in tropical regions worldwide and has been identified in more than 3,877 plant species. This mite poses a significant economic threat to at least 150 plant varieties (Le Goff et al., 2009, Migeon et al., 2010, Islam et al., 2017). Its feeding behavior involves puncturing the leaf tissues and extracting the contents of individual plant cells. With a consumption rate of 18–22 plant cells per minute, it hampers photosynthesis, transpiration, leaf chlorophyll levels, and leaf nitrogen content, consequently diminishing plant growth and productivity (Kiran et al., 2017). Additionally, it is involved in transmitting various viruses, including potato virus Y, tobacco mosaic virus, and tobacco ring spot virus (Sarwar, 2020). The current climate change scenario will undoubtedly impact the role of spider mites as agricultural pests. In arid and warm climates, T. urticae accelerates its life cycle, increases its yearly generational count, and expands its range of host plants (Ximénez-Embún et al., 2017).
Fungi have a global reputation for causing diseases in plants. They can infiltrate plants through various means, such as natural openings like stomata and injuries resulting from pruning, harvesting, hail, insects, and mechanical damage. Prompt management actions are necessary to prevent tomato diseases from turning fatal. Fungi, including crown and root rot, fusarium wilt, early blight, rhizoctonia, phoma, and others cause most tomato diseases. These diseases significantly impede tomato production, leading to substantial economic losses (Sanoubar and Barbanti, 2017). Botrytis cinerea leads to grey mould, a destructive disease in strawberries. This pathogen negatively impacts the fruit at different stages, including in the field, during storage, transportation, and even in the market. The existence of grey mould is the primary cause for growers, shippers, and consumers to reject affected fruits, resulting in significant economic losses (Petrasch et al., 2019).
Pesticides are artificial compounds employed for pest eradication. Their global consumption reaches approximately two million tons annually. Although pesticides can enhance agricultural productivity, their indiscriminate usage has detrimental impacts on soil quality, human and animal health, water purity, and the emergence of issues such as insect resistance, genetic alterations in plants, and toxic residues in food and animal feed. Therefore, advocating for using biopesticides instead of chemicals can benefit our planet and well-being. Biopesticides are naturally occurring plant-based substances that control pests through non-toxic and environmentally friendly mechanisms (Aktar et al., 2009, Hussien et al., 2022).
Essential oils are organic substances that possess intricate volatile properties that contribute to the unique scents of plants. They are produced as secondary metabolites and serve as a natural defense mechanism for plants against various threats such as bacteria, fungi, viruses, insects, and herbivorous (Akthar et al., 2014, Gaber et al., 2021, Abdel-Wahab et al., 2022). Recently, they have gained attention due to their diverse range of advantageous effects on pests and disease-causing organisms. Consequently, they are considered potential substitutes to synthetic chemical pesticides in crop protection and pest management applications. They are less harmful than pesticides and can quickly evaporate, leaving behind minimal residue (Isman, 2020, Hussien et al., 2022, Helmy et al., 2023, Yousef et al., 2023).
Hence, the current study aims to examine the efficacy of emulsifiable concentrate (10 % EC) formulations derived from B. carterii against two-spotted spider mites and fungal pathogens in vitro. Moreover, it is imperative to ascertain the active constituents of these substances through the utilization of chromatographic and spectroscopic techniques.
2. Materials and methods
2.1. Plant material
The B. carterii resin utilized in this study was acquired from authenticated Egyptian herbal shops. The plant material was finely crushed and kept in airtight plastic bags at normal room temperature until use.
2.2. Preparation of resin extracts
Fifty grams of dried powdered sample were subjected to separate maceration procedures using petroleum ether (60–80 °C) and ethyl acetate using an orbital shaker (150 rpm) at room temperature for 24 h. The resulting extracts were then filtered through Whatman No.1 filter paper. Residues were re-extracted twice with fresh aliquots of the same solvents. The filters of each solvent were evaporated at 40 °C using a rotary vacuum evaporator to obtain crude extracts of petroleum ether and ethyl acetate. The dried sample of each extract was weighed to determine the yield of soluble constituents and subsequently stored at 4 °C (Gupta et al., 2022).
2.3. Phytochemical screening of B. carterii crude extracts
The phytochemical screening of PE and EA derived from B. carterii was carried out according to (Iqbal et al., 2015).
2.4. Antioxidant activity evaluation
2.4.1. Determination of total phenol content
The content of total phenolic compounds in EA of B. carterii was determined using a colorimetric method described by (Velioglu et al., 1998), with gallic acid employed as the standard phenolic acid. The results were expressed in mg of gallic acid equivalent per gram of extract (mg GAE g−1).
2.4.2. Determination of total flavonoid content
The content of total flavonoid compounds in EA of B. carterii was determined through a colorimetric method described by (Jiao and Wang, 2000), with quercetin employed as the standard flavonoid.
2.4.3. DPPH antioxidant assay
The antioxidant activity of B. carterii FPEE and FEAE were assessed for their ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals according to Liu et al. (2010). The DPPH was calculated according to Eq. (1):
| (1) |
where: A0; the absorbance of DPPH, A1; the absorbance of the sample
2.5. Chemical composition determinations using gas-liquid chromatography (GLC)
2.5.1. Preparation of unsaponifiable matters (USMs) and fatty acids (FAs)
The BE of B. carterii (0.5 g) was refluxed with 0.5 N alcoholic sodium hydroxide for 2 hrs in a boiling water bath for saponification. The saponified extract underwent a cooling process, followed by its combination with an equivalent volume of distilled water, and subsequent extraction using diethyl ether. The diethyl ether extract was dehydrated using anhydrous sodium chloride, and the remaining residue was weighed and preserved for further analysis (Firsta et al., 2020). The USMs (%) was calculated according to Eq. (2):
| (2) |
The alkaline aqueous remaining solution was acidified with hydrochloric acid to liberate the FAs, which were separated using diethyl ether. The diethyl ether extract was dehydrated by evaporation, and the residue was subjected to methylation with acidified methanol for 1 hr. The methylated fatty acids were extracted with diethyl ether. The fatty acids methyl esters were identified by the GC apparatus.
2.5.2. Identification/separation of USMs and FAs by GLC technique
The measurements were performed at the National Research Center, Giza, Egypt. The identification of USM and FAs were performed using a Gas Chromatograph (Agilent Technologies 6890 N) by comparing the sample retention time with standards (Adams, 2007).
2.6. Preparation of B. carterii extracts as an emulsifiable concentrate (10 % EC) formulation
2.6.1. Tested materials
-
a)
Active ingredient: Resin crude extracted by petroleum-ether (PE) and ethyl-acetate (EA).
-
b)
Surface active agents: Potassium salt and polyethene glycol 600 monolaurate are supplied by the Egyptian Starch, Yeast & Detergents Co., Canal El Mahmoudeya St., Moharram Bey, Alexandria, Egypt. Tween 80 supplied by EL-Gomhoria Chemical Company, Cairo, Egypt.
2.6.2. Physicochemical properties of formulation basic constituents
2.6.2.1. Active ingredient
-
a)
The solubility or miscibility was calculated following an Eq. (3) developed by (Nelson and Fiero, 1954).
| (3) |
where: W; weight of active ingredient, V; volume of solvent required for complete solubility.
-
b)
Acidity/Alkalinity was determined following (WHO, 1979) guidelines.
2.6.2.2. Surface active agents
-
a)
Surface tension was measured using a Du-Nouy tensiometer (ASTM D-1331, 2001).
-
b)
Hydrophilic-lipophilic balance (HLB) was determined according to (Lynch and Griffin, 1974).
-
c)
Critical micelle concentration (CMC) was calculated using (Osipow, 1964).
-
d)
Acidity/Alkalinity was determined as previously mentioned.
2.6.3. Formulation of B. carterii extracts
The emulsifiable concentrate (10 % EC) of B. carterii was prepared following (Rao et al., 2000). A certain quantity of extract was obtained, dissolved in an appropriate solvent, and an emulsifier was introduced. The mixture was stirred for 1 h, after which it was transferred to a measuring flask and filled with the same solvent to achieve volume of 10 mL. The flask was shaken to homogenize the solution, and the physicochemical properties of the formulation were conducted.
-
a)
Emulsion stability test was performed according to (CIPAC MT 36.3, 2003).
-
b)
The storage stability test was conducted as per (CIPAC MT 46.3, 2000).
2.6.4. Physicochemical properties of spray solution at field dilution rate (0.5 %)
-
a)
Surface tension was determined, as mentioned before.
-
b)
Viscosity was assessed using a viscometer according to (ASTM D-2196, 2005).
-
c)
Electrical conductivity was determined as per (CIPAC MT 75.3, 2000) using a conductivity meter (Thermo Orion 115A + USA).
-
d)
pH was measured as per (CIPAC MT 75.3, 2000) using a pH meter (Hanna pH 211).
2.7. In vitro antifungal activity of formulated B. carterii extracts
2.7.1. Fungal pathogens tested
The fungal pathogens Rhizoctonia solani, Sclerotium rolfsii, Fusarium oxysporum, Fusarium semitectum, Fusarium solani, Alternaria alternata, Botrytis cinerea, and Phoma sp. were acquired from the Department of Fungicides, Bactericides, and Nematicides at the Central Agricultural Pesticides Laboratory. The strains were cultured on a potato dextrose agar (PDA) medium under controlled conditions at a temperature of 25 ± 2 °C to ensure their viability and stability for subsequent investigations.
2.7.2. In vitro antifungal activity
In vitro, the FPEE and FEAE of B. carterii at different concentrations (1000, 2000, 3000 and 4000 ppm) were used to study their efficacy against fungal pathogens using food-poisoned technique (Schmitz, 1930). Uniform® 390SE (Azoxy-strobin + mefenoxam, 650 mL 200L-1) was used as a reference control at different concentrations (40, 81, 162 and 325 ppm). Both extracts were individually combined with 50 mL of sterilized Potato Dextrose Agar (PDA) medium and evenly distributed into three Petri dishes, serving as replicates. Following this, a fungal culture disc with a diameter of 6 mm was introduced precisely at the central region of every Petri dish. A zero treatment was prepared for each fungus utilized as a control. The dishes were subjected to incubation at a temperature of 25 ± 2 °C. The subsequent evaluation involved measuring the radial expansion of the fungus, which was performed once the mycelia of the control had nearly enveloped the entire surface area of the Petri dishes. The percentage of inhibition of fungal growth was determined using the following equation (4):
| (4) |
where: C; hyphal growth (mm) under control, T; hyphal growth (mm) under treatment
2.8. Toxicological effect of formulated B. carterii extracts on two-spotted spider mite
2.8.1. Rearing of two-spotted spider mites (TSSM)
The two-spotted spider mite (TSSM), Tetranychus urticae was reared at the Central Agricultural Pesticides Laboratory, Bioassay department, Agricultural Research Center, Dokki, Giza, Egypt, under lab conditions at 25 ± 2 °C, 65 ± 5 % RH, and a photoperiod of 16 light: 8 Dark h according to the procedure of Dittrich (1962).
2.8.2. In vitro acaricidal activity
To assess the toxicity of FPEE and FEAE at different concentrations (62.5, 125, 250, 500, 1000, 2000 and 3000 ppm) on T. urticae, the leaf disc-dip technique was used (Siegler, 1947). Additionally, control discs were dipped in tap water only. A reference control, Abamectin (Vertimec® 1.8 % EC) was utilized at concentrations of 2.25, 4.5, 18, 36 and 54 ppm. Mortality (%) were counted utilizing the methodology established by Abbott (1925) following the Eq. (5). The calculation of the toxicity index for the tested compounds was performed in accordance with the methodology described by Sun (1950) following the Eq. (6).
| (5) |
| (6) |
where: T; mortality (%) of treatment, C; mortality (%) of control.
2.9. Statistical analysis
All experiments were conducted in a completely randomized design (CRD) with three replications for each treatment. The statistical analysis of all data was conducted utilizing the principles of analysis of variance (ANOVA) and Duncan's multiple-range test (Duncan, 1955). The formula proposed by (Abbott, 1925) was employed to ascertain the corrected mortality rate of mites. The LC50 and LC90 values for various formulations were determined by logarithmically transforming each concentration and its corresponding probit value, which represents the inhibition percentage (Finney, 1971, Lei and Sun, 2018).
3. Results
3.1. Chemical composition of B. carterii resin crude extracts
This work evaluated the efficacy of B. carterii resin as an emulsifiable concentrate formulation against Tetranychus urticae and some phytopathogenic fungi (tomato fungal diseases and grey mould mulberry) in vitro. As shown in Table 1, we obtained 46.0 % and 39.8 % yield of yellowish resin crude extracted by petroleum-ether (PE) and ethyl-acetate (EA), respectively.
Table 1.
Extraction yields (%) of B. carterii extracts.
| Extract* | Plant Wt (g) | Extract Wt (g) | Yields (%) | Color of matter |
|---|---|---|---|---|
| PE | 10 | 4.60 | 46.0 | Yellow |
| EA | 10 | 3.98 | 39.8 | Yellow |
PE; petroleum-ether, EA; ethyl-acetate.
The phytochemical screening of EA obtained from B. carterii revealed the existence of saponins, alkaloids, flavonoids, phenolic compounds and glycosides. On the other hand, in B. carterii PE only steroids and tannins were identified (Table 2).
Table 2.
Phytochemical screening of frankincense resin-extracts.
| Phytochemical constitutes* | PE extract | EA extract |
|---|---|---|
| Saponins | – | + |
| Alkaloids | – | + |
| Flavonoids | – | + |
| Steroids | + | – |
| Tannins | + | – |
| Phenolic compounds | – | + |
| Glycosides | – | + |
PE; petroleum-ether, EA; ethyl-acetate, +; presence, –; absence.
3.2. Total phenolic and flavonoids contents in B. carterii ethyl-acetate
Phenolic and flavonoids are secondary metabolites with redox properties contributing to their antioxidant capabilities. Total phenol and flavonoids content in B. carterii EA were assessed using a colorimetric technique (Table 3). The cumulative phenolic and flavonoid contents in B. carterii EA were recorded 3.28 mg GAE g−1 and 0.29 mg QE g−1, respectively.
Table 3.
Total phenolic and flavonoid contents in B. carterii ethyl acetate.
| Extract* |
Total phenol (mg/g, GAE) |
Total flavonoids (mg/g, QE) |
|---|---|---|
| EA | 3.28 | 0.29 |
EA; Ethyl-acetate, GAE; gallic acid equivalent, QE; quercetin equivalent.
3.3. Identification/separation of USMs and FAs by GLC
The saponification procedure was conducted on a 0.5 g sample of B. carterii PE, yielding 12.6 % saponified matters (SMs) and 87.2 % unsaponifiable matters (USMs). Analysis using gas–liquid chromatography (GLC) identified 12 compounds in the saponified matters (SMs) (Fig. 1 and Table 4). The chromatographic profile of the SMs indicated a notable concentration of unsaturated fatty acids, constituting 69.4 % of the total content, while saturated fatty acids accounted 30.6 %. The most abundant compounds observed were oleic acid (36.5 %), followed by α-linolenic acid (16 %) and linoleic acid (13.1 %), whereas arachidic acid had the lowest presence at 1.3 %. These findings imply that B. carterii PE contains a substantial quantity of volatile components, with oleic acid being the most prominent and arachidic acid being the least abundant.
Fig. 1.
GLC chromatogram of the saponified matters in B. carterii petroleum-ether crude extract.
Table 4.
Type and percent of saponifiable compounds identified in B. carterii petroleum-ether crude extract by GLC.
| Compounds* | Chemical Structure | Area (%) | Rt (min) |
|---|---|---|---|
| Lauric (C12) | 3.2 | 8.5 | |
| Myristic (C14) | 1.7 | 12.8 | |
| Palmetic (C16) | 5.2 | 17.4 | |
| Palmitoleic (C16.1) |  |
2.1 | 18.4 |
| 9,12 hexadecadienoic (C16.2) |  |
1.7 | 19.2 |
| Heptadecanoic (C17) | 6.1 | 20.2 | |
| Stearic (C18) | 7.9 | 21.2 | |
| Oleic (C18.1) |  |
36.5 | 22.3 |
| Linoleic (C18.2) |  |
13.1 | 22.7 |
| αLinolenic (C18.3) |  |
16.0 | 23.1 |
| Arachidic (C20) | 1.3 | 24.0 | |
| Arachidonic (C20.4) | 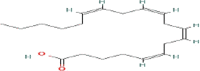 |
5.2 | 25.7 |
RT; Retention time obtained by chromatogram.
Fig. 2 and Table 5 display the chemical composition of unsaponifiable substances as determined by GLC analyses, confirming the presence of eight hydrocarbons (57.0 %) and four sterols (43.0 %). Among the components, the highest area under the curve was observed for n.henicosanoic (26.3 %), followed by squalene (17.1 %), β.sitosterol (15.5 %), and stigmasterol (14.7 %). Conversely, the lowest area under the curve was found for n.Docosane (0.9 %) and n.Tetracosane (0.6 %), indicates the most prominent and least abundant constituents in B. carterii PE, respectively.
Fig. 2.
GLC chromatogram of the unsaponified matters in B. carterii petroleum-ether crude extract.
Table 5.
Type and percent of unsaponifiable compounds identified in B. carterii petroleum-ether crude extract by GLC.
| Compounds* | Chemical Structure | Area (%) | Rt (min) |
|---|---|---|---|
| n.Nonadecane (C19) | 1.0 | 15.5 | |
| n.Eicosanoic (C20) | 1.2 | 16.1 | |
| n.Heneicosanoic (C21) | 26.3 | 17.7 | |
| n.Docosane (C22) | 0.90 | 19.1 | |
| n.Tetracosane (C24) | 0.6 | 21.5 | |
| n.Pentacosane (C25) | 7.4 | 23.5 | |
| Squalene (C30) | 17.1 | 24.1 | |
| n.Heptacosane (C27) | 2.5 | 25.2 | |
| Cholesterol like compound (C27) | 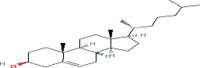 |
4.8 | 25.6 |
| Campesterol (C28) | 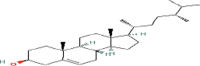 |
8.0 | 26.0 |
| Stigmasterol (C29) | 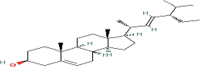 |
14.7 | 27.3 |
| β.sitosterol (C29) | 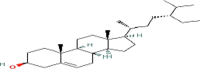 |
15.5 | 28.3 |
RT; Retention time obtained by chromatogram.
3.4. Characterization of formulation components
Table 6 displays the physicochemical characteristics of B. carterii PE and EA. The B. carterii PE demonstrated insolubility in water but solubility in xylene (83 %) and complete solubility in acetone (100 %), with an acidity level of 0.80. On the other hand, the B. carterii EA exhibited similar properties of insolubility in water but solubility in xylene (71 %) and complete solubility in acetone (100 %), showcasing the highest acidity level (3.43).
Table 6.
Physicochemical properties of B. carterii crude extracts as an active ingredient.
| Extract* |
Solubility % (W/V) |
Free acidity as H2SO4 | ||
|---|---|---|---|---|
| Water | Acetone | Xylene | ||
| PE | insoluble | 100 | 83 | 0.80 |
| EA | insoluble | 100 | 71 | 3.43 |
PE; petroleum-ether, EA; ethyl-acetate.
The results presented in Table 7 describe the physical and chemical characteristics of the surfactants utilized in the emulsifiable concentrate formulation of B. carterii resin. Both surfactants, namely Tween 80 and polyethene glycol 600 monolaurate (PEG 600ML) possessed hydrophilic-lipophilic balance values exceeding 13 and exhibited weakly acidic properties equivalent to 0.50 (Tween 80) and 0.88 (PEG 600ML), comparable to sulfuric acid. Additionally, both surfactants demonstrated low surface tension values, with Tween 80 and PEG 600ML measuring 39.2 and 30.64 dyne cm−1, respectively. Furthermore, the critical micelle concentration for Tween 80 and PEG 600 ML was determined to be 0.5 and 0.3 %, respectively.
Table 7.
Physicochemical properties of surfactants used for the preparation of B. carterii as an emulsifiable concentrate.
| Surfactants* | HLB | CMC | Free acidity as H2SO4 | Surface tension (Dyne cm−1) |
|---|---|---|---|---|
| Tween 80 | >13 | 0.5 | 0.50 | 39.2 |
| PEG 600ML | >13 | 0.3 | 0.88 | 30.6 |
HLB; hydrophilic-lipophilic balance, CMC; critical micelle concentration, PEG 600ML; polyethylene glycol 600 monolaurate.
The data in Table 8 provides an overview of the physical and chemical characteristics of emulsifiable concentrate (10 % EC) formulations. These formulations were subjected to three days of storage at a temperature of (54 ± 3 °C). The results indicate that the new formulations successfully passed in tests assessing the stability and spontaneity of the emulsions. No indications of oil separation, precipitation, or cream separation were observed in either soft or hard water. Before storage, the newly developed emulsifiable concentrates exhibited a mild acidity level, producing minimal foam in both types of water. However, there was a slight increase in free acidity for both formulations after accelerated storage.
Table 8.
Storage stability of B. carterii resin extracts prepared as an emulsifiable concentrate (10% EC).
| Storage | Physicochemical properties |
Extract |
||
|---|---|---|---|---|
| Petroleum Ether | Ethyl Acetate | |||
| Before storage | Spontaneity (%) | Hard | 100 | 100 |
| Soft | 100 | 100 | ||
| Emulsion stability | Hard | pass | pass | |
| Soft | pass | pass | ||
| Foam (cm3) | Hard | 4 | 3 | |
| Soft | 4 | 3 | ||
| Free acidity as % H2SO4 | 0.39 | 0.69 | ||
| After storage | Spontaneity (%) | Hard | 100 | 100 |
| Soft | 100 | 100 | ||
| Emulsion stability | Hard | pass | pass | |
| Soft | pass | pass | ||
| Foam (cm3) | Hard | 4 | 3 | |
| Soft | 4 | 3 | ||
| Free acidity as % H2SO4 | 0.49 | 0.78 | ||
Table 9 presented the physicochemical properties of B. carterii resin extracts formulated into an emulsifiable concentrate (10 % EC) and used at a field dilution rate (0.5 %). The formulated extracts exhibited characteristics such as low surface tension, high viscosity, high electrical conductivity, and low pH. The surface tension (dyne cm−1) of FPEE and FEAE was 37.02 %. The viscosity (cm poise-1) of FPEE and FEAE were 1.40 and 1.48, respectively. The electrical conductivity (µ mhos) of FPEE and FEAE were 317 and 323, respectively. Additionally, the pH of FPEE and FEAE were 6.90 and 6.80, respectively.
Table 9.
Physicochemical properties of B. carterii resin as emulsifiable concentrate formulation (10% EC) and used at a field dilution rate (0.5%).
| Extract* |
Physicochemical properties |
|||
|---|---|---|---|---|
|
Surface tension (dyne cm−1) |
Viscosity (cm poise-1) |
Electrical conductivity (µ mhos) |
pH | |
| FPEE | 37.02 | 1.40 | 317 | 6.90 |
| FEAE | 37.02 | 1.48 | 323 | 6.80 |
FPEE; formulated petroleum-ether extract, FEAE; formulated ethyl-acetate extract.
3.5. Antioxidant activity of B. carterii formulated as an emulsifiable concentrate
The antioxidant potential of B. carterii formulated as an emulsifiable concentrate at various concentrations, was determined by neutralizing the stable DPPH radical cation presented in Table 10. Compared to ascorbic acid (used as a reference), the findings demonstrate that the antioxidant activity (%) of FPEE and FEAE was increased with increasing concentration. At higher concentrations, FPEE (10 mg ml−1), FEAE (10 mg ml−1) and ascorbic acid (0.5 mg ml−1) exhibited an antioxidant activity (%) of 62 %, 90.8 %, and 92 %, respectively. In the DPPH assay, FPEE displayed low reducing power capacity, while FEAE showed good concentration-dependent activity. The FPEE displayed a favorable IC50 value of 8.68 mg ml−1, FEAE demonstrated a moderate activity of 4.15 mg ml−1, while ascorbic acid exhibited an excellent IC50 value of 0.19 mg ml−1.
Table 10.
Antioxidant activity (%) and DPPH scavenging activity of B. carterii FPEE and FEAE.
| Extract/Standard* | Concentration (mg/ml) | Optical Density | Antioxidant Activity (%) |
DPPH * (IC50 mg/ml) |
|---|---|---|---|---|
| FPEE | 2 | 0.72 | 10.3 | 8.68 |
| 4 | 0.63 | 21.4 | ||
| 6 | 0.56 | 30.0 | ||
| 8 | 0.44 | 44.9 | ||
| 10 | 0.30 | 62.0 | ||
| FEAE | 2 | 0.60 | 24.8 | 4.15 |
| 4 | 0.47 | 41.6 | ||
| 6 | 0.31 | 61.0 | ||
| 8 | 0.17 | 78.5 | ||
| 10 | 0.07 | 90.8 | ||
| AA | 0.1 | 0.58 | 27.5 | 0.19 |
| 0.2 | 0.48 | 40.4 | ||
| 0.3 | 0.24 | 70.6 | ||
| 0.4 | 0.10 | 87.0 | ||
| 0.5 | 0.06 | 92.0 |
FPEE; formulated petroleum-ether extract, FEAE; formulated ethyl-acetate extract, AA; ascorbic acid, DPPH; 2,2-diphenyl-1-picrylhydrazyl, IC50; half-maximal inhibitory concentration.
3.6. In vitro efficacy of B. carterii formulated as an emulsifiable concentrate
3.6.1. Antifungal activity
In vitro, the FPEE and FEAE at different concentrations (1000, 2000, 3000, and 4000 ppm) were tested for their ability to hinder the mycelial growth of fungal pathogens. Uniform® 390SE served as a reference control, with different concentrations (40, 81, 162 and 325 ppm). The antifungal activity of B. carterii resin extracts formulated as an emulsifiable concentrate on R. solani, S. rolfsii, F. solani, F. oxysporum, F. semitectium, B. cinerea, A. alternata and Phoma sp. were summarized in (Table 11 and Fig. 3) in comparison to the performance of Uniform® 390SE as illustrated in (Table 12 and Fig. 4). The FPEE displayed antifungal activity against tested fungal pathogens at different concentration, except for F. solani and F. oxysporum. The greatest suppression of fungal growth was observed against R. solani, S. rolfsii, F. semitectium, B. cinerea, and A. alternata, particularly at 4000 ppm concentration. Similarly, FEAE also exhibited antifungal activity against all tested fungal pathogens. At a concentration of 4000 ppm, it completely ceased the growth of R. solani, S. rolfsii, B. cinerea, and A. alternata. Interestingly, Phoma sp., unlike the other tested fungal pathogens, appeared resistant or more tolerant to both extracts.
Table 11.
In vitro antifungal activity of B. carterii formulated as an emulsifiable concentrate on some fungal pathogens.
| Fungi* | Extracts |
Mycelial growth inhibition (%) at concentration (ppm) |
EC50 | EC90 | Slope value | |||
|---|---|---|---|---|---|---|---|---|
| 1000 | 2000 | 3000 | 4000 | |||||
| R. solani | FPEE | 11 ± 0.00 | 32 ± 0.98 | 53 ± 1.70 | 67 ± 3.21 | 2826.71 | 8193.29 | 2.77+/-0.33 |
| FEAE | 36 ± 1.61 | 53 ± 1.70 | 68 ± 1.37 | 100 ± 0.00 | 1544.41 | 4413.31 | 2.81+/-0.31 | |
| S. rolfsii | FPEE | 19 ± 1.6 | 33 ± 0.00 | 44 ± 0.37 | 66 ± 0.64 | 3003.17 | 13621.93 | 1.95+/-0.31 |
| FEAE | 41 ± 1.61 | 47 ± 0.00 | 89 ± 0.00 | 100 ± 0.00 | 1426.81 | 4663.46 | 2.49+/-0.39 | |
| F. solani | FPEE | 0 | 0 | 0 | 0 | – | – | – |
| FEAE | 28 ± 3.21 | 41 ± 1.34 | 55 ± 0.37 | 70 ± 1.70 | 2312.62 | 12052.89 | 1.79+/-0.29 | |
| F. oxysporum | FPEE | 0 | 0 | 0 | 0 | – | – | – |
| FEAE | 20 ± 1.70 | 31 ± 1.85 | 43 ± 0.64 | 59 ± 1.61 | 3374.90 | 18829.26 | 1.71+/-0.30 | |
| F. semitectium | FPEE | 31 ± 1.61 | 42 ± 1.70 | 52 ± 1.28 | 70 ± 1.61 | 2321.54 | 15209.36 | 1.57+/-0.29 |
| FEAE | 41 ± 1.61 | 54 ± 1.34 | 68 ± 2.31 | 83 ± 2.89 | 1478.00 | 7505.06 | 1.82+/-0.29 | |
| B. cinerea | FPEE | 29 ± 1.70 | 40 ± 2.31 | 50 ± 0.00 | 77 ± 0.64 | 2305.28 | 11025.99 | 1.91+/-0.30 |
| FEAE | 32 ± 0.98 | 52 ± 1.28 | 70 ± 1.61 | 100 ± 0.00 | 1617.18 | 4172.38 | 3.11+/-0.32 | |
| A. alternata | FPEE | 30 ± 1.61 | 45 ± 2.59 | 65 ± 0.98 | 81 ± 1.61 | 1874.33 | 7186.39 | 1.86+/-0.29 |
| FEAE | 31 ± 2.06 | 54 ± 1.34 | 83 ± 0.74 | 100 ± 0.00 | 1540.61 | 3583.26 | 3.50+/-0.34 | |
| Phoma sp. | FPEE | 0 | 20 ± 1.70 | 23 ± 0.74 | 31 ± 1.70 | 5752.78 | 18837.85 | 2.49+/-0.43 |
| FEAE | 0 | 11 ± 0.00 | 18 ± 1.48 | 22 ± 0.00 | 7304.52 | 23947.37 | 2.49+/-0.50 | |
Values shown in the table are means ± standard error (n = 3), following Duncan’s multiple range test. FPEE; formulated petroleum-ether extract, FEAE; formulated ethyl-acetate extract, EC50; half maximal effective concentration, ppm; parts per million.
Fig. 3.
In vitro antifungal activity of B. carterii formulated as an emulsifiable concentrate on some fungal pathogens. A; Control, B; 1000 ppm, C; 2000 ppm, D; 3000 ppm, and E; 4000 ppm.
Table 12.
In vitro antifungal activity of Uniform® (a reference control) on some fungal pathogens.
| Fungi* |
Mycelial growth inhibition (%) at concentration (ppm) |
EC50 | EC90 | Slope value | |||
|---|---|---|---|---|---|---|---|
| 40 | 81 | 162 | 325 | ||||
| R. solani | 31 ± 1.70 | 48 ± 1.85 | 91 ± 1.28 | 100 ± 0.00 | 64.80 | 170.62 | 3.05+/-0.29 |
| S. rolfsii | 64 ± 1.61 | 72 ± 0.00 | 88 ± 0.64 | 100 ± 0.00 | 45.41 | 153.84 | 2.42+/-0.28 |
| F. solani | 44 ± 0.00 | 78 ± 0.98 | 100 ± 0.00 | 100 ± 0.00 | 45.17 | 98.32 | 3.79+/-0.44 |
| F. oxysporum | 35 ± 1.85 | 57 ± 2.31 | 77 ± 0.37 | 88 ± 0.74 | 64.16 | 335.88 | 1.78+/-0.22 |
| F. semitectium | 33 ± 0.74 | 54 ± 1.34 | 87 ± 1.70 | 100 ± 0.00 | 63.64 | 179.68 | 2.84+/-0.23 |
| B. cinerea | 64 ± 1.61 | 76 ± 1.85 | 86 ± 1.61 | 100 ± 0.00 | 27.71 | 151.15 | 1.74+/-0.26 |
| A. alternata | 26 ± 1.85 | 48 ± 1.85 | 65 ± 0.98 | 100 ± 0.00 | 81.28 | 267.06 | 2.48+/-0.24 |
| Phoma sp. | 0 | 0 | 0 | 0 | – | – | – |
Values shown in the table are means ± standard error (n = 3), following Duncan’s multiple range test. EC50; half maximal effective concentration, ppm; parts per million.
Fig. 4.
In vitro antifungal activity of Uniform® (a reference control) on some fungal pathogens. A; Control, B; 40 ppm, C; 81 ppm, D; 162 ppm and E; 325 ppm.
In vitro, Uniform® 390SE (a reference control) at different concentrations (40, 81, 162, and 325 ppm) was used to assess its ability to inhibit fungal pathogens (Table 12 and Fig. 4). The fungicide exhibited suppressive effects against all tested fungal pathogens, except for Phoma sp. At a concentration of 325 ppm, the application of Uniform® 390SE effectively and entirely inhibited the mycelial proliferation of various fungal species. Furthermore, it displayed a notable 89 % inhibition against F. oxysporum, while Phoma sp. did not exhibit any response to the fungicide at different concentrations.
3.6.2. Acaricidal activity
The acaricidal activity of FPEE and FEAE at various concentrations (62.5, 125, 250, 500, 1000, 2000 and 3000 ppm) was evaluated for their toxicity against the adult females of T. urticae using leaf disc-dip technique. Abamectin (Vertimec® 1.8 % EC) with concentrations (2.25, 4.5, 18, 36 and 54 ppm) was used as a reference control (Table 13). Concerning LC50 level, FPEE displayed the most potent acaricidal effect on T. urticae, with an LC50 value of 422.58 ppm and a corresponding toxicity index of 1.58 after 72 h of treatment. Conversely, FEAE exhibited moderate toxicity against T. urticae, with an LC50 value of 539.50 ppm and a toxicity index 1.23 after 72 h of treatment. These findings indicate that FPEE is 1.28 times more effective than FEAE against T. urticae adult female.
Table 13.
In vitro acaricidal activity of B. carterii formulated as an emulsifiable concentrate and Abamectin (a reference control) on T. urticae.
| Treatments* |
LC50 (ppm) (95 % Fiducial limits) |
Slope ± S.E | Toxicity index |
|---|---|---|---|
| Abamectin | 6.66 (2.04–12.51) | 1.23 ± 0.12 | 100 |
| FPEE | 422.58 (252.77–654.65) | 0.89 ± 0.14 | 1.58 |
| FEAE | 539.50 (408.11–698.11) | 1.92 ± 0.25 | 1.23 |
FPEE; formulated petroleum-ether extract, FEAE; formulated ethyl-acetate extract, LC50; lethal concentration 50, ppm; parts per million, S.E.; standard error.
4. Discussion
Modern agriculture extensively depends on pesticides to eradicate pests and disease control. However, their indiscriminate application negatively affects soil, water, and the overall ecosystem, thereby impacting animal and human health (Aktar et al., 2009). Recently, plant extracts and biostimulants have gained considerable attention as environmentally friendly remedies for plant diseases due to their natural properties, antimicrobial activities, ease of decomposition in the environment, and non-harmfulness to plants, animals and human health (Martínez, 2012, Abo-Elyousr et al., 2019, Ashmawy et al., 2020, Sánchez-Montesinos et al., 2021).
Current work was mainly conducted to assess the efficacy of B. carterii resin formulated as an emulsifiable concentrate (10 % EC) against T. urticae and some phytopathogenic fungi (tomato fungal diseases and grey mould mulberry). Our findings showed that B. carterii PE and EA yields were 45.9 % and 39.7 %, respectively (Table 1). In contrast, B. dalzielii yielded 16.08 %, 3.37 % and 0.03 % when methanol, hexane and ethyl-acetate were used as solvents (Kohoude et al., 2017). Additionally, Jansen et al. (2010) observed that B. dalzielii leaves produced yields of 4.7 %, 24.7 % and 33.4 % when subjected to solvents such as dichloromethane, methanol and water-based decoction, respectively.
The phytochemical screening of B. carterii PE and EA revealed the presence of various substances, including saponins, alkaloids, flavonoids, phenolic compounds, glycosides, steroids and tannins (Table 2). Additionally, total phenol and flavonoids in B. carterii EA were 3.28 mg GAE g−1 and 0.29 mg QE g−1, respectively (Table 3). These findings align with a previous study by Kohoude et al. (2017), who identified higher levels of phenolic (315.97 g GAE/kg dry mass) and flavonoid (37.19 g QE/kg dry mass) contents in methanol extract of B. dalzielii leaves. Further, previous research has reported that medicinal plants containing phenolic and flavonoid compounds exhibit antioxidative, antibacterial, antiviral, and anticancer properties (Tungmunnithum et al., 2018). The specific type and quantity of these compounds vary across different plant parts and are influenced by the choice of solvent for extraction (Vuddanda et al., 2016).
Analysis of B. carterii PE using GLC revealed the presence of 24 compounds in both the saponified and unsaponified fractions (Fig. 1, Fig. 2, and Table 4, Table 5). Numerous studies have explored the composition of B. carterii oil and have reported varying components. In the study conducted by Kubmarawa et al. (2006), a total of 29 compounds were discerned within the essential oil of B. dalzielii. Notably, the prevailing constituents were a-Pinene, accounting for 45.7 % of the composition, and a-terpinene, representing 11.5 % of the mixture. Another study by Van Vuuren et al. (2010) identified various components in the essential oils of B. carterii, encompassing α-pinene (2.0–64.7 %), α-thujene (0.3–52.4 %), β-pinene (0.3–13.1 %), myrcene (1.1–22.4 %), sabinene (0.5–7.0 %), limonene (1.3–20.4 %), p-cymene (2.7–16.9 %) and β-caryophyllene (0.1–10.5 %). Furthermore, Kohoude et al. (2017) unveiled a remarkable array of 50 distinct chemical compounds within the essential oil of B. dalzielii. Amongst these compounds, 3-carene (27.72 %) and a-pinene (15.18 %) were observed. Furthermore, the methanol extract was found to contain 2,5-dihydroxy acetophenone and b-D-xylopyranose.
Results displayed in Table 6, Table 7, Table 8, Table 9 depict the physicochemical properties of PE and EA extracts from B. carterii. These extracts exhibit insolubility in water but can be effectively dissolved in xylene and acetone. This suggests that both extracts can be effectively used in the formulation of emulsifiable concentrates (Abd-Alla and Hamouda, 2021). The emulsifiable concentrate formulation of B. carterii resin employs surfactants that possess qualities suitable for serving as dispersing agents during the creation of these emulsifiable concentrates as emulsifiers (Abd-Alla and Hamouda, 2021). Table 9 outlines the physicochemical properties of B. carterii resin extracts formulated into emulsifiable concentrate (10 % EC) and applied at a 0.5 % field dilution rate. The observations underscore that the resulting spray solution exhibits low surface tension, high viscosity, high conductivity, and a diminished pH value. The augmentation of the pesticide spray solution's efficacy in pest control is achieved through the reduction of surface tension, thereby facilitating its superior wetting, spreading, and adhesion to the surfaces of treated plants (Pereira et al., 2016, Safar et al., 2022). The manipulation of pH levels, coupled with the augmentation of electrical conductivity within the spray solution, serves to expedite the deionization process of pesticides. This, in turn, fosters their deposition and penetration into the surfaces subjected to treatment, ultimately amplifying their retention and overall efficacy (El-Sisi et al., 2011). The heightened viscosity of the spray solution also contributes to its enhanced biological efficacy by mitigating pesticide drift and enhancing adhesion to treated surfaces (Spanoghe et al., 2007).
The antioxidant potential of the formulated B. carterii was evaluated by neutralizing the stable free radical DPPH (Table 10). At higher concentrations, FPEE (10 mg ml−1), FEAE (10 mg ml−1) and ascorbic acid (0.5 mg ml−1) exhibited an antioxidant efficacy (%) of 62 %, 90.8 %, and 92 %, respectively. Compared to the potent antioxidant effect of ascorbic acid, FPEE exhibited limited capability in DPPH reduction, whereas FEAE demonstrated a moderate activity level. These results could be attributed to the high content of phenolic and flavonoid components in FEAE. These compounds may act as electron donors, interacting with free radicals to convert them into more stable substances, thereby terminating the radical chain reaction. In light of these findings, it can be inferred that FEAE holds significant potential as an antioxidant (Ruberto and Baratta, 2000, Mothana et al., 2011, Prakash et al., 2014).
The efficacy of B. carterii resin extracts against a diverse array of fungal strains has been presented in (Table 11, Table 12, and Fig. 3, Fig. 4). The results indicated that the FPEE showed strong antifungal activity against most of the tested fungal pathogens, except for F. solani and F. oxysporum. The highest inhibition of mycelial growth was observed in R. solani (67 %), S. rolfsii (66 %), F. semitectium (70 %), B. cinerea (77 %), and A. alternata (81 %) at a concentration of 4000 ppm. The findings of the FEAE experiment revealed a striking pattern, effectively impeding the mycelial expansion of at a concentration of 4000 ppm. However, Phoma sp. exhibited more resilience to growth inhibition caused by both extracts. Chemical analysis of B. carterii EA resin crude extracts revealed the presence of various secondary metabolites, such as flavonoids and phenolics, known for their antioxidant properties due to their redox abilities. Furthermore, the composition of B. carterii PE using GLC shows predominant components comprising oleic acid (36.5 %), α-linolenic acid (16 %), linoleic acid (13.1 %), n.henicosanoic (26.3 %), squalene (17.1 %), β.sitosterol (15.5 %), and stigmasterol (14.7 %). These findings are consistent with previous studies (Camarda et al., 2007, Goñi et al., 2009, El-Nagerabi et al., 2013, Prakash et al., 2014, Sadhasivam et al., 2016, Stupar et al., 2016, Chaurasia and Gharia, 2017, Venkatesh et al., 2017, Powers et al., 2018, Raveau et al., 2020), suggesting that the compounds present in B. carterii extracts have the potential to disrupt enzymatic reactions within fungal hyphae, thus impacting their metabolism and growth. This interference likely affects various aspects of fungal development, potentially leading to mechanisms such as cytoplasm retraction and hyphal wall disintegration.
The acaricidal activity of B. carterii FPEE and FEAE was assessed against adult females of T. urticae using the leaf disc-dip method (Table 13). Concerning LC50 level, FPEE exhibited higher toxicity against T. urticae (LC50 = 422.58 ppm) compared to FEAE (LC50 = 539.50 ppm) after 72 hrs of treatment. This could be attributed to the presence of various volatile components comprising oleic acid (36.5 %), α-linolenic acid (16 %), linoleic acid (13.1 %), n.henicosanoic (26.3 %), squalene (17.1 %), β.sitosterol (15.5 %), and stigmasterol (14.7 %) within FPEE, which were the most dominant. These compounds have the potential to block mite spiracles, leading to suffocation. Moreover, they can penetrate cell membranes, accumulate in the cytoplasm, cause cellular dehydration, and induce DNA condensation in the nucleus. Our findings align with a study conducted by Roh et al. (2011), who assessed the impact of B. carterii oil as an acaricide on two-spotted spider mites in a controlled laboratory setting. They recorded a 24.8 % mortality rate among mites and a reduction in egg laying, indicating a deterrent effect of the oil compared to the control.
In a study by Yoon and Tak (2018), the repellent and acaricidal properties of frankincense oil on adult two-spotted spider mites T. urticae were documented. Similarly, Kiran et al. (2017) found that essential oil from B. carterii is a potent insecticide against Callosobruchus chinensis and C. maculates, demonstrating no negative impact on seed germination. Furthermore, the remarkable efficacy of frankincense nanoemulsion in combating 2nd instar larvae of the spiny bollworm Earias insulana (Metayi et al., 2022). Their findings unequivocally demonstrate the enduring inhibitory effects on larvae, pupae, adult longevity, and reproductive capacity. According to Sabtharishi and Naveen, 2017, Jankowska et al., 2018, Boate and Abalis, 2020 and Yang et al. (2020), the toxicity exhibited by plant extracts might be attributed to their fatty acid components. The toxic effect of these fatty acids is due to their ability to penetrate the insect body covering and disrupt the lipoprotein matrix of the insect cellular membranes. Consequently, this disruption leads to the release of cellular contents, ultimately leading to cellular dehydration and death.
5. Conclusions and future perspective
The present study assessed the antifungal and acaricidal activities of B. carterii FPEE and FEAE in vitro. Our findings suggest that FEAE holds promise to serve as a natural antifungal remedy for preventing tomato fungal diseases and mulberry grey mould disease. Furthermore, FPEE exhibited specific capabilities in combating T. urticae, indicating its potential as an effective acaricidal agent. Nonetheless, further studies are necessary to assess the antifungal/acaricidal activities of B. carterii extracts in vivo and to deepen our comprehension of how these extracts impede fungal mycelia growth at a molecular level.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors also thank the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R182), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mai M.A. Gnedy, Email: mai.mokhtar71@arc.sci.eg.
Rania A.A. Hussien, Email: raniahussien187@arc.sci.eg.
Rasha A. Sleem, Email: Rashasleem@agr.asu.edu.eg.
Amr Elkelish, Email: aaelkelish@imamu.edu.sa.
Maha AlHarbi, Email: maalharbi@pnu.edu.sa.
Basmah M. Alharbi, Email: b.alharbi@ut.edu.sa.
Ali A.S. Sayed, Email: aas10@fayoum.edu.eg.
References
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18(2):265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Abd-Alla H.I., Hamouda S.E.S. Study of potential activity of clove oil 10% emulsifiable concentrate formulation on two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) J. Appl. Nat. Sci. 2021;13(4):1414–1419. doi: 10.31018/jans.v13i4.3124. [DOI] [Google Scholar]
- Abdel-Wahab B.A., Abd El-Kareem F., Alzamami A., Fahmy C.A., Elesawy B.H., Mahmoud M.M., Ghareeb A., El Askary A., Abo Nahas H.H., Attallah N.G.M., Altwaijry N., Saied E.M. Novel exopolysaccharide from marine Bacillus subtilis with broad potential biological activities: insights into antioxidant, anti-inflammatory, cytotoxicity, and anti-alzheimer activity. Metabolites. 2022;12(8):715. doi: 10.3390/metabo12080715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Elyousr K.A.M., Khalil Bagy H.M.M., Hashem M., Alamri S.A.M., Mostafa Y.S. Biological control of the tomato wilt caused by Clavibacter michiganensis subsp. michiganensis using formulated plant growth-promoting bacteria. Egypt J. Biol. Pest Control. 2019;29:54. doi: 10.1186/s41938-019-0152-6. [DOI] [Google Scholar]
- Adams R.P. fourth ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. Identification of essential oil components by gas chromatography/mass spectrometry. [Google Scholar]
- Ahmed H.H., Abd-Rabou A.A., Hassan A.Z., Kotob S.E. Phytochemical analysis and anticancer investigation of Boswellia serrata bioactive constituents in vitro. Asian Pac. J. Cancer Prev. 2015;16(16):7179–7188. doi: 10.7314/APJCP.2015.16.16.7179. [DOI] [PubMed] [Google Scholar]
- Aktar W., Sengupta D., Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2009;2(1):1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akthar M.S., Degaga B., Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: a review. Issues Biol. Sci. Pharm. Res. 2014;2(1):1–7. [Google Scholar]
- Al-Yasiry A.R., Kiczorowska B. Frankincense-therapeutic properties. Postep. Hig. Med. Dosw. 2016;70:380–391. doi: 10.5604/17322693.1200553. [DOI] [PubMed] [Google Scholar]
- Ashmawy N.A., Salem M.Z.M., El Shanhorey N., Al-Huqail A.A., Ali H.M., Behiry S.I. Eco-friendly wood-biofungicidal and antibacterial activities of various Coccoloba uvifera L. leaf extracts: HPLC analysis of phenolic and flavonoid compounds. BioRes. 2020;15(2):4165–4187. [Google Scholar]
- ASTM, 2001. Standard test method for surface and interfacial tension of solution, D-1331.
- ASTM, 2005. Standard test method for rheological properties of non-Newtonian materials by rotational (Brookfield type) Viscometer, D-2196, Bar Harbor Drive, West Conshohocken, PA 19248-2959, United States.
- Ayub M.A., Hanif M.A., Sarfraz R.A., Shahid M. Biological activity of Boswellia serrata Roxb. oleo gum resin essential oil: effects of extraction by supercritical carbon dioxide and traditional methods. Int. J. Food Prop. 2018;21(1):808–820. doi: 10.1080/10942912.2018.1439957. [DOI] [Google Scholar]
- Banno N., Akihisa T., Yasukawa K., Tokuda H., Tabata K., Nakamura Y., Nishimura R., Kimura Y., Suzuki T. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carterii. J. Ethnopharmacol. 2006;107(2):249–253. doi: 10.1016/j.jep.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Boate U.R., Abalis O.R. Review on the bio-insecticidal properties of some plant secondary metabolites: types, formulations, modes of action, advantages and limitations. Asian J. Res. Zool. 2020;3(4):27–60. doi: 10.9734/ajriz/2020/v3i430099. [DOI] [Google Scholar]
- Camarda L., Dayton T., Di Stefano V., Pitonzo R., Schillaci D. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp. (Burseraceae) Anal. Chim. 2007;97(9):837–844. doi: 10.1002/adic.200790068. [DOI] [PubMed] [Google Scholar]
- Chaurasia A., Gharia A. Antifungal activity of medicinal plant Boswellia serrata. J. Ultra Chem. 2017;13(4):88–90. doi: 10.22147/juc/130403. [DOI] [Google Scholar]
- CIPAC MT 36.3, 2003. Emulsion stability and re-emulsification. In: Dobrat, W., Martijn, A. (Eds.), CIPAC Handbook K. Physico-Chemical Methods for Technical and Formulated Pesticides, vol. 137. Collaborative International Pesticides Analytical Council Ltd, Harpenden, England.
- CIPAC MT 46.3, 2000. Accelerated storage procedure. In: Dobrat, W., Martijn, A. (Eds.), CIPAC Handbook J. Physico-Chemical Methods for Technical and Formulated Pesticides, vol. 128. Collaborative International Pesticides Analytical Council Ltd, Harpenden, England.
- CIPAC MT 75.3, 2000. Determination of pH. In: Dobrat, W., Martijn, A. (Eds.), CIPAC Handbook J. Physico-Chemical Methods for Technical and Formulated Pesticides. Collaborative International Pesticides Analytical Council Ltd, Harpenden, England, p. 131.
- Dittrich V. A comparative study of toxicological test methods on a population of the two-spotted spider mite (T. urticae) J. Econ. Entomol. 1962;55(5):644–648. [Google Scholar]
- Duncan D.B. Multiple ranges and multiple F. Test. Biomet. J. 1955;11:1–42. [Google Scholar]
- El-Nagerabi P.S., Elshafie A., AlKhanjari S., et al. Biological activities of Boswellia sacra extracts on the growth and aflatoxins secretion of two aflatoxigenic species of Aspergillus species. Food Control. 2013;34:763–769. doi: 10.1016/j.foodcont.2013.06.039. [DOI] [Google Scholar]
- El-Sisi A.G., El-Mageed A.E.M., El-Asawi T.F., El-Sharkawy R.A. Improvement the physico-chemical properties and efficiency of some insecticides formulation by using adjuvants against cotton leafworm Spodoptera littoralis (BOISD.) J. Plant Prot. Pathol. 2011;2(8):757–764. doi: 10.21608/JPPP.2011.86522. [DOI] [Google Scholar]
- Finney D.J. Probit Analysis. third ed. Cambridge University Press; New York, Ny: 1971. p. 10022. [Google Scholar]
- Firsta, N.C., Mentari, R.D., Salafiah, E.S., Estiasih, T., 2020. Preparation of unsaponifiable fraction from crude palm oil: a short review. In: IOP Conference Series: Earth and Environmental Science, Vol. 475, No. 1, International Conference on Green Agro-industry and Bioeconomy 26-27 August 2019, Malang East Java, Indonesia, p. 012032. 10.1088/1755-1315/475/1/012032. [DOI]
- Frank M.B., Yang Q., Osban J., Azzarello J.T., Saban M.R., Saban R., Ashley R.A., Welter J.C., Fung K.M., Lin H.K. Frankincense oil derived from Boswellia carterii induces tumor cell specific cytotoxicity. BMC Complement. Altern. Med. 2009;9:6. doi: 10.1186/1472-6882-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber A., Refat M.S., Belal A.A.M., El-Deen I.M., Hassan N., Zakaria R., Alhomrani M., Alamri A.S., Alsanie W.F., Saied E.M. New mononuclear and binuclear Cu (II), Co (II), Ni (II), and Zn (II) thiosemicarbazone complexes with potential biological activity: antimicrobial and molecular docking study. Molecules. 2021;26(8):2288. doi: 10.3390/molecules26082288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi P., López P., Sánchez C., Gómez-Lus R., Becerril R., Nerín C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009;116(4):982–989. doi: 10.1016/j.foodchem.2009.03.058. [DOI] [Google Scholar]
- Gupta M., Verma S.K., Singh S., Trivedi L., Rout P.K., Vasudev P.G., Luqman S., Darokar M.P., Bhakuni R.S., Misra L. Anti-proliferative and antibacterial activity of oleo-gum-resin of Boswellia serrata extract and its isolate 3-hydroxy-11-keto-β-boswellic acid. J. Herb. Med. 2022;32 doi: 10.1016/j.hermed.2022.100546. [DOI] [Google Scholar]
- Han X., Rodriguez D., Parker T.L. Biological activities of frankincense essential oil in human dermal fibroblasts. Biochim. Open. 2017;4:31–35. doi: 10.1016/j.biopen.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy Y.A., Taha-Abdelaziz K., Hawwas H.-A.-E.-H., Ghosh S., AlKafaas S.S., Moawad M.M.M., Saied E.M., Kassem I.I., Mawad A.M.M. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics. 2023;12(2):274. doi: 10.3390/antibiotics12020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.T., Badmaev V., Ding Y., Liu Y., Xie J.G., Ho C.T. Anti-tumor and anti-carcinogenic activities of triterpenoid, β-boswellic acid. Biofactors. 2000;13(1–4):225–230. doi: 10.1002/biof.5520130135. [DOI] [PubMed] [Google Scholar]
- Hussien R.A.A., Gnedy M.M.A., Sayed A.A.S., Bondok A., Alkhalifah D.H.M., Elkelish A., Tawfik M.M. Evaluation of the fungicidal effect of some commercial disinfectant and sterilizer agents formulated as soluble liquid against Sclerotium rolfsii infected tomato plant. Plants. 2022;11(24):3542. doi: 10.3390/plants11243542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal E., Salim K.A., Lim L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci. 2015;27(3):224–232. doi: 10.1016/j.jksus.2015.02.003. [DOI] [Google Scholar]
- Islam M.T., Jahan M., Gotoh T., Ullah M.S. Host-dependent life history and life table parameters of Tetranychus truncatus (Acari: Tetranychidae) Syst. Appl. Acarol. 2017;22(12):2068–2082. doi: 10.11158/saa.22.12.4. [DOI] [Google Scholar]
- Isman M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020;19:235–241. doi: 10.1007/s11101-019-09653-9. [DOI] [Google Scholar]
- Jankowska M., Rogalska J., Wyszkowska J., Stankiewicz M. Molecular targets for components of essential oils in the insect nervous system-A review. Molecules. 2018;23(1):34. doi: 10.3390/molecules23010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen O., Angenot L., Tits M., Nicolas J.P., De Mol P., Nikiéma J.B., Frederich M. Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J. Ethnopharmacol. 2010;130(1):143–150. doi: 10.1016/j.jep.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Jiao H., Wang S.Y. Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J. Agric. Food Chem. 2000;48(11):5672–5676. doi: 10.1021/jf000765q. [DOI] [PubMed] [Google Scholar]
- Karlina M.V., Pozharitskaya O.N., Kosman V.M., Ivanova S.A. Bioavailability of boswellic acids: in vitro/in vivo correlation. Pharm. Chem. J. 2007;41:569–572. doi: 10.1007/s11094-008-0017-x. [DOI] [Google Scholar]
- Kiran S., Kujur A., Patel L., Ramalakshmi K., Prakash B. Assessment of toxicity and biochemical mechanisms underlying the insecticidal activity of chemically characterized Boswellia carterii essential oil against insect pest of legume seeds. Pestic. Biochem. Physiol. 2017;139:17–23. doi: 10.1016/j.pestbp.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Kohoude M.J., Gbaguidi F., Agbani P., Ayedoun M.A., Cazaux S., Bouajila J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017;55(1):33–42. doi: 10.1080/13880209.2016.1226356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubmarawa D., Ogunwande I.A., Okorie D.A., Olawore N.O., Kasali A.A. Constituents of the essential oils of Boswellia dalzielii Hutch. from Nigeria. J. Ess. Oil Res. 2006;18(2):119–120. doi: 10.1080/10412905.2006.9699038. [DOI] [Google Scholar]
- Le Goff G., Mailleux A.C., Detrain C., Deneubourg J.L., Clotuche G., Hance T. Spatial distribution and inbreeding in Tetranychus urticae. C. R. Biol. 2009;332(10):927–933. doi: 10.1016/j.crvi.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Lei C., Sun X. Comparing lethal dose ratios using probit regression with arbitrary slopes. BMC Pharmacol. Toxicol. 2018;19:61. doi: 10.1186/s40360-018-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Wang H., Pang X., Yao W., Gao X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010;46:451–457. doi: 10.1016/j.ijbiomac.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lynch, M., Griffin, W., 1974. Food Emulsions in: Emulsion Technology, by Lissant KJ, Marcell Decker, Inc. New York. Mukerjee P. and KJ Mysels (1971) Critical Micelle Concentration of Aqueous Surfactant Systems. National Bureau of Standards Washington DC, 1-21.
- Martínez, J.A., 2012. Natural fungicides obtained from plants. In: Dhanasekaran, D., Thajuddin, N., Panneerselvam A., (Eds.) Fungicides for plant and animal diseases. InTech. ISBN: 978-953-307-804-5.
- Metayi M.H., El-Naby A., Shimaa S., El-Habal N.A., Fahmy H.H., Abdou M.S., Ali B., Abdel-Rheim K.H., Abdel-Megeed A. Omani Frankincense nanoemulsion formulation efficacy and its latent effects on biological aspects of the spiny bollworm Earias insulana (Boisd.) Front. Physiol. 2022;13:1001136. doi: 10.3389/fphys.2022.1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon A., Nouguier E., Dorkeld F. In: Trends in Acarology. Sabelis M., Bruin J., editors. Springer; Dordrecht: 2010. Spider Mites Web: A comprehensive database for the Tetranychidae; pp. 557–560. [DOI] [Google Scholar]
- Mishra S., Bishnoi R.S., Maurya R., Jain D. Boswellia serrata ROXB. A bioactive herb with various pharmacological activities. Asian J. Pharm. Clin. Res. 2020;13(11):33–39. doi: 10.22159/ajpcr.2020.v13i11.39354. [DOI] [Google Scholar]
- Mothana R.A.A., Hasson S.S., Schultze W., Mowitz A., Lindequist U. Phytochemical composition and in vitro antimicrobial and antioxidant activities of essential oils of three endemic Soqotraen Boswellia Species. Food Chem. 2011;126(3):1149–1154. doi: 10.1016/j.foodchem.2010.11.150. [DOI] [Google Scholar]
- Nelson F.C., Fiero G.W. Pesticide formulations, a selected aromatic fraction naturally occurring in petroleum as a pesticide solvent. J. Agric. Food Chem. 1954;2(14):735–737. doi: 10.1021/jf60034a005. [DOI] [Google Scholar]
- Osipow, L., 1964. Surface chemistry theory and application: Reinhold Publishing Crop, New York.
- Pereira V.J., da Cunha J.P.A.R., de Morais T.P., Ribeiro-Oliveira J.P., de Morais J.B. Physical-chemical properties of pesticides: concepts, applications, and interactions with the environment. Biosci. J. 2016;32(3):627–641. doi: 10.14393/BJ-v32n3a2016-31533. [DOI] [Google Scholar]
- Petrasch S., Knapp S.J., Van Kan J.A., Blanco-Ulate B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019;20(6):877–892. doi: 10.1111/mpp.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers C.N., Osier J.L., McFeeters R.L., Brazell C.B., Olsen E.L., Moriarity D.M., Satyal P., Setzer W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules. 2018;23(7):1549. doi: 10.3390/molecules23071549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash B., Mishra P.K., Kedia A., Dubey N.K. Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L. fruits. LWT-Food Sci. Technol. 2014;56(2):240–247. doi: 10.1016/j.lwt.2013.12.023. [DOI] [Google Scholar]
- Raja A.F., Ali F., Khan I.A., Shawl A.S., Arora D.S., Shah B.A., Taneja S.C. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011;11:54. doi: 10.1186/1471-2180-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, D.S., Srinivasa, S., Krishnasami, R., Chandrasek, G.M., 2000. Storage Stable Pesticide Formulations Containing Azadirachtin, Patent No: US 6811790 B1.
- Raveau R., Fontaine J., Lounès-Hadj Sahraoui A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: a review. Foods. 2020;9(3):365. doi: 10.3390/foods9030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh H.S., Lim E.G., Kim J., Park C.G. Acaricidal and oviposition deterring effects of santalol identified in sandalwood oil against two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) J. Pest. Sci. 2011;84:495–501. doi: 10.1007/s10340-011-0377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberto G., Baratta M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69(2):167–174. doi: 10.1016/S0308-8146(99)00247-2. [DOI] [Google Scholar]
- Sabtharishi S., Naveen N.C. Bioassay for monitoring insecticide toxicity in Bemisia tabaci populations. Protoc. Exch. 2017 doi: 10.1038/protex.2017.015. [DOI] [Google Scholar]
- Sadhasivam S., Palanivel S., Ghosh S. Synergistic antimicrobial activity of Boswellia serrata Roxb. ex Colebr. (Burseraceae) essential oil with various azoles against pathogens associated with skin, scalp and nail infections. Lett. Appl. Microbiol. 2016;63(6):495–501. doi: 10.1111/lam.12683. [DOI] [PubMed] [Google Scholar]
- Safar S.H.M., Gnedy M.M.A., Farag E.M. Acaricidal efficiency of solar 50% new emulsifiable concentrate formulation against the two-spotted spider mite (TSSM) Tetranychus urticae Koch (Acari: Tetranychidae) under laboratory and greenhouse conditions. J. Appl. Nat. Sci. 2022;14(4):1456–1464. doi: 10.31018/jans.v14i4.4061. [DOI] [Google Scholar]
- Sánchez-Montesinos B., Santos M., Moreno-Gavíra A., Marín-Rodulfo T., Gea F.J., Diánez F. Biological control of fungal diseases by trichoderma aggressivum f. europaeum and its compatibility with fungicides. J. Fungi. 2021;7:598. doi: 10.3390/jof7080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanoubar R., Barbanti L. Fungal diseases on tomato plant under greenhouse condition. Eur. J. Biol. Res. 2017;7(4):299–308. doi: 10.5281/zenodo.1011161. [DOI] [Google Scholar]
- Sarwar M. Applied Plant Virology. Academic Press; 2020. Mite (Acari Acarina) vectors involved in transmission of plant viruses; pp. 257–273. [DOI] [Google Scholar]
- Schmitz H. Poisoned food technique. Indust. Engin. Chem. Analyst. 1930;2:333–361. [Google Scholar]
- Siegler E.H. Leaf-Disk technique for laboratory tests of acaricides. J. Econ. Entomol. 1947;40(2):280. doi: 10.1093/jee/40.2.280. [DOI] [PubMed] [Google Scholar]
- Spanoghe P., De Schampheleire M., Van der Meeren P., Steurbaut W. Influence of agricultural adjuvants on droplet spectra. Pest Manag. Sci. 2007;63(1):4–16. doi: 10.1002/ps.1321. [DOI] [PubMed] [Google Scholar]
- Stupar M.Č., Kostić M.Z., Savković Ž.D., Unković N.D., Ljaljević-Grbić M.V., Vukojević J.B. Succeptibility of some fungi to Boswellia carteri Birdw. essential oil. Zbornik Matice Srpske Za Prirodne Nauke. 2016;130:19–27. doi: 10.2298/ZMSPN1630019S. [DOI] [Google Scholar]
- Sultan F.I. Phytochemical analysis and antibacterial activities of Frankincense of Boswellia serrate. Plant Arch. 2020;20(2):5219–5226. [Google Scholar]
- Sun Y.P. Toxicity index-An improved method of comparing the relative toxicity of insecticides. J. Econ. Entomol. 1950;43:45–53. [Google Scholar]
- Swallah M.S., Sun H., Affoh R., Fu H., Yu H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020 doi: 10.1155/2020/9081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vuuren S.F., Kamatou G.P.P., Viljoen A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010;76(4):686–691. doi: 10.1016/j.sajb.2010.06.001. [DOI] [Google Scholar]
- Velioglu Y.S., Mazza G., Gao L., Oomah B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998;46(10):4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Venkatesh H.N., Sudharshana T.N., Abhishek R.U., Thippeswamy S., Manjunath K., Mohana D.C. Antifungal and antimycotoxigenic properties of chemically characterised essential oil of Boswellia serrata Roxb. ex Colebr. Int. J. Food Prop. 2017;20(sup2):1856–1868. doi: 10.1080/10942912.2017.1354882. [DOI] [Google Scholar]
- Vuddanda P.R., Singh S., Velaga S. Boswellic acid-Medicinal use of an ancient herbal remedy. J. Herb. Med. 2016;6(4):163–170. doi: 10.1016/j.hermed.2016.08.002. [DOI] [Google Scholar]
- WHO, 1979. Specifications for pesticides used in public health: insecticides, molluscicides, repellents, methods. Geneva :[Albany, N.Y: World Health Organization ; sold by WHO Publications Centre, USA.
- Ximénez-Embún M.G., Castañera P., Ortego F. Drought stress in tomato increases the performance of adapted and non-adapted strains of Tetranychus urticae. J. Insect Physiol. 2017;96:73–81. doi: 10.1016/j.jinsphys.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Yang Y., Isman M.B., Tak J.-H. Insecticidal Activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects. 2020;11(8):474. doi: 10.3390/insects11080474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Tak J.H. Toxicity and repellent activity of plant essential oils and their blending effects against two spotted spider mites, Tetranychus urticae Koch. Korean J. Appl. Entomol. 2018;57(3):199–207. doi: 10.5656/KSAE.2018.08.0.032. [DOI] [Google Scholar]
- Yousef M.M., Zohri A.-N.-A., Darwish A.M.G., Shamseldin A., Kabeil S.A., Abdelkhalek A., Binsuwaidan R., Jaremko M., Alshwyeh H.A., Hafez E.E., Saied E.M. Exploring the antibacterial potential of plant extracts and essential oils against Bacillus thermophilus in beet sugar for enhanced sucrose retention: a comparative assessment and implications. Front. Microbiol. 2023;14:1219823. doi: 10.3389/fmicb.2023.1219823. [DOI] [PMC free article] [PubMed] [Google Scholar]







