Summary
Natural killer (NK)/T cell lymphoma (NKTCL) is a rare subtype of Epstein-Barr virus (EBV)-associated non-Hodgkin lymphoma characterized by poor clinical outcomes. It is more common in East Asian and Latin American countries. Despite the introduction of asparaginase/pegaspargase-based chemotherapy, the prognosis of patients with advanced NKTCL needs to be improved, and few salvage treatment options are available for relapsed/refractory patients who fail chemotherapy. Although many unknowns remain, novel treatment strategies to further improve outcomes are urgently needed. Immunotherapy has emerged and shown favorable antitumor activity in NKTCL, including monoclonal antibodies targeting immune checkpoint inhibitors, other receptors on the cellular membrane, and cellular immunotherapy, which could enhance immune cells attack on tumor cells. In this review, we provide an overview of recent immunotherapy in NKTCL, focusing on programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1), cytotoxic T lymphocyte-associated protein 4 (CTLA-4), chimeric antigen receptor (CAR) T cells, EBV-specific cytotoxic T lymphocytes, immunomodulatory agents, and other targeted agents, as well as the current progress and challenges in the field.
Subject areas: Haematology, Immunology, Cancer
Graphical abstract

Haematology; Immunology; Cancer
Introduction
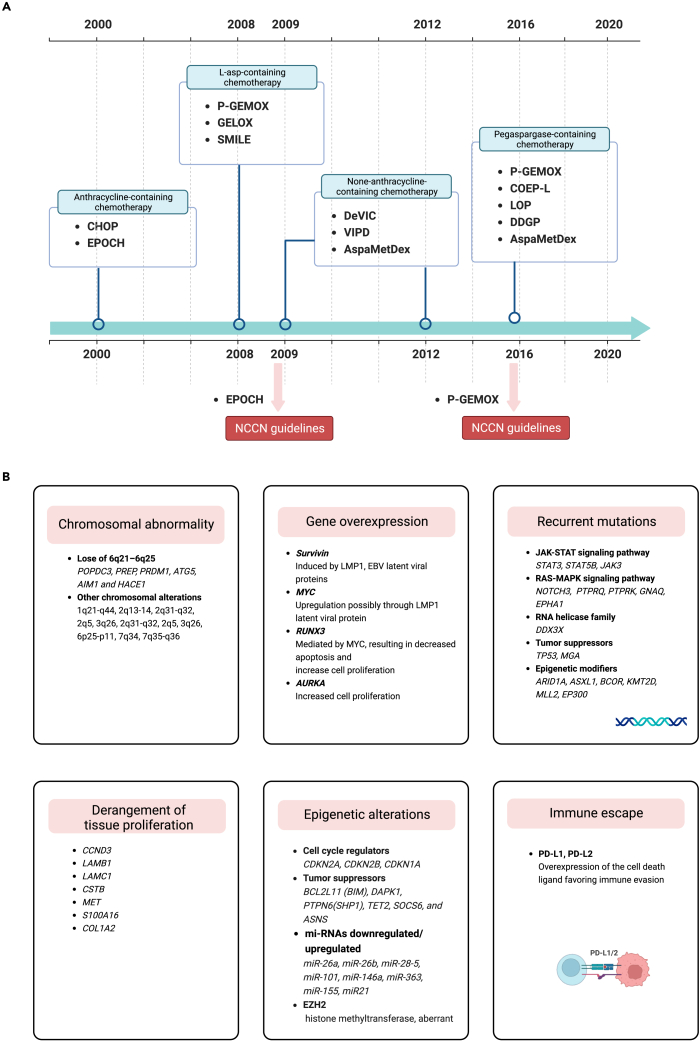
Natural killer/T cell lymphoma (NKTCL) is a rare and highly aggressive subtype of non-Hodgkin lymphoma (NHL) with a strong association with Epstein-Barr virus (EBV) infection.1,2,3 It is characterized by the malignant proliferation of mature NK/T cells and extranodal involvement, leading to distinct features such as prominent necrosis, vascular damage, and a cytotoxic phenotype.3,4 The incidence of NKTCL varies substantially by region, accounting for less than 1% in Western countries but exceeding 10% in Latin America and East Asia.5,6,7 The molecular pathogenic pathways and deregulated genes in EBV-associated NKTCL are mainly summarized in Figure 1.8,9,10
Figure 1.
Molecular pathogenic pathways and deregulated genes in Epstein-Barr virus associated NK/T cell lymphoma
Summary of the deregulated genes and key signaling pathways involved in the pathogenesis of Epstein-Barr virus (EBV)-related NK/T-cell lymphoma (NKTL), nasal type, that promote proliferation and survival of the lymphoma cells (A). This figure summarizes the pathogenic molecular mechanisms that are involved in activating JAK/STAT and NF-κB pathways, as well as alterations in deregulated genes, epigenetic dysregulation, and mechanisms of immune evasion (B).
Anthracycline regimens are largely ineffective for NKTCL due to overexpression of the multidrug resistance gene and its related P-glycoprotein (P-gp); therefore, asparaginase or pegaspargase-based regimens have been introduced as the primary treatment for NKTCL.11,12,13,14,15 At present, P-GEMOX, DDGP, SMILE, VIPD, and MESA regimens have shown high efficacy in treating early-stage NKTCL (Figure 2).16,17,18,19,20,21,22 Unfortunately, even with asparaginase regimens, the prognosis for patients with advanced disease remains bleak, with a 5-year overall survival (OS) rate of approximately 15%–25%.23,24,25,26 More than 10% of patients with NKTCL show primary resistance to asparaginase chemotherapy.27 The prognosis is even worse for relapsed/refractory (r/r) disease, and the available salvage therapies are limited. Therefore, a novel therapeutic strategy is needed to treat patients with NKTCL. Immunotherapy is emerging as a potential approach for advanced or r/r NKTCL and has demonstrated promising clinical efficacy.
Figure 2.
Milestones of chemotherapy and main genetic alterations in NKTCL
Timeline of the significant advancements in chemotherapy for managing NKTCL (A). Main genetic alterations in NKTCL (B). Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin; P-GEMOX, pegaspargase, gemcitabine and oxaliplatin; SMILE, dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide; AspaMetDex, L-asparaginase, methotrexate and dexamethasone; DDGP, dexamethasone, cisplatin, gemcitabine and pegaspargase; COEP-L, cyclophosphamide, vincristine, etoposide, prednisone and L-asparaginase.
In this review, we described the available immunotherapy options for patients with NKTCL focusing on immune checkpoint inhibitors (ICIs), including anti-programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) antibodies, anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) antibodies, and other monoclonal antibodies (mAbs) related to regulating immune function (brentuximab vedotin, daratumumab, alemtuzumab, and mogamulizumab). We also describe the current knowledge about cellular immunotherapeutic approaches involving antigen-specific EBV-cytotoxic T lymphocytes (EBV-CTLs) and chimeric antigen receptor (CAR)-T cells therapy (Table 1). Moreover, immunomodulatory agents are described in the section. A schematic representation of immunotherapy targets for NKTCL is depicted in Figure 3.
Table 1.
Summary of available targets for natural killer/T cell lymphoma
| Targets | Intervention/treatment | Signal crosslinking | Structure | Prognostic relevance | Percentage of NKTCL with positive expression | Research stage for NKTCL | Reference |
|---|---|---|---|---|---|---|---|
| PD-1 | Pembrolizumab | PD-1/PD-L1, LMP1, MAPK, JAK-STAT and NF-κB | Humanized IgG4 | NA | NA | clinical | Li et al.28 |
| Nivolumab | Humanized IgG4 | clinical | Chan et al.29 | ||||
| Tislelizumab | Humanized IgG4 | clinical | Tao et al.30 | ||||
| Sintilimab | Humanized IgG4 | clinical/preclinical | Yan et al., Wen et al.31,32 | ||||
| Toripalimab | Humanized IgG4 | clinical | |||||
| PD-L1 | Avelumab | PD-1/PDL1, LMP1, MAPK, JAK-STAT and NF-κB | Humanized IgG1 | Disputed | 39%–100% | clinical | Nagato et al., Huang et al., Kim et al., Jo et al., Panjwani et al., Han et al.33,34,35,36,37,38 |
| Sugemalimab | Humanized IgG4 | clinical | |||||
| IMC-001 | Humanized IgG1 | clinical | |||||
| CTLA-4 | Ipilimumab | Humanized IgG1 | NA | clinical | Hosseini et al.39 | ||
| CD25/IL-2Rα | Basiliximab | IL-2, LMP1, MAPK and NF-κB | Murine/human IgG1 | Negative | NA | clinical | Wang et al.40 |
| CD30 | Brentuximab vedotin | NF-κB, MAPKs | Humanized IgG1 | Disputed | 50%–70% | clinical | Kim et al., Zhang et al.41,42 |
| CD38 (TNFRSF8) | Daratumumab | calcium, BCR, TLR | Human IgGκ | Negative | 95% | clinical | Lund, Wang et al., Huang et al., Hari et al., Huang et al.43,44,45,46,47 |
| CD52 | Alemtuzumab | IL-15 | Humanized IgG1 | NA | clinical | Kim et al., Zhang et al.48,49 | |
| CD56 | huN901-DM1 | NA | NA | ∼100% | preclinical | Ishitsuka et al.50 | |
| CCR4 | Mogamulizumab | CCL17/CCR4, CCL22/CCR4 | Humanized IgG1 | NA | 47% | preclinical | Kumai et al., Kanazawa et al.51,52 |
| EBV antigens (LMP1/LMP2) | LMP-CTLs | NA | clinical | ||||
| CAR-T | anti-CD30 CAR-T | NA | clinical | ||||
| anti-CD38 CAR-T | NA | preclinical | Li et al.53 | ||||
| anti-CD7 CAR-T | NA | clinical | |||||
| anti-CD19 CAR-T | NA | clinical |
NKTCL, natural killer/T cell lymphoma; PD-1, programmed death 1; PD-L1, programmed cell death-ligand 1; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; IL-2Rα, IL-2 receptor alpha; EBV, Epstein-Barr virus; CTL, cytotoxic T lymphocyte; LMP1/2, Latent membrane protein 1/2; MAPK, Mitogen-activated protein kinases; JAK-STAT, Janus kinase-signal transducer and activator of transcription; NF-κB, Nuclear factor-κB; CCR4, C-C chemokine receptor 4; CAR-T, Chimeric antigen receptor T-cell; NA, data not available.
Figure 3.
Summary of immunotherapy drugs or cells in NKTCL and their respective cellular membrane targets
Anti-PD1/PD-L1 antibodies, such as pembrolizumab, nivolumab, sintilimab, sugemalimab, and avelumab. Monoclonal antibodies target cellular membrane antigens or receptors, which include brentuximab vedotin, basiliximab, alemtuzumab, and daratumumab. Latent membrane protein 1 (LMP1) is a transmembrane protein produced by Epstein-Barr virus (EBV), which subsequently activates the NF-κB pathway and leads to cell proliferation and lymphomagenesis. This in turn upregulates PD-L1, which makes immune checkpoint blockade an attractive target. Engineered chimeric antigen T-cells (CAR-T) and EBV-CTLs are feasible for the treatment of NKTCL.
Monoclonal antibody immunotherapy
Checkpoint inhibitors
New treatment approaches, such as ICIs, have demonstrated remarkable antitumor potential in several types of hematopoietic malignancies. Currently, an increasing number of trials of these drugs in lymphomas are underway. PD-1/PD-L1 and CTLA4 are negative regulators of T cell activation that may be potent therapeutic targets in NKTCL.54
Antibodies targeting programmed cell death-1/programmed cell death-ligand 1
PD-1 is a coinhibitory molecule that is critical to cancer cells escaping from immune surveillance. PD-L1, the ligand of PD-1, is universally upregulated in NKTCL by EBV-driven latent membrane protein 1 (LMP1). It triggers NF-κB and MAPK signaling pathways making anti-PD-1/PD-L1 axis a potent immunotherapy target.33,55,56 Evidence has shown that serum PD-L1 levels are associated with the prognosis of patients with NKTCL. Elevated baseline sPD-L1 levels were associated with an increased risk of worse OS and PFS.33,57 Recently, randomized clinical trials (RCTs) suggested that blocking the PD-1/PD-L1 axis is a safe and robust treatment for NKTCL, specifically showing potent activity in r/r disease.58,59
Pembrolizumab, a humanized anti-PD-1 IgG4 monoclonal antibody, exhibited favorable antitumor effects in seven patients with r/r NKTCL who had failed l-asparaginase-based regimens. The results suggested that the objective response rate (ORR) and complete remission (CR) rate were 100% and 71.4%, respectively. Regarding the treatment-related adverse events (TRAEs), only one patient had grade 2 skin graft-versus-host disease (GVHD).59 Similarly, two other studies utilizing pembrolizumab and nivolumab showed that these treatments were highly efficacious and well tolerated in patients with r/r NKTCL.28,29 In preclinical studies, sintilimab demonstrated superior antitumor activity compared to pembrolizumab or nivolumab.60 A multicenter, single-arm, phase 2 clinical trial, including 28 patients with r/r NKTCL, was designed to provide data on the clinical efficacy and safety of sintilimab. The results of the study illustrated that the ORR was 75.0%, 6 (21.4%) patients achieved CR, and 15 (53.6%) patients achieved PR. The 2-year OS rate was 78.6%, and grade 1-2 decreased lymphocyte count was the most common TRAE.58 Targeting PD-L1 also produced active antitumor efficacy. Avelumab is a human IgG1 mAb targeting PD-L1 that can enhance antibody-dependent cell-mediated cytotoxicity (ADCC). Kim et al. evaluated the efficacy and safety of avelumab in 21 patients with r/r NKTCL. The phase II study revealed that avelumab was active with an ORR of 38%, a CR rate of 24%, and no grade 4 adverse events occurred.61 Moreover, the efficacy of the anti-PD-L1 mAb sugemalimab in r/r NKTCL has also been revealed, which induces a high CR rate and durable response without significant toxicity. The results from the GEMSTONE-201 trial (NCT03595657) showed that in 80 eligible patients who received sugemalimab and were followed for a median of 18.7 months, the independent radiologic review committee-assessed ORR was 44.9%. Among them, 28 patients (35.9%) achieved a CR, and seven (9.0%) achieved a PR, with a 12-month duration of response rate of 82.5%. The most treatment-emergent adverse events were grade 1–2 in severity.34,62
Combining anti-PD1/PD-L1 antibodies with other innovative treatments, such as the combination of sintilimab and chidamide (a histone deacetylase inhibitor, HDACi), has demonstrated improved antitumor efficacy and safety in r/r NKTCL. A phase Ib/II study involving 45 patients with r/r NKTCL revealed encouraging outcomes, with an ORR of 58.3% (21 out of 36) and a CR rate of 44.4% (16 out of 36) among the evaluated subjects.63 Sun et al. conducted a retrospective study that assessed the efficacy and safety of combining anti-PD-1 antibodies (pembrolizumab/nivolumab/sintilimab) with anlotinib (small molecule tyrosine kinase inhibitor targeting VEGFR and other tyrosine kinase-mediated pathways), pegaspargase, and radiotherapy in eight patients with localized NKTCL. The results indicated that the 3-year OS and PFS rates of the combined regimen both were 100% (median follow-up, 35.5 months). There were no severe (grade 3/4) hematological toxicities reported.64
Overall, PD-1/PD-L1 axis blockade has demonstrated promising antitumor activity as a single agent or in combination in treating NKTCL.31,65,66 Evidence suggests that anti-PD-1/PD-L1 therapies might also work synergistically with cellular therapies or other therapeutic strategies. Combination regimens through variant antitumor mechanisms may improve the prognosis. Multiple clinical trials are currently underway, investigating therapies such as anti-PD1/PD-L1 inhibitors plus radiotherapy, antiangiogenesis treatment, anti-CD20 (rituximab), anti-CD30 (brentuximab vedotin), or CAR-T cells. Nonetheless, immunotherapy-related adverse events remain a major challenge, especially in the skin, gastrointestinal system, endocrine system, and liver.67 Trials seeking predictive biomarkers for identifying suitable patients are warranted to improve the response to checkpoint inhibitor-based immunotherapy. Table 2 summarizes the clinical trials of immune-related monoclonal antibodies in NKTCL.
Table 2.
Clinical trials of immune monoclonal antibodies in natural killer/T cell lymphoma
| Targets | Intervention/treatment | Disease | Trial name | Number of enrolled participants | Phase | Status | Trial identifier |
|---|---|---|---|---|---|---|---|
| PD-1 | Pembrolizumab | T cell Lymphoma or NK-Cell Lymphoma | Pembrolizumab for T/NK-cell lymphomas NK-cell Lymphomas | 33 | 2 | Unkonwn | NCT03021057 |
| NKTCL of the nasal cavity/nasopharynx | Study of Pembrolizumab in Patients With Early-Stage NK/T cell Lymphoma, Nasal Type | 19 | 2 | Recruiting | NCT03728972 | ||
| Pembrolizumab | Relapsed/Refractory PCTL include NKTCL | Pembrolizumab and Pralatrexate in Treating Patients With Relapsed or Refractory Peripheral T cell Lymphomas | 40 | 1/2 | Recruiting | NCT03598998 | |
| Pembrolizumab | Natural Killer/T cell Lymphoma, Nasal and Nasal-Type | Treatment of Relapsed or Refractory Natural Killer/T cell Lymphoma | 20 | 2 | Unkonwn | NCT03107962 | |
| Pembrolizumab, Copanlisib | NK and T cell non-Hodgkin’s lymphoma | Study of MK-3475 Alone or in Combination With Copanlisib in Relapsed or Refractory NK and T cell Non-Hodgkin Lymphoma | 19 | 1/2 | Active, not recruiting | NCT02535247 | |
| Tislelizumab | NK/T cell Lymphoma | Dexamethasone, Azacytidine, Pegaspargase and Tislelizumab for NK/T cell Lymphoma | 50 | 2 | Not yet recruiting | NCT04899414 | |
| Early-stage Extranodal NK-T-Cell Lymphoma, Nasal and Nasal-Type | Radiotherapy and Anti-PD-1 in Low-risk ES-ENKTCL | 30 | 2 | Recruiting | NCT05149170 | ||
| Sintilimab | Relapsed/Refractory ENKTL | Efficacy and Safety Evaluation of IBI308 in Patients With Extranodal NK/T cell Lymphoma Patients (ORIENT-4) | 28 | 2 | Completed | NCT03228836 | |
| Sintilimab, P-Gemox | NK/T cell Lymphoma Nos | Sintilimab With P-GemOx Regimen for Newly Diagnosed Advanced Extranodal Natural Killer/T cell Lymphoma, Nasal Type | 63 | 2 | Recruiting | NCT04127227 | |
| Sintilimab, Lenalidomide | NK/T cell Lymphoma Nos | Lenalidomide and Sintilimab for Relapsed/Refractory NK/T cell Lymphoma | 20 | 2 | Recruiting | NCT04231370 | |
| Sintilimab, Chidamide | Relapsed/Refractory ENKTL | Sintilimab in Combination With Chidamide in Refractory and Relapsed ENKTCL | 40 | 1/2 | Completed | NCT03820596 | |
| Sintilimab, Pegaspargase, Anlotinib | Early stage NKTL, nasal and nasal-Type | Combined Treatment of Sintilimab, Peg-aspargase Plus Anlotinib in NK/T cell Lymphoma | 30 | 2 | Recruiting | NCT03936452 | |
| Pegaspargase, Anti-PD-1 monoclonal antibody | ENKTCL, nasal type | Anti-PD-1 Antibody Combined With Pegaspargase in the Treatment of Advanced Stage NK/T cell Lymphoma | 22 | 2 | Recruiting | NCT04096690 | |
| PD-1 antibody, Chidamide, Lenalidomide, Etoposide | NK/T cell Lymphoma | PD-1 Antibody, Chidamide, Lenalidomide and Etoposide for Relapsed or Refractory NK/T cell Lymphoma | 50 | 4 | Unknown | NCT04038411 | |
| Nivolumab | Relapsed/Refractory PTCL | Nivolumab in Treating Patients With Relapsed or Refractory Peripheral T cell Lymphoma | 12 | 2 | Terminated | NCT03075553 | |
| Nivolumab | T cell and NK cell lymphomas, cutaneous squamous cell carcinoma, Merkel cell carcinoma, and other rare skin tumors | Talimogene Laherparepvec and Nivolumab in Treating Patients With Refractory Lymphomas or Advanced or Refractory Non-melanoma Skin Cancers | 68 | 2 | Recruiting | NCT02978625 | |
| SHR-1210 | Relapsed/Refractory ENKTCL, nasal type | SHR-1210 in Patients With Relapsed or Refractory Extranodal NK/T cell Lymphoma | 97 | 2 | Active, not recruiting | NCT03363555 | |
| SHR1210, Apatinib | Relapsed/Refractory NKTCL | PD1 Combined With Apatinib in Patients With Relapsed or Refractory NK/T cell Lymphoma | 61 | 2 | Unknown | NCT03701022 | |
| LEAP regimen (Sintilimab, Pegaspargase, Anlotinib) | Natural Killer/T cell Lymphoma, Nasal and Nasal-Type | Sintilimab, Pegaspargase and Anlotinib for Stage IV Natural Killer/T cell Lymphoma | 37 | 2 | Recruiting | NCT04004572 | |
| Toripalimab | Extranodal NK/T cell Lymphoma, Nasal Type | Maintenance Therapy With Anti-PD-1 Antibody for Patients With NK/T cell Lymphoma | 20 | 2 | Not yet recruiting | NCT04338282 | |
| Toripalimab, P-GemOx, IMRT | Early-Stage ENKTL | A Multicenter, Phase 3, Randomized Trial of Sequencial Chemoradiotherapy With or Without Toripalimab (PD-1 Antibody) in Newly Diagnosed Early-Stage Extranodal Natural Killer/T cell Lymphoma, Nasal Type (ENKTL) | 207 | 3 | Recruiting | NCT04365036 | |
| PD-L1 | Avelumab | Relapsed/Refractory ENKTL | Avelumab in Relapsed or Refractory Extranodal Natural Killer/T cell Lymphoma[AVENT STUDY] | 21 | 2 | Active, not recruiting | NCT03439501 |
| Sugemalimab (CS1001) | Extranodal Natural Killer/T cell Lymphoma | A Study of CS1001 in Subjects With Relapsed or Refractory Extranodal Natural Killer/T cell Lymphoma (ENKTL) | 80 | 2 | Active, not recruiting | NCT03595657 | |
| IMC-001 | Relapsed/Refractory ENKTL | A Study of IMC-001 in Subjects With Relapsed or Refractory Extranodal NK/T cell Lymphoma, Nasal Type | 20 | 2 | Recruiting | NCT04414163 | |
| Sugemalimab | Extranodal NK/T cell Lymphoma | An Expanded Access Program to Provide Sugemalimab for the Treatment of Relapsed or Refractory Extranodal Natural Killer/T cell Lymphoma (R/R ENKTL) | NA | NA | Available | NCT05131438 | |
| CTL-4 | Ipilimumab | Recurrent Melanoma, Non-Hodgkin Lymphoma, Colon, or Rectal Cancer, include Adult Nasal Type Extranodal NK/T cell Lymphoma | Ipilimumab and Local Radiation Therapy in Treating Patients With Recurrent Melanoma, Non-Hodgkin Lymphoma, Colon, or Rectal Cancer | 3 | 1/2 | Terminated (Planned Future Study) | NCT01769222 |
| CCR4 | Mogamulizumab (KW-0761) | Peripheral T/NK-cell Lymphoma | Study of KW-0761 in Subjects With CCR4-positive Peripheral T/NK-cell Lymphoma | 38 | 2 | Completed | NCT01192984 |
| KW-0761 | Adult T cell Leukemia and Lymphoma (ATL), Adult Peripheral T cell Lymphoma (PTCL) | Phase I Study of KW-0761 in Relapsed Patients With CCR4-Positive ATL and PTCL | 16 | 1 | Completed | NCT00355472 | |
| CD25 (IL-2Rα) | Basiliximab | Mature T cell and NK-Cell Non-Hodgkin Lymphoma, Recurrent Mature T- and NK-Cell Non-Hodgkin Lymphoma, Refractory Mature T cell and NK-Cell Non-Hodgkin Lymphoma, Recurrent Cutaneous T cell Non-Hodgkin Lymphoma, Refractory Cutaneous T cell Non-Hodgkin Lymphoma | Yttrium Y 90 Basiliximab and Combination Chemotherapy Before Stem Cell Transplant in Treating Patients With Mature T cell Non-Hodgkin Lymphoma | 20 | 1 | Active, not recruiting | NCT02342782 |
| Basiliximab, Pegaspargase | Extranodal NK/T cell Lymphoma | Combination of Basiliximab and Pegaspargase in the Treatment of ENKTCL | 20 | 2 | Not yet recruiting | NCT04337593 | |
| CD30 | B-MAD chemotherapy | Extranodal NK/T cell Lymphoma | B-MAD Chemotherapy in Newly-diagnosed Extranodal NK/ T cell Lymphoma | 36 | 1/2 | Active, not recruiting | NCT03246750 |
| Anti-CD30/CD16A Monoclonal Antibody AFM13 | Recurrent or refractory CD30-positive HL or NHL | Modified Immune Cells (AFM13-NK) and A Monoclonal Antibody (AFM13) in Treating Patients With Recurrent or Refractory CD30 Positive Hodgkin or Non-Hodgkin Lymphomas | 30 | 1 | Recruiting | NCT04074746 | |
| AFM 13 | Relapsed/Refractory cutaneous lymphomas | AFM13 in Relapsed/Refractory Cutaneous Lymphomas | 18 | 1/2 | Completed | NCT03192202 | |
| Brentuximab vedotin, Cyclophosphamide, Prednisone, Doxorubicin, Vincristine | Lymphoma, Large-Cell, AnaplasticLymphoma, NK-cellLymphoma, T cell | A Phase 1 Study of Brentuximab Vedotin Given Sequentially and Combined With Multi-Agent Chemotherapy for CD30-Positive Mature T cell and NK-Cell Neoplasms | 39 | 1 | Completed | NCT01309789 | |
| Brentuximab vedotin | Relapsed or Refractory EBV-and CD30-positive Lymphomas | Brentuximab Vedotin in Patients With Relapsed or Refractory EBV-and CD30-positive Lymphomas | 25 | 2 | Completed | NCT02388490 | |
| CD38 | Daratumumab | Relapsed or refractory NKTCL | A Study to Assess the Clinical Efficacy and Safety of Daratumumab in Participants With Relapsed or Refractory Natural Killer/T cell Lymphoma (NKTCL), Nasal Type | 32 | 2 | Completed | NCT02927925 |
| CD52 | Alemtuzumab (Campath), EPOCH | Lymphoma, T-CellLymphoma, Extranodal NK-T-Cell | Campath-1H and EPOCH to Treat Non-Hodgkin’s T- and NK-Cell Lymphomas | 31 | 2 | Completed | NCT00069238 |
| Alemtuzumab, CHOP | T cell LymphomaLymphoma, Non-Hodgkin’s | Study of CHOP + Campath for T cell, Null Cell, or Natural Killer (NK)-Cell Lymphoma | NA | 1 | Completed | NCT00161590 | |
| Alemtuzumab, CHOP | Aggressive T/NK-Cell Lymphomas | CHOP and Campath-1H in Previously Untreated Aggressive T/NK-Cell Lymphomas | 24 | 1 | Completed | NCT00323323 | |
| Alemtuzumab, CHOP-14 | Peripheral T cell Lymphoma, Unspecified Angioimmunoblastic Lymphadenopathy Extranodal NK/T cell Lymphoma | Immunotherapy in Peripheral T cell Lymphoma - the Role of Alemtuzumab in Addition to Dose Dense CHOP (A-CHOP-14) | 274 | 3 | Unknown | NCT00725231 |
NKTCL, natural killer/T cell lymphoma; ENKTL, extranodal NK/T-cell lymphoma, nasal type; R/R, Relapsed/Refractory; PTCL NOS, peripheral T cell lymphoma not otherwise specified; HL, Hodgkin lymphomas; NHL, non-Hodgkin lymphomas; PD-1, programmed death 1; PD-L1, programmed cell death-ligand 1; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; CCR4, C-C chemokine receptor 4; IL-2Rα, IL-2 receptor alpha; ORR, overall response rate; CR, complete response; PR, partial response; SD, stable disease; P-GemOx, pegaspargase, gemcitabine and oxaliplatin; IMRT, intensity-modulated radiotherapy; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone.
Antibodies targeting cytotoxic T-lymphocyte antigen 4
Cytotoxic T-lymphocyte antigen 4 (CTLA-4/CD152) is a transmembrane T cell inhibitory molecule that binds to B7 ligand expressed on antigen-presenting cells (APCs), triggering T cell anergy and negatively regulating the immune response.68 Ipilimumab, an anti-CTLA-4 monoclonal antibody, has demonstrated positive therapeutic outcomes in multiple malignancies, including malignant melanoma, lung cancer, and renal cancer.69,70,71 To date, a phase I/II clinical trial of patients with non-Hodgkin lymphoma, colorectal cancer, and recurrent melanoma (including NKTCL) was conducted to assess the safety of ipilimumab (NCT01769222). Although clinical data concerning anti-CTLA-4 monoclonal antibodies for NKTCL treatment are largely limited, CTLA-4 inhibitors may show promise. More evidence from preclinical and clinical studies is still needed to further explore the use of CTLA-4 inhibitors in treating hematological malignancies.
Targeting surface antigens
Novel monoclonal antibody-based therapies have emerged as essential strategies in NKTCL treatment. Monoclonal antibodies exert their effects primarily through ADCC, complement-dependent cytotoxicity, and apoptosis induction. Numerous ongoing clinical trials are dedicated to exploring monoclonal antibodies, either as standalone agents or in combination with other therapies, to enhance efficacy against NKTCL.
Anti-CD25 (IL-2Rα) monoclonal antibody
CD25, also known as IL-2Rα, is a subunit of the interleukin-2 receptor and plays a significant role in regulating the immune response. It is mainly expressed on the surface of the effector T cells, regulatory T cells (Tregs), Langerhans cells, and B cells, exerting proinflammatory effects.72,73 CD25 overexpression was induced by EBV-driven LMP1 in NKTCL through the activation of the NF-κB and MAPK pathways.74 Expression of CD25 was detected in 33.0%–53.8% of patients with NKTCL and was significantly correlated with B symptoms and elevated serum lactate dehydrogenase.40,75 The high-level serum soluble form of IL-2Rα (sIL-2Rα) was elevated and correlated with poor prognosis in various hematologic malignancies and solid tumors.76,77,78 The serum CD25 levels were elevated in NKTCL, especially in patients with hemophagocytic syndrome (HPS) or B symptoms. Data from preclinical and clinical studies suggest that CD25 may be a potential therapeutic target for CD25+ NKTCL. High sIL-2Rα level was significantly correlated with poor treatment response and survival for patients with NKTCL.40 In a case report, a patient with steroid dependent hemophagocytic syndrome was treated with daclizumab (anti-CD25 antibody), allowing successful withdrawal of corticosteroid therapy.79 Basiliximab, a recombinant chimeric mAb that specifically binds CD25 on the surface of activated T-lymphocytes, is an immunosuppressive agent. 131Iodine-labeled Basiliximab has shown promise in an early phase I trial, demonstrating CR or PR in patients with CD25+ lymphoma.80 A phase II trial is currently underway to evaluate the efficacy and safety of basiliximab with pegaspargase in patients with r/r NKTCL (NCT04337593).
Anti-CD56 monoclonal antibody
CD56 (neural cell adhesion molecule 1), a 200–220 kDa cell surface glycoprotein, is expressed highly in 76.1%–90% of NKTCL.81,82,83,84 IMGN901 (huN901-DM1) is an anti-CD56 mAb with high affinity binding to CD56. IMGN901 has shown safety and promising efficacy in CD56-positive malignancy in a phase I/II study.85 Ishitsuka et al. found that IMGN901 demonstrated a selective affinity for the NK-92 MI, a CD56-positive NK cell line. The antibody significantly inhibited the cells from growing when it bound to membrane CD56.50 Targeting CD56 with IMGN901 represents a potential therapeutic modality against NKTCL. Therefore, anti-CD56-based therapies, either as monotherapy or in combination, warrant further investigation on a larger scale in this patient population.
Anti-CD30 monoclonal antibody
CD30 (TNFRSF8) is a multifunctional transmembrane protein within the tumor necrosis factor receptor (TNFR) superfamily. It exhibits high expression on activated T- and B lymphocytes, as well as certain NHL cells. CD30 has been reported to stimulate T cells to promote the production of cytokines that include IL-2, IFN-γ, and TNF.86 Approximately 70% of patients with NKTCL exhibit CD30 overexpression, making it an ideal target for NKTCL immunotherapy.87 Brentuximab vedotin (BV) is an antibody-drug conjugate (ADC) combining CD30 mAb with the microtubule inhibitor monomethyl auristatin E (MMAE), which has been approved by the FDA for patients with CD30+ lymphoproliferative disorders, such as cutaneous anaplastic large cell lymphoma, peripheral T cell lymphomas, and mycosis fungoides. As mentioned earlier, CD30 is also proposed as a potential therapeutic target for NKTCL because of its high expression. In a later multicenter, open-labeled phase II trial study, data from 33 patients with r/r CD30+ NHL (including 7 subjects with NKTCL) who were treated with single-agent BV (1.8 mg/kg) were reported. Among this cohort, the ORR for NKTCL was 29%, with one case achieving CR and another obtaining PR, and the response lasted for more than one year.88 Subsequently, another phase II clinical study of BV (open-label, multicenter, investigator-initiated) demonstrated significant and durable clinical activity. Among a total of 25 patients with r/r EBV-positive lymphomas (including 22 cases of mature T/NK-cell neoplasms), the ORR was 48%, with a median follow-up of 20 months. For the intention-to-treat population, OS and PFS were 15.7 months and 6.2 months, respectively.41 In addition, two case reports have suggested that patients with refractory NKTCL achieved CR after BV therapy without significant toxicity.89,90 Recently, according to another RCT that recruited 25 patients with r/r CD30+ and EBV+ lymphomas, the ORR for mature NKTCL was 46% and it was even higher in patients with mature B-cell lymphomas (67%). The duration of response was 10.1 months and the most common TRAEs were peripheral neuropathy and neutropenia.41 Given its durable clinical activity and manageable toxicity, BV might have a promising future in treating r/r NKTCL as salvage therapy.
Anti-CD38 monoclonal antibody
CD38 is a type II transmembrane glycoprotein belonging to a complex family of enzymes on the cell surface and is related to the functions as a receptor and adhesion molecule.43,91 Previous gene expression profile data revealed that CD38 gene expression was upregulated, and the antigen CD38 was highly expressed in approximately 50% of NKTCL, which is associated with poorer outcomes.44,92 Due to its aberrant expression and involvement in regulating cellular metabolic pathways and immunomodulation, CD38 appears to be a promising target for treating NKTCL.93 Several novel monoclonal antibodies with specific targeting of CD38 have been successfully developed, including daratumumab, isatuximab (SAR650984), and MOR202.45,94 Hari and colleagues reported a case in which a patient with relapsed NKTCL achieved a sustained remission of 21 weeks as salvage therapy with single-agent daratumumab.46 In a multicenter phase II trial, daratumumab was administered to 32 patients with r/r NKTCL. The ORR observed was 25.0%, and the median OS reached 141.0 days (NCT02927925). Furthermore, it has been reported that a high CD38: complement inhibitory protein (CIP) ratio in NKTCL might potentially predict a more favorable response to daratumumab.95 In conclusion, while trials have provided initial insights, the available evidence remains limited, underscoring the need for further studies to inform the clinical application of anti-CD38 monoclonal antibodies.
Anti-CD52 monoclonal antibody
CD52 (CAMPATH-1 antigen) is a small glycoprotein expressed on lymphocytes, NK cells, dendritic cells (DCs), and macrophages. CD52 molecules provide costimulatory signals for lymphocyte activation and proliferation, as well as stimulate the production of IL-6, IFN, and TNF.96,97 The expression of CD52 has been reported in up to 47% of patients with NKTCL, thus representing an attractive target for immunotherapy of EBV-induced lymphoma.98 Alemtuzumab, a humanized IgG1 monoclonal antibody against CD52, was combined with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), producing 1 CR among 3 patients with newly diagnosed NKTCL.99 Kim et al. reported that different doses of alemtuzumab combined with dexamethasone, cytarabine, and cisplatin (DHAP) for treating r/r PTCL (including 8 NKTCL subjects). ORR was observed in 24 patients (50%), with five CR and seven PR. The most common adverse event was grade 3/4 leukopenia (79.2%). Given its antitumor efficacy and tolerability, combining alemtuzumab plus DHAP might be an effective salvage treatment.48 Findings from a phase II RCT (NCT00069238) suggested that alemtuzumab combined with etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) resulted in stable remission in patients with CD52-positive NKTCL; 24 (77.4%) patients among 31 subjects achieved a response: 17 patients with CR and 7 subjects with PR. More recently, a phase III clinical trial (NCT00725231) was conducted to investigate the value of adjuvant alemtuzumab in combination with dose-dense CHOP-14 in patients with previously untreated PTCL (including NKTCL), which might provide new evidence on alemtuzumab treatment.
The anti-CD52 mAb seems to be an attractive candidate for treatment optimization; however, significant treatment-related adverse events cannot be overlooked. More recent studies have focused on improving the safety profile of alemtuzumab and its efficacy in combination with other therapies. Trials involving the application of alemtuzumab in patients with NKTCL are currently being conducted (NCT00161590, NCT00323323, and NCT00118352).
Anti-CCR4 monoclonal antibody
C-C chemokine receptor 4 (CCR4) is expressed on Th2 cells, Tregs, or EBV-infected NK cells of EBV-related hematologic malignancies and belongs to the G-protein-coupled receptor family.100,101 CCR4 is commonly expressed in various NKTCL cell lines, such as SNK6, SNK10, SNK1, NKL, and HANK1.51,102,103 Elevated expression of the CCR4 ligands, CCL17 (TARC) and CCL22 (MDC), expression was reported in patients with NKTCL. Therefore, the CCL17/CCL22-CCR4 axis seems to be an attractive immune therapeutic target for aggressive NKTCL.51
Mogamulizumab, a monoclonal antibody of CCR4, targets CCR4-expressing malignant cells by ADCC and depletes Tregs in the tumor microenvironment.104 Kanazawa et al. confirmed that mogamulizumab induced ADCC against CCR4-expressing cells and prevented EBV-positive NK-cell lymphomas from growing in murine models.52 The trial findings of 26 aggressive adult patients with T cell leukemia-lymphoma suggested the potential antitumor activity and notable OS and PFS of mogamulizumab (NCT00920790).105 In 2018, mogamulizumab was approved by the FDA for treating mycosis fungoides or Sézary syndrome in patients who were treated with at least one prior systemic therapy. While anti-CCR4 monoclonal antibodies have primarily been studied in T cell NHL, further research is warranted to provide more evidence on antitumor efficacy and tolerability in NKTCL.
Adoptive T/natural killer-cell therapy
Epstein-Barr virus-related antigen-specific cytotoxic T lymphocytes
Antigen-specific cytotoxic T lymphocyte therapy can induce durable antitumor activity in specific tumors, such as lymphomas and melanoma.106,107 Previous reports have shown that over 90% of the patients with NKTCL have a latent infection of EBV in tumor cells.108,109 High expression of EBV proteins, LMP1/LMP2 and Epstein-Barr virus nuclear antigen 1 provides appealing targets for adoptive immunotherapy with EBV-CTLs in NKTCL.110,111 Data from one trial of patients with EBV-positive lymphomas (enrolled 11 patients with NKTCL) undergoing autologous EBV-specific T cell therapy reported sustained clinical responses at a median of 3.1 years in 13 of 21 patients with lymphoma, including CRs in 4 of 6 patients with r/r NKTCL.107 Another clinical trial reported that adoptive transfer of EBV LMP1/2a-specific CTLs for NKTCL is an effective post remission therapeutic approach without immediate or delayed toxicities. Ten of 11 subjects who received LMP1/2a CTLs achieved sustained CR after induction chemotherapy, with 100% 4-year OS and 90% PFS (median follow-up, 55.5 months).112 Another phase II trial revealed that patients with aggressive NKTCL who failed asparaginase-based regimens utilizing autologous EBV-specific T cells (47 subjects enrolled, of whom 15 patients were successfully generated and infused with baltaleucel T) achieved an ORR of 50.0% and a CR rate of 30.0%, with a median PFS of the patients was 12.3 months. Diarrhea, vomiting, pyrexia, and headache were the most frequent AEs.113
Although preclinical/clinical results demonstrated that adoptive EBV-CTL transfer is a promising treatment strategy in NKTCL, the clinical utility may be limited by the long turnaround time and high manufacturing failure rate. An off-the-shelf bank of donor-derived EBV-CTLs is being developed, and if the risk of graft versus host can be minimized, it might be an attractive and unprecedented option.114,115,116 Table 3 summarizes the clinical trials of cellular immunotherapy for NKTCL.
Table 3.
Clinical trials of cellular treatment in natural killer/T cell lymphoma
| Cellular treatment | Target | Intervention/treatment | Disease | Trial name | Number of enrolled participants | Phase | Status | Trial identifier | Dose |
|---|---|---|---|---|---|---|---|---|---|
| CAR-T cells | CD7 | CD7 CAR-T cells infusion | T-lymphoblastic Lymphoma, NK/T cell Lymphoma, Acute Lymphocytic Leukemia | CD7 CAR-T Cells for Patients With R/R CD7+ NK/T cell Lymphoma, T-lymphoblastic Lymphoma, and Acute Lymphocytic Leukemia | 10 | Phase 1 | Recruiting | NCT04004637 | 0.5-5×106/kg |
| CD7-specific CAR gene-engineered T cells | T cell Acute Lymphoblastic Leukemia T cell Acute Lymphoblastic Lymphoma Acute Myeloid Leukemia NK Cell Lymphoma |
Multi-CAR T cell Therapy Targeting CD7-positive Malignancies | 30 | Phase 1 and phase 2 | Recruiting | NCT04033302 | NA | ||
| CD7 UCAR-T cells | CD7+ T/NK Cell Hematologic Malignancies | Anti-CD7 U-CAR-T Cell Therapy for T/NK Cell Hematologic Malignancies | 30 | Early phase 1 | Recruiting | NCT04264078 | Three levels: 1×107 cells/kg, 3×107 cells/kg, 5 ×107 cells/kg | ||
| CD30 | Anti-CD30 CAR-T cells | Adult T cell Lymphoma/Leukemia, Anaplastic Large Cell Lymphoma, Angioimmunoblastic T cell Lymphoma, NK/T cell Lymphoma, Peripheral T cell LymphomaHodgkin Lymphoma | Anti-CD30 CAR-T Therapy in Patients With Refractory/Relapsed Lymphocyte Malignancies | 50 | Phase 1 | Recruiting | NCT04008394 | NA | |
| Anti-CD30 CAR-T cells, Cyclophosphamide, Fludarabine | Lymphoma, Large-Cell, Anaplasitc, Enteropathy-Associated T cell Lymphoma, Lymphoma, Large B-Cell, DiffuseLymphoma, Extranodal NK-T-Cell, Lymphoma, T cell, Peripheral | T Cells Expressing a Fully-Human Anti-CD30 Chimeric Antigen Receptor for Treating CD30-Expressing Lymphomas | 26 | Phase 1 | Completed | NCT03049449 | 0.3x106 cells/kg (up to a maximum dose of 18x106 CAR+ T cells/kg) | ||
| Anti-CD30 CAR T cells | Lymphomas | CAR T Cells Targeting CD30 Positive Lymphomas (4SCAR30273) | 20 | Phase 1 and phase 2 | Unknown | NCT02274584 | NA | ||
| Anti-CD30 CAR-T cells | CD30 positive NHL subtypes (ALCL, PTCL-NOS, ENKTL, DLBCL-NOS, PMBCL) | Phase 1 Study of Autologous CD30.CAR-T in Relapsed or Refractory CD30 Positive Non-Hodgkin Lymphoma (CERTAIN) | 21 | Phase 1 | Recruiting | NCT04526834 | Three levels: 2 x 108 cell/m2, 4 x 108 cell/m2, 6 x 108 cell/m2 | ||
| Anti CD30 CAR-T cells | Hodgkin Lymphoma, NK/T cell Lymphoma, Peripheral T cell Lymphoma, Unspecified; Angioimmunoblastic T cell Lymphoma, Anaplastic Large Cell Lymphoma, Diffuse Large B Cell Lymphoma, Mediastinal B-Cell Diffuse Large Cell Lymphoma, Gray Zone Lymphoma | An Exploratory Clinical Study Evaluating the Safety and Efficacy of Anti CD30 CAR T Cells in Patients With CD30+ Relapsed/Refractory Lymphoma | 9 | Early phase 1 | Not yet recruiting | NCT05208853 | Three levels: 1.5×107 cells, 1.5×108 cells, 5× 108 cells | ||
| Activated/stimulated T cells and LMP1/2- or LMP2-targeted strategies | Hodgkin Disease, Non Hodgkin Lymphoma, Lymphoepithelioma, Leiomyosarcoma | LMP-specific T-cells for Patients With Relapsed EBV-positive Lymphoma (ALCI) | 74 | Phase 1 | Completed | NCT00671164 | 2 x 107 cells/m2-2 x 108 cells/m2 | ||
| EBV-CTLs | LMP1/LMP2 (EBV antigens) | Baltaleucel-T cells | EBV Positive Extranodal NK/T cell Lymphoma | Cellular Immunotherapy Treatment Antigen-Directed for EBV Lymphoma (CITADEL) | 15 | Phase 2 | Terminated | NCT01948180 | 2x107 cells/m2 |
| EBViNT Cells | EBV Associated Extranodal NK/T cell Lymphoma, EBV-Associated Gastric Carcinoma | A Multi-center, Single-arm, Open, Phase I/IIa Clinical Trial to Evaluate the Efficacy and Safety of EBViNT Cell (EBV Specific Autologous CD8+ T cell) in Patients With Treatment Failed Epstein-Barr virus (EBV)-Positive Malignancies | 72 | Phase 1 and phase 2 | Recruiting | NCT03789617 | 1.4x109 cells/100mL | ||
| Epstein-Barr virus human cytotoxic T lymphocytes (EBV-CTLs) | Extranodal NK/T cell Lymphoma | VT-EBV-N for Treatment of Severe in EBV Positive Extranodal NK/T cell Lymphoma Patients | 48 | Phase 2 | Recruiting | NCT03671850 | NA |
CAR-T, Chimeric antigen receptor T-cell; NHL, non-Hodgkin lymphomas; Anaplastic Large Cell Lymphoma; PTCL-NOS, peripheral T cell lymphoma not otherwise specified; ENKTL, Extranodal NK/T-cell Lymphoma; DLBCL-NOS, Diffuse Large B Cell Lymphoma not otherwise specified; PMBCL, Primary Mediastinal Large B-Cell Lymphoma; LMP1/2, Latent membrane protein 1/2.
Chimeric antigen receptor cell-based immunotherapy
Chimeric antigen receptor T cell therapy
Chimeric antigen receptor T (CAR-T) cell therapy has been proven to be a successful immunotherapy that has achieved remarkable efficacy in some relapses or refractory hematologic malignancies.117 Tang et al. demonstrated that targeting LMP1 utilizing HELA/CAR cells might be a promising cellular therapy for treating EBV-positive malignancies.118 CD38-CAR-T cells exhibited substantial inhibition against diverse lymphocyte malignancies, including NKTCL, mantle cell lymphoma, and T cell acute lymphoblastic leukemia, in both in vitro and mouse xenograft models.119 In a recent preclinical study, four CAR-T cell lines (CD38-CAR, LMP1-CAR, CD38-LMP1 tandem CAR 1 and CD38-LMP1 tandem CAR 2) exhibited significant cytotoxicity against NKTCL cells both in vitro and in vivo.53 Another preclinical investigation suggested that NKTCL cell lines expressed high levels of B7-H3 homogeneously. Anti-B7-H3/CD3 bispecific T cell engaging and B7-H3-redirected CAR-T cells have demonstrated potent in vitro antitumor efficacy and induced tumor regression in NKTCL tumor mouse models.120 Currently, an ongoing phase I trial (NCT03049449) is designed to provide data on the safety and feasibility of utilizing fully human anti-CD30 CAR cells for the treatment of patients with advanced CD30-expressing lymphomas. While the data from clinical trials for CAR-T therapy in NKTCL are currently limited and in their nascent stages, the outlook is promising. Several research are underway to investigate the efficacy of the combination of anti-CD7/CD30 CAR-T cells and other treatments for NKTCL (NCT04004637, NCT04008394, NCT03049449, NCT02274584, NCT03910842, and NCT04033302).
Although CAR-T therapy is a powerful strategy for treating hematological malignancies, there are still many patients who do not respond to the therapy. Of note, life-threatening CAR-T cells therapy toxicities, especially cytokine release syndrome (CRS) and neurologic toxicity, should be addressed. To overcome the limitations of CAR-T cell therapy, researchers must identify the most suitable patients, improve clinical efficacy, and decrease the related toxicity, and innovative strategies and approaches are thus critical.
Immune system modulators
Immunomodulatory drugs (IMiDs) demonstrate potential benefits via anti-inflammatory properties, immunomodulatory effects, and anti-proliferative effects. IMiDs (e.g., thalidomide and lenalidomide) exhibited antitumor activity in multiple lymphomas and demonstrated a potential therapeutic activity in NKTCL (Table 4). Previous studies have shown that the immunomodulatory activity of lenalidomide depends on increasing levels of IL-2 and antiangiogenic. Wang et al. revealed that one NKTCL patient previously treated with an asparaginase-containing regimen in combination with radiotherapy exhibited no response to autologous stem cell transplantation, but it was a successful salvage therapy in which the patient was treated with lenalidomide monotherapy and obtained a CR.128 Additionally, the combination of thalidomide with the CHOP regimen has demonstrated increased efficacy and the potential to decrease the occurrence of adverse gastrointestinal side effects.127 Du et al. reported three patients with r/r NKTCL treated with the combination regimen of toripalimab, chidamide, etoposide, and thalidomide. Two patients achieved CR and another patient obtained PR, with controllable TRAEs, such as grade 2/3 leukocytopenia and anemia.129 The combination of thalidomide with dexamethasone-containing therapy has also been shown to have potential therapeutic benefits. The V140A variant of the evolutionarily conserved signaling intermediate in the Toll pathway (ECSIT) activated the NF-κB pathway, leading to hyperinflammation, and promoted hemophagocytic syndrome (HPS) in NKTCL. Thalidomide prevented the NF-κB pathway from binding to the DNA promoters of the target genes, revealing the feasibility of combination with dexamethasone-containing therapy in two patients with NKTCL with HPS.130 To date, data from studies regarding the efficacy of IMiDs are still limited. In a phase III RCT (NCT02085655), the efficacy and safety of P-Gemox in combination with thalidomide was compared with AspaMetDex (pegaspargase, methotrexate, and dexamethasone) in previously untreated or patients with r/r NKTCL. Other trials to further explore the use of IMiDs in NKTCL are ongoing (NCT04231370, NCT05058755, NCT04038411, and NCT03054532). Collectively, findings from the IMiDs provide mechanistic insights and a potential therapeutic strategy for NKTCL.
Table 4.
Selected reports of immunotherapy for natural killer/T cell lymphoma
| Targets | Intervention/treatment | Phase/Study design | Disease | Sample size | Dosing | Treatment | ORR (%) | CRR (%) | Common AE | Median follow-up, mo (range) | Trial identifier | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-1/PD-L1 | Pembrolizumab | Retrospective | Relapsed or refractory after SMILE-like therapy | 7 | 2 mg/kg every 3 weeks | Single agent | 100% | 71.40% | Grade 2 rush | 6 (2–10) | Kwong et al.59 | |
| Retrospective | Relapsed or refractory NKTCL | 7 | 100 mg every 3 weeks | Single agent | 57.10% | 28.6% | All-grade AEs 71.4% | NA | Li et al.28 | |||
| Retrospective | Relapsed or refractory NHL (include 14 NKTCL) | 30 | 100 mg or 200mg every 3 weeks | Single agent | NKTCL: 44% | – | Grade II skin rash, bowel perforation | NA | Kim et al.121 | |||
| Nivolumab | Retrospective | Relapsed or refractory after SMILE-like therapy | 3 | 40 mg (the smallest vial available) every 2 weeks | Single agent, low dose | 100.00% | 66.70% | NA | NA | Chan et al.29 | ||
| Sintilimab | Phase 2 | Relapsed or refractory ENKTL | 28 | 200mg every 3 weeks | Single agent | 75.0% | 21.4% | Decreased lymphocyte count (46.5%), pyrexia (42.9%), and decreased white blood cell count (35.7%) | 30.4 (27.5–31.9) | NCT03228836 | Tao et al.58 | |
| Avelumab | Phase 2 | Relapsed or refractory ENKTL | 21 | 10 mg/kg on days 1 and 15 of a 28-day cycle | Single agent | 38.0% | 24.0% | Fever (29%), anorexia (10%), Infusion-related reaction (19%) | 15.7 (95% CI: 14.5–16.9) | NCT03439501 | Kim et al.61 | |
| Sugemalimab | Phase 2 | Relapsed or refractory ENKTL | 80 | 1200 mg | Single agent | 45.6% | 30.4% | – | 18.7 | NCT03595657 | Huang et al.34 | |
| CCR4 | Mogamulizumab (KW-0761) | Phase 2 | CCR4-positive Peripheral T/NK-cell Lymphoma | 37 | 1.0 mg/kg once weekly for 8 weeks | Single agent | 35.0% | 14.0% | Lymphocytopenia (81%), neutropenia (38%), leukocytopenia (43%), and pyrexia (30%) | NA | NCT01192984 | Ogura et al.122 |
| Relapsed/refractory peripheral T cell lymphoma | 35 | 1.0 mg/kg once weekly for 4 weeks | Single agent | 11.40% | 3.00% | Drug eruption (34.2%), pyrexia (23.7%), diarrhea (18.4%), and pruritus (18.4%) | NA | – | Zinzani et al.123 | |||
| CD30 | Brentuximab vedotin | Phase 1 | Refractory or relapsed HL or CD30+ NHL | 24 | SGN-30 at 4 dose levels (2, 4, 8, or 12 mg/kg) weekly for 6 consecutive weeks | Single agent | Modest efficacy | 4.16% | Nausea (13.0%), fatigue (13.0%), and fever (13.0%) | NA | NCT00051597 | Bartlett et al.124 |
| Phase 1 | Relapsed or refractory CD30-positive hematologic cancers | 45 | 0.1 to 3.6 mg/kg every 3 weeks | Single agent | 67.0% | 24.4% | Fatigue (36%), pyrexia (33%), and diarrhea, nausea, neutropenia, and peripheral neuropathy (22% each) | NA | NCT00430846 | Younes et al.125 | ||
| Phase 2 | Relapsed/refractory CD30+ NHL | 34 | 1.8 mg/kg every 3 weeks | Single agent | 41.0% | 23.5% | Neutropenia (14%), peripheral sensory neuropathy, and hyperkalemia (9% each) | 2.7 (0.3–17.3) | NCT01421667 | Horwitz et al.126 | ||
| Cases | ENKTL with CD30 expression | 2 | 1.8 mg/kg every 3 weeks | Single-agent | – | – | Grade 2 toxicity dyspnea | – | – | Kim et al., Poon et al.89,90 | ||
| Phase 2 | Relapsed or Refractory EBV-and CD30-positive Lymphomas | 25 | 1.8 mg/kg every 3 weeks | Single-agent | 48.0% | 20.0% | Peripheral neuropathy (48%), neutropenia (44%), thrombocytopenia (20%), and rash (16%) | 20 (1.7–30.4) | NCT02388490 | Kim et al.41 | ||
| CD38 | Daratumumab | Phase 2 | Relapsed or refractory ENKTL | 32 | 16 mg/kg once weekly for Cycles 1 and 2, every other week for Cycles 3 through 6, and every 4 weeks thereafter, all cycles were 28 days | Single agent | 25.0% | – | Thrombocytopenia (25.0%), neutropenia (18.8%), and anemia and leukopenia (15.6%) | 10.2 months | NCT02927925 | Huang et al.45 |
| Case | A patient with stage IV NKTCL | 1 | 16 mg/kg weekly | Single agent | Patients reached a maximum sustained remission period of 21 weeks. | – | Multiple infectious complications (were considered unrelated to daratumumab) | NA | – | Hari et al.46 | ||
| CD52 | Alemtuzumab | Phase 2 | Newly diagnosed PTCL (inlude 3 ENKTL) | 3 | 10 mg i.v. on day 1 and 20 mg i.v. on day 2 in the Wrst cycle, then 30 mg i.v. on day 1 in the subsequent cycles | Combined with CHOP | 80.0% | 65.0% | Neutropenia (90%), febrile neutropenia (55.0%), cytomegalovirus reactivation (25%) | 7 (1–12) | – | Kim et al.99 |
| Phase 2 | Relapsed or refractory PTCL after first-line therapy | 8 | 70 mg or 40 mg | Combined with DHAP | 50.0% | 29.1% | Leukopenia (79.2%) | NA | – | Kim et al.48 | ||
| EBV antigens (LMP1/LMP2) | Activated/stimulated T cells | Phase 1 | EBV-associated lymphoma | 50 | 2 x 107-3 x 108 cells/m2 | LMP-Specific CTLs As Adjuvant Therapy | – | – | There were no clinical toxicities associated with the CTLs | NA | NCT00671164 | Bollard et al.107 |
| IMiDs | Thalidomide | Prospective, single center | T-NHL (include 21 NKTCL) | 46 | 200 mg (range, 150–400 mg) every night | Combined with chemotherapy | 79.2% | 50.0% | Without reported severe side effects | NA | – | Wu et al.,127 |
PD-1, programmed death 1; PD-L1, programmed cell death-ligand 1; C-C chemokine receptor 4; SMILE, dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide; DHAP, dexamethasone, cytarabine, and cisplatin; HL, Hodgkin lymphomas; NHL, non-Hodgkin lymphomas; NKTCL, natural killer/T cell lymphoma; ENKTL, extranodal NK/T-cell lymphoma; AEs, adverse events; CCR4, C-C chemokine receptor 4; PTCL, peripheral T cell lymphoma; IMiDs, immunomodulatory drugs; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; ORR, objective response rate; CRR, complete response rate; OS, overall survival; NE, not estimable; 95% CI, 95% confidence interval; NA, data not available.
Conclusion
Although the outcomes of early-stage diseases have been remarkably improved due to asparaginase-based regimens, there remains an urgent need for novel and additional therapies in relapsed/refractory NKTCL. Immunotherapy, including monoclonal antibodies and adoptive T cell therapy, has rapidly transformed the treatment landscape, offering unprecedented therapeutic efficacy. Challenges persist, including selecting patients who could respond well to the treatment and identifying molecular markers to predict further prognoses, still need to be solved. Results of the underway preclinical and clinical research may help to better understand the safety and efficacy of immunotherapy. Collectively, with deeper insight into immunotherapy for NKTCL, it is hoped that future studies will further optimize immunotherapies for patients with NKTCL to achieve better therapeutic responses and minimize side effects.
Acknowledgments
The work was supported by the National Key Research and Development Program of China (2021YFE0206600), National Natural Science Foundation of China (82172842 and 81672386), the Sichuan Province Science and Technology Support grant and TianFu Laboratory (2022SYSX0064, 2021YFSY0008, 2020YFS0276), West China Nursing Discipline Development Special Fund Project (HXHL21008), the Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH119), and Translational medicine fund of West China Hospital (2020HXBH119 and CGZH19002). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the article. Figures 1 and 2 were created with BioRender.com.
Author contributions
LH and NC wrote the review, XCP and LD provided critical suggestions, LH refined the final draft of the review.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Lei Dai, Email: dailei2016@scu.edu.cn.
Xingchen Peng, Email: pxx2014@163.com.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Xiong J., Cui B.W., Wang N., Dai Y.T., Zhang H., Wang C.F., Zhong H.J., Cheng S., Ou-Yang B.S., Hu Y., et al. Genomic and Transcriptomic Characterization of Natural Killer T Cell Lymphoma. Cancer Cell. 2020;37:403–419.e6. doi: 10.1016/j.ccell.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Tse E., Kwong Y.L. The diagnosis and management of NK/T-cell lymphomas. J. Hematol. Oncol. 2017;10:85. doi: 10.1186/s13045-017-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th Edition) IARC; 2017. [Google Scholar]
- 5.Cao C., Feng J., Gu H., Tang H., Xu L., Dong H., Dong B., Shu M., Bai Q., Liang R., et al. Distribution of lymphoid neoplasms in Northwest China: Analysis of 3244 cases according to WHO classification in a single institution. Ann. Diagn. Pathol. 2018;34:60–65. doi: 10.1016/j.anndiagpath.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Perry A.M., Diebold J., Nathwani B.N., MacLennan K.A., Müller-Hermelink H.K., Bast M., Boilesen E., Armitage J.O., Weisenburger D.D. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244–1250. doi: 10.3324/haematol.2016.148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haverkos B.M., Pan Z., Gru A.A., Freud A.G., Rabinovitch R., Xu-Welliver M., Otto B., Barrionuevo C., Baiocchi R.A., Rochford R., Porcu P. Extranodal NK/T Cell Lymphoma, Nasal Type (ENKTL-NT): An Update on Epidemiology, Clinical Presentation, and Natural History in North American and European Cases. Curr. Hematol. Malig. Rep. 2016;11:514–527. doi: 10.1007/s11899-016-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvarajan V., Osato M., Nah G.S.S., Yan J., Chung T.H., Voon D.C.C., Ito Y., Ham M.F., Salto-Tellez M., Shimizu N., et al. RUNX3 is oncogenic in natural killer/T-cell lymphoma and is transcriptionally regulated by MYC. Leukemia. 2017;31:2219–2227. doi: 10.1038/leu.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong G., Liu X., Wang L., Yin W., Bouska A., Gong Q., Shetty K., Chen L., Sharma S., Zhang J., et al. Genomic profiling identifies distinct genetic subtypes in extra-nodal natural killer/T-cell lymphoma. Leukemia. 2022;36:2064–2075. doi: 10.1038/s41375-022-01623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez Barca E., Tomás-Roca L., Esteve A., Rodriguez M., Gato L., Alonso-Alonso R., Martin Garcia-Sancho A., Cordoba R., Monter-Rovira A., Bastos-Oreiro M., et al. Extranodal natural killer/T-cell lymphoma nasal type in a western population: Molecular profiling identifies new therapeutic targets. Am. J. Hematol. 2023;98:E134–E138. doi: 10.1002/ajh.26904. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J., Ma J., Union for China Lymphoma Investigators of Chinese Society of Clinical Oncology Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for malignant lymphoma 2021 (English version) Chin. J. Cancer Res. 2021;33:289–301. doi: 10.21147/j.issn.1000-9604.2021.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi S.N., Yang Y., Song Y.Q., Wang Y., He X., Hu C., Zhang L.L., Wu G., Qu B.L., Qian L.T., et al. First-line non-anthracycline-based chemotherapy for extranodal nasal-type NK/T-cell lymphoma: a retrospective analysis from the CLCG. Blood Adv. 2020;4:3141–3153. doi: 10.1182/bloodadvances.2020001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tse E., Zhao W.L., Xiong J., Kwong Y.L. How we treat NK/T-cell lymphomas. J. Hematol. Oncol. 2022;15:74. doi: 10.1186/s13045-022-01293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam Y.S., Im K.I., Kim N., Song Y., Lee J.S., Jeon Y.W., Cho S.G. Down-regulation of intracellular reactive oxygen species attenuates P-glycoprotein-associated chemoresistance in Epstein-Barr virus-positive NK/T-cell lymphoma. Am. J. Transl. Res. 2019;11:1359–1373. [PMC free article] [PubMed] [Google Scholar]
- 15.Drénou B., Lamy T., Amiot L., Fardel O., Caulet-Maugendre S., Sasportes M., Diebold J., Le Prisé P.Y., Fauchet R. CD3- CD56+ non-Hodgkin's lymphomas with an aggressive behavior related to multidrug resistance. Blood. 1997;89:2966–2974. [PubMed] [Google Scholar]
- 16.Xu P.P., Xiong J., Cheng S., Zhao X., Wang C.F., Cai G., Zhong H.J., Huang H.Y., Chen J.Y., Zhao W.L. A Phase II Study of Methotrexate, Etoposide, Dexamethasone and Pegaspargase Sandwiched with Radiotherapy in the Treatment of Newly Diagnosed, Stage IE to IIE Extranodal Natural-Killer/T-Cell Lymphoma, Nasal-Type. EBioMedicine. 2017;25:41–49. doi: 10.1016/j.ebiom.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong Y.L., Kim W.S., Lim S.T., Kim S.J., Tang T., Tse E., Leung A.Y.H., Chim C.S. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Wang Y., Li X., Li L., Wang X., Sun Z., Wu J., Fu X., Zhang X., Yu H., et al. Radiotherapy vs sequential pegaspargase, gemcitabine, cisplatin and dexamethasone and radiotherapy in newly diagnosed early natural killer/T-cell lymphoma: A randomized, controlled, open-label, multicenter study. Int. J. Cancer. 2021;148:1470–1477. doi: 10.1002/ijc.33329. [DOI] [PubMed] [Google Scholar]
- 19.Zhong H., Cheng S., Zhang X., Xu B., Chen J., Jiang X., Xiong J., Hu Y., Cui G., Wei J., et al. Etoposide, dexamethasone, and pegaspargase with sandwiched radiotherapy in early-stage natural killer/T-cell lymphoma: A randomized phase III study. Innovation. 2023;4 doi: 10.1016/j.xinn.2023.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Doesum J.A., Niezink A.G.H., Huls G.A., Beijert M., Diepstra A., van Meerten T. Extranodal Natural Killer/T-cell Lymphoma, Nasal Type: Diagnosis and Treatment. Hemasphere. 2021;5:e523. doi: 10.1097/hs9.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S.J., Kim K., Kim B.S., Kim C.Y., Suh C., Huh J., Lee S.W., Kim J.S., Cho J., Lee G.W., et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J. Clin. Oncol. 2009;27:6027–6032. doi: 10.1200/jco.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Ma S., Cai J., Yang Y., Jing H., Shuang Y., Peng Z., Li B., Liu P., Xia Z., et al. Sequential P-GEMOX and radiotherapy for early-stage extranodal natural killer/T-cell lymphoma: A multicenter study. Am. J. Hematol. 2021;96:1481–1490. doi: 10.1002/ajh.26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox C.P., Civallero M., Ko Y.H., Manni M., Skrypets T., Pileri S., Kim S.J., Cabrera M.E., Shustov A.R., Chiattone C.S., et al. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet. Haematol. 2020;7:e284–e294. doi: 10.1016/s2352-3026(19)30283-2. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., Zhang Y.J., Zhu Y., Cao J.Z., Yuan Z.Y., Xu L.M., Wu J.X., Wang W., Wu T., Lu B., et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. 2015;29:1571–1577. doi: 10.1038/leu.2015.44. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi M., Suzuki R., Oguchi M., Asano N., Amaki J., Akiba T., Maeda T., Itasaka S., Kubota N., Saito Y., et al. Treatments and Outcomes of Patients With Extranodal Natural Killer/T-Cell Lymphoma Diagnosed Between 2000 and 2013: A Cooperative Study in Japan. J. Clin. Oncol. 2017;35:32–39. doi: 10.1200/jco.2016.68.1619. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Wang J.W. Extranodal natural-killer T-cell lymphoma: experience from China. Lancet. Haematol. 2020;7:e441. doi: 10.1016/s2352-3026(20)30103-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Wang Z.H., Chen X.Q., Li Y.J., Wang K.F., Xia Y.F., Xia Z.J. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119:348–355. doi: 10.1002/cncr.27752. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Cheng Y., Zhang M., Yan J., Li L., Fu X., Zhang X., Chang Y., Sun Z., Yu H., et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J. Hematol. Oncol. 2018;11:15. doi: 10.1186/s13045-018-0559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan T.S.Y., Li J., Loong F., Khong P.L., Tse E., Kwong Y.L. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann. Hematol. 2018;97:193–196. doi: 10.1007/s00277-017-3127-2. [DOI] [PubMed] [Google Scholar]
- 30.Tao R., Liu C., Zhang W., Zhu Y., Ma Y., Hao S. Selinexor With Anti-PD-1 Antibody as a Potentially Effective Regimen for Patients With Natural Killer/T-Cell Lymphoma Failing Prior L-Asparaginase and PD-1 Blockade. Oncol. 2023:oyad241. doi: 10.1093/oncolo/oyad241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Z., Yao S., Liu Y., Zhang J., Li P., Wang H., Chu J., Zhao S., Yao Z. Durable Response to Sintilimab and Chidamide in a Patient With Pegaspargase- and Immunotherapy-Resistant NK/T-Cell Lymphoma: Case Report and Literature Review. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.608304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen T., Sun G., Jiang W., He X., Shi Y., Ma F., Liu P. Histone deacetylases inhibitor chidamide synergizes with humanized PD1 antibody to enhance T-cell chemokine expression and augment Ifn-γ response in NK-T cell lymphoma. EBioMedicine. 2023;87 doi: 10.1016/j.ebiom.2022.104420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagato T., Ohkuri T., Ohara K., Hirata Y., Kishibe K., Komabayashi Y., Ueda S., Takahara M., Kumai T., Ishibashi K., et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol. Immunother. 2017;66:877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H., Tao R., Hao S., Yang Y., Cen H., Zhou H., Guo Y., Zou L., Cao J., Huang Y., et al. Sugemalimab Monotherapy for Patients With Relapsed or Refractory Extranodal Natural Killer/T-Cell Lymphoma (GEMSTONE-201): Results From a Single-Arm, Multicenter, Phase II Study. J. Clin. Oncol. 2023;41:3032–3041. doi: 10.1200/jco.22.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim W.Y., Jung H.Y., Nam S.J., Kim T.M., Heo D.S., Kim C.W., Jeon Y.K. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. 2016;469:581–590. doi: 10.1007/s00428-016-2011-0. [DOI] [PubMed] [Google Scholar]
- 36.Jo J.C., Kim M., Choi Y., Kim H.J., Kim J.E., Chae S.W., Kim H., Cha H.J. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2017;96:25–31. doi: 10.1007/s00277-016-2818-4. [DOI] [PubMed] [Google Scholar]
- 37.Panjwani P.K., Charu V., DeLisser M., Molina-Kirsch H., Natkunam Y., Zhao S. Programmed death-1 ligands PD-L1 and PD-L2 show distinctive and restricted patterns of expression in lymphoma subtypes. Hum. Pathol. 2018;71:91–99. doi: 10.1016/j.humpath.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Han L., Liu F., Li R., Li Z., Chen X., Zhou Z., Zhang X., Hu T., Zhang Y., Young K., et al. Role of programmed death ligands in effective T-cell interactions in extranodal natural killer/T-cell lymphoma. Oncol. Lett. 2014;8:1461–1469. doi: 10.3892/ol.2014.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosseini A., Gharibi T., Marofi F., Babaloo Z., Baradaran B. CTLA-4: From mechanism to autoimmune therapy. Int. Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2020.106221. [DOI] [PubMed] [Google Scholar]
- 40.Wang L., Liao D.Z., Zhang J., Xia Z.J., Peng X.W., Lu Y. Clinical significance of serum soluble interleukin-2 receptor-α in extranodal natural killer/T-cell lymphoma (ENKTL): a predictive biomarker for treatment efficacy and valuable prognostic factor. Med. Oncol. 2013;30:723. doi: 10.1007/s12032-013-0723-4. [DOI] [PubMed] [Google Scholar]
- 41.Kim M., Lee J.O., Koh J., Kim T.M., Lee J.Y., Jeon Y.K., Keam B., Kim D.W., Lee J.S., Heo D.S. A phase II study of brentuximab vedotin in patients with relapsed or refractory Epstein-Barr virus-positive and CD30-positive lymphomas. Haematologica. 2021;106:2277–2280. doi: 10.3324/haematol.2021.278301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang P., Shi C., Song Y., Li Z., Zhang M., Jin M. Brentuxinmab vedotin, alone or combine with bendamustine in the treatment of natural killer T cell lymphoma. Hematol. Oncol. 2022;40:941–952. doi: 10.1002/hon.3042. [DOI] [PubMed] [Google Scholar]
- 43.Lund F.E. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol. Med. 2006;12:328–333. doi: 10.2119/2006–00099.Lund. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Wang H., Li P.F., Lu Y., Xia Z.J., Huang H.Q., Zhang Y.J. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann. Hematol. 2015;94:1381–1388. doi: 10.1007/s00277-015-2359-2. [DOI] [PubMed] [Google Scholar]
- 45.Huang H., Zhu J., Yao M., Kim T.M., Yoon D.H., Cho S.G., Eom H.S., Lim S.T., Yeh S.P., Song Y., et al. Daratumumab monotherapy for patients with relapsed or refractory natural killer/T-cell lymphoma, nasal type: an open-label, single-arm, multicenter, phase 2 study. J. Hematol. Oncol. 2021;14:25. doi: 10.1186/s13045-020-01020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hari P., Raj R.V., Olteanu H. Targeting CD38 in Refractory Extranodal Natural Killer Cell-T-Cell Lymphoma. N. Engl. J. Med. 2016;375:1501–1502. doi: 10.1056/NEJMc1605684. [DOI] [PubMed] [Google Scholar]
- 47.Huang H.-q., Kim W.-S., Yao M., Kim T.M., Yoon D., Cho S.-G., Eom H.-S., Yeh S.-P., Lim S.T., Song Y., et al. Daratumumab Monotherapy for Patients with Relapsed or Refractory (R/R) Natural Killer/T-Cell Lymphoma (NKTCL), Nasal Type: Updated Results from an Open-Label, Single-Arm, Multicenter Phase 2 Study. Blood. 2019;134:1568. doi: 10.1182/blood-2019-123446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S.J., Kim K., Park Y., Kim B.S., Huh J., Ko Y.H., Park K., Suh C., Kim W.S. Dose modification of alemtuzumab in combination with dexamethasone, cytarabine, and cisplatin in patients with relapsed or refractory peripheral T-cell lymphoma: analysis of efficacy and toxicity. Invest. New Drugs. 2012;30:368–375. doi: 10.1007/s10637-010-9523-2. [DOI] [PubMed] [Google Scholar]
- 49.Zhang M., Wen B., Anton O.M., Yao Z., Dubois S., Ju W., Sato N., DiLillo D.J., Bamford R.N., Ravetch J.V., Waldmann T.A. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc. Natl. Acad. Sci. USA. 2018;115:E10915–E10924. doi: 10.1073/pnas.1811615115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishitsuka K., Jimi S., Goldmacher V.S., Ab O., Tamura K. Targeting CD56 by the maytansinoid immunoconjugate IMGN901 (huN901-DM1): a potential therapeutic modality implication against natural killer/T cell malignancy. Br. J. Haematol. 2008;141:129–131. doi: 10.1111/j.1365-2141.2008.07000.x. [DOI] [PubMed] [Google Scholar]
- 51.Kumai T., Nagato T., Kobayashi H., Komabayashi Y., Ueda S., Kishibe K., Ohkuri T., Takahara M., Celis E., Harabuchi Y. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer immunology, immunotherapy. CII. 2015;64:697–705. doi: 10.1007/s00262-015-1675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanazawa T., Hiramatsu Y., Iwata S., Siddiquey M., Sato Y., Suzuki M., Ito Y., Goshima F., Murata T., Kimura H. Anti-CCR4 monoclonal antibody mogamulizumab for the treatment of EBV-associated T- and NK-cell lymphoproliferative diseases. Clin. Cancer Res. 2014;20:5075–5084. doi: 10.1158/1078-0432.Ccr-14-0580. [DOI] [PubMed] [Google Scholar]
- 53.Li H., Song W., Wu J., Shi Z., Gao Y., Li J., Han L., Zhang J., Li Z., Li Y., Zhang M. CAR-T cells targeting CD38 and LMP1 exhibit robust antitumour activity against NK/T cell lymphoma. BMC Med. 2023;21:330. doi: 10.1186/s12916-023-03040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchbinder E.I., Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016;39:98–106. doi: 10.1097/coc.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng S.B., Chung T.H., Kato S., Nakamura S., Takahashi E., Ko Y.H., Khoury J.D., Yin C.C., Soong R., Jeyasekharan A.D., et al. Epstein-Barr virus-associated primary nodal T/NK-cell lymphoma shows a distinct molecular signature and copy number changes. Haematologica. 2018;103:278–287. doi: 10.3324/haematol.2017.180430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muhamad H., Suksawai N., Assanasen T., Polprasert C., Bunworasate U., Wudhikarn K. Programmed Cell Death 1 and Programmed Cell Death Ligands in Extranodal Natural Killer/T Cell Lymphoma: Expression Pattern and Potential Prognostic Relevance. Acta Haematol. 2020;143:78–88. doi: 10.1159/000500974. [DOI] [PubMed] [Google Scholar]
- 57.Wang H., Wang L., Liu W.J., Xia Z.J., Huang H.Q., Jiang W.Q., Li Z.M., Lu Y. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget. 2016;7:33035–33045. doi: 10.18632/oncotarget.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao R., Fan L., Song Y., Hu Y., Zhang W., Wang Y., Xu W., Li J. Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: a multicenter, single-arm, phase 2 trial (ORIENT-4) Signal Transduct. Target. Ther. 2021;6:365. doi: 10.1038/s41392-021-00768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwong Y.L., Chan T.S.Y., Tan D., Kim S.J., Poon L.M., Mow B., Khong P.L., Loong F., Au-Yeung R., Iqbal J., et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 60.Wang J., Fei K., Jing H., Wu Z., Wu W., Zhou S., Ni H., Chen B., Xiong Y., Liu Y., et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs. 2019;11:1443–1451. doi: 10.1080/19420862.2019.1654303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim S.J., Lim J.Q., Laurensia Y., Cho J., Yoon S.E., Lee J.Y., Ryu K.J., Ko Y.H., Koh Y., Cho D., et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020;136:2754–2763. doi: 10.1182/blood.2020007247. [DOI] [PubMed] [Google Scholar]
- 62.Huang H.-q., Tao R., Zou L., Cen H., Guo Y., Huang Y., Qian W., Zhang L., Zhou H., Yang Y., et al. Preliminary Results from a Multicenter, Single-Arm, Phase 2 Study of CS1001, an Anti-Programmed Death-Ligand 1 (PD-L1) Human Monoclonal Antibody (mAb), in Patients (pts) with Relapsed or Refractory Extranodal Natural Killer/T Cell Lymphoma (rr-ENKTL) Blood. 2019;134:2833. doi: 10.1182/blood-2019-121865. [DOI] [Google Scholar]
- 63.Gao Y., Huang H., Wang X., Bai B., Zhang L., Xiao Y., Liu X., Li W., Xu W., Feng R., et al. Anti-PD-1 Antibody (Sintilimab) Plus Histone Deacetylase Inhibitor (Chidamide) for the Treatment of Refractory or Relapsed Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (r/r-ENKTL): Preliminary Results from a Prospective, Multicenter, Single-Arm, Phase Ib/II Trial (SCENT) Blood. 2020;136:39–40. doi: 10.1182/blood-2020-134665. [DOI] [Google Scholar]
- 64.Sun P., Wang Y., Yang H., Chen C., Nie M., Sun X.-Q., He X.-H., Huang K.-M., Huang J.-J., Li Z.-M. Combination of Anti-PD-1 Antibody, Anlotinib and Pegaspargase "Sandwich" With Radiotherapy in Localized Natural Killer/T Cell Lymphoma. Front. Immunol. 2022;13:766200. doi: 10.3389/fimmu.2022.766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai J., Liu P., Huang H., Li Y., Ma S., Zhou H., Tian X., Zhang Y., Gao Y., Xia Y., et al. Combination of anti-PD-1 antibody with P-GEMOX as a potentially effective immunochemotherapy for advanced natural killer/T cell lymphoma. Signal Transduct. Target. Ther. 2020;5:289. doi: 10.1038/s41392-020-00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun P., Li Y., Li C., Ren K., Wang Y., Yang H., Jiang W., Zou L., Yang H., Zhou H., Li Z.M. A phase II study of sintilimab, anlotinib, and pegaspargase sandwiched with radiotherapy as first-line therapy in patients with newly diagnosed, stage I-II extranodal natural-killer/T-cell lymphoma. Am. J. Hematol. 2023;98:1043–1051. doi: 10.1002/ajh.26922. [DOI] [PubMed] [Google Scholar]
- 67.Anderson R., Theron A.J., Rapoport B.L. Immunopathogenesis of Immune Checkpoint Inhibitor-Related Adverse Events: Roles of the Intestinal Microbiome and Th17 Cells. Front. Immunol. 2019;10:2254. doi: 10.3389/fimmu.2019.02254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stamper C.C., Zhang Y., Tobin J.F., Erbe D.V., Ikemizu S., Davis S.J., Stahl M.L., Seehra J., Somers W.S., Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 69.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perrier A., Didelot A., Laurent-Puig P., Blons H., Garinet S. Epigenetic Mechanisms of Resistance to Immune Checkpoint Inhibitors. Biomolecules. 2020;10 doi: 10.3390/biom10071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flynn M.J., Hartley J.A. The emerging role of anti-CD25 directed therapies as both immune modulators and targeted agents in cancer. Br. J. Haematol. 2017;179:20–35. doi: 10.1111/bjh.14770. [DOI] [PubMed] [Google Scholar]
- 73.Malek T.R., Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L., Bi X.W., Zhu Y.J., He Y.Z., Lai Q.Y., Xia Z.J., Cai Q.Q. IL-2Rα up-regulation is mediated by latent membrane protein 1 and promotes lymphomagenesis and chemotherapy resistance in natural killer/T-cell lymphoma. Cancer Commun. 2018;38:62. doi: 10.1186/s40880-018-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Mel S., Li J.B., Abid M.B., Tang T., Tay H.M., Ting W.C., Poon L.M., Chung T.H., Mow B., Tso A., et al. The utility of flow cytometry in differentiating NK/T cell lymphoma from indolent and reactive NK cell proliferations. Cytometry B Clin. Cytom. 2018;94:159–168. doi: 10.1002/cyto.b.21529. [DOI] [PubMed] [Google Scholar]
- 76.Tartour E., Mosseri V., Jouffroy T., Deneux L., Jaulerry C., Brunin F., Fridman W.H., Rodriguez J. Serum soluble interleukin-2 receptor concentrations as an independent prognostic marker in head and neck cancer. Lancet (London, England) 2001;357:1263–1264. doi: 10.1016/s0140-6736(00)04420-2. [DOI] [PubMed] [Google Scholar]
- 77.Goto N., Tsurumi H., Goto H., Shimomura Y.I., Kasahara S., Hara T., Yasuda I., Shimizu M., Murakami N., Yoshikawa T., et al. Serum soluble interleukin-2 receptor (sIL-2R) level is associated with the outcome of patients with diffuse large B cell lymphoma treated with R-CHOP regimens. Ann. Hematol. 2012;91:705–714. doi: 10.1007/s00277-011-1363-4. [DOI] [PubMed] [Google Scholar]
- 78.Gupta M., Stenson M., O'Byrne M., Maurer M.J., Habermann T., Cerhan J.R., Weiner G.W., Witzig T.E. Comprehensive serum cytokine analysis identifies IL-1RA and soluble IL-2Rα as predictors of event-free survival in T-cell lymphoma. Ann. Oncol. 2016;27:165–172. doi: 10.1093/annonc/mdv486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olin R.L., Nichols K.E., Naghashpour M., Wasik M., Shelly B., Stadtmauer E.A., Vogl D.T. Successful use of the anti-CD25 antibody daclizumab in an adult patient with hemophagocytic lymphohistiocytosis. Am. J. Hematol. 2008;83:747–749. doi: 10.1002/ajh.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dancey G., Violet J., Malaroda A., Green A.J., Sharma S.K., Francis R., Othman S., Parker S., Buscombe J., Griffin N., et al. A Phase I Clinical Trial of CHT-25 a 131I-Labeled Chimeric Anti-CD25 Antibody Showing Efficacy in Patients with Refractory Lymphoma. Clin. Cancer Res. 2009;15:7701–7710. doi: 10.1158/1078-0432.Ccr-09-1421. [DOI] [PubMed] [Google Scholar]
- 81.Yang J., Li P., Piao Y., Liu X., Wei L., Sang W., Zhang L., Wang L. CD56-Negative Extranodal Natural Killer/T-Cell Lymphoma: A Retrospective Study in 443 Patients Treated by Chemotherapy With or Without Asparaginase. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.829366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pongpruttipan T., Sukpanichnant S., Assanasen T., Wannakrairot P., Boonsakan P., Kanoksil W., Kayasut K., Mitarnun W., Khuhapinant A., Bunworasate U., et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am. J. Surg. Pathol. 2012;36:481–499. doi: 10.1097/PAS.0b013e31824433d8. [DOI] [PubMed] [Google Scholar]
- 83.Gualco G., Domeny-Duarte P., Chioato L., Barber G., Natkunam Y., Bacchi C.E. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am. J. Surg. Pathol. 2011;35:1195–1203. doi: 10.1097/PAS.0b013e31821ec4b5. [DOI] [PubMed] [Google Scholar]
- 84.Li S., Feng X., Li T., Zhang S., Zuo Z., Lin P., Konoplev S., Bueso-Ramos C.E., Vega F., Medeiros L.J., Yin C.C. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am. J. Surg. Pathol. 2013;37:14–23. doi: 10.1097/PAS.0b013e31826731b5. [DOI] [PubMed] [Google Scholar]
- 85.Chanan-Khan A.A., Jagannath S., Munshi N.C., Schlossman R.L., Anderson K.C., Lee K., DePaolo D., Miller K.C., Zildjian S., Fram R.J., Qin Phase I Study of huN901-DM1 (BB-10901) in Patients with Relapsed and Relapsed/Refractory CD56-Positive Multiple Myeloma. Blood. 2007;110:1174. doi: 10.1182/blood.V110.11.1174.1174. [DOI] [Google Scholar]
- 86.Gruss H.J., Boiani N., Williams D.E., Armitage R.J., Smith C.A., Goodwin R.G. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood. 1994;83:2045–2056. [PubMed] [Google Scholar]
- 87.Sabattini E., Pizzi M., Tabanelli V., Baldin P., Sacchetti C.S., Agostinelli C., Zinzani P.L., Pileri S.A. CD30 expression in peripheral T-cell lymphomas. Haematologica. 2013;98:e81–e82. doi: 10.3324/haematol.2013.084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S.J., Yoon D.H., Kim J.S., Kang H.J., Lee H.W., Eom H.S., Hong J.Y., Cho J., Ko Y.H., Huh J., et al. Efficacy of Brentuximab Vedotin in Relapsed or Refractory High-CD30-Expressing Non-Hodgkin Lymphomas: Results of a Multicenter, Open-Labeled Phase II Trial. Cancer Res. Treat. 2020;52:374–387. doi: 10.4143/crt.2019.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim H.K., Moon S.M., Moon J.H., Park J.E., Byeon S., Kim W.S. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 2015;50:254–256. doi: 10.5045/br.2015.50.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poon L.M., Kwong Y.L. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann. Hematol. 2016;95:847–849. doi: 10.1007/s00277-016-2627-9. [DOI] [PubMed] [Google Scholar]
- 91.Li W., Liang L., Liao Q., Li Y., Zhou Y. CD38: An important regulator of T cell function. Biomed. Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113395. [DOI] [PubMed] [Google Scholar]
- 92.Ng S.B., Selvarajan V., Huang G., Zhou J., Feldman A.L., Law M., Kwong Y.L., Shimizu N., Kagami Y., Aozasa K., et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J. Pathol. 2011;223:496–510. doi: 10.1002/path.2823. [DOI] [PubMed] [Google Scholar]
- 93.Calabretta E., Carlo-Stella C. The Many Facets of CD38 in Lymphoma: From Tumor-Microenvironment Cell Interactions to Acquired Resistance to Immunotherapy. Cells. 2020;9:802. doi: 10.3390/cells9040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Overdijk M.B., Verploegen S., Bögels M., van Egmond M., Lammerts van Bueren J.J., Mutis T., Groen R.W.J., Breij E., Martens A.C.M., Bleeker W.K., Parren P.W.H.I. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7:311–321. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mustafa N., Nee A.H.F., Chooi J.Y., Toh S.H.M., Hee Y.T., Selvarajan V., Zhou L., Yang J., Chng W.J. CD55 and CD59 Can Limit the Anti-Tumor Efficacy of Daratumumab in Natural Killer/T-Cell Lymphoma. Blood. 2018;132:1663. doi: 10.1182/blood-2018-99-115566. [DOI] [Google Scholar]
- 96.Zhao Y., Su H., Shen X., Du J., Zhang X., Zhao Y. The immunological function of CD52 and its targeting in organ transplantation. Inflamm. Res. 2017;66:571–578. doi: 10.1007/s00011-017-1032-8. [DOI] [PubMed] [Google Scholar]
- 97.Waldmann H., Hale G. CAMPATH: from concept to clinic. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1707–1711. doi: 10.1098/rstb.2005.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang S.T., Lu C.L., Chuang S.S. CD52 expression in non-mycotic T- and NK/T-cell lymphomas. Leuk. Lymphoma. 2007;48:117–121. doi: 10.1080/10428190601016167. [DOI] [PubMed] [Google Scholar]
- 99.Kim J.G., Sohn S.K., Chae Y.S., Cho Y.Y., Yang D.H., Lee J.J., Kim H.J., Shin H.J., Chung J.S., Cho G.J., et al. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother. Pharmacol. 2007;60:129–134. doi: 10.1007/s00280-007-0469-9. [DOI] [PubMed] [Google Scholar]
- 100.Sugiyama D., Nishikawa H., Maeda Y., Nishioka M., Tanemura A., Katayama I., Ezoe S., Kanakura Y., Sato E., Fukumori Y., et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshie O., Matsushima K. CCR4 and its ligands: from bench to bedside. Int. Immunol. 2015;27:11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]
- 102.Berahovich R.D., Lai N.L., Wei Z., Lanier L.L., Schall T.J. Evidence for NK cell subsets based on chemokine receptor expression. J. Immunol. 2006;177:7833–7840. doi: 10.4049/jimmunol.177.11.7833. [DOI] [PubMed] [Google Scholar]
- 103.Kitadate A., Ikeda S., Abe F., Takahashi N., Shimizu N., Matsue K., Tagawa H. Histone deacetylase inhibitors downregulate CCR4 expression and decrease mogamulizumab efficacy in CCR4-positive mature T-cell lymphomas. Haematologica. 2018;103:126–135. doi: 10.3324/haematol.2017.177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilcox R.A. Mogamulizumab: 2 birds, 1 stone. Blood. 2015;125:1847–1848. doi: 10.1182/blood-2015-02-625251. [DOI] [PubMed] [Google Scholar]
- 105.Ishida T., Utsunomiya A., Jo T., Yamamoto K., Kato K., Yoshida S., Takemoto S., Suzushima H., Kobayashi Y., Imaizumi Y., et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. 2017;108:2022–2029. doi: 10.1111/cas.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mackensen A., Meidenbauer N., Vogl S., Laumer M., Berger J., Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J. Clin. Oncol. 2006;24:5060–5069. doi: 10.1200/jco.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 107.Bollard C.M., Gottschalk S., Torrano V., Diouf O., Ku S., Hazrat Y., Carrum G., Ramos C., Fayad L., Shpall E.J., et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 2014;32:798–808. doi: 10.1200/jco.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kwong Y.L., Anderson B.O., Advani R., Kim W.S., Levine A.M., Lim S.T., Asian Oncology Summit Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1093–1101. doi: 10.1016/s1470-2045(09)70265-7. [DOI] [PubMed] [Google Scholar]
- 109.Asano N., Kato S., Nakamura S. Epstein-Barr virus-associated natural killer/T-cell lymphomas. Best practice & research. Clinical haematology. 2013;26:15–21. doi: 10.1016/j.beha.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Chiang A.K., Tao Q., Srivastava G., Ho F.C. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease. Int. J. Cancer. 1996;68:285–290. doi: 10.1002/(sici)1097-0215(19961104)68:3<285::Aid-ijc3>3.0.Co;2-y. [DOI] [PubMed] [Google Scholar]
- 111.Tse E., Kwong Y.L. Epstein Barr virus-associated lymphoproliferative diseases: the virus as a therapeutic target. Exp. Mol. Med. 2015;47:e136. doi: 10.1038/emm.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho S.G., Kim N., Sohn H.J., Lee S.K., Oh S.T., Lee H.J., Cho H.I., Yim H.W., Jung S.E., Park G., et al. Long-term Outcome of Extranodal NK/T Cell Lymphoma Patients Treated With Postremission Therapy Using EBV LMP1 and LMP2a-specific CTLs. Mol. Ther. 2015;23:1401–1409. doi: 10.1038/mt.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim W.S., Oki Y., Kim S.J., Yoon S.E., Ardeshna K.M., Lin Y., Ruan J., Porcu P., Brammer J.E., Jacobsen E.D., et al. Autologous EBV-specific T cell treatment results in sustained responses in patients with advanced extranodal NK/T lymphoma: results of a multicenter study. Ann. Hematol. 2021;100:2529–2539. doi: 10.1007/s00277-021-04558-0. [DOI] [PubMed] [Google Scholar]
- 114.Prockop S., Doubrovina E., Suser S., Heller G., Barker J., Dahi P., Perales M.A., Papadopoulos E., Sauter C., Castro-Malaspina H., et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J. Clin. Invest. 2020;130:733–747. doi: 10.1172/jci121127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vickers M.A., Wilkie G.M., Robinson N., Rivera N., Haque T., Crawford D.H., Barry J., Fraser N., Turner D.M., Robertson V., et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br. J. Haematol. 2014;167:402–410. doi: 10.1111/bjh.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanley P.J., Lam S., Shpall E.J., Bollard C.M. Expanding cytotoxic T lymphocytes from umbilical cord blood that target cytomegalovirus, Epstein-Barr virus, and adenovirus. J. Vis. Exp. 2012:e3627. doi: 10.3791/3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F., et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang X., Zhou Y., Li W., Tang Q., Chen R., Zhu J., Feng Z. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J. Biomed. Res. 2014;28:468–475. doi: 10.7555/jbr.28.20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X., Yu X., Li W., Neeli P., Liu M., Li L., Zhang M., Fang X., Young K.H., Li Y. Expanding anti-CD38 immunotherapy for lymphoid malignancies. J. Exp. Clin. Cancer Res. 2022;41:210. doi: 10.1186/s13046-022-02421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng M., Yu L., Hu J., Zhang Z., Wang H., Lu D., Tang X., Huang J., Zhong K., Wang Z., et al. Efficacy of B7-H3-Redirected BiTE and CAR-T Immunotherapies Against Extranodal Nasal Natural Killer/T Cell Lymphoma. Transl. Oncol. 2020;13 doi: 10.1016/j.tranon.2020.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim S.J., Hyeon J., Cho I., Ko Y.H., Kim W.S. Comparison of Efficacy of Pembrolizumab between Epstein-Barr Virus‒Positive and ‒Negative Relapsed or Refractory Non-Hodgkin Lymphomas. Cancer Res. Treat. 2019;51:611–622. doi: 10.4143/crt.2018.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ogura M., Ishida T., Hatake K., Taniwaki M., Ando K., Tobinai K., Fujimoto K., Yamamoto K., Miyamoto T., Uike N., et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. 2014;32:1157–1163. doi: 10.1200/jco.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 123.Zinzani P.L., Karlin L., Radford J., Caballero D., Fields P., Chamuleau M.E.D., d'Amore F., Haioun C., Thieblemont C., González-Barca E., et al. European phase II study of mogamulizumab, an anti-CCR4 monoclonal antibody, in relapsed/refractory peripheral T-cell lymphoma. Haematologica. 2016;101:e407–e410. doi: 10.3324/haematol.2016.146977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bartlett N.L., Younes A., Carabasi M.H., Forero A., Rosenblatt J.D., Leonard J.P., Bernstein S.H., Bociek R.G., Lorenz J.M., Hart B.W., Barton J. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111:1848–1854. doi: 10.1182/blood-2007-07-099317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Younes A., Bartlett N.L., Leonard J.P., Kennedy D.A., Lynch C.M., Sievers E.L., Forero-Torres A. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N. Engl. J. Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 126.Horwitz S.M., Advani R.H., Bartlett N.L., Jacobsen E.D., Sharman J.P., O'Connor O.A., Siddiqi T., Kennedy D.A., Oki Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095–3100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu H., Zhao C., Gu K., Jiao Y., Hao J., Sun G. Thalidomide plus chemotherapy exhibit enhanced efficacy in the clinical treatment of T-cell non-Hodgkin's lymphoma: A prospective study of 46 cases. Mol. Clin. Oncol. 2014;2:695–700. doi: 10.3892/mco.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L., Wang Z.H., Chen X.Q., Wang K.F., Huang H.Q., Xia Z.J. First-line combination of GELOX followed by radiation therapy for patients with stage IE/IIE ENKTL: An updated analysis with long-term follow-up. Oncol. Lett. 2015;10:1036–1040. doi: 10.3892/ol.2015.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Du L., Zhang L., Li L., Li X., Yan J., Wang X., Fu X., Sun Z., Zhang X., Li Z., et al. Effective Treatment with PD-1 Antibody, Chidamide, Etoposide, and Thalidomide (PCET) for Relapsed/Refractory Natural Killer/T-Cell Lymphoma: A Report of Three Cases. OncoTargets Ther. 2020;13:7189–7197. doi: 10.2147/ott.S262039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wen H., Ma H., Cai Q., Lin S., Lei X., He B., Wu S., Wang Z., Gao Y., Liu W., et al. Recurrent ECSIT mutation encoding V140A triggers hyperinflammation and promotes hemophagocytic syndrome in extranodal NK/T cell lymphoma. Nat. Med. 2018;24:154–164. doi: 10.1038/nm.4456. [DOI] [PubMed] [Google Scholar]