Abstract
The effect of vertebral osteoporosis on disc degeneration is still debated. The purpose of this study was to provide a systematic review of studies in this area to further reveal the relationship between the two. Relevant studies were searched in electronic databases, and studies were screened according to inclusion and exclusion criteria, and finally, basic information of the included studies was extracted and summarized. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A total of 34 publications spanning 24 years were included in our study. There were 19 clinical studies, including 12 prospective studies and 7 retrospective studies. Of these, 7 considered vertebral osteoporosis to be positively correlated with disc degeneration, 8 considered them to be negatively correlated, and 4 considered them to be uncorrelated. Two cadaveric studies were included, one considered the two to be negatively correlated and one considered them not to be correlated. Seven animal studies were included, of which five considered a positive correlation between vertebral osteoporosis and disc degeneration and two considered a negative correlation between the two. There were also 6 studies that used anti-osteoporosis drugs for intervention, all of them were animal studies. Five of them concluded that vertebral osteoporosis was positively associated with disc degeneration, and the remaining one concluded that there was no correlation between the two. Our systematic review shows that the majority of studies currently consider an association between vertebral osteoporosis and disc degeneration, but there is still a huge disagreement whether this association is positive or negative. Differences in observation time and follow-up time may be one of the reasons for the disagreement. A large number of clinical and basic studies are still needed in the future to further explore the relationship between the two.
Keywords: Disc degeneration, Endplate, Osteoporosis, Systematic review
Introduction
Intervertebral disc degeneration is a pathological change that occurs when the lumbar disc is damaged or ages with age. It can be induced by a variety of factors, such as mechanical loading, genetics, obesity, smoking, and aging. Although the mechanism remains unclear, degeneration and death of disc cells due to reduced nutrient supply, imbalance in microenvironmental homeostasis, infiltration of inflammatory factors, and altered mechanical loading are its main causes [1]. It is associated with a variety of common lumbar spine diseases, such as low back pain, lumbar disc herniation, lumbar spinal stenosis. It can be said that disc degeneration is the initiating factor of these degenerative lumbar spine diseases. The intervertebral disc is a special structure that begins to age around early adolescence, and as age increases, the incidence of disc degeneration increases. It is estimated that about 10% of the population at the age of 50 has disc degeneration, and this number will increase to about 60% in the population at the age of 70 [2].
Osteoporosis is a systemic bone disease characterized by reduced bone mass and damage to bone microarchitecture, leading to increased bone fragility and susceptibility to fracture [3]. Like disc degeneration, osteoporosis is an age-related disease and, in fact, most elderly patients with disc degeneration suffer from concomitant osteoporosis. Since disc degeneration is associated with reduced nutrient supply, and blood supply from the vertebral body is the main source of disc nutrition, changes in vertebral body and endplate structure after osteoporosis may have an impact on the nutrient supply and mechanical loading of the disc. However, there is still no consensus on the exact mechanism of this effect. Some argue that denser vertebral bone accelerates disc degeneration, whereas lower vertebral bone density delays disc degeneration [4–6]. Another view is the exact opposite, that is, that a less dense vertebral body accelerates disc degeneration, while a healthy vertebral bone structure protects the disc [7–9]. Still others believe that the bone density of the vertebral body does not correlate with disc degeneration [10, 11].
A recent study based on quantitative Dixon and GRAPPATINI T2 mapping techniques [4] found that disc degeneration caused by mechanical stress or aging often first manifests as loss of annulus fibrosus integrity, whereas the effect of osteoporosis on the lumbar disc is concentrated in the nucleus pulposus rather than the annulus fibrosus, suggesting that osteoporosis may directly trigger nucleus pulposus degeneration through the longitudinal structures (vertebral body-osseous endplates-bone marrow contact channels—cartilage endplates—nucleus pulposus), a pathological process completely different from disc degeneration caused by mechanical stress or aging.
Therefore, we believe that starting from the alteration of vertebral bone structure may provide a deeper understanding of the mechanisms of disc degeneration and may even lead to a potential means of intervention in the future. Over the last 20 years, there have been numerous clinical and animal studies to investigate the correlation between vertebral osteoporosis and disc degeneration. Unfortunately, no one has yet performed a systematic review of these studies. We believe that a systematic summary and evaluation of these studies is necessary. On the one hand, it helps one to understand the current research status in the field, and on the other hand, it helps to identify some shortcomings in the current research, which can provide guidance for future research design. Moreover, we may be able to find some common patterns from the existing research results, which can help to provide new ideas and entry points for future research.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12].
Literature search strategy and selection criteria
We conducted a literature search using the following search terms: osteoporosis, bone density, intervertebral disc degeneration, and their combined forms in several electronic databases, PubMed(1966 to January 1, 2023), Cochrane Library (1966 to January 1, 2023), and Embase (1980 to January 1, 2023). Target articles were identified step by step based on title, abstract, and full text.
Ultimately, studies that met the following criteria were included: (1) studies exploring the relationship between vertebral osteoporosis or reduced bone density and intervertebral discs or endplates, including clinical studies and animal studies, with no restriction on the level of evidence, country and language; (2) clinical studies could be prospective or retrospective. There was no restriction on the duration of observation and the imaging means used; (3) there was also no restriction on the type of animals, strain, modelling method, intervention method, or observation time. Reviews, commentaries, letters to the editor, unpublished articles, and retracted articles were excluded. For studies that met the inclusion criteria, we also traced their references to identify potential studies. Also, some high-quality reviews were important references and basis for this study. The literature search and screening was conducted independently by two researchers. Any disagreements that arose during that process were resolved through discussion.
Data extraction
According to the purpose of our study, we extracted the following information from the included studies: first author, year of publication, type of study, imaging method and indicator, type of animal, modeling method, intervention method, observation time, results, and conclusions. This work was done independently by two researchers.
Quality assessment of the literature
We evaluated the quality of the included retrospective and prospective clinical studies using the Newcastle–Ottawa Scale (NOS) and the quality of the included animal studies using the initial Stroke Therapy Academic Industry Roundtable list (STAIR).
Results
Literature search results
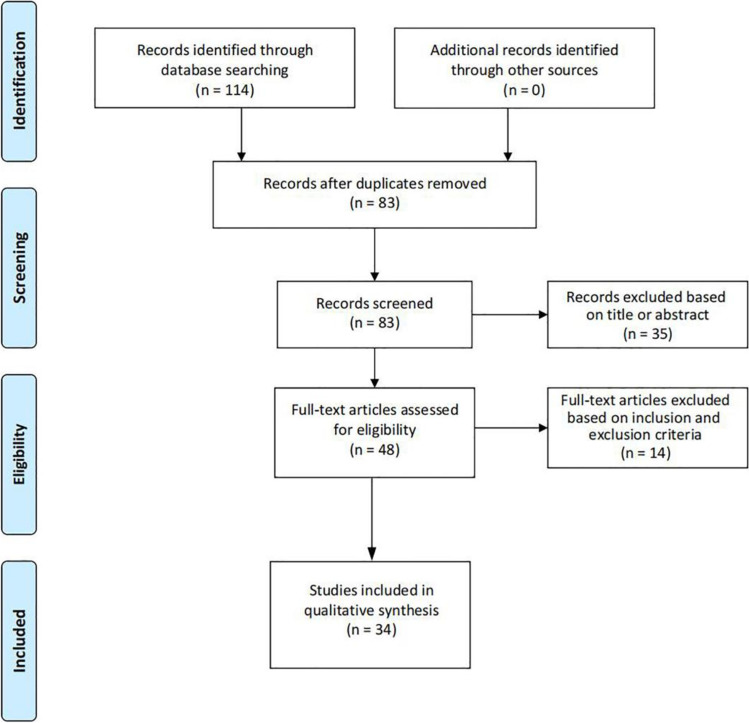
According to the search strategy, we obtained a total of 114 relevant studies, and 83 after removing duplicates. After screening by title and abstract, a total of 35 papers were excluded. The remaining 48 studies were evaluated in full text, 14 of which were excluded because they did not meet the inclusion criteria, and 34 studies were finally included. The literature screening process is shown in Fig. 1. Basic information on the included clinical studies is shown in Table 1, basic information on the included animal studies is shown in Table 2, and basic information on the included drug therapy-related studies is shown in Table 3. Of the 34 studies, 17 considered positive correlation between vertebral osteoporosis and disc degeneration, 11 considered negative correlation, and 6 considered no correlation.
Fig. 1.
Literature screening process
Table 1.
Basic information about the included clinical and cadaveric studies
| Author | Year | Study type |
Basic information of the sample | Imaging tool and indicator | Evaluation time | Result | Conclusion | Relevance |
|---|---|---|---|---|---|---|---|---|
| Liang et al. [7] | 2022 | Retrospective |
324 patients Male: 162 Female: 162 Average age: 53.3 ± 12.7 |
CT: HU value MRI: Pfirrmann grading system |
When joining the study | As disc degeneration increases, vertebral HU values tend to decrease | Decreased vertebral bone density and osteoporosis may trigger or exacerbate disc degeneration | Positive correlation |
| Zhao et al. [8] | 2021 | Retrospective |
15 OVX patients Average age: 62.40 ± 10.64 60 non-OVX patients Average age:62.85 ± 6.46 |
MRI: modified Pfirrmann grading system | When joining the study | Pfirmann grade of discs in OVX group were higher than that in non-OVX group | OVX accelerates disc degeneration | Positive correlation |
| Zhuang et al. [9] | 2021 | Retrospective |
156 patients Male: 79 Female: 77 Average age: 63.2 ± 12.2 |
CT: HU value MRI: TEPS |
When joining the study | TEPS in low bone density group was significantly higher than that in control group | Osteoporosis is positively correlated with endplate injury | Positive correlation |
| Fabreguet et al. [13] | 2015 | Prospective | 261 males |
DEXA: BMD X-ray |
When joining the study | The incidence of vertebral fractures in men with osteoporosis was 26.4% | The prevalence of lumbar degeneration is higher among men with osteoporosis | Positive correlation |
| Ou-Yang et al. [14] | 2015 | Prospective | 186 patients | CTP: blood flow; blood volume; permeability; time to peak; mean transit time | When joining the study | Vertebral blood flow, blood volume, and permeability are strongly positively correlated with bone density and strongly negatively correlated with the incidence of disc degeneration | Decreased lumbar bone marrow microcirculation perfusion precedes decreased bone density and disc degeneration, and there may be a causal relationship between the two | Positive correlation |
| Kwok et al. [15] | 2012 | prospective |
359 adults Male: 150 Average age: 73.3 Female: 209 Average age: 72.9 |
DEXA: BMD MRI: Ha, Hm, Hp, and AP dimension |
When joining the study | Low bone density was associated with a decrease in Ha, Hp, and AP and an increase in Hm. The biconvexity index of the vertebral body was increased. Lower bone density was associated with reduced disc volume | Lower bone density in older subjects correlates with a tendency to reduce disc volume | Positive correlation |
| Livshits et al. [16] | 2010 | Prospective |
2256 women (age range 18–84) comprising 378 and 716 pairs of MZ and DZ twins |
DEXA: BMD MRI: LDD summary score |
When joining the study | Disc degeneration was significantly and positively correlated with lumbar and hip bone density | There was a clear, significant, and independent association between hip and lumbar bone density and disc degeneration | Positive correlation |
| Li et al. [4] | 2022 | Retrospective |
105 postmenopausal women Average age: 65 ± 7 (range 55–75) |
QCT: BMD MRI (Q-Dixon and GRAPPATINI T2 mapping sequences) |
When joining the study | BMFF of L1–L3 vertebrae was significantly negatively correlated with disc T2 values, and L4–5 segments were weakly correlated | Osteoporosis is associated with disc degeneration | Negative correlation |
| Kaiser et al. [5] | 2018 | Prospective |
377 patients Male: 181 Female: 196 Age range 41–83 |
QCT: Int.BMD; trabecular BMD; rBMD ratios (anterior/posterior, superior/mid-transverse, inferior/mid-transverse, and central/outer) | When joining the study | Higher integral or trabecular bone density is associated with increased incidence of disc height loss | Bone distribution within the vertebral body varies between ages and correlates with disc degeneration | Negative correlation |
| Tosun et al. [6] | 2012 | Retrospective |
64 patients Male: 7 Female: 57 |
DEXA: BMD MRI: Modic classification system; Schmorl nodes; gas within disc; anterior, mid, and posterior parts of the disc heights; Griffith eight-grade system |
When joining the study | The incidence of bone marrow abnormalities and intervertebral disc degeneration at the lower endplate of L1 was lower in the osteoporotic group than in the normal and osteoporotic groups | Denser vertebral bone is associated with more severe disc degeneration | Negative correlation |
| Wang et al.[17] | 2010 | Prospective |
359 elderly patients (average age: 73.2, range 67–89) Male: 163 Average age: 73.5 ± 4.3 Female: 196 Average age: 73.2 ± 4.1 |
DEXA: BMD QCT: BMD; MRI: eight-level grading system |
When joining the study | The higher the vertebral bone density, the narrower the vertebral space. This is more pronounced in the mid-lumbar segment (L3/4 and L4/5) | Lower lumbar bone density is associated with milder disc degeneration | Negative correlation |
| Pye et al.[18] | 2005 | Prospective |
585 patients Male: 286 Female: 299 |
DEXA: BMD X-ray: presence and severity of osteophytes; endplate sclerosis; disc space narrowing |
When joining the study | Imaging grading of disc degeneration increases with increasing bone density in the lumbar spine | Imaging features of disc degeneration are associated with increased bone density in the spine | Negative correlation |
| Nanjo et al.[19] | 2003 | Prospective |
90 women Age range 22–74 |
DEXA: BMD MRI: Thompson’s classification |
When joining the study | When the disc degeneration is severe, the bone density is high not only in the lumbar spine, but also in other parts of the spine | Lumbar bone density is closely associated with disc degeneration. Bone density of the heel and radius also correlates closely with disc degeneration | Negative correlation |
| Miyakoshi et al.[20] | 2002 | Prospective | 104 postmenopausal women older than 60 years |
DEXA: BMD X-ray: semiquantitative osteophyte score; disc score; number of vertebral fracture |
When joining the study | Disc score is positively correlated with bone density | Osteoporosis is negatively correlated with lumbar spine lesions | Negative correlation |
| Harada et al. [21] | 1998 | Prospective | 110 postmenopausal women (average age: 67.2, range 49–86) |
DEXA: BMD MRI: disc area; disc bulge ratio |
When joining the study | Whole-body bone density and lumbar bone density were negatively correlated with disc area | Bone density is negatively correlated with disc degeneration | Negative correlation |
| Wang et al. [22] | 2011 | Cadaver study | 137 cadaveric lumbar vertebrae (L2–L5) from 48 male human with a mean age of 50 years (range 21 to 64 years) |
Discography assess the degree of disc degeneration Micro–CT: volume; BMC; BMD; measurements for the posterior elements, osteophytes, endplates, and the vertebral body without osteophytes and endplates |
When joining the study | The greater the vertebral bone density, the more severe the disc degeneration. This correlation persisted after further exclusion of osteophytes and endplates | High bone density associated with more severe disc degeneration | Negative correlation |
| Geng et al.[10] | 2021 | Retrospective | 754 healthy patients aged 20–60 years | QCT: Trab.vBMD | When joining the study | No statistically significant difference in volumetric bone density between those without LDH and those with LDH | No association between lumbar disc herniation and volumetric bone density was observed in either men or women | Irrelevant |
| Pan et al. [11] | 2017 | Retrospective |
512 subjects 165 (F/M: 135/30, average age 69.9 ± 8.7)with various degenerative lumbar spine disorders 161 (F/M: 44/117, average age 48.8 ± 8.6) of health assessment 186 (F/M: 77/109, average age 50.8 ± 15.5) randomly selected |
DEXA: BMD MRI: Pfirrmann grading system; facet joint osteoarthritis |
When joining the study | No statistical association between bone density and disc degeneration was observed. Small joint osteoarthritis increases bone density measurements and may confound the association between the two | Bone density may not be a risk factor for disc degeneration in the Chinese population | Irrelevant |
| Salo et al. [23] | 2014 | Prospective | 168 postmenopausal women aged 63.3–75.0 years, mean 68.6 years |
DEXA: BMD MRI: Pfirrmann grading system |
When joining the study、5、10、15 years | The higher the lumbar bone density, the more severe the disc degeneration, but it may be a pseudo-increase in bone density due to local degenerative changes | No significant correlation between bone density and disc degeneration in the lumbar and femoral neck | Irrelevant |
| Yang et al. [24] | 2009 | Prospective |
90 patients Male: 21 Female: 69 Average age: 72.5 ± 4.2 |
X-ray MRI: Pfirrmann grading system |
When joining the study | Osteoporosis is associated with expansion of the middle of the disc and collapse of the vertebral body, but not with disc degeneration | Osteoporosis was not correlated with disc degeneration | Irrelevant |
| Wang et al. [25] | 2011 | Cadaver study |
150 cadaveric lumbar vertebrae (L1–L5) and 209 adjacent discs (L1/2–L5/S1) from 48 male human spines The mean age for the sample is 50 years (range 21–64) |
Discography: assess the degree of disc degeneration Micro-CT: endplate thickness; BMD |
When joining the study | Lumbar endplate thickness and bone density were not associated with age. There was no evidence of an association between more severe disc degeneration and increased endplate bone density | Endplate sclerosis may not be a risk factor for disc disease in men | Irrelevant |
Table 2.
Basic information about the included animal studies
| Author | Year | Imaging tool and indicator | Animal and modeling method | Evaluation time | Result | Conclusion | Relevance |
|---|---|---|---|---|---|---|---|
| Xiao et al. [26] | 2019 | Micro-CT: BV/TV; BS/TV; Tb.N; Tb.Th; Tb.Sp; SMI; BMDtv |
C57BL/6 J mice Female a total of 40, 10 per group OVX |
12 weeks | OVX can cause vertebral trabecular thinning, cartilage endplate ossification, and angiogenesis | OVX can cause disc degeneration | Positive correlation |
| Xiao et al. [27] | 2018 |
Micro-CT: BMDtv; BV/TV; Tb.N; Tb.Th; Tb.Sp; Tb.Pf; CONN.D; SMI; Po.N(cl); PO(op); Po.V(tot) |
C57BL/6 J mice female a total of 30, 15 per group OVX |
12 weeks | A large number of osteoclasts appeared in the subchondral bone and cartilage endplates in the OVX group, and the expression of OSX, OPN, and VEGF in the endplates was elevated | OVX induces vertebral osteoporosis and endplate osteochondral remodeling, promoting angiogenesis and increased porosity of the osteochondral surface | Positive correlation |
| Li et al. [28] | 2016 | Micro-CT: build the 3D images to show the bottom of L4 vertebrae and top of L5 vertebrae, evaluate the newly formed bone marrow cavities | Opg KO mice | 8, 12, 24 weeks | New bone formation in endplate cartilage and increased VEGF-A, CD31, VE-cadherin, CD34, IL-1β, and TNF-α protein expression in endplate of Opg knockout mice | Increased expression of neovascularization and inflammatory cytokines in the lumbar disc of Opg knockout mice | Positive correlation |
| Zhong et al. [29] | 2016 |
DEXA: BMD MRI: Pfirrmann grading system Micro-CT: BV/TV; Tb.N; Tb.Th; Tb.Sp; SMI |
rhesus monkey female a total of 12, 6 per group OVX + PYM injection |
12 months | PYM injection caused disc degeneration, which was more pronounced when combined with OVX. Endplate calcification was more severe in the OVX + PYM group, and endplate vascularization was lower than in the other groups | Osteoporosis can lead to accumulation of calcification in the endplate and reduced vascularization | Positive correlation |
| Ding et al. [30] | 2014 |
Micro-CT: BV/TV; SMI; Tb.N; Tb.Th; Tb.Sp; Mdtv |
SD rats female a total of 52 OVX + CMS |
12, 18, 24 weeks | The CMS-OVX group showed cartilage endplate wear at 12 weeks postoperatively and in situ calcification at 18 and 24 weeks postoperatively | CMS combined with OVX causes more severe cartilage endplate damage than OVX alone. The cartilage endplates wear early and calcify in situ later | Positive correlation |
| Zhang et al. [31] | 2012 | DEXA: BMD |
SD rats female a total of 100, 50 per group natural aging |
22, 28 months | Indicators of osteoporosis are negatively correlated with indicators of disc degeneration | Osteoporosis was negatively correlated with disc degeneration | Negative correlation |
| Wang et al. [32] | 2004 | DEXA: BMD |
SD rats female a total of 30, 10 per group OVX |
6 months | There was a significant negative correlation between the bone mineral density and Grade II discs in the OVX rats | Cartilage endplate degeneration as a possible causative factor for disc degeneration | Negative correlation |
Table 3.
Basic information on studies using pharmacological interventions
| Author | Year | Medication | Study type | Imaging tool and indicator | Animal and modeling method | Conclusion | Relevance |
|---|---|---|---|---|---|---|---|
| Sun et al. [33] | 2021 | Denosumab | Animal experiment | Micro-CT: DHI; BV/TV; Tb.Th; Tb.N; Tb.Sp; Po. N (cl);Po (op); Po. V (tot) |
SD rats female a total of 60, 12 per group OVX |
Denosumab delays the degeneration of adjacent discs after lumbar fusion. This protective effect is mainly achieved by inhibiting osteoporosis and endplate cartilage remodeling | Positive correlation |
| Luo et al. [34] | 2013 | Alendronate | Animal experiment |
DEXA: BMD Micro-CT: BV/TV; Tb.Th; Tb.N; Tb.Sp; SMI |
SD rats female a total of 30, 10 per group OVX |
Alendronate delays the progression of disc degeneration in OVX rats. The mechanism may be related to the protection of the structural integrity of the vertebral body and endplate | Positive correlation |
| Liu et al. [35] | 2018 | 17β-estradiol | Animal experiment | Micro-CT: IDH; VEL; BV/TV; Tb.N; Tb.Th; Tb.Sp |
SD rats female a total of 72 OVX |
Estrogen deficiency exacerbates disc degeneration caused by spinal instability, and estrogen supplementation reduces the progression of disc degeneration | Positive correlation |
| Luo et al. [36] | 2014 | Salmon calcitonin | Animal experiment | Micro-CT: BV/TV; Tb.Th; Tb.N; Tb.Sp; SMI |
SD rats female a total of 30, 10 per group OVX |
Salmon calcitonin delays the progression of disc degeneration in OVX rats. The mechanism may be related to the protection of the structural integrity of the vertebral body and the regulation of the expression of MMPs, aggrecan and type II collagen | Positive correlation |
| Chen et al. [37] | 2018 | Sintered dicalcium pyrophosphate | Animal experiment |
Micro-CT: BV/TV; Tb.Th; Tb.Sp; Tb.N; BMD |
SD rats female a total of 60, 20 per group OVX |
Sintered dicalcium pyrophosphate prevents osteoporosis and improves disc degeneration in rats | Positive correlation |
| Luo et al. [38] | 2018 | Parathyroid hormone 1–34 | Animal experiment |
DEXA: BMD Micro-CT: BV/TV; Tb.N; Tb.Th; Tb.Sp; SMI |
SD rats female a total of 30, 15 per group OVX |
Parathyroid hormone 1–34 has a significant promotion effect on bone mass and bone strength in OVX rats, but no significant effect on disc degeneration | Irrelevant |
Clinical and cadaveric studies
We included a total of 21 clinical and cadaveric studies, including 12 prospective studies, 7 retrospective studies, and 2 cadaveric studies. Among the clinical studies, 7 studies considered vertebral osteoporosis to be positively associated with disc degeneration, 8 studies considered them to be negatively associated, and 4 studies considered them not to be associated. Of the cadaveric studies, 1 concluded that they were negatively correlated and 1 concluded that they were not correlated. The earliest of these studies was published in 1998 [21] and the most recent in 2022 [7]. The largest sample size was the study by Livshits et al. [16] and the smallest sample size was the study by Tosun et al. [6]. The middle-aged and older groups of men and women were the main subjects of clinical studies. In terms of imaging tools, the most used tool for measuring bone mineral density was dual-energy X-ray absorptiometry (DEXA), while some studies also used quantitative computed tomography (QCT). One study [14] used the dynamic computed tomographic perfusion (CTP) technique to detect indicators related to bone marrow microcirculation perfusion, and two other studies [7, 9] used Hounsfield Unit (HU) values from computed tomography (CT) instead of bone density. Magnetic resonance imaging (MRI) is a widely used tool to assess disc degeneration. Its indices are more diverse and include Pfirrmann grading (the most used), intervertebral space height, degree of endplate damage, bone redundancy formation, disc volume, and disc bulge rate. Two cadaveric studies [22, 25] used discography to assess the degree of disc degeneration and Micro-CT to measure bone density and endplate related parameters. With regard to the timing of assessment, most studies performed only one assessment of bone density and disc at sample entry, and only Salo et al. [23] performed three follow-up visits 5, 10, and 15 years after entry.
Animal studies
We included a total of 7 animal studies, of which 5 concluded that vertebral osteoporosis was positively associated with disc degeneration and 2 concluded that they were negatively associated. The earliest of these animal studies was published in 2004 [32] and the most recent study [26] was published in 2019. Rats and mice were the most used animals, with only one study using rhesus monkeys [29]. In terms of imaging tools, most animal studies used Micro-CT to measure bone trabeculae and endplate related parameters. The main parameters related to bone trabeculae are bone volume/total volume (BV/TV), bone surface density/total volume (BS/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), structural model index (SMI), total volume of bone mineral density (BMDtv), and the parameters related to endplates are number of closed pores (Po.N(cl)), open porosity (PO(op)), total volume of pore space (Po.V(tot)), which mainly reflect the porosity of endplates. In terms of modeling methods, apart from the osteoprotegerin (Opg) knockout model [28] and the natural aging model [31], ovariectomy (OVX) is the most commonly used method for modeling osteoporosis. In addition, there are studies using composite models. For example, Zhong et al. [29] combined pingyangmycin endplate injection with ovariectomy, and Ding et al. [30] combined paravertebral muscle dissection with ovariectomy. In terms of observation time, Zhang et al. [31] performed up to 28 months, and Xiao et al. [26, 27] had the shortest observation time of 12 weeks.
Drug therapy-related studies
We also included 6 studies in which anti-osteoporosis drugs were used, all of them animal studies. Five of the six studies concluded that vertebral osteoporosis was positively associated with disc degeneration, and the remaining one concluded that there was no correlation between the two. All of these studies used Micro-CT to measure bone trabeculae and endplate related parameters. Five studies inhibited abnormal bone remodeling of the endplate, maintained the structural integrity of the endplate, and delayed disc degeneration by using anti-osteoporosis drugs such as denosumab [33], alendronate [34], 17β-estradiol [35], salmon calcitonin [36], and sintered dicalcium pyrophosphate [37]. One other study [38] found that parathyroid hormone 1–34 enhanced bone mass in rats, but did not find that it had a significant effect on disc degeneration.
Literature quality evaluation results
In the retrospective studies, the relevant item was not scored because most studies did not report non-response. Most of the included prospective studies did not score on the relevant items because the outcome indicators to be observed were already identified at the beginning of the study. In addition, most prospective studies did not have a long enough follow-up period and therefore did not score on the follow-up-related items. There were also several prospective studies [13, 20, 21] with unrepresentative exposure group populations, and therefore no score was given for relevant items. In animal studies, all studies did not specify sample size calculation and inclusion and exclusion criteria, did not hide the animal grouping scheme, and did not perform blinded evaluation of outcomes, so these items were not scored. Detailed information on the quality evaluation of the literature is shown in Tables 4, 5, and 6.
Table 4.
Quality evaluation results of retrospective clinical studies
| Study | Selection of study population | Comparability between groups | Measurement of exposure factors | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Appropriateness of case determination | Representativeness of cases | Selection of controls | Identification of the control | Determination of exposure factors | The same method was used to determine the exposure factors for the case and control groups | Non-response rate | |||
| Liang et al. [7] | * | * | * | * | * | * | * | 7 | |
| Zhao et al. [8] | * | * | * | * | * | * | * | 7 | |
| Zhuang et al. [9] | * | * | * | * | * | * | * | 7 | |
| Li et al. [4] | * | * | * | * | * | * | * | 7 | |
| Tosun et al. [6] | * | * | * | * | * | * | 6 | ||
| Geng et al. [10] | * | * | * | * | * | * | * | 7 | |
| Pan et al. [11] | * | * | * | * | ** | * | * | 8 | |
Table 5.
Quality evaluation results of prospective clinical studies
| Study | Selection of study population | Comparability between groups | Outcome measurement | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representation of the exposed group | Selection method of non-exposure group | Methods for determining exposure factors | There were no outcome indicators to observe at study entry | Adequacy of the evaluation of the results | Whether the follow-up period after the occurrence of the outcome is long enough | Adequacy of follow-up visits | |||
| Fabreguet et al. [13] | * | * | * | * | 4 | ||||
| Ou-Yang et al. [14] | * | * | * | * | * | 5 | |||
| Kwok et al. [15] | * | * | * | * | * | 5 | |||
| Livshits et al. [16] | * | * | * | * | * | * | 6 | ||
| Kaiser et al. [5] | * | * | * | * | * | 5 | |||
| Wang et al. [17] | * | * | * | * | * | 5 | |||
| Pye et al. [18] | * | * | * | * | * | 5 | |||
| Nanjo et al. [19] | * | * | * | * | 4 | ||||
| Miyakoshi et al. [20] | * | * | * | * | 4 | ||||
| Harada et al. [21] | * | * | * | * | 4 | ||||
| Salo et al. [23] | * | * | * | * | * | * | * | 7 | |
| Yang et al. [24] | * | * | * | * | * | 5 | |||
Table 6.
Quality evaluation results of animal experiments
| Study | Sample size calculation | Inclusion and exclusion criteria | Random sequence generation | Hidden animal grouping scheme | Reasons for excluding animals from the analysis are reported | Blinded evaluation of outcome indicators | Statement of potential conflicts of interest and funding |
|---|---|---|---|---|---|---|---|
| Xiao et al. [26] | U | N | Y | N | Y | N | Y |
| Xiao et al. [27] | U | N | Y | N | N | N | Y |
| Li et al. [28] | U | N | N | N | Y | N | Y |
| Zhong et al. [29] | U | N | Y | N | N | N | Y |
| Ding et al. [30] | U | N | Y | N | N | N | Y |
| Zhang et al. [31] | U | N | N | N | N | N | Y |
| Wang et al. [32] | U | N | Y | N | N | N | Y |
| Sun et al. [33] | U | N | N | N | N | N | Y |
| Luo et al. [34] | U | N | Y | N | N | N | Y |
| Liu et al. [35] | U | N | Y | N | Y | N | Y |
| Luo et al. [36] | U | N | Y | N | N | N | Y |
| Chen et al. [37] | U | N | N | N | Y | N | Y |
| Luo et al. [38] | U | N | Y | N | N | N | Y |
U unclear, N no, Y yes.
CT computed tomography, HU Hounsfield Unit, MRI magnetic resonance imaging, TEPS total endplate score, DEXA dual-energy X-ray absorptiometry, BMD bone mineral density, CTP dynamic computed tomographic perfusion, Ha anterior height, Hm middle height, Hp posterior height, AP anteriorposterior dimension, MZ monozygotic, DZ dizygotic, LDD lumbar disc degeneration, QCT quantitative computed tomography, Q-Dixon quantitative Dixon, Int.BMD integral BMD, Tb.BMD trabecular BMD, Trab.vBMD trabecular volumetric BMD, M male, F female, BMC bone mineral content, BV bone volume, TV total volume, BV/TV percentage bone volume, BS bone surface density, Tb.N trabecular number, Tb.Th trabecular thickness, Tb.Sp trabecular separation, SMI structural model index, BMDtv total volume of bone mineral density, Tb.Pf trabecular pattern factor, CONN.D connectivity density, Po.N(cl) number of closed pores, PO(op) open porosity, Po.V(tot) total volume of pore space, Mdtv mean density of TV, DHI disc height index, IDH intervertebral disc height, VEL vertebral endplates lesions, SD Sprague Dawley, OVX ovariectomy, Opg osteoprotegerin, KO knockout, VEGF-A vascular endothelial growth factor-A, IL-1β interleukin-1β, TNF-α tumor necrosis factor-α, PYM pingyangmycin, CMS cervical muscle section.
Discussion
The physiological and pathological characteristics of intervertebral discs, such as their anatomical structure, nutritional pathways, and mechanical properties, have been studied quite thoroughly as early as the last century. In recent years, although clinical and basic research on discs is still gradually increasing, there seems to be few groundbreaking findings. For disc degeneration, there is also still no recognized effective intervention and remains one of the most urgent challenges to be solved. When it comes to vertebral osteoporosis, more attention is often paid to the vertebral fractures caused by it and its impact on lumbar spine surgery, such as on the stability of intervertebral fusion devices and pedicle screws. There are not many studies examining the effects of vertebral osteoporosis on disc degeneration, and the findings are divergent. Therefore, we designed this study. To our knowledge, this is the first time in the last two decades that a systematic and comprehensive review and summary of relevant studies has been performed. This helps one to understand the points of controversy and the problems with the current study, so that the next studies can be improved in a targeted manner.
In this systematic review, we have made a comprehensive and detailed summary and presentation of the basic information of 34 studies, such as study type, sample information, imaging methods, modeling methods, observation time, results, and conclusions, and we have also evaluated the quality of these studies. Of the 34 studies, 17 concluded that vertebral osteoporosis was positively correlated with disc degeneration, 11 concluded that they were negatively correlated, and 6 concluded that they were not correlated. Most of the retrospective clinical studies we included were cross-sectional studies, which had a high risk of bias and a limited level of evidence. Prospective studies were more convincing than retrospective studies, but these studies lacked long follow-up. Moreover, the included clinical studies commonly used DEXA to measure BMD, and their results are susceptible to interference from other tissues [20]. In addition, these clinical studies could not be explored in greater depth at the level of mechanism of action. The animal studies we included were small in number and each had a small sample size, but they provided a wealth of histological evidence in addition to imaging findings. Moreover, these animal studies commonly used Micro-CT to measure bone trabecular structures as well as endplate microstructure, which is more accurate than DEXA. Encouragingly, several studies [26–29, 33–36] have observed and histologically confirmed abnormal bone remodeling in the endplate after vertebral osteoporosis using Micro-CT. This abnormal bone remodeling process is mainly characterized on imaging by increased porosity on the endplate surface, cavities inside the endplate, endplate edge defects or bone redundancy formation. Histologically, it is characterized by calcification of the endplate, active osteoclasts, and neovascularization. Moreover, similar abnormal bone remodeling was also found in primates [29]. We also included studies [33–36] that achieved delayed disc degeneration by inhibiting abnormal bone remodeling of the endplate and maintaining the integrity of the endplate through the use of anti-osteoporosis drugs. The above studies provide new ideas to further reveal the mechanism of action of vertebral osteoporosis and disc degeneration. By combing through the 34 included studies, we believe that the current debate about the relationship between vertebral osteoporosis and disc degeneration focuses on abnormal bone remodeling of the endplate, endplate vascularization, and biomechanics, and we will discuss these aspects in depth below.
Nutritional pathways of the disc
Reduced nutrient supply is now recognized as one of the major causes of disc degeneration. The maximum cell density within the disc is regulated by the nutritional status, and a decrease in nutrient supply will reduce the number and activity of surviving cells within the disc, leading to disc degeneration [39]. The intervertebral disc is a special structure with two main nutritional pathways: (1) the annulus fibrosus pathway: the blood vessels on the surface of the annulus fibrosus provide nutrition to the outer layer of the annulus; (2) the endplate pathway: nutrition from the blood vessels in the vertebral body diffuses through the bone marrow cavity—blood sinusoid—endplate interface to the disc, providing nutrition to the nucleus pulposus and the inner layer of the annulus fibrosus. It is now widely accepted that the endplate pathway is the main nutritional route of the disc [40–42]. Zhu et al. [43] used a finite element approach to simulate the effects of different nutritional pathway damage on disc degeneration patterns. The results showed that the critical levels of nutrition for the endplate pathway, the annulus fibrosus pathway, and the common endplate-annulus pathway were about 30%, 20%, and 50% of the reference values, respectively. Below this critical level, the disc will degenerate. Wu et al. [44] evaluated the solute diffusion rates of glucose and lactate in healthy cartilage endplates and found a region-dependent solute diffusion rate that was highest in the central region of the cartilage endplate, further suggesting that the cartilage endplate is the gateway for solute diffusion into the disc.
The vertebral body is connected to the disc by the endplate. The endplate consists of the cartilaginous endplate and the subchondral bone. The subchondral bone, also known as the bony endplate, is a general term for the vascularized cortical and trabecular layer located between the cartilage endplate and the vertebral body [45]. Endplate degeneration is often seen as the beginning of disc degeneration. One study [46] found that when endplate perfusion was inhibited, perfusion in the nucleus pulposus was suppressed by nearly 50%. A study by Jackson et al. [47] also found that when endplate calcification was present, the minimum glucose concentration in the disc could be reduced by 17% compared to the normal group, with the nucleus pulposus being the most significantly involved site, with an average glucose concentration reduction of 30.6%, while the annulus fibrosus was reduced by only 6.4%. The relationship between cartilage endplate degeneration and disc degeneration has been confirmed by numerous studies. However, there seems to be little research on bony endplates and disc degeneration. The bony endplate is also an important link in the nutritional pathway of the disc. The osseous endplate region contains a large number of arteries and veins, whose tiny branches coiled around each other at the bone-chondral interface through bone marrow contact channels, forming a vascular bed that nourishes the disc [48]. A study by Benneker et al. [49] demonstrated a significant indirect correlation between the density of openings in the bony endplates and the morphology and partial degenerative grading of the disc. Moreover, this correlation was strongest in the region where the nucleus pulposus is located. When these openings are blocked, they may deprive the cells of nutrients and lead to disc degeneration. Laffosse et al. [42] suggested that the sparseness of the capillary network may be a key factor affecting the effective permeability of the bony endplate, while alterations in the vascular canal wall, subchondral osteosclerosis and the initiation of cartilage endplate calcification may be amplifying factors. Thus, the bony structure of the vertebral body and the disc are not separate entities and are closely linked in terms of trophic pathways. What we want to focus on is how the microstructure of the bony endplates as well as bone marrow contact channels (e.g., number of pores, thickness, vascular density) changes when the vertebral body is in a state of osteoporosis, and whether such changes have an impact on the nutritional pathways of the disc.
Abnormal bone remodeling of the endplate
The phenomenon of abnormal bone remodeling in the endplate is a new entry point to explore the correlation between vertebral osteoporosis and intervertebral disc degeneration in recent years, which may be related to the availability of some new technologies (e.g., Micro-CT). The results of these studies are impressive and convincing.
Remodeling is the result of the interaction between osteoblast bone formation and osteoclast bone destruction. In the normal process of bone remodeling, bone absorption and formation are dynamically balanced, with old bones constantly being replaced by new ones [50]. In the osteoporotic state, this balance is disturbed and the bone resorption effect of osteoclasts is greater than the bone formation effect of osteoblasts, causing abnormal bone remodeling [51]. One of the main reasons is estrogen deficiency. Estrogen can stimulate osteoblast proliferation and reduce its apoptosis, reduce the responsiveness of osteoclast progenitor cells to RANKL, thus preventing the formation of osteoclast and shortening the life span of osteoclast [52]. Several animal studies [26–29, 33–36] that we included identified abnormal bone remodeling of the endplate in the osteoporotic state on imaging and histology. According to these studies, the abnormal bone remodeling of the endplate was mainly reflected in imaging in terms of increased surface porosity, internal cavity formation, endplate defects, and bone redundancy formation. Histologically, the above imaging manifestations were corroborated by calcification of the cartilage endplate, thinning of the cartilage endplate, and active osteoclasts, as observed by the use of safranin O—fast green staining, tartrate acid phosphatase staining, and immunohistochemistry. These studies showed that vertebral osteoporosis accelerates disc degeneration.
Pharmacological studies [33–36] also confirmed that when anti-osteoporosis drugs were used, abnormal bone remodeling of the endplates was inhibited, maintaining the structural integrity of the endplates and achieving a delay in disc degeneration. These drugs are mainly inhibitors of bone resorption such as denosumab, alendronate, 17β-estradiol, and salmon calcitonin. However, parathyroid hormone 1–34, a drug that promotes bone formation, was not found to have a protective effect on the disc [38]. The authors suggest that this may be related to the application of parathyroid hormone 1–34 in larger doses and for longer periods of time, resulting in excessive increase in vertebral bone mass, decrease in vertebral bone marrow perfusion, and decrease in nutrient supply to the disc [38].
Although the previously mentioned studies found increased porosity and cavities in the endplates, this does not imply an increase in nutrient infiltration. Because they are a poor bone microstructure formed by abnormal bone remodeling, their ability to provide an ideal growth environment for nutrient vessels as well as maintain normal disc hydrostatic pressure is questionable. According to Benneker et al. [49], these apparently enlarged pores were not reported to contain capillary sprouts. In addition to this, we found that abnormal endplate remodeling manifested itself differently at different times. In some studies with a relatively short observation period, the endplate pores, cavities, and defects were increased, whereas in studies with a longer observation period, the pore cavities of the endplate gradually disappeared, the defects were gradually filled, and the endplate was eventually extensively sclerotic. This suggests that abnormal bone remodeling of the endplate is a dynamically changing long-term process. Early on, osteoclast-dominated bone resorption predominates, causing destruction of the endplate bone structure and a decrease in the loading capacity to stress. In order to adapt to the stress changes, osteoblast activity increases and bone trabeculae become dense from sparse and porous, which eventually hardens and thickens the endplate. Therefore, from the perspective of abnormal bone remodeling of the endplate, the available evidence suggests that vertebral osteoporosis may accelerate disc degeneration.
Endplate vascularization
During the development, maturation, and degeneration of the spine, the disc undergoes vascularization, de-vascularization, and re-vascularization. In infancy, vascular tissue exists in the cartilaginous plate, and the disc receives nutrition through the outer fibrous ring and the rich vascular network of the endplate. In adulthood, the vascular tissue closes in order to maintain homeostasis within the nucleus pulposus of the disc, as well as to resist shock and decompression functions. Only the outermost fibrous annulus and the longitudinal ligament of the normal intervertebral disc have vascular distribution [40, 53]. During disc degeneration, the calcified layer of cartilage is stiffer than the non-calcified cartilage, so the stress transfer process creates a greater pressure at the tideline and the calcified layer of cartilage develops microfissures due to fatigue under repeated stress [54, 55]. In order to repair the damaged structure, the disc is revascularized. The fibrovascular invasion into the cartilage through the microfissures causes infiltration of inflammatory factors, macrophages and mast cells, accompanied by a large number of growth factors and cytokines and enzymes, causing an imbalance in the synthesis and catabolism of the extracellular matrix and causing further degeneration and differentiation of the disc cells [56–58]. In addition, it has also been found that myeloid cells highly express vascular endothelial growth factor (VEGF) and its cell membrane receptor and that the former maintains the survival of myeloid cells in an autocrine or paracrine manner. In a pathological hypoxic environment, hypoxic factors are activated and VEGF activity is increased and expressed in high amounts, promoting endothelial cell migration, nodulation and chemotaxis, and promoting neovascular outgrowth [59]. Therefore, most studies consider vascularization of the disc as the pathogenetic basis and key pathological changes of disc degeneration. The phenomenon of vascularization can occur in the nucleus pulposus, the fibrous annulus and the cartilage endplate regions, especially in the junctional regions [56–58]. Some of our included animal studies [26–28] found high expression of angiogenesis-related markers, such as VEGF-A, CD31, VE-cadherin, and CD34, in cartilage endplates of osteoporotic animals, suggesting that abnormal bone remodeling in the endplates is accompanied by neovascularization and that the localization of neovascularization is the same as that of abnormal bone remodeling, indicating that the two are a coupled process [27].
There are two controversies regarding the relationship between vertebral osteoporosis and endplate vascularization. One is whether the new blood vessels will provide more nutrition to the intervertebral discs or will they bring more inflammatory factors and immune exposure? Second, will osteoporosis accelerate endplate vascularization or decrease it? Regarding the first controversy, Seol et al. [60] argued that the vascularization of the cartilage endplates, with its enhanced function of transporting nutrients, oxygen and water substances and outward transport of acidic metabolites, could serve to slow down disc degeneration. However, as mentioned earlier, more studies [56–58] support the opposite view that neovascularization causes infiltration of inflammatory factors, macrophages and mast cells, while bringing in a large number of growth factors and cytokines as well as enzymes, which disrupt the internal environmental homeostasis and cause further degeneration and differentiation of disc cells. Moreover, from the results of some of the animal studies we included, it appears that although endplate vascularization occurs, the final outcome of the disc is still degenerative. Therefore, the latter view seems to be more convincing. However, since neovascularization arises from the repair of damaged structures, we cannot exclude the possibility that these neovascularizations may have both destructive and reparative effects. Since the repairing effect is overshadowed by the destructive effect, it is not possible to reverse the outcome of disc degeneration. This is a question that needs to be investigated more further. Regarding the second controversy, Mattei et al. [61] argued that the decrease in vertebral bone density provides more space for vascular growth and reduces endplate resistance, which facilitates nutrient transport and diffusion. In contrast, pharmacological treatments that increase bone density reduce the number and size of vascular buds, significantly reducing endplate vascularization and adversely affecting intervertebral disc nutrition [62]. Since bone loss is a long process, even if Mattei's view is correct, then this phenomenon may only occur in the early stages when the bone structure has not yet deteriorated. This is because, according to the current view, it is questionable whether the endplate can provide an ideal environment for the survival of new blood vessels when the bone structure deteriorates. Firstly, the process of vertebral osteoporosis is accompanied by infiltration of inflammatory factors within the vertebral body [63]. Second, according to Armas et al. [64], a higher than normal proportion of newly formed bone from abnormal remodeling is under-mineralized and therefore less resistant to bending, and the vessels are prone to twist and narrow due to compression. Moreover, according to Zhong et al. [29], in the late stage of vertebral osteoporosis, the endplate is extensively calcified, exceeding the compensatory capacity of the vasculature, and the vascularization gradually decreases until it disappears. Therefore, further studies are still needed to verify whether there is a relationship between vertebral osteoporosis and endplate vascularization and what this relationship is.
Bone marrow perfusion
Regarding the nutritional pathways of the disc, attention is often focused on the endplate pathway, but the impact of altered bone marrow microcirculation within the vertebral body as a higher level nutritional pathway on the disc seems to be underappreciated. Indeed, not only bone marrow perfusion of the vertebral body, but also impaired blood supply due to lumbar artery and even abdominal aorta atherosclerosis has been shown to be associated with lumbar disc degeneration [65, 66]. Burkhardt et al. [67] showed an age-dependent decrease in blood sinusoids in the vertebral bone marrow. The number of blood sinuses is highest in early adulthood, a period when bone marrow function is stable. Decreased blood sinusoids are common in old age and primary bone loss, both of which are accompanied by a decrease in hematopoietic cells and an increase in adipocytes. A study based on CTP [14] showed a decrease in blood flow, volume and permeability in the lumbar vertebral body with age, while the time to peak and mean passage time increased, indicating reduced bone marrow perfusion and diffusion. A study by Griffith et al. [68] found reduced bone perfusion in men with reduced bone mass and further reduced bone perfusion in men with osteoporosis compared to men with normal bone density. Conversely, vertebral bone marrow fat content was increased in men with reduced bone mass or osteoporosis. Another study [69] similarly found a significant increase in vertebral bone marrow fat content and a significant decrease in vertebral bone marrow perfusion index in the osteoporotic group. And the reduction in perfusion index occurred only within the vertebral body, no reduction in perfusion index occurred in the paravertebral tissue supplied by the same artery. Reduced bone marrow perfusion may reduce the nutrient supply to the disc at its source. Using dynamic contrast-enhanced MRI (DCE-MRI), Liu et al. [70] found that when lumbar bone marrow perfusion is reduced, the supply of nutrients to the lumbar discs through the intact endplates and the excretion of waste products are also reduced. The vertebral bone marrow between two degenerated discs has 14% less perfusion than the vertebral bone marrow between two normal discs, and most of these changes are the result of cellular metabolic changes that occurred months to years ago [71]. Based on the above studies, although there is a lack of evidence for a causal relationship between bone marrow perfusion and osteoporosis, the reduced bone marrow perfusion that accompanies osteoporosis may reduce the nutrient supply to the discs, which in turn accelerates disc degeneration. This may be one of the reasons why some studies have observed accelerated disc degeneration after vertebral osteoporosis. It is worth mentioning that this is disc degeneration due to reduced bone marrow perfusion and not directly due to vertebral osteoporosis.
Biomechanics
Tosun et al. [6] and Harada et al. [21] suggested that under the same load, discs with dense subchondral bone may be subjected to increasing mechanical stress, while osteoporotic vertebrae may act as a buffer and reduce the stress on the disc. Mattei [61] similarly suggested that the osteoporotic bone may act as a cushion to protect and reduce the stress on the cartilage, thereby slowing disc degeneration. A study by Anthony et al. [72] found an increase in disc height in subjects with reduced bone density, suggesting a tendency for the disc to expand by pushing into the endplate. Wang et al. [17] concluded that this tendency to expand toward the endplate is conducive to increasing the contact area between the disc and the endplate and dissipating the stresses exerted on the disc, thus helping to reduce disc injury. From the perspective of nutrient infiltration, Mattei [61] concluded that the rate of glucose diffusion into the disc significantly decreases as the static compressive strain of the disc increases. When the vertebral bone density decreases, the endplate resistance decreases and the compressive strain of the disc decreases, which facilitates the diffusion of glucose and delays the degeneration of the disc.
Contrary to the above, other evidence suggests that the vertebral body after osteoporosis does not act as a stress buffer, but rather increases the mechanical stresses on the endplate and disc. Finite element models [73] showed that when the modulus of trabeculae decreased by 50%, the peak stress on the endplate increased by 74%, eventually leading to endplate thinning, calcification [74], and even collapse [25, 75, 76]. Histological evidence also suggests the presence of many active small chondrocytes within the cartilage endplate adjacent to the nucleus pulposus, which is considered an indication of abnormal endplate loading [77]. The overgrowth of bony flaps at the edges of the vertebral body in osteoporotic rats is also an evidence of increased disc stress [78]. We believe that the force patterns of the lumbar spine are different in humans and experimental animals. Humans walk upright, whereas experimental animals are crawlers. Therefore, clinical studies may reach different conclusions than animal studies, which may be one of the reasons for the disagreement. In addition, the difference in observation time between the above studies may also contribute to the disagreement. Bone loss is a long-term process. In the early stages, a mild decrease in vertebral bone density may serve to cushion the pressure to protect the disc, but this cushioning effect may be difficult to maintain as the bone structure gradually deteriorates. Unfortunately, no one has yet observed the dynamic effects of vertebral bone density on the disc at different times. Therefore, this question still needs to be answered by more future studies.
Disc height
Changes in disc height after reduction in vertebral bone density were observed in several animal experiments and clinical studies in our included studies [6, 15, 17, 24]. Exceptionally, disc height changes under osteoporosis are more often reflected in an increase in the intermediate disc height [6, 24], whereas the anterior and posterior disc heights do not seem to change significantly [24]. Biomechanical studies have shown that osteoporosis leads to a concentration of stress in the center of the vertebral endplate, and the increased pressure leads to trabecular compression and axial bulging of the endplate [79]. This is therefore manifested on imaging as an increased concavity of the endplate [24], leading to an increase in the intermediate height of the disc. However, it is important to point out that this is a secondary change due to the additional expansion space created by the increased concavity of the endplate and the compression of the vertebral body, and is not a change in the state of the disc itself. It is also believed a loss of vertebral body height due to osteoporosis causes instability of the spinal motion segment and accelerates degeneration of the articular eminence joint and disc [17, 80]. We suggest that when exploring disc height, humans and animals similarly show different results depending on different walking patterns. Changes in disc height may be more pronounced in humans who walk upright relative to animals who crawl. Therefore, the plausibility of using animal disc height changes to model human disc degeneration seems to be debatable.
Imaging tools
DEXA is the most widely used method for screening and diagnosis of osteoporosis. It has the advantage of being technically mature, easy to perform, and inexpensive, but it has limitations in terms of spatial resolution and is susceptible to interference from endplate sclerosis, bone redundancy, and ligamentous calcification, leading to falsely elevated measurements [20]. Therefore, it has been suggested [23, 26] that the conclusion that higher BMD is associated with more severe disc degeneration may be related to the falsely elevated BMD values caused by DEXA. Furthermore, DEXA does not allow for the quantitative analysis of bone tissue in the target region. In view of this limitation, more and more studies have started to use Q-CT and Micro-CT to quantify the microstructure of bone trabeculae in the target region after differentiating between cortical and cancellous bone to reduce the interference of other structures. Micro-CT can clearly scan bone trabeculae in the target area at the micron level, calculate bone structural parameters precisely and in three dimensions, and detect early changes in bone microstructure. Some impressive studies [25, 29] used Micro-CT to precisely delineate the target area, segment the endplate, and directly measure the calcified area, thickness, and porosity of the endplate to observe structural changes in the endplate from a more microscopic perspective. However, Micro-CT is not as popular as DEXA due to its high price, complicated operation, and high radiation dose, and is currently used more for basic research. There is also an alternative method of assessing BMD using the HU value, which is based on the existence of a strong positive correlation between HU values and BMD [9]. As an alternative method, no additional examination costs are required and the procedure is relatively simple. In the previously mentioned section on bone marrow perfusion, functional CTP imaging has been used to quantify the spatio-temporal distribution patterns of bone marrow microcirculation perfusion and bone density, which is also an emerging technical tool for the assessment of hemodynamics by continuous measurement of multiplanar imaging [14]. As for the assessment of disc degeneration, it is evident from the included studies that MRI is still the most commonly used imaging method to assess the disc.
Animal models of osteoporosis
Cost is an important factor influencing the choice of experimental animals. In our included studies, rats and mice remained the most used animals due to their lower cost. Primates share anatomical, physiological and biomechanical similarities with humans and are higher up in the phylogenetic tree. Of the studies we included, only one study [29] used rhesus monkeys for the study. We believe that large-scale use of primates may remain elusive due to high costs. Accelerated bone turnover due to estrogen deficiency is the most common cause of osteoporosis. Therefore, the animal model of osteoporosis caused by estrogen deficiency is the most used animal model in our included studies. This model was constructed by bilateral oophorectomy, which is a mature technique, simple to perform, and has a high modeling rate. Other studies have used models of natural aging, which have a longer experimental period and higher time cost. OPG exerts an inhibitory effect on the differentiation, activation and survival of osteoblasts. One study [28] used a mouse model with OPG knockout, again limited by cost and difficult to apply on a large scale. Other studies have used composite models, which add to the OVX model by removing paravertebral muscles to exacerbate lumbar instability [30] or by injecting drugs into the endplate to block the nutrient supply to the disc [29], achieving a more significant disc degeneration effect than the OVX model alone and shortening the experimental period.
Limitations
We have the following limitations in this study. First, the included studies were highly variable with few common outcome indicators, so we were unable to perform a quantitative meta-analysis of the valuable outcome indicators to increase the persuasiveness. Second, because disc degeneration is the result of a combination of factors and there is no ideal experimental model to date that can model any one of these factors alone [14]. Therefore, the studies we included only illustrate the correlation between vertebral osteoporosis and disc degeneration without revealing a causal relationship between the two. In addition, the relationship between vertebral osteoporosis and disc degeneration may not be one-sided, but rather an interactive relationship. Disc degeneration can likewise have an impact on vertebral bone density. Some studies [6, 10, 23] have shown that when the disc degenerates, the load shifts from the nucleus pulposus to the annulus fibrosus and the stress on the vertebral wall and posterior structures increases, leading to a decrease in core bone density and an increase in bone density in the vertebral wall and posterior structures. However, due to space limitations, we did not explore the effect of disc degeneration on vertebral bone density. Finally, osteoporosis is a systemic disease, and more in-depth studies on the relationship between vertebral and extremity bone density, as well as the relationship between disc degeneration and extremity bone density, are still pending [16, 18, 81, 82], which is our next step to focus on.
Conclusion
Our systematic review shows that the majority of studies currently consider an association between vertebral osteoporosis and intervertebral disc degeneration, but there is still a huge disagreement whether this association is positive or negative. Differences in observation time and follow-up time may be one of the reasons for the disagreement. Our view is that vertebral osteoporosis may have a bidirectional effect on disc degeneration. At different stages in the progression of osteoporosis, the effect on disc degeneration shows different trends. Therefore, for future clinical and animal studies, we not only recommend the use of more precise imaging tools such as QCT and Micro-CT, but also the setting of different observation time points to explore the dynamic relationship between vertebral osteoporosis and disc degeneration.
Data availability
All data generated or analyzed during this study are included in this article.
Declarations
Conflict of interest
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
He Zhao and Xing Yu contributed equally to this work.
Contributor Information
He Zhao, Email: zhaohe.2018@tsinghua.org.cn.
Xing Yu, Email: yuxingbucm@sina.com.
References
- 1.Xin J, Wang Y, Zheng Z, Wang S, Na S, Zhang S. Treatment of intervertebral disc degeneration. Orthop Surg. 2022;14(7):1271–1280. doi: 10.1111/os.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13:173–8. doi: 10.1097/00007632-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma AY, Meskers CG, Westendorp RG, Maier AB. Chronology of age-related disease definitions: osteoporosis and sarcopenia. Ageing Res Rev. 2012;11(2):320–324. doi: 10.1016/j.arr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Xie Y, Lu R, Zhang Y, Li Q, Kober T, Hilbert T, Tao H, Chen S. Q-Dixon and GRAPPATINI T2 mapping parameters: a whole spinal assessment of the relationship between osteoporosis and intervertebral disc degeneration. J Magn Reson Imaging. 2022;55(5):1536–1546. doi: 10.1002/jmri.27959. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser J, Allaire B, Fein PM, Lu D, Jarraya M, Guermazi A, Demissie S, Samelson EJ, Bouxsein ML, Morgan EF. Correspondence between bone mineral density and intervertebral disc degeneration across age and sex. Arch Osteoporos. 2018;13(1):123. doi: 10.1007/s11657-018-0538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosun O, Fidan F, Erdil F, Tosun A, Karaoğlanoğlu M, Ardıçoğlu O. Assessment of lumbar vertebrae morphology by magnetic resonance imaging in osteoporosis. Skeletal Radiol. 2012;41(12):1583–1590. doi: 10.1007/s00256-012-1435-0. [DOI] [PubMed] [Google Scholar]
- 7.Liang X, Liu Q, Xu J, Ding W, Wang H. Hounsfield unit for assessing bone mineral density distribution within cervical vertebrae and its correlation with the intervertebral disc degeneration. Front Endocrinol. 2022;13:920167. doi: 10.3389/fendo.2022.920167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Wang H, Li Z, Wang Z, Huo Y, Yang S, Ding W. Lumbar disk degeneration in female patients with and without ovariectomy: a case-control study. World Neurosurg. 2021;156:68–75. doi: 10.1016/j.wneu.2021.09.080. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang C, Wang Z, Chen W, Tian B, Li J, Lin H. Osteoporosis and endplate damage correlation using a combined approach of hounsfield unit values and total endplate scores: a retrospective cross-sectional study. Clin Interv Aging. 2021;5(16):1275–1283. doi: 10.2147/CIA.S315213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng J, Wang L, Li Q, Huang P, Liu Y, Blake GM, Tian W, Cheng X. The association of lumbar disc herniation with lumbar volumetric bone mineral density in a cross-sectional Chinese study. Diagnostics. 2021;11(6):938. doi: 10.3390/diagnostics11060938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J, Lu X, Yang G, Han Y, Tong X, Wang Y. Lumbar disc degeneration was not related to spine and hip bone mineral densities in Chinese: facet joint osteoarthritis may confound the association. Arch Osteoporos. 2017;12(1):20. doi: 10.1007/s11657-017-0315-6. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):123–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Fabreguet I, Fechtenbaum J, Briot K, Paternotte S, Roux C. Lumbar disc degeneration in osteoporotic men: prevalence and assessment of the relation with presence of vertebral fracture. J Rheumatol. 2013;40(7):1183–1190. doi: 10.3899/jrheum.120769. [DOI] [PubMed] [Google Scholar]
- 14.Ou-Yang L, Lu GM. Dysfunctional microcirculation of the lumbar vertebral marrow prior to the bone loss and intervertebral discal degeneration. Spine. 2015;40(10):593–600. doi: 10.1097/BRS.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok AW, Wang YX, Griffith JF, Deng M, Leung JC, Ahuja AT, Leung PC. Morphological changes of lumbar vertebral bodies and intervertebral discs associated with decrease in bone mineral density of the spine: a cross-sectional study in elderly subjects. Spine. 2012;37(23):1415–21. doi: 10.1097/BRS.0b013e31826f561e. [DOI] [PubMed] [Google Scholar]
- 16.Livshits G, Ermakov S, Popham M, Macgregor AJ, Sambrook PN, Spector TD, Williams FM. Evidence that bone mineral density plays a role in degenerative disc disease: the UK Twin Spine study. Ann Rheum Dis. 2010;69(12):2102–2106. doi: 10.1136/ard.2010.131441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YX, Griffith JF, Ma HT, Kwok AW, Leung JC, Yeung DK, Ahuja AT, Leung PC. Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: a study in elderly subjects using an eight-level MRI-based disc degeneration grading system. Osteoporos Int. 2011;22(1):91–96. doi: 10.1007/s00198-010-1200-y. [DOI] [PubMed] [Google Scholar]
- 18.Pye SR, Reid DM, Adams JE, Silman AJ, O'Neill TW. Radiographic features of lumbar disc degeneration and bone mineral density in men and women. Ann Rheum Dis. 2006;65(2):234–238. doi: 10.1136/ard.2005.038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanjo Y, Morio Y, Nagashima H, Hagino H, Teshima R. Correlation between bone mineral density and intervertebral disk degeneration in pre- and postmenopausal women. J Bone Miner Metab. 2003;21(1):22–27. doi: 10.1007/s007740300004. [DOI] [PubMed] [Google Scholar]
- 20.Miyakoshi N, Itoi E, Murai H, Wakabayashi I, Ito H, Minato T. Inverse relation between osteoporosis and spondylosis in postmenopausal women as evaluated by bone mineral density and semiquantitative scoring of spinal degeneration. Spine. 2003;28(5):492–5. doi: 10.1097/01.BRS.0000048650.39042.58. [DOI] [PubMed] [Google Scholar]
- 21.Harada A, Okuizumi H, Miyagi N, Genda E. Correlation between bone mineral density and intervertebral disc degeneration. Spine. 1998;23(8):857–61. doi: 10.1097/00007632-199804150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Boyd SK, Battié MC, Yasui Y, Videman T. Is greater lumbar vertebral BMD associated with more disk degeneration? A study using µCT and discography. J Bone Miner Res. 2011;26(11):2785–2791. doi: 10.1002/jbmr.476. [DOI] [PubMed] [Google Scholar]
- 23.Salo S, Leinonen V, Rikkonen T, Vainio P, Marttila J, Honkanen R, Tuppurainen M, Kröger H, Sirola J. Association between bone mineral density and lumbar disc degeneration. Maturitas. 2014;79(4):449–455. doi: 10.1016/j.maturitas.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Griffith JF, Leung PC, Lee R. Effect of osteoporosis on morphology and mobility of the lumbar spine. Spine. 2009;34(3):115–21. doi: 10.1097/BRS.0b013e3181895aca. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Battié MC, Boyd SK, Videman T. The osseous endplates in lumbar vertebrae: thickness, bone mineral density and their associations with age and disk degeneration. Bone. 2011;48(4):804–809. doi: 10.1016/j.bone.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Xiao ZF, Su GY, Hou Y, Chen SD, Zhao BD, He JB, Zhang JH, Chen YJ, Lin DK. Mechanics and biology interact in intervertebral disc degeneration: a novel composite mouse model. Calcif Tissue Int. 2020;106(4):401–414. doi: 10.1007/s00223-019-00644-8. [DOI] [PubMed] [Google Scholar]
- 27.Xiao ZF, He JB, Su GY, Chen MH, Hou Y, Chen SD, Lin DK. Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice. Arthritis Res Ther. 2018;20(1):207. doi: 10.1186/s13075-018-1701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XF, Xue CC, Zhao YJ, Cheng SD, Zhao DF, Liang QQ, Chen L, Wang Q, Lu S, Shi Q, Wang YJ, Shu B. Deletion of Opg leads to increased neovascularization and expression of inflammatory cytokines in the lumbar intervertebral disc of mice. Spine. 2017;42(1):8–E14. doi: 10.1097/BRS.0000000000001701. [DOI] [PubMed] [Google Scholar]
- 29.Zhong R, Wei F, Wang L, Cui S, Chen N, Liu S, Zou X. The effects of intervertebral disc degeneration combined with osteoporosis on vascularization and microarchitecture of the endplate in rhesus monkeys. Eur Spine J. 2016;25(9):2705–2715. doi: 10.1007/s00586-016-4593-2. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y, Jiang J, Zhou J, Wu X, Huang Z, Chen J, Zhu Q. The effects of osteoporosis and disc degeneration on vertebral cartilage endplate lesions in rats. Eur Spine J. 2014;23(9):1848–1855. doi: 10.1007/s00586-014-3324-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Xia J, Qiu Y, Bai Y. Correlation between osteoporosis and degeneration of intervertebral discs in aging rats. J Huazhong Univ Sci Technolog Med Sci. 2012;32(2):210–215. doi: 10.1007/s11596-012-0037-3. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Zhang L, Huang C, Cheng AG, Dang GT. Relationship between osteopenia and lumbar intervertebral disc degeneration in ovariectomized rats. Calcif Tissue Int. 2004;75(3):205–213. doi: 10.1007/s00223-004-0240-8. [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Tian FM, Liu F, Fang JK, Hu YP, Lian QQ, Zhou Z, Zhang L. Denosumab alleviates intervertebral disc degeneration adjacent to lumbar fusion by inhibiting endplate osteochondral remodeling and vertebral osteoporosis in ovariectomized rats. Arthritis Res Ther. 2021;23(1):152. doi: 10.1186/s13075-021-02525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y, Zhang L, Wang WY, Hu QF, Song HP, Su YL, Zhang YZ. Alendronate retards the progression of lumbar intervertebral disc degeneration in ovariectomized rats. Bone. 2013;55(2):439–448. doi: 10.1016/j.bone.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Wang X, Hua Y, Kong G, Wu X, Huang Z, Huang Z, Liu J, Yang Z, Zhu Q. Estrogen deficiency exacerbates intervertebral disc degeneration induced by spinal instability in rats. Spine. 2019;44(9):510–519. doi: 10.1097/BRS.0000000000002904. [DOI] [PubMed] [Google Scholar]
- 36.Luo Y, Zhang L, Wang WY, Hu QF, Song HP, Zhang YZ. The inhibitory effect of salmon calcitonin on intervertebral disc degeneration in an ovariectomized rat model. Eur Spine J. 2015;24(8):1691–1701. doi: 10.1007/s00586-014-3611-5. [DOI] [PubMed] [Google Scholar]
- 37.Chen CH, Chen WC, Lin CY, Chen CH, Tsuang YH, Kuo YJ. Sintered dicalcium pyrophosphate treatment attenuates estrogen deficiency-associated disc degeneration in ovariectomized rats. Drug Des Devel Ther. 2018;18(12):3033–3041. doi: 10.2147/DDDT.S170816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo Y, Li SY, Tian FM, Song HP, Zhang YZ, Zhang L. Effects of human parathyroid hormone 1–34 on bone loss and lumbar intervertebral disc degeneration in ovariectomized rats. Int Orthop. 2018;42(5):1183–1190. doi: 10.1007/s00264-018-3821-2. [DOI] [PubMed] [Google Scholar]
- 39.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26(23):2543–9. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 40.Nerlich AG, Schaaf R, Wälchli B, Boos N. Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J. 2007;16(4):547–555. doi: 10.1007/s00586-006-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(2):30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 42.Laffosse JM, Accadbled F, Molinier F, Bonnevialle N, de Gauzy JS, Swider P. Correlations between effective permeability and marrow contact channels surface of vertebral endplates. J Orthop Res. 2010;28(9):1229–1234. doi: 10.1002/jor.21137. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Q, Gao X, Levene HB, Brown MD, Gu W. Influences of nutrition supply and pathways on the degenerative patterns in human intervertebral disc. Spine. 2016;41(7):568–76. doi: 10.1097/BRS.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Cisewski SE, Wegner N, Zhao S, Pellegrini VD, Jr, Slate EH, Yao H. Region and strain-dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J Biomech. 2016;49(13):2756–2762. doi: 10.1016/j.jbiomech.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez AG, Rodriguez-Soto AE, Burghardt AJ, et al. Morphology of the human vertebral endplate. J Orthop Res. 2012;30(2):280–287. doi: 10.1002/jor.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Lenart BA, Lee JK, et al. Histological features of endplates of the mammalian spine: from mice to men. Spine. 2014;39(5):312–317. doi: 10.1097/BRS.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Werf M, Lezuo P, Maissen O, van Donkelaar CC, Ito K. Inhibition of vertebral endplate perfusion results in decreased intervertebral disc intranuclear diffusive transport. J Anat. 2007;211(6):769–774. doi: 10.1111/j.1469-7580.2007.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson AR, Huang CY, Brown MD, Gu WY. 3D finite element analysis of nutrient distributions and cell viability in the intervertebral disc: effects of deformation and degeneration. J Biomech Eng. 2011;133(9):091006. doi: 10.1115/1.4004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oki S, Matsuda Y, Shibata T, et al. Morphologic differences of the vascular buds in the vertebral endplate: scanning electron microscopic study. Spine. 1996;21(2):174–177. doi: 10.1097/00007632-199601150-00003. [DOI] [PubMed] [Google Scholar]
- 50.Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005;30(2):167–73. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 51.Frost HM. Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolff's law: the remodeling problem. Anat Rec. 1990;226(4):414–22. doi: 10.1002/ar.1092260403. [DOI] [PubMed] [Google Scholar]
- 52.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 53.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 54.Roberts S, Evans H, Trivedi J, et al. Histology and pathology of thehuman intervertebral disc. J Bone Joint Surg Am. 2006;88(2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 55.Burr DB, Radin EL. Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin North Am. 2003;29(4):675–685. doi: 10.1016/s0889-857x(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 56.Peng B, Chen J, Kuang Z, Li D, Pang X, Zhang X. Expression and role of connective tissue growth factor in painful disc fibrosis and degeneration. Spine. 2009;34(5):178–82. doi: 10.1097/BRS.0b013e3181908ab3. [DOI] [PubMed] [Google Scholar]
- 57.Ye F, Lyu FJ, Wang H, Zheng Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine. 2022;5(1):1196. doi: 10.1002/jsp2.1196.。. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koike Y, Uzuki M, Kokubun S, Sawai T. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003;28(17):1928–33. doi: 10.1097/01.BRS.0000083324.65405.AE. [DOI] [PubMed] [Google Scholar]
- 59.Tapia-Perez H. Intervertebral disc pathologies from an immunologicalperspective. Revista de neurologia. 2008;46(12):751–7. [PubMed] [Google Scholar]
- 60.Fujita N, Imai J, Suzuki T, Yamada M, Ninomiya K, Miyamoto K, Iwasaki R, Morioka H, Matsumoto M, Chiba K, Watanabe S, Suda T, Toyama Y, Miyamoto T. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun. 2008;372(2):367–372. doi: 10.1016/j.bbrc.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 61.Seol D, Choe H, Zheng H, et al. Selection of reference genes fornormalization of quantitative real-time PCR in organ culture of therat and rabbit intervertebral disc. BMC Res Notes. 2011;4:162. doi: 10.1186/1756-0500-4-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mattei TA. Osteoporosis delays intervertebral disc degeneration by increasing intradiscal diffusive transport of nutrients through both mechanical and vascular pathophysiological pathways. Med Hypotheses. 2013;80(5):582–586. doi: 10.1016/j.mehy.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 63.Turgut M, Uslu S, Uysal A, Yurtseven ME, Ustün H. Changes in vascularity of cartilage endplate of degenerated intervertebral discs in response to melatonin administration in rats. Neurosurg Rev. 2003;26(2):133–138. doi: 10.1007/s10143-003-0259-8. [DOI] [PubMed] [Google Scholar]
- 64.Silverman NE, Nicklas BJ, Ryan AS. Addition of aerobic exercise to aweight loss program increases BMD, with an associated reduction in in-flammation in overweight postmenopausal women. Calcif TissueInt. 2009;84:257–265. doi: 10.1007/s00223-009-9232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armas LA, Recker RR. Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am. 2012;41(3):475–486. doi: 10.1016/j.ecl.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Kauppila LI, Penttila A, Karhunen PJ, et al. Lumbar disc degeneration and atherosclerosis of the abdominal aorta. Spine. 1994;19:923–929. doi: 10.1097/00007632-199404150-00010. [DOI] [PubMed] [Google Scholar]
- 67.Kurunlahti M, Tervonen O, Vanharanta H, et al. Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine. 1999;24:2080–2084. doi: 10.1097/00007632-199910150-00003. [DOI] [PubMed] [Google Scholar]
- 68.Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8(3):157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 69.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236(3):945–951. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 70.Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, Leung PC. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241(3):831–838. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 71.Liu YJ, Huang GS, Juan CJ, Yao MS, Ho WP, Chan WP. Intervertebral disk degeneration related to reduced vertebral marrow perfusion at dynamic contrast-enhanced MRI. AJR Am J Roentgenol. 2009;192(4):974–979. doi: 10.2214/AJR.08.1597. [DOI] [PubMed] [Google Scholar]
- 72.Urban JP, Winlove CP. Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging. 2007;25:419–432. doi: 10.1002/jmri.20874. [DOI] [PubMed] [Google Scholar]
- 73.Anthony W.L. Kwok; James F. Griffith; Heather Ting Ma; Yixiang Wang; Ping Chung Leung; Jason Leung; David K.W. Yeung (2008). Estimated volume of both vertebral body and disc decreases as BMD decreases though this effect is seen more in the vertebral body than the disc 43 66. 10.1016/j.bone.2008.08.079
- 74.Mizrahi J, Silva MJ, Keaveny TM, Edwards WT, Hayes WC. Finite-element stress analysis of the normal and osteoporotic lumbar vertebral body. Spine. 1993;18(14):2088–96. doi: 10.1097/00007632-199310001-00028. [DOI] [PubMed] [Google Scholar]
- 75.Anderson DD, Brown TD, Radin EL. The influence of basal cartilage calcification on dynamic juxtaarticular stress transmission. Clin Orthop Relat Res. 1993;286:298–307. doi: 10.1097/00003086-199301000-00043. [DOI] [PubMed] [Google Scholar]
- 76.Haschtmann D, Stoyanov JV, Gédet P, Ferguson SJ. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J. 2008;17(2):289–299. doi: 10.1007/s00586-007-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Videman T, Battié MC. ISSLS prize winner: Lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine. 2012;37(17):1490–6. doi: 10.1097/BRS.0b013e3182608ac4. [DOI] [PubMed] [Google Scholar]
- 78.Peng B, Shi Q, Shen P, Wang Y, Jia L. The relationship between cartilage end-plate calcification and disc degeneration: an experimental study. Zhonghua Wai Ke Za Zhi. 1999;37(10):613–616. [PubMed] [Google Scholar]
- 79.Kurowski P, Kubo A. The relationship of degeneration of the intervertebral disc to mechanical loading conditions on lumbar vertebrae. Spine. 1986;11(7):726–31. doi: 10.1097/00007632-198609000-00012. [DOI] [PubMed] [Google Scholar]
- 80.Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37(2):213–221. doi: 10.1016/s0021-9290(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 81.Margulies JY, Payzer A, Nyska M, Neuwirth MG, Floman Y, Robin GC. The relationship between degenerative changes and osteoporosis in the lumbar spine. Clin Orthop Relat Res. 1996;324:145–152. doi: 10.1097/00003086-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 82.Li R, Zhang W, Xu Y, Ma L, Li Z, Yang D, Ding W. Vertebral endplate defects are associated with bone mineral density in lumbar degenerative disc disease. Eur Spine J. 2022;31(11):2935–2942. doi: 10.1007/s00586-022-07329-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.