Abstract
Sphingomonas xenophaga Bayram, isolated from the activated sludge of a municipal wastewater treatment plant, was able to utilize 4-(1-ethyl-1,4-dimethylpentyl)phenol, one of the main isomers of technical nonylphenol mixtures, as a sole carbon and energy source. The isolate degraded 1 mg of 4-(1-ethyl-1,4-dimethylpentyl)phenol/ml in minimal medium within 1 week. Growth experiments with five nonylphenol isomers showed that the three isomers with quaternary benzylic carbon atoms [(1,1,2,4-tetramethylpentyl)phenol, 4-(1-ethyl-1,4-dimethylpentyl)phenol, and 4-(1,1-dimethylheptyl)phenol] served as growth substrates, whereas the isomers containing one or two hydrogen atoms in the benzylic position [4-(1-methyloctyl)phenol and 4-n-nonylphenol] did not. However, when the isomers were incubated as a mixture, all were degraded to a certain degree. Differential degradation was clearly evident, as isomers with more highly branched alkyl side chains were degraded much faster than the others. Furthermore, the C9 alcohols 2,3,5-trimethylhexan-2-ol, 3,6-dimethylheptan-3-ol, and 2-methyloctan-2-ol, derived from the three nonylphenol isomers with quaternary benzylic carbon atoms, were detected in the culture fluid by gas chromatography-mass spectrometry, but no analogous metabolites could be found originating from 4-(1-methyloctyl)phenol and 4-n-nonylphenol. We propose that 4-(1-methyloctyl)phenol and 4-n-nonylphenol were cometabolically transformed in the growth experiments with the mixture but that, unlike the other isomers, they did not participate in the reactions leading to the detachment of the alkyl moiety. This hypothesis was corroborated by the observed accumulation in the culture fluid of an as yet unidentified metabolite derived from 4-(1-methyloctyl)phenol.
Technical nonylphenol is a mixture of >22 4-nonylphenol isomers (30, 32), which differ in the structure of the nonyl moiety attached to the phenol ring. It also contains isomers of 2-nonylphenol and 4-decylphenol as by-products (29). The technical product is mainly used as an intermediate for manufacturing nonylphenol polyethoxylates, commercially important surfactants that are widely used in cleaning products and as industrial process aids. The spectrum of application of these compounds ranges from dispersing agents for paper and pulp production to emulsifying agents in latex paints and pesticide formulations, flotation agents, industrial cleaners (metal surfaces, textile processing, and food industry), cold cleaners for cars, and household cleaners (29). The majority of nonylphenol polyethoxylates are used in aqueous solutions; therefore, they are discharged into municipal and industrial wastewaters and subsequently into sewage treatment plants. Once there, they are rapidly but only incompletely degraded, and more recalcitrant metabolites such as short-chain nonylphenol ethoxylates, their carboxylic derivatives, and 4-nonylphenol occur in treated sewage effluents (1, 2, 33, 34). Anaerobically stabilized sewage sludges contain extraordinarily high concentrations of 4-nonylphenol, indicating that its formation and accumulation are favored under mesophilic anaerobic conditions (11).
4-Nonylphenol was shown to accumulate in aquatic organisms and to be acutely toxic for them (12, 18, 19, 23). In chronic toxicity tests, concentrations with no observable effects are as low as 3.7 μg/liter for invertebrates (23). The fact that 4-nonylphenol also mimics estrogenic activity in mammals (24), fishes (13, 21), and other species has caused increasing concern about its impact on wildlife and human health and has resulted in calls for a better understanding of how it is further degraded in the environment.
The estrogenicity of alkylphenols depends on the structure of the aliphatic chain (14, 22). In particular, compounds with a threefold-substituted benzylic position are much more potent (22). In agreement with these findings, the most effective isomer isolated from a mixture of technical nonylphenol proved to be about 10 times more estrogenic than the least potent isomer (14). For an understanding of how microbial degradation affects the estrogenicity of mixtures of different 4-nonylphenol isomers, it is therefore necessary to know which isomers are preferentially degraded.
4-Nonylphenol can be found in all environmental compartments that directly or indirectly receive nonylphenol polyethoxylates (1, 2, 4, 7, 15, 20). Several studies indicate that further degradation is possible under aerobic conditions (5, 6, 8, 26, 31). Two bacteria that are able to degrade 4-nonylphenol efficiently and use it as a sole carbon and energy source have recently been isolated from activated sludge (9, 28). Both strain TTNP3 (28) and Sphingomonas cloacae (9) belong to the sphingomonads, a group of gram-negative bacteria which are widely distributed in nature and are well known for their extraordinary metabolic capabilities of degrading many otherwise recalcitrant chemicals (16). Both strains seem to transform 4-nonylphenols in a similar way. By an unknown mechanism, the alkyl moiety is released into the medium as a volatile alcohol derivative with an otherwise unchanged structure (3, 8), and most likely only the aryl part is utilized as a source of carbon and energy. Likewise, 2,4,4-trimethylpentan-2-ol has been reported to accumulate when octylphenoxyacetic acid and octylphenol are transformed by enriched cultures from groundwater (10) and by strain TTNP3 (27), respectively.
We report here the isolation of a Sphingomonas strain which efficiently degrades 4-(1-ethyl-1,4-dimethylpentyl)phenol. Our degradation experiments with five different 4-nonylphenol isomers showed that degradability is strongly dependent on the structure of the alkyl substituent.
MATERIALS AND METHODS
4-Nonylphenol isomers.
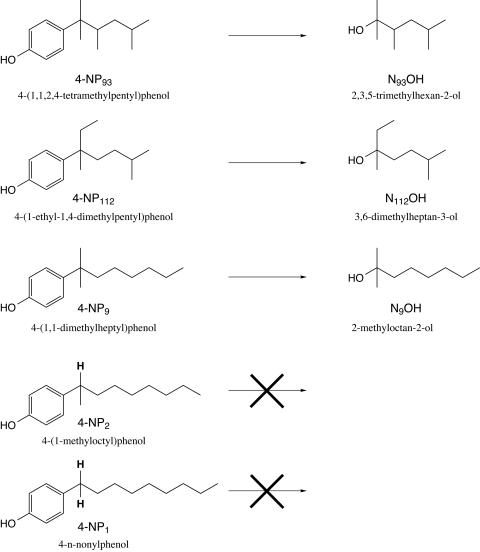
The abbreviations used for the various 4-nonylphenol isomers (see Fig. 3) are based on the systematic numbering system proposed by Guenther and coworkers (K. Guenther, E. Kleist, and B. Thiele, submitted for publication). 4-n-Nonylphenol (4-NP1; 99.5% pure, as determined by gas chromatography [GC]-flame ionization detection) was purchased from Ehrentorfer (Augsburg, Germany). The synthesis of the racemic nonylphenol isomers 4-(1,1,2,4-tetramethylpentyl)phenol (4-NP93), 4-(1-ethyl-1,4-dimethylpentyl)-phenol (4-NP112), 4-(1,1-dimethylheptyl)phenol (4-NP9), and 4-(1-methyloctyl)-phenol (4-NP2) will be described elsewhere (K. Guenther, R. Boehme, B. Andries, B. Thiele, and K.-H. Doetz, unpublished data). Chemical structures were confirmed by GC-mass spectrometry (GC-MS) and by one- and two-dimensional nuclear magnetic resonance (NMR) techniques. The purity of the synthetic products was determined by comparing relative signal intensities in the 1H-NMR spectra. 4-NP112, 4-NP9, and 4-NP2 were >99% pure. 4-NP93 contained 17% 4-(1,1,3,4-tetramethylpentyl)phenol (4-NP95) and, at most, 2% of an unknown substance as side products.
FIG. 3.
Structural requirements for detachment of the nonyl moieties of 4-nonylphenol isomers as C9 alcohols during incubation with S. xenophaga Bayram. Only nonylphenol isomers with three alkyl groups at the benzylic position are metabolized to the corresponding C9 alcohols and serve as growth substrates. Isomers with benzylic hydrogens (bold) do not undergo detachment of the nonyl moiety and do not serve as growth substrates. The numbering system is based on a proposal by Guenther et al. (submitted).
To check whether the available isomers were contained in commercial nonylphenol mixtures, we analyzed the pure isomers and a technical mixture (85%; Fluka, Buchs, Switzerland) by GC-MS under different temperature conditions and compared their retention times and mass spectra. 4-NP93 and 4-NP112 seemed to be important isomers of the analyzed charge, whereas 4-NP2 and 4-NP1 were clearly absent (data not shown). According to Thiele et al. (30), both 4-NP112 and 4-NP9 are contained in technical mixtures, with 4-NP112 being one of the main isomers.
Media and growth conditions.
Media were prepared with ultrapure water that was produced with a Seradest LFM 20 apparatus (Seral, Ransbach-Baumbach, Germany). Luria-Bertani (LB) medium contained the following reagents (per liter): 10 g of tryptone, 5 g of yeast extract (both from Biolife, Milan, Italy), 5 g of NaCl (Fluka), and for solid medium, 15 g of agar (bacteriological agar; Biolife). The medium was adjusted with NaOH (Fluka) to pH 7.2 and then autoclaved. For a minimal medium, we used 0.2-μm-pore-size filter-sterilized Bacto yeast nitrogen base without amino acids (Difco, Detroit, Mich.) supplemented with an appropriate carbon source. To enrich 4-nonylphenol-degrading bacteria, we added small amounts of yeast extract and peptones to the medium (17 to 50 μg of each/ml) at the beginning. The pH of the medium was adjusted with NaOH to a value of 7.0. Solid minimal media contained 15 or 20 g of Bacto agar (Difco)/liter and 1 g of technical nonylphenol (Pestanal; Riedel de Häen, Buchs, Switzerland)/liter. For preparations of these media, 0.2-μm-pore-size filter-sterilized medium and nonsterile nonylphenol (taken up with a sterile syringe) were added to the liquid agar, which had been autoclaved separately. In order to disperse the nonylphenol to obtain turbidity, we intensively stirred the hot agar. All manipulations involving sterile media or autoclaved culture vials were done inside a sterile flow bench.
Due to its low solubility in aqueous media, most of the nonylphenol added to the culture medium remained as droplets on the liquid surface or on the walls of the culture vials. To monitor the amount of nonylphenol in a degradation experiment, we set up a series of identical vessels, with each being sacrificed on the appropriate day. Unless specified otherwise, nonsterile nonylphenol dissolved in n-hexane was added to empty, autoclaved culture vials (screw-cap cylindrical glass vials; 2.5 by 8.3 cm). The n-hexane was then evaporated in a sterile laminar flow bench, and sterile liquid medium (3 ml) was added. After inoculation, the screw caps were only loosely tightened to allow air diffusion. Regular culture vials and appropriate controls were incubated on a rotary shaker (240 rpm) at 25°C. The incubations were stopped by shock freezing at −80°C. The samples were then stored at −25°C until workup.
For degradation experiments, the medium was inoculated with 100 μl (3.33%) of a preculture of Sphingomonas xenophaga Bayram, unless stated otherwise. The precultures were maintained by incubating the bacteria on minimal medium (3 ml), which was generally amended with very small amounts of yeast extract and peptones (10 μg of each/ml) and with technical nonylphenol (1 mg/ml; ∼85% 4-nonylphenols) (Fluka) as the sole carbon source. Transfer (100 μl) to fresh medium was done regularly after 16 days, and the ages of the inocula varied according to the starting date of the experiment (6 to 11 days).
Analytical procedures.
The frozen cultures were thawed, acidified with 300 μl of 1 N HCl, and extracted twice with 2 ml of CH2Cl2, unless stated otherwise. Before concentrating the CH2Cl2 extract under a gentle flow of N2, we set aside an aliquot for GC-MS analysis. The dry extracts were dissolved in appropriate amounts of isopropanol and analyzed in duplicate by high-performance liquid chromatography (HPLC) with UV detection. The amounts of the individual 4-nonylphenol isomers were determined by external calibration and corrected for the withdrawal of the GC-MS aliquot (a separate calibration curve was used for each isomer). In a degradation experiment with 4-NP112 in minimal medium, 50 μl of an internal standard solution (0.5 mg of 4-n-nonylphenol in propan-2-ol) was added to the culture prior to CH2Cl2 extraction, and the HPLC-UV results were corrected for the efficacy of the CH2Cl2 extraction. The recovery of the internal standard ranged from 92 to 100%, with a mean value of 96% and a standard deviation of 2.9%.
For HPLC analysis, we used a Gynkotek HPLC system consisting of a model M480 gradient pump, a UV/VIS UVD3404 diode array detector, and a Gina 50 autosampler (Dionex, Olten, Switzerland). Samples of 20 μl were injected. All chromatographic separations were performed on a reversed-phase column protected by a precolumn (CC 250/4 and CC8/4 Nucleosil 100-5 C18 HD, respectively; Macherey-Nagel, Oensingen, Switzerland), with a flow rate of 1 ml min−1. Gradient systems I and II were used to quantify 4-NP112 and the isomer mixture, respectively. With gradient system I, the column was equilibrated for 5 min with solvent B (methanol [MeOH]-H2O [8:2]), and then a linear gradient to 100% solvent C (MeOH-H2O [9:1]) was applied over 10 min. Elution with 100% solvent C was maintained for 7 min. With gradient system II, the column was equilibrated for 5 min with solvent A (MeOH-H2O [7:3]), and then a linear gradient to 40% solvent C was applied over 17 min followed by a gradient to 100% solvent C over 8 min. Elution with 100% solvent C was maintained for 12 min followed by a linear gradient back to 100% solvent A over 3 min. With both gradient systems, 4-NP95 coeluted with 4-NP93.
The aliquots from the CH2Cl2 extracts were diluted appropriately with the same solvent and analyzed by GC-MS on a GC 8060 gas chromatograph (Fisons Instruments, Milan, Italy) directly coupled to an MD 800 quadrupole mass spectrometer (Fisons Instruments). The injector was operated in splitless mode at 270°C. Volumes of 2 μl were injected by an A200S autosampler (CTC Analytics, Zwingen, Switzerland). Separations were performed on a DB-17 MS capillary column (30 m long, 0.25-mm internal diameter, and 0.25-μm film thickness; J&W Scientific, Folsom, Calif.) linked to deactivated pre- and transfer columns (ca. 2 m each), with the following gradient temperature program: 40°C for 0 min, ramp 3°C/min, and 220°C for 10 min. Helium was used as a carrier gas, with a flow rate of 1.2 ml/min for all oven temperatures. The interface and source temperatures were set at 250 and 200°C, respectively. Detection was performed in the electron impact mode (70 eV), and data were acquired in the full scan mode from m/z 20 to 241.
Measurements of CFU, cell count, optical density, and dry weight.
Twenty-microliter aliquots of the cultures were diluted serially 1:10 with 0.9% NaCl. From appropriate dilutions, 20-μl samples were transferred to LB agar plates. CFU were counted after 3 days of incubation. Direct cell counts were determined in a counting chamber by a glycerol dilution technique. Sixty microliters of the 20-fold diluted liquid culture was mixed with 80 μl of glycerol solution (87%) and then added to the counting chamber (Thoma Assistant with a depth of 0.1 mm; Glaswarenfabrik Karl Hecht KG, Sondheim, Germany). Cells were counted at a magnification of 400-fold by use of a phase-contrast microscope equipped with a camera and a monitor screen.
Optical densities of cultures were measured at 546 nm by use of a Uvikon 860 photometer (Kontron AG, Zürich, Switzerland).
Six individual culture vials were pooled to determine the dry weight of the biomass. After centrifugation, the pellet was taken up in NaCl solution (0.85%), and the bacterial suspension was filtered through a preweighed filter (Nuclepore polycarbonate; Costar, Cambridge, Mass.) with a diameter of 25 mm and a pore size of 0.2 μm. The filter, with the biomass, was dried at 85°C for 72 h prior to weighing.
Enrichment and isolation of 4-nonylphenol-degrading S. xenophaga Bayram.
Enrichment started from activated sludge (3 ml) to which 4-NP112 (3 mg) had been added. This shaking culture was used to inoculate (100 μl) several vials containing minimal medium with 4-NP112 as the carbon source (3 mg in 3 ml). Further transfers to minimal medium were performed. The degradation of 4-nonylphenol was evaluated by observing the growth of bacteria (turbidity) and the disappearance of the nonylphenol droplets. After about eight transfers, shaking cultures were plated on minimal medium agar containing an emulsion of technical nonylphenol. Colonies with different morphologies were picked to inoculate into liquid minimal medium and were checked for 4-nonylphenol-degrading activity.
RESULTS
Isolation of S. xenophaga Bayram.
Sludge samples from a municipal wastewater treatment plant were incubated with the nonylphenol isomer 4-NP112 (0.3 mg/ml) to check for 4-nonylphenol-degrading activity. In such experiments, 4-NP112 disappeared nearly completely within 6 days of incubation.
To obtain pure cultures, we enriched nonylphenol-degrading bacteria by successive transfers. Cultures of stable lineages were plated on minimal medium agar plates with technical nonylphenol as the carbon source. Single colonies were picked and tested for 4-NP112-degrading activity. It appeared that even after several transfers in amendment-free liquid medium, a large majority of bacteria in the culture community did not degrade 4-nonylphenol. 4-Nonylphenol-degrading colonies finally revealed themselves by a transparent halo that developed in the turbid agar during 4 weeks of incubation. Under a binocular microscope, slightly yellow, large, distinctive colonies with diameters of about 1 mm could be observed. Such colonies were picked and transferred to agar plates of the same type, and eventually a 4-nonylphenol-degrading bacterium, designated strain Bayram, was isolated in pure form by picking one of the colonies under the view of the binocular microscope. Intensively yellow-orange colonies formed on LB agar.
The taxonomic identification of strain Bayram was performed by the German Collection of Microorganisms and Cell Cultures. The strain forms gram-negative rods with widths of 0.6 to 0.8 μm and lengths of 2.0 to 4.0 μm. The fatty acid profile was typical for the genus Sphingomonas, and the analyzed partial 16S rRNA gene was identical to that of S. xenophaga. The classification of the isolate as S. xenophaga Bayram agreed well with its physiological characteristics (25). In contrast to S. cloacae (9), Bayram was able to utilize d-glucose, l-arabinose, and maltose as growth substrates.
Degradation of 4-NP112 by strain Bayram.
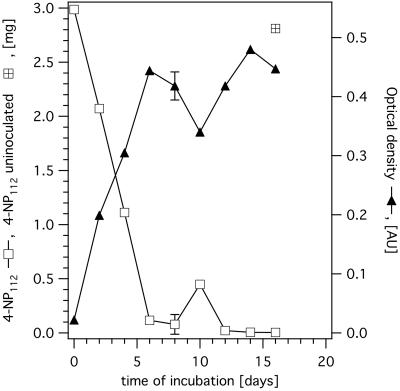
To show that the isolated strain was able to grow with 4-NP112 as the sole carbon source, we inoculated minimal medium (3 ml) supplemented with 1 mg of 4-NP112/ml with 50 μl of a 6-day-old culture (optical density, 0.50) grown on 4-NP112-containing minimal medium and then incubated this culture. The amount of nonylphenol and the optical density as well as the number of CFU and the direct cell count were monitored. Over time, the nonylphenol droplets on top of the medium became smaller and slowly developed a yellow-pink and then a red-brown coloration. This was not observed in the case of uninoculated controls. The medium itself remained colorless.
Most of the 4-NP112 (96%) disappeared within 6 days, while the optical density rose to a plateau of 0.44, which corresponded to a direct cell count of about 6.5 × 108/ml. After 14 days, only 5 μg (1.7‰) of the substrate remained (Fig. 1) (compared to 94% after 16 days for the uninoculated control).
FIG. 1.
Degradation of 4-NP112 by S. xenophaga Bayram in minimal medium. The error bars for day 8 represent the standard deviations (0.09 mg and 0.024 absorbance units [AU], respectively) for three independent measurements.
Unfortunately, the CFU could not be determined after day 4, since colonies no longer formed. At one specific time point (day 12), the dry weight was determined. The yield coefficient amounted to 0.11 mg of dry weight/mg of nonylphenol or 0.14 mg of dry weight/mg of nonylphenol carbon.
No trace of a metabolite could be detected by HPLC-UV. An analysis of the liquid CH2Cl2 extract by GC-MS showed the presence of 3,6-dimethylheptan-3-ol, the C9 alcohol with the same structure as the 4-NP112 alkyl chain.
Degradation of a mixture of five 4-nonylphenol isomers by S. xenophaga Bayram.
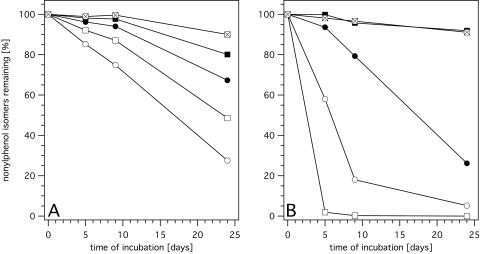
We set up experiments with five single isomers (1 mg/ml) and with an isomer mixture (1 mg of each isomer/ml) in vials with 3 ml of minimal medium to determine which 4-nonylphenol isomers could serve as growth substrates and to evaluate the influence of the structure of the nonyl moiety on the degradability of the various 4-nonylphenol isomers. Two isomers contained a strongly branched alkyl substituent (4-NP93 and 4-NP112), while the other three had a less ramified (4-NP9 and 4-NP2) or a linear (4-NP1) one (see Fig. 3).
In the mixture, all five isomers were transformed to a certain extent (Fig. 2A). Remarkably, the degradation rate proved to be higher and the disappearance of the substrate was more complete as the alkyl substituent was more strongly branched. It is noteworthy that the least branched isomers, 4-NP2 and 4-NP1, were also degraded to a certain extent; 0.6 mg of 4-NP2 (20%) and 0.3 mg of 4-NP1 (10%) disappeared within 24 days. In the uninoculated control, the amounts of all isomers remained constant (Fig. 2A).
FIG. 2.
Degradation of five nonylphenol isomers (4-NP93 [○], 4-NP112 [□], 4-NP9 [•], 4-NP2 [▪], and 4-NP1 [⊠]) by S. xenophaga Bayram in minimal medium. The isomers were added as a mixture (A) and as individual isomers (B) at a concentration of 1 mg/ml for each isomer. Recoveries of nonylphenol isomers from uninoculated controls were between 100 and 101% for experiment A and between 91 and 104% for experiment B.
Interestingly, when tested individually, 4-NP93 and 4-NP112 were transformed in the inverse order. The degradation of these isomers was fast, and the amounts that disappeared within 5 (1.3 and 2.94 mg, respectively) and 9 days (2.5 and 2.96 mg, respectively) (Fig. 2B) were markedly larger than the total amount of nonylphenol isomers degraded in the mixture (0.9 and 1.4 mg after 5 and 9 days, respectively) (Fig. 2A). In the experiment in which 4-NP9 was added as a single isomer, only a small percentage was transformed during the first few days, but the degradation accelerated, and about 65% of the compound disappeared between days 5 and 24. As for the vials with 4-NP2 and 4-NP1 as sole carbon sources, no bacterial growth was observed, and in contrast to the incubation of the mixture, no degradation occurred (Fig. 2B). The turbidity of the medium was regularly assessed by visual inspection and corresponded well to the amount of nonylphenol metabolized.
By means of HPLC-UV analyses, several potential polar metabolites were found in cultures with the isomer mixture. One of these metabolites was detected in the experiment with 4-NP2 but was not found in those with other isomers as single carbon sources. The experiment with the mixture was repeated with a lower substrate concentration (0.33 mg of each isomer/ml). The same order of degradation was observed. For this experiment, all of the liquid CH2Cl2 culture extracts were also analyzed by GC-MS. The GC-MS traces revealed the presence of three C9 alcohols, 2,3,5-trimethylhexan-2-ol (N93OH), 3,6-dimethylheptan-3-ol (N112OH), and 2-methyloctan-2-ol (N9OH). N9OH was only detected on days 9 and 24, whereas the other two C9 alcohols were also present on day 5. The GC-electron impact ionization-MS (GC-EI-MS) data are shown in Table 1.
TABLE 1.
GC-EI-MS data for the C9 alcohols formed during incubation of S. xenophaga Bayram with a mixture of five nonylphenol isomers (0.33 mg of each isomer/ml)
| Metabolite | m/z of prominent ions (relative abundance [%] and interpretation) |
|---|---|
| 2,3,5-Trimethyl-hexan-2-ol (N93OH) | 129 (2.9, M·+ − ·CH3), 111 (3.4, M·+ − ·CH3 − H2O), 87 (0.4), 85 (1.4), 83 (1.6), 73 (0.5), 71 (2.1), 70 (3.0), 69 (8.3), 67 (0.8), 60 (0.6), 59 (100, M·+ − ·C6H13), 57 (4.9), 55 (5.8), 53 (1.1), 45 (1.9), 44 (0.4), 43 (15.7), 41 (11.8, M·+ − ·C6H13 − H2O), 39 (3.2) |
| 3,6-Dimethyl-heptan-3-ol (N112OH) | 129 (4.8, M·+ − ·CH3), 115 (13.5, M·+ − ·C2H5), 111 (2.2, M·+ − ·CH3 −H2O), 98 (1.2), 97 (21.1, M·+ − ·C2H5 −H2O), 83 (1.5), 74 (2.4), 73 (100, M·+ − ·C5H11), 71 (5.2), 70 (4.2), 69 (22.8), 59 (4.4), 58 (3.3), 57 (17.9), 55 (58.2, M·+ − ·C5H11 − H2O), 53 (2.8), 45 (9.3), 43 (33.9), 41 (20.1), 39 (6.0) |
| 2-Methyl-octan-2-ol (N9OH) | 130 (0.5), 129 (8.0, M·+ − ·CH3), 111 (0.8, M·+ − ·CH3 −H2O), 85 (1.0), 83 (0.4), 71 (2.7), 70 (0.7), 69 (13.5), 67 (0.6), 60 (0.7), 59 (100, M·+ − ·C6H13), 57 (3.5), 56 (3.8), 55 (7.2), 53 (1.1), 45 (3.7), 44 (0.5), 43 (18.1), 41 (12.0, M·+ − ·C6H13 − H2O), 39 (3.2) |
DISCUSSION
A bacterium utilizing 4-(1-ethyl-1,4-dimethylpentyl)phenol (4-NP112), one of the main isomers of technical nonylphenol mixtures, as the sole carbon and energy source was isolated from activated sludge from a municipal wastewater treatment plant. The isolate, designated strain Bayram, was classified as an S. xenophaga strain. Different transfer procedures starting from several enrichment lineages always led to the same type of 4-nonylphenol-degrading colony, indicating that Bayram was probably the dominant bacterium in the activated sludge that was able to grow with 4-NP112 as the sole carbon source.
In minimal medium with 1 mg of 4-NP112/ml, Bayram transformed about 50% of the substrate within 3 days, and only 0.7% remained after 12 days. This degradation rate is similar to the one observed for the transformation of 4-(1-ethyl-1,3-dimethylpentyl)phenol (4-NP111, a mixture of two diastereoisomers; 1 mg/ml) by strain TTNP3 (3). In our hands, incubations of LB agar plates that were inoculated with appropriately diluted shaking cultures that were older than 4 days did not lead to colony formation. However, when the plates were inoculated with an undiluted culture by means of a loop, colonies still formed. Similar observations were made with S. xenophaga BN6T and S. xenophaga N,N, for which it was reported that cells rapidly lose the ability to form colonies on solid medium after reaching the stationary growth phase (25).
Our experiments clearly show that S. xenophaga Bayram differentially transforms five selected 4-nonylphenol isomers. The degradation rate showed a positive correlation with the branching degree of the alkyl substituent—the more highly branched the substituent, the faster the degradation. These findings are in contrast to what has been reported for the degradation of technical nonylphenol by a community of soil microorganisms (31) and by strain TTNP3 (3, 28). In those previous experiments, a preferential degradation of certain isomers could not be shown. However, the same authors observed that in an experiment with 4-(1-ethyl-1,3-dimethylpentyl)phenol (4-NP111) as the sole carbon source, strain TTNP3 consumed the substrate more rapidly than in another experiment with technical nonylphenol as the sole carbon source. Also, 4-NP111 was completely degraded, in contrast to technical nonylphenol, of which one-fifth remained (3). This is an indication that strain TTNP3 might also differentiate between isomers during degradation.
By means of GC-MS, the C9 alcohols N93OH (retention time, 11.2 min), N112OH (12.4 min), and N9OH (13.8 min), derived from the nonylphenol isomers 4-NP93 (46.0 min), 4-NP112 (46.2 min), and 4-NP9 (48.1 min), respectively, could be detected in an experiment in which a mixture of five isomers was incubated (Fig. 3). The alcohols N93OH and N9OH showed nearly identical EI-MS spectra, with a base peak at m/z 59. Both alcohols exhibit an α,α-dimethyl structure, and the major fragmentation corresponded to the loss of the C6H13 group attached to the α position. The experiments with 4-NP93 and 4-NP9 as single substrates, in which only N93OH and N9OH, respectively, were detected, provided confirmed assignments of the GC-MS signals. However, no traces of nonan-2-ol (N2OH) and nonan-1-ol (N1OH), potential metabolites derived from 4-NP2 and 4-NP1, could be detected in any experiment, although 4-NP2 and 4-NP1 were partially degraded, e.g., 35% of the 4-NP2 disappeared in the experiment in which the mixture (0.33 mg of each isomer/ml) was incubated. This indicates that the nonylphenols 4-NP2 and 4-NP1 are metabolized in a different way than the more highly branched isomers. We propose that 4-NP2 and 4-NP1 did engage in the main degradation pathway but were incompletely degraded, as they were unable to undergo the reaction in which the alkyl group was detached from the aromatic part of the molecule (Fig. 3). This hypothesis was corroborated by the detection of a UV-active metabolite that accumulated in the medium and that most likely derived from 4-NP2. Our data further indicate that the aromatic ring needs to be cleaved from the aliphatic substituent in order to be degraded and utilized. In our experiments, the alkyl chains of 4-NP2 and 4-NP1 were not released, so the bacterium was unable to utilize these isomers as growth substrates. Nevertheless, significant amounts of 4-NP2 and 4-NP1 were cometabolically transformed when they were supplied together with 4-NP93, 4-NP112, and 4-NP9, which did serve as growth substrates.
Like strain TTNP3 (3), Bayram probably cannot utilize the alkyl part of 4-nonylphenol as a source of carbon and energy. The yield coefficient of 0.14 mg of dry weight/mg of carbon was markedly lower than that reported for benzoate (1.0) (17), indicating that only part of the molecule was completely metabolized. Unfortunately, the experimental setup did not allow the determination of a complete mass balance.
On the one hand, an important factor determining whether the nonyl moiety is released as a C9 alcohol seems to be the presence of three alkyl groups at the benzylic position. However, the observation that Bayram did not grow with tert-butylphenol (data not shown) indicates that the sizes of the alkyl groups attached to the α-carbon atom are also crucial. We believe that three sufficiently large α-alkyl groups are needed to effectively stabilize the transiently activated α-carbon atom during the detachment of the nonyl moiety from the aromatic part. The activated α-carbon atom may be positively charged, negatively charged, or a radical species. Such an atom could then form an ether bond with a nucleophilic, electrophilic, or radical oxygen atom that had been previously introduced onto the aromatic ring. The ether bond would then finally be cleaved to release the respective C9 alcohol.
It has been shown by others that the branching at the α-carbon atom also greatly affects the estrogenicity of 4-alkylphenols (22). Compounds with a quaternary benzylic carbon atom are generally much more estrogenic than those with a tertiary or a secondary one (22). In a hypothetical mixture containing α-tri-, di-, and mono-alkyl-substituted 4-nonylphenol isomers, strain Bayram should more efficiently transform the threefold-substituted constituents, i.e., those that are more estrogenic. Hence, degradation should reduce the overall estrogenicity of the mixture.
Future studies will aim at investigating how the degradative activity of Bayram affects the estrogenicity of commercial nonylphenol mixtures and will try to elucidate the degradation pathways involved. For confirmation of the dominant role of the benzylic substitution in determining the degradation behavior of the various 4-nonylphenol isomers, experiments using isomers with alkyl groups that are structurally similar in the vicinity of the α-carbon atom but different farther away are planned.
Acknowledgments
This research was supported by the Swiss National Science Foundation within the framework of the National Research Programme “Endocrine Disruptors: Relevance to Humans, Animals, and Ecosystems.”
We thank H. Singer, A. Lück, and C. Schaffner (EAWAG) for technical help with the GC-MS apparatus and procedures and D. Rentsch, Swiss Federal Laboratories for Materials Testing and Research (EMPA; Dübendorf, Switzerland), for NMR measurements and analyses of the NMR spectra of the nonylphenol isomers.
REFERENCES
- 1.Ahel, M., W. Giger, and M. Koch. 1994. Behavior of alkylphenol polyethoxylate surfactants in the aquatic environment. I. Occurrence and transformation in sewage treatment. Water Res. 28:1131-1142. [Google Scholar]
- 2.Brunner, P. H., S. Capri, A. Marcomini, and W. Giger. 1988. Occurrence and behaviour of linear alkylbenzenesulphonates, nonylphenol, nonylphenol mono- and nonylphenol diethoxylates in sewage and sewage sludge treatment. Water Res. 22:1465-1472. [Google Scholar]
- 3.Corvini, P. F. X., R. Vinken, G. Hommes, B. Schmidt, and M. Dohmann. 2004. Degradation of the radioactive and non-labelled branched 4(3′,5′-dimethyl-3′-heptyl)-phenol nonylphenol isomer by Sphingomonas TTNP3. Biodegradation 15:9-18. [DOI] [PubMed] [Google Scholar]
- 4.Dachs, J., D. A. Van Ry, and S. J. Eisenreich. 1999. Occurrence of estrogenic nonylphenols in the urban and coastal atmosphere of the lower Hudson River estuary. Environ. Sci. Technol. 33:2676-2679. [Google Scholar]
- 5.Dutka, B. J., D. Liu, A. Jurkovic, R. McInnis, H.-B. Lee, F. Onuska, and S. S. Rao. 1998. Observations from a six month study on the effect of biodegradation processes in sediment on the toxicity potential of targeted chemicals. Environ. Toxicol. Water Qual. 13:313-322. [Google Scholar]
- 6.Ekelund, R., Å. Grandmo, K. Magnusson, and M. Berggren. 1993. Biodegradation of 4-nonylphenol in seawater and sediment. Environ. Pollut. 79:59-61. [DOI] [PubMed] [Google Scholar]
- 7.Field, J. A., and R. L. Reed. 1996. Nonylphenol polyethoxy carboxylate metabolites of nonionic surfactants in U.S. paper mill effluents, municipal sewage treatment plant effluents, and river waters. Environ. Sci. Technol. 30:3544-3550. [Google Scholar]
- 8.Fujii, K., N. Urano, H. Ushio, M. Satomi, H. Iida, N. Ushio-Sata, and S. Kimura. 2000. Profile of a nonylphenol-degrading microflora and its potential for bioremedial applications. J. Biochem. 128:909-916. [DOI] [PubMed] [Google Scholar]
- 9.Fujii, K., N. Urano, H. Ushio, M. Satomi, and S. Kimura. 2001. Sphingomonas cloacae sp. nov., a nonylphenol-degrading bacterium isolated from wastewater of a sewage-treatment plant in Tokyo. Int. J. Syst. Evol. Microbiol. 51:603-610. [DOI] [PubMed] [Google Scholar]
- 10.Fujita, Y., and M. Reinhard. 1997. Identification of metabolites from the biological transformation of the nonionic surfactant residue octylphenoxyacetic acid and its brominated analog. Environ. Sci. Technol. 31:1518-1524. [Google Scholar]
- 11.Giger, W., P. H. Brunner, and C. Schaffner. 1984. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science 225:623-625. [DOI] [PubMed] [Google Scholar]
- 12.Giger, W., E. Stephanou, and C. Schaffner. 1981. Persistent organic chemicals in sewage effluents. I. Identifications of nonylphenols and nonylphenolethoxylates by glass capillary gas chromatography/mass spectrometry. Chemosphere 10:1253-1263. [Google Scholar]
- 13.Jobling, S., and J. P. Sumpter. 1993. Detergent components in sewage effluent are weakly oestrogenic to fish: an in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 27:361-372. [Google Scholar]
- 14.Kim, Y.-S., T. Katase, S. Sekine, T. Inoue, M. Makino, T. Uchiyama, Y. Fujimoto, and N. Yamashita. 2004. Variation in estrogenic activity among fractions of a commercial nonylphenol by high performance liquid chromatography. Chemosphere 54:1127-1134. [DOI] [PubMed] [Google Scholar]
- 15.Kvestak, R., S. Terzic, and M. Ahel. 1994. Input and distribution of alkylphenol polyethoxylates in a stratified estuary. Mar. Chem. 46:89-100. [Google Scholar]
- 16.Laskin, A. I., and D. C. White. 1999. Preface to special issue Sphingomonas. J. Ind. Microbiol. Biotechnol. 23:231. [DOI] [PubMed] [Google Scholar]
- 17.Linton, J. D., and R. J. Stephenson. 1978. A preliminary study on growth yields in relation to the carbon and energy content of various organic growth substrates. FEMS Microbiol. Lett. 3:95-98. [Google Scholar]
- 18.McLeese, D. W., V. Zitko, C. D. Metcalfe, and D. B. Sergeant. 1980. Lethality of Aminocarb and the components of the Aminocarb formulation to juvenile Atlantic salmon, marine invertebrates and a freshwater clam. Chemosphere 9:79-82. [Google Scholar]
- 19.McLeese, D. W., V. Zitko, D. B. Sergeant, L. Burridge, and C. D. Metcalfe. 1981. Lethality and accumulation of alkylphenols in aquatic fauna. Chemosphere 10:723-730. [Google Scholar]
- 20.Potter, T. L., K. Simmons, J. Wu, M. Sanchez-Olivera, P. Kostecki, and E. Calabrese. 1999. Static die-away of a nonylphenol ethoxylate surfactant in estuarine water samples. Environ. Sci. Technol. 33:113-118. [Google Scholar]
- 21.Routledge, E. J., and J. P. Sumpter. 1996. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. 15:241-248. [Google Scholar]
- 22.Routledge, E. J., and J. P. Sumpter. 1997. Structural features of alkylphenolic chemicals associated with estrogenic activity. J. Biol. Chem. 272:3280-3288. [DOI] [PubMed] [Google Scholar]
- 23.Servos, M. R. 1999. Review of the aquatic toxicity, estrogenic responses and bioaccumulation of alkylphenols and alkylphenol polyethoxylates. Water Qual. Res. J. Can. 34:123-177. [Google Scholar]
- 24.Soto, A. M., H. Justicia, J. W. Wray, and C. Sonnenschein. 1991. p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ. Health Perspect. 92:167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolz, A., C. Schmidt-Maag, E. B. M. Denner, H.-J. Busse, T. Egli, and P. Kämpfer. 2000. Description of Sphingomonas xenophaga sp. nov. for strains BN6T and N,N which degrade xenobiotic aromatic compounds. Int. J. Syst. Evol. Microbiol. 50:35-41. [DOI] [PubMed] [Google Scholar]
- 26.Tanghe, T., G. Devriese, and W. Verstraete. 1998. Nonylphenol degradation in lab scale activated sludge units is temperature dependent. Water Res. 32:2889-2896. [Google Scholar]
- 27.Tanghe, T., W. Dhooge, and W. Verstraete. 2000. Formation of the metabolic intermediate 2,4,4,-trimethyl-2-pentanol during incubation of a Sphingomonas sp. strain with the xeno-estrogenic octylphenol. Biodegradation 11:11-19. [DOI] [PubMed] [Google Scholar]
- 28.Tanghe, T., W. Dhooge, and W. Verstraete. 1999. Isolation of a bacterial strain able to degrade branched nonylphenol. Appl. Environ. Microbiol. 65:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiele, B., K. Günther, and M. J. Schwuger. 1997. Alkylphenol ethoxylates: trace analysis and environmental behavior. Chem. Rev. 97:3247-3272. [DOI] [PubMed] [Google Scholar]
- 30.Thiele, B., V. Heinke, E. Kleist, and K. Guenther. 2004. Contribution to the structural elucidation of 10 isomers of technical p-nonylphenol. Environ. Sci. Technol. 38:3405-3411. [DOI] [PubMed] [Google Scholar]
- 31.Topp, E., and A. Starratt. 2000. Rapid mineralization of the endocrine-disrupting chemical 4-nonylphenol in soil. Environ. Toxicol. Chem. 19:313-318. [Google Scholar]
- 32.Wheeler, T. F., J. R. Heim, M. R. LaTorre, and A. B. Janes. 1997. Mass spectral characterization of p-nonylphenol isomers using high-resolution capillary GC-MS. J. Chromatogr. Sci. 35:19-30. [Google Scholar]
- 33.White, G. F. 1993. Bacterial biodegradation of ethoxylated surfactants. Pestic. Sci. 37:159-166. [Google Scholar]
- 34.White, G. F., N. J. Russell, and E. C. Tidswell. 1996. Bacterial scission of ether bonds. Microbiol. Rev. 60:216-232. [DOI] [PMC free article] [PubMed] [Google Scholar]