Key Points
-
•
NGS MRD evaluation in B-cell precursor acute lymphoblastic leukemia is highly concordant with qPCR while being providing more specific results.
-
•
Frontline NGS MRD evaluation is a viable alternative to qPCR assays in future MRD-based acute lymphoblastic leukemia treatment protocols.
Visual Abstract
Abstract
We compared minimal/measurable residual disease (MRD) levels evaluated by routinely used real-time quantitative polymerase chain reaction (qPCR) patient-specific assays and by next-generation sequencing (NGS) approach in 780 immunoglobulin (IG) and T-cell receptor (TR) markers in 432 children with B-cell precursor acute lymphoblastic leukemia treated on the AIEOP-BFM ALL 2009 protocol. Our aim was to compare the MRD-based risk stratification at the end of induction. The results were concordant in 639 of 780 (81.9%) of these markers; 37 of 780 (4.7%) markers were detected only by NGS. In 104 of 780 (13.3%) markers positive only by qPCR, a large fraction (23/104; 22.1%) was detected also by NGS, however, owing to the presence of identical IG/TR rearrangements in unrelated samples, we classified those as nonspecific/false-positive. Risk group stratification based on the MRD results by qPCR and NGS at the end of induction was concordant in 76% of the patients; 19% of the patients would be assigned to a lower risk group by NGS, largely owing to the elimination of false-positive qPCR results, and 5% of patients would be assigned to a higher risk group by NGS. NGS MRD is highly concordant with qPCR while providing more specific results and can be an alternative in the front line of MRD evaluation in forthcoming MRD-based protocols.
Two articles focus on the use of next generation sequencing (NGS) for the assessment of minimal residual disease (MRD). In the first article, Hengeveld et al report on a novel, academically developed immunoglobulin sequence-based NGS assay for quantitative detection of MRD in chronic lymphocytic leukemia (CLL). This technique, made available to other laboratories, has a range of detection that is more sensitive than conventional techniques and provides accurate prognostic stratification of patients with CLL. In the second article, Svaton et al compared allele-specific oligonucleotide polymerase chain reaction to NGS assay of immunoglobulin or T-cell receptor markers in pediatric B-cell acute lymphoblastic leukemia (ALL), again confirming that this is a reliable alternative for assessing MRD.
Introduction
Together with multiparameter flow cytometry, quantitation of clonal immunoglobulin (IG) and T-cell receptor (TR) gene rearrangements represent the current standard for the detection of minimal/measurable residual disease (MRD) in treatment protocols for pediatric patients with acute lymphoblastic leukemia (ALL). The increasing availability of next-generation sequencing (NGS) has permitted its growing use for MRD detection in lymphoid malignancies, and the methodology for NGS MRD detection has been published by our group and others, demonstrating its relevance in clinical use.1, 2, 3, 4, 5, 6, 7, 8 However, one of the main unresolved questions is the accuracy of MRD quantification and the correlation with patient-specific quantitative polymerase chain reaction (qPCR), which is currently used for risk stratification in most European ALL treatment protocols for children and adults. Our goal was to determine whether NGS can be used successfully for MRD-based risk stratification in the same way as qPCR on day 33 (end of induction), as the principal stratification time point, in a cohort of children with B-cell precursor ALL treated on the AIEOP-BFM ALL 2009 protocol.
Study design
In total, 458 patients treated on the AIEOP-BFM ALL 2009 protocol diagnosed with B-cell precursor ALL in the Czech Republic between 2010 and 2018 were included in this study. We excluded 19 patients who either had no usable IG/TR MRD marker identified at diagnosis with the required sensitivity of 10−4 detectable by qPCR or for whom DNA from day-33 bone marrow aspirate was not available (Figure 1A). The study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Second Faculty of Medicine, Charles University, Prague, Czech Republic.
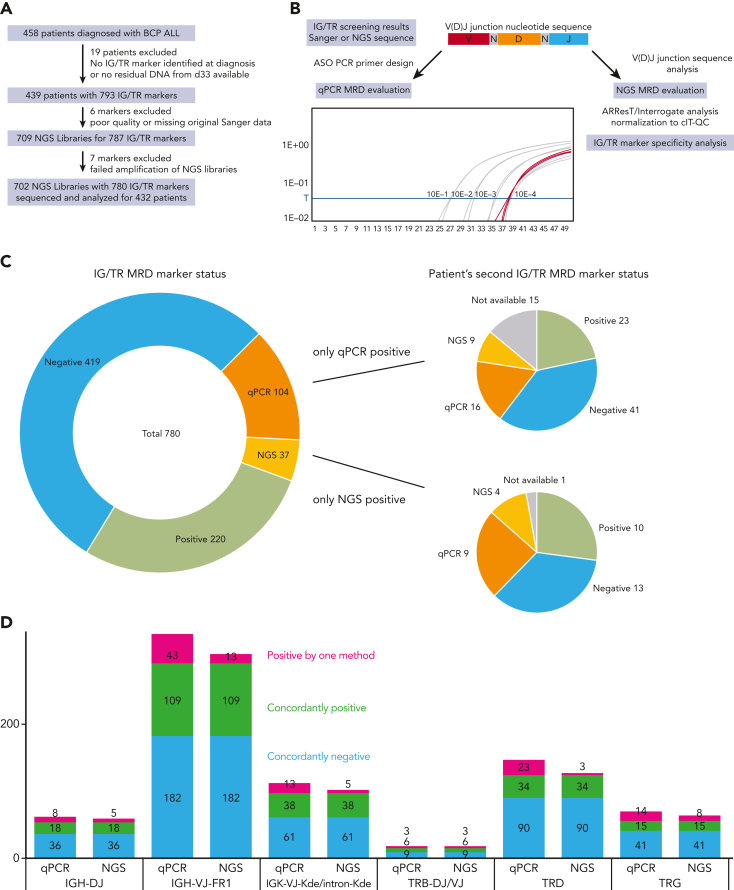
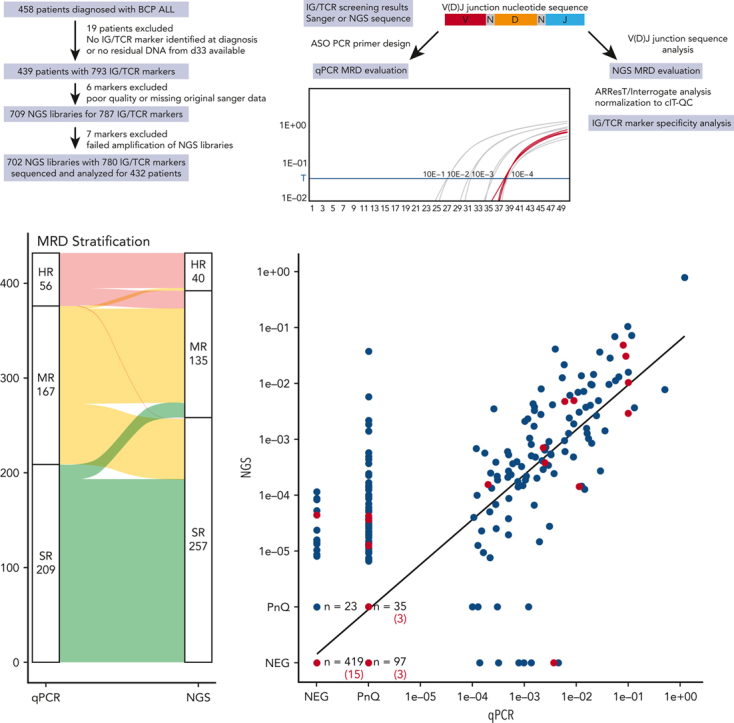
Figure 1.
Schematic diagram of the study and classification of the MRD markers by qPCR and NGS. (A) Flow diagram of the study showing the exclusion criteria and the number of patients and samples analyzed in the study. (B) Diagram of the IG/TR MRD marker selection process and analysis from the time of diagnosis to the MRD evaluation at day 33 using both qPCR and NGS. (C) Results of IG/TR MRD marker analysis by qPCR and NGS. Markers positive only by qPCR (orange) or only by NGS (yellow) are broken down into subcategories based on the results of the second MRD marker evaluated for the same patient and marked by the same colors or gray, if the second MRD marker was not available. (D) Breakdown of all the evaluated MRD markers with their results based on the genetic locus of the IG/TR rearrangement and NGS library preparation primer mix used. Owing to a small number of samples, the IGK-VJ-Kde and intron-Kde were merged together as were TRB-DJ and TRB-VJ rearrangements. cIT-QC, central in-tube quality/quantification control; V(D)J, variable diversity joining.
IG/TR MRD markers were identified according to the EuroMRD guidelines as described previously,9, 10, 11, 12, 13, 14, 15 and ideally 2 independent qPCR assays with the best sensitivity and quantitative range (QR) were used for MRD evaluation. DNA samples isolated from bone marrow aspirates taken at day 33 (Figure 1B) were evaluated and MRD positivity below the QR was assessed as positive nonquantifiable (PnQ).2,16 NGS libraries were prepared according to the EuroClonality-NGS Working Group protocols.17 From the total of 709 NGS libraries, 702 (99%) were successfully amplified and sequenced and only 7 had to be excluded owing to errors in NGS library preparation (Figure 1A).
Sequencing data were analyzed using an in-house data processing pipeline and further evaluated using the ARResT/Interrogate pipeline.18 NGS MRD results were normalized and quantified based on the EuroClonality-NGS central in-tube quality/quantification control19 and for the total DNA sample input for each library. NGS MRD levels below the theoretical QR were evaluated as PnQ. V(D)J junction nucleotide sequences were extracted from the original Sanger sequencing data and used for the identification of patient-specific IG/TR MRD markers in the NGS libraries (Figure 1B). A minimum of 3 reads with the corresponding sequence were required for any NGS positivity.
Results and discussion
We sequenced a total of 702 NGS libraries with a median coverage of 1 629 389 reads and evaluated 780 unique IG/TR MRD markers of 432 patients by both qPCR and NGS. Because the sensitivity and specificity of the qPCR MRD assays are strongly influenced by the characteristics of each selected IG/TR marker and the complexity of its V(D)J junction, their usability for MRD evaluation is determined by criteria based on nonspecific amplification in a polyclonal nonmalignant background. To ascertain the specificity of all selected markers for NGS MRD detection, we analyzed their junctional nucleotide sequences and determined their specificity as described in the supplemental Methods (available on the Blood website), and identified 54 markers that did not meet the criteria of sufficient specificity.
We obtained concordant results for 419 negative markers (53.7%) and 220 positive markers (28.2%) by both methods. Another 104 markers (13.3%) were positive only by qPCR, a vast majority of them (97; 93.3%) were PnQ, with nonspecific amplification of the polyclonal controls used in the qPCR evaluation observed with 55 of these markers (52.9%). More importantly, although 23 out of these 104 markers (22.1%) were also detected using the NGS approach, they were classified as nonspecific (as described above) and their poor specificity was further supported by nonspecific qPCR amplification of the polyclonal controls, albeit with higher Ct values (not affecting qPCR MRD positivity as per the EuroMRD guidelines) in 18 of them. Similarly, 23 out of the 37 markers (62%) positive only by NGS were PnQ, and the mean NGS MRD value of the remaining 14 quantifiable markers was 3.62 × 10−5 (Figure 1C-D).
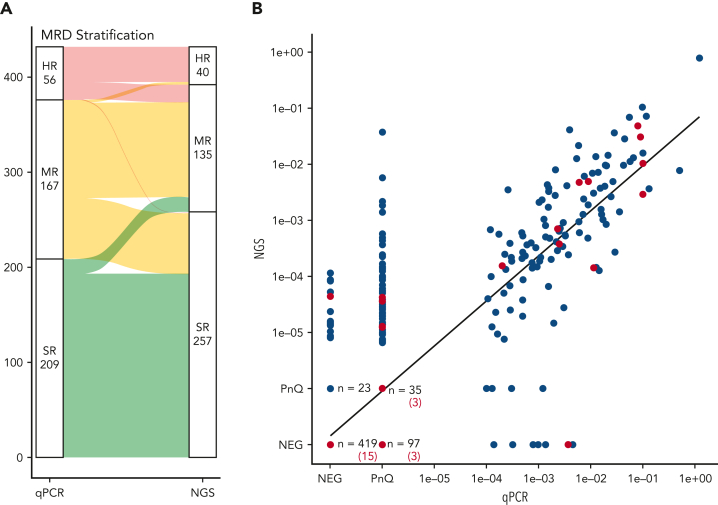
The stratification of patients based on day-33 MRD results was concordant in 76% of the patients by both qPCR and NGS, with 193 patients (44.7%) assigned to the standard risk (SR) group, 100 patients (23.1%) to the medium risk (MR) group, and 37 (8.6%) to the high risk (HR) group (Figure 2A). A change in assignment to a higher risk group according to the NGS result occurred in 19 patients (4.4%) and to a lower risk group in 82 patients (19%), mainly owing to the elimination of false-positive results (Figure 2A). One patient was assigned to the SR group by NGS, having only 1 T-cell receptor gamma (TRG) MRD marker that was highly positive by qPCR and not detected in the NGS data. However, a major clonal TRG rearrangement identified upon NGS rescreening confirmed the HR stratification of this patient (supplemental Results).
Figure 2.
Patient MRD-based risk stratification and quantitative comparison of the MRD markers. (A) Alluvial plot of the risk stratification of all patients based on their qPCR or NGS MRD levels at day 33 with the threshold MRD value of 10−3 used for HR MRD stratification in the AIEOP-BFM ALL 2009 protocol. A change in assignment to a higher risk group based on the NGS MRD result occurred for 16 patients (3.7%) from the SR to the MR group, and for 3 patients (0.7%) from the MR to the HR group. An opposite change from the MR to the SR group occurred for 64 patients (14.8%) and from the HR group to the MR group for 18 patients (4.2%). (B) Comparison of the MRD levels as measured by qPCR (x-axis) or NGS (y-axis). All markers of patients that relapsed are marked in red. NEG, negative.
We also observed a significant correlation of the calculated MRD values by both methods (R = 0.83, Figure 2B). Considering the differences in MRD quantitation between qPCR and NGS, a perfect numerical correlation of both methods cannot be expected. In addition, NGS MRD also offers a much higher QR than the traditionally used qPCR assays and is therefore more informative.
Even though the focus on high sensitivity of MRD detection is often emphasized in commercially available assays, the true sensitivity of any MRD assay is determined by the number of evaluated cells20 and the desired sensitivity is not reached for a large number of samples evaluated in MRD studies (eg, 40% of samples reported by Wood et al).6 It is equally important to maintain a very high specificity and implement sufficient checks to avoid false-positive results that would lead to therapy intensification and may be the cause of serious treatment-related toxicity, especially in pediatric patients. We analyzed the V(D)J junction segmentation of all 54 nonspecific IG/TR markers with the majority belonging to the immunoglobulin kappa (IGK), T-cell receptor delta (TRD), and TRG gene loci and found that the N regions of their junctions are significantly shorter (supplemental Results), thus, challenging their prioritization during the selection process for patient-specific qPCR system design.21
Because of its higher specificity compared with the qPCR assays, thus, eliminating the falsely positive qPCR results with high confidence, NGS improves the correct risk stratification of patients with undetectable MRD into the SR group in the setting of an MRD-based clinical protocol. These patients can then be safely considered for reduction of treatment. In contrast, even very low MRD levels detected specifically by NGS must be considered unambiguously as a true value and the patients should be treated with according intensity.7,8 Frontline NGS MRD evaluation as developed by the EuroClonality-NGS Working Group can be used as an alternative to traditional qPCR-based MRD quantitation in future MRD-based treatment protocols. Moreover, this method can be immediately implemented in off-protocol treatment monitoring and independent evaluation of PnQ results of qPCR, typically after stem cell transplant.
Conflict-of-interest disclosure: M.B. received personal fees from Incyte (advisory board) and Roche Pharma AG, financial support for reference diagnostics from Affimed and Regeneron, grants and personal fees from Amgen (advisory board, speakers bureau, and travel support), and personal fees from Janssen (speaker’s bureau), all outside of the submitted work. The remaining authors declare no competing financial interests.
Acknowledgments
This work was supported by grants from the Charles University (project GA UK No. 534120) and the Ministry of Health of the Czech Republic (NU20-03-00284). Institutional support was provided by the project National Institute for Cancer Research (Project No. LX22NPO5102), funded by the European Union–Next Generation EU.
Authorship
Contribution: M.S. and A.S. performed the research, analyzed the data, and wrote the manuscript; L.R., A.R., and T.V. performed the research and analyzed the data; N.D. provided critical analytical tools for data analysis; M.K., V.H.J.v.d.V., M.B., and A.W.L. provided critical reagents, interpreted the data, and reviewed and edited the manuscript; J.Z. and J.S. contributed and interpreted the data; J.T. and E.F. designed and supervised the study, interpreted the data, and wrote the manuscript; and all authors revised and approved the final version of the manuscript.
Footnotes
Data are available on request from author, Michael Svaton (michael.svaton@lfmotol.cuni.cz). All NGS data are publicly available in the Sequence Read Archive database under the accession PRJNA892500.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Kotrova M, Muzikova K, Mejstrikova E, et al. The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL. Blood. 2015;126(8):1045–1047. doi: 10.1182/blood-2015-07-655159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotrova M, van der Velden VHJ, van Dongen JJM, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant. 2017;52(7):962–968. doi: 10.1038/bmt.2017.16. [DOI] [PubMed] [Google Scholar]
- 3.Pulsipher MA, Carlson C, Langholz B, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015;125(22):3501–3508. doi: 10.1182/blood-2014-12-615757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Jiang N, Lim EH, et al. Identifying IGH disease clones for MRD monitoring in childhood B-cell acute lymphoblastic leukemia using RNA-Seq. Leukemia. 2020;34(9):2418–2429. doi: 10.1038/s41375-020-0774-4. [DOI] [PubMed] [Google Scholar]
- 5.Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–5180. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood B, Wu D, Crossley B, et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood. 2018;131(12):1350–1359. doi: 10.1182/blood-2017-09-806521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulsipher MA, Han X, Maude SL, et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov. 2022;3(1):66–81. doi: 10.1158/2643-3230.BCD-21-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotrová M, Koopmann J, Trautmann H, et al. Prognostic value of low-level MRD in adult acute lymphoblastic leukemia detected by low- and high-throughput methods. Blood Adv. 2022;6(10):3006–3010. doi: 10.1182/bloodadvances.2021006727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pongers-Willemse M, Seriu T, Stolz F, et al. Primers and protocols for standardized detection of minimal residual disease in acute lymphoblastic leukemia using immunoglobulin and T cell receptor gene rearrangements and TAL1 deletions as PCR targets: report of the BIOMED-1 CONCERTED ACTION: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13(1):110–118. doi: 10.1038/sj.leu.2401245. [DOI] [PubMed] [Google Scholar]
- 10.van Dongen JJM, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 11.Pongers-Willemse M, Verhagen O, Tibbe G, et al. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia. 1998;12(12):2006–2014. doi: 10.1038/sj.leu.2401246. [DOI] [PubMed] [Google Scholar]
- 12.Langerak AW, Wolvers-Tettero ILM, van Gastel-Mol EJ, Oud MECM, van Dongen JJM. Basic helix-loop-helix proteins E2A and HEB induce immature T-cell receptor rearrangements in nonlymphoid cells. Blood. 2001;98(8):2456–2465. doi: 10.1182/blood.v98.8.2456. [DOI] [PubMed] [Google Scholar]
- 13.van der Velden V, Wijkhuijs J, Jacobs D, van Wering E, van Dongen J. T cell receptor gamma gene rearrangements as targets for detection of minimal residual disease in acute lymphoblastic leukemia by real-time quantitative PCR analysis. Leukemia. 2002;16(7):1372–1380. doi: 10.1038/sj.leu.2402515. [DOI] [PubMed] [Google Scholar]
- 14.van der Velden V, Willemse M, van der Schoot C, et al. Immunoglobulin kappa deleting element rearrangements in precursor-B acute lymphoblastic leukemia are stable targets for detection of minimal residual disease by real-time quantitative PCR. Leukemia. 2002;16(5):928–936. doi: 10.1038/sj.leu.2402475. [DOI] [PubMed] [Google Scholar]
- 15.Verhagen O, Willemse M, Breunis W, et al. Application of germline IGH probes in real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia. Leukemia. 2000;14(8):1426–1435. doi: 10.1038/sj.leu.2401801. [DOI] [PubMed] [Google Scholar]
- 16.van der Velden VHJ, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21(4):604–611. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
- 17.Brüggemann M, Kotrová M, Knecht H, et al. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia. 2019;33(9):2241–2253. doi: 10.1038/s41375-019-0496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bystry V, Reigl T, Krejci A, et al. ARResT/Interrogate: an interactive immunoprofiler for IG/TR NGS data. Bioinformatics. 2017;33(3):435–437. doi: 10.1093/bioinformatics/btw634. [DOI] [PubMed] [Google Scholar]
- 19.Knecht H, Reigl T, Kotrová M, et al. Quality control and quantification in IG/TR next-generation sequencing marker identification: protocols and bioinformatic functionalities by EuroClonality-NGS. Leukemia. 2019;33(9):2254–2265. doi: 10.1038/s41375-019-0499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen MH, Cédile O, Larsen TS, Abildgaard N, Nyvold CG. Perspective: sensitive detection of residual lymphoproliferative disease by NGS and clonal rearrangements-how low can you go? Exp Hematol. 2021;98:14–24. doi: 10.1016/j.exphem.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Theunissen PMJ, de Bie M, van Zessen D, et al. Next-generation antigen receptor sequencing of paired diagnosis and relapse samples of B-cell acute lymphoblastic leukemia: Clonal evolution and implications for minimal residual disease target selection. Leuk Res. 2019;76:98–104. doi: 10.1016/j.leukres.2018.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.