Key Points
-
•
The development of EBV-associated infectious mononucleosis depends on the host HLA-E allele and HLA-E–restricted CD8+ T-cell responses.
-
•
The risk for EBV+PTLD is highly associated with the HLA-E/LMP-1/NKG2A axis and depends on specific EBV and host variations in this pathway.
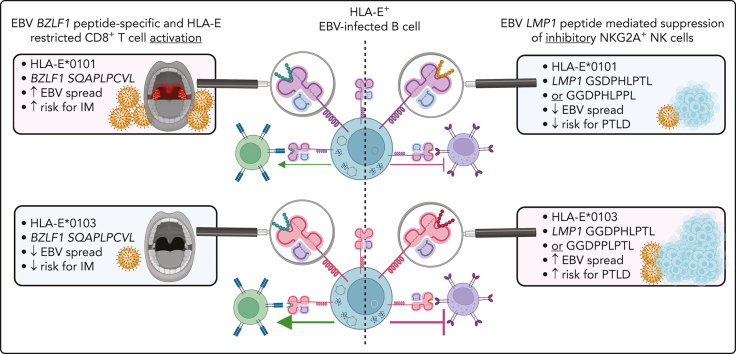
Visual Abstract
Abstract
Primary Epstein-Barr virus (EBV) infections may cause infectious mononucleosis (IM), whereas EBV reactivations in solid organ and hematopoietic stem cell transplant recipients are associated with posttransplantation lymphoproliferative disorders (PTLDs). It is still unclear why only a minority of primary EBV-infected individuals develop IM, and why only some patients progress to EBV+PTLD after transplantation. We now investigated whether nonclassic human leukocyte antigen E (HLA-E)–restricted immune responses have a significant impact on the development of EBV diseases in the individual host.
On the basis of a large study cohort of 1404 patients and controls as well as on functional natural killer (NK) and CD8+ T-cell analyses, we could demonstrate that the highly expressed HLA-E∗0103/0103 genotype is protective against IM, due to the induction of potent EBV BZLF1-specific HLA-E–restricted CD8+ T-cell responses, which efficiently prevent the in vitro viral dissemination.
Furthermore, we provide evidence that the risk of symptomatic EBV reactivations in immunocompetent individuals as well as in immunocompromised transplant recipients depends on variations in the inhibitory NKG2A/LMP-1/HLA-E axis. We show that EBV strains encoding for the specific LMP-1 peptide variants GGDPHLPTL or GGDPPLPTL, presented by HLA-E, elicit strong inhibitory NKG2A+ NK and CD8+ T-cell responses. The presence of EBV strains encoding for both peptides was highly associated with symptomatic EBV reactivations. The further progression to EBV+PTLD was highly associated with the presence of both peptide-encoding EBV strains and the expression of HLA-E∗0103/0103 in the host. Thus, HLA-E–restricted immune responses and the NKG2A/LMP-1/HLA-E axis are novel predictive markers for EBV+PTLD in transplant recipients and should be considered for future EBV vaccine design.
Over 95% of the population has been infected by Epstein-Barr virus, but why only some patients develop symptomatic infectious mononucleosis (IM) and why only a small subset of patients develop posttransplant lymphoproliferative disease (PTLD) after stem cell transplantation are unknown. Vietzen et al describe an interplay of genetic determinants in host and virus involving variants in host HLA-E that interact with specific latent membrane protein 1 peptide variants to modulate T-cell and natural killer cell responses that predict for IM and PTLD.
Introduction
Epstein-Barr virus (EBV) infects >90% of the adult human population worldwide. Primary EBV infection may result in an infectious mononucleosis (IM), which is hallmarked by fever, lymphadenopathy, and tonsillitis. In the absence of a licensed EBV vaccine, cases of IM result in a high economic burden for public health services.1 However, the cumulative risk of developing IM on primary EBV infection is estimated to be only between 13.3% and 22.4%,2 and it is so far an unresolved question why during primary EBV infection some patients develop clinically evident IM, whereas others remain asymptomatic.
After primary infection, EBV establishes a persistent infection in B cells, from which sporadic reactivations may occur. EBV is associated with the development of malignant diseases, resulting worldwide in >137 900 annual deaths.3 In solid organ and hematopoietic stem cell transplant recipients, EBV may cause posttransplant lymphoproliferative disorders (EBV+PTLDs), which are associated with poor survival.4 As these complications occur only in a part of the EBV-seropositive patients, it was hypothesized that there are distinct, individually determined factors in the human immune response that may control EBV replication and eliminate EBV infected and transformed cells.
The EBV-specific immune responses are hallmarked by potent cytotoxic CD8+ T and natural killer (NK) cell responses.5, 6, 7 Among the broad EBV-specific CD8+ T-cell responses, a small subset of CD8+ T cells bind with their αβ T-cell receptor to the nonclassic human leukocyte antigen (HLA) molecule, HLA-E.8 HLA-E is highly conserved in European populations, and only 2 allelic variants, the high-expressing HLA-E∗0103 and the low-expressing HLA-E∗0101, are prevalent.9 The limited polymorphism results in a restricted set of EBV-derived peptides, which can be presented via HLA-E on the surface of EBV-infected cells. It was shown that HLA-E is stabilized by the conserved EBV-encoded BZLF1 peptide or by polymorphic EBV LMP-1–derived peptides.10 HLA-E further binds to the inhibitory NKG2A receptor, which is expressed on distinct CD8+ T and NK cell subsets. By their peptides presented via HLA-E, EBV infections elicit the expansion of NKG2A+ NK cells, an NK cell subset that responds to EBV-infected cells by the secretion of proinflammatory cytokines and cellular cytotoxicity.6,11
As HLA-E–mediated immune responses are key factors in antiviral immune responses,9,12, 13, 14, 15 we hypothesized in the present study that EBV-specific, HLA-E–restricted CD8+ T– and HLA-E–mediated NKG2A+ cell responses play an important role in the control of EBV infections and in the prevention of EBV-associated diseases. By combining genetic association approaches with functional NK and CD8+ T-cell activation and EBV-dissemination assays, we could demonstrate that HLA-E–restricted immune responses have a substantial impact in mediating protection from IM and EBV+PTLD.
Patients and methods
In our study, 1404 individuals were included, who were diagnosed for their EBV status and EBV-associated diseases as specified in the supplemental Patients and methods, available on the Blood website. The cohort consisted of 578 patients with IM; 206 EBV-seropositive individuals, who had never experienced IM; 28 older persons with symptomatic EBV reactivation; and 180 transplant recipients with symptomatic EBV reactivation, 36 of these with EBV+PTLD. In addition, 412 healthy controls were included, independent of the EBV status. The HLA-E, LMP-1, and BZLF1 genotyping, functional immunologic assays, and the statistical analyses are provided in detail in the supplemental Patients and methods. All voluntary blood donors provided informed consent under the Declaration of Helsinki guidelines and agreed to sample collection, storage, and analysis. The study was approved by the ethics committee of the Medical University of Vienna (institutional review board number 1159/2022).
Results
The low-expressing HLA-E∗0101/0101 variant is overrepresented in patients with IM
To analyze the impact of HLA-E–restricted immune responses on the prevention of clinically evident IM, we first compared the prevalence of the HLA-E variants between patients with IM and EBV-seropositive persons who had, despite a past EBV infection, no history of IM. We recruited 578 patients with acute IM, 412 adolescent or adult and 166 pediatric subjects. IM was diagnosed clinically by the triad of fever, lymphadenopathy, and tonsillitis and was confirmed serologically by the detection of EBV–viral-capsid antigen (VCA)–specific IgM, in the absence of EBV–EBV nuclear antigen 1–specific IgG antibodies. In all patients with IM, EBV DNA was detected in plasma. Furthermore, we recruited 206 EBV-IgG–positive, VCA-IgM–negative healthy persons, who had a past but clinically inapparent EBV infection (asymptomatic EBV-seropositive individuals). These individuals were matched to the adolescent/adult cohort with IM in regard to age and sex (Table 1). In addition, we included a control cohort of 412 persons with unknown EBV serostatus, also matched to the adolescent/adult cohort with IM, to reflect the overall distribution of HLA-E∗0101/0103 variants in the population (control).
Table 1.
Characteristics of the study cohort
| Characteristic | Study cohort |
|||||
|---|---|---|---|---|---|---|
| Primary EBV infection |
EBV reactivation |
|||||
| Immunocompetent |
Immunocompromised Transplant recipients |
|||||
| Asymptomatic EBV-seropositive patients (N = 206) | Adolescent/adult patients with IM (N = 412) | Pediatric patients with IM (N = 166) |
Older patients (N = 28) | Patients without PTLD (N = 144) | Patients with EBV+PTLD (N = 36) | |
| Female sex, No. (%) | 90 (43.6) | 181 (43.9) | 73 (43.9) | 11 (39.2) | 61 (42.4) | 14 (38.9) |
| Age, median (min-max), y | 22.5 (18-40) | 22.1 (15-35) | 5.8 (1-14) | 71.7 (60-92) | 37.8 (14-91) | 30.2 (14-74) |
| EBV status | ||||||
| EBV– RT-qPCR | Neg. | Pos. | Pos. | Pos. | Pos. | Pos. |
| VCA IgM | Neg. | Pos. | Pos. | Pos. | Pos. | Pos. |
| VCA IgG | Pos. | Pos. | Pos. | Pos. | Pos. | Pos. |
| EBNA IgG | Pos. | Neg. | Neg. | Pos. | Pos. | Pos. |
| Clinical diagnosis | None | IM∗ | IM∗ | Unspecific symptoms† | Unspecific symptoms† | EBV+PTLD |
| Transplantation type, No. (%) | ||||||
| HSCT | 15 (10.4) | 16 (44.4) | ||||
| SOT | 129 (89.6) | 20 (55.6) | ||||
| First EBV episode after transplantation, median (range), d |
90 (29-312) | |||||
| EBV+PTLD diagnosis after transplantation, median (range), d | 99 (35-302) | |||||
| PTLD type, No. (%) | ||||||
| Early lesions | 4 (11.1) | |||||
| Monomorphic B-cell PTLD | 21 (58.3) | |||||
| Polymorphic PTLD | 11 (30.6) | |||||
EBNA, EBV virus nuclear antigen 1; HSCT, hematopoietic stem cell transplantation; max, maximum; min, minimum; Neg., negative; Pos., positive; RT-qPCR, real-time quantitative polymerase chain reaction; SOT, solid organ transplantation.
As defined by the triad of fever, lymphadenopathy, and tonsillitis.
Unspecific symptoms include fever, weight loss, lymphadenopathy, night sweats, or fatigue.
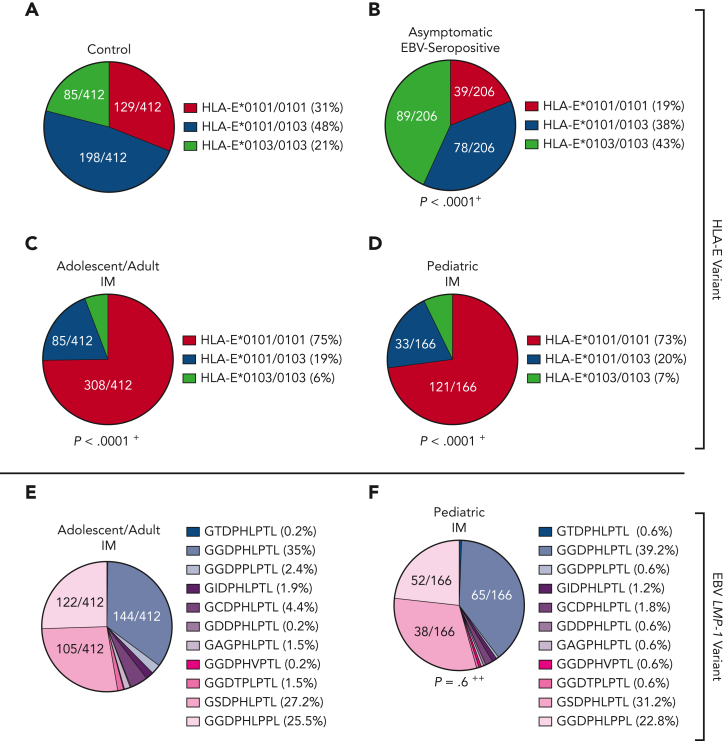
All groups were tested for their HLA-E∗0101/0103 variants. Asymptomatic EBV-seropositive individuals exhibited a significantly higher frequency of the HLA-E∗0103/0103 genotype and a lower frequency of the HLA-E∗0101 allele compared with the control (P < .0001; odds ratio [OR], 2.9; Figure 1A-B). In contrast, the patients with IM showed a significantly higher frequency of the HLA-E∗0101/0101 genotype than the overall population (adolescent/adult IM: P < .0001; OR, 6.4 [Figure 1C]; pediatric IM: P < .0001; OR, 5.9 [Figure 1D]), whereas the HLA-E∗0103 allele occurred rarely in both groups with IM. Thus, the high-expressing HLA-E∗0103/0103 variant may be a protective factor against symptomatic IM.
Figure 1.
HLA-E and EBV LMP-1 variants in patients with IM and asymptomatic EBV-seropositive individuals. (A-D) Distribution of HLA-E variants between control persons (N = 412) (A), asymptomatic EBV-seropositive persons (N = 206) (B), adolescent/adult patients with IM (N = 412) (C), and pediatric patients with IM (N = 166) (D). Fractions represent the relative frequency of HLA-E∗0101/0101, HLA-E∗0101/0103, and HLA-E∗0103/0103 variants. (E-F) LMP-1 peptide variants in adolescent/adult patients with IM (N = 412) (E) and pediatric patients with IM (N = 166) (F). Fractions represent the relative frequency of the LMP-1 peptide GGDPHLPTL, GSDPHLPTL, GGDPHLPPL, GGDPPLPTL, GCDPHLPTL, GIDPHLPTL, GAGPHLPTL, GGDTPLPTL, GDDPHLPTL, GGDPHVPTL, and GTDPHLPTL variants. +The frequency of the HLA-E genotypes was compared with the control cohort by the χ2 test. ++The frequency of the LMP-1 variants was compared with the adolescent/adult cohort with IM by the χ2 test.
IM is hallmarked by a high LMP-1 peptide diversity
In EBV infections, the HLA-E upregulation is dependent on 2 EBV peptides: a highly polymorphic LMP-1 and a more conserved BZLF1-derived peptide. We then analyzed the LMP-1 and BZLF1-derived peptide sequences of the EBV strains infecting the individual patients with IM. Overall, 11 different LMP-1 peptide variants were identified (supplemental Figure 1A). Among these, the GGDPHLPTL (N = 242 [41.9%]), GSDPHLPTL (N = 147 [25.4%]), and GGDPHLPPL (N = 127 [22%]) variants were the most frequent ones, whereas the 8 remaining variants only rarely occurred (Figure 1E-F). The distribution of LMP-1 peptide variants was similar in adolescent/adult and pediatric patients with IM. In contrast, the BZLF1-derived SQAPLPCVL peptide was highly conserved in all EBV strains (supplemental Figure 1B).
EBV-specific, HLA-E–restricted CD8+ T-cell responses are dependent on the HLA-E variants
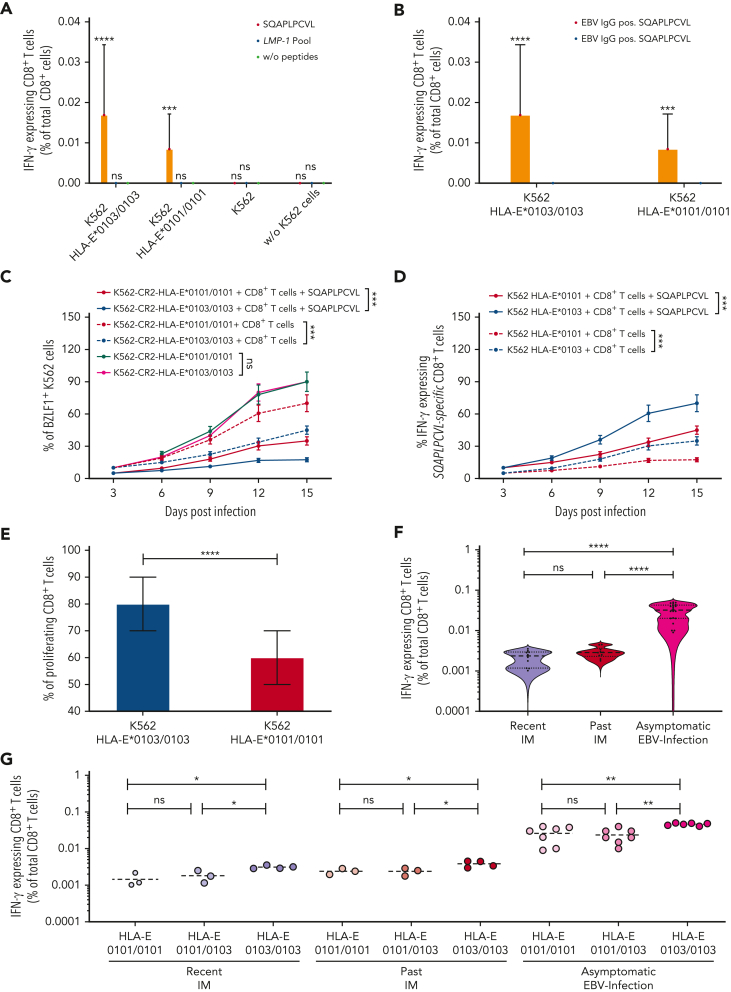
We then analyzed the capacity of the BZLF1-derived SQAPLPCVL and of all LMP-1 peptide variants to elicit EBV-specific, HLA-E–restricted CD8+ T-cell responses. We first isolated CD8+ T cells from 12 EBV-seropositive and 6 EBV-seronegative healthy individuals and cocultured these cells with K562-HLA-E∗0103/0103 or K562-HLA-E∗0101/0101 cells, pulsed with SQAPLPCVL or with a peptide pool including all 11 LMP-1-peptide variants, respectively. An EBV-specific, HLA-E–restricted CD8+ T-cell response was detected in EBV-seropositive persons, but only against SQAPLPCVL-pulsed K562-HLA-E–expressing cells, and not in response to the LMP-1 pool, in the absence of HLA-E–expressing K562 cells, or in EBV-seronegative individuals (Figure 2A-B).
Figure 2.
BZLF1-dervied SQAPLPCVL and HLA-E–restricted CD8+T-cell responses prevent the development of IM. (A-B) Analysis of SQAPLPCVL-specific and HLA-E–restricted CD8+ T-cell responses, evaluated between 12 healthy EBV-seropositive individuals and 6 healthy EBV-seronegative blood donors by flow cytometry. Enriched CD8+ T cells were stimulated with K562-HLA-E∗0103/0103 cells, K562-HLA-E∗0101/0101 cells, or K562 cells or without cells and either 300 μM of the SQAPLPCVL peptide, 300 μM of the LMP-1 peptide pool (consisting of equal concentrations of 11 LMP-1 peptide variants), or without peptides. (C-D) Evaluation of the viral spread and the activation of SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells against EBV-infected K562-CR2-HLA-E∗0103/0103 cells or K562-CR2-HLA-E∗0101/0101 cells. EBV-infected K562-CR2-HLA-E∗0103/0103 cells or EBV-infected K562-CR2-HLA-E∗0101/0101 cells were cultured either alone or together with SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells from 12 healthy EBV-seropositive individuals. (C) K562-CR2-HLA-E∗0103/0103 or K562-CR2-HLA-E∗0101/0101 cells were analyzed for the expression of EBV-BZLF1 after 3, 6, 9, 12, and 15 days after infections by flow cytometry. (D) CD8+ T cells were then analyzed for the expression of IFN-γ after 3, 6, 9, 12, and 15 days after infections by flow cytometry. (A-D) Repeated measures 1-way analysis of variance (with the Geisser-Greenhouse correction) was used to compare differences between the respective groups. Plots represent the mean (±SD) of 12 independent biological replicates. (E) Analysis of proliferating SQAPLPCVL-specific and HLA-E–restricted CD8+ T cells. Enriched and CFSE-stained CD8+ T cells from 12 healthy EBV-seropositive individuals were cocultured together with SQAPLPCVL-peptide pulsed K562-HLA-E∗0103/0103 or K562-HLA-E∗0101/0101 cells. The percentage of proliferating (CFSElow) CD8+ T cells was analyzed by flow cytometry and compared by the Wilcoxon signed-rank test. (F-G) Percentage of SQAPLPCVL-specific and HLA-E–restricted CD8+ T cells in patients with a recent IM (1-8 years; HLA-E∗0101/0101: N = 3; HLA-E∗0101/0103: N = 3; HLA-E∗0103/0103: N = 4) or past IM (10-23 years; HLA-E∗0101/0101: N = 3; HLA-E∗0101/0103: N = 3; HLA-E∗0103/0103: N = 4) or asymptomatic EBV-infected individuals (HLA-E∗0101/0101: N = 7; HLA-E∗0101/0103: N = 7; HLA-E∗0103/0103: N = 6). The percentage of SQAPLPCVL-specific and HLA-E–restricted CD8+ T cells was compared between the groups by the Kruskal-Wallis test and the Dunn posttest. P < .05 was considered significant. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. IFN-γ, interferon gamma; neg., negative; ns, not significant; pos., positive; w/o, without.
We further compared SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell responses between K562-HLA-E∗0103/0103– and K562-HLA-E∗0101/0101–expressing cells. The SQAPLPCVL peptide led to a stable upregulation of HLA-E and to HLA-E–restricted CD8+ T-cell responses; this effect was, however, significantly stronger on HLA-E∗0103/0103– compared with HLA-E∗0101/0101–expressing K562 cells (supplemental Figure 2).
We then analyzed to which extent the SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell response is able to prevent the EBV spread in vitro. As K562-HLA-E cells cannot be infected by EBV,16 we established K562-CR2-HLA-E∗0103/0103 and K562-CR2-HLA-E∗0101/0101 cell lines by CRISPR gene editing (supplemental Figure 3). We infected both K562-CR2-HLA-E cell lines with the EBV B95-8 isolate and cultured the infected cells together with sorted SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells of the healthy 12 EBV-seropositive donors, in the presence or absence of supplementary SQAPLPCVL peptides. The K562-CR2-HLA-E cells were then analyzed for the expression of EBV-BZLF1 (supplemental Figure 4), and the CD8+ T cells were tested for the activation marker interferon gamma (IFN-γ) by flow cytometry. As shown in Figure 2C, SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells efficiently inhibited the viral spread, as demonstrated by a reduction of BZLF1+K562-CR2-HLA-E cells in the presence of SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells.
We then compared the viral spread and the CD8+ T-cell activation levels between cells, expressing either the HLA-E∗0103/0103 or the HLA-E∗0101/0101 variant. SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells prevented the viral spread to a significantly higher extent in K562-CR2-HLA-E∗0103/0103, compared with K562-CR2-HLA-E∗0101/0101, cells (Figure 2C). Vice versa, K562-CR2-HLA-E∗0103/0103 cells elicited a significantly higher level of CD8+ T-cell activation, as reflected by more IFN-γ–expressing cells, compared with K562-CR2-HLA-E∗0101/0101 cells (Figure 2D).
To analyze whether the HLA-E variants also have an impact on the expansion of SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells, we cultured K562-HLA-E∗0103/0103 or K562-HLA-E∗0101/0101 cells together with sorted carboxyfluorescein succinimidyl ester-stained SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells and subsequently analyzed the proliferating CD8+ T-cell subsets. Stimulation with K562-HLA-E∗0103/0103 cells led to a significantly higher proliferation of SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells, compared with stimulation with K562-HLA-E∗0101/0101 cells (Figure 2E).
Overall, the data demonstrate that the HLA-E∗0103/0103 variant is better stabilized by the BZLF1-derived SQAPLPCVL peptide, which further leads to a robust proliferation and activation of EBV-specific, HLA-E–restricted CD8+ T cells.
High levels of EBV-specific, HLA-E–restricted T cells are present in asymptomatic EBV-seropositive persons
On the basis of these results, we hypothesized that SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell responses are a key factor providing protection against symptomatic primary EBV infections and may differ between individuals with and without IM.
We first gained blood samples from 20 randomly selected patients in whom the primary EBV infection has occurred asymptomatically somewhere in the past. To enable a comparison between this patient group and patients with IM, we also gained blood samples from 20 randomly selected patients with past IM. From 10 patients with IM, samples were obtained 1 to 8 years after the diagnosis of IM (recent IM); and from 10 patients, samples were obtained 10 to 23 years after the diagnosis of IM (past IM). We then compared the SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell levels of those 3 groups. Patients with asymptomatic EBV infections showed significantly higher SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell levels, compared with patients with earlier IM (Figure 2F). Patients with IM and patients with asymptomatic EBV infection, encoding for the HLA-E∗0103/0103 genotype, had always significantly higher SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell levels, compared with respective individuals of the same group, encoding for the HLA-E∗0101 allele (Figure 2G).
NKG2A+SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells prevent EBV spread less efficiently than NKG2A–SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells
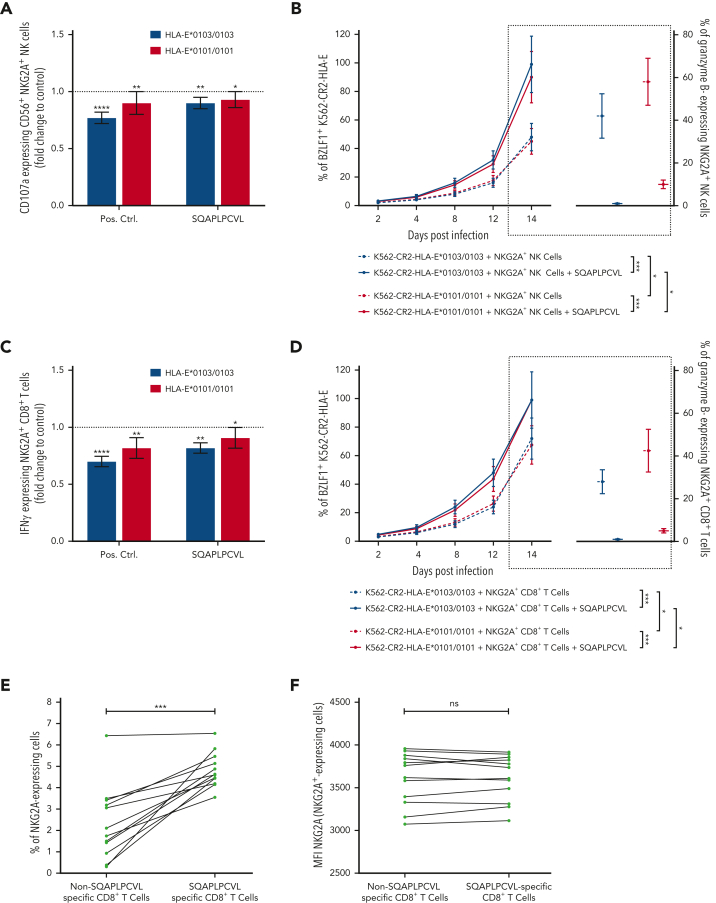
Because the SQAPLPCVL peptide has an important role in the stabilization of HLA-E, which, in turn, leads to stimulation of the inhibitory NKG2A+ NK and NKG2A+CD8+ T cells, we next analyzed the capacity of SQAPLPCVL to inhibit NKG2A+ NK and NKG2A+CD8+ T cells. Therefore, we isolated NK and CD8+ T cells from 12 healthy EBV-seropositive donors and cocultured the preactivated cells together with SQAPLPCVL-pulsed K562-HLA-E∗0101/0101 or K562-HLA-E∗0103/0103 cells.
SQAPLPCVL-pulsed K562-HLA-E∗0103/0103, and to a lower extent also SQAPLPCVL-pulsed K562-HLA-E∗0101/0101, cells led to a significant inhibition of NKG2A+ NK cells, as reflected by a decrease of CD107a-expressing cells in comparison to nonpeptide pulsed cells (Figure 3A; supplemental Figure 5). Similarly, we observed a significant inhibition of NKG2A+CD8+ T cells (Figure 3C).
Figure 3.
BZLF1-dervied SQAPLPCVL peptides are a potent inhibitor of NKG2A+ cells. (A-D) NKG2A+ inhibition assays: K562-HLA-E∗0103/0103 or K562-HLA-E∗0101/0101 cells were first incubated together with 300 μM of the positive control (VMAPRTLIL) or the BZLF1-dervied SQAPLPCVL peptide and then incubated together with (A-B) preactivated enriched CD56+ NK or (C-D) preactivated enriched CD8+ T cells. The percentage of CD107-expressing NKG2A+ NK cells (A) or IFN-γ–expressing NKG2A+ CD8+ T cells (C) was assessed by flow cytometry. Each peptide was compared with the negative control (dashed black line) (ie, K562-HLA-E∗0103/0103 or K562-HLA-E∗0101/0101 cells without peptides) using the Mann-Whitney test. (B,D) EBV dissemination assay: K562-CR2-HLA-E∗0103/0103 or K562-CR2-HLA-E∗0101/0101 cells were infected with the EBV B95-8 isolate and cultured together with sorted NKG2A+ NK cells (B) or sorted NKG2A+ CD8+ T cells (D) for 2, 4, 8, 12, or 14 days. The percentage (B,D) of BZLF1+ K562-CR2-HLA-E∗0103/0103 or K562-CR2-HLA-E∗0101/0101 cells was assessed after 2, 4, 8, 12, or 14 days, and granzyme B–expressing NKG2A+ NK cells (B) or NKG2A+ CD8+ T cells (D) were assessed by flow cytometry after 14 days of coculture. Repeated measures 1-way analysis of variance (ANOVA) (with the Geisser-Greenhouse correction) was used to analyze differences between the peptides and the granzyme B–expressing NKG2A+ NK (B) or NKG2A+ CD8+ T cells (D) without peptides. (A,C) Plot represents the mean (±SD) of 12 independent biological replicates. (E-F) Analysis of the percentage (E) and the expression level (mean fluorescence intensity [MFI]) (F) of NKG2A+ expressing non–SQAPLPCVL-specific CD8+ T cells and NKG2A+ expressing SQAPLPCVL-specific T cells. The NKG2A MFI was assessed in both NKG2A+ CD8+ T-cell subsets in 12 EBV-seropositive blood donors by flow cytometry and compared using the Wilcoxon matched t-test. (G) EBV dissemination assay: K562-CR2-HLA-E∗0103/0103 or K562-CR2-HLA-E∗0101/0101 cells were infected with the EBV B95-8 isolate and cultured together with sorted NKG2A+ expressing SQAPLPCVL-specific T cells or sorted NKG2A–SQAPLPCVL-specific T cells for 2, 4, 8, 12, or 14 days. The percentage of BZLF1+ K562-CR2-HLA-E∗0103/0103 or K562-CR2-HLA-E∗0101/0101 cells was assessed by flow cytometry after 2, 4, 8, 12, or 14 days of coculture. RM 1-way ANOVA (with the Geisser-Greenhouse correction) was used to analyze differences between the cell subsets. P < .05 was considered significant. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. Pos. Ctrl., positive control.
We then evaluated whether the SQAPLPCVL peptide facilitates the EBV spread via the inhibition of NKG2A+ NK or CD8+ T cells. We cultured EBV-infected K562-CR2-HLA-E cells together with sorted NKG2A+ NK or NKG2A+ CD8+ T cells in the presence or absence of additional SQAPLPCVL. The presence of SQAPLPCVL resulted in a significantly higher EBV spread and significantly fewer granzyme B–expressing NKG2A+ NK (Figure 3B) and CD8+ T cells (Figure 3D).
As our data demonstrate that the SQAPLPCVL peptide can activate HLA-E–restricted CD8+ T cells, but also inhibits the subpopulation of NKG2A+CD8+ T cells, we next analyzed to which extent SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells express NKG2A. Therefore, we analyzed sorted SQAPLPCVL-specific and all non–SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell subsets of the 12 seropositive persons for their NKG2A expression. Only a small fraction of both, non–SQAPLPCVL-specific (>6.3%) and SQAPLPCVL-specific (>6.5%), HLA-E–restricted CD8+ T cells expressed NKG2A (Figure 3E). A comparable level of NKG2A expression was observed between both CD8+ T-cell subsets (Figure 3F).
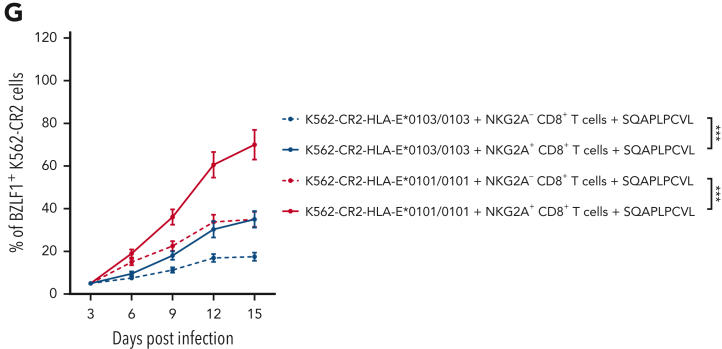
We then evaluated whether SQAPLPCVL-specific, HLA-E–restricted NKG2A+CD8+ T cells can prevent EBV spread. We subjected the sorted NKG2A+ and NKG2A– SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells from 12 EBV-seropositive donors to EBV-dissemination assays. SQAPLPCVL-specific, HLA-E–restricted NKG2A+CD8+ T cells were able to prevent viral spread in K562-CR2-HLA-E cells, but to a significantly lower extent than HLA-E–restricted NKG2A–CD8+ T cells, which had no inhibition via the HLA-E/NKG2A axis (Figure 3G).
In summary, the data provide evidence that the BZLF-1–derived SQAPLPCVL peptide can activate SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells, but also inhibit the minority of SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells that express NKG2A. However, also, these cells can overcome to some extent the NKG2A-mediated inhibition and prevent the EBV dissemination.
HLA-E and LMP-1 peptide variants are associated with symptomatic EBV reactivations in older persons
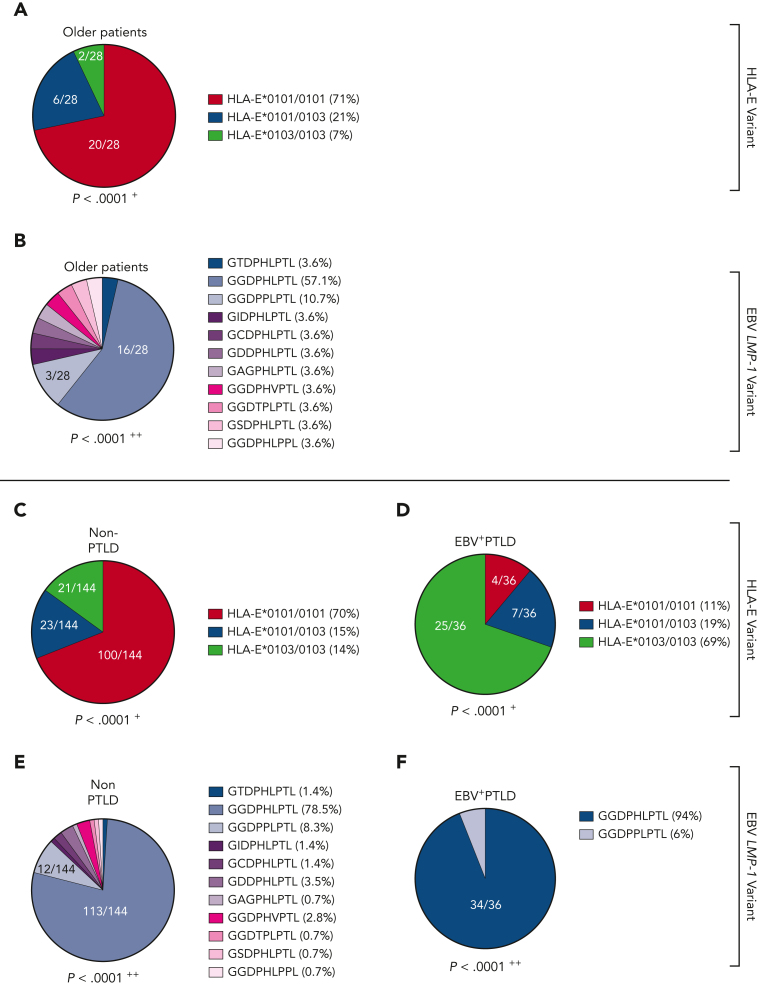
On the basis of the findings that HLA-E–restricted immune responses play an important role in primary EBV infections, we further analyzed the impact of host HLA-E∗0101/0103 and EBV LMP-1 peptide variants in 28 older patients with symptomatic EBV reactivations. All patients had fever, detectable EBV-viremia (>1000 copies/mL plasma), and EBV-VCA–specific IgM and EBV-VCA– and EBV nuclear antigen 1–specific IgG antibodies (Table 1). In these patients, the HLA-E∗0101/0101 genotype was significantly overrepresented, compared with the control cohort (Figure 1A), whereas the HLA-E∗0103 allele rarely occurred (P < .0001; OR, 5.5; Figure 4A).
Figure 4.
Distribution of HLA-E and LMP-1 peptide variants in primary and reactivating EBV infections. (A-B) Distribution of HLA-E (A) and LMP-1 (B) variants in immunocompetent older patients with EBV reactivations (N = 28). (C-F) Distribution of HLA-E (C,D) and (E,F) LMP-1 (E,F) variants in immunosuppressed non-PTLD (N = 149) (C,E) and EBV+PTLD (N = 36) (D,F) transplant patients with EBV reactivations. (A,C,D) Fractions represent the relative frequency of HLA-E∗0101/0101, HLA-E∗0101/0103, and HLA-E∗0103/0103. (B,E,F) Fractions represent the relative frequency of the LMP-1 peptide GGDPHLPTL, GSDPHLPTL, GGDPHLPPL, GGDPPLPTL, GCDPHLPTL, GIDPHLPTL, GAGPHLPTL, GGDTPLPTL, GDDPHLPTL, GGDPHVPTL, and GTDPHLPTL variants. +The frequency of the HLA-E genotypes was compared with the control cohort by the χ2 test. ++The frequency of the LMP-1 variants was compared with the adolescent/adult cohort with IM by the χ2 test.
We then assessed the LMP-1 peptide variants of the patients’ EBV strains identified during the reactivation episodes. The patients showed a significantly different LMP-1 peptide profile compared with patients with IM, characterized by a higher frequency of the GGDPHLPTL and GGDPPLPTL variant (P = .02; OR, 2.7; Figure 4B; supplemental Figure 1).
HLA-E variants and LMP-1 peptide EBV reactivations in transplant recipients with and without EBV+PTLD
EBV reactivations in transplant recipients may result in development of life-threatening EBV+PTLD.4 We, therefore, assessed whether there is an association between host HLA-E and EBV LMP-1 peptide variants, EBV reactivation, and EBV+PTLD in transplant recipients. We included 180 EBV–polymerase chain reaction–positive patients after hematopoietic stem cell transplant (N = 31) or solid organ transplant (N = 149), who were followed up for 3 years after transplantation (Table 1). Patients were tested within this period for EBV-DNA, either because of unspecific symptoms or in case of PTLD diagnosis, according to international guidelines.17, 18, 19 Of all transplant recipients, 36 developed EBV+PTLD, whereas in 144 recipients, EBV-viremia was detected without progressing to EBV+PTLD in the follow-up (Table 1).
Similar to the older persons, PTLD-free transplant recipients with symptomatic EBV reactivations encoded significantly more frequently for the HLA-E∗0101/0101 genotype, compared with the controls (P < .0001; OR, 5.2; Figure 4C).
Compared with PTLD-free transplant recipients, patients with EBV+PTLD, however, carried significantly more often the HLA-E∗0103/0103 genotype, whereas the HLA-E∗0101 allele rarely occurred (P < .0001; OR, 31.25; Figure 4D; supplemental Figure 6). This implicates that a strong HLA-E–mediated immune response, as reflected by the high-expressing HLA-E∗0103/0103 variant, may be associated with the risk for EBV+PTLD.
We then assessed the viral LMP-1 peptides in the EBV strains, infecting patients with and without PTLD. In comparison to patients with IM (Figure 1E), patients without PTLD (P < .0001; OR, 8.4; Figure 4E) and patients with EBV+PTLD (P < .0001; OR, 94.7; Figure 4F) were infected with EBV strains, which encoded especially for the GGDPHLPTL and GGDPPLPTL variants. In patients with EBV+PTLD, only the GGDPHLPTL or the GGDPPLPTL LMP-1 variant was found (PTLD vs non-PTLD: P = .02; OR, 11.3; supplemental Figure 6).
Individual EBV LMP-1 peptide variants elicit altered NKG2A+ NK and CD8+ T-cell responses
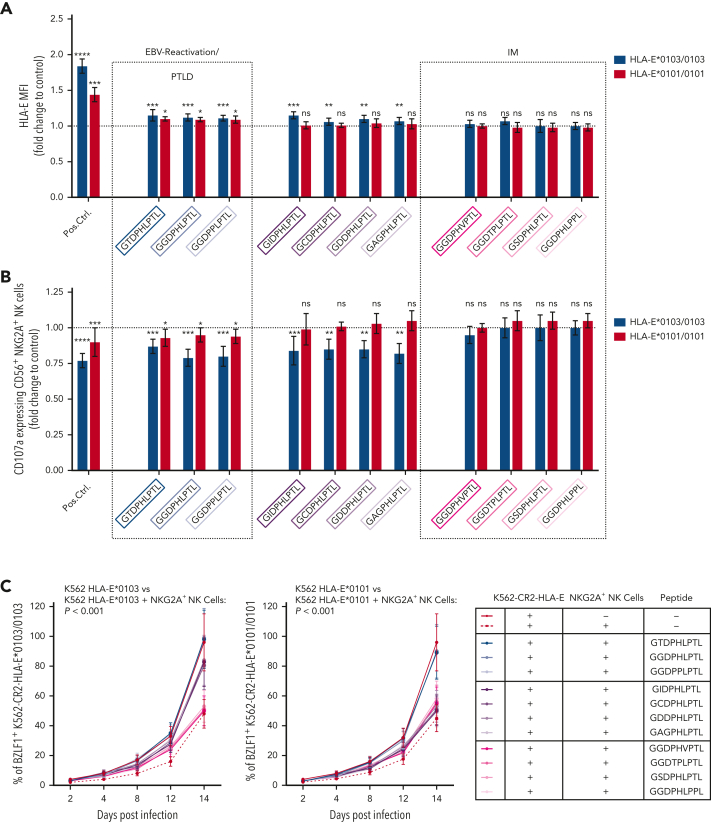
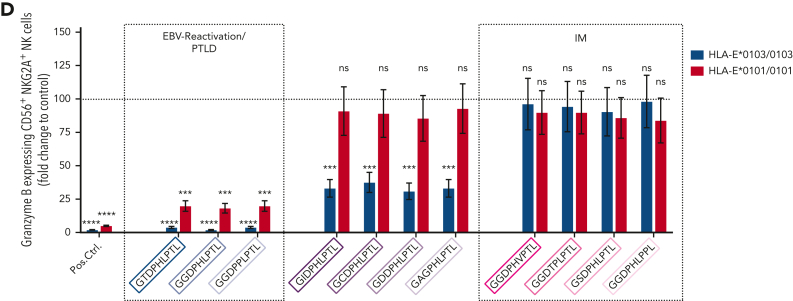
NKG2A+ NK cell–mediated effector functions can prevent the B-cell transformation of EBV-infected cells,6,11 but NKG2A+ cells can also be inhibited by EBV-derived peptides, presented on HLA-E. Thus, we further analyzed whether the individual LMP-1–derived peptides differ in their ability to stabilize HLA-E and to inhibit NKG2A+ cells. We, therefore, isolated CD56+ NK and CD8+ T cells from 12 healthy EBV-seropositive donors and cocultured the cells together with K562-HLA-E cells, independently pulsed with each of the 11 different LMP-1 peptide variants. The LMP-1–derived peptides caused varying degrees of HLA-E stabilization (Figure 5A), as well as of inhibition of NKG2A+ NK (Figure 5B) and of NKG2A+ CD8+ T cells (supplemental Figure 7). Especially the GGDPHLPTL and GGDPPLPTL peptides, which are found preferably in EBV strains causing reactivation and EBV+PTLD, led to potent inhibition of NKG2A+ NK and NKG2A+ CD8+ T cells, when stimulated with K562-HLA-E cells, and this may allow for an increased immune evasion of these EBV strains.
Figure 5.
LMP-1–derived peptides are a potent inhibitor of NKG2A+NK cells. (A) HLA-E stabilization assay: K562-HLA-E∗0103/0103 or K562-HLA-E∗0101/0101 cells were incubated together with 300 μM of the positive control (VMAPRTLIL) or the LMP-1–derived GGDPHLPTL, GSDPHLPTL, GGDPHLPPL, GGDPPLPTL, GCDPHLPTL, GIDPHLPTL, GAGPHLPTL, GGDTPLPTL, GDDPHLPTL, GGDPHVPTL, and GTDPHLPTL peptides. The surface expression of HLA-E was then assessed after 16 hours of coculture by flow cytometry. Box plot represents the mean (±SD) of 3 independent replicates. Each peptide was compared with the negative control (dashed black line) (ie, K562-HLA-E∗0103/0103 cells or K562-HLA-E∗0101/0101 cells without peptides) using the Mann-Whitney test. (B) NKG2A+ inhibition assay: K562-HLA-E∗0103/0103 or K562-HLA-E∗0101/0101 cells were first incubated together with 300 μM of the positive control (VMAPRTLIL) or the LMP-1–derived GGDPHLPTL, GSDPHLPTL, GGDPHLPPL, GGDPPLPTL, GCDPHLPTL, GIDPHLPTL, GAGPHLPTL, GGDTPLPTL, GDDPHLPTL, GGDPHVPTL, and GTDPHLPTL peptides and then incubated together with preactivated enriched CD56+ NK cells. The percentage of CD107-expressing NKG2A+ NK cells was assessed by flow cytometry. Plots represent the mean (±SD) of 12 independent biological replicates. Each peptide was compared with the negative control (dashed black line) (ie, K562-HLA-E∗0103/0103 cells or K562-HLA-E∗0101/0101 cells without peptides) using the Mann-Whitney test. (C-D) EBV dissemination assay: K562-CR2-HLA-E∗0103/0103 or K562-CR2-HLA-E∗0101/0101 cells were infected with the EBV B95-8 isolate and cultured together with sorted NKG2A+ NK cells for 2, 4, 8, 12, or 14 days. The percentage of BZLF1+ K562-CR2-HLA-E∗0103/0103 or K562-CR2-HLA-E∗0101/0101 cells (C) or granzyme B–expressing NKG2A+ NK cells (D) was assessed by flow cytometry after 2, 4, 8, 12, or 14 days of coculture. The dashed black line indicates the percentage of granzyme B–expressing NKG2A+ NK cells in the absence of any peptides. (C) Plots represent the mean (±SD) of 12 independent biological replicates. Repeated measures 1-way analysis of variance (with the Geisser-Greenhouse correction) was used to analyze differences between the peptides. (D) Box plot represents the mean (±SD) of 12 independent replicates. Each peptide was compared with the negative control (dashed black line) (ie, K562-HLA-E∗0103/0103 cells or K562-HLA-E∗0101/0101 cells without peptides) using the Mann-Whitney test. P < .05 was considered significant. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. MFI, mean fluorescence intensity; ns, not significant; Pos. Ctrl., positive control.
Other peptide variants (namely, the GSDPHLPTL and GGDPHLPPL peptides), which were frequently found in EBV strains during IM, but rarely in EBV reactivations, elicited neither significant HLA-E upregulation nor inhibition of NKG2A+ NK or NKG2A+ CD8+ T cells (Figure 5B; supplemental Figure 7).
To investigate the different impact of the individual peptides on the prevention of EBV spread, we further tested the LMP-1 peptides in EBV-dissemination assays, using sorted NKG2A+ NK or NKG2A+CD8+ T cells. NKG2A+ NK (Figure 5C) and, to a lower extent, NKG2A+CD8+ T cells (supplemental Figure 7B) inhibited efficiently the spread of EBV in the absence of LMP-1–derived peptides.
We then performed the EBV-dissemination assay, including K562-CR2-HLA-E∗0103/0103 and K562-CR2-HLA-E∗0101/0101 target cells pulsed by each of the 11 LMP-1 peptide variants. Only the LMP-1 GGDPHLPTL, GGDPPLPTL, and the only rarely detected GTDPHLPTL peptide variants caused the inhibition of NKG2A+ NK (Figure 5C-D) and NKG2A+ CD8+ T cells (supplemental Figure 7B-C), which further lead to an increased viral spread in K562-CR2-HLA-E∗0103/0103 and K562-CR2-HLA-E∗0101/0101 cells.
As shown in Figure 5C and Figure 5D, 4 peptides inhibited the viral spread only in K562-CR2-HLA-E∗0103/0103, but not in K562-CR2-HLA-E∗0101/0101, cells. The remaining 4 peptides, including the GGDPHLPPL and GSDPHLPTL variants, which were observed frequently in IM but not in EBV reactivations, exhibited no inhibitory effect against the NKG2A+ NK or NKG2A+CD8+ T-cell–mediated prevention of the viral spread.
Thus, our data confirm that the LMP-1 GGDPHLPTL and GGDPPLPTL variants, which are prevalent in patients developing EBV+PTLD, are associated with a potent inhibition of NKG2A+ NK and T cells, especially in response to HLA-E∗0103/0103-expressing cells. Thus, EBV strains which encode either of these peptides possess a specific NKG2A+-mediated immune evasion strategy.
Discussion
In the present study, we reveal that the EBV-disease development is significantly associated with variations in HLA-E–restricted immune responses. We provide evidence that the HLA-E∗0101 allele is a risk factor for development of IM. In contrast, the homozygous HLA-E∗0103/0103 genotype was significantly overrepresented in individuals who did not develop IM during primary EBV infections, suggesting that this genotype may contribute to a more efficient protection against EBV-associated diseases. Our finding is supported by earlier in vitro studies, which demonstrated that the HLA-E∗0103/0103 provides a more efficient assembly with β2-microglobulin and a faster endoplasmic reticulum egress, compared with the HLA-E∗0101/0101 genotype.9 This high-level expression of HLA-E∗0103/0103 may also prevent, to some degree, the development of IM during primary EBV infection. Previously published studies have already described familial aggregations of IM and concluded that a genetic susceptibility may exist for IM.20,21 Although recent observational studies suggested that there might be a certain relation between classic HLA polymorphisms and the occurrence of IM,22,23 we now identified the HLA-E∗0101 allele as a novel factor, which is highly associated with development of IM.
In the search for the functional background of these findings, we could show that it is the HLA-E–restricted immune response by CD8+ T cells, which is a key factor for the limitation of the viral replication in primary EBV infections. Using in vitro and ex vivo analyses, we uncovered that a small subset of EBV-BZLF1 SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells efficiently prevent the in vitro EBV spread. So far, most studies focused on the role of total EBV-specific CD8+ T-cell responses in the defense against primary EBV infections24 but could not find a correlation between large expansions of classic, highly activated EBV-specific CD8+ T cells and freedom from IM.7,25, 26, 27 In contrast, we found that the small subset of protective SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells is, in fact, highly prevalent in EBV-seropositive individuals, who had not undergone IM during primary infection. Such SQAPLPCVL-specific, HLA-E–restricted T cells were earlier found in patients with multiple sclerosis5 and in in vitro studies,15 but their functional impact on the EBV spread was unknown. More important, we revealed that the SQAPLPCVL peptide is highly conserved among different EBV strains, and it is thus likely that SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells mediate cross-strain protection during primary EBV infections.
In addition, we could provide further evidence that HLA-E∗0103/0103-expressing cells are, in comparison to HLA-E∗0101/0101-expressing cells, associated with an especially stable SQAPLPCVL-mediated upregulation of HLA-E as well as an especially efficient inhibition of EBV spread in vitro and with a pronounced activation and proliferation of these SQAPLPCVL-specific, HLA-E–restricted CD8+ T cells. On the basis of our results, it thus appears that a potent SQAPLPCVL-specific, HLA-E–restricted CD8+ T-cell response limits the EBV spread, especially when presented via the HLA-E∗0103/0103 genotype. Consequently, individuals encoding the HLA-E∗0103/0103 genotype may possess a lower risk for IM.
Not only the highly conserved BZLF-1–derived SQAPLPCVL, but also a highly polymorphic LMP-1–derived peptide is presented via HLA-E. In patients with IM, we found 11 variants of this peptide in the infecting EBV strains. Interestingly, the LMP-1 peptide profile of the infecting EBV strains was significantly different in older persons, who developed symptomatic EBV reactivations. On reactivation, the LMP-1 peptide profile became more restricted toward 2 peptides (namely, GGDPHLPTL and GGDPPLPTL). Our functional analyses revealed that these peptides show a particularly strong inhibition of NKG2A+ NK and NKG2A+ CD8+ T cells. Our findings thus suggest that EBV strains carrying 1 of these 2 specific peptides may, to some degree, evade the human immune response by the efficient inhibition of NKG2A-expressing cells, thereby enabling the reactivation from latency. These data were further confirmed by our analyses of EBV-reactivation events in transplant recipients, in whom an even higher frequency of EBV strains carrying either of the LMP-1–derived peptides, GGDPHLPTL or GGDPPLPTL, was observed.
EBV reactivations are a main concern in transplant recipients, and EBV+PTLD is a life-threatening malignant complication following EBV reactivations.4 Although our data now suggest that the occurrence of reactivation events is highly associated with the LMP-1 peptide variant of the infecting EBV strain, the question remains why some of these reactivating patients progress toward EBV+PTLD, whereas in others, the reactivation episode is limited. So far, the degree of immunosuppression, recipient age and ethnicity, allograft type, and distinct host genetic variations were considered as contributing to the risk for EBV+PTLD,28 but cannot fully explain the progression toward this complication. We now uncovered that it is the combination of the viral LMP-1 peptide variant of the infecting EBV strain (namely, GGDPHLPTL and GGDPPLPTL) and the high-expressing HLA-E∗0103/0103 genotype of the host that are associated with a particularly high risk for EBV+PTLD in transplant recipients.
We further revealed that both LMP-1 peptides, if presented by the high-level expressing HLA-E∗0103/0103 variant, confer an especially potent inhibition of NKG2A+ NK cells. Thereby, distinct EBV strains, encoding for distinct LMP-1 variants, may efficiently escape the human immune responses, which otherwise would prevent the development of EBV+PTLD. Our data are supported by a recent study of others, who identified a distinct NKG2A+ NK cell subset in the tonsils of EBV carriers, which produced large amounts of IFN-γ and prevented the malignant B-cell transformation.29 These data implicate that distinct NKG2A+ NK cell subsets may provide important immune responses preventing EBV+PTLD. Other authors showed that NKG2A+ NK cells provide potent cytotoxicity against latently EBV-infected B cells.6 Our data now further reveal that a potent inhibition of NKG2A+ NK cells by specific viral peptides can also inhibit the NKG2A+ NK cell effector functions, thereby increasing the risk for EBV+PTLD.
These data are of special interest as the identification of EBV LMP-1 peptides, combined with the analysis of the HLA-E variant of a transplant recipient, may possibly allow the early identification of patients at high risk for PTLD and may serve as prognostic biomarkers.
Interestingly, a recently published study identified the HLA-E∗0101 variant as a protective factor for EBV-related classic Hodgkin lymphoma.30 This not only supports our findings but also suggests that the LMP-1 peptide-mediated upregulation of HLA-E may play a role in EBV-associated lymphoproliferative diseases in general. In combination with our data, this highlights the need for further assessment of the potential of LMP-1 variant and HLA-E analysis to identify patients generally at risk for EBV-associated lymphoproliferative diseases.
Overall, our study reveals that potent HLA-E–restricted CD8+ T-cell responses against the BZLF1-derived SQAPLPCVL peptide prevent the development of IM, whereas the HLA-E and LMP-1 peptide-mediated inhibition of NKG2A+ cells facilitates the development of EBV+PTLD. This is in agreement with earlier data showing that the lytic life cycle of EBV during IM is characterized by a high level of BZLF1 and LMP-1 expression, whereas during the EBV latency type III status, found in patients with EBV+PTLD, mainly the LMP-1 is expressed.31,32 Thus, EBV-specific, HLA-E–restricted CD8+ T-cell responses play an especially important role during primary EBV infections, but do not prevent the development of PTLD in transplant recipients. In addition, transplant recipients receive immunosuppressive therapy that mainly targets T-cell responses,33 and this may hinder the development of potent HLA-E–restricted immune responses.
In summary, we have identified that HLA-E–restricted immune responses and the NKG2A/LMP-1/HLA-E axis are important factors, which contribute to clinically evident EBV-associated diseases. Further extended studies are needed to evaluate whether the analysis of individual variations in the HLA-E–restricted immune response may serve as a prognostic marker for malignant EBV-associated diseases. In addition, the presented data may substantially contribute to the development of efficient protective EBV vaccines.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgment
The study was funded by the Center for Virology, Medical University of Vienna, Vienna, Austria.
Authorship
Contribution: H.V. and E.P.-S. conceptualized the study and wrote the original draft; H.V. and P.L.F. curated data, performed formal analysis, and performed methods; E.P.-S. acquired funding and supervised the study; H.V., P.L.F., and E.P.-S. performed investigations; E.P.-S. performed project administration; J.J.C., G.A.B., and P.J. obtained resources; and H.V. performed validation and visualization.
Footnotes
Deidentified data are available on request by contacting the corresponding author (hannes.vietzen@meduniwien.ac.at).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Hocqueloux L, Causse X, Valery A, et al. The high burden of hospitalizations for primary EBV infection: a 6-year prospective survey in a French hospital. Clin Microbiol Infect. 2015;21(11) doi: 10.1016/j.cmi.2015.07.015. 1041.e1-7. [DOI] [PubMed] [Google Scholar]
- 2.Rostgaard K, Balfour HH, Jr., Jarrett R, et al. Primary Epstein-Barr virus infection with and without infectious mononucleosis. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0226436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong Y, Meehan MT, Burrows SR, Doolan DL, Miles JJ. Estimating the global burden of Epstein–Barr virus-related cancers. J Cancer Res Clin Oncol. 2022;148(1):31–46. doi: 10.1007/s00432-021-03824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearse WB, Vakkalagadda CV, Helenowski I, et al. Prognosis and outcomes of patients with post-transplant lymphoproliferative disorder: a single center retrospective review. Blood. 2020;136(Suppl 1):9–10. [Google Scholar]
- 5.Jørgensen PB, Livbjerg AH, Hansen HJ, Petersen T, Höllsberg P. Epstein-Barr virus peptide presented by HLA-E is predominantly recognized by CD8(bright) cells in multiple sclerosis patients. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0046120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatton O, Strauss-Albee DM, Zhao NQ, et al. NKG2A-expressing natural killer cells dominate the response to autologous lymphoblastoid cells infected with Epstein-Barr virus. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez OM, Krams SM. The immune response to Epstein Barr virus and implications for posttransplant lymphoproliferative disorder. Transplantation. 2017;101(9):2009–2016. doi: 10.1097/TP.0000000000001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietra G, Romagnani C, Manzini C, Moretta L, Mingari MC. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/907092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rölle A, Jäger D, Momburg F. HLA-E peptide repertoire and dimorphism—centerpieces in the adaptive NK cell puzzle? Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.02410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbiribindi B, Pena JK, Arvedson MP, et al. Epstein–Barr virus peptides derived from latent cycle proteins alter NKG2A + NK cell effector function. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-76344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azzi T, Lünemann A, Murer A, et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 2014;124(16):2533–2543. doi: 10.1182/blood-2014-01-553024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vietzen H, Hartenberger S, Aberle SW, Puchhammer-Stöckl E. Dissection of the NKG2C NK cell response against Puumala Orthohantavirus. PLoS Neglected Trop Dis. 2021;15(12) doi: 10.1371/journal.pntd.0010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vietzen H, Rückert T, Hartenberger S, et al. Extent of cytomegalovirus replication in the human host depends on variations of the HLA-E/UL40 axis. mBio. 2021;12(2) doi: 10.1128/mBio.02996-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vietzen H, Zoufaly A, Traugott M, Aberle J, Aberle SW, Puchhammer-Stöckl E. Deletion of the NKG2C receptor encoding KLRC2 gene and HLA-E variants are risk factors for severe COVID-19. Genet Med. 2021;23:1–5. doi: 10.1038/s41436-020-01077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romagnani C, Pietra G, Falco M, et al. Identification of HLA-E-specific alloreactive T lymphocytes: a cell subset that undergoes preferential expansion in mixed lymphocyte culture and displays a broad cytolytic activity against allogeneic cells. Proc Natl Acad Sci USA. 2002;99(17):11328–11333. doi: 10.1073/pnas.172369799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carel JC, Frazier B, Ley TJ, Holers VM. Analysis of epitope expression and the functional repertoire of recombinant complement receptor 2 (CR2/CD21) in mouse and human cells. J Immunol. 1989;143(3):923–930. [PubMed] [Google Scholar]
- 17.van Esser JW, van der Holt B, Meijer E, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell--depleted SCT. Blood. 2001;98(4):972–978. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- 18.Knowles DM, Cesarman E, Chadburn A, et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85(2):552–565. [PubMed] [Google Scholar]
- 19.Shah N, Eyre TA, Tucker D, et al. Front-line management of post-transplantation lymphoproliferative disorder in adult solid organ recipient patients — a British Society for Haematology Guideline. Br J Haematol. 2021;193(4):727–740. doi: 10.1111/bjh.17421. [DOI] [PubMed] [Google Scholar]
- 20.Hwang AE, Hamilton AS, Cockburn MG, et al. Evidence of genetic susceptibility to infectious mononucleosis: a twin study. Epidemiol Infect. 2012;140(11):2089–2095. doi: 10.1017/S0950268811002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rostgaard K, Wohlfahrt J, Hjalgrim H. A genetic basis for infectious mononucleosis: evidence from a family study of hospitalized cases in Denmark. Clin Infect Dis. 2014;58(12):1684–1689. doi: 10.1093/cid/ciu204. [DOI] [PubMed] [Google Scholar]
- 22.McAulay KA, Higgins CD, Macsween KF, et al. HLA class I polymorphisms are associated with development of infectious mononucleosis upon primary EBV infection. J Clin Investig. 2007;117(10):3042–3048. doi: 10.1172/JCI32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houldcroft CJ, Kellam P. Host genetics of Epstein-Barr virus infection, latency and disease. Rev Med Virol. 2015;25(2):71–84. doi: 10.1002/rmv.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long HM, Meckiff BJ, Taylor GS. The T-cell response to Epstein-Barr virus–new tricks from an old dog. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangye SG, Palendira U, Edwards ESJ. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017;214(2):269–283. doi: 10.1084/jem.20161846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunmire SK, Grimm JM, Schmeling DO, Balfour HH, Jr., Hogquist KA. The incubation period of primary Epstein-Barr virus infection: viral dynamics and immunologic events. PLoS Pathog. 2015;11(12) doi: 10.1371/journal.ppat.1005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187(9):1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013;8(3):173–183. doi: 10.1007/s11899-013-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lünemann A, Vanoaica LD, Azzi T, Nadal D, Münz C. A distinct subpopulation of human NK cells restricts B cell transformation by EBV. J Immunol. 2013;191(10):4989–4995. doi: 10.4049/jimmunol.1301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín P, Krsnik I, Navarro B, et al. HLA allele E∗01:01 is associated with a reduced risk of EBV-related classical Hodgkin lymphoma independently of HLA-A∗01/∗02. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0135512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayee R, Ofori MEO, Wright E, Quaye O. Epstein Barr virus associated lymphomas and epithelia cancers in humans. J Cancer. 2020;11(7):1737–1750. doi: 10.7150/jca.37282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imajoh M, Hashida Y, Murakami M, et al. Characterization of Epstein-Barr virus (EBV) BZLF1 gene promoter variants and comparison of cellular gene expression profiles in Japanese patients with infectious mononucleosis, chronic active EBV infection, and EBV-associated hemophagocytic lymphohistiocytosis. J Med Virol. 2012;84(6):940–946. doi: 10.1002/jmv.23299. [DOI] [PubMed] [Google Scholar]
- 33.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.