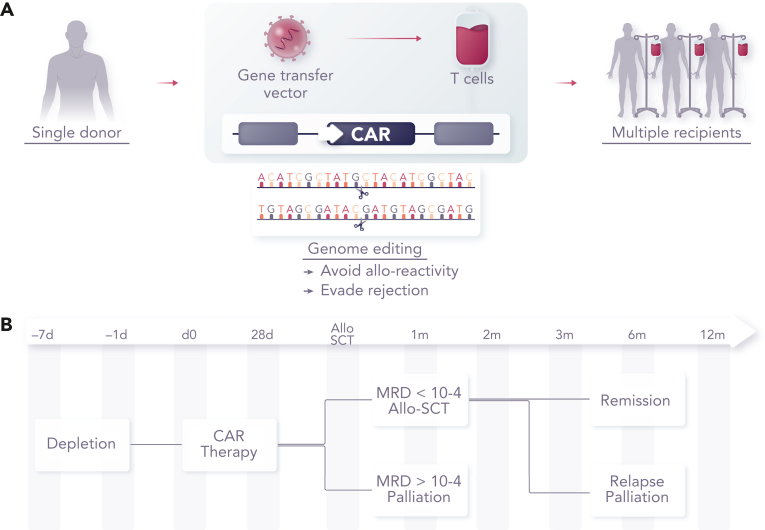
Figure 2.
Concept of banked universal CAR T cells. (A) CAR T cells can be generated from healthy allogeneic donors for use in multiple recipients after genome editing to remove endogenous TCRαβ to prevent GVHD, disruption of HLA to reduce rejection or removal of CD52 to allow cells to persist in the presence of alemtuzumab, a serotherapy used as a part of augmented lymphodepletion. (B) Therapeutic effects sufficient to induce molecular remission are achievable within a period of 2 to 4 weeks, offering a bridge of consolidation with allogeneic SCT.