Dear Editors,
We read with great interest the article by Beck and colleagues entitled “Autopsy Case of Progressive Supranuclear Palsy Treated with Monoclonal Antibody Against Tau.”1 Their patient with progressive supranuclear palsy (PSP) treated with experimental medication, tilavonemab (ABBV-8E12, a humanized monoclonal subclass of the immunoglobulin antibody against human microtubule-associated protein tau) died during the trial. On brain examination, there were no additional pathological abnormalities other than PSP. Their findings align with two previously reported patients treated with the same drug whose brains were pathologically examined.2 In Beck’s study, the patient died during the clinical trial and therefore had a shorter interval between the last dose and death compared with the patients reported in our study.2 Since our initial report, another PSP patient who participated in the same medication trial came to autopsy. This patient died approximately 2 years after the last dose of tilavonemab. This allowed us to examine the potential long-term effects of this experimental compound.
The patient was a 69-year-old man who had a 7-year history of Richardson subtype of PSP, characterized by levodopa non-responsive parkinsonism, frequent falls, vertical gaze palsy, slow saccades, eyelid opening apraxia, difficulty swallowing, and personality changes. He was enrolled in the phase 2 trial of tilavonemab (M15–562: “A Study to Assess Efficacy, Safety, Tolerability, and Pharmacokinetics of ABBV-8E12 in Subjects With Progressive Supranuclear Palsy”) at age 66 years but received a placebo over a 48-week period.3 After completing the trial, he was enrolled in the extension study (M15–563: “An Extension Study of ABBV-8E12 in Progressive Supranuclear Palsy”) and received 12 doses of tilavonemab at 4000 mg every 28 days. No clinical side effects were observed during or after the treatment, and no improvement in his clinical symptoms was noted. His neurological state continued to deteriorate, as seen in the majority of PSP patients. Brain MRI was performed before and after the trial, showing no significant changes during the trial, except for midbrain atrophy, which was already present before the trial. He died of pneumonia at age 69 years, 117 weeks after the last infusion.
Brain autopsy was performed with the consent of the legal next-of-kin. Macroscopic findings included severe atrophy of the subthalamic nucleus, midbrain, superior cerebellar peduncle, and cerebellar dentate nucleus. Microscopically, subthalamic nucleus had neuronal loss and gliosis, with globose tangles and coiled bodies with tau immunohistochemistry using anti-phosphorylated-tau antibody CP13. The substantia nigra had moderate-to-marked neuronal loss in the ventrolateral cell group, associated with extraneuronal neuromelanin, and frequent globose tangles, tufted astrocytes, and coiled bodies. The distribution of neuronal and glial tau pathology in the substantia nigra, subthalamic nucleus, globus pallidus, motor and premotor cortex, ventral thalamus, corpus striatum, and the olivopontocerebellar system was consistent with typical PSP (Figure 1). Perivascular vesicular astrocytes reported as a neuropathological finding related to experimental therapy with yet another anti-tau antibody, gosuranemab, were not observed in the cortex and subcortical nuclei.4 No significant cerebrovascular pathology, such as microbleeds, was observed.
Figure 1:
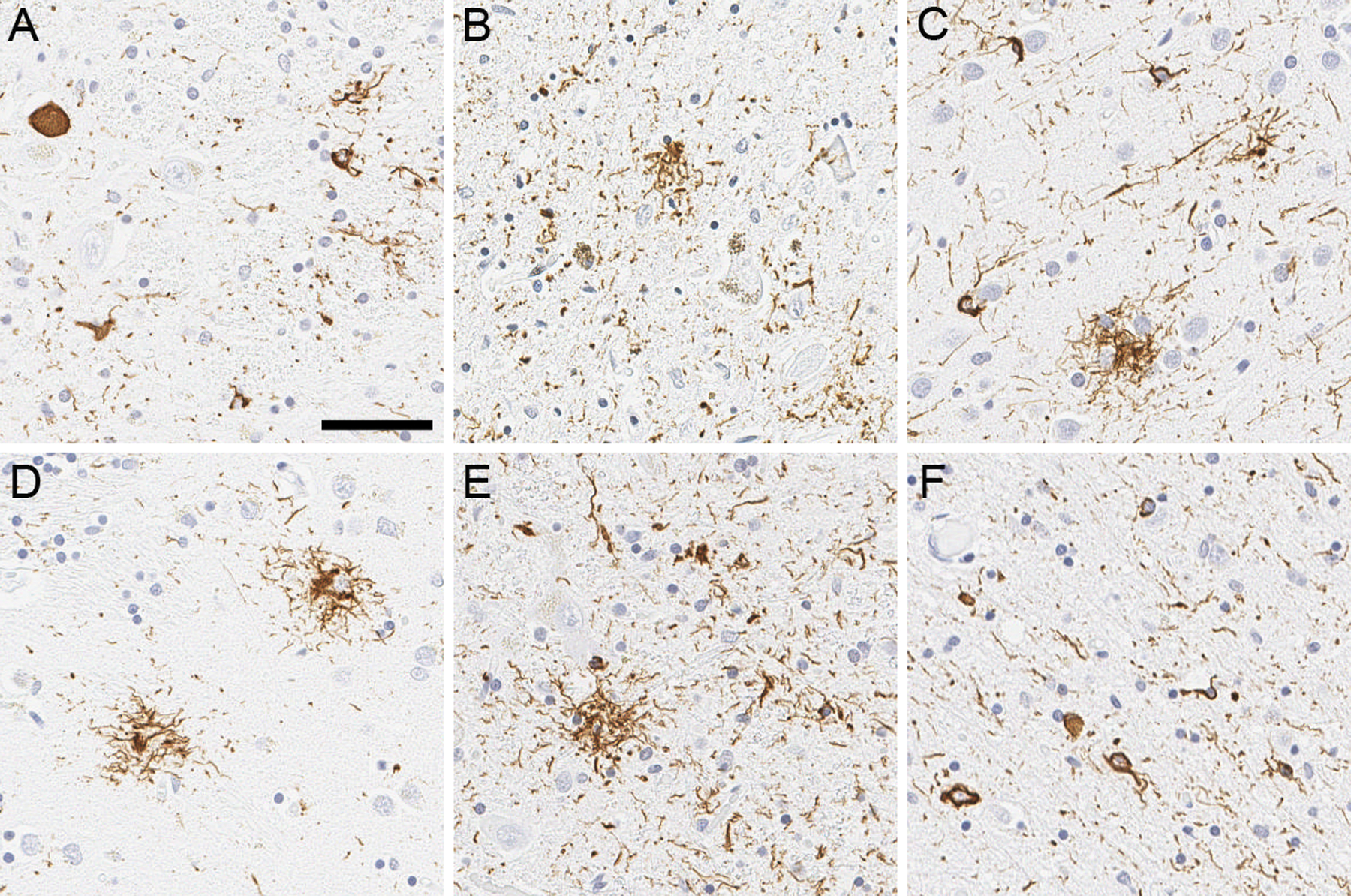
Representative images of tau immunohistochemistry. Immunohistochemistry using anti-phospho-tau antibody reveals globose tangles, coiled bodies, and threads in the subthalamic nucleus (A). Neuronal loss with extraneural pigment, globose tangles, tufted astrocyte, and coiled bodies are observed in the ventrolateral cell group of substantia nigra (B). Tufted astrocytes are frequent in the motor cortex (C), caudate nucleus (D), and red nucleus (E), accompanied with coiled bodies and threads. Midbrain tectum shows numerous coiled bodies and threads (F). Scale bar = 50 μm.
This new case provides additional evidence that there are no long-term detrimental effects of exposure to large total doses and multiple infusions of tilavonemab. As shown in Table 1, the postexposure time of observation in our new case was more than double that of our previous cases, which emphasizes the safety of this treatment over long time periods. There were no undue effects of medication exposure in neural, glial, or connective tissues.
Table 1:
Clinical information of reported and the present cases.
| Patient 12 | Patient 22 | Patient 31 | Patient 4 | |
|---|---|---|---|---|
| Age, years | 67 | 87 | 73 | 69 |
| Sex | Male | Male | Male | Male |
| Disease duration, years | 5 | 5 | 4 | 7 |
| Total dose, mg | 54,000 | 4,510 | 28,000 | 48,000 |
| Interval, weeks | 57 | 50 | 2 | 117 |
The interval indicates the duration between the last infusion and death.
In conclusion, our findings further support the safety of tilavonemab for the treatment of PSP. Unfortunately, this compound has not provided any substantial benefit for PSP patients; nevertheless, it is important to report pathological observations for this and other similar trials, so they can be used for the future development of disease-modifying compounds.
Acknowledgements
We would like to thank the patient and his family who donated the brain to help further the scientific understanding of neurodegeneration. The authors would like to acknowledge Dr. Peter Davies (Feinstein Institute for Medical Research, LIJ-North Shore Health System, NY) for the monoclonal anti-tau antibody CP13, Nicholas B. Martin (Mayo Clinic) for scanning glass slides, Virginia Phillips (Mayo Clinic) for histologic support, and Monica Castanedes-Casey (Mayo Clinic) for immunohistochemistry support. We also would like to thank Audrey Strongosky (Mayo Clinic) for her assistance in communication with the patient and his family and in brain retrieval process.
Financial Disclosures
Dr. Koga is partially supported by the State of Florida Ed and Ethel Moore Alzheimer’s Disease Research Program and Mayo Clinic ADRC.
Dr. Dickson receives support from the NIH (U54 NS100693, UG3-NS104095, R01 AG062348), and Tau Consortium.
Dr. Wszolek is partially supported by the NIH/NIA and NIH/NINDS (1U19AG063911, FAIN: U19AG063911), Mayo Clinic Center for Regenerative Medicine, the gifts from the Donald G. and Jodi P. Heeringa Family, the Haworth Family Professorship in Neurodegenerative Diseases fund, and The Albertson Parkinson’s Research Foundation. He serves as PI or Co-PI on Biohaven Pharmaceuticals, Inc. (BHV4157-206), Neuraly, Inc. (NLY01-PD-1), and Vigil Neuroscience, Inc. (VGL101-01.002, VGL101-01.201, PET tracer development protocol, Csf1r biomarker and repository project, and ultra-high field MRI in the diagnosis and management of CSF1R-related adult-onset leukoencephalopathy with axonal spheroids and pigmented glia) projects/grants. He serves as Co-PI of the Mayo Clinic APDA Center for Advanced Research and as an external advisory board member for the Vigil Neuroscience, Inc., and as a consultant on neurodegenerative medical research for Eli Lilli & Company. He served as Mayo Clinic Florida site PI for the protocols: AbbVie, Inc. M15–562 (“A Study to Assess Efficacy, Safety, Tolerability, and Pharmacokinetics of ABBV-8E12 in Subjects With Progressive Supranuclear Palsy (PSP)”), AbbVie, Inc. M15–563 (“An Extension Study of ABBV-8E12 in Progressive Supranuclear Palsy (PSP)”), and C2N Diagnostics C2N-8E12-WW-104 (“Safety, Tolerability, and Pharmacokinetics of C2N-8E12 in Subjects With Progressive Supranuclear Palsy”).
Abbreviations:
- PSP
progressive supranuclear palsy
Footnotes
Disclosure of Ethical Statements
Approval of the research protocol: The brain bank operates under procedures approved by the Mayo Clinic Institutional Review Board. De-identified studies of autopsy samples are considered exempt from human subject research by the Mayo Clinic Institutional Review Board.
Informed Consent: The brain autopsy on this patient (Case 1) was performed after consent of the legal next-of-kin.
Registry and the Registration No. of the study/trial: N/A
Animal Studies: N/A
Research involving recombinant DNA: N/A
References
- 1.Beck G, Yamashita R, Kido K, Ikenaka K, Chiba T, Yonenobu Y, et al. An autopsy case of progressive supranuclear palsy treated with monoclonal antibody against tau. Neuropathology 2023. [DOI] [PubMed] [Google Scholar]
- 2.Koga S, Dickson DW, Wszolek ZK. Neuropathology of progressive supranuclear palsy after treatment with tilavonemab. Lancet Neurol 2021; 20: 786–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoglinger GU, Litvan I, Mendonca N, Wang D, Zheng H, Rendenbach-Mueller B, et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: a phase 2, randomised, placebo-controlled trial. Lancet Neurol 2021; 20: 182–92. [DOI] [PubMed] [Google Scholar]
- 4.Kim B, Mikytuck B, Suh E, Gibbons GS, Van Deerlin VM, Vaishnavi SN, et al. Tau immunotherapy is associated with glial responses in FTLD-tau. Acta Neuropathol 2021; 142: 243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]


