Abstract
Background:
Patients with hepatocellular carcinoma (HCC) have been overprioritized in the deceased donor liver allocation system. The United Network for Organ Sharing (UNOS) adopted a policy in May 2019 that limited HCC exception points to median model for end-stage liver disease (MELD) at transplant in the listing region minus 3 (MMaT-3). We hypothesized this policy change would increase the likelihood to transplant marginal quality livers into HCC patients.
Methods:
Retrospective cohort study of a national transplant registry, including adult deceased donor liver transplant recipients with and without HCC from 5/18/2017-5/18/2019 (pre-policy) to 5/19/2019-3/1/2021 (post-policy). Transplanted livers were considered marginal quality if they met ≥1 of the following: 1) donation after circulatory death; 2) donor age ≥ 70; 3) macrosteatosis ≥ 30%; 4) donor risk index≥ 95th percentile. We compared characteristics across policy periods and by HCC status.
Results:
23,164 patients were included (11,339 pre-policy, 11,825 post-policy), 22.7% of whom received HCC exception points (pre-vs. post-policy: 26.1% vs. 19.4%; p=0.03). The percentage of transplanted donor livers meeting marginal quality criteria decreased for non-HCC (17.3% vs. 16.0% p<0.001) but increased for HCC (17.7% vs. 19.4%; p<0.001) pre- vs. post-policy. After adjusting for recipient characteristics, HCC recipients had 28% higher odds of being transplanted with marginal quality liver independent of policy period (OR: 1.28, CI: 1.09 – 1.50; p<0.01).
Conclusion:
The MMaT-3 policy limited exception points and decreased the quality of livers received by HCC patients.
INTRODUCTION
The goal of the liver transplant (LT) allocation system is to ensure fair and equitable access to LT according to the principle of “sickest first”, while attempting to reduce wait-list mortality and improve post-LT outcomes. To prioritize LT candidates based on medical urgency, the Organ Procurement and Transplantation Network (OPTN) / United Network for Organ Sharing (UNOS) introduced the Model for End-Stage Liver Disease (MELD) score in 2002.1 Following MELD score adoption, waitlist mortality was reduced2–3 and the median waiting time for LT decreased 3–5. For patients with hepatocellular carcinoma (HCC) – one of the leading indications for LT - MELD does not accurately capture severity of liver disease, and therefore candidates listed with HCC were eligible for MELD exception points to assign additional priority 6–9.
In the United States, LT candidates with HCC must undergo a thorough assessment before applying for standardized MELD exception. First, based on the Milan Criteria, LT is restricted to candidates with the following characteristics: 1) single tumor with diameter less than 5 cm; 2) no more than 3 tumors, each one not exceeding 3 cm; 3) no angioinvasion; 4) no extrahepatic involvement10. In general, candidates become eligible for HCC MELD exception points if they have an AFP level of less than or equal to 1000 ng/mL and either: 1) one T2 lesion ≥2 cm and ≤5 cm in size; 2) two or three lesions each ≥1 cm and ≤3 cm in size.11 Patients with AFP levels above 1000 ng/mL are eligible to be treated with locoregional therapy to reduce it to below 500 ng/mL, at which point they may be granted MELD exception. Beyond these criteria, candidates can apply for exception points by appealing to the National Liver Review Board for individual case review. The presence of metastatic disease, extrahepatic spread, or macrovascular involvement excludes the candidate from receiving exception points for HCC.11
The adoption of these HCC MELD exception points has provided waitlisted HCC candidates with an advantage over non-exception candidates12. In particular, candidates with HCC were less likely to die or be removed from the wait list and more likely to be transplanted than candidates without exception points13,14. There have been several policy changes to address this inequity. Between January 2005 and October 2015, all LT candidates with HCC received MELD of 22 with an increase in points every 3 months. A 2015 policy adopted a 6-month waiting period prior to awarding MELD exception points to patients with HCC and capped the MELD exception score at 34. Although HCC patients continued to benefit from higher rates of LT than non-exception candidates after the policy was enacted, the risk of waitlist mortality and dropout was found to be comparable between the two groups15. However, regional inequities in access to HCC exception points persisted. Specifically, candidates with HCC listed in OPTN/UNOS regions with long wait times had a 1.7-fold probability of dropout or delisting when compared to short-wait regions patients16
In response to these disparities, OPTN implemented a new policy in May 2019 that capped the HCC exception points score at the median MELD at transplant in the listing region minus 3 points (MMaT-3). Alongside this was the start of the national liver review board (NLRB) that led to central review of all HCC exception requests and decreased the regional variability in HCC exception points. Under the current policy, a candidate who meets the requirements for HCC MELD exception and who is at least 18 years old at the time of registration will appear in the match run according to the calculated MELD score after the initial exception request and the first extension request. After the first extension, eligible HCC candidates receive a MELD score of MMaT-3.
MMaT-3 appears to have successfully narrowed the disparity in the probability of LT between HCC and non-HCC patients across all regions17–18. Given that HCC patients now face de-prioritization, particularly in lower MELD regions, we hypothesized that transplant centers may change their approach toward marginal quality livers (e.g., DCD, older donors, steatosis) to preserve access to LT for HCC patients who now face longer wait times. However, to date, the impact of the MMaT-3 policy on the quality of transplanted livers has not been determined. Therefore, using the U.S. national transplant registry, we evaluated the impact of this policy change on the quality of transplanted livers for patients with and without HCC, hypothesizing that marginal quality livers would be used with higher frequency post-policy implementation for HCC patients.
MATERIALS AND METHODS
Study cohort
We conducted a retrospective cohort study of adult patients (≥18 years old) with and without HCC exception points who underwent deceased donor liver transplant in the United States between May 18, 2017 and March 1, 2021. This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes de-identified data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The study was approved by the Institutional Board Review (IRB) of Mass General Brigham with a waiver of informed consent (#2022P001585).
We defined the ‘HCC’ cohort as all patients who received exception points for HCC. All other patients, i.e., those not receiving exception points for HCC, were included in the non-HCC cohort. Patients were excluded if they had a prior LT, were listed as status 1A, received a living donor LT, were listed at more than one transplant center, or if they were granted MELD exception points for non-HCC indications.
The policy period (pre-policy vs. post-policy) was the main exposure variable. The MMaT-3 policy took effect on May 19, 2019. Patients transplanted between May 18, 2017 and May 18, 2019 were in the pre-policy group, whereas the post-policy policy cohort consisted of patients who were transplanted between May 19, 2019 and March 1, 2021. The pre-policy start date was chosen to allow for balanced pre- and post-policy cohort sizes. The post-policy end date was selected to be March 1, 2021 based on availability of data. The COVID-19 pandemic started midway through the post-policy period and unavoidably confounded the findings. For a subset analysis, we further divided the post-policy period into two eras: 1) pre-COVID-19 (May 19, 2019-March 11, 2020) and COVID-19 (March 12, 2020 - March 1, 2021). March 12, 2020 was the date that the World Health Organization (WHO) declared COVID-19 a pandemic.
Marginal Quality Liver Definition
Our main outcome measure was donor liver quality—specifically, whether a transplanted liver was a marginal quality donor liver. We classified transplanted livers as marginal if they met ≥ 1 of the following: 1) donation after circulatory death19–20 (DCD); 2) donor age ≥ 7021–23 3) biopsy-proven macrosteatosis ≥ 30%24–29 4) donor risk index (DRI) ≥ 95th percentile. In this manner, our primary dependent variable was a binary variable. Since the definition of macrosteatosis requires a liver biopsy, any liver without a biopsy would not be considered marginal in quality unless one or more of the other criteria was met. For descriptive purposes, we also categorized steatosis as 0-14.9%, 15-29.9%, and ≥ 30% steatosis.
Analysis
We first compared recipient and donor characteristics across policy periods and by HCC status. We compared the following recipient characteristics: demographic data, lab and match MELD, primary diagnosis, waiting time, BMI, ABO blood type, comorbidities. We then compared the following donor characteristics: demographic data, donor’s COD, BMI, DCD, donor age ≥ 70, percentage of organs shared nationally, DRI, cold and warm ischemia times, percentage of biopsied livers and macrovesicular fat, and comorbidities. For all categorical variables, we used Pearson chi-squared and Fisher exact tests, while for continuous variables, we used two-sample t-tests (if the data were normally distributed) and rank-sum Wilcoxon tests (if the data were not normally distributed).
Next, as a subset analysis, we divided the post-policy era by timing relative to the COVID-19 pandemic (post-policy/pre-COVID-19 and post-policy/COVID-19) and again compared donor and recipient characteristics across policy periods and by HCC status.
To address the regional variability in the use of marginal quality livers, we compared the main outcome measure of marginal quality donor liver across policy periods and by HCC status in all eleven OPTN regions. Despite the introduction of the Acuity Circles (AC) policy in February 2020, we conducted the regional analyses using the previous system of regional boundaries because of the dynamically changing nature of the AC policy and the fact that it came into effect midway through the post-policy era and guided allocation during most of the study timeframe.
To account for differences in recipient characteristics before and after the MMaT-3 policy change, we performed a multivariable logistic regression to estimate the covariate-adjusted association of policy period with our primary outcome of marginal quality transplanted liver. In other words, this model estimated the odds that a transplanted liver in the post policy period was marginal in quality compared to a transplanted liver in the pre-policy period, after adjusting for other differences between the pre- and post-policy period recipient cohorts. We did not adjust for other donor characteristics in this model since our outcome—marginal quality liver—was defined by donor characteristics. Model covariate selection was guided by univariable analysis and clinical relevance. We first conducted a univariable logistic regression for each of the recipient characteristics. Any variable with a P<0.200 was included as a covariate in the final multivariable model. Age, gender, race, and ethnicity were included in the final multivariable model for their clinical relevance regardless of statistical significance.
To determine if the effect of the MMaT-3 policy change on transplanted liver quality was different for HCC vs. non-exception patients, i.e., effect modification, we tested an interaction term between policy-period and HCC status. We also tested the interaction between OPTN region and policy period to ascertain whether the effect of policy-period on transplanted liver quality differed by region.
Finally, we compared alpha-fetoprotein (AFP) values and tumor characteristics of HCC patients across the policy periods, including maximum and most recent AFP values; mean number of tumors at the time of exception points and pre-transplant; and maximum tumor size while waitlisted and at the time of exception points. We also compared the frequency of pre-transplant surgical resection and locoregional therapy by policy-period. Locoregional therapies recorded in the SRTR primarily represent chemoembolization, while other types (i.e., chemical, cryotherapy, and radiofrequency ablation) are not well recorded.
Sensitivity Analysis
We then performed an additional sensitivity analysis, where we sought to evaluate the effect of the AC policy on the primary outcome. The AC policy was introduced on 4 February 2020 and replaced the Donor Service Area (DSA) as the unit of allocation, in favor of 250 nautical mile allocation circles centered on the donor hospital, to address regional disparities in access to transplant and prioritize patients with higher MELD or illness acuity. For this analysis, we redefined pre-policy era to include patients who received exception points for HCC between the start of the study timeframe (5/19/2017) and the beginning of the AC (2/4/2020). We calculated the covariate-adjusted percentages of HCC and non-HCC patients that met the criteria for marginal quality liver.
RESULTS
Donor and Recipient Characteristics
A total of 23,164 LT recipients met the inclusion criteria, (11,339 [49.0%] pre-policy period; 11,825 [51.0%] post-policy period). Of these patients, 5,246 (22.6%) received HCC exception points (2,954 [56.3%] pre-policy; 2,292 [43.7%] post-policy). The percentage of candidates with HCC exception points decreased from 26.1% in the pre-policy period to 19.4% in the post-policy period (pre-vs. post-policy: 26.1% vs. 19.4%; p=0.03).
Demographic and clinical characteristics of LT recipients are shown in Table 1a. When compared to pre-policy era, non-HCC candidates in the post-policy period had a shorter waiting time for LT (158.4 days vs. 172.8 days; p<0.001), were less likely to be on life support at transplant (8.9% vs. 12.1%; p<0.001), and less likely to undergo dialysis within 1 week pre-transplant (22.0% vs. 23.3%; p=0.04). In addition, the percentage of female non-HCC LT recipients increased in the post-policy era (37.9% vs. 36.3%; p=0.03). In the HCC cohort, there were fewer white (63.2% vs. 66.2%; p=0.02) and more Latino candidates (21.1% vs. 17.4%; p<0.001) in the post-policy era. Furthermore, HCC LT recipients had a higher lab MELD (13.3 vs. 12.7; p<0.001), but a lower match MELD at transplant after the policy was enacted (25.5 vs. 28.2; p<0.001).
Table 1a. Demographic and clinical characteristics of liver transplant recipients with and without exception points for hepatocellular carcinoma in pre- and post-MMaT-3 periods.
Numbers represent total count (%) and means (SD).
| non-HCC | HCC | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | pre-MMaT-3 N=8,385 |
post-MMaT-3 N=9,533 |
p-value | pre-MMaT-3 N=2,954 |
post-MMaT-3 N=2,292 |
p-value |
| Age | 54.40 (10.84) | 54.04 (11.33) | 0.03 | 62.06 (6.74) | 62.57 (7.02) | 0.01 |
| Gender: | 0.03 | 0.77 | ||||
| Male | 5,341 (63.7%) | 5,922 (62.1%) | 2,282 (77.2%) | 1,762 (76.9%) | ||
| Female | 3,044 (36.3%) | 3,611 (37.9%) | 673 (22.8%) | 530 (23.1%) | ||
| Race | 0.89 | 0.02 | ||||
| White | 6,090 (72.6%) | 6,915 (72.5%) | 1,957 (66.2%) | 1,449 (63.2%) | ||
| Non-White | 2,295 (27.4%) | 2,618 (27.5%) | 997 (33.8%) | 843 (36.8%) | ||
| Ethnicity | 0.09 | <0.001 | ||||
| Latino | 1,279 (15.3%) | 1,542 (16.2%) | 513 (17.4%) | 484 (21.1%) | ||
| Non-Latino | 7,106 (84.7%) | 7,991 (83.8%) | 2,441 (82.6%) | 1,808 (78.9%) | ||
| Lab MELD | 23.18 (7.02) | 23.62 (9.87) | 0.68 | 12.69 (6.17) | 13.29 (6.74) | <0.001 |
| Match MELD at Transplant | 28.36 (8.70) | 28.27 (8.53) | 0.48 | 28.15 (4.99) | 25.54 (5.23) | <0.001 |
| Primary Diagnosis | <0.001 | <0.001 | ||||
| HCV | 808 (9.6%) | 503 (5.3%) | 783 (26.5%) | 452 (19.7%) | ||
| Alcohol | 3,431 (40.9%) | 4,421 (46.4%) | 407 (13.8%) | 325 (14.2%) | ||
| NASH | 1,951 (23.3%) | 2,227 (23.4%) | 283 (9.6%) | 298 (13.0%) | ||
| HCC | 151 (1.8%) | 149 (1.6%) | 1,241 (42.0%) | 1,024 (44.7%) | ||
| Other | 2,044 (24.4%) | 2,233 (23.4%) | 240 (8.1%) | 193 (8.4%) | ||
| Waiting Time (Days) | 172.75 (410.39) | 153.38 (383.44) | 0.001 | 372.93 (483.86) | 344.66 (407.99) | 0.19 |
| BMI: | 29.16 (9.23) | 28.51 (9.98) | 0.43 | 29.72 (9.08) | 29.08 (9.76) | 0.70 |
| ABO Blood Type | 0.35 | 0.31 | ||||
| A | 3,033 (36.2%) | 3,498 (36.7%) | 1,131 (38.3%) | 821 (35.8%) | ||
| AB | 454 (5.4%) | 505 (5.3%) | 125 (4.2%) | 107 (4.7%) | ||
| B | 1,196 (14.3%) | 1,274 (13.4%) | 393 (13.3%) | 318 (13.9%) | ||
| O | 3,702 (44.2%) | 4,256 (44.6%) | 1,305 (44.2%) | 1,046 (45.6%) | ||
| On Life Support at Transplant | 1,016 (12.1%) | 852 (8.9%) | <0.001 | 30 (1.0%) | 30 (1.3%) | 0.32 |
| Dialysis Within 1 Week Pre-Transplant | 1,955 (23.3%) | 2,098 (22.0%) | 0.04 | 72 (2.4%) | 68 (3.0%) | 0.24 |
| Prior Abdominal Surgery | 3,639 (43.4%) | 3,915 (41.1%) | 0.002 | 1,342 (45.4%) | 1,037 (45.2%) | 0.89 |
| Diabetes | 2,347 (28.0%) | 2,603 (27.6%) | 0.52 | 1,104 (37.4%) | 909 (39.7%) | 0.09 |
| Preoperative Portal Vein Thrombosis | 708 (8.4%) | 822 (8.6%) | 0.67 | 231 (7.8%) | 173 (7.5%) | 0.71 |
| Region | 0.005 | 0.011 | ||||
| 1 | 279 (3.3%) | 252 (2.6%) | 105 (3.6%) | 85 (3.7%) | ||
| 2 | 956 (11.4%) | 1,022 (10.7%) | 390 (13.2%) | 310 (13.5%) | ||
| 3 | 1,520 (18.1%) | 1,797 (18.9%) | 424 (14.4%) | 325 (14.2%) | ||
| 4 | 767 (9.1%) | 876 (9.2%) | 336 (11.4%) | 236 (10.3%) | ||
| 5 | 1,256 (15.0%) | 1,367 (14.3%) | 407 (13.8%) | 374 (16.3%) | ||
| 6 | 265 (3.2%) | 345 (3.6%) | 142 (4.8%) | 104 (4.5%) | ||
| 7 | 672 (8.0%) | 670 (7.0%) | 295 (10.0%) | 208 (9.1%) | ||
| 8 | 590 (7.0%) | 737 (7.7%) | 156 (5.3%) | 145 (6.3%) | ||
| 9 | 286 (3.4%) | 331 (3.5%) | 121 (4.1%) | 101 (4.4%) | ||
| 10 | 830 (9.9%) | 987 (10.4%) | 203 (6.9%) | 181 (7.9%) | ||
| 11 | 964 (11.5%) | 1,149 (12.1%) | 375 (12.7%) | 223 (9.7%) | ||
p-value compares non-HCC to HCC LT recipients in a given policy period.
Demographic and clinical characteristics of LT donors are shown in Table 1b. In the post-policy period, deceased donors whose livers were transplanted into HCC candidates were older when compared to pre-policy (44.8 vs. 42.9; p<0.001). In the non-HCC group, patients transplanted post-policy received more livers from donors who were HCV Ab+ (10.5% vs. 8.8%; p<0.001) and HCV NAT+ (6.6% vs. 5.8%; p=0.03) In both cohorts, the percentage of organs shared nationally increased significantly in the post-MMaT-3 era.
Table 1b. Demographic and clinical characteristics of liver transplant donors whose livers were transplanted into HCC or non-HCC recipients in pre- and post-MMaT-3 periods.
Numbers represent total count (%) and means (SD).
| non-HCC | HCC | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | pre-MMaT-3 N=8,385 |
post-MMaT-3 N=9,533 |
p-value | pre-MMaT-3 N=2,954 |
post-MMaT-3 N=2,292 |
p-value | |
| Marginal Liver Characteristics | |||||||
|
| |||||||
| Donation After Cardiac Death | 657 (7.8%) | 873 (9.2%) | 0.002 | 243 (8.2%) | 331 (14.4%) | <0.001 | |
| Donor Aged 70 or More | 279 (3.3%) | 269 (2.8%) | 0.05 | 145 (4.9%) | 136 (5.9%) | 0.1 | |
| DRI | 1.45 (0.41) | 1.48 (0.41) | 0.001 | 1.46 (0.41) | 1.56 (0.43) | <0.001 | |
| Macrosteatosis ≥ 30% | 340 (4.1%) | 340 (3.6%) | 0.09 | 117 (4.0%) | 100 (4.4%) | 0.47 | |
|
| |||||||
| Age | 41.23 (15.66) | 40.83 (15.30) | 0.08 | 42.97 (16.60) | 44.79 (16.24) | <0.001 | |
| Gender | 0.35 | 0.88 | |||||
| Male | 5,125 (61.1%) | 5,891 (61.8%) | 1,783 (60.4%) | 1,388 (60.6%) | |||
| Female | 3,260 (38.9%) | 3,642 (38.2%) | 1,171 (39.6%) | 904 (39.4%) | |||
| Race | 0.79 | 0.28 | |||||
| White | 5,365 (64.0%) | 6,081 (63.8%) | 1,938 (65.6%) | 1,471 (64.2%) | |||
| Non-White | 3,020 (36.0%) | 3,452 (36.2%) | 1,016 (34.4%) | 821 (35.8%) | |||
| Ethnicity | 0.74 | 0.65 | |||||
| Latino | 1,222 (14.6%) | 1,406 (14.7%) | 397 (13.4%) | 318 (13.9%) | |||
| Non-Latino | 7,163 (85.4%) | 8,127 (85.3%) | 2,557 (86.6%) | 1,974 (86.1%) | |||
| Donor/s Cause of Death | <0.001 | 0.002 | |||||
| Anoxia | 3,517 (41.9%) | 4,319 (45.3%) | 1,248 (42.2%) | 1,026 (44.8%) | |||
| CVA | 2,265 (27.0%) | 2,388 (25.0%) | 884 (29.9%) | 698 (30.5%) | |||
| Head Trauma | 2,390 (28.5%) | 2,589 (27.2%) | 758 (25.7%) | 499 (21.8%) | |||
| CNS Tumor | 30 (0.4%) | 24 (0.3%) | 13 (0.4%) | 7 (0.3%) | |||
| Other | 183 (2.2%) | 213 (2.2%) | 51 (1.7%) | 62 (2.7%) | |||
| BMI | 28.28 (6.71) | 28.22 (6.77) | 0.53 | 28.37 (6.63) | 29.02 (7.28) | 0.001 | |
| Organ Shared Nationally | 3,315 (39.5%) | 5,218 (54.7%) | <0.001 | 721 (24.4%) | 979 (42.7%) | <0.001 | |
| Cold Ischemia Time (hours) | 5.92 (2.12) | 5.96 (2.02) | 0.11 | 5.90 (2.24) | 5.93 (2.03) | 0.64 | |
| Warm Ischemia Time (minutes) | 14.88 (10.60) | 12.28 (10.78) | <0.001 | 15.92 (9.69) | 14.51 (9.21) | 0.12 | |
| Donor HCV Ab | 736 (8.8%) | 1,001 (10.5%) | <0.001 | 327 (11.1%) | 235 (10.3%) | 0.34 | |
| Donor HCV NAT | 484 (5.8%) | 625 (6.6%) | 0.03 | 224 (7.6%) | 145 (6.3%) | 0.07 | |
| Diabetes | 1,076 (12.8%) | 1,230 (12.9%) | 0.89 | 443 (15.0%) | 357 (15.6%) | 0.56 | |
| High Risk of Disease Transmission | 2,458 (29.3%) | 2,868 (30.1%) | 0.26 | 878 (29.7%) | 633 (27.6%) | 0.095 | |
| Liver Biopsied | 3,298 (39.3%) | 3,859 (40.5%) | 0.032 | 1,311 (44.4%) | 1,122 (49.3%) | <0.001 | |
| % Macrovesicular Fat on Biopsy | 0.23 | 0.04 | |||||
| No Biopsy | 5,087 (60.7%) | 5,861 (59.5%) | 1,643 (55.5%) | 1,221 (50.7%) | |||
| No result/Result Unavailable | 97 (1.0%) | 187 (1.9%) | 34 (1.2%) | 51 (4.5%) | |||
| 0-14.9% Steatosis | 2,461 (29.4%) | 2,888 (30.3%) | 1,012 (34.3%) | 827 (36.1%) | |||
| 15-29.9% Steatosis | 400 (4.8%) | 444 (4.7%) | 148 (5.0%) | 144 (6.3%) | |||
| 30+% Steatosis | 340 (4.1%) | 340 (3.6%) | 117 (4.0%) | 100 .4%) | |||
p-value compares non-HCC to HCC LT recipients in a given policy period.
When the post-policy era was divided into two periods to account for the COVID-19 pandemic, no major changes in donor and recipient demographic characteristics were found between the pre- and post-COVID portions of the post-policy era (Supplemental Tables 1a and 1b). Of note, the percentage of nationally shared organs increased significantly in the post-policy/COVID era for all patients when compared to the pre-policy and post-policy/pre-COVID eras.
Donor liver quality
In the era after the policy was enacted, older donor livers were less often transplanted into non-HCC patients (3.3% vs.2.8%, p=0.05) but were equally likely to be transplanted into HCC patients (4.9% vs. 5.9%, p=0.11). Both HCC (1.46 vs. 1.56; p<0.001) and non-HCC (1.45 vs. 1.48; p=0.001) recipients were found to have received donor livers with a significantly higher donor risk index in the post-policy era when compared to pre-policy era. Similarly, both HCC (8.2% vs. 14.4%; p<0.001) and non-HCC (7.8% vs. 9.2%; p=0.002) had a significantly higher DCD donors in the post-policy period. There were no significant pre- vs. post-policy differences in the percentage of recipients who received livers with macrosteatosis ≥ 30% (Table 1b). After adjusting for key recipient characteristics, the percentage of transplanted donor livers meeting the criteria for a marginal quality liver after the policy was introduced decreased for non-HCC (17.3% vs. 16.0%; p<0.001) and increased for HCC (17.7% vs. 19.4%; p<0.001; Figure 1).
Figure 1. Comparison of adjusted percentages of liver transplant (LT) with a marginal quality liver among HCC and non-HCC recipients in pre- and post-MMaT-3 periods.
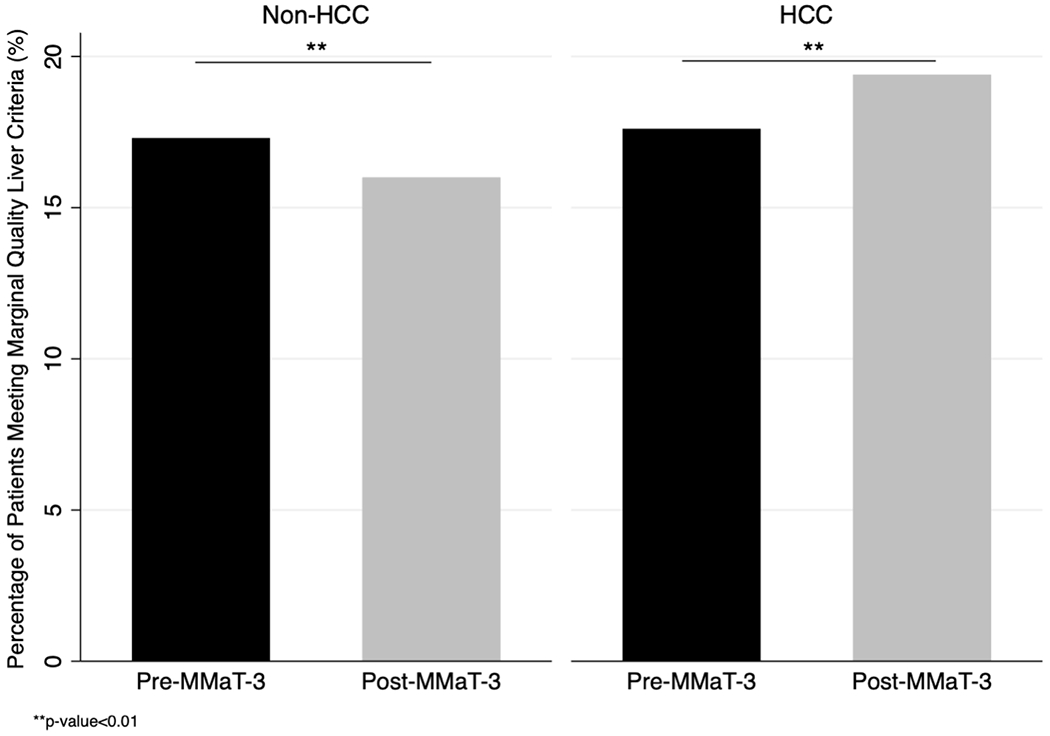
Transplanted livers were classified as marginal if they met ≥1 of the following: 1) donation after circulatory death; 2) donor age≥70; 3) macrosteatosis ≥ 30%; 4) donor risk index≥ 95th percentile.
Table 2 shows the results of the logistic regression modelling of donor liver marginal quality. Following the adjustment for key recipient covariates, recipients with HCC exception points had 28% higher odds of being transplanted with a liver of marginal quality (OR: 1.28; CI: 1.09 – 1.50; p<0.01). Additionally, both non-White (OR: 0.88: CI: 0.79 – 0.99; p=0.03) and Hispanic (OR: 0.86; CI: 0.75 – 0.99; p=0.04) LT recipients were found to have lower likelihood of receiving a marginal quality liver in the post-policy era. For all LT recipients in the study timeframe, there was no difference in the likelihood of transplantation with a marginal quality liver (OR:1.03; CI:0.95 – 1.12; p=0.45).
Table 2. Univariable and multivariable logistic regression model of recipient factors associated with marginal quality liver.
Clinically relevant variables or variables with p-value < 0.2 in the univariable analysis were entered into the multivariable model. Odds ratio (OR) can be interpreted as the change in odds a transplanted liver being marginal quality for a given change in the explanatory variable.
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| HCC Status | 1.38 | 1.28 - 1.49 | <0.001 | 1.28 | 1.09 - 1.50 | 0.002 |
| Policy Period | 1.13 | 1.06 - 1.21 | <0.001 | 1.03 | 0.95 - 1.12 | 0.45 |
| Age | 1.01 | 1.01 - 1.02 | <0.001 | 1.01 | 1.01 - 1.02 | <0.001 |
| Male | 0.99 | 0.91 - 1.05 | 0.42 | 1.07 | 0.99 - 1.15 | 0.08 |
| Race: Non-White | 0.85 | 0.77 - 0.92 | 0.001 | 0.88 | 0.79 - 0.99 | 0.03 |
| Ethnicity: Latino | 1.05 | 0.96 - 1.15 | 0.31 | 0.86 | 0.75 – 0.99 | 0.04 |
| Lab MELD | 0.95 | 0.94 - 0.96 | 0.001 | 0.98 | 0.97 - 0.99 | <0.001 |
| Match MELD at Transplant | 0.92 | 0.91 - 0.93 | <0.001 | 0.97 | 0.96 - 0.97 | <0.001 |
| Primary Diagnosis | ||||||
| HCV | 1.08 | 0.96 - 1.21 | 0.18 | 0.92 | 0.81 - 1.06 | 0.19 |
| Alcohol | 0.68 | 0.61 - 0.76 | 0.001 | 0.86 | 0.67 - 1.06 | 0.87 |
| NASH | 0.89 | 0.79 - 1.01 | 0.06 | 0.96 | 0.85 - 1.08 | 0.45 |
| Waiting Time (Log-transformed Days) | 1.14 | 1.08 - 1.18 | <0.001 | 1.01 | 0.98 - 1.03 | 0.48 |
| BMI: | 1 | 0.99 - 1.01 | 0.29 | |||
| ABO Blood Type | ||||||
| A | 1 | 1 | ||||
| AB | 0.63 | 0.52 – 0.76 | <0.001 | 0.44 | 0.36 – 0.52 | <0.001 |
| B | 0.74 | 0.66 - 0.83 | 0.001 | 0.88 | 0.77 - 0.99 | 0.03 |
| O | 1.11 | 1.04 - 1.21 | 0.04 | 1.14 | 1.06 - 1.24 | 0.001 |
| On Life Support at Transplant | 3.24 | 2.70 - 3.99 | <0.001 | 1.06 | 0.87 - 1.31 | 0.53 |
| Dialysis Within 1 Week Pre-Transplant | 3.62 | 3.19 - 4.12 | <0.001 | 1.61 | 1.39 - 1.87 | <0.001 |
| Prior Abdominal Surgery | 0.94 | 0.79 – 1.09 | 0.37 | |||
| Diabetes | 1.26 | 1.17 – 1.35 | <0.001 | 1.02 | 0.95 - 1.11 | 0.51 |
| Preoperative Portal Vein Thrombosis | 0.92 | 0.79 - 1.03 | 0.12 | 0.95 | 0.84 - 1.10 | 0.52 |
| Region | ||||||
| 1 (Ref) | 1 | 1 | ||||
| 2 | 1.16 | 0.92 – 1.46 | 0.21 | 1.01 | 0.79 - 1.28 | 0.94 |
| 3 | 1.02 | 0.82 - 1.28 | 0.21 | 0.74 | 0.58 – 0.93 | 0.01 |
| 4 | 1.12 | 0.89 - 1.42 | 0.34 | 0.96 | 0.74 - 1.21 | 0.73 |
| 5 | 1.49 | 1.19 - 1.80 | 0.001 | 1.64 | 1.30 - 2.08 | 0.001 |
| 6 | 1.11 | 0.85 - 1.47 | 0.44 | 0.93 | 0.71 - 1.25 | 0.66 |
| 7 | 1.66 | 1.31 - 2.09 | <0.001 | 1.51 | 1.23 – 2.01 | <0.001 |
| 8 | 1.29 | 1.01 – 1.64 | 0.04 | 0.88 | 0.69 - 1.14 | 0.34 |
| 9 | 1.23 | 0.94 – 1.62 | 0.13 | 1.49 | 1.11 – 1.98 | 0.006 |
| 10 | 1.43 | 1.13 – 1.80 | 0.002 | 0.99 | 0.77 – 1.26 | 0.92 |
| 11 | 1.01 | 0.81 - 1.21 | 0.76 | 0.60 – 0.98 | 0.03 | |
We tested the interaction between the HCC status and policy period and found it was statistically significant (p<0.001), which indicated that the effect of the policy period on liver quality differed for HCC vs. non-HCC patients.
Regional analysis
Figure 2 shows the map of the United States with OPTN regions and corresponding median MELD at transplant scores. We compared unadjusted proportions of marginal quality transplanted livers pre/post policy by HCC status and by region (Figure 3). Region 6 had a significantly higher post-MMaT-3 percentage of marginal quality liver use in both HCC (13.2% vs. 27.3%; p<0.001) and non-HCC (12.1% vs. 18.9%; p<0.05). Post-policy, region 2 (19.7% vs. 26.4%; p<0.05), region 3 (15.9% vs. 25.8%; p<0.001), region 8 (13.5% vs. 32.5%; p<0.001), region 10 (17.1% vs. 30.0%; p<0.01), and region 11 (12.7% vs. 24.9%; p<0.001) all had a significantly higher use of marginal quality livers for HCC patients only. After adjustment for recipient characteristics, utilizing an interaction term between policy period and region to allow the effect of the policy change to differ by region, we found that only HCC recipients in region 6 (OR: 2.5, CI:1.4 – 3.9, p<0.01), region 8 (OR: 3.6, CI:1.7 – 6.1, p<0.001), region 11 (OR: 3.2; CI: 2.0 – 5.2; p<0.001) were more likely to receive a marginal quality liver.
Figure 2.
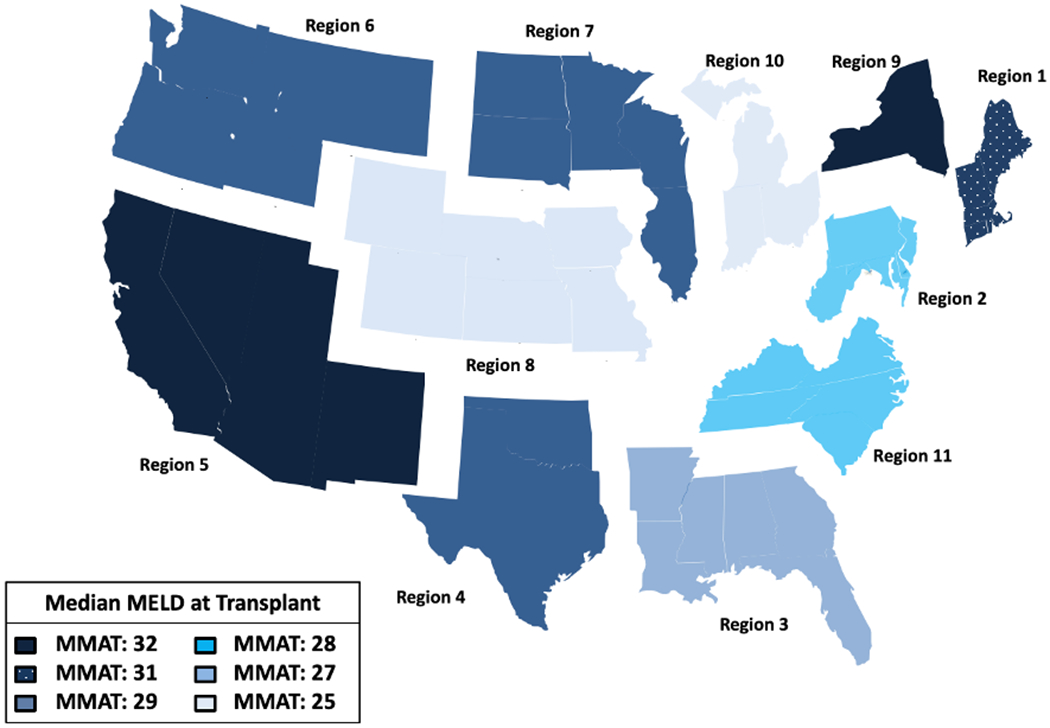
The breakdown of the United States into 11 OPTN regions with pre-MMaT-3 median MELD at transplant scores.
HCC patients waitlisted in regions 5 and 9 are now assigned a MELD score of 29. Under the current policy, they are not deprioritized until they first receive exception for extension approval, which would have increased their MELD above 29 in the pre-MMaT-3 era. Patients in region 5 now face an identical challenge. The remaining patients listed in regions with MMAT<31 are now deprioritized across the board because they will never attain as high a MELD score as they would have under the previous policy.
Figure 3. Comparison of regional differences in unadjusted frequencies of marginal quality liver transplant (LT) among HCC and non-HCC patient in pre- and post-MMaT-3 period.
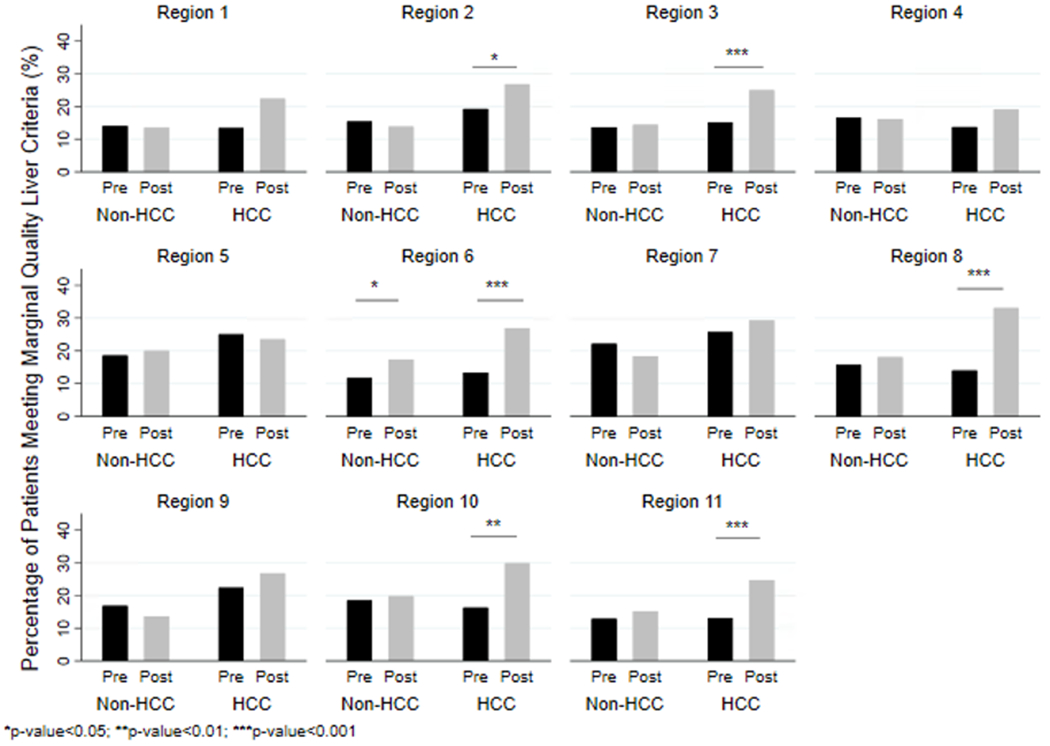
Transplanted livers were classified as marginal if they met ≥1 of the following: 1) donation after circulatory death; 2) donor age ≥ 70; 3) macrosteatosis ≥ 30%; 4) donor risk index ≥ 95th percentile.
HCC LT Recipient Characteristics:
Table 3 shows tumor characteristics, prevalence of surgical resection, and prevalence of locoregional therapy use for transplant recipients in the pre- vs. post-policy groups. Among HCC LT recipients, both mean (51.4 vs. 27.0; p=0.003) and most recent AFP (49.3 vs. 26.2; p<0.001) decreased significantly after the MMaT-3 policy came into the effect. In addition, post-policy, we found that the mean tumor size while waitlisted was significantly higher (1.11 vs. 1.52; p<0.001). After the policy was enacted, fewer HCC candidates underwent locoregional therapies compared to pre policy (32.1% vs. 19.8%; p=0.004).
Table 3.
Tumor characteristics of patients with hepatocellular carcinoma (HCC) exception points in pre- and post-MMaT-3 periods.
| pre-MMaT-3 N=2,954 |
post-MMaT-3 N=2,292 |
p-value | |
|---|---|---|---|
|
| |||
| Max AFP while waitlisted | 71.82 ± 425.87 | 41.9 ± 112.96 | 0.11 |
| Last AFP while waitlisted | 49.26 ± 355.43 | 26.23 ± 78.26 | <0.001 |
| Mean number tumors at time of exception points | 1.15 ± 0.45 | 1.14 ± 0.44 | 0.62 |
| Maximum tumor size at time of exception points | 1.61 ± 0.92 | 1.85 ± 0.81 | 0.19 |
| Maximum tumor size while waitlisted (cm) | 1.11 ± 1.35 | 1.52 ± 1.47 | <0.001 |
| Surgical resection | 48 (1.6%) | 43 (1.9%) | 0.76 |
| Locoregional Therapies | 948 (32.1%) | 453 (19.8%) | 0.004 |
Sensitivity Analysis:
In the sensitivity analysis, where we considered the effect of the AC policy on the donor liver quality in non-HCC and HCC patients, older donor livers continued to be less often transplanted into non-HCC patients in the post-policy era (3.4% vs. 2.4%; p=0.02) and DCD livers continued to be more often transplanted into both HCC (9.2% vs. 16.2%; p<0.001) and non-HCC patients (8.1% vs. 9.%; p=0.002). Contrary to the pre- vs. post-MMaT-3 analysis, HCC recipients received more livers with macrosteatosis >30% in the post-AC period (3.9% vs. 4.9%;p=0.007; Supplemental Table 2) After adjusting for key recipient characteristics, the percentage of transplanted donor livers that met the criteria for a marginal organ after the adoption of AC Policy was lower for non-HCC (17.2% vs. 16.3%; p<0.001) and higher for HCC (18.1% vs. 20.9%; p<0.001; Supplemental Figure 1).
DISCUSSION
In the present study, we assessed the impact of the MMaT-3 policy on the quality of transplanted deceased donor livers in recipients with and without HCC exception points. The policy that attempted to deprioritize LT for candidates with HCC has led to adverse changes in the quality of livers transplanted into patients with HCC. Non-HCC patients became less likely to receive a liver of marginal quality in the post-policy era, though the absolute difference between the two groups was modest. Further, the implementation of the policy resulted in regional differences in the likelihood of transplanting marginal quality livers into HCC patients. In particular, the likelihood of receiving a marginal quality liver increased for HCC patients in regions 6, 8, and 11, whereas no region was noted to have a significant pre- vs. post-policy difference in marginal quality liver use among non-HCC candidates.
The increasing prevalence of marginal quality livers among candidates with HCC reported in this study confirms our hypothesis that the implementation of the MMaT-3 national policy would result in a heightened utilization of these organs in the post-policy era. Changes in the individual components of marginal quality criteria selected here could be responsible for our findings. Recent studies suggest that the adoption of the MmaT-3 policy has reduced the disparity in access to LT between HCC and non-HCC candidates. Bernards et al.18 reported nearly identical incidence rates of waitlist dropout among patients with and without HCC post-policy, while the probability of LT within 1 year of listing among HCC candidates in shorter waiting regions decreased from 73.0% to 55.4%. A subsequent study by Shaikh et al.17 corroborated these results by demonstrating that HCC patients had an 80% higher LT probability than non-HCC in the pre-policy era, which decreased to 14% higher probability in the MmaT-3 era.
In the advent of machine perfusion (MP) technologies, a more robust utilization of DCD organs without compromised graft outcomes has been reported.30–33 Although the current dataset provides only limited information on the use of MP across transplant centers in the US, the heightened need to rely on DCD livers is the likely reason why HCC patients continue to exhibit comparable probabilities of LT to non-HCC patients. A larger absolute increase in the use of DCD livers among HCC candidates when compared to non-HCC patients could be driving the overall difference in the percentage of organs meeting the composite marginal liver quality criteria between the two groups. Alarmingly, United States DCD LT has been shown to be driven by a few high-utilization transplant centers. In fact, Hobeika et al. (2020) reported that between 2013 and 2017, only 11 centers performed ≥ 50 DCD LT, which amounted to nearly half of national DCD LTs.34 These centers were primarily concentrated in regions 5 and 7, both of which also exhibited the highest baseline utilization of marginal quality livers in the pre-MMaT-3 era in our investigation. Regarding the increased percentage of post-policy use of marginal quality livers among HCC LT candidates in regions 6, 8, and 11, it can be argued this may be reflective of a higher aggressiveness of organ procurement in a few centers in these regions, perhaps coinciding with changes in center philosophy regarding marginal quality organ use or broader acceptance of MP techniques after the implementation of MMaT-3. Overall, though a successful use of marginal quality grafts should be celebrated as a means of improving organ quality, not all centers are willing or able to transplant marginal organs or utilize machine perfusion yet, and thus patients with HCC may not have equal access to these livers.
The increase in the donor DRI in both HCC and non-HCC patients could be explained by the fact that the calculation for DRI classifies nationally shared livers as more marginal. By nature of broader organ sharing under the AC Policy, reflected by a higher proportion of nationally shared organs in the post-MMaT-3 era, a marked increase in the DRI had been expected. However, it is important to note that the DRI should be overestimated equally for both HCC and non-HCC patients. Given the efforts of the MMaT-3 policy to deprioritize HCC candidates from accessing LT, we had not expected to find the waiting time for LT to decrease from pre- to post-policy periods for both HCC and non-HCC patients. Though surprising, this finding could be explained by the effect of the AC Policy which has allowed broader organ sharing across larger geographic areas. This seems to be supported by the steep increase in the proportion of nationally shared organs in the post-MMaT-3 when compared to the pre-MMaT-3 era.
Our results are consistent with a recent study by Shah et al. (2022)35 who evaluated the impact of four significant policy changes in LT on the proportion of donor service area (DSA)-level transplants for HCC as well as the characteristics of organ quality, including DCD and DRI. Though the authors defined the MMaT-3 era as the time period from 5/2019-2/2020, thereby not including the population of HCC patients that were transplanted during the COVID-19 pandemic (after 2/2020), we showed very similar percentages of DCD donors and almost identical DRI values across both policy periods in HCC candidates. Our findings and those by Shah and colleagues are consistent in that the quality of transplanted livers into HCC candidates has decreased after the recent allocation policy changes. Additional strengths of our study include a large sample size of nearly 25,000 LT recipients and the assessment of the regional variability in the use of marginal quality livers among HCC and non-HCC patients before and after the policy went into effect.
The use of marginal quality organs has attracted attention in recent years. In the setting of a gap between organ demand and supply in the United States, exacerbated by high rates of liver graft discards19–20, there is a pressing need to expand the pool of transplantable livers. To rectify this shortage, a broader utilization of marginal quality organs with careful selection of both donor and recipient risks has become more prevalent. While a standardized definition of what constitutes a marginal liver has not been adopted, recent evidence suggests that many grafts historically regarded as suboptimal quality can in fact be transplanted with good outcomes. For example, well-selected older donor livers (e.g., ages 60-80) can be safely used in the right recipients21–23. Recent reports have also shown improving outcomes for transplanted livers with ≥30% macrosteatosis on biopsy, with similar graft and patient survival in steatotic compared to non-steatotic livers24–29. Given mounting evidence that using marginal livers does not result in inferior outcomes, efforts to optimize the procurement and transplantation of marginal livers may improve access to life-saving LT when transplanted into appropriate recipients. In general, a more aggressive approach toward transplanting marginal quality organs is one strategy to improve access to LT. Since patients with HCC are less physiologically ill, as reflected by their lower calculated MELD scores, they may potentially be an ideal group of candidates to benefit from an expanded use of marginal organs, including the DCD organs, provided that they are listed in centers which practice a more liberal approach to marginal organ utilization. However, the potential of allocation policies to lead to systematic increases in use of marginal quality livers in certain groups should be monitored. Therefore, it is particularly critical to ensure that future changes to allocation policies do not negatively impact post-transplant outcomes for HCC patients, whether it be regionally or nationally, thereby creating additional disparities.
Our study was limited by a short follow-up time for the candidates transplanted in the post-policy era. The MMaT-3 policy took effect shortly before the onset of the COVID-19 pandemic, and thus it is difficult to discern the effect of the policy change from the effects of the concurrent pandemic. In an exploratory analysis in which we divided the post-policy era into two periods based on the COVID-19 pandemic, we did not observe significant differences in donor and recipient demographic characteristics between the two eras. Consistent with recent reports in the literature regarding the effect of the pandemic on the LT volume36–37, our data show that the total number of LT from March 2020 to March 2021 was not adversely impacted among both HCC and non-HCC recipients. In terms of the individual characteristics of marginal liver quality, non-HCC liver donors were significantly younger in the post-MMaT-3, COVID period than in the other two eras and were transplanted with significantly fewer livers from donors aged 70 or more. In both groups, the DRI was highest in the post-MMaT-3, COVID period. Both groups also saw the highest use of DCD livers in the post-MMaT-3, COVID era.
Other policy revisions that came into effect during the study time frame should be considered. The national liver review board (NLRB) was implemented at the time of the MMaT-3 policy change, which limited our ability to ascertain whether the introduction of NLRB contributed to our findings. In February 2020, to address regional disparities in access to transplant and prioritize patients with higher MELD or illness acuity, the AC policy replaced the Donor Service Area (DSA) as the unit of allocation. Despite this change, we had elected to perform the regional analyses using the previous system of regional boundaries. This is not only due to the dynamically changing nature of the AC policy, including the recent introduction of the concentric circles around the donor hospital for assessing candidate’s appropriateness for allocation38, but also the fact that the DSA guided allocation practices throughout most of the study timeframe.
The AC policy had originally been approved to go into effect alongside the MMaT-3 policy in May 2019; yet the transition to this new liver distribution model was temporarily halted by the federal court15. As a consequence, the liver allocation system reverted back to DSA-based, and the MMaT-3 was computed using the DSA-level median MELD scores and patient characteristics. Given the fact that the implementation of the AC policy took place in close proximity to the adoption of the MMaT-3 and the NLRB, as well as coincided with the COVID-19 pandemic, we were unable to fully assess its distinct effect on the organ quality in HCC and non-HCC recipients. That said, in our sensitivity analysis, the use of marginal quality livers continued to be lower for non-HCC patients and higher for HCC candidates in the post-AC period. It is likely that a significant increase in the percentage of nationally shared livers in both groups in the post-policy/COVID era reflects the transition from the distribution system based on DSA to distance. A recent study by Chan et al. 39 revealed that the usage of non-ideal liver donors, defined by a set of criteria, including donor age ≥ 60, BMI ≥ 35, DCD, peak bilirubin ≥ 2, peak AST and ALT ≥ 2000, split liver, and positive HBV Ab, HBV NAT, HCV Ab, HCV NAT, increased only in low MELD centers (MELD ≤ 28) after the implementation of AC policy. When compared to high MELD centers (MELD > 28), low MELD centers were also characterized by significantly higher DCD LT volume post-AC. Most recently, in June 2022, the OPTN transitioned to calculating the MMaT scores around the donor hospital instead of the transplant center where a candidate is listed. In the context of this change, patients with MELD exception points no longer have a single MELD score. Rather, the final MELD exception score fluctuates based on organ offers from the donor hospital where the match is run.40 The recency of this change has thus far precluded viable analyses of its effect on the waitlisted LT candidates. Nevertheless, future studies should monitor the ongoing impact of the AC policy and recent updates to the MMaT-3 national policy on organ quality in LT to identify best practices of aggressive donor utilization.
In summary, the policy that had intended to deprioritize HCC candidates for LT did indeed adversely impact the quality of livers transplanted into HCC patients. Though the difference was modest, HCC candidates became more likely to receive a marginal quality liver after the MMaT-3 policy change took effect. Nevertheless, the ongoing impact of the MMaT-3 policy change on organ quality in LT will require further monitoring.
Supplementary Material
Disclosure:
RDM, LAD, HY, DCC: Have not received any specific grant from funding agencies from public, commercial and not-for-profit sectors.
DCC: Dr. Cron is supported by National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number F32DK128981
Abbreviations:
- HCC
Hepatocellular Carcinoma
- LT
Liver Transplantation
- MELD
Model for End-Stage Liver Disease
- MMaT-3
Median MELD at Transplant minus 3
- NLRB
National Liver Review Board
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
- DCD
Donation after Circulatory Death
- SRTR
Scientific Registry of Transplant Recipients
- HRSA
Health Resources and Services Administration
- WHO
World Health Organization
- AC
Acuity Circles
- AFP
Alpha-Fetoprotein
- HCV
Hepatitis C Virus
Footnotes
Meeting Presentation:
103rd Annual Meeting of the New England Surgical Society – September 16-18, 2022, Boston, MA
Conflicts of Interest: Authors have no conflicts of interest.
References
- 1.Wiesner RH, McDiarmid SV, Kamath PS, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7(7):567–580. Doi: 10.1053/jlts.2001.25879 [DOI] [PubMed] [Google Scholar]
- 2.Kanwal F, Dulai GS, Spiegel BM, et al. A comparison of liver transplantation outcomes in the pre- vs. post-MELD eras. Aliment Pharmacol Ther. 2005;21(2):169–177. Doi: 10.1111/j.1365-2036.2005.02321.x [DOI] [PubMed] [Google Scholar]
- 3.Freeman RB, Wiesner RH, Edwards E, et al. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10(1):7–15. Doi: 10.1002/lt.20024 [DOI] [PubMed] [Google Scholar]
- 4.Berg CL, Steffick DE, Edwards EB, et al. Liver and intestine transplantation in the United States 1998-2007. Am J Transplant. 2009;9(4 Pt 2):907–931. Doi: 10.1111/j.1600-6143.2009.02567.x [DOI] [PubMed] [Google Scholar]
- 5.Desai NM, Mange KC, Crawford MD, et al. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77(1):99–106. Doi: 10.1097/01.TP.0000101009.91516.FC [DOI] [PubMed] [Google Scholar]
- 6.Yang JD, Larson JJ, Watt KD, et al. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin Gastroenterol Hepatol. 2017;15(5):767–775.e3. Doi: 10.1016/j.cgh.2016.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimbach JK. United States liver allocation. Curr Opin Organ Transplant. 2020;25(2):104–109. Doi: 10.1097/MOT.0000000000000740 [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Balan V, Hernandez JL, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10(1):36–41. Doi: 10.1002/lt.20012 [DOI] [PubMed] [Google Scholar]
- 9.Elwir S, Lake J. Current Status of Liver Allocation in the United States. Gastroenterol Hepatol (N Y). 2016;12(3):166–170. [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. Doi: 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 11.OPTN Policies - OPTN: Organ Procurement and Transplantation Network - OPTN. (2022). Retrieved December 3, 2022. Available at https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf
- 12.Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61(5):1643–1650. Doi: 10.1002/hep.27704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washburn K, Edwards E, Harper A, et al. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10(7):1643–1648. Doi: 10.1111/j.1600-6143.2010.03127.x [DOI] [PubMed] [Google Scholar]
- 14.Massie AB, Caffo B, Gentry SE, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11(11):2362–2371. Doi: 10.1111/j.1600-6143.2011.03735.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishaque T, Massie AB, Bowring MG, et al. Liver transplantation and waitlist mortality for HCC and non-HCC candidates following the 2015 HCC exception policy change. Am J Transplant. 2019;19(2):564–572. Doi: 10.1111/ajt.15144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brondfield MN, Dodge JL, Hirose R, et al. Unfair Advantages for Hepatocellular Carcinoma Patients Listed for Liver Transplant in Short-Wait Regions Following 2015 Hepatocellular Carcinoma Policy Change. Liver Transpl. 2020;26(5):662–672. Doi: 10.1002/lt.25701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaikh A, Goli K, Rich NE, et al. Early Impact of MMaT-3 Policy on Liver Transplant Waitlist Outcomes for Hepatocellular Carcinoma. Transplant Direct. 2022;8(5):e1313. Published 2022. Apr 12. Doi: 10.1097/TXD.0000000000001313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernards S, Hirose R, Yao FY, et al. The Impact of Median Model for End-Stage Liver Disease at Transplant Minus 3 National Policy on Waitlist Outcomes in Patients With and Without Hepatocellular Carcinoma. Liver Transpl. 2022;28(3):376–385. Doi: 10.1002/lt.26368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015;21(8):1040–1050. Doi: 10.1002/lt.24160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orman ES, Barritt AS 4th, Wheeler SB, et al. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver Transpl. 2013;19(1):59–68. Doi: 10.1002/lt.23547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haugen CE, Thomas AG, Garonzik-Wang J, et al. Minimizing Risk Associated With Older Liver Donors by Matching to Preferred Recipients: A National Registry and Validation Study. Transplantation. 2018;102(9):1514–1519. Doi: 10.1097/TP.0000000000002190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez-Romero C, Clemares-Lama M, Manrique-Municio A, et al. Long-term results using old liver grafts for transplantation: sexagenerian versus liver donors older than 70 years. World J Surg. 2013;37(9):2211–2221. Doi: 10.1007/s00268-013-2085-7 [DOI] [PubMed] [Google Scholar]
- 23.Cescon M, Grazi GL, Cucchetti A, et al. Improving the outcome of liver transplantation with very old donors with updated selection and management criteria. Liver Transpl. 2008;14(5):672–679. Doi: 10.1002/lt.21433 [DOI] [PubMed] [Google Scholar]
- 24.Jackson KR, Bowring MG, Holscher C, et al. Outcomes After Declining a Steatotic Donor Liver for Liver Transplant Candidates in the United States. Transplantation. 2020;104(8):1612–1618. Doi: 10.1097/TP.0000000000003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croome KP, Lee DD, Taner CB. The “Skinny” on Assessment and Utilization of Steatotic Liver Grafts: A Systematic Review. Liver Transpl. 2019;25(3):488–499. Doi: 10.1002/lt.25408 [DOI] [PubMed] [Google Scholar]
- 26.Jackson KR, Motter JD, Haugen CE, et al. Temporal trends in utilization and outcomes of steatotic donor livers in the United States. Am J Transplant. 2020;20(3):855–863. Doi: 10.1111/ajt.15652 [DOI] [PubMed] [Google Scholar]
- 27.Linares I, Hamar M, Selzner N, et al. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation. 2019;103(1):78–90. Doi: 10.1097/TP.0000000000002466 [DOI] [PubMed] [Google Scholar]
- 28.Zhang QY, Zhang QF, Zhang DZ. The Impact of Steatosis on the Outcome of Liver Transplantation: A Meta-Analysis. Biomed Res Int. 2019;2019:3962785. Published 2019. May 14. Doi: 10.1155/2019/3962785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T, Dunson J, Kanwal F, et al. Trends in Outcomes for Marginal Allografts in Liver Transplant [published online ahead of print, 2020 Aug 5] [published correction appears in JAMA Surg. 2020 Oct 1;155(10):1002]. JAMA Surg. 2020;155(10):926–932. Doi: 10.1001/jamasurg.2020.2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz P, Gastaca M, Bustamante FJ, et al. Favorable Outcomes After Liver Transplantation With Normothermic Regional Perfusion From Donors After Circulatory Death: A Single-center Experience. Transplantation. 2019;103(5):938–943. Doi: 10.1097/TP.0000000000002391 [DOI] [PubMed] [Google Scholar]
- 31.Markmann JF, Abouljoud MS, Ghobrial RM, et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157(3):189–198. Doi: 10.1001/jamasurg.2021.6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scalea JR, Redfield RR, Foley DP. Liver transplant outcomes using ideal donation after circulatory death livers are superior to using older donation after brain death donor livers. Liver Transpl. 2016;22(9):1197–1204. Doi: 10.1002/lt.24494 [DOI] [PubMed] [Google Scholar]
- 33.Bohorquez H, Seal JB, Cohen AJ, et al. Safety and Outcomes in 100 Consecutive Donation After Circulatory Death Liver Transplants Using a Protocol That Includes Thrombolytic Therapy. Am J Transplant. 2017;17(8):2155–2164. Doi: 10.1111/ajt.14261 [DOI] [PubMed] [Google Scholar]
- 34.Hobeika MJ, Menser T, Nguyen DT, et al. United States donation after circulatory death liver transplantation is driven by a few high-utilization transplant centers. Am J Transplant. 2020;20(1):320–321. Doi: 10.1111/ajt.15629 [DOI] [PubMed] [Google Scholar]
- 35.Shah RH, Chyou D, Goldberg DS. Impact of major hepatocellular carcinoma policy changes on liver transplantation for hepatocellular carcinoma in the United States. Liver Transpl. 2022;28(12):1857–1864. Doi: 10.1002/lt.26509 [DOI] [PubMed] [Google Scholar]
- 36.Strauss AT, Boyarsky BJ, Garonzik-Wang JM, et al. Liver transplantation in the United States during the COVID-19 pandemic: National and center-level responses. Am J Transplant. 2021;21(5):1838–1847. Doi: 10.1111/ajt.16373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.United Network for Organ Sharing. COVID-19 and solid organ transplant. (2021). Retrieved April 11, 2022. Available at https://unos.org/covid/
- 38.Wey A, Noreen S, Gentry S, et al. The Effect of Acuity Circles on Deceased Donor Transplant and Offer Rates Across Model for End-Stage Liver Disease Scores and Exception Statuses. Liver Transpl. 2022;28(3):363–375. Doi: 10.1002/lt.26286 [DOI] [PubMed] [Google Scholar]
- 39.Chan E, Logan AJ, Sneddon JM, et al. Dynamic impact of liver allocation policy change on donor utilization. Am J Transplant. 2022;22(7):1901–1908. Doi: 10.1111/ajt.17006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United Network for Organ Sharing. Median MELD at transplant now based around donor hospital. (2022). Retrieved February 3, 2023. Available at https://unos.org/news/median-meld-at-transplant-based-donor-hospital-june-28/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


