Abstract
The gene man5K encoding the mannanase Man5K from Clostridium cellulolyticum was cloned alone or as an operon with the gene cipC1 encoding a truncated scaffoldin (miniCipC1) of the same origin in the solventogenic Clostridium acetobutylicum. The expression of the heterologous gene(s) was under the control of a weakened thiolase promoter Pthl. The recombinant strains of the solventogenic bacterium were both found to secrete active Man5K in the range of milligrams per liter. In the case of the strain expressing only man5K, a large fraction of the recombinant enzyme was truncated and lost the N-terminal dockerin domain, but it remained active towards galactomannan. When man5K was coexpressed with cipC1 in C. acetobutylicum, the recombinant strain secreted almost exclusively full-length mannanase, which bound to the scaffoldin miniCipC1, thus showing that complexation to the scaffoldin stabilized the enzyme. The secreted heterologous complex was found to be functional: it binds to crystalline cellulose via the carbohydrate binding module of the miniscaffoldin, and the complexed mannanase is active towards galactomannan. Taken together, these data show that C. acetobutylicum is a suitable host for the production, assembly, and secretion of heterologous minicellulosomes.
Cellulolytic Clostridia species, such as Clostridium cellulolyticum, produce and secrete large cellulolytic complexes called cellulosomes that efficiently degrade cellulose and related plant cell wall polysaccharides (for a review, see reference 1). These complexes contain various enzymes, which are tightly bound to a large protein devoid of enzymatic activity called scaffoldin. The scaffoldin produced by C. cellulolyticum is a modular protein bearing a carbohydrate binding module (CBM), two X2 domains of unknown function, and eight cohesin modules that bind to the complementary dockerin domain of the catalytic subunits (21). The interaction between the cohesins of the scaffoldins and the dockerins of the enzymes, which are responsible for cellulosome assembly, is calcium dependent and of high affinity (Ka ≈ 109 M−1) (8). The catalytic subunits participating with cellulosomes, and therefore appended with a dockerin domain, comprise mainly cellulases (2) but also hemicellulases such as xylanases (16), a mannanase (25), and at least one pectinase (23). As do most species of cellulolytic bacteria, C. cellulolyticum grows rather slowly, even on cellobiose, the major product released by cellulosomes on cellulose. Its catabolic pathways are adapted to low carbon flow (11, 12), which is characteristic of growth on cellulose, and the main products of the metabolism are acetate, lactate, and ethanol (10).
On the contrary, Clostridium acetobutylicum is unable to grow on crystalline cellulose, although its genome contains a large cluster of genes encoding cellulolytic enzymes and a scaffoldin (19, 28). Interestingly, it has been shown that the bacterium produces small amounts of a 665-kDa cellulosome devoid of activity towards crystalline cellulose and is poorly active on carboxymethyl cellulose or phosphoric acid-swollen cellulose (28). On the other hand, C. acetobutylicum grows much faster than C. cellulolyticum, rapidly consumes cellobiose, and produces substantial amounts of solvents of industrial interest such as butanol (9).
C. cellulolyticum and C. acetobutylicum are thus two anaerobic bacteria that display interesting complementary properties. The combination of both cellulolytic and solventogenic phenotypes in a single bacterium is an attractive challenge. The strategy chosen by our group aims at cloning and expressing the genes encoding a functional minicellulosome in C. acetobutylicum. It has been shown that the cohesin-dockerin interaction is species specific, at least between C. cellulolyticum and Clostridium thermocellum (20); this specificity was further exploited to build chimeric miniscaffoldins containing one cohesin from each species and an optional CBM(s) (5, 7). These hybrid scaffoldins were used to perform in vitro reconstitution of minicellulosomes containing one cellulase bearing a C. cellulolyticum dockerin and one cellulase possessing a C. thermocellum dockerin. Such a complex was found to display enhanced activity towards crystalline cellulose compared to a free-enzyme system (5, 7). Our goal is now to produce and assemble in vivo the most efficient hybrid minicellulosome in C. acetobutylicum. The first step towards this goal was achieved recently with the production and secretion of two different heterologous miniscaffoldins by C. acetobutylicum: miniCipC1, a truncated form of CipC from C. cellulolyticum containing the family 3A CBM (CBM3A); the first X2 domain; the first cohesin domain; and the chimeric miniscaffoldin Scaf3, which contains an additional cohesin domain from CipA of C. thermocellum fused at the C terminus of miniCipC1 (26). Both miniscaffoldins were found to be secreted at approximately 10 to 15 mg/liter, and their domains (CBM and cohesins) were found to be fully operational (26).
In the present study, we tested the capacity of C. acetobutylicum to produce and secrete a heterologous complex mimicking a cellulosome. For this purpose, we chose to clone and express the gene encoding Man5K from C. cellulolyticum (25), since the culture supernatant of C. acetobutylicum displays weak but measurable cellulase activity (28) but no detectable mannanase activity (this study). Furthermore, it has been shown that antisera raised against C. cellulolyticum cellulases did recognize the homologous enzymes secreted by C. acetobutylicum (28), whereas no protein secreted by C. acetobutylicum was detected by the antiserum raised against Man5K from C. cellulolyticum (this study).
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli SG-13009, harboring pREP4 repressor plasmid (QIAGEN, Venlo, The Netherlands) was used as a host for recombinant expression vectors, whereas E. coli strain ER-2275 carrying the pAN1 methylating plasmid (15) was used to in vivo methylate the recombinant plasmids prior to transformation of C. acetobutylicum ATCC 824 (18). E. coli strains were grown in Luria-Bertani medium supplemented with ampicillin (100 μg/ml) and kanamycin (50 μg/ml) [E. coli SG-13009(pREP4); derivatives of pSOS95] or with ampicillin (100 μg/ml) and chloramphenicol (34 μg/ml) [E. coli ER-2275(pAN1); derivatives of pSOS95]. C. acetobutylicum was routinely grown anaerobically at 37°C in 2YT medium (containing 16 g of Bacto tryptone/liter, 10 g of yeast extract/liter, 4 g of NaCl/liter, and 1 mM CaCl2) supplemented with cellobiose (5 g/liter). Recombinant C. acetobutylicum strains carrying derivatives of pSOS95 were grown in 2YT-cellobiose supplemented with 40 μg of erythromycin/ml. To prepare for spore suspension, C. acetobutylicum was grown at 37°C for 7 to 10 days in 10 ml of synthetic medium (30) supplemented with erythromycin (40 μg/ml) for recombinant strains. The culture was then aliquoted and frozen at −20°C.
Expression vectors and cloning of the man5K gene and the operon cipC1-man5K in C. acetobutylicum.
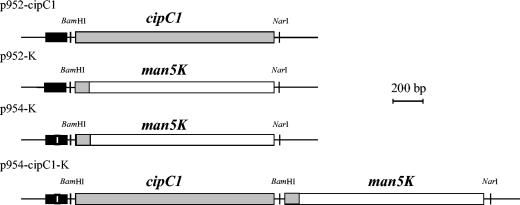
The vectors p952, p954, and p952-cipC1 were described previously (25, 26). Briefly, p952 was generated from the E. coli-C. acetobutylicum shuttle vector pSOS95 (kindly provided by P. Soucaille, INSA, Toulouse, France). This vector was constructed for cloning genes between the strong constitutive promoter of the thiolase gene and the transcriptional terminator of the adc gene (encoding acetoacetate-decarboxylase) with BamHI and NarI sites. Two lac operators upstream and downstream of the thiolase promoter region were introduced to down regulate the level of expression of the cloned genes in E. coli (26). The expression vector p954 (Fig. 1) was generated from p952 by introducing a single mutation (A→T) in the −35 box to weaken the thiolase promoter and induce a lower level of expression of the cloned gene (25). The plasmid p952-cipC1 (Fig. 1) was obtained by cloning the gene encoding miniCipC1 between the BamHI and NarI sites of p952. The vectors p952-K and p954-K (Fig. 1) were generated by cloning an engineered man5K gene between the BamHI and NarI sites of p952 and p954, respectively (25). The engineered man5K gene is a chimeric gene in which the sequence encoding the leader peptide of Man5K was replaced by the DNA encoding the leader peptide of miniCipC1 (25). Construction of p954-cipC1-K (Fig. 1) was performed as follows: the cipC1 gene encoding miniCipC1 was amplified by PCR from p952-cipC1 with the forward primer SOSdir (5′-ACTATTGGTTGGAATGGCGTG-3′) that matches a sequence located upstream of the thiolase promoter and the reverse primer Ciprev (5′-GGTGGGGATCCTTCGAACTACTCGAGTTCCTTTGTAGGTTGAGTACC-3′) introducing a BamHI site (underlined) at the 3′ extremity of the gene encoding miniCipC1. The 1.6-kb amplified DNA was digested by BamHI, and the largest fragment (1.35 kb) containing cipC1 was purified and cloned in BamHI-linearized p954-K, thus generating p954-cipC1-K. The orientation of the cipC1 in E. coli SG-13009(pREP4)-positive clones was checked by PCR with the primers SOSdir and Ciprev, and the p954-cipC1-K selected was subsequently verified by DNA sequencing. p952-K, p954-K, and p-954-cipC1-K were methylated in vivo in E. coli ER-2275(pAN1) and used to transform C. acetobutylicum by electrotransformation (18). Plasmids from recombinant C. acetobutylicum strains were prepared from one clone grown in 2YT-cellobiose supplemented with 40 μg of erythromycin/ml, until absorbance at 620 nm reached 0.9. Cells from 4.5 ml were harvested and submitted to a modified alkalin lysis miniprep protocol to purify the plasmid (18). The DNA solution was used to transform E. coli strain ER-2275(pAN1). The plasmid was purified, and it was checked by restriction analysis and DNA sequencing so that no modification of the cloned genes occurred in C. acetobutylicum.
FIG. 1.
Schematic representation of the cloned genes and promoter region. The black box indicates the thiolase promoter region (Pthl). The white bar (p954-K and p954-cipC1-K) indicates the mutation in the −35 box of the thiolase promoter. Grey and white boxes indicate the cipC1 gene and the man5K gene, respectively. The grey box at the beginning of the man5K gene indicates the DNA region encoding the leader peptide of miniCipC1.
Batch fermentations of C. acetobutylicum.
pH-controlled fermentations of recombinant C. acetobutylicum strains were performed with a 2-liter fermentor (B. Braun Biotech International, Allentown, Pa.) at 37°C in 2YT-cellobiose medium supplemented with 40 μg of erythromycin/ml. A continuous flow of sterile N2 gas was used to maintain anaerobic conditions. The culture was agitated at an impeller speed of 50 rpm. The pH was maintained at 5.5 with controlled additions of 2 M NaOH. A 1% (vol/vol) inoculum of a mid-exponential-phase culture grown in selective 2YT-cellobiose medium was used for all cultures. Samples (each, 2 ml) were removed at intervals for the measurement of culture turbidity at 620 nm. Cultures were stopped at the late exponential phase (optical density at 620 nm [OD620] ranged from 2.9 to 3.1), and centrifuged for 30 min at 10,000 × g. The supernatant was aliquoted and frozen at −20°C.
Analysis of C. acetobutylicum supernatants.
Tris-HCl (pH 8.0) and potassium acetate were added to 90 ml of culture supernatant (batch fermentations) at final concentrations of 25 mM and 1.0 M, respectively. The sample was then loaded onto 4 ml of phenyl-Sepharose resin (Amersham Bioscience, Little Chalfont, United Kingdom) equilibrated in the same buffer. The bound proteins were eluted with 50 ml of distilled water and concentrated to 0.5 ml by ultrafiltration (PM10 membrane, cutoff 10 kDa; Millipore, Billerica, Mass.). The concentration of protein was determined by the method of Lowry (14) with bovine serum albumin as the standard. Samples (each, 100 μl) were subjected to gel filtration chromatography by injection onto a 30-ml Superdex 200 GL column (Amersham Bioscience), equilibrated in 30 mM potassium phosphate-0.15 M NaCl (pH 7) with an Akta-FPLC system (Amersham Bioscience). The flow rate was 0.5 ml/min, and 0.5-ml fractions were collected.
Enzyme assays.
Mannanase activity was assayed on locust bean gum (LBG; Fluka, Buchs, Switzerland), a linear polymer of mannose linked by β-1,4 bonds and displaying α-1,6 galactose side chains with a mannose/galactose ratio of 4:1. LBG (15 g) was dissolved in 10 ml of ethanol, and 1 liter of distilled water was subsequently added. The suspension was boiled for 15 min and washed four times by centrifugation (10,000 × g; 10 min) with distilled water. The last pellet was resuspended in 200 ml of distilled water, and the amount of LBG remaining was estimated by dry weight. The solution was finally adjusted to 1% (wt/vol) LBG in 25 mM Tris-maleate, pH 6.0. Enzymatic assays were performed at 37°C by mixing 2 ml of substrate with 0.5 ml of an appropriate dilution of mannanase sample. At specific intervals, aliquots were pipetted and centrifuged for 20 min (13,000 × g) at 4°C, and the supernatants were examined for soluble reducing sugar content by the ferricyanide method (24) with mannose as the standard. The specific activity of whole and truncated Man5K purified from the E. coli-overproducing strain on this LBG suspension was estimated to be 103 and 569 IU/μmol, respectively. Untreated supernatants of batch cultures of C. acetobutylicum contain large amounts of residual cellobiose that interferes with the determination of soluble reducing sugar content. Therefore, supernatant samples (each, 0.5 ml) had to be dialyzed four times against 25 mM Tris-maleate (pH 6.0) by ultrafiltration with microconcentrators (10-kDa cutoff; Vivascience, Hannover, Germany) prior to the enzyme assay with LBG.
Cellulose binding assays.
Untreated supernatants (each, 10 ml) of C. acetobutylicum cultures (batch fermentor) were incubated for 30 min at 25°C with 40 mg of crystalline cellulose Avicel PH 101 (Fluka) under mild shaking (60 rpm.). The sample was centrifuged for 10 min (8,000 × g; 4°C), and the pellet was washed twice with 50 mM Tris-maleate (pH 6.0)-50 mM NaCl-10 mM CaCl2. The last pellet was resuspended in 50 μl of denaturing loading buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and boiled for 10 min. The sample was subsequently centrifuged for 10 min at 13,000 × g, and the supernatant was analyzed by SDS-PAGE.
Polyacrylamide gel electrophoresis and Western blot analysis.
SDS-PAGE (12% polyacrylamide) was performed with a vertical electrophoresis apparatus (Amersham Bioscience). Gels were stained by Coomassie blue or electrotransferred onto a nitrocellulose BA 83 membrane (Schleicher and Schuell, Dassel, Germany). After saturation, membranes were probed with polyclonal antibodies raised against Man5K or miniCipC1 purified from E. coli-overproducing strains. Antibodies were detected using anti-rabbit horseradish peroxidase conjugate and a chemiluminescent substrate (Amersham Bioscience). The dockerin-containing forms of Man5K were detected by incubating blots with biotin-labeled miniCipC1 (22), streptavidin-conjugated peroxidase (Roche Applied Science, Rotkreuz, Switzerland), and a chemiluminescent substrate as described above.
RESULTS
Investigation of C. acetobutylicum endogenous mannanase activity.
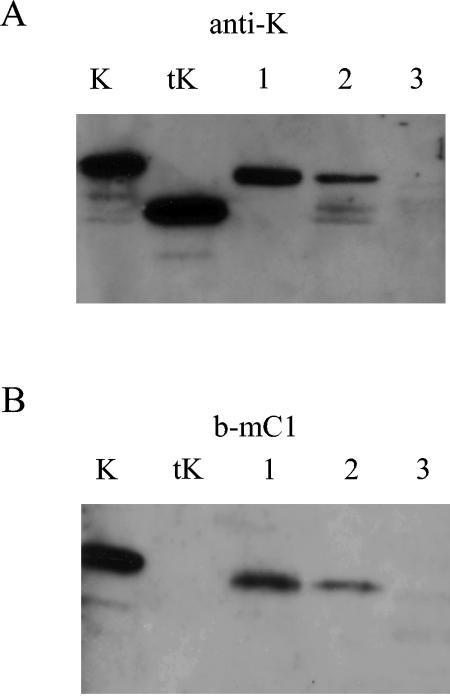
Prior to cloning and expressing the C. cellulolyticum man5K gene in C. acetobutylicum, it was verified that wild-type C. acetobutylicum and the recombinant strain carrying p952-cipC1 (26) grown on cellobiose did not secrete any detectable mannanase activity. Batch fermentions of both strains were performed at pH 5.5 and at the late exponential phase (OD620, ≈3), the cultures were centrifuged, and the supernatants of both strains were dialyzed and concentrated by ultrafiltration. The mannanase activity was investigated on LBG, a galactomannan commonly used to screen this type of activity and efficiently degraded by Man5K (13, 25). No reducing sugar was released, even after prolonged incubation of the concentrated supernatants with the substrate, thus indicating the lack of endogenous activity that would interfere with that of the heterologous Man5K. Western blotting performed on the concentrated supernatant of C. acetobutylicum(p952-cipC1) (Fig. 2A, lane 3) and the wild-type strain (data not shown) with antiserum raised against Man5K failed to detect any protein, thus confirming that no homologous mannanase is secreted by C. acetobutylicum under the culture conditions used.
FIG. 2.
Production of Man5K by C. acetobutylicum. Western blot analysis of SDS-PAGE with antiserum raised against Man5K (anti-K) (A) or biotin-labeled miniCipC1 (b-mC1) (B) K, whole form of Man5K produced in E. coli (0.1 mg/ml); tK, truncated form of Man5K produced in E. coli (0.1 mg/ml); lane 1, concentrated supernatant of C. acetobutylicum(p954-cipC1-K); lane 2, concentrated supernatant of C. acetobutylicum(p954-K); lane 3, concentrated supernatant of C. acetobutylicum (p952-cipC1).
Cloning and expression of man5K alone.
In a former study, it was shown that transformation of C. acetobutylicum with the expression vector p952-cipC1 (26) led to a recombinant strain which produces and secretes the miniscaffoldin miniCipC1. This report thus showed that the strong constitutive promoter Pthl was suitable for heterologous expression and that the leader peptide of the scaffoldin CipC from C. cellulolyticum induced efficient secretion of miniCipC1. Based on these data, a similar strategy was selected to express the gene man5K: the coding sequence of mature miniCipC1 in p952-cipC1 was replaced by the Man5K sequence, leaving the Pthl and the miniCipC1 leader peptide intact, and generating the vector p952-K (Fig. 1). Despite several attempts, no colonies were selected on erythromycin-containing medium after electrotransformation of C. acetobutylicum with p952-K, whereas transformation with p952-cipC1 performed simultaneously generated 20 to 150 clones. The lack of transformants with p952-K suggested that high expression levels of man5K were indeed toxic for Clostridium. To circumvent this difficulty, a modified Pthl promoter, mutated in the −35 box (TGATAA→TGATTA) (the site of the mutation is in italics) and presumably leading to lower expression levels in C. acetobutylicum, was chosen to control the expression of man5K (p954-K expression vector) (Fig. 1). Electrotransformation of C. acetobutylicum with this vector generated a similar number of colonies (52 clones), like the reference expression vector p952-cipC1. The plasmid p954-K was purified from a culture of one individual C. acetobutylicum transformant, and the suspension was used to transform the E. coli strain ER-2275(pAN1). Restriction analysis as well as sequencing of the Pthl-man5K region revealed that no modification of the vector occurred in C. acetobutylicum.
A batch fermentation of the recombinant strain was performed in a 2-liter fermentor at pH 5.5. The culture was stopped at an OD620 value of ≈3 and centrifuged. After dialysis, the activity on LBG was estimated to be approximately 8.0 mIU/ml of supernatant. Concentrated supernatant was also analyzed by SDS-PAGE and Western blotting with antibodies raised against Man5K. The full-length Man5K and a truncated form lacking the N-terminal dockerin, which were purified from the E. coli-overproducing strain, were loaded on the same gel (Fig. 2, lanes K and tK) (25). As shown in Fig. 2A, lane 2, the C. acetobutylicum supernatant contained three distinct bands which were probed by the antiserum: the upper and lower bands corresponded to the intact enzyme (45 kDa) and a truncated form of mass (39 kDa) similar to that purified from E. coli, respectively. An additional band of intermediate mass was also detected by the antiserum. Only the whole form of the mannanase was probed by the biotin-labeled miniCipC1 (Fig. 2B, lane 2), thus confirming that the truncated mannanases secreted by C. acetobutylicum(p954-K) lost the N-terminal dockerin domain, similarly to the truncated form purified from E. coli-overproducing strain (Fig. 2B, lane tK).
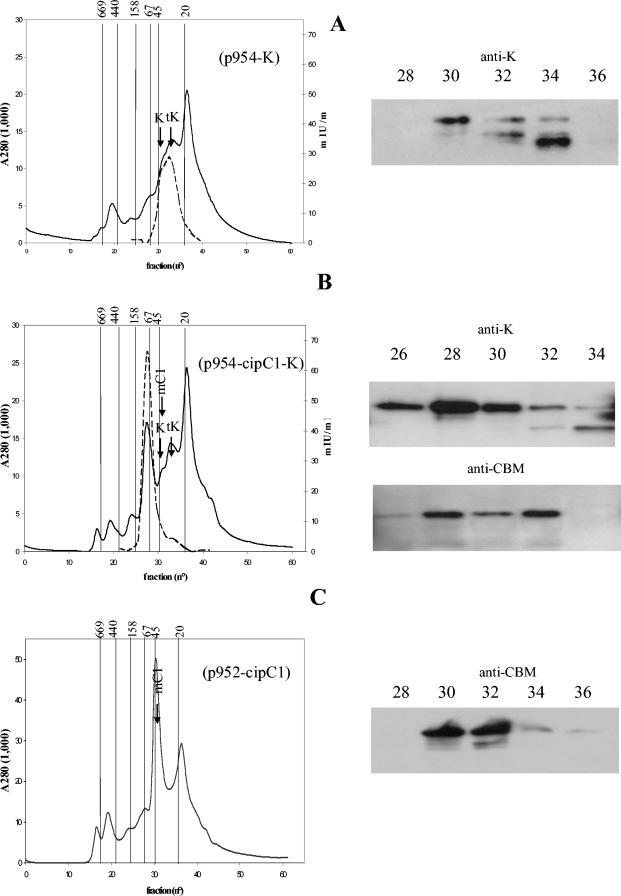
Ninety milliliters of supernatant were loaded on phenyl-Sepharose resin, and the bound proteins were eluted with distilled water. Mannanase activity was completely recovered in the latter fraction, which was subsequently concentrated and subjected to gel filtration. The obtained chromatogram is shown in Fig. 3A. Mannanase activity was mainly found in fractions 30 to 34, and Western blot analysis performed on these fractions confirmed the presence of whole Man5K in fractions 30 to 32, whereas the smallest truncated form was essentially detected in fraction 34. The elution profile of the two main forms matched perfectly with the whole and truncated Man5K purified from E. coli. Thus, as observed in the case of the E. coli strain overproducing Man5K, partial proteolysis of the recombinant enzyme occurred in C. acetobutylicum. Whereas the entire form seemed predominant in the concentrated supernatant (Fig. 2A, lane 2), the proportion of truncated form increased in the sample subjected to gel filtration. Most probably, proteolysis continued during the preparation of the sample.
FIG. 3.
Examination of the supernatants of C. acetobutylicum recombinant strains. The supernatants of C. acetobutylicum(p954-K) (A), C. acetobutylicum(p954-cipC1-K) (B), and C. acetobutylicum(p952-cipC1) (C) were examined by gel filtration (left) and subsequent Western blot analysis (right) with antisera raised against Man5K (anti-K) or against CBM3A of miniCipC1 (anti-CBM). Protein elution was followed by absorbance at 280 nm (solid lines). Activity (dashed lines) was measured on 0.8% LBG and expressed in international milliunits per milliliter. Vertical lines indicate the elution volume of molecular mass markers: tyroglobulin, 669 kDa; ferritin, 440 kDa; aldolase, 158 kDa; bovine serum albumin, 67 kDa; ovalbumin, 45 kDa; and trypsin inhibitor, 20 kDa. Arrows indicate the elution volume of purified Man5K (whole [K] and truncated [tK] forms) and miniCipC1 (mC1) from E. coli-overproducing strains. The numbers on top of the Western blots indicate fraction numbers.
Accurate determination of the amount of secreted mannanase on the basis of the activity is difficult, since whole and truncated forms (produced in E. coli) display very different specific activities on 0.8% LBG (103 and 569 IU/μmol, respectively), but activity was estimated to be in the range of 0.5 to 1 mg/liter.
Cloning and expression of an operon cipC1-man5K.
The gene encoding miniCipC1 was cloned upstream of man5K in p954-K, generating the plasmid p954-cipC1-K (Fig. 1). In this construction, the expression of both genes was under the control of mutated Pthl. Electrotransformation of C. acetobutylicum generated 19 clones. As described above, the plasmid was extracted from one recombinant C. acetobutylicum clone and checked for the absence of sequence alteration.
A batch fermentation of the recombinant strain was performed, and the culture supernatant was analyzed. The activity of the dialyzed supernatant on LBG was found to be 4.5 mIU/ml. An aliquot of the supernatant was concentrated 10 times and subjected to SDS-PAGE and Western blot analysis with anti-Man5K (Fig. 2A) or antibodies raised against CBM3A of miniCipC1 (data not shown). As shown in Fig. 2A, lane 1, the supernatant contained almost exclusively the whole form of Man5K. As a control experiment, a concentrated supernatant of the strain carrying p952-cipC1 was also loaded on the same gel to verify that anti-Man5K was unable to recognize miniCipC1 (lane 3). The scaffoldin was also detected in the supernatant by anti-CBM3A (data not shown). As expected, the entire Man5K reacted with the biotin-labeled miniCipC1 (Fig. 2B, lane 1). A sample (90 ml) of the supernatant was prepared as described above and analyzed by gel filtration fast-performance liquid chromatography (Fig. 3B). Mannanase activity was essentially found in fractions 26 to 30, with a maximum in fraction 28. The elution volume corresponds to a mass of approximately 87 kDa, in good agreement with the theoretical mass of 89 kDa for the Man5K-miniCipC1 complex (Man5K, 45 kDa; miniCipC1, 44 kDa). Western blot analysis performed on these fractions confirmed the presence of both miniCipC1 and the entire form of Man5K in the most active fraction. Small amounts of truncated Man5K were also detected in fraction 34. The elution profile of the proteolyzed form remained unchanged, compared to that obtained with the recombinant strain carrying plasmid p954-K. In addition to fraction 28, miniCipC1 was also found in fraction 32. Gel filtration performed on the supernatant of the recombinant strain producing only miniCipC1 (Fig. 3C) showed that free miniCipC1 produced by C. acetobutylicum was eluted in the same volume (fractions 30 to 32). This result indicates that the strain carrying p954-cipC1-K produces an excess of miniCipC1 compared to Man5K, since a fraction of the scaffoldin is found in the free state.
The mannanase secreted by the strain harboring p954-cipC1-K was found to be almost exclusively in complex with the scaffoldin. The specific activity on 0.8% LBG of the entire form of Man5K (produced in E. coli) in complex with miniCipC1 was twofold that of the free (entire) Man5K: 205 IU/μmol (4.5 IU per mg of Man5K). Therefore, on the basis of the mannanase activity of the supernatant (4.5 IU/liter), it is concluded that the recombinant strain secretes approximately 1 mg of the enzyme/liter, which corresponds to 2 mg of active minicellulosome/liter.
Cellulose binding assays.
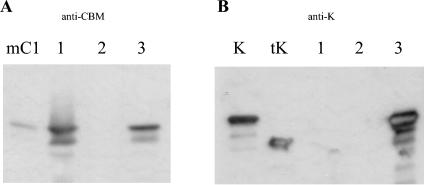
Supernatant samples from the strains carrying p954-K, p954-cipC1-K, and p952-cipC1 were incubated with 40 mg of crystalline cellulose. The adsorbed proteins were eluted by boiling the cellulose in SDS-containing buffer. The suspension was centrifuged, and the supernatants were subsequently analyzed by SDS-PAGE and Western blotting with antisera raised against Man5K and the CBM3A of miniCipC1 (Fig. 4). As expected, no mannanase was adsorbed to the cellulose in the supernatant of the recombinant strain carrying p954-K (Fig. 4B, lane 2). On the contrary, the mannanase was detected in the cellulose-adsorbed fraction in the strain coexpressing cipC1 and man5K (Fig. 4B, lane 3), thus indicating that the complex miniCipC1-Man5K produced by C. acetobutylicum did bind to the crystalline cellulose. Cellulose-adsorbed miniCipC1 was indeed detected with anti-CBM in the case of the strains harboring p954-cipC1-K and p952-cipC1 (Fig. 4A).
FIG. 4.
Cellulose binding assay. The proteins adsorbed on Avicel were analyzed by Western blotting with antisera raised against the CBM3A of miniCipC1 (A) or against Man5K (B). (A) mC1, purified miniCipC1 (produced in E. coli); lane 1, cellulose-adsorbed fraction of C. acetobutylicum(p952-cipC1) supernatant; lane 2, cellulose-adsorbed fraction of C. acetobutylicum(p954-K) supernatant; lane 3, cellulose-adsorbed fraction of C. acetobutylicum(p954-cipC1-K) supernatant. (B) K, whole Man5K purified from E. coli; tK, purified truncated Man5K produced by E. coli; lane 1, cellulose-adsorbed fraction of C. acetobutylicum(p952-cipC1) supernatant; lane 2, cellulose-adsorbed fraction of C. acetobutylicum(p954-K) supernatant; lane 3, cellulose-adsorbed fraction of C. acetobutylicum(p954-cipC1-K) supernatant.
DISCUSSION
The present study shows that a two-component heterologous minicellulosome can be produced, assembled, and secreted by C. acetobutylicum. Man5K from C. cellulolyticum was chosen as the enzymatic component, since C. acetobutylicum does not secrete any detectable mannanase activity in the growth medium used (cellobiose as the carbone source), although its genome contains at least one mannanase-encoding gene (28).
In a former study, the cipC1 gene encoding miniCipC1 was successfully expressed in C. acetobutylicum under the control of the constitutive promoter Pthl (26), and the recombinant scaffoldin was secreted at 15 mg/ml. It was suggested that the same strategy could be used to express or coexpress genes encoding heterologous cellulosomal enzymes in the solventogenic Clostridium. This approach, however, failed for the expression of man5K, since the transformation of C. acetobutylicum by p952-K was clearly toxic for the bacterium, although the same leader peptide for miniCipC1 was used (26). Furthermore, the transformation of C. cellulolyticum with the same p952-K vector was not toxic for the cellulolytic bacterium and led to a stable recombinant strain producing mannanase-enriched cellulosomes (25). Nevertheless, the Pthl promoter might be less strong in C. cellulolyticum than in C. acetobutylicum. On the basis of these observations, we hypothesized that the toxicity might be due to a blockage of the secretion machinery of C. acetobutylicum by large amounts of Man5K precursor. To test this hypothesis and circumvent the toxicity, we thus used a Pthl promoter weakened by mutation in the −35 box to reduce the level of expression of man5K. This strategy was successful, and a recombinant strain secreting Man5K was obtained. No accumulation of the precursor was observed in the cells (data not shown), and the secretion yield in the culture supernatant was in the range of milligrams per liter.
However, a fraction of the recombinant enzyme secreted by C. acetobutylicum(p954-K) was proteolyzed, generating a truncated form which was still active but lost the N-terminal dockerin domain. Such phenomenon is generally observed with cellulosomal enzymes appended with a dockerin domain purified from E. coli-overproducing strains upon purification or storage above 0°C (4, 6, 27). Proteolysis of Man5K produced in E. coli was also reported (25). This spontaneous cleavage usually takes place in the linker region connecting the catalytic and the dockerin domains (4, 6, 27).
Coexpression of the gene encoding the scaffoldin miniCipC1 led to the secretion of a minicellulosome, miniCipC1-Man5K. The mannanase secreted by C. acetobutylicum was found almost exclusively in the complexed form. Furthermore, the complexation induced a stabilization of the enzyme, since only trace amounts of truncated enzyme were detected. The mannanase remained active towards LBG in the complexed state, whereas the whole complex was capable to bind to crystalline cellulose via the CBM3A of the scaffoldin.
Comparable work was recently performed with the cellulase EngB from Clostridium cellulovorans, the gene for which was cloned alone (17) or as an operon with the gene encoding a truncated form of the C. cellulovorans scaffoldin CbpA (3) in the aerobic bacterium Bacillus subtilis. Expression had to be carried out in an engineered strain of B. subtilis deficient in eight different proteases to prevent the proteolysis of the heterologous proteins and at 30°C to avoid the formation of inclusion bodies (17). Compared to the present study, the aerobic bacterium secreted approximately 10-fold-smaller amounts of minicellulosomes (0.2 mg/liter); nevertheless, the complexes secreted by B. subtilis were also found to be functional (i.e., capable of binding to cellulose and displaying activity toward soluble cellulose). It has been shown that active minicellulosomes can be assembled in vitro simply by mixing stoichiometric amounts of each component (5, 7). Recently published data (3) and the present study suggest that neither C. cellulolyticum nor C. cellulovorans absolutely requires a specific protein(s) to mediate the assembly of their cellulosomes in vivo, since C. acetobutylicum and B. subtilis, which are noncellulolytic, were capable of assembling and secreting active heterologous minicellulosomes. It is therefore likely that in both cases the two recombinant proteins are secreted separately in the external medium, where complexation occurs simply because of the high affinity of the cohesin-dockerin interaction. The traces of truncated Man5K detected in the case of the C. acetobutylicum strain (p954-cipC1-K), which produces an excess of miniCipC1 compared to Man5K, are consistent with this hypothesis (Fig. 3). Some proteolysis may have occurred during the secretion of the enzyme and before the complexation to the miniscaffoldin.
To date, the miniCipC1-Man5K complex is the first example of heterologous production, assembly, and secretion of a fully operational minicellulosome by a solventogenic Clostridium. Homologous overproduction of such a complex in C. acetobutylicum, however, was recently achieved by cloning the DNA encoding the CBM and the two first cohesins of the endogenous scaffoldin (29), thus leading to the formation of a minicellulosome containing the recombinant miniscaffoldin and mainly the endogenous cellulase Cel48A (29). Nevertheless, the homologous production of the complex did not improve the activity towards carboxymethyl cellulose or phosphoric acid-swollen cellulose, which remained low and similar to that of the wild-type strain. This observation was not surprising, since Cel48A is the most abundant catalytic subunit of the cellulosomes produced by wild-type C. acetobutylicum, which display no activity towards crystalline cellulose and low levels of activity on substituted or swollen celluloses. The present report, however, suggests that the noncellulolytic phenotype of C. acetobutylicum might be corrected by heterologous production and secretion of minicellulosomes active towards crystalline cellulose. To reach this goal, studies are under way to find the most suitable promoter (wild type or mutated) and the most appropriate leader peptide(s) that would allow the secretion by C. acetobutylicum of a chimeric minicellulosome containing the hybrid scaffoldin Scaf3 (26) and the cellulases Cel9G and Cel48F from C. cellulolyticum appended with suitable dockerin domains. These two enzymes were found to be the most active cellulase pair in the complexed state (5).
Acknowledgments
We are grateful to Philippe Soucaille, Pascale de Philip, and Sandrine Pagès for fruitful discussions. We thank Odile Valette and Marielle Bauzan for expert technical assistance.
We acknowledge the financial support received from the Centre National de la Recherche Scientifique, the Conseil Général des Bouches du Rhône, and the Région Provence-Alpes-Côte d'Azur.
REFERENCES
- 1.Bayer, E. A., Bélaïch J.-P., Shoham, Y., Lamed, R. 2004. The cellulosomes: multienzymes machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58:521-524. [DOI] [PubMed] [Google Scholar]
- 2.Bélaïch, J. P., C. Tardif, A. Bélaïch, and C. Gaudin. 1997. The cellulolytic system of Clostridium cellulolyticum. J. Biotechnol. 57:3-14. [DOI] [PubMed] [Google Scholar]
- 3.Cho, H.-Y., H. Yukawa, M. Inui, R. H. Doi, and S.-L. Wong. 2004. Production of minicellulosomes from Clostridium cellulovorans in Bacillus subtilis WB800. Appl. Environ. Microbiol. 70:5704-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fierobe, H. P., C. Bagnara-Tardif, C. Gaudin, F. Guerlesquin, P. Sauve, A. Bélaïch, and J. P. Bélaïch. 1993. Purification and characterization of endoglucanase C from Clostridium cellulolyticum. Catalytic comparison with endoglucanase A. Eur. J. Biochem. 217:557-565. [DOI] [PubMed] [Google Scholar]
- 5.Fierobe, H. P., E. A. Bayer, C. Tardif, M. Czjzek, A. Mechaly, A. Bélaïch, R. Lamed, Y. Shoham, and J. P. Bélaïch. 2002. Degradation of cellulose substrates by cellulosome chimeras. Substrate targeting versus proximity of enzyme components. J. Biol. Chem. 277:49621-49630. [DOI] [PubMed] [Google Scholar]
- 6.Fierobe, H. P., C. Gaudin, A. Bélaïch, M. Loutfi, E. Faure, C. Bagnara, D. Baty, and J. P. Bélaïch. 1991. Characterization of endoglucanase A from Clostridium cellulolyticum. J. Bacteriol. 173:7956-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fierobe, H. P., A. Mechaly, C. Tardif, A. Bélaïch, R. Lamed, Y. Shoham, J. P. Bélaïch, and E. A. Bayer. 2001. Design and production of active cellulosome chimeras. Selective incorporation of dockerin-containing enzymes into defined functional complexes. J. Biol. Chem. 276:21257-21261. [DOI] [PubMed] [Google Scholar]
- 8.Fierobe, H. P., S. Pagès, A. Bélaïch, S. Champ, D. Lexa, and J. P. Bélaïch. 1999. Cellulosome from Clostridium cellulolyticum: molecular study of the dockerin/cohesin interaction. Biochemistry 38:12822-12832. [DOI] [PubMed] [Google Scholar]
- 9.Fischer, R. J., J. Helms, and P. Dürre. 1993. Cloning, sequencing, and molecular analysis of the sol operon of Clostridium acetobutylicum, a chromosomal locus involved in solventogenesis. J. Bacteriol. 175:6959-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giallo, J., C. Gaudin, J. P. Bélaïch, E. Petitdemange, and F. Caillet-Mangin. 1983. Metabolism of glucose and cellobiose by cellulolytic mesophilic Clostridium sp. strain H10. Appl. Environ. Microbiol. 45:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guedon, E., M. Desvaux, S. Payot, and H. Petitdemange. 1999. Growth inhibition of Clostridium cellulolyticum by an inefficiently regulated carbon flow. Microbiology 145:1831-1838. [DOI] [PubMed] [Google Scholar]
- 12.Guedon, E., M. Desvaux, and H. Petitdemange. 2002. Improvement of cellulolytic properties of Clostridium cellulolyticum by metabolic engineering. Appl. Environ. Microbiol. 68:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogg, D., G. Pell, P. Dupree, F. Goubet, S. M. Martin-Orue, S. Armand, and H. J. Gilbert. 2003. The modular architecture of Cellvibrio japonicus mannanases in glycoside hydrolase families 5 and 26 points to differences in their role in mannan degradation. Biochem. J. 371:1027-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 15.Mermelstein, L. D., and E. T. Papoutsakis. 1993. In vivo methylation in Escherichia coli by the Bacillus subtilis phage φ3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 59:1077-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohand-Oussaid, O., S. Payot, E. Guedon, E. Gelhaye, A. Youyou, and H. Petitdemange. 1999. The extracellular xylan degradative system in Clostridium cellulolyticum cultivated on xylan: evidence for cell-free cellulosome production. J. Bacteriol. 181:4035-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murashima, K., C.-L. Chen, A. Kosugi, Y. Tamaru, R. H. Doi, and S.-L. Wong. 2002. Heterologous production of Clostridium cellulovorans engB, using protease-deficient Bacillus subtilis, and preparation of active recombinant cellulosomes. J. Bacteriol. 184:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakotte, S., S. Schaffer, M. Bohringer, and P. Dürre. 1998. Electroporation of, plasmid isolation from and plasmid conservation in Clostridium acetobutylicum DSM 792. Appl. Microbiol. Biotechnol. 50:564-567. [DOI] [PubMed] [Google Scholar]
- 19.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagès, S., A. Bélaïch, J. P. Bélaïch, E. Morag, R. Lamed, Y. Shoham, and E. A. Bayer. 1997. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: prediction of specificity determinants of the dockerin domain. Proteins 29:517-527. [PubMed] [Google Scholar]
- 21.Pagès, S., A. Bélaïch, H. P. Fierobe, C. Tardif, C. Gaudin, and J. P. Bélaïch. 1999. Sequence analysis of scaffolding protein CipC and ORFXp, a new cohesin-containing protein in Clostridium cellulolyticum: comparison of various cohesin domains and subcellular localization of ORFXp. J. Bacteriol. 181:1801-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagès, S., A. Bélaïch, C. Tardif, C. Reverbel-Leroy, C. Gaudin, and J. P. Bélaïch. 1996. Interaction between the endoglucanase CelA and the scaffolding protein CipC of the Clostridium cellulolyticum cellulosome. J. Bacteriol. 178:2279-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagès, S., O. Valette, L. Abdou, A. Bélaïch, and J. P. Bélaïch. 2003. A rhamnogalacturonan lyase in the Clostridium cellulolyticum cellulosome. J. Bacteriol. 185:4727-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, J. T., and M. J. Johnson. 1949. A submicrodetermination of glucose. J. Biol. Chem. 181:149-151. [PubMed] [Google Scholar]
- 25.Perret, S., A. Bélaïch, H. P. Fierobe, J. P. Bélaïch, and C. Tardif. 2004. Towards designer cellulosomes in Clostridio: mannanase enrichment of the cellulosomes produced by Clostridium cellulolyticum. J. Bacteriol. 186:6544-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perret, S., L. Casalot, H. P. Fierobe, C. Tardif, F. Sabathe, J. P. Bélaïch, and A. Bélaïch. 2004. Production of heterologous and chimeric scaffoldins by Clostridium acetobutylicum ATCC 824. J. Bacteriol. 186:253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reverbel-Leroy, C., S. Pagès, A. Bélaïch, J. P. Bélaïch, and C. Tardif. 1997. The processive endocellulase CelF, a major component of the Clostridium cellulolyticum cellulosome: purification and characterization of the recombinant form. J. Bacteriol. 179:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabathe, F., A. Bélaïch, and P. Soucaille. 2002. Characterization of the cellulolytic complex (cellulosome) of Clostridium acetobutylicum. FEMS Microbiol. Lett. 217:15-22. [DOI] [PubMed] [Google Scholar]
- 29.Sabathe, F., and P. Soucaille. 2003. Characterization of the CipA scaffolding protein and in vivo production of a minicellulosome in Clostridium acetobutylicum. J. Bacteriol. 185:1092-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasconcelos, I., L. Girbal, and P. Soucaille. 1994. Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J. Bacteriol. 176:1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]