Abstract
To identify genes that are important for class IIa bacteriocin interaction and resistance in Listeria species, transposon Tn917 knockout libraries were constructed for Listeria innocua strain Lin11 and screened for mutants that are resistant to pediocin AcH. A highly resistant mutant (G7) (MIC > 20 μg/ml; 1,000-fold less susceptible than the wild type), in which the transposon integrated into the putative promoter of the lin0142 gene, was isolated. lin0142 is located immediately upstream of the mpt operon (mptA/mptC/mptD) that encodes the mannose-specific phosphoenolpyruvate-dependent phosphotransferase system permease EIItMan, which serves as a docking protein for class IIa bacteriocins. The transcription of the mpt operon is known to be positively controlled by σ54 factor and ManR (a σ54-associated activator). Transcripts for lin0142 and mpt were undetectable in the G7 mutant, based on quantitative real-time reverse transcriptase PCR analysis. When the wild-type lin0142 gene was expressed at a 7.9-fold-elevated level in the mutant via a multicopy-number plasmid, the level of mpt mRNA became 70% higher than that in the wild-type strain. In addition, the complementation strain reverted back to the pediocin AcH-susceptible phenotype. The levels of manR and rpoN (σ54) mRNAs were not directly influenced by the level of lin0142 transcription. lin0142 is the only one of the three mpt regulatory genes whose transcription is induced, albeit slightly (1.2-fold), by glucose. The combined results show that the lin0142 gene encodes a novel activator of the mpt operon. The Lin0142 protein contains a winged-helix DNA-binding motif and is distantly related to the Crp-Fnr family of transcription regulators.
Bacteriocins are small, cationic, antimicrobial peptides produced by lactic acid bacteria (12, 16, 27, 28). These peptides are grouped into three major classes: lantibiotics (class I); small, heat-stable peptides functioning alone (class IIa) or in pairs (class IIb); and large, heat-labile proteins (class III) (28). Pediocin AcH (also known as pediocin PA-1) is a 44-amino-acid class IIa bacteriocin, produced primarily by Pediococcus species (40). The peptide is active against many types of lactic acid bacteria and has the highest activity against Listeria species of any class IIa bacteriocin (15). Largely because of their antilisterial activities, class IIa bacteriocins have provoked considerable interest for use as biopreservatives (12, 16, 40).
Class IIa bacteriocins (also known as YGNGV bacteriocins) have a common secondary structure (19, 48, 51) and mechanism of action (16). Initially, these peptides are thought to bind to negatively charged molecules, such as teichoic acids in the cell wall (7). Subsequently, they transfer to the cytoplasmic membrane, where they interact with anionic phospholipids (9) and a phosphoenolpyruvate-dependent phosphotransferase system (PTS) permease of the mannose structural family (EIItMan) (38). Once they are bound to cell membranes, class IIa bacteriocins fold into a βα conformation (19, 48, 51) in which the nonpolar α-helical region is inserted into the phospholipid bilayer (32). Ultimately, ion conductance pores are formed, resulting in cell death due to the dissipation of the membrane electrochemical gradient (10).
One concern regarding the use of bacteriocins as antimicrobial agents is the development of resistance in target bacterial strains (13, 34). In Listeria species, low-level resistance (two- to fourfold) to class IIa bacteriocins is caused by alterations in membrane lipid composition (13, 49, 50). High-level resistance (1,000-fold) in Listeria monocytogenes and Enterococcus faecalis results primarily from the loss of EIItMan, which is encoded by the mpt (mannose permease two) operon (14, 26, 38). High-level resistance in L. monocytogenes is also caused by the loss of σ54 factor (encoded by rpoN) (43) and the σ54-associated activator ManR (14), which together positively regulate the mpt operon. ManR activity also may be regulated by reversible phosphorylation of its two PTS regulation domains (14, 46).
Listeria uses both low-affinity proton motive force-dependent and high-affinity PTS transporters for glucose uptake (11). This genus appears to lack transporters of the glucose PTS structural family (ListiList database [http://genolist.pasteur.fr/ListiList]) (21). The high-affinity glucose transporter in Listeria probably is the EIItMan permease, whose expression is induced by glucose (14). It is not unusual that mannose PTS permeases participate in glucose transport, as they often display relatively broad substrate specificities (37). The interaction of class IIa bacteriocins with L. monocytogenes is highly dependent on EIItMan, which contains three subunits (IIABMan, IICMan, and IIDMan). Deletion analysis indicates that the IICMan subunit encoded by the mpt operon is needed for permease-peptide recognition (39). Inactivation of the mpo (mannose permease one) operon, which encodes a permease (EIIoMan) with four subunits (IIAMan, IIBMan, IICMan, and IIDMan), causes moderate resistance to class IIa peptides in L. monocytogenes due to a slight reduction in mpt expression (3). One common finding is that a β-glucoside-specific PTS (EIIBgl) is overexpressed in strains that are highly resistant to class IIa peptides (23, 24). This outcome may result from the absence of EIItMan and consequent relief of catabolite control protein (CcpA)-mediated repression of the transcription of the EIIBgl operon (2, 24, 42).
To identify additional genes that are important for class IIa bacteriocin-Listeria interaction and to learn more about basic mechanisms of resistance to these peptides, we have used Tn917 mutagenesis to isolate mutants resistant to pediocin AcH in Listeria innocua strain Lin11. L. innocua was used as a model system because mpt-based resistance mechanisms are thought to be operative in this species (24). In one mutant designated G7, transcription of the lin0142 gene is eliminated. The present study shows that the inactivation of lin0142 is responsible for pediocin AcH resistance in G7 and that lin0142 is required for the transcription of the mpt operon.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Tn917 libraries were constructed in L. innocua strain Lin11 (Jean Richard, Institut National de la Recherche Agronomique, Paris, France). Escherichia coli strain DH5 (supE44 hsd17 recA1 endA1 gyrA96 thi1 relA1) was used in cloning procedures (25). L. innocua strains were grown in brain heart infusion (BHI) (Difco, Sparks, Md.) or Luria-Bertani (LB) medium at 30°C with shaking. E. coli strains were grown in LB medium at 37°C with shaking.
Isolation of Tn917 insertion mutants.
Gene knockout libraries were constructed using plasmid pLTV3 to introduce Tn917 into L. innocua Lin11 (8). To identify mutants, ∼10,000 cells from a transposon library were screened at ∼100 cells per well in 96-well microtiter plates containing pediocin AcH. Pediocin AcH was purified by cell adsorption and desorption and by reversed-phase high-pressure liquid chromatography steps (51, 53). The screening medium was purple base broth (PBB) (Difco) with 1% lactose, 1 μg of erythromycin/ml, 25 μg of lincomycin/ml, and 0.05 μg of pediocin AcH/ml. Wells with resistant cells were identified based on a change in color from purple to yellow due to lactose metabolism after incubating 48 to 72 h at 30°C. Mutants were isolated by streaking on antibiotic plates and cultured through five or more passages to confirm the stability of the resistance phenotype.
Identification of the Tn917 insertion site in the G7 mutant.
DNA flanking the Tn917 insertion site in a mutant designated G7 was cloned by digesting genomic DNA with KpnI restriction enzyme, self-ligating the DNA (which contains a portion of the original pLTV3 vector with its ColE1 E. coli replication origin and an insert derived from chromosomal DNA), and transforming the ligation reaction into E. coli DH5. The insert in the resulting plasmid designated pG7 was sized by restriction enzyme analysis, and 262 bp of the chromosomal DNA adjacent to the transposon was sequenced by use of a primer (5′-AACTCACAATAGAGAGATGTCACCG-3′) complementary to the lacZ gene of pLTV3. All primers used in this study were obtained from Integrated DNA Technologies, Inc. (Coralville, Iowa). The L. innocua CLIP 11262 and L. monocytogenes EGD-e databases available at the Institut Pasteur website were used to conduct BLAST-N searches to locate the Tn917 insertion site in G7 (ListiList) (21).
Southern blot analysis.
Genomic DNA was isolated from the L. innocua G7 mutant by using standard procedures (18). A DNA-shearing step was added to improve the migration of genomic DNA in agarose gels. Plasmid DNA was purified from E. coli DH5/pG7 as a control by using the QIAfilter plasmid midi kit (QIAGEN, Valencia, Calif.). Plasmid and genomic DNAs were digested with KpnI restriction enzyme and analyzed on a 0.6% agarose gel. After electrophoresis, DNA was transferred to Zeta-Probe GT blotting membranes (Bio-Rad, Hercules, Calif.), and the DNA was fixed to the membranes by baking at 80°C for 30 min in a vacuum oven. Hybridization was performed at 43°C using probes complementary to the pLTV3 cat and ble genes at probe concentrations of 10 ng/ml. The sequence of the cat probe was 5′-CTAACTCTCCGTCGCTATTGTAACC-3′, and the sequence of the ble probe was 5′-GCACAGATAGCGTGGTCCGGCC-3′. Probes were labeled at the 3′ ends with fluorescein-dUTP. Labeling, hybridization, and washing were performed by following the instructions in the Gene Images 3′-oligolabeling module kit (Amersham Biosciences, Piscataway, N.J.). Membranes were first washed at a low stringency in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate at room temperature and then at a high stringency in 1× SSC and 0.1% sodium dodecyl sulfate at 52°C. DNA bands were visualized with the Gene Images ECL detection kit (Amersham Biosciences). X-ray films were exposed for 1 h when the cat probe was used and for 2 h when the ble probe was used.
Complementation testing.
The lin0142 gene was amplified by PCR using L. innocua Lin11 genomic DNA as a template and cloned into the shuttle vector, pAM401 (52). BamHI and SalI restriction sites were incorporated into the forward and reverse PCR primers for cloning purposes. The forward primer sequence was 5′-CGGGATCCATTGAAAGAATAGTTTCGG-3′, and the reverse primer sequence was 5′-ACGCGTCGACTTAATTTCATATTAACCC-3′. BamHI and SalI restriction sites are underlined. The BamHI-SalI fragment was ligated into the corresponding sites of the gram-negative tetracycline resistance gene in pAM401, and the construct was transformed into E. coli DH5. The gram-negative and gram-positive chloramphenicol resistance genes are preserved in the recombinant plasmid, which is named pJX0142. The sequence of the lin0142 gene was determined by DNA sequencing.
pJX0142 was isolated from E. coli DH5/pJX0142 grown in LB medium containing 20 μg of chloramphenicol/ml and transformed into L. innocua Lin11 and G7 by electroporation (33). Transformation mixtures were plated on BHI plates containing 30 μg of chloramphenicol/ml at 30°C. pAM401 also was transformed into the strains for controls. Plasmid isolation and restriction enzyme digestion were performed to confirm that all transformations were successful.
Pediocin AcH susceptibility testing.
The MICs of pediocin AcH were measured by use of a microtiter plate assay. Freshly grown cultures in PBB-lactose-antibiotic media were diluted and inoculated at ∼100 cells per well into microtiter plate wells containing 200 μl of PBB-lactose broth plus chloramphenicol as needed and a range of 0 to 20 μg of high-pressure liquid chromatography-purified pediocin AcH/ml. Lin11 was grown in PBB-1% lactose without antibiotics, Lin11/pAM401 and Lin11/pJX0142 were grown in PBB-1% lactose with 10 μg of chloramphenicol/ml, G7 was grown in PBB-1% lactose with 5 μg of chloramphenicol/ml, and G7/pAM401 and G7/pJX0142 were grown in PBB-1% lactose with 40 μg of chloramphenicol/ml. Higher chloramphenicol concentrations were used for G7 strains transformed with plasmids because G7 carries a copy of the cat gene from pLTV3. Plates were incubated at 30°C, and growth was scored between 48 to 72 h.
Sequencing of lin0142.
The BamHI-SalI PCR product described above was used to sequence the lin0142 gene. In addition, a PCR fragment spanning the lin0142 promoter region was sequenced to verify the results obtained with the larger PCR fragment. The forward primer used in amplification of the promoter region was 5′-ACCTGGTAAGCAAAAGACAGCAAC-3′, and the reverse primer was 5′-CGTTCCTCTGGATAAGCCGAC-3′. The PCR products were treated with ExoSAP-IT (U.S. Biochemical Corp., Cleveland, Ohio) to remove primers and nucleotides, and sequencing was then performed using the ABI Prism dRhodamine terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, Calif.). The same primers used for PCR amplification were used for the sequencing of both DNA strands. Dye terminators were removed from reactions by use of AGTC gel filtration cartridges (Edge BioSystems, Gaithersburg, Md.). Electrophoresis was performed using an Applied Biosystems model 3100 genetic analyzer.
RNA isolation and transcript analysis by quantitative real-time RT-PCR.
Strains were grown at 30°C in BHI or in LB medium supplemented with or without 2 g of d-glucose/liter to optical densities at 600 nm of 0.4 to 0.6. RNA from 3-ml cultures was stabilized using the RNAprotect bacterial reagent (QIAGEN) and was purified with the RNeasy mini kit (QIAGEN). Residual genomic DNA in the RNA samples was eliminated by DNase I (Invitrogen) treatment. cDNA was synthesized from total RNA by use of SuperScript II reverse transcriptase (RT) and random hexamers (both supplied by Invitrogen). To standardize the amount of RNA added to cDNA synthesis reactions performed with different RNA preparations, 16S rRNA was amplified as an endogenous control. Additional controls included a no-RT control, to assess contamination from genomic DNA, and no-template controls for each primer pair to measure interference due to primer dimer formation.
Real-time RT-PCRs were performed in 25-μl mixtures containing 12.5 μl of iQ SYBR green supermix (Bio-Rad), 10 ng of cDNA, and a 0.2 μM concentration of each of the forward and reverse primers (Table 1). Amplification steps were performed with an iCycler iQ real-time PCR detection system and an iCycler thermal cycler (Bio-Rad). The thermal cycler was programmed to initially hold at 95°C for 3.5 min and then to complete 40 cycles of 95°C for 15 s, 60°C for 30 s, and 68°C for 1 min. To carry out a melting curve analysis of PCR products, the temperature of samples was raised from 55 to 95°C by increases of 0.5°C per 10 s. The data were analyzed by using the comparative CT method, where CT is the cycle number at which the fluorescence emission due to PCR products exceeds the threshold. The threshold was set within the linear range of the PCR (44). The CT values for PCR products from strains Lin11/pJX0142, G7, and G7/pJX0142 were compared to those for products from strain Lin11 in order to determine differences (n-fold) in CT values. The REST program, which uses the pairwise fixed reallocation randomization test, was used to perform statistical analyses (35). A P value of <0.05 was considered significant.
TABLE 1.
Primers used for real-time RT-PCR
| Primera | Sequenceb |
|---|---|
| 16S rRNA | |
| F | AAGCAACGCGAAGAACCTTA |
| R | TGCACCACCTGTCACTTTGT |
| lin0142 | |
| F | CGGGTCAGAATGGTTTTGAG |
| R | AGACGGAACATTTTGCGAAC |
| lin0143 | |
| F | TTTGCCAATGCTGATTGAAG |
| R | GCGGATACCTTCTTGAGCTG |
| manR | |
| F | GCCATCAAAGTCATCCCAAT |
| R | GGATCTTGAATTCCGACAGC |
| rpoN | |
| F | TTCGTCCAAAAGCAACAACA |
| R | TGCCACTAAATCAGCTGTCG |
F, forward primer; R, reverse primer.
Primers were designed using published sequences available at the ListiList server (http://genolist.pasteur.fr/ListiList).
Database searches and sequence analyses.
Searches for promoter locations and prediction of the lin0142 transcription start point were performed with the Neural Network program (http://www.fruitfly.org/seq_tools/promoter.html) (41). Listeria genome analyses were performed using the ListiList server (http://genolist.pasteur.fr/ListiList) (21). GenBank database searches were carried out using the National Center for Biotechnology Information BLAST web server (http://www.ncbi.nlm.nih.gov/BLAST) (1). Protein sequences were analyzed for functionally important motifs and domains by use of the InterProScan server (http://www.ebi.ac.uk/InterProScan) (4, 6, 17, 22, 30). Multiple sequence alignments were carried out with the Tcoffee software package (http://igs-server.cnrs-mrs.fr/Tcoffee) (36).
RESULTS
Pediocin AcH resistance in the G7 mutant is caused by loss of lin0142 function.
Gene knockout libraries were constructed for L. innocua Lin11 by using transposon Tn917 introduced into the strain on plasmid pLTV3 (8). Altogether, 11 mutants which had various levels of resistance to pediocin AcH were isolated. Mutants were recovered from libraries at frequencies of 1 in 2,000 to 1 in 10,000 cells, depending on the library. One mutant, G7, is 1,000-fold less susceptible to the peptide (MIC > 20 μg/ml) than is the wild type. For the wild-type Lin11 strain, the MIC of pediocin AcH is only 0.02 μg/ml.
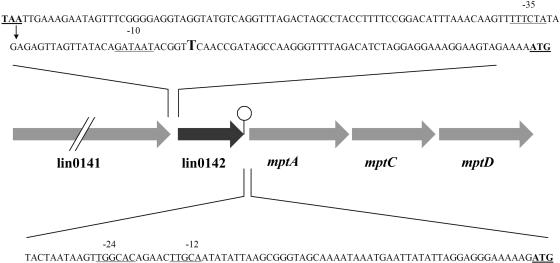
To determine where Tn917 resides in G7, the pG7 plasmid was constructed from genomic DNA and cloned in E. coli DH5. DNA sequencing of the pG7 insert showed that the transposon integrated between the lin0141 and lin0142 genes that have been mapped by sequencing the entire L. innocua CLIP 11262 genome (ListiList) (21) (Fig. 1). lin0141 encodes a putative cell wall protein with an LPXTG sortase cleavage motif (31). The function of the protein encoded by lin0142 is listed as unknown. lin0142 is just upstream of the mpt operon that contains mptA (IIABMan), mptC (IICMan), and mptD (IIDMan) (Fig. 1). A putative rho-independent terminator occurs between lin0142 and mptA (ListiList) (21), which suggests that lin0142 and the mpt operon are not cotranscribed.
FIG. 1.
Physical map of the Tn917 insertion site in the G7 mutant. The insertion site is shown by an arrow. The gene products are as follows: putative peptidoglycan bound protein with LPXTG motif (from lin0141), a protein of unknown function (from lin0142), EIItMan component IIAB (from mptA), EIItMan component IIC (from mptC), and EIItMan component IID (from mptD). The stem-loop symbol denotes a rho-independent terminator (ListiList). The likely −35 and −10 and −24 and −12 sequences of the respective lin0142 and mpt operon promoters are underlined. The putative transcription start point for the lin0142 mRNA is shown in larger font. The stop codon of lin0141, the start codon of lin0142, and the start codon of mptA also are underlined. Note that only part of the sequence between the lin0142 and mptA genes is shown.
Southern blotting was performed with KpnI-digested genomic DNA to determine the number of Tn917 insertions in G7. pG7 plasmid DNA was used as a size control, since this plasmid was constructed directly from KpnI-digested genomic DNA. Southern blots obtained by using a probe complementary to the pLTV3 ble gene showed only one hybridization band (∼19.5 kb) in G7 genomic DNA (data not shown). This band was the same size as that of the pG7 plasmid, and one band of this size also was obtained using a probe complementary to the pLTV3 cat gene. The results indicate that the G7 mutant carries a single copy of the Tn917 transposon. They also eliminate the possibility that the phenotype of G7 is caused instead by insertion of a second copy of the transposon elsewhere in the genome.
To determine if the pediocin AcH-resistant phenotype of G7 is caused by disruption of lin0142 transcription per se or is due to a polar effect on mpt transcription, complementation testing was performed using plasmid pJX0142. This plasmid contains lin0142 and its putative promoter and terminator and none of the mpt operon or promoter sequences. As noted above, the MIC of pediocin AcH for strain G7 (>20 μg/ml) is 1,000-fold greater than the MIC (0.02 μg/ml) for the Lin11 wild-type strain. When the pJX0142 plasmid was introduced into G7, the MIC (0.02 μg/ml) decreased to the level of the wild-type strain. However, the MIC (>20 μg/ml) for the G7/pAM401 vector-only control was not reduced. This result indicates that the wild-type lin0142 gene complements the pediocin AcH-resistant phenotype of G7 in trans. It also suggests that the loss of lin0142 transcription causes resistance, as only this gene is required for complementation. Further support for this conclusion was obtained via real-time RT-PCR analysis of lin0142 transcription in G7 and the G7/pJX0142 complementation strain (see below).
Promoter analysis performed with the Neural Network program (41) indicates that the Tn917 insertion site may reside within the lin0142 promoter (Fig. 1). The insertion point (between coordinates 11065 and 11066 relative to the L. innocua CLIP 11262 sequence) is 77 bp upstream of the lin0142 start codon and between the −35 and −10 sequences of a promoter predicted by the Neural Network algorithm (probability score, 0.94). The predicted initiation site for the lin0142 mRNA is a T residue at coordinate 11092. Real-time RT-PCR analysis of lin0142 mRNA level in G7 indicates that the Tn917 insertion may indeed have inactivated the lin0142 promoter.
lin0142 regulates mpt transcription.
Previous work has shown that 1,000-fold-increased resistance to class IIa bacteriocins in L. monocytogenes and E. faecalis results from the loss of mpt expression (14, 24, 26, 38). However, this fact has not yet been demonstrated directly for L. innocua. Because lin0142 is closely linked to the mpt operon, we tested whether lin0142 is involved in mpt control. We also tested whether lin0142 is needed for the transcription of the rpoN and manR genes, which positively regulate mpt transcription in L. monocytogenes. In this regard, searches conducted at the ListiList website (21) indicate that orthologs of all genes known to be important for class IIa bacteriocin resistance in L. monocytogenes are present in L. innocua. In both species, manR (lin0778/lmo0785) is located immediately upstream of the mpo operon. rpoN genes (sigL) also are present in both species. In addition, L. monocytogenes contains a lin0142 homolog (lmo0095) located immediately upstream of the mpt operon, which is composed of the mptA, mptC, and mptD genes.
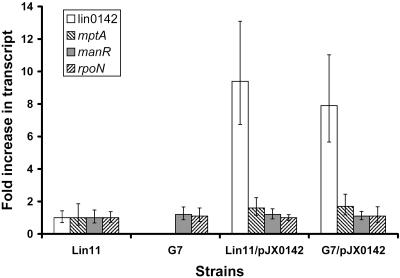
To study the effects of lin0142 inactivation on gene expression, real-time RT-PCR was performed using the Lin11, G7, Lin11/pJX0142, and G7/pJX0142 strains grown in BHI medium. Real-time RT-PCR results indicate that the transcription of lin0142 is abolished in the G7 mutant (Fig. 2). Furthermore, lin0142 inactivation decreased mptA mRNA to an undetectable level. In contrast, the 7.9-fold overexpression of lin0142 from plasmid pJX0142 led to an increase in the mpt mRNA level in the G7/pJX0142 complementation strain (1.7-fold higher, P < 0.05). rpoN and manR transcript levels were not significantly affected (P > 0.05) by differences in lin0142 mRNA levels in the four strains grown in BHI medium.
FIG. 2.
Real-time RT-PCR analysis of the relative levels of the lin0142, mptA, manR, and rpoN mRNAs in Lin11, G7, and both strains transformed with pJX0142. The averages of results obtained from three independent RNA preparations are shown. All transcript levels were measured in triplicate for each RNA preparation. Error bars represent standard deviations from the means. All strains were grown in BHI medium.
Regulation of gene expression in Lin11 and G7 by glucose.
Experiments were performed to investigate the role of glucose in the regulation of genes involved in mpt expression in the Lin11 and G7 strains. First, we determined whether transcription of the mpt operon is induced in the presence of glucose in L. innocua, as it is in L. monocytogenes (14). Second, the effects of glucose supplementation on manR, rpoN, and lin0142 mRNA levels were studied to evaluate whether glucose controls the transcription of these genes. Because BHI medium contains glucose, these experiments were performed in LB medium (14).
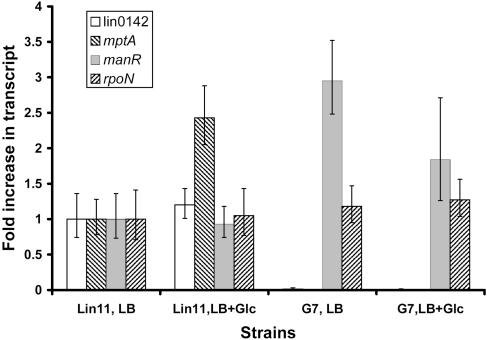
To determine whether expression of the mpt operon is regulated by glucose in L. innocua, real-time RT-PCR was performed using RNA isolated from Lin11 grown in LB medium with or without 2 g of glucose/liter. As shown in Fig. 3, the expression of the mptA gene was induced 2.4-fold (P < 0.01) by glucose in Lin11. Because transcription of mpt was elevated by glucose, it can be concluded that this operon also plays a specific role in glucose transport in L. innocua (14, 37).
FIG. 3.
Real-time RT-PCR analysis of the relative levels of the lin0142, mptA, manR, and rpoN mRNAs in Lin11 and G7 grown in LB medium with or without glucose (Glc). Averages of results obtained from four independent RNA preparations are shown. All transcript levels were measured in triplicate for each RNA preparation. Error bars represent standard deviations from the means.
The effects of glucose on rpoN, manR, and lin0142 mRNA levels also were studied by real-time RT-PCR analysis (Fig. 3). rpoN transcript levels were found to be the same in all media tested. manR mRNA levels in Lin11 were unchanged by the addition of glucose to the media. However, manR transcripts in G7 increased 2.7-fold (P < 0.01) compared to those in Lin11 when the strains were grown without glucose. Interestingly, the addition of glucose to the media reduced the level of manR mRNA in G7 by 40% (P < 0.01). Finally, lin0142 mRNA levels were increased 1.2-fold (P < 0.05) in Lin11 when glucose was added to the media. Transcription of lin0142 was undetectable in G7, regardless of whether glucose was present. In summary, lin0142 is the only one of the three genes for which expression is modulated by glucose, albeit slightly, in the wild-type strain.
Sequence alignment of Lin0142 homologs.
To gain insight into the function of the Lin0142 protein, its amino acid sequence was used as a query in BLAST-P searches conducted with the National Center for Biotechnology Information website and ListiList databases. It should be noted that the sequences of the lin0142 promoter, coding region, and terminator in strain Lin11 were determined and were found to be identical to the corresponding regions of the lin0142 gene present in L. innocua CLIP 11262.
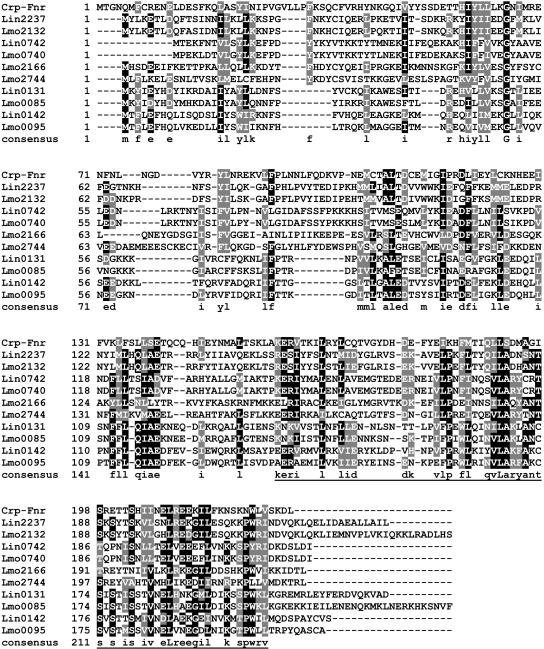
Lin0142 was found to be weakly similar to the Crp-Fnr family of transcription regulators (29). The Crp-Fnr family member from other bacterial genera that shows the highest percent identity (27%) to Lin0142 is a protein of unknown function from Staphylococcus epidermidis ATCC 12228 (GenBank accession no. NP765769) (Fig. 4). The most similar proteins come from Listeria species. For example, the lin0142 ortholog, lmo0095, displays 59% identity, and the lin0131 protein and its ortholog, lmo0085, show 37 and 32% identity, respectively. In no case is the regulatory function of any of these proteins apparent from BLAST search information.
FIG. 4.
Alignment of the amino acid sequences of Lin0142 and its homologs and orthologs in L. innocua and L. monocytogenes. The Crp-Fnr family protein from S. epidermidis ATCC 12228 (NP 765769) (labeled Crp-Fnr) also was included in the alignment. Black shading, amino acids that are identical in at least 50% of the sequences; grey shading, amino acids that are similar in at least 50% of the sequences. The putative winged-helix DNA-binding region is underlined.
Crp-Fnr regulators have two main sequence features: an N-terminal cyclic nucleotide-binding domain and a C-terminal helix-turn-helix DNA-binding domain (29). An InterProScan search was conducted to look for these and other sequence elements in Lin0142 and its homologs. This server uses SUPERFAMILY, PFAM, SMART, PROFILE, and other algorithms to identify potential signature sequences within a queried sequence. Lin0142 and its homologs were all found to contain C-terminal winged-helix DNA-binding motifs (Table 2) (20). In contrast, only some Lin0142 homologs, and not Lin0142 itself, contain cyclic nucleotide-binding domains. Although the E values for some of the matches are poor, the fact that these sequences were often detected by several algorithms adds to the significance of the findings.
TABLE 2.
BLAST-P results from ListiList and InterProScan searches
| Proteina | Domain type | Algorithm | Locationb | E value |
|---|---|---|---|---|
| Lin0142 | Winged-helix DNA binding | SUPERFAMILY | 136-196 | 1.1E−4 |
| Lmo0095 | Winged-helix DNA binding | SUPERFAMILY | 133-196 | 6.7E−6 |
| Lin0131 | Winged-helix DNA binding | SUPERFAMILY | 133-202 | 6.3E−5 |
| Lmo0085 | Winged-helix DNA binding | SUPERFAMILY | 133-202 | 2.3E−6 |
| Lin2237 | Cyclic nucleotide binding | SMART | 16-130 | 1.2 |
| Cyclic nucleotide binding | PROFILE | 36-115 | 9.361 | |
| Cyclic nucleotide binding | SUPERFAMILY | 3-126 | 1.5E−13 | |
| Winged-helix DNA binding | SUPERFAMILY | 146-224 | 9.5E−7 | |
| Lmo2166 | Cyclic nucleotide binding | PFAM | 29-122 | 0.5 |
| Cyclic nucleotide binding | SMART | 7-132 | 1.8 | |
| Cyclic nucleotide binding | PROFILE | 7-134 | 10.835 | |
| Cyclic nucleotide binding | SUPERFAMILY | 28-132 | 9.8E−13 | |
| Winged-helix DNA binding | SUPERFAMILY | 152-223 | 9.3E−6 | |
| Lin0742 | Cyclic nucleotide binding | SUPERFAMILY | 14-133 | 3.0E−10 |
| Winged-helix DNA binding | SUPERFAMILY | 142-212 | 3.0E−6 | |
| Lmo0740 | Cyclic nucleotide binding | SUPERFAMILY | 1-117 | 1.6E−10 |
| Winged-helix DNA binding | SUPERFAMILY | 142-212 | 3.4E−6 | |
| Lmo2132 | Cyclic nucleotide binding | PROFILE | 38-115 | 8.41 |
| Cyclic nucleotide binding | SUPERFAMILY | 3-126 | 2.0E−12 | |
| Winged-helix DNA binding | SUPERFAMILY | 151-224 | 2.0E−6 | |
| Lmo2744 | Cyclic nucleotide binding | PFAM | 29-129 | 0.4 |
| Cyclic nucleotide binding | PROFILE | 29-96 | 8.507 | |
| Cyclic nucleotide binding | SUPERFAMILY | 28-146 | 2.3E−14 | |
| Winged-helix DNA binding | SUPERFAMILY | 157-225 | 9.5E−11 |
Proteins are named according to the ListiList server (http://genolist.pasteur.fr/ListiList). The GenBank accession number for Listeria sequences is NC003212. Proteins are listed in decreasing order of identity to Lin0142.
Locations of amino acid sequences containing the identified domains.
Previously, only one of these proteins (Lmo2132) was classified by phylogenetic analysis as a Crp-Fnr protein (within the D cluster of the family) (29). Lin0142 and the other proteins shown in Fig. 4 may have escaped classification due to the lack of cyclic nucleotide-binding domains or due to weak similarity of their DNA-binding domains to those in more typical Crp-Fnr proteins (29).
DISCUSSION
We have demonstrated that the lin0142 gene clearly is needed for the sensitivity of L. innocua to pediocin AcH. When lin0142 is not expressed, the bacterium becomes highly resistant to pediocin AcH. After lin0142 is reintroduced into the mutant, the bacterium is once again sensitive. We also have shown that lin0142 is required for transcription of the mpt operon, which encodes the EIItMan permease. This transporter is thought to be a docking protein for class IIa bacteriocins in L. monocytogenes and E. faecalis (14, 26, 38, 39). The level of mpt mRNA was very low in the G7 mutant and increased to greater than wild-type levels in the G7/pJX0142 strain due to the elevated transcription of lin0142 caused by the gene dosage effect of the multicopy-number plasmid. These data show for the first time that lin0142 controls the expression of the EIItMan permease and is needed for sensitivity of L. innocua to a class IIa bacteriocin.
Work performed here and bioinformatics analyses indicate that the basic aspects of EIItMan control are similar in L. monocytogenes and L. innocua. First, all genes implicated in the regulation of mpt in L. monocytogenes are present in L. innocua. Second, the expression of the mpt operon is induced by glucose in both species. Third, the L. innocua mpt operon contains an upstream sequence that precisely matches the −24 and −12 σ54-controlled promoter consensus sequence, YTGGCACGrNNNTTGCW (Fig. 1) (5, 45), and therefore is likely to be controlled by σ54 and ManR. Fourth, L. monocytogenes contains a lin0142 homolog (lmo0095) immediately upstream of its mpt operon. In addition, EIIBgl mRNA levels were found to be down-regulated when Lin11 was grown in LB medium supplemented with glucose (data not shown). This result is most likely caused by elevation of EIItMan levels in the presence of glucose (47).
Because the expression of the mpt operon is dependent on rpoN and manR in L. monocytogenes (2, 14, 43), we investigated the role played by lin0142 in the transcription of these two genes. lin0142 was found to be unnecessary for the transcription of either gene. The levels of rpoN and manR transcripts remained the same and increased, respectively, when the G7 mutant was grown in LB medium. The second of these observations might seem to indicate that lin0142 is a repressor of the manR gene. However, it is more likely that manR mRNA levels are elevated in the mutant due to the loss of mpt expression. In this regard, the level of manR mRNA remains unchanged when lin0142 mRNA is overexpressed from pJX0142 in both the wild-type and mutant strains grown in BHI medium. It therefore appears that lin0142 is not normally involved in regulating the transcription of rpoN and manR.
We also examined whether glucose regulates the transcription of the lin0142, rpoN, and manR genes. Based on the finding that the levels of rpoN and manR mRNAs were not affected by glucose in the wild-type strain, it appears that these genes are not normally controlled by glucose. While manR transcript levels were found to decrease in response to glucose in the G7 mutant, this outcome could be an abnormal regulatory consequence caused by the loss of mpt expression. In the case of lin0142, it was found that glucose stimulated the transcription of this gene in the wild-type strain. We speculate that an increase in the Lin0142 protein level may contribute to the induction of the mpt operon in glucose media. However, the phosphorylation state and associated activation state of ManR are likely to be controlled by glucose, and this also may play an important role in the induction of the operon by glucose (14, 46).
Although Lin0142 is not strongly homologous to Crp-Fnr transcription factors (29), it is distantly related to this family of proteins. In addition, it contains a winged-helix DNA-binding motif (20). The winged-helix motif is a compact α/β structure that contains two wings (large loops), three α-helices, and three β-strands. The third α-helix typically participates in DNA binding, and winged-helix proteins often form complexes with other transcription factors (20). Sequence alignments of Lin0142 homologs indicate that the C-terminal region, where the winged-helix motif is located, is more conserved than the N-terminal region of these proteins. With regard to mpt promoter activation, Lin0142 is not a canonical σ54-associated activator, as it does not contain a σ54 interaction module. It also lacks PTS regulation domains, which are common in PTS regulators (46). At this stage of the work, it is unknown whether the Lin0142 protein plays a direct or indirect role in activation of the mpt operon. However, the data suggest that Lin0142 may bind to DNA while carrying out its regulatory functions.
In conclusion, the results show that the lin0142 gene is a novel activator of mpt transcription in L. innocua. It is possible that additional regulators of EIItMan permease expression may be discovered by further screening for resistance to class IIa bacteriocins.
Acknowledgments
This work was supported by a grant to K.W.M. from the Wyoming Agricultural Experiment Station.
We thank Daniel Portnoy for kindly providing the pLTV3 and pAM401 plasmids.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arous, S., C. Buchrieser, P. Folio, P. Glaser, A. Namane, M. Hebraud, and Y. Hechard. 2004. Global analysis of gene expression in an rpoN mutant of Listeria monocytogenes. Microbiology 150:1581-1590. [DOI] [PubMed] [Google Scholar]
- 3.Arous, S., K. Dalet, and Y. Hechard. 2004. Involvement of the mpo operon in resistance to class IIa bacteriocins in Listeria monocytogenes. FEMS Microbiol. Lett. 238:37-41. [DOI] [PubMed] [Google Scholar]
- 4.Attwood, T. K., P. Bradley, D. R. Flower, A. Gaulton, N. Maudling, A. L. Mitchell, G. Moulton, A. Nordle, K. Paine, P. Taylor, A. Uddin, and C. Zygouri. 2003. PRINTS and its automatic supplement, prePRINTS. Nucleic Acids Res. 31:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrios, H., B. Valderrama, and E. Morett. 1999. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res. 27:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhunia, A. K., M. C. Johnson, B. Ray, and N. Kalchayanand. 1991. Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. J. Appl. Bacteriol. 70:25-30. [Google Scholar]
- 8.Camilli, A., D. A. Portnoy, and P. Youngman. 1990. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J. Bacteriol. 172:3738-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y., R. D. Ludescher, and T. J. Montville. 1998. Influence of lipid composition on pediocin PA-1 binding to phospholipid vesicles. Appl. Environ. Microbiol. 64:3530-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikindas, M. L., M. J. Garcia-Garcera, A. J. Driessen, A. M. Ledeboer, J. Nissen-Meyer, I. F. Nes, T. Abee, W. N. Konings, and G. Venema. 1993. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl. Environ. Microbiol. 59:3577-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, D. P., and R. W. Hutkins. 1994. Glucose uptake by Listeria monocytogenes Scott A and inhibition by pediocin JD. Appl. Environ. Microbiol. 60:3870-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleveland, J., T. J. Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 13.Crandall, A. D., and T. J. Montville. 1998. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl. Environ. Microbiol. 64:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalet, K., Y. Cenatiempo, P. Cossart, and Y. Hechard. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 15.Eijsink, V. G., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 17.Falquet, L., M. Pagni, P. Bucher, N. Hulo, C. J. A. Sigrist, K. Hofmann, and A. Bairoch. 2002. The PROSITE database, its status in 2002. Nucleic Acids Res. 30:235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flamm, R. K., D. J. Hinrichs, and M. F. Thomashow. 1984. Introduction of pAMβ1 into Listeria monocytogenes by conjugation and homology between native L. monocytogenes plasmids. Infect. Immun. 44:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fregeau-Gallagher, N. L., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 20.Gajiwala, K. S., and S. K. Burley. 2000. Winged helix proteins. Curr. Opin. Struct. Biol. 10:110-116. [DOI] [PubMed] [Google Scholar]
- 21.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 22.Gough, J., K. Karplus, R. Hughey, and C. Chothia. 2001. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J. Mol. Biol. 313:903-919. [DOI] [PubMed] [Google Scholar]
- 23.Gravesen, A., P. Warthoe, S. Knochel, and K. Thirstrup. 2000. Restriction fragment differential display of pediocin-resistant Listeria monocytogenes 412 mutants shows consistent overexpression of a putative β-glucoside-specific PTS system. Microbiology 146:1381-1389. [DOI] [PubMed] [Google Scholar]
- 24.Gravesen, A., M. Ramnath, K. B. Rechinger, N. Andersen, L. Jansch, Y. Hechard, J. W. Hastings, and S. Knochel. 2002. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361-2369. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 26.Hechard, Y., C. Pelletier, Y. Cenatiempo, and J. Frere. 2001. Analysis of σ54-dependent genes in Enterococcus faecalis: a mannose PTS permease (EIIMan) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575-1580. [DOI] [PubMed] [Google Scholar]
- 27.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 29.Korner, H., H. J. Sofia, and W. G. Zumft. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27:559-592. [DOI] [PubMed] [Google Scholar]
- 30.Letunic, I., L. Goodstadt, N. J. Dickens, T. Doerks, J. Schultz, R. Mott, F. Ciccarelli, R. R. Copley, C. P. Ponting, and P. Bork. 2002. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res. 30:242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazmanian, S. K., G. Liu, H. Ton-That, and O. Schneewind. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285:760-763. [DOI] [PubMed] [Google Scholar]
- 32.Miller, K. W., R. Schamber, O. Osmanagaoglu, and B. Ray. 1998. Isolation and characterization of pediocin AcH chimeric protein mutants with altered bactericidal activity. Appl. Environ. Microbiol. 64:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, S. F., and G. S. A. B. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 34.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 35.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirot, O., E. O'Toole, and C. Notredame. 2003. Tcoffee@igs: a web server for computing, evaluating and combining multiple sequence alignments. Nucleic Acids Res. 31:3503-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramnath, M., M. Beukes, K. Tamura, and J. W. Hastings. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramnath, M., S. Arous, A. Gravesen, J. W. Hastings, and Y. Hechard. 2004. Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663-2668. [DOI] [PubMed] [Google Scholar]
- 40.Ray, B., and K. W. Miller. 2000. Pediocins, p. 525-566. In A. S. Naidu (ed.), Natural food antimicrobial systems. CRC Press, New York, N.Y.
- 41.Reese, M. G. 2001. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput. Chem. 26:51-56. [DOI] [PubMed] [Google Scholar]
- 42.Reizer, J., C. Hoischen, F. Titgemeyer, C. Rivolta, R. Rabus, J. Stulke, D. Karamata, M. H. Saier, Jr., and W. Hillen. 1998. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol. Microbiol. 27:1157-1169. [DOI] [PubMed] [Google Scholar]
- 43.Robichon, D., E. Gouin, M. Debarbouille, P. Cossart, Y. Cenatiempo, and Y. Hechard. 1997. The rpoN (σ54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J. Bacteriol. 179:7591-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmittgen, T. D., B. A. Zakrajsek, A. G. Mills, V. Gorn, M. J. Singer, and M. W. Reed. 2000. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 285:194-204. [DOI] [PubMed] [Google Scholar]
- 45.Studholme, D. J., and R. Dixon. 2003. Domain architectures of σ54-dependent transcriptional activators. J. Bacteriol. 185:1757-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stulke, J., M. Arnaud, G. Rapoport, and I. Martin-Verstraete. 1998. PRD-a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol. Microbiol. 28:865-874. [DOI] [PubMed] [Google Scholar]
- 47.Titgemeyer, F., and W. Hillen. 2002. Global control of sugar metabolism: a gram-positive solution. Antonie Leeuwenhoek 82:59-71. [PubMed] [Google Scholar]
- 48.Uteng, M., H. H. Hauge, P. R. Markwick, G. Fimland, D. Mantzilas, J. Nissen-Meyer, and C. Muhle-Goll. 2003. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide sakacin P and a sakacin P variant that is structurally stabilized by an inserted C-terminal disulfide bridge. Biochemistry 42:11417-11426. [DOI] [PubMed] [Google Scholar]
- 49.Vadyvaloo, V., J. W. Hastings, M. J. van der Merwe, and M. Rautenbach. 2002. Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl. Environ. Microbiol. 68:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vadyvaloo, V., J. L. Snoep, J. W. Hastings, and M. Rautenbach. 2004. Physiological implication of class IIa bacteriocin resistance in Listeria monocytogenes strains. Microbiology 150:335-340. [DOI] [PubMed] [Google Scholar]
- 51.Watson, R. M., R. W. Woody, R. V. Lewis, D. S. Bohle, A. H. Andreotti, B. Ray, and K. W. Miller. 2001. Conformational changes in pediocin AcH upon vesicle binding and approximation of the membrane-bound structure in detergent micelles. Biochemistry 40:14037-14046. [DOI] [PubMed] [Google Scholar]
- 52.Wirth, R., F. Y. An, and D. B. Clewell. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, R., M. C. Johnson, and B. Ray. 1992. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 58:3355-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]