Abstract
17α-estradiol has recently been shown to extend healthspan and lifespan in male mice through multiple mechanisms. These benefits occur in the absence of significant feminization or deleterious effects on reproductive function, which makes 17α-estradiol a candidate for translation into humans. However, human dosing paradigms for the treatment of aging and chronic disease are yet to be established. Therefore, the goals of the current studies were to assess tolerability of 17α-estradiol treatment, in addition to evaluating metabolic and endocrine responses in male rhesus macaque monkeys during a relatively short treatment period. We found that our dosing regimens (0.30 and 0.20 mg/kg/day) were tolerable as evidenced by a lack of GI distress, changes in blood chemistry or complete blood counts, and unaffected vital signs. We also found that the higher dose did elicit mild benefits on metabolic parameters including body mass, adiposity, and glycosylated hemoglobin. However, both of our 17α-estradiol trial doses elicited significant feminization to include testicular atrophy, increased circulating estrogens, and suppressed circulating androgens and gonadotropins. We suspect that the observed level of feminization results from a saturation of the endogenous conjugation enzymes, thereby promoting a greater concentration of unconjugated 17α-estradiol in serum, which has more biological activity. We also surmise that the elevated level of unconjugated 17α-estradiol was subjected to a greater degree of isomerization to 17β-estradiol, which is aligned with the sevenfold increase in serum 17β-estradiol in 17α-estradiol treated animals in our first trial. Future studies in monkeys, and certainly humans, would likely benefit from the development and implementation of 17α-estradiol transdermal patches, which are commonly prescribed in humans and would circumvent potential issues with bolus dosing effects.
Supplementary information
The online version contains supplementary material available at 10.1007/s11357-023-00767-9.
Keywords: Aging, Dihydrotestosterone, Endocrinology, Follicle stimulating hormone, Luteinizing hormone, Testosterone
Introduction
17α-estradiol (17α-E2) is one of the more recently studied pro-longevity compounds to demonstrate efficacy for beneficially modulating health outcomes in mice. The NIA Interventions Testing Program (ITP) found that 17α-E2 substantially extends median lifespan in male mice when treatment is initiated in mid-life [1, 2] and late-life [3]. The magnitude of lifespan extension with 17α-E2 treatment in male mice is similar to that of calorie restriction [4] and rapamycin administration [5], which indicates 17α-E2 elicits potent effects and may hold therapeutic utility for treating humans. We have been exploring potential mechanisms by which 17α-E2 may improve healthspan and extend lifespan in a sex-specific manner. We have reported that 17α-E2 administration reduces calorie intake and regional adiposity in combination with significant improvements in several metabolic measures including glucose tolerance, insulin sensitivity, and ectopic lipid deposition in obese and aged male mice [6–10]. Other groups have also reported that 17α-E2 treatment elicits similar beneficial effects on glucose tolerance, mTORC2 signaling, hepatic urea cycling, markers of neuroinflammation, and sarcopenia [11–15]. We have shown that 17α-E2-mediated declines in food intake are not required for metabolic benefits during treatment [7], suggesting that 17α-E2 modulates metabolism and potentially aging through multiple mechanisms. It remains unclear if 17α-E2 elicits benefits by altering reproductive function, although we recently demonstrated that chronic 17α-E2 treatment does not adversely affect sperm parameters or fertility in breeding age male mice [16].
To date, the vast majority of studies evaluating 17α-E2 have been performed in culture and rodent models. The few human studies that have been done focused predominantly on pharmacokinetic parameters of 17α-E2 or were short-term Phase 1 tolerability trials [17]. At this juncture, there is no available data indicating that 17α-E2 treatment elicits significant adverse effects or feminizing activity in humans when provided as a single dose or repeated daily oral doses [17]. However, human dosing paradigms of 17α-E2 for the treatment of aging and multimorbidity are yet to be established. Given these unknowns, we surmised that a study evaluating the effects of 17α-E2 in a species closely related to humans was a more realistic goal for initially determining the potential translatability of 17α-E2 into higher order mammals.
The work outlined in this report sought to determine tolerable dosing of 17α-E2 in rhesus monkeys in addition to evaluating metabolic and endocrine responses during a relatively short treatment period. We chose to use rhesus monkeys because they closely recapitulate human aging and chronic disease onset [18]. The rhesus macaque genome shares roughly 93% sequence homology with the human genome [19], and numerous aspects of their anatomy, physiology, neurology, endocrinology, and immunology directly parallel those of humans [20, 21]. Therefore, they are an excellent model for testing efficacy and toxicity of pharmacotherapies [22]. Moreover, rhesus monkeys develop obesity- and age-related metabolic disorders similarly to humans [23–25], which provides an additional advantage due to the aforementioned effects of 17α-E2 on metabolic homeostasis in mice. Herein, we present data from two independent, short-term 17α-E2 dosing studies in male rhesus monkeys. We found that 17α-E2 treatment did elicit mild benefits on metabolic parameters in our first trial, but these benefits were accompanied by a significant degree of feminization, which far exceeded what is typically observed in mice. Our second trial, which used a lower dose of 17α-E2, also induced feminization. We conclude that additional studies that employ alternative dosing regimens and potentially delivery methods will be needed to determine if 17α-E2 can be effectively translated into higher order mammals for the treatment of aging and chronic disease.
Methods
Subjects:
Twenty-eight adult (7–24 years of age) male rhesus monkeys (Macaca mulatta) were used in our studies (Table 1). All monkeys were housed in standard nonhuman primate caging under established conditions (temperature, 22–28 °C; humidity, 50–60%; 12:12 light–dark) at the NIH Animal Center as previously described [26, 27]. Throughout the studies, all monkeys received commercially prepared monkey chow (Lab Diet #5038, LabDiet Inc., St. Louis, MO) which was distributed twice/day along with daily food enrichment and ad libitum access to water. All monkeys had extensive visual, auditory, and olfactory but limited tactile contact with monkeys housed in the same room. Monkeys were monitored minimally 3 times daily by trained care staff. All procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals in an AALAC-accredited facility and approved by the NIA Institutional Animal Care and Use Committee.
Table 1.
Rhesus macaques evaluated in trials 1 and 2
| Animal | sex | Age at Trial(y) | Mass at Trial Start (kg) | Trial | Treatment | Treatment Dose (mg/kg/day) |
|---|---|---|---|---|---|---|
| 1 | Male | 16 | 18.44 | 1 | CON | - |
| 2 | Male | 7 | 11.85 | 1 | CON | - |
| 3 | Male | 19 | 14.53 | 1 | CON | - |
| 4 | Male | 15 | 16.16 | 1 | CON | - |
| 5 | Male | 18 | 19.35 | 1 | CON | - |
| 6 | Male | 23 | 16.06 | 1 | CON | - |
| 7 | Male | 24 | 14.08 | 1 | 17ɑ -E2 | 0.30 |
| 8 | Male | 19 | 12.53 | 1 | 17ɑ -E2 | 0.30 |
| 9 | Male | 17 | 17.48 | 1 | 17ɑ -E2 | 0.30 |
| 10 | Male | 17 | 15.01 | 1 | 17ɑ -E2 | 0.30 |
| 11 | Male | 15 | 17.45 | 1 | 17ɑ -E2 | 0.30 |
| 12 | Male | 19 | 16.32 | 1 | 17ɑ -E2 | 0.30 |
| 13 | Male | 17 | 12.41 | 1 | 17ɑ -E2 | 0.30 |
| 14 | Male | 8 | 12.03 | 1 | 17ɑ -E2 | 0.30 |
| 15 | Male | 13 | 11.4 | 2 | CON | - |
| 16 | Male | 21 | 18.32 | 2 | CON | - |
| 17 | Male | 19 | 16.37 | 2 | CON | - |
| 18 | Male | 10 | 14.96 | 2 | CON | - |
| 19 | Male | 16 | 12.34 | 2 | CON | - |
| 20 | Male | 22 | 14.48 | 2 | CON | - |
| 21 | Male | 9 | 14.73 | 2 | 17ɑ-E2 | 0.20 |
| 22 | Male | 19 | 17.75 | 2 | 17ɑ -E2 | 0.20 |
| 23 | Male | 12 | 15.5 | 2 | 17ɑ -E2 | 0.20 |
| 24 | Male | 19 | 14.33 | 2 | 17ɑ -E2 | 0.20 |
| 25 | Male | 12 | 15.14 | 2 | 17ɑ -E2 | 0.20 |
| 26 | Male | 20 | 12.42 | 2 | 17ɑ -E2 | 0.20 |
| 27 | Male | 22 | 14.23 | 2 | 17ɑ -E2 | 0.20 |
| 28 | Male | 18 | 15.96 | 2 | 17ɑ -E2 | 0.20 |
17α-E2 Dosing and Delivery:
Our previous studies in mice have established that the average dietary intake of 17α-E2 ranges from 30–45 µg/day [6–8]. Normalizing these intake levels by body mass revealed that male mice generally consumed 0.92–1.15 mg/kg/day during our studies. These data were used to calculate dosing paradigms in rhesus monkeys through allometric scaling. Allometric scaling is used to calculate pharmacological dosing paradigms because it accounts for differences in metabolic rate and surface area between species [28, 29]. These calculations determined that 17α-E2 doses ranging from 0.20–0.30 mg/kg/day would be roughly equivalent to what mice ingest on a daily basis. 17α-E2 was purchased from Steraloids (Newport, RI) and reconstituted in EtOH at a concentration of 10 mg/ml. This stock solution was created fresh each week and then aliquoted into prelabeled cryovials to account for daily individualized dosing based on body mass, which was determined biweekly for dosing purposes. Delivery of 17α-E2 was achieved by instilling the appropriate volume of stock solution to 5–6 PRIMA-Treat® wafers (Bio-Serv, Flemington, NJ) each day, which were then provided to each respective subject in their home cage during the morning feeding period. Each day control animals were provided an equivalent number of PRIMA-Treat® wafers without 17α-E2 so that calorie intake and exposure to alternative food sources could be normalized across groups. Monkeys were monitored to ensure they consumed their entire allotment of PRIMA-Treat® wafers. Calorie intake from PRIMA-Treat® wafers was included in daily calorie consumption calculations.
Trial 1:
Fourteen male subjects were assigned to control or 17α-E2 (0.30 mg/kg/day) treatment groups for a 12-week intervention. Subjects ranged in age from 7–24 years (16.6 ± 1.3) with initial body masses ranging from 11.9–19.4 kg (15.3 ± 0.7). Subjects receiving 17α-E2 were monitored several times each day for signs of vomiting, diarrhea, and/or constipation, which are initial indicators of poor tolerability of an orally delivered pharmacotherapy in rhesus monkeys. Food intake, another measure of oral compound tolerability, was also monitored daily throughout the duration of the study. Animals were anesthetized at weeks 0, 6, 10, and 12 with intramuscular ketamine (10 mg/kg) or Telazol (2–6 mg/kg) following an overnight fast in preparation for blood collection and the assessment of body temperature, respiration rate, heart rate, and blood pressure. Additional procedures were performed at these timepoints including dual x-ray absorptiometry (DEXA) scanning, intravenous glucose tolerance testing (IVGTT), and gonadal ultrasound measurements as described below.
Trial 2:
Fourteen male subjects were assigned to control or 17α-E2 (0.20 mg/kg/day) treatment groups for an 11-week intervention. Subjects ranged in age from 9–22 years (16.6 ± 1.2) with initial body masses ranging from 11.4–18.3 kg (14.9 ± 0.5). Subjects receiving 17α-E2 were monitored several times each day for signs of vomiting, diarrhea, and/or constipation, which are initial indicators of poor tolerability of an orally delivered pharmacotherapy in rhesus monkeys. Similar to Trial 1, animals were anesthetized at weeks 0 and 11 with intramuscular ketamine (10 mg/kg) or Telazol (2–6 mg/kg) following an overnight fast in preparation for blood collection and the assessment of body temperature, respiration rate, and heart rate. DEXA scanning and gonadal ultrasounds were also performed on animals under anesthesia during Trial 2.
Food Intake:
Food intake was monitored throughout Trial 1. Each animal received half of its daily calorie allotment twice daily, which was based on body mass and estimated from the National Research Council Guidelines [30]. Monkeys were part of an established colony and weight stable prior to the onset of the study. Daily chow allotments were also adjusted based on the number of PRIMA-Treat® wafers consumed during vehicle and drug delivery. Any chow remaining prior to the next week feeding period was subtracted from the amount provided to determine daily consumption.
Blood Collection and Analyses:
Blood samples were collected under anesthesia following an overnight fast. Glycosylated hemoglobin (HbA1C) was measured in whole blood using a Siemens DCA Vantage Analyzer (Siemens Medical Solutions, Malvern, PA) for Trial 1. Whole blood and serum were sent to Antech Diagnostics (Irvine, CA, USA) for assessment of blood chemistry and complete blood count values for Trial 1. Serum was sent to Mayo Clinic (Rochester, MN) for assessment of circulating 17α-E2, 17β-estradiol (17β-E2), estrone, testosterone, and dihydrotestosterone (DHT) for Trials 1 & 2 as described previously [6]. Additional serum was sent to the Endocrine Technologies Core at the Oregon National Primate Research Center (Beaverton, OR) for assessment of circulating follicle stimulating hormone (FSH) and luteinizing hormone (LH) by radioimmunoassay for Trial 1 as described elsewhere [31].
DEXA Scanning:
Scanning for fat mass, lean mass, and bone mineral content (BMC) was performed under anesthesia for Trials 1 & 2 using a GE Lunar Prodigy DEXA (GE Healthcare, Chicago, IL).
IVGTT Assessments:
Testing was performed under anesthesia at baseline and during week 10 of Trial 1. Intravenous dextrose administration and blood sampling procedures were done as described previously [32]. In brief, 50% dextrose solution at a dose of 300 mg/kg was administered through the saphenous vein and blood samples were collected at 0, 1, 3, 5, 7, 10, 15, 20, 30, and 40 min. Glucose values were determined via an Ascensia Breeze 2 glucometer (Bayer Healthcare, Mishawaka, IN) and plasma insulin levels were determined via an ELISA from Novus Biologicals (Centennial, CO).
Gonadal Ultrasounds:
Testicle size was determined under anesthesia for Trials 1 & 2 using a Siemens Acuson S2000 ultrasound system (Siemens Medical Solutions, Malvern, PA) by a trained technician blinded to group designation. Two dimensional measures were recorded for both the left and right testis.
Statistical Analyses:
Results are presented as mean ± SEM with p values less than 0.05 considered to be significantly different. Analyses of differences between groups were performed by two-way ANOVA, two-way repeated measures ANOVA, or Student’s t-test where appropriate using Graphpad Prism 9.0 Software.
Results
Oral dosing of 17α-E2 at 0.30 mg/kg/day appears tolerable
In our first trial, we sought to determine if oral administration of 17α-E2 at 0.30 mg/kg/day would be tolerated in male rhesus monkeys. Initial outcomes indicated that this dose was indeed tolerable as evidenced by a lack of vomiting, diarrhea, or constipation. Food intake remained constant in the 17α-E2 treatment group throughout the trial, which is consistent what is shown in during the first 5 weeks (Fig. 1). This observation provides additional evidence that oral 17α-E2 administration did not adversely affect feeding behavior. Blood chemistry and complete blood count values, which were independently evaluated by veterinary staff, were predominantly unaffected by 12 weeks of 17α-E2 administration (Supplemental Table 1). Although observed differences from week 0 to 12 were deemed to be biologically insignificant by the attending veterinarian staff, it should be noted that 17α-E2 did modestly, but significantly, reduce red blood cells (p = 0.011), hemoglobin (p = 0.007), and hematocrit (p = 0.047) following 12 weeks of treatment. The biological significance of these declines remains unknown, but these variables should at least be monitored during future experimentation in monkeys and/or humans. Other secondary markers of tolerability including body temperature, heart rate, respiration rate, and blood pressure, which were recorded while under anesthesia, were unchanged by 6 and 12 weeks of 17α-E2 administration (Supplemental Table 2). Collectively, these findings indicate that oral administration of 17α-E2 at a dose of 0.30 mg/kg/day does not induce acute illness or symptoms typically associated with poor tolerability. We subsequently sought to determine if this dosing paradigm would elicit benefits commonly observed in male rodents.
Fig. 1.
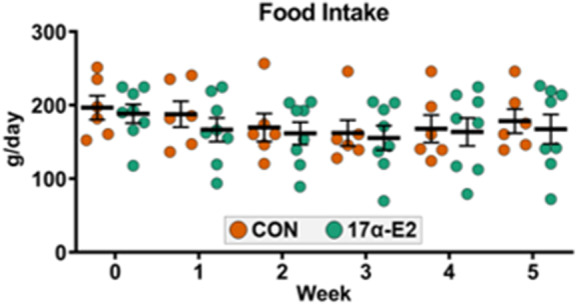
Food intake is unaffected by 17α-E2 treatment in male rhesus monkeys. Average daily food consumption from weeks 0 to 5 during Trial 1. All data are presented as mean ± SEM and were analyzed by two-way repeated measures ANOVA with Sidak post-hoc comparisons. Controls (CON): n = 6; 17α-E2 (0.30 mg/kg/day): n = 8
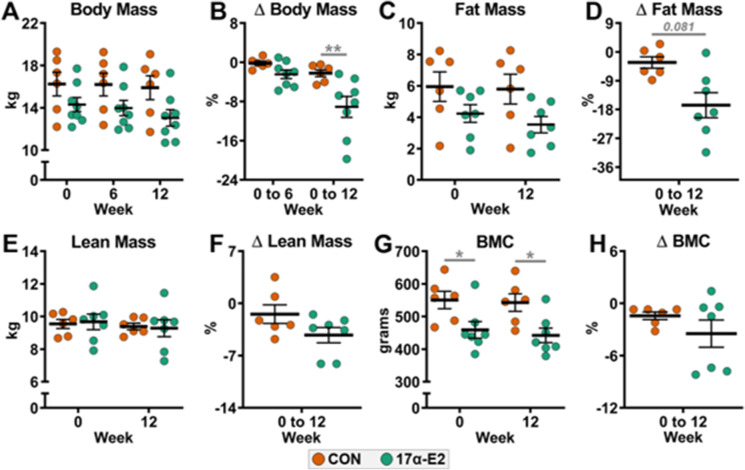
Oral dosing of 17α-E2 at 0.30 mg/kg/day mildly reduces body mass and adiposity
To our surprise, 17α-E2 treatment in male rhesus monkeys elicited only modest reductions in body mass and adiposity at the prescribed dose of 0.30 mg/kg/day. Body mass between treatment groups was not found to be different at baseline, 6 weeks, or 12 weeks treatment (Fig. 2A). Since the treatment groups starting body mass was moderately different, we also expressed the data as a percent changes from baseline. We found that 12 weeks of treatment with 17α-E2 did elicit a greater percent change in body mass when compared the controls (p = 0.009; Fig. 2B). Percent change in whole body adiposity also declined with 17α-E2 treatment, but this effect was not statistically different from controls. (p = 0.081; Fig. 2C-D). Lean mass and BMC were unaffected by treatment in this study (Fig. 2E-H).
Fig. 2.
17α-E2 treatment mildly reduces body mass and adiposity in male rhesus monkeys. (A) Body mass at weeks 0, 6, and 12, (B) Percent change in body mass from week 0 to 6 and 0 to 12, (C) Fat mass at weeks 0 and 12, (D) Percent change in fat mass from week 0 to 12, (E) Lean mass at weeks 0 and 12, (F) Percent change in lean mass from week 0 to 12, (G) Bone mineral content (BMC) at weeks 0 and 12, and (H) Percent change in BMC from week 0 to 12 in control (CON) and 17α-E2 treated monkeys during Trial 1. All data are presented as mean ± SEM and were analyzed by two-way repeated measures ANOVA with Sidak post-hoc comparisons (Panels A, C, E, G) or Student’s t-test (Panels B, D, F, H). CON: n = 6; 17α-E2 (0.30 mg/kg/day): n = 8. *p < 0.05, **p < 0.01
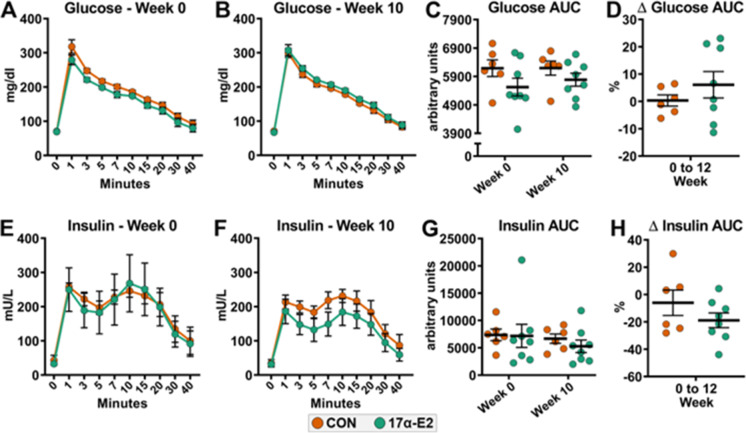
Oral dosing of 17α-E2 at 0.30 mg/kg/day does not improve glucose homeostasis
17α-E2 treatment at a daily dose of 0.30 mg/kg/day did not improve responsiveness to a glucose challenge during an IVGTT in male rhesus monkeys (Fig. 3A-D). We also evaluated plasma insulin levels during the IVGTT because 17α-E2 treatment reduces insulin resistance [6, 8], and therefore insulin production [6], which commonly results in lower first-phase insulin response levels during a glucose challenge [33]. Ten weeks of 17α-E2 treatment mildly reduced insulin levels during the IVGTT but these changes were found to be nonsignificant (Fig. 3E–H). It should be noted that all the animals enrolled in our studies were metabolically healthy, with average HbA1c values being under 4.7 percent (Supplemental Fig. 1). Interestingly, 12-weeks of 17α-E2 treatment did significantly reduce HbA1c levels (p = 0.012), which is similar to what we have previously reported in mice, although the biological significance of improving something already well within normal levels remains unclear.
Fig. 3.
17α-E2 treatment does not improve glucose homeostasis in male rhesus monkeys. (A) Glucose responsiveness during an intravenous glucose tolerance test (IVGTT) at week 0, (B) Glucose responsiveness during an IVGTT at week 10, (C) Glucose responsiveness area under the curve (AUC) during an IVGTT at weeks 0 and 10, (D) Percent change in glucose responsiveness AUC during an IVGTT from week 0 to 10, (E) Insulin responsiveness during an IVGTT at week 0, (F) Insulin responsiveness during an IVGTT at week 10, (G) Insulin responsiveness AUC during an IVGTT at weeks 0 and 10, and (H) Percent change in insulin responsiveness AUC during an IVGTT from week 0 to 10 in control (CON) and 17α-E2 treated monkeys during Trial 1. All data are presented as mean ± SEM and were analyzed by two-way repeated measures ANOVA with Sidak post-hoc comparisons (Panels A, B, C, E, F, G) or Student’s t-test (Panels D, H). CON: n = 6; 17α-E2 (0.30 mg/kg/day): n = 8
Oral dosing of 17α-E2 at 0.30 mg/kg/day elicits feminizing characteristics
Oral administration of 17α-E2 at 0.30 mg/kg/day was found to induce feminization in male rhesus monkeys (Table 2). Testis atrophy ranging from 30–38% was observed following 12 weeks of treatment (left testis, p = 0.001; right testis p = 0.001). These changes were mirrored by significant increases in circulating 17β-E2 (p = 0.002) and estrone (p = 0.006) by the 6 week treatment timepoint, which remained elevated throughout the 12-week treatment period. As expected, circulating 17α-E2 was also significantly higher in animals receiving the hormone by the 6 week treatment timepoint (p = 0.011). Unexpectedly, circulating 17α-E2 levels were elevated several-fold over what we have previously reported in male mice being treated with 17α-E2 [6, 16], providing evidence that a daily dose of 0.30 mg/kg is excessive in rhesus monkeys. Additionally, circulating testosterone (p = 0.005) and DHT (p = 0.001) were significantly reduced by 17α-E2 treatment by the 6 week treatment timepoint, and these remained suppressed throughout the 12-week treatment period. We also evaluated circulating FSH and LH due to their essential roles in controlling testicular function. FSH predominantly stimulates testicular growth and spermatogenesis by inducing Sertoli cell proliferation, whereas LH stimulates testosterone production in Leydig cells [34]. We found that both FSH and LH were reduced by nearly 50% with 17α-E2 treatment by the 6 week treatment timepoint (FSH, p = 0.038; LH, p = 0.271), where they remained throughout the 12-week treatment period. Due to significant variation in the hormone levels, likely due to pulsatility patterns, these changes were found to be generally nonsignificant, although we surmise they do indeed represent a significant decline in biological function; which is congruent with the observation of testicular atrophy. Given the unanticipated magnitude of feminization observed in Trial 1, we subsequently performed a second trial at a lower dose, but within the presumed effective dose range, in which we predominately evaluated tolerability and feminization characteristics.
Table 2.
Testis size serum sex hormone levels at baseline and following 6 & 12 weeks of treatment during Trial 1
| CON | 17a-E2 | CON | 17ɑ-E2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks 0 | Week 6 | Week 12 | Week 0 | Week 6 | Week 12 | 0 vs 6 | 0 vs 12 | 6 vs 12 | 0 vs 6 | 0 vs 12 | 6 vs 12 | |
| Right Testis ø(cm) | 2.85 ± 0.13 | 2.69 ± .011 | 1.54 ± 0.05 | ns | ** | |||||||
| Left Testis ø (cm) | 2.75 ± 0.16 | 2.48 ± 0.07 | 1.63 ± 0.09 | ns | ** | |||||||
| 17ɑ-estradiol (pg/ml) | 5.1 ± 2.3 | 4.1 ± 0.8 | 17.6 ± 7.2 | 2.8 ± 0.1 | 633.9 ± 63.3 | 368.9 ± 66.3 | ns | ns | ns | *** | ** | ns |
| 17β-estradiol (pg/ml) | 13.7 ± 0.8 | 14.3 ± 1.6 | 10.5 ± 0.4 | 10.4 ± 1.1 | 72.6 ± 11.6 | 76.8 ± 24.1 | ns | ns | ns | *** | ns | ns |
| Estrone (pg/ml) | 9.9 ± 2.3 | 7.1 ± 0.8 | 8.5 ± 1.0 | 7.2 ± 1.7 | 105.3 ± 20.6 | 97.1 ± 26.4 | ns | ns | ns | ** | * | ns |
| Testosterone (ng/dl) | 294.8 ± 26.6 | 340.3 ± 69.9 | 253.0 ± 26.8 | 386.0 ± 91.6 | 52.8 ± 34.3 | 43.2 ± 19.5 | ns | ** | ns | ** | * | ns |
| DHT (pg/ml) | 1344.0 ± 221.5 | 1223.0 ± 175.1 | 1172.0 ± 148.2 | 1471.0 ± 165.8 | 72.8 ± 21.3 | 162.5 ± 73.0 | ns | ns | ns | *** | *** | ns |
| FSH (pg/ml0) | 220.5 ± 68.7 | 250.2 ± 85.9 | 247.0 ± 51.4 | 191.6 ± 36.7 | 553.4 ± 59.4 | 12,101 ± 16.3 | ns | ns | ns | * | ns | ns |
| LH (pg/ml) | 1109.0 ± 297.9 | 655.0 ± 78.4 | 1101.0 ± 243.6 | 1094.0 ± 336.9 | 556.5 ± 37.2 | ns | ns | ns | ns | ns | ns | |
Date are shown as mean ± SEM and were compared by two-way ANOVA with specific emphasis on differences over time within treatments groups.*p < 0.01,***p < 0.01,**P < 0.005,ns = not signficant
Oral dosing of 17α-E2 at 0.20 mg/kg/day appears tolerable but does not reduce mass or adiposity
In this trial, we sought to determine if oral administration of 17α-E2 at 0.20 mg/kg/day would be tolerated in a separate cohort of male rhesus monkeys than were evaluated in Trial 1. Similar to Trial 1, this dose was also tolerated as evidenced by a lack of of vomiting, diarrhea, or constipation. Food intake, blood chemistry, and complete blood count values were not evaluated in this study due to the higher dose eliciting minimal if any changes in these variables. Body temperature, heart rate, and respiration rate were also unchanged by 11 weeks of 17α-E2 administration in Trial 2 (Supplemental Table 3). These observations indicate that oral administration of 17α-E2 at a dose of 0.20 mg/kg/day also does not induce acute illness or symptoms typically associated with poor tolerability. In contrast to Trial 1, the dose of 17α-E2 administered in Trial 2 did not elicit even minor reductions in body mass or adiposity over the 11-week intervention period (Fig. 4A-B). Lean mass was also unaffected by treatment in this study (Fig. 4C).
Fig. 4.
A diminished treatment dose of 17α-E2 does not reduce body mass or adiposity in male rhesus monkeys. (A) Body mass at weeks 0 and 11, (B) Fat mass at weeks 0 and 11, and (C) Lean mass at weeks 0 and 11 in control (CON) and 17α-E2 treated monkeys during Trial 2. All data are presented as mean ± SEM and were analyzed by two-way repeated measures ANOVA with Sidak post-hoc comparisons. CON: n = 4–5; 17α-E2 (0.20 mg/kg/day): n = 8
Oral dosing of 17α-E2 at 0.20 mg/kg/day elicits feminizing characteristics
In this study, oral administration of 17α-E2 at 0.20 mg/kg/day was found to induce feminization in male rhesus monkeys (Table 3). Testis atrophy with 17α-E2 treatment ranged from 22–24% in this 11-week trial (left testis, p = 0.001; right testis p = 0.001). Similar to Trial 1, 17α-E2 treatment again significantly increased in circulating 17β-E2 (p = 0.002) and estrone (p = 0.001) by the 11 week timepoint. Circulating 17α-E2 was also significantly higher in 17α-E2 treated animals (p = 0.001), with concentrations being lower than observed in Trial 1, but still significantly higher than in our prior mouse studies [6, 16]. Circulating testosterone (p = 0.059) and DHT (p = 0.011) were also dramatically reduced by 17α-E2 treatment in this trial, although robust variability limited statistical significance despite being reduced by nearly 50%. We did not evaluate LH or FSH in this trial due to the variability observed in samples from Trial 1. Collectively, this trial provided additional evidence that our daily dosing paradigms are excessive for preventing 17α-E2-mediated feminization.
Table 3.
Testis size and serum sex hormone levels at baseline and following 11 weeks of treatment during Trial 2
| CON | 17 ɑ-E2 | CON | 17 ɑ-E2 | |||
|---|---|---|---|---|---|---|
| Week 0 | Week 11 | Week 0 | Week 11 | 0 vs 11 | 0 vs 11 | |
| Right Testis ø (cm) | 4.14 ± 0.24 | 3.66 ± 0.29 | 3.83 ± 0.11 | 2.93 ± 0.14 | * | ** |
| Left Testis ø (cm) | 3.89 ± 0.18 | 4.19 ± 0.19 | 3.89 ± 0.18 | 3.05 ± 0.13 | * | *** |
| 17ɑ-estradiol (pg/ml) | 8.7 ± 0.3 | 9.7 ± 0.3 | 10.5 ± 1.6 | 197.3 ± 33.8 | ns | *** |
| 17β-estradiol (pg/ml) | 10.6 ± 1.5 | 12.2 ± 1.3 | 13.0 ± 1.4 | 24.9 ± 3.2 | ns | *** |
| Estrone (pg/ml) | 7.1 ± 0.7 | 9.0 ± 1.0 | 8.7 ± 1.3 | 22.9 ± 2.6 | ns | ** |
| Testosterone (ng/dl) | 218.0 ± 52.1 | 281 ± 76.7 | 421.3 ± 95.0 | 170.8 ± 46.2 | ns | Ns |
| DHT (pg/ml) | 843.3 ± 175.9 | 1077.0 ± 195.2 | 982.4 ± 86.3 | 562.5 ± 11.6 | ns | * |
Dates ae shown as mean ± SEM and were compared by two-way ANOVA with Specific emphasis on differenced over time within treatment groups. *p < 0.05,**p < 0.005, ns = not signficant
Discussion
Previous work has established that 17α-E2 administration extends lifespan and attenuates several mechanisms underlying the aging process and chronic disease burden almost exclusively in male mice [1–3, 6–15, 35]. Importantly, male-specific benefits occur without significant feminization of the sex hormone profiles [6] or reproductive function [16]. Intact female mice are generally unresponsive to 17α-E2 treatment [11–15, 36, 37], unless subjected to chronic high-fat feeding over several months (unpublished observation). Regardless, any benefits observed in intact female mice are marginal in comparison to those observed in their male counterparts. Ovariectomy renders female mice more responsive to the metabolic benefits of 17α-E2 treatment [38], but chronic administration in ovariectomized females does little to curtail pro-aging mechanisms as observed in male mice [11–15]. With consideration given to the findings described above, coupled with minimal data regarding the translatability of 17α-E2 into higher order mammals, the studies outlined herein sought to determine tolerable dosing of 17α-E2 in male rhesus monkeys. We also evaluated metabolic and endocrine parameters as a means of assessing efficacy and effects on feminization. A few anticipated, and several unanticipated, outcomes were observed through these trials, which we discuss below. Our findings provide critical insights for ongoing and future studies related to the translatability of 17α-E2.
When treatments were initiated our first objective was to determine if the two oral doses of 17α-E2 employed in our studies induced acute signs of illness and/or behavioral distress. We observed no indications of illness or behavior changes with either dose trial. We anticipated this would be the case for a variety of reasons. First, 17α-E2 is naturally present in both mammalian sexes [39–41] and a minor constituent of several FDA-approved menopausal-related hormone replacement therapies, including Premarin (2.5–9.5%) [17]. Therefore, millions of female humans have been chronically exposed to exogenous 17α-E2 for decades without adverse effects. Secondly, 17α-E2 is commonly prescribed to men as a topical treatment for androgenic alopecia [42] in Germany, Argentina, Brazil, and Mexico, with no significant side effects reported. Lastly, 17α-E2 is not currently approved in the United States for human use as a single compound, but a few Phase 1 trials have been completed with very few issues tolerability issues reported. Specifically, Phase 1 trials in women (~ 1–3 mg/day) have found no evidence of vaginal bleeding or breast tenderness and all biochemistry, hematology, urinalysis outcomes, and vital signs remained within normal ranges [17, 39]. A Phase 1 trial in men (2 mg/day) revealed no signs of gynecomastia or breast tenderness with only a single patient reporting a reduction in libido [17, 39]. Our initial tolerability outcomes in the current studies were aligned with these previous reports and provided the justification to continue with additional weeks of treatment.
We subsequently evaluated metabolic parameters with the anticipation that 17α-E2 would elicit metabolic benefits similarly in male rhesus monkeys as previously established in male mice [6–10]. To our surprise, reductions in body mass and adiposity with 17α-E2 treatment were much subtler in male rhesus monkeys than commonly observed in male mice. Changes over time in actual body mass and adiposity were not different in our studies, although the percent changes over time were trending or significant in Trial 1. This likely stems from natural variability in the data due to macaque genetic heterogeneity [43] and our use of a wide range of ages in our studies. However, these circumstances cannot entirely explain the modest effects of 17α-E2 treatment on these variables in male monkeys, particularly since the animals do not appear to split into groups of responders and non-responders. It is plausible that expectations of significant reductions in mass and/or adiposity were unrealistic in the healthy cohort used for our studies. Despite this possibility our current interpretation is that 17α-E2 treatment at these doses for these interventional periods only mildly affect mass and adiposity.
In alignment with surprising outcomes related to mass and adiposity, we also found that 17α-E2 treatment failed to improve glucose tolerance in Trial 1. We hypothesized that 17α-E2 would at least modestly improve glucose tolerance due to the overwhelming data demonstrating these improvements in obese and aged male mice [6–10]. Ten weeks of 17α-E2 treatment had no effect on glucose or insulin responsiveness to an IV glucose challenge in Trial 1, and the data was generally consistent, thereby eliminating potential batch effects due to genetic differences or ages between animals. One important caveat to this finding is that all the animals in this study were metabolically healthy as evidenced by average HbA1c levels being lower than 4.7 percent at study initiation [44]. Despite healthy HbA1c levels when the study began, 17α-E2 treatment still significantly reduced HbA1c levels by significant amounts in male monkeys, which is congruent with our previous work in male mice [6, 8]. It remains unclear how best to interpret the lack of benefits during the IVGTT procedures, while concomitantly observing improvements in HbA1c outcomes. We surmise that future studies may provide clearer outcomes if obese, pre-diabetic, and/or older monkeys are evaluated.
The most unexpected outcome from our studies was the magnitude of feminization observed during our trials. Both doses tested were found to significantly elicit testicular atrophy, increased circulating estrogens, and suppressed circulating androgens. Circulating gonadotropins were also suppressed by approximately half with 17α-E2 treatment. These findings were unanticipated due to the nonfeminizing nature of 17α-E2 treatment in male mice [6, 16], coupled with the fact that we calculated our dosing regimens for monkeys through allometric scaling based on 17α-E2 intake in our mouse studies. Our findings are also at odds with a prior report that indicates 12 weeks of daily 17α-E2 administration at 2 mg/day in middle-aged men had no significant effects on serum 17β-E2, estrone, testosterone, or sex hormone binding globulin (SHBG) [17, 39]. However, it should be noted that the dosing regimens employed in our monkey trials would scale to much higher human doses than have previously been tested. For reference, scaling the 0.20 and 0.30 mg/kg/day monkey doses to a 90 kg man, the current average mass for a man over 20 years of age in the United States, would result in daily dosing levels ranging from 11.5 to 17.3 mg/day, which is roughly 6–8 times higher than the aforementioned Phase 1 clinical trial in men.
We suspect that the observed level of feminization results from a saturation of the endogenous conjugation enzymes, thereby promoting a greater concentration of unconjugated 17α-E2 in serum, which has more biological activity [45]. Our suspicion stems from the fact that prior studies have shown that over 99% of orally administered 17α-E2 is rapidly and extensively conjugated within 24 h of ingestion [46]. Moreover, peak concentrations of serum 17α-E2 are proportional to the dose administered and the elimination half-life is also dose-dependent [17, 39]; therefore higher dosing, particularly when provided chronically over a period of weeks, has a high probability of overwhelming enzymatic conjugation capacity. The unconjugated form of 17α-E2 is also more likely to be subjected to isomerization to 17β-E2, which likely explains the sevenfold increase in serum 17β-E2 in the 17α-E2-treated monkeys in Trial 1. This dramatic increase in 17β-E2 also likely contributes to the magnitude of feminization in our studies. Another factor that should be considered if future monkey studies are undertaken is the method of 17α-E2 delivery. Dietary administration in mice results in slower ingestion rates due to the intermittent nibbling behavior of mice [47], whereas the monkeys received their entire daily dose of 17α-E2 during a single feeding of 5–6 PRIMA-Treat® wafers, which are typically consumed in less than 30 min. This ‘bolus’ effect almost certainly contributes to the higher serum levels of 17α-E2 seen in our studies and subsequent overwhelming of conjugation capability. Future studies in monkeys, and certainly humans, would likely benefit from the development and implementation of 17α-E2 transdermal patches, which are commonly used to administer hormone therapies [48].
There are a few notable caveats to the current studies that should be acknowledged. First, all the animals evaluated were apparently healthy without obesity or metabolic perturbations. Secondly, our animals also had a wide range of ages and our numbers per group were relatively low given the magnitude of genetic heterogeneity in rhesus macaques. Third, oral administration of hormone therapies is somewhat of an antiquated approach; thus, alternative delivery approaches should be considered during future studies. Transdermal patches would provide a slow-release option that could potentially mitigate some of the aforementioned issues with bolus delivery. Despite these limitations, the overarching goals of our trials were to assess 17α-E2 tolerability and metabolic/endocrine responses over a relative short treatment window, which was successful with the animals we had at our disposal. Our studies clearly demonstrate that the doses we employed are excessive, at least when administered as a single bolus. We suspect that the dramatic level of feminization resulted from significant suppression of the hypothalamus-pituitary–gonadal axis due to agonism of estrogen receptor α in the hypothalamus and pituitary, which occurs not only in females but males as well [49].
In summary, the data presented herein is the first to show 17α-E2 treatment effects in male rhesus macaques. Our studies clearly demonstrate that doses of 0.30 and 0.20 mg/kg/day are highly feminizing and only elicit modest benefits on metabolic parameters when administered in a bolus fashion. We conclude that future studies that use alternative delivery methods, such as transdermal patches, will be needed in an effort to establish tolerable dosing paradigms that minimize feminization so that longer treatment durations can be evaluated for effects on aging and chronic disease burden.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplemental Figure 1. 17α-E2 treatment improves glycosylated hemoglobin levels in male rhesus monkeys. Glycosylated hemoglobin (HbA1c) levels at weeks 0 and 12 in control (CON) and 17α-E2 treated monkeys during Trial 1. All data are presented as mean ± SEM and were analyzed by two-way repeated measures ANOVA with Sidak post-hoc comparisons CON: n=6; 17α-E2 (0.30 mg/kg/day): n=8. *p<0.05.(PNG 19 KB)
Acknowledgements
We thank Drs. Rick Herbert and Ruth Woodward and the NIA NHP Core team including Edward Tilmont, Kielee Toepfer, Sarah Thomas, and Caryn Seward for assistance with animal colony management and sample collection.
Author contributions
M.B.S., K.L.V., and J.A.M. conceived the project and designed the experiments. K.L.V., B.W. and J.A.M. performed the experiments with contributions from M.B.S. and S.N.M. M.B.S., J.V.V.I., and S.N.M. analyzed the data and created figures with contributions from J.M.H., H.L.P., and W.M.F. M.B.S. wrote the manuscript and all authors edited and approved the final version.
Funding
This work was supported by the Intramural Research Program of the National Institute on Aging, NIH, and extramural grants from the National Institutes of Health (R00 AG51661 & R01 AG069742 to M.B.S.). The Endocrine Technologies Core at the Oregon National Primate Research Center, which analyzed gonadotropins for these studies, is also supported by the National Institutes of Health (P51 OD011092).
Data Availability
The datasets generated through this work are available upon reasonable request from the corresponding authors.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
†These authors contributed equally to the work presented herein.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael B. Stout, Email: michael-stout@omrf.org
Julie A. Mattison, Email: julie.mattison@nih.gov
References
- 1.Strong R, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison DE, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison DE, et al. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell. 2021;20(5):e13328. doi: 10.1111/acel.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turturro A, et al. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–501. doi: 10.1093/gerona/54.11.B492. [DOI] [PubMed] [Google Scholar]
- 5.Miller RA, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stout MB, et al. 17alpha-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization. J Gerontol A Biol Sci Med Sci. 2017;72(1):3–15. doi: 10.1093/gerona/glv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steyn FJ et al 17alpha-estradiol acts through hypothalamic pro-opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell, 2018. 17(1). [DOI] [PMC free article] [PubMed]
- 8.Mann SN, et al Health benefits attributed to 17alpha-estradiol, a lifespan-extending compound, are mediated through estrogen receptor alpha. Elife, 2020. 9. [DOI] [PMC free article] [PubMed]
- 9.Miller BF, et al. Short-term Calorie Restriction and 17alpha-Estradiol Administration Elicit Divergent Effects on Proteostatic Processes and Protein Content in Metabolically Active Tissues. J Gerontol A Biol Sci Med Sci. 2020;75(5):849–857. doi: 10.1093/gerona/glz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidhom S, et al. 17alpha-Estradiol Modulates IGF1 and Hepatic Gene Expression in a Sex-Specific Manner. J Gerontol A Biol Sci Med Sci. 2021;76(5):778–785. doi: 10.1093/gerona/glaa215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garratt M, et al. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017;16(6):1256–1266. doi: 10.1111/acel.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garratt M, et al. Male lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell. 2018;17(4):e12786. doi: 10.1111/acel.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garratt M, et al. 17-alpha estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell. 2019;18(2):e12920. doi: 10.1111/acel.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garratt M, Stout MB. Hormone actions controlling sex-specific life-extension. Aging (Albany NY) 2018;10(3):293–294. doi: 10.18632/aging.101396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debarba LK, et al. 17-alpha-Estradiol Has Sex-Specific Effects on Neuroinflammation That Are Partly Reversed by Gonadectomy. J Gerontol A Biol Sci Med Sci. 2022;77(1):66–74. doi: 10.1093/gerona/glab216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isola JVV, et al, 17α-estradiol does not adversely affect sperm parameters or fertility in male mice: implications for reproduction-longevity trade-offs. GeroScience, 2022. [DOI] [PMC free article] [PubMed]
- 17.Moos WH, et al. Review of the Effects of 17 alpha-Estradiol in Humans: A Less Feminizing Estrogen With Neuroprotective Potential. Drug Dev Res. 2009;70(1):1–21. doi: 10.1002/ddr.20284. [DOI] [Google Scholar]
- 18.Colma, RJ, Non-human primates as a model for aging. Biochim Biophys Acta Mol Basis Dis, 2018. 1864(9 Pt A): p. 2733–2741. [DOI] [PMC free article] [PubMed]
- 19.Rhesus Macaque Genome S, et al Evolutionary and biomedical insights from the rhesus macaque genome. Science, 2007; 316(5822): 222–34. [DOI] [PubMed]
- 20.Bowden DM, Williams DD. Aging. Adv Vet Sci Comp Med. 1984;28:305–341. doi: 10.1016/B978-0-12-039228-5.50015-2. [DOI] [PubMed] [Google Scholar]
- 21.Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age (Omaha) 1997;20(1):1–13. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding JD. Nonhuman Primates and Translational Research: Progress, Opportunities, and Challenges. ILAR J. 2017;58(2):141–150. doi: 10.1093/ilar/ilx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodkin NL, Metzger BL, Hansen BC. Hepatic glucose production and insulin sensitivity preceding diabetes in monkeys. Am J Physiol. 1989;256(5 Pt 1):E676–E681. doi: 10.1152/ajpendo.1989.256.5.E676. [DOI] [PubMed] [Google Scholar]
- 24.Bodkin NL, et al. Central obesity in rhesus monkeys: association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? Int J Obes Relat Metab Disord. 1993;17(1):53–61. [PubMed] [Google Scholar]
- 25.Hansen BC, Bodkin NL. Beta-cell hyperresponsiveness: earliest event in development of diabetes in monkeys. Am J Physiol. 1990;259(3 Pt 2):R612–R617. doi: 10.1152/ajpregu.1990.259.3.R612. [DOI] [PubMed] [Google Scholar]
- 26.Bernier M, et al. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8(5):899–916. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattison JA, et al. Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging. 2005;26(7):1117–1127. doi: 10.1016/j.neurobiolaging.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 28.West GB, Brown JH. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol. 2005;208(Pt 9):1575–1592. doi: 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- 29.White CR, Seymour RS. Allometric scaling of mammalian metabolism. J Exp Biol. 2005;208(Pt 9):1611–1619. doi: 10.1242/jeb.01501. [DOI] [PubMed] [Google Scholar]
- 30.Knapka J, Nutrient Requirements of Nonhuman Primates: Second Revised Edition. Lab Animal, 2003. 32(10): p. 26–26.
- 31.Niswender GD, Spies HG. Serum levels of luteinizing hormone, follicle-stimulating hormone and progesterone throughout the mentrual cycle of rhesus monkeys. J Clin Endocrinol Metab. 1973;37(2):326–328. doi: 10.1210/jcem-37-2-326. [DOI] [PubMed] [Google Scholar]
- 32.Vaughan KL, et al. Comparison of anesthesia protocols for intravenous glucose tolerance testing in rhesus monkeys. J Med Primatol. 2014;43(3):162–168. doi: 10.1111/jmp.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas DD, et al. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J Endocr Soc. 2019;3(9):1727–1747. doi: 10.1210/js.2019-00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaprara A, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism. 2018;86:3–17. doi: 10.1016/j.metabol.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Ali Mondal S, et al 17alpha-estradiol, a lifespan-extending compound, attenuates liver fibrosis by modulating collagen turnover rates in male mice. Am J Physiol Endocrinol Metab, 2022. [DOI] [PMC free article] [PubMed]
- 36.Isola JVV, et al. Mild calorie restriction, but not 17alpha-estradiol, extends ovarian reserve and fertility in female mice. Exp Gerontol. 2022;159:111669. doi: 10.1016/j.exger.2021.111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isola JVV, et al. 17alpha-Estradiol promotes ovarian aging in growth hormone receptor knockout mice, but not wild-type littermates. Exp Gerontol. 2020;129:110769. doi: 10.1016/j.exger.2019.110769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann SN, et al. 17α-Estradiol prevents ovariectomy-mediated obesity and bone loss. Exp Gerontol. 2020;142:111113. doi: 10.1016/j.exger.2020.111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dykens JA, Moos WH, Howell N. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci. 2005;1052:116–135. doi: 10.1196/annals.1347.008. [DOI] [PubMed] [Google Scholar]
- 40.Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 41.Courant F, et al. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J Clin Endocrinol Metab. 2010;95(1):82–92. doi: 10.1210/jc.2009-1140. [DOI] [PubMed] [Google Scholar]
- 42.Orfanos CE and L Vogels Local therapy of androgenetic alopecia with 17 alpha-estradiol. A controlled, randomized double-blind study. Dermatologica, 1980. 161(2): p. 124–32. [PubMed]
- 43.Hobson W. Safety assessment studies in nonhuman primates. Int J Toxicol. 2000;19(2):141–147. doi: 10.1080/109158100224962. [DOI] [Google Scholar]
- 44.Vaughan KL, Mattison JA. Obesity and Aging in Humans and Nonhuman Primates: A Mini-Review. Gerontology. 2016;62(6):611–617. doi: 10.1159/000445800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raftogianis R, et al. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000;27:113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- 46.Hobe G, et al. Some new aspects of 17alpha-estradiol metabolism in man. Steroids. 2002;67(11):883–893. doi: 10.1016/S0039-128X(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 47.Ellacott KL, et al. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010;12(1):10–17. doi: 10.1016/j.cmet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galzote RM, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health. 2017;9:315–321. doi: 10.2147/IJWH.S102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chimento A, et al Role of estrogen receptors and g protein-coupled estrogen receptor in regulation of hypothalamus-pituitary-testis axis and spermatogenesis. Front Endocrinol (Lausanne), 2014. 5: p. 1. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplemental Figure 1. 17α-E2 treatment improves glycosylated hemoglobin levels in male rhesus monkeys. Glycosylated hemoglobin (HbA1c) levels at weeks 0 and 12 in control (CON) and 17α-E2 treated monkeys during Trial 1. All data are presented as mean ± SEM and were analyzed by two-way repeated measures ANOVA with Sidak post-hoc comparisons CON: n=6; 17α-E2 (0.30 mg/kg/day): n=8. *p<0.05.(PNG 19 KB)
Data Availability Statement
The datasets generated through this work are available upon reasonable request from the corresponding authors.