Abstract
Salmonella enterica subsp. enterica serovar Newport resistant to the extended-spectrum cephalosporins (ESCs) and other antimicrobials causes septicemic salmonellosis in humans and animals and is increasingly isolated from humans, animals, foods, and environmental sources. Mechanisms whereby serovar Newport bacteria become resistant to ESCs and other classes of antimicrobials while inhabiting the intestinal tract are not well understood. The present study shows that 25.3% of serovar Newport strains isolated from the turkey poult intestinal tract after the animals were dosed with Escherichia coli harboring a large conjugative plasmid encoding the CMY-2 β-lactamase and other drug resistance determinants acquired the plasmid and its associated drug resistance genes. The conjugative plasmid containing the cmy-2 gene was transferred not only from the donor E. coli to Salmonella serovar Newport but also to another E. coli serotype present in the intestinal tract. Laboratory studies showed that the plasmid could be readily transferred between serovar Newport and E. coli intestinal isolates. Administration of a single dose of ceftiofur, used to prevent septicemic colibacillosis, to 1-day-old turkeys did not result in the isolation of ceftiofur-resistant E. coli or Salmonella serovar Newport. There was a remarkable association between serotype, drug resistance, and plasmid profile among the E. coli strains isolated from the poults. This study shows that Salmonella serovar Newport can become resistant to ESCs and other antibiotics by acquiring a conjugative drug resistance plasmid from E. coli in the intestines.
Salmonella enterica subsp. enterica serovar Newport infections increased as a proportion of reported infections by all Salmonella serovars in humans in the United States from 4.6 to 10% during the 1997-2001 period (15), and in Canada they rose from 2.2 to 3.6% during the years 2002 and 2003 (2). The percentages of Salmonella serovar Newport isolates in particular but also of other Salmonella serovars and Escherichia coli that are resistant to the extended-spectrum cephalosporins (ESCs) are increasing (15, 37, 43, 45, 46). Human infections with multidrug-resistant serovar Newport isolates are often food borne, may result from direct exposure to infected dairy cows and calves (15), and have occurred in people handling contaminated pet treats (32). Multidrug-resistant Salmonella serovars cause severe and septicemic salmonellosis more frequently than those that are not resistant (15, 16). The taking of antimicrobials by patients within a month of the onset of salmonellosis may aggravate the severity of disease symptoms and result in increased hospitalization rates (42). Contributing mechanisms are thought to include selection for the resistant pathogen (23, 42), concomitant killing of protective normal flora (23), and increased colonization of the enterocytes by the offending bacteria (3). Commensal E. coli bacteria may play a contributory role in the acquisition of antimicrobial resistance by Salmonella serovars (10, 30, 31). For example, in one clinical study, antimicrobial-susceptible E. coli and S. enterica serovar Anatum were initially isolated from a hospitalized diabetic patient with wound and urinary tract infections. However, 2 weeks after the initiation of ceftriaxone (CRO) therapy, CRO-resistant E. coli and Salmonella serovar Anatum bacteria were isolated, and the patient eventually died of sepsis caused by the CRO-resistant Salmonella isolate (43). Ceftiofur (CTF), a veterinary ESC, is the only cephalosporin approved for systemic use in food-producing animals in the United States. It was first approved in 1988 as an injectable therapeutic agent for the treatment of acute bovine respiratory disease and has been subsequently approved for use in other food animal species, including pigs, sheep, chickens, and turkeys (47). In Canada, CTF has been approved to prevent colibacillosis (which causes early poult mortality) associated with E. coli infection in day-old poults (5), whereas in the United States, CTF has been approved for the same purpose in turkey poults and additionally in day-old chicks to prevent early chick mortality when they are infected with E. coli (44). Little is known about the transfer of antimicrobial resistance-encoding genes and the mechanisms and frequencies of the acquisition of these genes by members of the family Enterobacteriaceae in the intestinal tracts of animals and humans. Therefore, the purpose of the present study was to examine the mechanisms and frequency of transfer of resistance to ESCs by E. coli and the acquisition of such resistance by antimicrobial-susceptible Salmonella serovar Newport bacteria in the turkey poult intestinal tract.
(A preliminary account of part of this work has appeared previously [C. Poppe, L. Martin, C. Gyles, K. Rekker, R. Reid-Smith, P. Boerlin, S. McEwen, J. Prescott, and K. Forward, Speaker Abstr., ASM Conferences, Salmonella: Pathogenesis, Epidemiology, and Vaccine Development, p. 23, 2003].)
MATERIALS AND METHODS
Bacterial strains.
Salmonella serovar Newport strain 02-6203 was isolated from an environmental swab of a truck that transported turkeys. The strain was susceptible to all 24 antimicrobials in the antimicrobial testing panel (Table 1). E. coli MPS57 is a nonverotoxigenic E. coli O168:H7 strain previously isolated from uncooked chicken wings obtained at a grocery store in Nova Scotia, Canada (13). It was resistant to ampicillin (AMP), amoxicillin-clavulanic acid (AMC), cephalothin (CEF), ceftriaxone (CRO) at 8 μg/ml, CTF, cefoxitin (FOX), chloramphenicol (CHL), spectinomycin (SPT), and sulfisoxazole (SUL).
TABLE 1.
Antimicrobials and concentrations used to test susceptibility of E. coli and Salmonella isolates
| Antimicrobial (abbreviation) | Breakpoint concn (μg/ml)a
|
|
|---|---|---|
| Susceptibility | Resistance | |
| Amikacin (AMK) | 16 | ND |
| Ampicillin (AMP) | ND | 32 |
| Amoxicillin-clavulanate (AMC) | ND | 64/16b |
| Apramycin (APR)c | ND | 32d |
| Carbadox (CRB)c | ND | 30e |
| Cephalothin (CEF) | ND | 32 |
| Ceftriaxone (CRO) | 8 | ND |
| Ceftiofur (CTF) | ND | 8 |
| Cefoxitin (FOX) | ND | 32 |
| Chloramphenicol (CHL) | ND | 32 |
| Ciprofloxacin (CIP) | 0.125f | ND |
| Florfenicol (FLO)c | ND | 16g |
| Gentamicin (GEN) | ND | 16 |
| Kanamycin (KAN) | ND | 64 |
| Nalidixic acid (NAL) | ND | 32 |
| Neomycin (NEO)c | ND | 16d |
| Nitrofurantoin (NIT) | ND | 64h |
| Spectinomycin (SPT)c | ND | 64d |
| Streptomycin (STR)c | ND | 32d |
| Sulfisoxazole (SUL) | ND | 512 |
| Sulfamethoxazole-trimethoprim (SXT) | ND | 76/4 |
| Tetracycline (TET) | ND | 16 |
| Tobramycin (TOB) | ND | 8 |
| Trimethoprim (TMP) | ND | 16 |
The breakpoint concentrations to determine susceptibility and resistance are those specified by NCCLS standards M31-A (26) and M100-S12 (27). ND, not determined.
The strains were considered resistant when growing on agar plates with amoxicillin-clavulanic acid at 64 and 16 μg/ml; respectively.
There are no interpretive criteria specified by NCCLS standards M31-A (26) or M100-S12 (27) for these drugs.
Strains were considered resistant to apramycin, neomycin, spectinomycin, and streptomycin at 32, 16, 64, and 32 μg/ml; respectively.
Strains were considered resistant to carbadox, a veterinary growth promoter for pigs, at 30 μg/ml (12).
A 0.125-μg/ml concentration of ciprofloxacin determines reduced sensitivity to ciprofloxacin (34).
Strains were considered resistant to florfenicol at the level of 16 μg/ml (36).
Strains were considered resistant to nitrofurantoin at 64 μg/ml; human urinary tract isolates are considered resistant at 128 μg/ml (27).
Study design.
Twelve or 13 1-day-old turkey poults, obtained from a Salmonella-free primary breeder turkey flock, were placed in each of four isolation units (Table 2). Antimicrobials had not been administered to the flocks of origin at the farm, and the newly hatched poults had not been treated with antimicrobials at the hatchery. The poults were housed, fed, watered, cared for, and treated in accordance with recommendations of the Canadian Council on Animal Care (6, 7). The treatment of the poults is described in Table 2. Briefly, the poults in pen 1 were dosed orally with the antimicrobial-susceptible serovar Newport strain 02-6203 on day 1 and injected subcutaneously with 0.17 mg of CTF in 0.2 ml of saline (5) on day 2; poults in pen 2 were dosed with the antimicrobial-susceptible serovar Newport on day 1 and injected subcutaneously with 0.2 ml of saline on day 2; poults in pen 3 were dosed orally with the antimicrobial-susceptible serovar Newport and the E. coli strain MPS57 encoding resistance to the ESCs and other antimicrobials on a self-transmissible 72-MDa plasmid but were neither treated with antimicrobials nor injected with saline; and poults in pen 4 received no treatment. The trial was repeated once with poults from a different Salmonella-free turkey flock from the same supplier. The sample collection scheme is shown in Table 3. To examine possible early transfer of drug resistance genes between E. coli and Salmonella serovar Newport, the cloacal swabs during the second trial were taken on day 2, after the poults were dosed on day 1 with serovar Newport in pens 1, 2, and 3 and with the ESC-resistant E. coli in pen 3 but before treatment on day 2 of the poults with CTF in pen 1 and with saline in pen 2 (Table 3). Health and safety procedures for working with strains of serovar Newport in a risk level-2 biohazard isolation unit facility were described, approved, and carried out. Protective clothing and footwear available in the anteroom of each isolation unit were worn during each procedure, and disinfection procedures were carried out before entering and upon leaving the isolation unit.
TABLE 2.
Experimental design of study—dosing and treatment of poults
| Day | Dosing and treatment for poults in pena:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 | Dosing with antibiotic-susceptible serovar Newport | Dosing with antibiotic-susceptible serovar Newport | Dosing with antibiotic-susceptible serovar Newport and ESC-resistant E. coli | No treatment |
| 2 | Administration of CTF (0.17 mg in 0.2 ml of saline) subcutaneouslyb | Administration of saline (0.2 ml) subcutaneously | No treatment with antimicrobials | No treatment |
The pens were isolation units in an animal isolation building. Pens 1 and 4 contained 12 poults; pens 2 and 3 contained 13 poults. Poults given serovar Newport were dosed orally with 4.2 × 106 (trial 1) or 3.8 × 106 (trial 2) serovar Newport 02-6203 bacteria, an antimicrobial-susceptible strain that did not harbor any plasmids. The poults in pen 3 were also dosed with 2.5 × 106 (trial 1) or 1.4 × 106 (trial 2) bacteria of the ESC-resistant E. coli strain MPS57 that is resistant to AMP, AMC, CEF, CFT, CRO at 8 μg/ml; FOX; CHL, SPT, and SUL and that contains two plasmids of 66 and 72 MDa; the latter plasmid is self-transmissible, contains the cmy-2 gene, and encodes resistance to all the above-mentioned antimicrobials. Saline, phosphate-buffered saline.
Administered subcutaneously at a dose and route indicated for the prevention of colibacillosis in 1-day-old poults (5).
TABLE 3.
Experimental design of the study—sample collection
| Day | Trial(s) | Sample type (no. of samples) and time of collectiona |
|---|---|---|
| 1 | 1 and 2 | Shavings (4), feed (1), water (1); before poults were placed in pens |
| 1 | 1 | Cloacal swabsb (12 or 13; 1/poult); when poults were placed in pens and before they were dosed with serovar Newport (pens 1 to 3) and E. coli (pen 3) |
| 2 | 2 | Cloacal swabs (12 or 13; 1/poult); after poults were dosed with serovar Newport (pens 1 to 3) and E. coli (pen 3) on day 1 but before CTF (pen 1) or saline (pen 2) treatment on day 2 |
| 4 | 1 and 2 | Litter (4), feed with litter (1), water (1), cloacal swabs (12 or 13; 1/poult) |
| 10 | 1 and 2 | Litter (4), feed with litter (1), water (1), cecal contentsc (12 or 13; 1/poult) |
Samples were taken from 12 or 13 poults in each of four pens.
Cloacal swabs were taken by inserting a cotton swab wetted with BPW into the cloaca.
Upon necropsy; cecal contents were taken and examined for the presence of Salmonella and E. coli; one isolate each of E. coli and serovar Newport per sample, if present, was further characterized.
Isolation and identification of Salmonella and E. coli.
The numbers and types of samples collected are described in Table 3. The feed samples taken from different sites of the feeder each weighed approximately 5 g, the samples of shavings taken at four different sites in the pen each weighed approximately 5 g, 1 ml of water was taken from the drinker with a sterile syringe, the litter samples taken at four sites in the pen each weighed about 5 g, the cloacal swabs each gained approximately 0.3 to 0.4 g in weight when collected, and approximately 0.5 to 1.5 g of cecal contents was collected upon necropsy of each of the birds. The samples were collected in sterile plastic bags or tubes and mixed at a 1:10 (wt/vol) ratio with buffered peptone water (BPW; Difco). To isolate E. coli, 1 drop of the mixture was streaked on each of two MacConkey (MC; Difco) agar plates. The MC agar plates and the bags and tubes with the samples in BPW were incubated overnight (ON) at 37°C. One lactose-fermenting colony that resembled typical E. coli bacteria (a medium-sized colony, purple to pink in color, with a slight dip in the center) was picked per sample from either of the MC agar plates and streaked onto Luria-Bertani (LB; Difco) agar, which was then incubated ON at 37°C. Putative E. coli colonies were picked from the LB agar plate and stabbed into Simmons citrate agar in a petri dish, incubated ON at 37°C, and examined for the use of citrate as a sole carbon source. The colonies were also examined for the production of indole by streaking them onto filter paper wetted with 1% p-dimethyl-aminobenzaldehyde in 10% HCl. Lactose-fermenting, citrate-negative, indole-producing colonies with a typical appearance on MC agar were considered to be E. coli. A subset of 1 to 2 environmental and 4 to 10 animal (cloacal-swab and cecal-content) isolates of E. coli from samples taken on each sampling date from the poults and their environments in each pen were examined at the E. coli typing laboratory at the Laboratory for Foodborne Zoonoses in Guelph, Ontario, Canada, to determine the serotype and to verify that they indeed typed as E. coli. One hundred microliters of the preenriched sample in BPW was dropped onto the periphery of a modified semisolid Rappaport-Vassiliadis (MSRV; Oxoid) agar plate and incubated for 24 to 48 h at 41.5°C. Whenever putative Salmonella bacteria had, by means of selective motility in MSRV agar, moved more than 2 cm from the site of inoculation, a loopful was taken from the outer edge of the area of growth and streaked onto LB agar plates that were then incubated ON at 37°C. Colonies were examined by slide agglutination tests with polyvalent Salmonella O antisera (serogroups A to I and Vi) and O-8 antiserum to determine that they were indeed Salmonella bacteria, and, like serovar Newport, belonged to serogroup C2 and C3, respectively (33). One Salmonella isolate per sample was further characterized. A subset of 1 to 2 environmental Salmonella isolates and 4 to 12 animal isolates (derived from cloacal swabs or cecal contents), when recovered from samples on each occasion from each pen, were serotyped at the OIÉ Reference Laboratory for Salmonellosis at the Laboratory for Foodborne Zoonoses in Guelph.
Antimicrobial susceptibility testing.
Antimicrobial susceptibilities of all serovar Newport and E. coli strains and their transconjugants and transformants were determined at resistant and, for certain antimicrobials (amikacin, ceftriaxone, and ciprofloxacin), at susceptible breakpoint levels (26, 27) with the agar dilution test using Mueller-Hinton (MH) agar (Table 1). To determine resistance to florfenicol (FLO), Aquaflor (Schering Plough, Animal Health, Point Claire, Quebec, Canada) containing 50% florfenicol was dissolved in dimethylformamide and added to MH agar (36).
Plasmids.
Plasmid DNA preparations were made by the alkaline lysis method (9), electrophoresed in a horizontal 0.7% agarose gel in Tris-acetate buffer, and stained and visualized as described previously (35). The plasmids used as molecular mass standards were pDT285, a 96-MDa plasmid (34); p97-1028, an S. enterica subsp. enterica serovar Typhimurium virulence-associated 62-MDa plasmid (36); pDT369, a 23-MDa plasmid (34); and the eight plasmids of E. coli V517 with molecular masses of 1.4 to 35.8 MDa (22).
Conjugation.
To determine if the plasmids were self-transmissible and transferred resistance genes, the strains were conjugated as described by Provence and Curtiss (38) with recipient E. coli C600 or other E. coli and serovar Newport isolates previously selected for resistance to nalidixic acid (NAL) (11). The recipient was grown for 15 to 20 min at 45°C prior to mating (38). Briefly, the donor and recipient cells were conjugated for 60 min at 37°C, and then 250 μl of the mating mixture was transferred to 2.25 ml of prewarmed LB broth containing 50 μg of NAL/ml, incubated at 37°C for 30 min, and plated onto LB agar containing a 32-μg/ml concentration of NAL and any of the antimicrobials to which the donor strain was resistant. Alternatively, E. coli donor strains were conjugated with an antimicrobial-susceptible, plasmid-free serovar Newport isolate in LB broth for 60 min at 37°C, and then 0.1 ml of the mixture was plated on MSRV medium. After incubation and outgrowth of the Salmonella isolate in the MSRV medium, a loopful was streaked onto MH containing the antimicrobial to which the donor strain was resistant.
Transformation.
Plasmid DNA, prepared as described above, was used to transform E. coli DH10B (Gibco BRL, Burlington, Ontario, Canada) by electroporation (40) to determine if plasmids that could not be transferred by conjugation, and were therefore likely not self-transmissible or mobilizable, encoded drug resistance determinants. Standard conditions (2.5 kV, 200 Ω, and 25 μF) were used (28). In case the donor strain contained more than one plasmid, the transformed cells were plated on LB agar plates containing one of the antimicrobials to which the donor strain was resistant in order to select for transformants that might have the plasmid expected to encode the antimicrobial resistance. Transformants that grew on plates containing the antimicrobial were then picked, and their plasmid profiles and antimicrobial susceptibility profiles were examined.
Amplification, sequencing, and hybridization of the cmy-2 gene sequence.
The Citrobacter freundii blaAmpC primers (25) were used to examine the E. coli and serovar Newport isolates for the presence of the cmy-2 gene encoding the CMY-2 β-lactamase. The sequences of the oligonucleotide primers were as follows: for the forward primer, 5′-ATA ACC ACC CAG TCA CGC-3′; and for the reverse primer, 5′-CAG TAG CGA GAC TGC GCA-3′. The annealing temperature was 60°C, the size of the amplicon product was 631 bp, and the GenBank accession number of the Salmonella Senftenberg extended-spectrum beta-lactamase blaampC gene sequence from which the primers were derived is U77414 (20). PCR using the above-described primers was performed with parent strains, transconjugants, and transformants expected to contain the cmy-2 gene. Plasmid DNA preparations of parents, transconjugants, and transformants were electrophoresed, and Southern blots were made. A digoxigenin (DIG)-labeled probe was generated using a PCR DIG probe synthesis kit (Roche), and hybridizations with the probe were performed as described previously (1). PCR mixtures were cleaned prior to DNA sequencing by use of a MinElute PCR purification kit (QIAGEN, Mississauga, Ontario, Canada). DNA sequences were determined using a DYEnamic ET terminator cycle sequencing kit and a MegaBACE 500 sequencing system (Amersham Pharmacia, Piscataway, N.J.).
RESULTS
Acquisition of resistance to the ESCs by conjugation and transformation under laboratory experimental conditions.
Upon conjugation of E. coli MPS57 with NAL-resistant E. coli C600N and Salmonella serovar Newport strain 02-6203, and by transformation of E. coli DH10B, the 72-MDa plasmid encoding resistance to AMP, AMC, CEF, CRO (at 8 μg/ml), CTF, FOX, CHL, SPT, and SUL was transferred to the recipient strains. PCR using the cmy-2 primers showed that the parent, transconjugants, and transformant all produced the expected 631-bp amplification products. Sequencing showed that the amplification products of the parent, transconjugants, and transformant had complete sequence identity with the plasmidic Salmonella cmy-2 gene first reported for an S. enterica subsp. enterica serovar Senftenberg strain identified by using the ampC primers (20). Hybridization studies using the DIG-labeled cmy-2 probe revealed that the 72-MDa plasmid in the parent strain, the transconjugants, and the transformant contained the cmy-2 gene (Fig. 1, lanes 1 to 3).
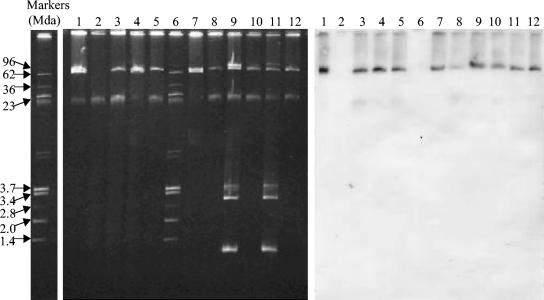
FIG. 1.
Plasmids with the cmy-2 gene in E. coli transferred by conjugation to serovar Newport in the intestinal tracts of poults. Lanes 1, E. coli MPS57 (O168:H7) with 72-MDa plasmid (containing cmy-2 gene) and 66-MDa plasmid; lanes 2, Salmonella serovar Newport 02-6203 before conjugation; lanes 3, serovar Newport 02-6203 after conjugation with E. coli MPS57 containing the 72-MDa plasmid; lanes 4 and 5, E. coli CE332-1 (O168:H7; 72- and 66-MDa plasmids) and serovar Newport CE332-1 (72-MDa plasmid), isolated from the cecal contents of the same poult; lanes 6, reference plasmids of 96, 62, 36, 23, 3.7, 3.4, 2.8, 2.0, and 1.4 MDa; lanes 7 and 8, E. coli CE3313-1 (O168:H7; 72- and 66-MDa plasmids) and serovar Newport CE3313-1 (72-MDa plasmid) isolated from the cecal contents of the same poult; lanes 9 and 10, E. coli CE331-2 (O101:H9; 80-, 72-, 3.6-, 3.0-, and 1.0-MDa plasmids) and serovar Newport CE331-2 (72-MDa plasmid) isolated from the cecal contents of the same poult; lanes 11 and 12, E. coli CE338-2 (O101:H9; 96-, 72-, 3.6-, 3.0-, and 1.0-MDa plasmids) and serovar Newport CE338-2 (72-MDa plasmid) isolated from the cecal contents of the same poult. The isolates with the suffix “-1” were isolated during the first trial; those with the suffix “-2” were isolated during the second trial.
Trials to determine the acquisition of antimicrobial resistance by antimicrobial-susceptible serovar Newport in the poult intestinal tract.
Neither E. coli nor Salmonella bacteria were isolated from the environmental samples taken during trials 1 and 2 before the poults were placed in the pens. During the first trial, all 196 E. coli isolates from samples taken from all four pens were resistant to antimicrobials (Table 4). The most common resistance patterns among the E. coli isolates were AMP-CIP-NAL-STR-TET (95 isolates), KAN-NEO-SPT-STR-SUL-TET (52 isolates), and AMP-STR (34 isolates) (see Table 1 for antimicrobial abbreviations). Isolates with these three patterns of resistance were recovered from all four pens. E. coli isolates (15 in total) resistant to the ESCs, including the cephamycins, with the resistance pattern AMP-AMC-CEF-CRO-CTF-FOX-SPT-SUL were isolated only from pen 3 on sampling days 4 and 10 (Table 4). Six of these strains were isolated from 6 of 13 cloacal swabs on day 4, one strain was isolated from 1 of 5 environmental samples on day 10, and eight strains were isolated from the cecal contents of 8 of 13 poults on day 10. Thus, 15 (39.5%) of the total of 38 E. coli strains isolated on days 4 and 10 after administration of the donor E. coli on day 1 were resistant to the ESCs and showed the same resistance pattern as that of the donor strain. All of the strains serotyped as E. coli O168:H7 and were of the same serotype as the donor (Table 6).
TABLE 4.
Trial 1 antimicrobial resistance patterns and isolation rates of E. coli and Salmonella serovar Newport
| Pen | Day | Resistance patterns (no. of isolates) ofa:
|
|
|---|---|---|---|
| E. coli | Serovar Newport | ||
| 1 | 1 | AMP-STR (6), AMP-CIP-NAL-(STR)-TET (5), KAN-NEO-SPT-STR-SUL-TET (2) | NIb |
| 4 | AMP-STR (1), AMP-CIP-NAL-(STR)-(SUL)-TET (15), KAN-NEO-SPT-STR-(SUL)-TET (2) | Susc.c (18) | |
| 10 | AMP-(AMC)-(CEF)-CIP-NAL-(NIT)-(STR)-TET (16), (AMP)-KAN-NEO-SPT-STR-SUL-TET (2) | Susc. (16), SPT-STR-SUL (2) | |
| 2 | 1 | AMP-STR (6), AMP-CIP-NAL-(STR)-TET (4), (AMP)-KAN-NEO-SPT-STR-SUL-TET (3) | NI |
| 4 | AMP-STR (4), AMP-CIP-NAL-STR-(TET) (10), KAN-NEO-SPT-STR-SUL-TET (4) | Susc. (18) | |
| 10 | AMP-STR (4), AMP-CIP-NAL-(STR)-TET (12), KAN-NEO-STR-SUL-TET (2) | Susc. (11), SPT-(STR)-SUL (6) | |
| 3 | 1 | AMP-STR (5), AMP-CIP-NAL-(STR)-TET (6), KAN-NEO-SUL-TET (2) | NI |
| 4 | AMP-STR (2), AMP-CIP-NAL-STR-TET (3), KAN-NEO-(STR)-SUL-TET (8), AMP-(AMC)-CEF-(CRO)-(CTF)-FOX-SPT-SUL (7) | Susc. (18), AMP-CEF-CRO-CTF-FOX-CHL-SPT-SUL (1) | |
| 10 | AMP-STR (2), AMP-CIP-NAL-(STR)-TET (3), KAN-NEO-(SPT)-(STR)-SUL-TET (6), AMP-(AMC)-CEF-CRO-(CTF)-FOX-SPT-SUL (8) | Susc. (11), AMP-(AMC)-CEF-(CRO)-(CTF)-FOX-CHL-SPT-SUL (8) | |
| 4 | 1 | AMP-STR (2), AMP-CIP-NAL-(STR)-TET (4), (AMP)-(GEN)-KAN-NEO-SPT-STR-SUL-TET (6) | NI |
| 4 | AMP-STR (2), AMP-CIP-NAL-(STR)-TET (9), (AMP)-(CIP)-KAN-(NAL)-NEO-(SPT)-STR-SUL-TET (7) | NI | |
| 10 | AMP-STR (1), AMP-CIP-NAL-(SPT)-STR-TET (8), (AMP)-KAN-NEO-SPT-STR-SUL-TET (8) | Susc. (1) | |
See Table 1 for abbreviations for antimicrobials. Isolates were considered resistant to CIP when growth occurred on MH agar containing 0.125 μg of CIP/ml. Antimicrobials shown in parentheses are those to which the isolates may or may not be additionally resistant.
NI, none isolated.
Susc., susceptible.
TABLE 6.
Relationships among serovars, antimicrobial resistances, and plasmid profiles of E. coli isolates
| Trial | Pen | E.coli serovara | Antimicrobial resistance patternb | Plasmid profile (MDa)c |
|---|---|---|---|---|
| 1 | 1 | O8:H9 | AMP-STR | 2.8, 1.0 |
| O77:H18 | AMP-CIP-NAL-(STR)-(SUL)-TET | 96, 60, 30, (1.0) | ||
| O92:NM | (AMP)-KAN-NEO-SPT-STR-SUL-TET | 80, 66, 18, 4.8, 2.0 | ||
| 2 | O8:H9 | AMP-STR | (80), (66), 2.8, 1.0 | |
| O77:H18 | AMP-CIP-NAL-(STR)-(TET) | 96, 60, 30 | ||
| O92:NM | (AMP)-KAN-NEO-SPT-STR-SUL-TET | 80, 66, 18, 4.8, 2.0 | ||
| 3 | O8:H9 | AMP-STR | 80, 66, 2.8, 1.0 | |
| O17:H18 | KAN-NEO-SUL-TET | NONE | ||
| O77:H18 | AMP-CIP-NAL-(STR)-TET | 96, 60, 30 | ||
| O168:H7 | AMP-AMC-CEF-CRO-(CFT)-FOX-SPT-SUL | 72, 66 | ||
| 4 | O8:H9 | AMP-STR | 2.8, 1.0 | |
| O77:H18 | AMP-CIP-NAL-STR-TET | 96, 60, 30, (1.0) | ||
| O92:NM | (AMP)-(GEN)-KAN-NEO-(SPT)-STR-SUL-TET | 80, 66, 18, 4.8, 2.0 | ||
| 2 | 1 | O8:H9 | AMP-STR | 2.8, 1.0 |
| O159:H28 | Susc. | None | ||
| 2 | O11:H11 | SPT-SUL | 90, 84 | |
| 3 | O101:H9 | Susc. | 96, 3.6, 3.0, 1.0 | |
| O101:H9 | AMP-AMC-CEF-FOX-SPT-SUL | 96 or 80, 72, 3.6, 3.0, 1.0 | ||
| O168:H7 | AMP-AMC-CEF-CRO-(CTF)-FOX-SPT-SUL | 72, 66 | ||
| 4 | O8:H9 | AMP-STR | 80, 66, 24, 2.8, 1.0 | |
| O17:H18 | KAN-NEO-SUL-TET | None | ||
| O17:H18 | AMP-CIP-NAL-TET | 96, 60, 30 | ||
| O92:NM | KAN-NEO-(SPT)-STR-SUL-TET | 80, 66, 18, 2.0 |
None of the E. coli isolates were verotoxigenic. NM, nonmotile.
Antimicrobials shown in parentheses are those to which the isolates may or may not be additionally resistant.
Those plasmids indicated in parentheses may or may not be present. The 72-MDa plasmid indicated in bold was the only one that hybridized with the cmy-2 probe.
All Salmonella isolates that were serotyped were identified as serovar Newport. Ninety-three of the 110 Salmonella isolates were susceptible to all antimicrobials (Table 4). Salmonella isolates resistant to the ESCs and other antimicrobials were isolated only from pen 3 on day 4 (one isolate from 1 of 5 environmental samples) and day 10 (eight isolates from 8 of 13 cecal content samples). Hence, 9 of the 38 (23.7%) serovar Newport isolates from samples taken on days 4 and 10 after the poults were dosed with the ESC-resistant E. coli on day 1 acquired resistance to the ESCs and other antimicrobials. ESC-resistant serovar Newport and E. coli O168:H7 were both isolated from the same samples of cecal contents from 5 of 13 poults in pen 3 (Fig. 1).
During the second trial, poults from the same Salmonella-free primary turkey breeder operation but from a different flock than that employed during the first trial were placed in the isolation pens. The first set of samples were taken after the poults in pens 1, 2, and 3 were dosed with the drug-sensitive serovar Newport and, for those in pen 3, also with the drug-resistant E. coli donor strain but before treatment with CTF in pen 1 and with saline in pen 2 (Table 3). Ninety-one (86.6%) of a total of 105 E. coli isolates were resistant to two or more classes of antimicrobials (Table 5). The AMP-AMC-CEF-CRO-CTF-FOX-SPT-SUL resistance pattern was found in 36 (34.3%) of the isolates, all of which were isolated from samples taken from pen 3 on days 2, 4, and 10.
TABLE 5.
Trial 2 antimicrobial resistance patterns and isolation rates of E. coli and Salmonella serovar Newport
| Pen | Day | Resistance pattern(s) (no. of isolates) ofa:
|
|
|---|---|---|---|
| E. coli | Serovar Newport | ||
| 1 | 2 | NIb | Susc.c(12) |
| 4 | NI | Susc. (17) | |
| 10 | Susc. (1), AMP-STR (1) | Susc. (16), SUL (1) | |
| 2 | 2 | NI | Susc. (13) |
| 4 | NI | Susc. (18) | |
| 10 | SPT-SUL (18) | Susc. (16), SUL (2) | |
| 3 | 2 | AMP-AMC-CEF-CRO-(CTF)-FOX-SPT-SUL (13) | Susc. (13) |
| 4 | Susc. (7), AMP-(AMC)-CEF-(CRO)-(CTF)-(FOX)-SPT-SUL-(TET) (11) | Susc. (18) | |
| 10 | Susc. (6), AMP-(AMC)-CEF-(CRO)-(CTF)-FOX-SPT-SUL (12) | Susc. (5), AMP-(AMC)-CEF-(CRO)-(CTF)-(FOX)-CHL-SPT-SUL (13) | |
| 4 | 2 | AMP-CIP-NAL-TET (2) | NI |
| 4 | AMP-CIP-NAL-(STR)-TET (15), KAN-NEO-SUL-TET (2) | NI | |
| 10 | AMP-STR (4), AMP-CIP-NAL-(SPT)-STR-SUL-TET (10), KAN-NEO-(SPT)-STR-SUL-TET (3) | NI | |
See Table 1 for abbreviations for antimicrobials. Antimicrobials shown in parentheses are those to which the isolates may or may not be additionally resistant.
NI, none isolated.
Susc., susceptible.
Among a total of 144 Salmonella strains isolated during the second trial, 12 of 13 strains isolated from the cecal contents of poults in pen 3 and 1 of 5 strains isolated from environmental samples of poults in pen 3 had become resistant to the ESCs and had acquired the other resistance determinants carried by the E. coli donor strain (Table 5). In total, 13 (26.5%) of 49 serovar Newport strains isolated after administration of the E. coli resistant to ESCs and other antimicrobials to the poults in pen 3 on day 2 did become resistant to the ESCs and other antimicrobials. Ten pairs of ESC-resistant serovar Newport and E. coli isolates were each isolated from the same samples of cecal contents from 10 of the 13 poults in pen 3 (Fig. 1). The E. coli and serovar Newport pairs isolated from the same cecal contents consisted of either serovar Newport and E. coli O168:H7 (the donor E. coli strain) or serovar Newport and E. coli O101:H9 (Table 6).
Serotype, drug resistance, and plasmid profiles of the E. coli isolates.
Determination of the relationships among serotypes, antimicrobial resistances, and plasmid profiles of E. coli isolates showed a remarkably consistent relationship among the three characteristics (Table 6). Thus, all E. coli O8:H9 isolates were resistant to AMP and STR and harbored 2.8- and 1.0-MDa plasmids and, occasionally, others. Similarly, all E. coli O77:H18 isolates were resistant to AMP, CIP, NAL, TET, and sometimes also to STR and SUL and possessed plasmids of 96, 60, and 30 MDa. Two patterns were observed with the E. coli O101:H9 strains isolated from samples taken from pen 3 during the second trial; they were either susceptible to antimicrobials and possessed plasmids of 96, 3.6, 3.0, and 1.0 MDa or were resistant to AMP, AMC, CEF, FOX, SPT, and SUL and contained plasmids of 96 or 80, 72, 3.6, 3.0, and 1.0 MDa (Table 6; Fig. 1).
Conjugation.
Conjugation of ESC-resistant E. coli O101:H9 strains isolated during the second trial from the cecal contents of poults in pen 3 with serovar Newport strain 02-6203 and selection of the serovar Newport strain from MSRV medium and plating on MC agar with CTF resulted in the isolation of serovar Newport strains that contained the 72-MDa plasmid and were resistant to the ESCs. Mating of ESC-resistant E. coli O101:H9 strains with ESC-susceptible NAL-resistant E. coli O101:H9 and serovar Newport 02-6203 strains resulted in the isolations of NAL- and ESC-resistant E. coli O101:H9 and serovar Newport 02-6203 strains containing an additional 72-MDa plasmid. Conversely, conjugation of an ESC-resistant, 72-MDa plasmid containing serovar Newport isolated from the cecal contents of a poult from pen 3 with ESC-susceptible E. coli O101:H9 strains isolated from poults in the same pen and plating on MC agar with CTF resulted in the isolation of ESC-resistant E. coli O101:H9 strains that contained an additional 72-MDa plasmid.
Hybridization.
Southern hybridization studies of pairs of the donor E. coli O168:H7 and recipient serovar Newport strains isolated from the cecal contents of the same poults in pen 3 during trials 1 and 2 showed that the 72-MDa plasmid of the ESC-resistant E. coli O168:H7 and serovar Newport strains hybridized with the cmy-2 probe (Fig. 1, lanes 4 and 5 and lanes 7 and 8). Similarly, the 72-MDa plasmids of pairs of ESC-resistant E. coli O101:H9 and serovar Newport strains isolated from the cecal contents of the same poults from pen 3 during the second trial hybridized with the cmy-2 probe (Fig. 1, lanes 9 and 10 and lanes 11 and 12).
DISCUSSION
This study shows that the 72-MDa conjugative plasmid of an avian E. coli isolate harboring the cmy-2 gene and other antimicrobial resistance determinants was transferred from the E. coli donor to 23.7 and 26.5% of Salmonella serovar Newport strains isolated from the intestinal tract of poults during trials 1 and 2, respectively. It is the first demonstration of the ease with which transfer of these resistance genes can occur between E. coli and Salmonella serovar Newport within the intestines of turkeys in the absence of antimicrobial selection. The conjugative plasmid transferred not only from the E. coli O168:H7 donor to the serovar Newport recipient but also to another E. coli serotype (O101:H9) that occurs naturally in the intestinal tract. The latter E. coli serotype, having acquired the conjugative plasmid from E. coli O168:H7, may in turn have transferred the plasmid to the drug-sensitive serovar Newport since pairs of E. coli O101:H9 and serovar Newport harboring the same plasmid were isolated from the cecal contents of the same poults. Salmonella serovar Newport isolates, having acquired the 72-MDa conjugative plasmid in the intestinal tract, may have in turn by conjugation transferred the plasmid to ESC-sensitive E. coli O101:H9 cecal isolates. These observations suggest that conjugation might be a common and important route whereby Salmonella serovar Newport acquires resistance to the ESCs and that commensal E. coli might be an important source of the cmy-2 gene encoding the β-lactamase CMY-2 and of other antimicrobial resistance genes.
One of the limitations in concluding that conjugation might be a common and important route by which serovar Newport acquired the cmy-2 gene and other resistance genes is that a chicken E. coli isolate possessing a conjugative plasmid containing the cmy-2 gene and other resistance genes was purposely administered to the poults. The frequency of commensal E. coli harboring a conjugative plasmid containing the cmy-2 gene is, however, not known. During the present study, we purposely did not favor the isolation of E. coli strains resistant to the ESCs or other antimicrobials but used nonselective MC agar plates without antimicrobials for the initial isolation of E. coli, and, for the isolation of Salmonella, we used preenrichment in BPW followed by selective enrichment in MSRV medium and plating on LB agar without antimicrobials. Since we did not treat poults that were dosed with the E. coli donor strain containing the conjugative plasmid and the susceptible serovar Newport with CTF, we do not know whether such treatment might have augmented the percentage of recipient serovar Newport and commensal E. coli strains resistant to the ESCs and other antimicrobials.
We do not know the role transduction may play in the acquisition by serovar Newport of genes encoding resistance to the ESCs. Schmieger and Schicklmaier (39) showed that the chromosomally carried resistance genes in serovar Typhimurium DT104 could be efficiently transduced by the P22-like phage ES18 and by phage PDT17 that was released from all DT104 isolates examined. However, the occurrence and efficacy of transfer by bacteriophages of genes encoding resistance to the ESCs and associated resistances located on large plasmids as found in ESC-resistant serovar Newport isolates (14) have not been determined. The occurrence of bacteriophages that would harbor the cmy-2 gene upon inducing Salmonella serovar Newport and other members of the Enterobacteriaceae isolated from litter taken from chicken or turkey barns or from the feces of calves and cows on farms where animals had experienced infection with multidrug-resistant serovar Newport bacteria is not known.
Recently, Lu and coworkers (21) found substantial bacterial DNA contamination, including nucleotide sequences consisting of the three genes vanH, vanA, and vanX that are required for high-level resistance to the glycopeptides in animal feed-grade avoparcin. They suggested that the prolonged use of avoparcin in agriculture may have led to the uptake of glycopeptide resistance genes by commensal bacteria in animals, which were subsequently transferred to humans. In this study, we did not examine the possible transfer of the cmy-2 gene by transformation.
The possible transfer of the cmy-2 gene and other resistance determinants located on large conjugative plasmids by commensal E. coli to serovar Newport and other Salmonella serovars may be a significant factor contributing to the increased isolation of Salmonella serovars producing the ampC-encoded β-lactamase CMY-2. Winokur et al. (46) found a higher percentage (15.6%) of clinical bovine and porcine E. coli isolates harboring the cmy-2 gene on a large plasmid than of clinical isolates of Salmonella (5.1%) isolated from the same animal species (45). None of the large plasmids that harbored the cmy-2 gene were transferable in vitro by conjugation to a recipient E. coli HB101 strain (45). However, other researchers showed that an ESC-resistant E. coli isolated from ground chicken transferred the ESC resistance phenotype encoded by the cmy-2 gene in vitro by conjugation to two E. coli O157:H7 recipient strains (47).
Our findings that 20 of 22 ESC-resistant serovar Newport strains were isolated from cecal contents and that pairs of ESC-resistant E. coli and serovar Newport strains were most commonly isolated from cecal contents suggest that transfer of the 72-MDa conjugative ESC resistance-encoding plasmid occurred predominantly in the intestinal tract and much less frequently in the environment. These findings would agree with the observation of Brownell et al. (4), who showed that the ceca are the most common site for the isolation of Salmonella serovar Typhimurium. With Salmonella bacteria being the most prevalent and numerous in the cecum, these organisms would therefore most frequently conjugate with commensal E. coli bacteria at that site. This would further agree with the finding that even though 1-day-old chicks and poults are highly susceptible to infection by Salmonella (24) and shed the organisms in large numbers in their feces during the first 2 to 14 days of life (41), only two ESC-resistant serovar Newport strains were isolated from environmental samples in our study. The body temperature of the poults (about 41°C) (18) may also have favored the ready transfer of the self-transmissible resistance-encoding plasmid (38). The plasmid may belong to one of the incompatibility groups of plasmids that have a broad host range among gram-negative bacteria (29), since it transferred between E. coli and Salmonella organisms.
An examination of the relationship among serotype, antimicrobial resistance, and plasmid profiles of E. coli isolates showed a remarkably consistency among them (Table 6). This finding is reminiscent of the serovar and phage type specificity of antimicrobial resistance-associated plasmid profiles among isolates of different Salmonella serovars (34, 35) and suggests that host factors are important in the ability of bacteria to acquire resistance elements.
A high percentage (95%) of E. coli strains isolated from the poults in pens 1, 2, and 4 were resistant to antimicrobials other than the ESCs and showed the same AMP-STR, AMP-CIP-NAL-STR-TET, and KAN-NEO-SPT-STR-SUL-TET resistance patterns (Tables 4 and 5). To prevent a selection bias, we purposely did not select for antimicrobial-resistant E. coli or Salmonella during the isolation procedures. Even though the poults in pen 1 had been treated with CTF, no ESC-resistant E. coli or serovar Newport strains were isolated from the poults. It appears therefore that neither the E. coli bacteria naturally occurring in the intestinal tract of poults nor the serovar Newport bacteria infecting the poults readily became resistant to the ESCs or were selected for by the single administration of CTF. However, such a finding should not be taken to suggest that a single injection or prolonged administration of CTF would not select for and augment the number of enterobacteria resistant to the ESCs if such enterobacteria were present in the host or in the environment (43). Almost all E. coli isolates from poults and those taken from the environment in all pens were resistant to two or more classes of antimicrobials other than the ESCs, even though the poults in three of the pens had not been treated with antimicrobials. This finding is reminiscent of earlier unexplained findings of extensive antimicrobial resistance among E. coli isolates from calves during the first weeks after birth even though the calves had not been treated with antimicrobials (17, 19).
This study has demonstrated the ease with which a cmy-2-carrying plasmid can be transferred between Salmonella serovar Newport and E. coli within the intestinal tracts of turkeys, even in the absence of antimicrobial drug selection pressure. The work of Carattoli et al. (8) suggests that there are three major plasmid families carrying cmy-2 and other resistance genes in Salmonella bacteria. Further studies are required to examine the basis of the rapid transfer of plasmids carrying this gene, including the impact of antimicrobial drug selection pressure, the effect of the host (E. coli and Salmonella serovar and phage type specificity), and the extent to which these plasmids may transfer to other species among the enteric microbial flora. Understanding the factors responsible for the rapid transfer and maintenance of these plasmids may suggest ways to reduce their transfer, and the model developed here may be useful in examining practices that reduce such plasmid transfer.
Acknowledgments
We thank Nathan Larson and Sara Hahn, co-op students at the University of Guelph, and Kristen Rekker for assisting in the isolation and identification of the isolates. We thank Arpad Ferencz of Hybrid Turkeys for freely donating the turkey poults, Anne Muckle, Linda Cole, and Betty Wilkie for serotyping the Salmonella isolates, and Irene Yong for serotyping the E. coli isolates.
This work was supported by Genome Canada grant 256177, administered by the Office of Biotechnology Strategies of the Government of Canada.
REFERENCES
- 1.Allen, K. J., and C. Poppe. 2002. Occurrence and characterization of resistance to extended-spectrum cephalosporins mediated by β-lactamase CMY-2 in Salmonella isolated from food-producing animals in Canada. Can. J. Vet. Res. 66:137-144. [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2003. Unpublished data. National Laboratory for Enteric Pathogens, Winnipeg, Manitoba, Canada.
- 3.Bohnhoff, M., and C. P. Miller. 1962. Enhanced susceptibility to Salmonella infection in streptomycin-treated mice. J. Infect. Dis. 111:117-127. [DOI] [PubMed] [Google Scholar]
- 4.Brownell, J. R., W. W. Sadler, and M. J. Fanelli. 1969. Factors influencing the intestinal infection of chickens with Salmonella Typhimurium. Avian Dis. 13:804-816. [PubMed] [Google Scholar]
- 5.Canadian Animal Health Institute. 2001. Compendium of veterinary products, 7th ed., p. 514-516. North American Compendiums Ltd., Hensall, Ontario, Canada.
- 6.Canadian Council on Animal Care. 1984. Guide to the care and use of experimental animals, vol. 1. Canadian Council on Animal Care, Ottawa, Ontario, Canada.
- 7.Canadian Council on Animal Care. 1984. Guide to the care and use of experimental animals, vol. 2. Canadian Council on Animal Care, Ottawa, Ontario, Canada.
- 8.Carattoli, A., F. Tosini, W. P. Giles, M. E. Rupp, S. H. Hinrichs, F. J. Angulo, T. J. Barrett, and P. D. Fey. 2002. Characterization of plasmids carrying CMY-2 from expanded-spectrum cephalosporin-resistant Salmonella strains isolated in the United States between 1996 and 1998. Antimicrob. Agents Chemother. 46:1269-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croza, J. H., and S. Falkow. 1981. Plasmids, p. 269-270. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods for general bacteriology, American Society for Microbiology, Washington, D.C.
- 10.DeFrancesco, K. A., R. N. Cobbold, D. H. Rice, T. E. Besser, and D. D. Hancock. 2004. Antimicrobial resistance of commensal Escherichia coli from dairy cattle associated with recent multi-resistant salmonellosis outbreaks. Vet. Microbiol. 98:55-61. [DOI] [PubMed] [Google Scholar]
- 11.Dickson, J. S., and D. G. Olson. 2001. Growth rates of Salmonella and Escherichia coli O157:H7 in irradiated beef. J. Food Prot. 64:1828-1831. [DOI] [PubMed] [Google Scholar]
- 12.Dunlop, R. H., S. A. McEwen, A. H. Meek, W. D. Black, R. C. Clarke, and R. M. Friendship. 1998. Individual and group antimicrobial usage rates on 34 farrow-to-finish swine farms in Ontario, Canada. Prev. Vet. Med. 34:247-264. [DOI] [PubMed] [Google Scholar]
- 13.Forward, K. R., K. M. Matheson, M. Hiltz, H. Musgrave, and C. Poppe. 2004. Recovery of cephalosporin-resistant Escherichia coli and Salmonella from pork, beef and chicken marketed in Nova Scotia. Can. J. Infect. Dis. Med. Microbiol. 15:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giles, W. P., A. K. Benson, M. E. Olson, R. W. Hutkins, J. M. Whichard, P. L. Winokur, and P. D. Fey. 2004. DNA sequence analysis of regions surrounding blaCMY-2 from multiple Salmonella plasmid backbones. Antimicrob. Agents Chemother. 48:2845-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, A., J. Fontana, C. Crowe, B. Bolstorff, A. Stout, S. Van Duyne, M. P. Hoekstra, J. M. Whichard, T. J. Barrett, and F. J. Angulo. 2003. Emergence of multidrug-resistant Salmonella enterica serotype Newport infections resistant to expanded-spectrum cephalosporins in the United States. J. Infect. Dis. 188:1707-1716. [DOI] [PubMed] [Google Scholar]
- 16.Helms, M., P. Vastrup, P. Gerner-Smidt, and K. Mølbak. 2002. Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerg. Infect. Dis. 8:490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyle, D. V., H. I. Knight, D. J. Shaw, K. Hillman, M. C. Pearce, J. C. Low, G. J. Gunn, and M. E. J. Woolhouse. 2004. Acquisition and epidemiology of antibiotic-resistant Escherichia coli in a cohort of newborn calves. J. Antimicrob. Chemother. 53:867-871. [DOI] [PubMed] [Google Scholar]
- 18.Hutt, F. B., and R. D. Crawford. 1960. On breeding chicks resistant to pullorum disease without exposure thereto. Can. J. Genet. Cytol. 2:357-370. [Google Scholar]
- 19.Khachatryan, A. R., D. D. Hancock, T. E. Besser, and D. R. Call. 2004. Role of calf-adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves. Appl. Environ. Microbiol. 70:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeck, J.-L, G. Arlet, A. Philippon, S. Basmaciogullari, H. V. Thien, Y. Buisson, and J.-D. Cavallo. 1997. A plasmid-mediated CMY-2 β-lactamase from an Algerian clinical isolate of Salmonella senftenberg. FEMS Microbiol. Lett. 152:255-260. [DOI] [PubMed] [Google Scholar]
- 21.Lu, K., R. Asano, and J. Davies. 2004. Antimicrobial resistance gene delivery in animal feeds. Emerg. Infect. Dis. 10:679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macrina, F. L., K. J. Kopecko, K. R. Jones, D. J. Ayers, and S. M. McCowen. 1987. A multiple plasmid-containing E. coli strain: convenient source of size reference plasmid molecules. Plasmid 1:417-420. [DOI] [PubMed] [Google Scholar]
- 23.Miller, C. P., and M. Bohnhoff. 1963. Changes in the mouse's enteric microflora associated with enhanced susceptibility to Salmonella infection following streptomycin treatment. J. Infect. Dis. 113:59-66. [DOI] [PubMed] [Google Scholar]
- 24.Milner, K. C., and M. F. Shaffer. 1952. Bacteriological studies of experimental Salmonella infections in chicks. J. Infect. Dis. 90:81-96. [DOI] [PubMed] [Google Scholar]
- 25.M'Zali, F. H., J. Heritage, D. M. Gascoyne-Binzi, M. Denton, N. J. Todd, and P. M. Hawkey. 1997. Transcontinental importation into the UK of Escherichia coli expressing a plasmid-mediated AmpC-type β-lactamase exposed during an outbreak of SHV-5 extended-spectrum β-lactamase in a Leeds hospital. J. Antimicrob. Chemother. 40:823-831. [DOI] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A. NCCLS. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 27.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing; eighth informational supplement. Document M100-S12. NCCLS, Wayne, Pa.
- 28.O'Callaghan, D., and A. Charbit. 1990. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol. Gen. Genet. 223:156-158. [DOI] [PubMed] [Google Scholar]
- 29.Old, R. W., and S. B. Primrose. 1985. Principles of gene manipulation. An introduction to genetic engineering, 3rd ed. Blackwell Scientific Publications, Oxford, United Kingdom.
- 30.Oppegaard, H., T. M. Steinum, and Y. Wasteson. 2001. Horizontal transfer of a multi-drug resistance plasmid between coliform bacteria of human and bovine origin in a farm environment. Appl. Environ. Microbiol. 67:3732-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Österblad, M., A. Hakanen, R. Manninen, T. Leistevuo, R. Peltonen, O. Meurman, P. Huovinen, and P. Kotilainen. 2000. A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora. Antimicrob. Agents Chemother. 44:1479-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitout, J. D. D., M. D. Reisbig, M. Mulvey, L. Chui, M. Louie, L. Crowe, D. L. Church, S. Elsayed, D. Gregson, R. Ahmed, P. Tilley, and N. D. Hanson. 2003. Association between handling of pet treats and infection with Salmonella enterica serotype Newport expressing the AmpC β-lactamase, CMY-2. J. Clin. Microbiol. 41:4578-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popoff, M. Y. 2001. Antigenic formulas of the Salmonella serovars, 8th ed. W. H. O. Collaborating Centre for Reference and Research on Salmonella, Paris, France.
- 34.Poppe, C., M. Ayroud, G. Ollis, M. Chirino-Trejo, N. Smart, S. Quessy, and P. Michel. 2001. Trends in antimicrobial resistance of Salmonella isolated from animals, foods of animal origin, and the environment of animal production in Canada, 1994-1997. Microb. Drug Resist. 7:197-212. [DOI] [PubMed] [Google Scholar]
- 35.Poppe, C., K. A. McFadden, and W. H. B. Demczuk. 1996. Drug resistance, plasmids, biotypes and susceptibility to bacteriophages of Salmonella isolated from poultry in Canada. Int. J. Food Microbiol. 30:325-344. [DOI] [PubMed] [Google Scholar]
- 36.Poppe, C., K. Ziebell, L. Martin, and K. Allen. 2002. Diversity in antimicrobial resistance and other characteristics among Salmonella Typhimurium DT104 isolates. Microb. Drug Resist. 8:107-122. [DOI] [PubMed] [Google Scholar]
- 37.Prats, G., B. Mirelis, E. Miró, F. Navarro, T. Llovet, J. R. Johnson, N. Camps, Á. Domínguez, and L. Salleras. 2003. Cephalosporin-resistant Escherichia coli among summer camp attendees with salmonellosis. Emerg. Infect. Dis. 9:1273-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Provence, D. A., and R. Curtiss III. 1994. Gene transfer in gram-negative bacteria, p. 337-345. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular biology. American Society for Microbiology, Washington, D.C.
- 39.Schmieger, H., and P. Schicklmaier. 1999. Transduction of multiple drug resistance of Salmonella enterica serovar typhimurium DT104. FEMS Microbiol. Lett. 170:251-256. [DOI] [PubMed] [Google Scholar]
- 40.Sheng, Y.-L., V. Mancino, and B. Birren. 1995. Transformation of Escherichia coli with large DNA molecules by electroporation. Nucleic Acids Res. 23:1990-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snoeyenbos, G. H., V. L. Carlson, C. F. Smyser, and O. M. Olesiuk. 1969. Dynamics of Salmonella infection in chicks reared on litter. Avian Dis. 13:72-83. [PubMed] [Google Scholar]
- 42.Spika, J. S., S. H. Waterman, G. W. Soo Hoo, M. E. St. Louis, R. E. Pacer, S. M. James, M. L. Bissett, L. W. Mayer, J. Y. Chiu, M. L. Cohen, and P. A. Blake. 1987. Chloramphenicol-resistant Salmonella Newport traced through hamburger to dairy farms. N. Engl. J. Med. 316:565-570. [DOI] [PubMed] [Google Scholar]
- 43.Su, L.-H., C.-H. Chiu, C. Chu, M.-H. Wang, J.-H. Chia, and T.-L. Wu. 2003. In vivo acquisition of ceftriaxone resistance in Salmonella enterica serotype Anatum. Antimicrob. Agents Chemother. 47:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veterinary Healthcare Communications. 2001. Veterinary pharmaceuticals and biologicals, 12th ed., p. 1747-1749. Veterinary Healthcare Communications, Lenexa, Kans.
- 45.Winokur, P. L., A. Brueggemann, D. L. DeSalvo, L. Hoffmann, M. D. Apley, E. K. Uhlenhopp, M. A. Pfaller, and G. V. Doern. 2000. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob. Agents Chemother. 44:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winokur, P. L., D. L. Vonstein, L. J. Hoffman, E. K. Uhlenhopp, and G. V. Doern. 2001. Evidence of transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob. Agents Chemother. 45:2716-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, S., D. G. White, P. F. McDermott, S. Friedman, L. English, S. Ayers, J. Meng, J. J. Maurer, R. Holland, and R. D. Walker. 2001. Identification and expression of cephamycinase blaCMY genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob. Agents Chemother. 45:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]