Abstract
α(1-3) glucan is a main component of the Aspergillus fumigatus cell wall. In spite of its importance, synthesis of this amorphous polymer has not been investigated to date. Two genes in A. fumigatus, AGS1 and AGS2, are highly homologous to the AGS genes of Schizosaccharomyces pombe, which encode putative α(1-3) glucan synthases. The predicted Ags proteins of A. fumigatus have an estimated molecular mass of 270 kDa. AGS1 and AGS2 were disrupted in A. fumigatus. Both Δags mutants have similar altered hyphal morphologies and reduced conidiation levels. Only Δags1 presented a reduction in the α(1-3) glucan content of the cell wall. These results showed that Ags1p and Ags2p were functionally different. The cellular localization of the two proteins was in agreement with their different functions: Ags1p was localized at the periphery of the cell in connection with the cell wall, whereas Ags2p was intracellularly located. An original experimental model of invasive aspergillosis based on mixed infection and quantitative PCR was developed to analyze the virulence of A. fumigatus mutant and wild-type strains. Using this model, it was shown that the cell wall and morphogenesis defects of Δags1 and Δags2 were not associated with a reduction in virulence in either mutant. This result showed that a 50% reduction in the content of the cell wall α(1-3) glucan does not play a significant role in A. fumigatus pathogenicity.
The fungal cell wall of Aspergillus fumigatus is mainly composed of polysaccharides that can be separated into two fractions, depending on their solubility in hot sodium hydroxide (7). The alkali-insoluble (AI) fraction constitutes the wall skeleton. It is composed of a branched β(1-3) glucan to which are attached chitin, β(1-3) and β(1-4) glucan, and galactomannan (7). The alkali-soluble (AS) fraction contains mainly α(1-3) glucans and galactomannan (1). Galactomannan has been shown to be associated with host immune reactions against A. fumigatus (18). In A. fumigatus, α(1-3) glucan is the major polysaccharide of the cell wall (40% [dry weight] of the cell wall) (7). The role of α(1-3) glucan in A. fumigatus pathogenicity has not been investigated, although its role in virulence has been demonstrated in Histoplasma capsulatum, Paracoccidioides brasiliensis, and Blastomyces dermatitidis. In these species, mutants with a reduction of α(1-3) glucan in their cell wall are avirulent (13, 16, 30). The construction of a mutant with a reduced amount of α(1-3) glucan in the cell wall of A. fumigatus and the study of its virulence in a mouse model are the aims of this paper.
The synthesis of α(1-3) glucan has been studied only in Schizosaccharomyces pombe. In this fungal species, α(1-3) glucan synthesis is controlled by a single gene, AGS1 [for α(1-3) glucan synthase], which is an essential gene (12, 15). α(1-3) glucan synthesis is essential for correct cell wall morphogenesis, since the mutant Δags1 with a reduced α(1-3) glucan in its cell wall shows a loss of cell polarity at restrictive temperatures (12, 15).
In this paper, we describe two AGS genes of A. fumigatus (AGS1 and AGS2), highly homologous to those of S. pombe, with different functions. Mutant analysis shows that the Δags1 mutant has a reduced cell wall α(1-3) glucan and altered cell polarity and conidiation. In contrast, Δags2 has no cell wall modification but altered cell polarity and reduced conidiation. In vivo virulence studies using an original method of mixed infection showed that these altered phenotypes were not associated with a modification of virulence.
MATERIALS AND METHODS
Strains and culture conditions.
The wild-type (WT) A. fumigatus strain CBS 144-89 (Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands) was used in this study. This strain was maintained on 2% malt agar slants, and transformants were maintained on 2% malt slants supplemented with 0.1 mg of hygromycin B (Sigma)/ml. Mycelia for DNA extraction were grown for 18 h at 37°C in Sabouraud medium (2% glucose and 1% mycopeptone; Biokar). The defined medium used was Brian's medium (3). Growth inhibition studies were conducted with defined RPMI medium (Sigma) supplemented with glutamine (0.3 mg/ml) (RPMI-glu) on microtiter plates. For transformation experiments, minimal medium (1% glucose, 0.092% ammonium tartrate, 0.052% KCl, 0.052% MgSO4 · 7H2O, 0.152% KH2PO4, 1 ml of trace elements solution/liter [pH 6.8]) was used. Escherichia coli strain DH5α (Biolabs) was used for cloning procedures with pBluescript SK(+) plasmid (Stratagene).
A. fumigatus AGS1 and AGS2 isolation.
Genes were isolated before the genome sequence of A. fumigatus was available. To clone AGS genes, degenerated oligonucleotide primers (Table 1) were designed, based on conserved amino acid sequences of Ags1p and Ags3p of S. pombe (HNAEFQG and PSRDEPFGL, respectively) (Fig. 1) and codon usage in A. fumigatus. Primers were used in PCR assays with the genomic DNA of A. fumigatus as a template. Genomic DNA of A. fumigatus strains was prepared according to Girardin et al. (9). An amplified fragment of 790 bp was cloned, sequenced, and subsequently used to screen a cosmid genomic library of A. fumigatus (kindly provided by P. Borgia, Southern Illinois University School of Medicine, Springfield, Ill.), which was immobilized on a nylon membrane (Hybond N+; Amersham). The membranes were hybridized with the [α32-P]dCTP-labeled 790-bp PCR fragment under low-stringency conditions (hybridization at 42°C and washing at 50°C) (22). Positive clones were isolated, and the cosmid was purified. Agarose gel electrophoresis of restricted cosmids and Southern blotting and cloning of the positive bands in a pBluescript SK(+) plasmid were performed according to standard protocols (29). To clone AGS2, a 59-bp fragment was obtained by PCR with the primers for AGS2 isolation (Table 1). This fragment was cloned and used to screen the same cosmid genomic library under high-stringency conditions (hybridization and washings at 65°C). With this probe, positive cosmids were identified that contained an AfAGS1-homologous gene called AfAGS2. Sequencing of AGS1 and AGS2 from genomic DNA and cDNA was performed at ESGS (Cybergène, Evry, France). DNA sequence data were analyzed using the University of Wisconsin Genetics Computer Group programs. Hydropathy and pI determination profiles were done following TopPred analysis (4). For all sequences, nucleotide 1 is the A of the ATG of the open reading frame of the gene.
TABLE 1.
Primers and probes used
| Target | Primer name | Primer sequencea | Molecular beacon |
|---|---|---|---|
| AGS isolation | CACAAYGCYGARTTCCARGG | ||
| AGRCCGAARGGCTCRTCNCGNGA | |||
| AGS2 isolation | GCCAACTAGGCAATCTGA | ||
| AATTCTCCGCGACTGGCC | |||
| AGS1 deletion | ags1DisCa | CCAAGCTTGGCCTGCCCAACTAC | |
| ags1DisCb | GGGAGCTCCTTTTGGACAGGTC | ||
| ags1DisNa | TTGCGGCCGCAGCTCCTTAAGTTCC | ||
| ags1DisNb | GCTGATATCATGCTCGAA | ||
| AGS2 deletion | ags2DisCa | CCAAGCTTGTCGGCGCCAAGAC | |
| ags2DisCb | GGGAGCTCCAAGCCAGAGCCAC | ||
| ags2DisNa | TTGCGGCCGCAGCCCAACCAATATC | ||
| ags2DisNb | CTGATATCAGAGAATGAACTGC | ||
| AfAGS1 mutant | CCACTCCACATCTCCACTC | CGCGATCCCAACTACTACCGCCAGAGTCCTGATCGCG | |
| AACACCGACCGATAGAAGG | |||
| AfAGS2 mutant | ATTCAACATCATGCTGCTGGTC | CGCGATGCTATTCTACCAACTGGGGCAGTTATCGCG | |
| AGTTCTTCTCGGCGTTCTGG | |||
| AfAGS1 wild type | GATTACTTCGGTTCCATCTAC | CGCGATCGACAGCAGAACCAGTGGCTTCCGGATCGCG | |
| AACGCAGCACTATCAAGG | |||
| AfAGS2 wild type | CAAAGCAGGTCAGCATTCAA | CGCACAGAAGGGACATTAAAGCGGAGTGTGCG | |
| CATTGTGGCAATCAGGACAG | |||
| Mouse GAPDH | GCCTTCCGTGTTCCTACC | CGCGATCTGACGTGCCGCCTGGAGAAATCGCG | |
| GCCTGCTTCACCACCTTC |
Underlined nucleotides in the primers for AGS1 and AGS2 deletion correspond to the following restriction enzyme sites: for ags1DisCa and ags2DisCa, HindIII; for ags1DisCb and ags2DisCb, XhoI; for ags1DisNa and ags2DisNa, NotI; and for ags1DisNb and ags2DisNb, EcoRV.
FIG. 1.
Box-shade representation of the amino acid sequence similarities of the Ags proteins of A. fumigatus (Af) and S. pombe (Sp). AfAgs1p, aa 1321 to 1582; AfAgs2p, aa 1316 to 1575; SpAgs1p, aa 1300 to 1558; SpAgs2p, aa 1302 to 1556; SpAgs3p, aa 1328 to 1585; SpAgs4p, aa 1308 to 1566; and Sp Ags5p, aa 411 to 667 (note that this protein lacks the N-terminal region). Amino acid sequences used for degenerate oligonucleotide primers are bracketed.
Expression of AGS genes.
For AGS gene expression, the three strains (the WT and the two Δags mutants) were grown in a flask for 16 h at 37°C and with rotary shaking at 150 rpm in Sabouraud medium. Total RNA was extracted following the manufacturer's instructions (RNeasy kit; QIAGEN). Single-stranded cDNA was synthesized from total RNA with the reverse transcriptase (RT) Moloney murine leukemia virus superscript (Invitrogen): 5 to 30 μg of total RNA was incubated for 10 min at 70°C in the presence of 1 μg of oligo(dT)15. Then, 6 μl of strand buffer (5×), 3 μl of dithiothreitol (0.1 M), 1.5 μl of deoxynucleoside triphosphate (16 mM), and 2 μl of RT (200 U) were added to the RNA-oligo(dT)15 mixture, followed by incubation for 2 h at 42°C.
Transcript analysis of the AfAGS genes was performed by PCR with AfAGS1 and wild-type AfAGS2 as primers (Table 1).
Deletion of the AfAGS genes.
A three-step strategy outlined in Fig. 2 was used to produce A. fumigatus strains with a nonfunctional, disrupted AfAGS gene. The knockout pΔags plasmids were constructed with pBluescript SK(+) (Stratagene) and contained two fragments of AfAGS separated by the E. coli hygromycin B phosphotransferase gene (HPH), flanked by the TRPC terminator and the GPD promoter of Aspergillus nidulans (HPH fragment). The HPH fragment (3.7-kb StuI-HindIII fragment) was obtained from the plasmid pAN 7.1 (a kind gift of P. Punt and C. A. M. J. J. van den Hondel) (25). The two AfAGS fragments were obtained by PCR with A. fumigatus genomic DNA as a template and two sets of primers flanked by restriction enzymes for cloning into pBluescript SK(+). The sets of primers for pΔags1 and pΔags2 are presented in Table 1. The first PCR fragments, ags1DisC (1.4 kb) and ags2DisC (1.5 kb), were obtained with the primer pairs ags1DisCa and ags1DisCb and ags2DisCa and ags2DisCb, respectively. They were cloned in pBluscript SK(+) at the HindIII-XhoI sites. The second PCR fragments, ags1DisN (1.2 kb) and ags2DisN (1.6 kb), were obtained using the primer pairs ags1DisNa and ags1DisNb and ags2DisNa and ags2DisNb, respectively. They were cloned into the previously associated agsDisC-Sk(+) plasmids at the NotI-EcoRV site. The third HPH fragment was then inserted between agsDisN and agsDisC at the EcoRV-HindIII sites. The strategy for pΔags1 is shown in Fig. 2A.
FIG. 2.
Characterization of Δags mutants. (A) Construction of the knockout plasmid pΔags1. (1) First integration of the HindIII-XhoI fragment of ags1DisC in pBluscript SK(+) digested at HindIII and XhoI; (2) second integration of the NotI-EcoRV fragment of ags1DisN in Sk(+)-ags1DisC digested at NotI and EcoRV; and (3) third integration of the StuI-HindIII fragment of the HPH fragment in Sk(+)-ags1DisC-ags1DisN digested at EcoRV and HindIII. The same strategy was used for the knockout plasmid pΔags2. (B) Schematic representation showing the predicted disruption events resulting from homologous integration of the AfAGS1 and AfAGS2 deletion constructs into the A. fumigatus genome. (C) Southern blot analysis of Δags1 and Δags2 mutants compared to the WT. Genomic DNA was digested with NcoI and hybridized with ags1DisC or ags2DisC, labeled “probe” in panel B.
The transformation of A. fumigatus using pΔags1 or pΔags2 was done by the protoplast procedure described previously (24). After overnight expression of the HPH gene, transformants were selected on minimal medium supplemented with 1 M sucrose and containing 200 μg of hygromycin B/ml.
Complementation of the Δags mutants was obtained by a cotransformation strategy using a WT copy of the genes with a plasmid containing another resistance marker. To perform this complementation, the pAN 8.1 plasmid (5.9 kb; 1 to 5 μg) containing the phleomycin resistance gene (19) and the cosmid containing the AGS gene (40 kb; 1 to 5 μg) (isolated as described in Materials and Methods) were cotransformed in the respective A. fumigatus Δags mutants. The presence of the WT copy of the genes was confirmed by PCR (data not shown).
Growth characteristics. (i) Growth on liquid media.
Fungal growth was quantified in Brian's medium in a 2-liter fermentor under 500-rpm agitation and 50 liters of air/min for 48 h at 25°C. At different sampling times, 10-ml (each) culture aliquots were taken, and dry weight was measured after extensive water washing of the mycelium.
(ii) Conidiation measurement.
Aliquots of 100 μl (each) containing 104 conidia were inoculated into five tubes of malt medium (10 ml of medium/tube). After 1 week at 25°C, the conidia were recovered with 1.5 ml of H2O containing 0.01% Tween 20 and counted with a hemacytometer.
Analysis of mutant cell walls.
For cell wall extraction, the fungus was grown in a fermentor in defined Brian's medium under the growth conditions defined above. Preparation of cell wall extracts was done as previously described (23), except that sodium borohydride (500 mM) was added to the 1 N NaOH solution for extraction for the AS and AI fractions. Borohydride was removed after neutralization with acetic acid and evaporation in the presence of methanol. Determination of total hexose was done by the phenol sulfuric acid procedure with glucose as a standard (6). Total hexosamine was measured after hydrolysis with 4 N HCl for 4 h at 100°C (14). Monosaccharide determinations and analysis of the interglycosidic linkages were done as previously described (23). Both hexose and hexosamine concentrations were given as percentages of the total cell wall. Statistical analysis of the results was done by one-way analysis of variance, followed by Duncan's multiple range test (SuperAnova software; Abacus Concepts, Inc., Berkeley, Calif.).
Release of reducing sugars after incubation of the AS fraction with recombinant α(1-3) glucanase (a kind gift of C. Fulgslang, Novozymes) or β(1-3) glucanase (Quantazyme; Quantum) was measured by the p-amino-hydroxybenzoic acid hydrazide method as previously described (8, 27). Briefly, 90 μg (dry weight) of AS was incubated with 0.2 μg of α(1-3) glucanase in 100 μl of 50 mM sodium acetate buffer (pH 5.6) and with 0.2 μg of β(1-3) glucanase in 100 μl of 100 mM imidazole-acetic acid (pH 7.4). The enzymatic reaction lasted 8 h at 37°C.
To study the sensitivity of the mutants to various inhibitors, 104 conidia were inoculated in 200 μl of RPMI-glu medium containing a range of concentrations (final amounts in 200 μl are given in parentheses following each ingredient) of sodium dodecyl sulfate (0 to 250 μg), mulundocandin (0 to 20 μg) (a kind gift from Aventis), nikkomycin Z (0 to 125 μg) (Sigma), Congo red (0 to 100 μg) (Geigy), calcofluor white (0 to 100 μg) (Sigma), β-glucanase mixture (0 to 1 mg) (lytic enzyme from Sigma), α(1-3) glucanase (0 to 200 μg), or amphotericin B (0 to 1 μg), in enzyme-linked immunosorbent assay plates. Plates were incubated at 37°C for 48 h, and the MICs were determined by quantification of the growth estimated as the optical density at 600 nm (absorbance) in a microtiter plate reader.
Immunolocalization of AfAgs1 and AfAgs2 proteins.
Anti-Ags1p is a polyclonal antibody that was raised by Eurogentec (Herstal, Belgium) in rabbit against two internal peptides of Ags1p (WLPDPSQGPKMTNES, amino acids [aa] 366 to 380; and QNNPQSQSGRATPRE, aa 1803 to 1817) coupled to an m-maleimido-benzoyl-N-hydroxy-succimide ester through a cysteine residue.
Similar anti-Ags2p polyclonal antibody was raised against the internal peptide of Ags2p (QAGSDDNQNSTTDVE, aa 1750 to 1764) by Eurogentec following the same method.
Immunolabeling of permeabilized germ tubes of WT, Δags1, and Δags2 was done as previously described (2), with the anti-Agsp antiserum and preimmune serum diluted 1/50 and goat anti-rabbit-fluorescein isothiocyanate conjugate (Sigma) diluted 1/50. Germ tubes were observed under a TCS4D Leica confocal laser scanning microscope with the same exposure times for the control and the anti-Agsp antiserum assays.
Analysis of mutant virulence. (i) Mixed infections.
Mutants and the WT strain (CBS 144-89) were used for experimental infections. Ten female Swiss mice (each, 16 to 18 g) were infected with equal amounts of one mutant and the WT strain. A total of 2 × 106 conidia (in 25 μl of phosphate-buffered saline [PBS]-0.1% Tween 20) were administered intranasally to mice which received intraperitoneal injections of cortisone acetate (5 mg/mouse on days −3, 0, and +2). Two different methods of quantification were used. (i) The first method was as described previously (31). Briefly, the lungs from euthanized mice were plated on 2% agar medium with 0.05% choramphenicol. After conidiation, the lungs were floated with PBS-0.01% Tween 20, and successive dilutions of the conidial suspension were applied to 2% malt extract medium with or without hygromycin B (0.1 mg/ml). The proportion of hygromycin-resistant mutant (Δags1 or Δags2) to hygromycin-sensitive WT was based on the number of colonies growing in the two media. (ii) The second method is based on DNA quantification of each strain in vivo. For this method, the lungs from euthanized mice were disrupted in a mortar under liquid nitrogen, and the total DNA was prepared (9). The DNA extracts were further diluted 10-fold in sterile double-distilled H2O for real-time PCR. Molecular beacon quantitative real-time PCR (QPCR) was performed for detection and quantification of the different strains in each mouse. Molecular beacon probes and primers were designed to amplify and detect each AfAGS mutant and WT strain. A molecular beacon was also designed for the mouse housekeeping gene GAPDH, as an internal control for all QPCR experiments (Table 1). All primer and probe designs were done with Beacon Designer 2 software (Premier Biosoft). Molecular beacon oligonucleotide probes were 5′ end labeled with phosphoramidites FAM (AfAGS mutant and WT) or HEX (GAPDH) and 3′ end quenched with DABCYL (Biosearch Technologies). Each 50 μl of QPCR mixture included 2 μl of DNA, 1 μl of each primer (25 μM) (Sigma-Genosys), 2 μl of molecular beacon probes (50 ng/μl), 19 μl of double-distilled H2O, and 25 μl of IQ supermix (2×) (Bio-Rad). QPCR amplification was performed using an iCycler thermal cycler (Bio-Rad) with the following parameters: 10 min at 95°C and 45 cycles of three steps, each consisting of 30 s at 95°C, 30 s at 50°C, and 30 s at 72°C. Cycle threshold (CT) values were analyzed by the paired t test with JMP5 software (SAS, Cary, N.C.).
(ii) Single infection.
The same procedure as above was followed, except that 2 × 106 conidia of each strain were used in separate experiments on four mice per strain. After 24 h of infection, mice were sacrificed, and the right lungs were prepared for histological studies while the left lungs were used to quantify galactomannan.
Tissues were fixed in 10% neutral buffered formalin and processed routinely for histopathology. Briefly, tissues were dehydrated with graded alcohols, lipids were removed with xylene, and tissues were infiltrated with paraffin and placed in a tissue block. Sections were cut on a rotary microtome at a 5-μm thickness and stained with hematoxylin-eosin and by the Gomori methenamine silver method (10). Representative slides from each lung section were evaluated and graded according to a five-point pulmonary infarct score that incorporated necrosis, inflammation, polymorphonuclear neutrophil recruitment, and presence of hyphae. Scores ranged from 0 to 5, with the score roughly equivalent to the percentage of tissue involvement (0 = 0%, 1 = 10%, 2 = 20%, 3 = 30%, 4 = 40%, and 5 = 50% involvement).
Galactomannan was quantified in aliquots of lungs homogenized in 5 ml of 0.01% Tween 20 aqueous solution. After centrifugation of the homogenate for 10 min at 1,000 × g, the supernatant was diluted 1/100 in PBS containing 0.01% Tween 20 and 1% bovine serum albumin in a Platelia enzyme-linked immunosorbent assay plate (Bio-Rad) following the manufacturer's instructions. Galactomannan concentration was calculated based on a galactomannan standard calibration curve.
Multiple response fitting with mouse data as the repeated measure was analyzed with MANOVA from JMP5 software.
Nucleotide sequence accession numbers.
DNA sequences of A. fumigatus AfAGS1 and AfAGS2 are available in the GenBank database under accession numbers AF435120 and AF432352, respectively.
RESULTS
AGS sequence analysis.
After hybridization under low-stringency conditions using the 790-bp fragment as a probe, we isolated the AfAGS1 gene. After sequencing, we found that the sequence of the 790-bp PCR fragment was homologous but not identical to the AfAGS1 sequence, suggesting the presence of another homologous gene not recovered in our initial hybridization screen. To search for this homolog, a short 59-bp fragment specific for the 790-bp PCR fragment and absent from the AfAGS1 sequence was used.
The genomic sequences of AfAGS1 and AfAGS2 open reading frames of A. fumigatus were 7.26 and 7.28 kb long, respectively. AfAGS1 and AfAGS2 encoded predicted proteins of 2,421 and 2,427 aa with estimated molecular masses of 273 and 272 kDa and pIs of 6.43 and 6.83, respectively. The amino acid sequences of AfAGS1 and AfAGS2 were highly similar (72% identity). The AfAgsp proteins were also highly similar (40 to 48% identity) to the sequences of the Agsp proteins found in S. pombe (accession numbers are as follows: SpAGS1/MOK1, AF061180; SpAGS2/MOK11, AB018380; SpAGS3/MOK12, ABO18381; SpAGS4/MOK13, AB018382; and SpAGS5/MOK14, AB018383). The highest identity between all AGS genes was found in the region shown in Fig. 1. In this region, where the amino acid sequences used for degenerate oligonucleotide primers were selected, identity between all A. fumigatus and S. pombe genes reached 80%. This region was homologous to plant and bacterial glycogen synthase (22 to 32% identity).
RT-PCR data showed that both genes are expressed during mycelial growth of A. fumigatus (data not shown).
Disruption of AfAGS genes.
Disruption of the two AfAGS genes was undertaken following the strategy outlined in Fig. 2. Selection of transformants was initially done by PCRs with different sets of primers, with one oligonucleotide primer inside the AGS fragment of the deletion cassette and another one in the AGS gene fragment normally removed by the homologous recombination event (data not shown). Integration of the drug resistance gene at the correct locus for Δags1 and Δags2 was confirmed by Southern blot data with DNA digested by NcoI (Fig. 2) or BamHI (data not shown). The lack of ectopic integration of DNA fragments from the deletion was verified by PCR, with primers from throughout the AGS gene sequences (data not shown). The lack of expression of the deleted gene in each mutant, seen with RT-PCR, validated the disruption of each AGS gene (data not shown).
Mycelial growth and conidiation of Δags mutants.
The growth rate of Δags1 and Δags2 was similar to that of the WT strain in Brian’s medium (data not shown). However, Δags1 and Δags2 showed altered hyphal morphology compared to the WT (Fig. 3 and data not shown for Δags1). The WT strain produced long hyphae with unique apices at the ends (Fig. 3b), whereas Δags1 and Δags2 produced hyphae with excessive branching and dichotomous apices (Fig. 3a; data not shown for Δags1).
FIG. 3.
Hyphal morphology of Δags2 and WT strains grown for 16 h in Brian’s medium at 25°C. Note the dichotomous branching of the apex of Δags2 (a) compared to the normal apical growth of the WT (b). Magnification, ×1,400.
Both Δags mutants had reduced conidiation (Fig. 4A). On malt medium, the amounts of conidia produced by both mutants were similar to each other but 3.5 times lower than the amount produced by the WT strain (Fig. 4A). This reduction in conidiation is due to the formation of altered phialides on the Aspergillus heads of Δags1 and Δags2 (Fig. 4B). Complementation of the Δags mutants restored the WT phenotype.
FIG. 4.
Conidiation of Δags1 and Δags2 mutants and the parental WT strain of A. fumigatus after 1 week of growth on malt medium at 25°C. (A) Bar, standard error of the mean calculated for at least three replicates. (B) A. fumigatus head and phialides of WT (a), Δags2 (b), and Δags1 (c). Magnification, ×2,800.
Cell wall characteristics of the mutants.
Hexose and hexosamine contents of the AS and AI cell wall extracts of the WT and Δags1 and Δags2 strains were measured. No qualitative or quantitative differences were seen in the hexosamine content of the AI or AS fractions of the two mutants and WT strains. Galactosamine was the only hexosamine found in AS fractions and represented 1.2% ± 0.4% (in milligrams) of the content of the cell wall in all strains. In AI fractions, glucosamine and galactosamine accounted for 8.5% ± 1.2% and 2.5% ± 0.5% (in milligrams), respectively, of the cell wall. Glucose, galactose, and mannose were the hexoses found in the AS and AI fractions of WT and Δags2 strains, with similar cell wall concentrations in the two strains: 10% ± 1.2%, 3% ± 1.2%, and 0.9% ± 0.4% for AS glucose, galactose, and mannose; and 14% ± 1.8%, 1.5% ± 0.4% and 0.9% ± 0.15% for AI glucose, galactose, and mannose, respectively (Fig. 5). In contrast, the repartition of total hexose concentration in the cell wall of Δags1 was different than that of the WT. A 50% reduction in the AS fraction/AI fraction ratio of total hexose concentration was seen, whereas no modification of this ratio was observed in the WT and Δags2 mutants (Fig. 5A). The low AS/AI hexose ratio was due to a reduction in the glucose content in the AS fraction; the amount of glucose in Δags1 was equal to half of the glucose content of the WT strain (Fig. 5B). Galactose and mannose contents in Δags1 cell wall were not significantly different than those in the WT (Fig. 5B). Incubation of AS fractions of Δags1, Δags2, and WT strains with α(1-3) or β(1-3) glucanases confirmed that the glucan fraction of AS contained only α(1-3) glucan, as seen by the release of reducing sugars after α(1-3) glucanase treatment (data not shown). In contrast, the addition of β(1-3) glucanase did not result in the release of reducing sugar. This result showed that AS fractions from WT or mutant strains did not contain β(1-3) glucan (data not shown). Consequently, the deletion of AfAGS1 resulted in significant reduction in the α(1-3) glucan content of the cell wall. In contrast, deletion of AfAGS2 did not result in a modification of the synthesis of α(1-3) glucan. Modification in the cell wall composition of Δags1 was associated with a higher sensitivity of this strain to some cell wall inhibitors. MICs of nikkomycin Z and Congo red were reduced for Δags1 (nikkomycin Z, 8 to 16 μg/ml; Congo red, 3 to 4 μg/ml), while they were identical for Δags2 and WT (nikkomycin Z, >125 μg/ml; Congo red, 16 to 30 μg/ml). No differences were observed in the sensitivities of Δags1, Δags2 and WT strains to sodium dodecyl sulfate, mulundocandin, calcofluor white, β-glucanase, α(1-3) glucanase, or amphotericin B (data not shown).
FIG. 5.
(A) Total hexose content of the AS and AI fractions for the parental strain (WT) and the Δags1 and Δags2 mutants. The hexose concentration was estimated as milligram equivalents of glucose. (B) Comparison of the monosaccharide composition of AS and AI fractions of the cell wall from parental (WT) and Δags1. glc, glucose; gal, galactose; man, mannose. As an example, the value for glucose in the AI fraction was calculated as: 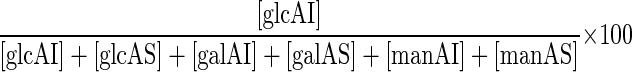 where [glcAI] is the concentration in milligram equivalents of glucose in the AI fraction. Bar, standard error of the mean calculated for at least three replicates.
where [glcAI] is the concentration in milligram equivalents of glucose in the AI fraction. Bar, standard error of the mean calculated for at least three replicates.
Immunolocalization of Ags1p and Ags2p.
Examination of the intracellular localization of Ags1p and Ags2p by immunofluorescence showed that each protein had a different distribution. In WT and Δags2 strains, Ags1p was present at the cell wall level of the germinating conidium and on the apical region of the germ tube. The septum was also labeled (Fig. 6B). No labeling was detected in Δags1 with the anti-Ags1p antibody (data not shown). In WT and Δags1 strains, Ags2p was located intracellularly in the spores and the germ tube and at the apex (Fig. 6C). No labeling was detected in Δags2 with the anti-Ags2p antibody (data not shown).
FIG. 6.
Immunolocalization of Ags1p and Ags2p in germ tubes of A. fumigatus WT, using anti-Agsp antiserum or preimmune serum and goat anti-rabbit-fluorescein isothiocyanate-conjugated antibodies. A TCS4D Leica confocal laser scanning microscope was used, and the same exposure time was used for control and assay experiments. Bar, 5 μm. (A) Control preimmune serum of two different germinated conidia (panels 1 and 2 and 3 and 4, respectively). (B) Immunofluorescence microscopy of two different germinated conidia (panels 1 and 2 and 3 and 4, respectively) using anti-Ags1p antibody. (C) Immunofluorescence microscopy of two different germinated conidia (panels 1 and 2 and 3 and 4, respectively) using anti-Ags2p antibody. A1, A3, B1, B3, C1, and C3 are shown by phase-contrast light; A2, A4, B2, B4, C2, and C4 are shown by epifluorescence.
Virulence of Δags transformants in mice.
Quantification of the mutant and WT strains by estimation of the number of colonies issued from conidia of sporulating lungs obtained after mixed infection showed that around 80% of WT conidia were recovered, whereas around 20% of mutant conidia were present on selective medium (plus hygromycin).
Since the mutants were roughly sporulating in vitro at a rate 3.5 times lower than that of the WT strain, these data suggested that mutant and WT strains had similar virulence in mice. The data also indicated that this method was difficult to use in the case of mutants with altered conidiation.
To palliate this difficulty, QPCR assays were undertaken. During mixed infections, Δags1, in spite of a low level of α(1-3) glucan in the cell wall, and Δags2 demonstrated the same virulence as the WT: there was no significant difference in the cycle threshold (CT) values between the two strains (paired t test; probability of t = >0.5 for a df of 39 for Δags1/WT, probability of t = >0.17 for a df of 33 for Δags2/WT), indicating that in the same mouse, equivalent amounts of DNA of both mutant and WT strains were present. This result demonstrated that Δags1, in spite of a low level of α(1-3) glucan in the cell wall, and Δags2 demonstrated the same virulence as the WT.
The same observation was made during single infections. MANOVA analysis of the inflammation and fungal filamentation indices (as defined in Materials and Methods) and lung galactomannan levels showed no differences between each mutant and its parental strain 24 h after the initiation of the infection (probability of F of >0.87 for Δags1 versus WT and probability of F of >0.35 for Δags2 versus WT).
These results indicated the following. (i) The quantification of the amount of DNA from a specific mutant and its parental strain was an accurate and reliable method to estimate their respective growth in the same mouse and could be used to compare strain virulence following joint infections. (ii) A reduction in half of the α(1-3) glucan content or altered morphology did not induce a decrease in the virulence of A. fumigatus.
DISCUSSION
Modification of the α(1-3) glucan content of the cell wall of A. fumigatus was not associated with a reduction in the virulence of this fungus. In contrast, alterations in the α(1-3) glucan content of other human fungal pathogens affect their pathogenicity. The reasons for a reduction in fungal virulence remain mainly hypothetical. In Cryptococcus neoformans, a unique AGS gene exists (28), and inactivation of this gene leads to a significant decrease in the α(1-3) glucan content of the cell wall. The ags1 mutant of this basidiomycete yeast grows poorly at 37°C and was not encapsulated because the capsule usually anchors on the α(1-3) glucan (28). Although the virulence of this ags1 mutant has not been tested, the lack of a capsule as well as thermosensitivity consecutively to AGS disruption should lead to an avirulent phenotype in C. neoformans (28). The avirulence of spontaneous α(1-3) glucan mutants of H. capsulatum and P. brasiliensis could be due to a higher sensitivity to phagocytes (16, 30). A similar explanation was suggested for the α(1-3) glucan mutant of B. dermatitidis: lack of masking of the major surface antigen WI-I by α(1-3) glucan would result in a more efficient phagocytosis (13). The lack of difference in virulence in the Δags1 mutant and the WT could indicate that α(1-3) glucan has a different function in A. fumigatus and in other fungal pathogens. In A. fumigatus, this polysaccharide may only play a role as a cement for other constituents of the cell wall without anchoring or masking surface molecules playing a putative role in fungal pathogenicity.
With S. pombe, in which a single AGS was active, RT-PCR data showed that AfAGS1 and AfAGS2 were expressed during vegetative growth. However, Δags1 was the only mutant with a cell wall phenotype. The deletion of AfAGS1 resulted in a 50% decrease in the α(1-3) glucan content of the cell wall and in higher sensitivity to polysaccharide assembly inhibitors such as nikkomycin Z and Congo red (17, 26). The remaining α(1-3) glucans present in this mutant may be associated with activity of Ags2p and Ags3p, the latter encoded by a gene identified in the genome sequence of A. fumigatus (available at TIGR [http://tigrblast.tigr.org/]). The localization of Ags1p at the cell wall level is in agreement with a role of this protein in the biosynthesis of the cell wall α(1-3) glucan. The intracellular localization of Ags2p also confirmed a function different from that of Ags1p. The phenotype of the Δags1 mutant of S. pombe is reminiscent of the phenotype observed with the Δags1 of A. fumigatus, since the deletion of AGS1 in S. pombe resulted in a 69% decrease in α(1-3) glucan at restrictive temperatures and a loss of cell polarity (12, 14).
Induction of dichotomous branching at the apex and reduction of conidiation of either the Δags1 or Δags2 mutant of A. fumigatus suggest that both genes have an essential function in unilateral mycelial growth, as in S. pombe (14). This phenotype is reminiscent of the ΔchsG mutant of A. fumigatus, which is characterized by an excessive dichotomous branching (21). This phenotype is also reminiscent of the sepA mutation of A. nidulans (11).
The reason for the different functions of Ags1p, which acts directly on the α(1-3) glucan synthesis of the cell wall, and of Ags2p, which does not, remains unexplained. Numerous examples of functional discrepancy for two gene products in spite of very similar sequences have been found among cell wall gene families both in yeast and in molds. In A. fumigatus chitin synthesis, disruption of the CHSE and CHSD genes resulted in a decrease in chitin content of the cell wall mutant, whereas disruption of the CHSG gene did not (20). In Saccharomyces cerevisiae, three genes encode FKS homologs. Only fks1 and fks2 mutants showed a decrease in the β(1-3) glucan content of the cell wall, indicating that Fks1p and Fks2p are involved in β(1-3) glucan synthesis, whereas Fks3p is not (5). As for Ags2p, the role of ChsGp and Fks3p in the chitin and β(1-3) glucan synthesis remains unknown. Unfortunately, no biochemical assays have been developed for α(1-3) glucan synthase activity to quantify this enzymatic activity, a research area that is actively being pursued in our laboratory.
Acknowledgments
We are very grateful to Emmanuelle Perret in the Centre d'Imagerie Dynamique at the Pasteur Institute for all the confocal data, to Jean-Paul Debeaupuis for his informatics help, and to P. Silar for suggesting the complementation strategy used here.
REFERENCES
- 1.Beauvais, A., and J. P. Latgé. 2001. Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist. Updates. 4:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Beauvais, A., J. M. Bruneau, P. C. Mol, M. J. Buitrago, R. Legrand, and J. P. Latgé. 2001. Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brian, P. W., A. W. Dawkins, J. F. Grove, H. G. Hemming, D. Lowe, and G. L. Norris. 1961. Phytotoxic compounds produced by Fusarium equiseti. J. Exp. Bot. 12:1-7. [Google Scholar]
- 4.Claros, M. G., and G. van Heijne. 1994. TopRed II: an improved software for membrane protein structure predictions. Comput. Appl. Biol. Sci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 5.Dijkgraaf, G. J. P., M. Abe, Y. Ohya, and H. Bussey. 2002. Mutations in Fks1p affect the cell wall content of β-1,3- and β-1,6-glucan in Saccharomyces cerevisiae. Yeast 19:671-690. [DOI] [PubMed] [Google Scholar]
- 6.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric methods for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 7.Fontaine, T., C. Simenel, G. Dubreucq, O. Adam, M. Delepierre, J. Lemoine, C. E. Vorgias, M. Diaquin, M., and J. P. Latgé. 2000. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275:27594-27607. [DOI] [PubMed] [Google Scholar]
- 8.Fontaine, T., R. Hartland, A. Beauvais, M. Diaquin, and J. P. Latgé. 1997. Purification and characterization of an endo-1,3-β-glucanase from Aspergillus fumigatus. Eur. J. Biochem. 243:315-321. [DOI] [PubMed] [Google Scholar]
- 9.Girardin, H., J. P. Latgé, T. Skirantha, B. Morrow, and D. Soll. 1993. Development of DNA probes for fingerprinting Aspergillus fumigatus. J. Clin. Microbiol. 31:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grocott, R. G. 1955. A stain for fungi in tissue sections and smears using Gomori's methenamine-silver nitrate technic. Am. J. Clin. Pathol. 25:975-979. [DOI] [PubMed] [Google Scholar]
- 11.Harris, S. D., J. L. Morrell, and J. E. Hamer. 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136:517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochstenbach, F., F. M. Klis, H. van den Ende, E. van Donselaar, P. J. Peters, and R. D. Klausner. 1998. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA 95:9161-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan, L. H., and B. S. Klein. 1994. Altered expression of surface α-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect. Immun. 62:3543-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, A. R. 1971. Improved method of hexosamine determination. Anal. Biochem. 44:628-635. [DOI] [PubMed] [Google Scholar]
- 15.Katayama, S., D. Hirata, M. Arellano, P. Pérez, and T. Toda. 1999. Fission yeast α-glucan synthase mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J. Cell Biol. 144:1173-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klimpel, K. R., and W. E. Goldman. 1988. Cell walls from avirulent variants of Histoplasma capsulatum lack α-(1,3)-glucan. Infect. Immun. 56:2997-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopecka, M., and M. Gabriel. 1992. The influence of Congo red on the cell wall and (1→3)-β-d-glucan microfibril biogenesis in Saccharomyces cerevisiae. Arch. Microbiol. 158:115-126. [DOI] [PubMed] [Google Scholar]
- 18.Latgé, J. P., H. Kobayashi, J. P. Debeaupuis, M. Diaquin, J. Sarfati, J. M. Wieruszeski, E. Parra, and B. Fournet. 1994. Chemical and immunological characterization of the galactomannan secreted by Aspergillus fumigatus. Infect. Immun. 62:5424-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattern, I. E., P. J. Punt, and C. A. M. J. J. Van den Hondel. 1988. A vector of Aspergillus transformation conferring phleomycin resistance. Fungal Genet. Newsl. 35:25. [Google Scholar]
- 20.Mellado, E., G. Dubreucq, P. Mol, J. Sarfati, S. Paris, M. Diaquin, D. W. Holden, J. L. Rodriguez-Tudela, and J. P. Latgé. 2003. Cell wall biogenesis in a double chitin synthase mutant (chsG−/chsE−) of Aspergillus fumigatus. Fungal Genet. Biol. 38:98-109. [DOI] [PubMed] [Google Scholar]
- 21.Mellado, E., A. Aufauvre-Brown, N. A. R. Gow, and D. W. Holden. 1996. The Aspergillus fumigatus chsC and chsG genes encode class III chitin synthases with different functions. Mol. Microbiol. 20:667-679. [DOI] [PubMed] [Google Scholar]
- 22.Monod, M., G. Togni, B. Hube, and D. Sanglard. 1994. Multiplicity of genes encoding secreted aspartic proteinase in Candida species. Mol. Microbiol. 13:357-368. [DOI] [PubMed] [Google Scholar]
- 23.Mouyna, I., R. P. Hartland, T. Fontaine, M. Diaquin, C. Simenel, M. Delepierre, B. Henrissat, and J. P. Latgé. 1998. A β(1-3) glucanosyltransferase isolated from the cell wall of Aspergillus fumigatus is a homologue of the yeast Bgl2p. Microbiology 144:3171-3180.9846753 [Google Scholar]
- 24.Paris, S. 1994. Isolation of protease-negative mutants of Aspergillus fumigatus by insertion of a disrupted gene, p. 49-55. In B. Maresca and G. S. Kobayashi (ed.), Molecular biology of pathogenic fungi: a laboratory manual. Telos Press, New York, N.Y.
- 25.Punt, P. J., R. P. Oliver, M. A. Dingemanse, P. H. Pouwels, and C. A. M. J.J. van den Hondel. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117-124. [DOI] [PubMed] [Google Scholar]
- 26.Raclavsky, V., R. Novotny, J. Smigova, and Z. Vojkuvka. 1999. Nikkomycin Z counteracts Rylux BSU and Congo red inhibition of Saccharomyces cerevisiae growth but does not prevent formation of aberrant cell walls. Folia Microbiol. 44:663-668. [DOI] [PubMed] [Google Scholar]
- 27.Ram, S. P., K. H. Hynes, L. K. Romana, M. G. Shepherd, and P. A. Sullivan. 1988. The β-glucanases and β-glucosidase of Candida albicans. Life Sci. Adv. 7:379-383. [Google Scholar]
- 28.Reese, J. A., and T. L. Doering. 2003. Cell wall α1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 50:1401-1409. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.San-Blas, G., F. San-Blas, and L. E. Serrano. 1977. Host-parasite relationships in the yeastlike form of Paracoccidioides brasiliensis strain IVIC Pb9. Infect. Immun. 15:343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarfati, J., M. Diaquin, J. P. Debeaupuis, A. Schmidt, D. Lecaque, A. Beauvais, and J. P. Latgé. 2002. A new experimental murine aspergillosis model to identify strains of Aspergillus fumigatus with reduced virulence. Nippon Ishinkin Gakkai Zasshi 43:203-213. [DOI] [PubMed] [Google Scholar]








