Abstract
Impulsive-compulsive behaviour (ICB) is a frequently observed non-motor symptom in early Parkinson’s disease after initiating dopamine replacement therapy. At the opposite end of the motivated behaviour spectrum, apathy occurs in early Parkinson’s disease even before dopamine replacement is started. The co-occurrence of these behavioural conditions in Parkinson’s disease raises questions about their relationship and underlying pathophysiological determinants. In previous imaging or genetic studies, both conditions have been associated with the limbic dopaminergic system. The risk variant of the Ser9Gly polymorphism of the dopamine receptor D3 (DRD3) is linked to increased dopamine affinity in the limbic striatum. With this in mind, we investigated how ICB expression is explained by apathy and DRD3 polymorphisms and their effects on grey matter volume and dopamine synthesis capacity. Fifty-four patients with early Parkinson’s disease took part in anatomical T1-weighted MRI. Forty of them also underwent dynamic PET imaging using [18F]DOPA to measure striatal dopamine synthesis capacity. Further, Ser9Gly (rs6280) gene polymorphism influencing the DRD3 dopamine-binding affinity was determined in all patients. The severity of impulsive-compulsive behaviour and apathy was assessed using the Questionnaire for Impulsive-Compulsive Disorders Rating Scale and the Apathy Evaluation Scale. ICB and the severity of apathy were indeed positively correlated. Apathy and the DRD3 polymorphism were interactive risk factors for ICB severity. Apathy was significantly linked to atrophy of the bilateral putamen. Patients with the DRD3 risk type had reduced dopamine synthesis capacity in the putamen and limbic striatum, apathy was associated with reduced dopamine synthesis capacity in the limbic striatum. The results of [18F]DOPA reached only trend significance. Apathy in drug-naïve PD patients might be a consequence of impaired striatal dopaminergic tone. This may represent a predisposing factor for the development of ICB after the initiation of dopamine replacement therapy. The risk type of DRD3 could further amplify this predisposition due to its higher affinity to dopamine.
Subject terms: Parkinson's disease, Risk factors, Neurodegeneration
Introduction
Impulsive-compulsive behaviours (ICB) are among the most disabling and frequent non-motor symptoms in patients with early Parkinson’s disease (PD)1,2. In particular, ICB also comprises subsyndromal impulsive-compulsive behaviour and not only severely manifest impulse control disorders (ICD), such as pathological gambling, compulsive shopping, binge eating, and hypersexuality. ICB symptoms are an extreme burden for patients and their surroundings. Therefore, identifying risk factors for ICB and understanding its pathophysiology is critical for the development of treatment strategies.
We previously conceptualised a vulnerability-stress model for the development of ICB in PD. In this model, vulnerability is underpinned by a hypodopaminergic state in the striatum, which could be genetically modified, while stress is induced by dopamine replacement therapy3.
The Ser9Gly single nucleotide polymorphism (rs6280) of the dopamine receptor D3 (DRD3) constitutes a candidate for a genetic risk modifier (vulnerability), since the serine (Ser) to glycine (Gly) substitution increases dopamine-binding affinity4. The DRD3 receptor is primarily located in the limbic part of the striatum and primarily acts as a presynaptic autoreceptor inhibiting tonic dopamine release5. Therefore, as previously described in ICB6, a reduced dopamine synthesis capacity in the limbic striatum would dovetail with a higher affinity for dopamine. Indeed, carriers of the risk allele are more prone to develop ICB. Moreover, the DRD3 risk variant is associated with impulsive decision-making in the general population7,8.
Another behavioural marker for altered dopaminergic function is the presence of apathy. Apathy is a very frequent non-motor symptom in early PD, but unlike ICB, often predates the initiation of dopamine replacement therapy9. Recent findings indicate that apathy and ICB co-occur in many patients with PD10,11. Apathy is a significant part of dopamine agonist withdrawal syndrome (DAWS), which occurs in patients with ICB12. Besides, patients with preoperative ICB are prone to develop apathy after subthalamic nucleus deep brain stimulation (STN-DBS), while those with preoperative apathy are at risk of developing ICB after surgery13. Apathy in PD seems to be associated with striatal atrophy14 and a reduction of dopamine transporter (DAT) density15 in the striatum. Indeed, patients with ICB also showed a reduced DAT density in the ventral striatum16,17.
Therefore, apathy might be a specific risk factor for developing ICB when dopamine replacement therapy is initiated.
We hypothesised that apathy and the DRD3 polymorphism might be synergistic determinants for ICB severity. The aim of the current study was to investigate the impact of these determinants on the phenotypic expression of ICB. Furthermore, we were interested in the structural and functional imaging differences linked to these determinants in terms of grey matter volume and dopamine synthesis capacity. We hypothesised that carriers of DRD3 risk variants would show a relatively reduced dopamine synthesis capacity in the limbic striatum since DRD3 receptors are primarily located in the limbic striatum, and ICB is linked to reduced dopaminergic signalling in this area6. We expected that apathy is linked to atrophy and reduced dopamine synthesis capacity in the striatum.
Results
Link between severity of apathy and ICB
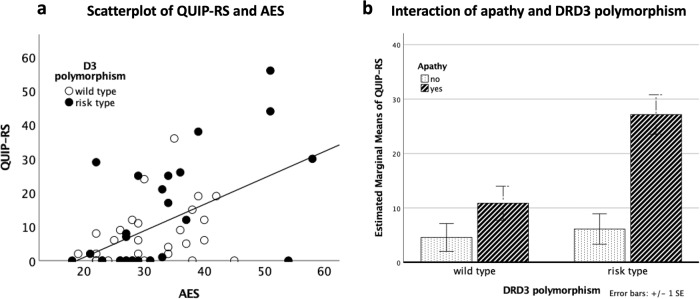
There was a significant positive correlation between QUIP-RS and AES (rho = 0.446, P < 0.001) (Fig. 1a), which means that patients with more severe apathy had more severe ICB. Four patients had an AES score higher than 50. The correlation analysis remained significant even after the removal of these patients (rho = 0.406, p = 0.003). In the healthy controls, there was no significant correlation between the severity of apathy and ICB (rho = −0.244, p = 0.381). In patients, severity of apathy and depression were strongly correlated (rho = 0.600, p < 0.001), but we did not find an effect of severity of depression on ICB that was independent of apathy severity by using a partial rank correlation (rho = 0.148, p = 0.291).
Fig. 1. The link between ICB, apathy and DRD3 polymorphism.
a Significant positive correlation between AES and QUIP-RS. b Bar diagram of the interaction analysis of DRD3 polymorphism and apathy status. The error bars refer to the standard error of the mean.
Distribution of ICB, apathy and DRD3 polymorphism
The distribution of ICB, apathy and DRD3 polymorphism was as follows: 23 patients presented at least one ICB and 15 of them had multiple ICBs. The distribution among the different ICB categories was as follows: 2 pathological gambling, 9 hypersexuality, 3 excessive buying, 8 binge eating, 14 hobbyism, 12 punding and 17 dopamine dysregulation syndrome. 21 patients scored positive for apathy. 15 patients reported apathy and ICB. Figure 2 shows the distribution of apathy and ICB in our cohort. Genotyping for DRD3 resulted in 30 carriers of DRD3− (wild type) and 24 carriers of DRD3+ (risk type). Among the healthy controls, 5 subjects suffered from ICB and 3 of them from multiple ICBs. ICB categories were as follows: 0 pathological gambling, 6 hypersexuality, 2 excessive buying, 3 binge eating, 3 hobbyism, 1 punding and 0 dopamine dysregulation syndrome. The healthy controls did not fulfil the criteria for apathy. DRD3 polymorphism was not assessed in controls. Therefore, the ANOVA and imaging analyses could not be replicated in the healthy controls.
Fig. 2. Distribution of apathy and ICB in our cohort.
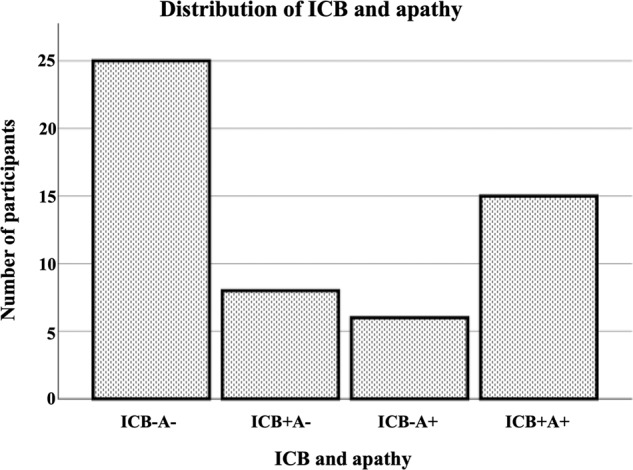
No ICB and no apathy (ICB−A−). ICB and no apathy, (ICB+A−), no ICB and apathy (ICB−A+), ICB and apathy (ICB+A+).
ANOVA of apathy status and DRD3 polymorphism
The model was statistically significant (F(6,48) = 13.14, P < 0.001). The main effects of apathy (F(1, 48) = 20.13, P < 0.001, BCa 95% CI [−32.28, −8.657]) and of DRD3 polymorphism (F(1, 48) = 8.33, P = 0.006, BCa 95% CI [14.79, 57.19]) significantly determined QUIP-RS. The interaction of DRD3 polymorphism and apathy status (F(1, 48) = 5.79, P = 0.02, BCa 95% CI [.536, 27.93]) also significantly determined QUIP-RS (see Fig. 1b).
Comparisons between DRD3− and DRD3+ and Apathy− and Apathy+
Group comparisons between carriers of DRD3− and DRD3+ as well as Apathy− and Apathy+ are presented in Table 1. Patients with apathy showed more severe symptoms of ICB, impulsivity (BIS) and depression. Additionally, motor complications were more severe in A+ than in A-. Nine patients had an UPDRS IV higher than 4 indicating at least moderate motor complications, eight of them were apathetic18.
Table 1.
Group comparison between DRD3 wild type and DRD3 risk type as well as non-apathetic and apathetic PD patients.
| All patients | Apathy | DRD3 polymorphism | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | p value | Wild type | Risk type | p value | ||
| Number of subjects | 54 | 33 | 21 | ∅ | 30 | 24 | ∅ |
| Age | 70 (13) | 70 (15) | 70 (10) | 0.993 | 70.5 (13) | 68.5 (11) | 0.280 |
| Male | 35 | 21 | 14 | 0.82 | 19 | 16 | 0.799 |
| Right-handed | 50 | 31 | 19 | 0.443 | 28 | 22 | 0.496 |
| AES | 30 (11) | 27 (7) | 38 (8) | <0.001 | 29.5 (13 | 30 (11) | 0.807 |
| QUIP-RS | 6 (18) | 1 (8) | 15 (24) | <0.001 | 4.5 (11) | 7.5 (26) | 0.297 |
| PANDA | 23 (7) | 23 (6) | 22 (8) | 0.593 | 23.5 (9) | 23 (6) | 0.523 |
| MMSE | 29 (2) | 29 (2) | 28 (3) | 0.165 | 29 (2) | 29 (2) | 0.726 |
| BDI-II | 8 (9) | 6 (6) | 14 (9) | <0.001 | 8 (9) | 7 (9) | 0.402 |
| BIS | 59 (9) | 56 (7) | 62 (10) | <0.001 | 60 (7) | 58 (11) | 0.438 |
| UPDRS III (off) | 26 (11) | 25 (12) | 26 (11) | 0.186 | 26 (10) | 24 (14) | 0.644 |
| UPDRS IV | 2 (4) | 0 (2) | 4 (4) | <0.001 | 2 (4) | 1 (3) | 0.170 |
| LED (mg) | 430 (354) | 425 (325) | 450 (376) | 0.936 | 453.5 (304) | 400 (462.5) | 0.365 |
| Intake of DA-agonists | 46 | 29 | 17 | 0.697 | 24 | 22 | 0.277 |
| DA-LED (mg) | 100 (142) | 120 (125) | 80 (110) | 0.101 | 100 (152.5) | 150 (122.5) | 0.294 |
| Disease duration | 4 (5) | 4 (5) | 3 (5) | 0.675 | 4 (5) | 3 (6) | 0.285 |
Median and interquartile range (in brackets). P values based on Mann–Whitney U test or Fisher’s exact test. The median and interquartile range for the complete group were added for better readability. UPDRS III Unified Parkinson’s Disease Rating Scale part 3, UPDRS IV, Unified Parkinson’s Disease Rating Scale part 4, QUIP-RS Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease Rating Scale, AES Apathy Evaluation Scale, BDI-II Beck Depression Inventory version 2, BIS Barratt Impulsiveness Scale, MMSE Mini-Mental State Examination, PANDA Parkinson Neuropsychometric Dementia Assessment, LED Levodopa equivalent dose, DA-LED dopamine agonist equivalent dose.
Significant values in bold.
Imaging—VBM
There was a significant negative effect of apathy on grey matter volume in the left (left: x = −22, y = 8, z = −2, pseudo-t = 4.09) and right putamen (x = 24, y = 6, z = −2, pseudo-t = 3.62). The results survived FWE correction on the cluster level. The DRD3 polymorphism was not associated with any volumetric differences in the striatum. See Fig. 3.
Fig. 3. The link between apathy and grey matter volume.
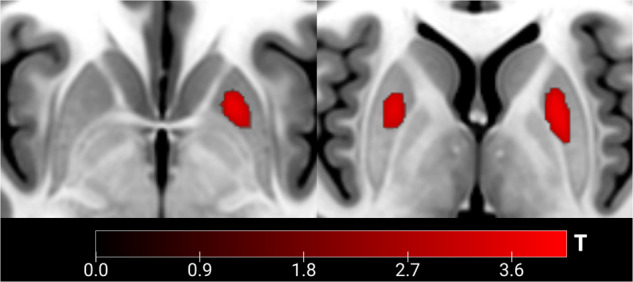
Significant negative effect of apathy on grey matter volume in bilateral putamen. Axial view.
Imaging—[18F]DOPA-PET
The following results are only trend significant and reported with a liberal threshold of p < 0.001 uncorrected. The risk type of the DRD3 polymorphism was associated with a reduced dopamine synthesis capacity in the right limbic striatum (x = 16, y = 20, z = −4, pseudo-t = 3.11), and bilateral putamen (right: x = 28, y = 12, z = −2, pseudo-t = 3.45; left: x = −28, y = −6, z = 0, pseudo-t = 2.8). Apathy was linked to a reduced dopamine synthesis capacity in the right limbic striatum (x = 10, y = 20, z = −4, pseudo-t = 3.23).
Discussion
We found that apathy and risk type of DRD3 polymorphism are interactive risk factors for the severity of ICB. Apathy was linked to significant atrophy of the putamen, while the risk type of the DRD3 polymorphism was not associated with any volumetric differences. PET imaging revealed at trend significance that the DRD3 risk type and apathy had a negative effect on dopamine synthesis capacity in the limbic striatum and putamen.
Consistent with previous work10, apathy and ICB co-occurred in our early-stage PD cohort to a similar extent. In a cross-sectional study with 887 PD patients, 61% of the patients with ICB symptoms also reported apathy, whereas 41.3% of the apathetic PD patients also reported ICB11. A study with a large population sample of young adults showed that the severity of apathy and impulsivity were positively correlated19. We could replicate this finding in a group of patients with PD. Given this positive correlation, we speculate that apathy might constitute a risk factor for ICB, based on a striatal vulnerability and its modulation by dopaminergic replacement therapy. It is worth noticing that apathetic patients scored higher on the BIS. A previous study demonstrated that impulsivity measured by BIS did not lead to a higher prevalence of ICB in PD, but increased their severity20. It is worth speculating that impulsivity goes along with similar imaging changes like ICB, i.e., a reduction of dopaminergic terminals to the ventral striatum16. Notably, this seemingly counterintuitive behavioural co-expression of ICB and apathy may result from patients experiencing increased sensitivity for immediate reward while experiencing difficulties in pursuing long-term goals due to apathy and discounting21. In controls, we could not find a correlation between the severity of ICB and apathy. Controls with ICB did not suffer from apathy. It is tempting to speculate that this link between apathy and ICB might be more specific for PD. Interestingly, apathy was associated with more severe motor complications. This is in line with the fact that apathy predicted motor complications in PD patients in a previous study22. However, against our expectations, dopamine dysregulation syndrome was not associated with motor complications. Furthermore, there was no effect of depression on ICB that was independent of severity of apathy. Therefore, we assume that apathy and depression are similar sides of the same hypodopaminergic “coin”.
We report that apathy was linked to atrophy of the bilateral putamen and this effect was independent of the DRD3 polymorphism. This region is associated with action-reward association learning and the storage of motor memories emphasising the link between apathy and ICB. Our finding goes along with striatal atrophy in non-demented apathetic PD patients14,23. In addition, apathetic elderly without PD also had a reduced volume of the putamen24. Apathy was not only associated with atrophy but was also linked to microstructural alterations in the anterior striatum in de novo PD patients25. We therefore speculate that—independent of the development of PD—apathy is causally linked with anatomical changes in the putamen.
We further found that the dopamine synthesis rate in the limbic striatum was reduced in patients with apathy independent of the DRD3 polymorphism. However, these results only reached trend significance, because they did not survive FWE correction. Therefore, they have to be interpreted with caution. This finding is in line with a previous raclopride study revealing that apathetic PD patients had a significantly reduced dopamine release in the entire striatum26. Our result is further supported by the fact that DAT density in the striatum was reduced in apathetic PD patients in a previous study15. Furthermore, apathetic PD patients demonstrated, compared to controls, a widespread dopaminergic and serotonergic degeneration in the caudate, putamen, and limbic striatum27,28. A study on PD patients with deep brain stimulation further emphasises a reduced function of the limbic striatum in apathy: A reduced preoperative glucose metabolism in the limbic striatum was associated with an increase in apathy after surgery29. We conclude that atrophy and reduced dopaminergic signalling in the striatum may promote the development of apathy. For further reading, we recently highlighted the role of the limbic striatum in PD in a systematic review30.
Interestingly, our behavioural results demonstrate that the severity of ICB increases together with apathy. However, not only apathy, but also the risk type of DRD3 polymorphism led to a more pronounced ICB phenotype. DRD3 are G-protein coupled receptors belonging to the DRD2-like family of dopamine receptors. Activation of these receptors inhibits adenylate cyclase and reduces cyclic adenosine monophosphate (cAMP) levels, which may affect dopamine synthesis directly. DRD3 is mainly found in the limbic striatum and the ventral tegmental area (VTA), where they act primarily as autoreceptors, resulting in negative feedback on presynaptic dopamine signalling, enhanced for risk type with a higher affinity for dopamine7,31,32. Here, we observed the trend that carriers of the risk allele have reduced dopaminergic synthesis capacity in the (limbic) striatum independent of apathy on a trend level. We conclude that this reduction in dopamine levels might be due to the enhanced affinity of those presynaptic receptors to dopamine and dopamine agonists7. Furthermore, we previously demonstrated that the severity of ICB was associated with a reduced dopamine synthesis capacity in the limbic striatum6. Taken together, these studies suggest that the DRD3 risk variant might be an important modulator to develop ICB via influencing the limbic dopaminergic tone.
Additionally, the postsynaptic effects of DRD3 in the limbic striatum could also contribute to the severity of ICB. A pharmacological fMRI design showed that dopamine agonists prevent pauses in dopamine transmission, thus limiting learning from negative feedback33. This effect might be amplified in carriers of the DRD3 risk type, since it has a higher affinity for dopamine and maybe also for dopamine agonists7. As a result of this tonic overstimulation of the postsynaptic membrane, dips in dopaminergic signalling might not be registered anymore, leading to a reduced sensitivity to negative consequences of actions. Note that a neuropathological study demonstrated that DRD3 levels in the limbic striatum were associated with ICB, with downregulation of the dopamine D3 receptor levels possibly occurring either as a consequence of the degenerative process or as a pre-morbid trait34.
To our knowledge, no other study could show the interactive effect of apathy and the DRD3 risk type on the severity of ICB. Indeed, apathy and the DRD3 risk type were associated with impaired function and structure of the striatum. The results of this study follow and extend the recently proposed vulnerability-stress model for ICB3. We speculate that apathy, especially in drug-naïve PD patients, is based on impaired function of the striatum. These apathetic patients might be especially vulnerable for ICB with the initiation of dopamine replacement therapy. This effect is amplified in patients with the risk type of DRD3 due to higher affinity to dopamine and especially dopamine agonists.
There are several limitations to consider. QUIP-RS and AES scores may reflect symptom severity over a relatively long time. Therefore, we cannot claim that symptoms of apathy and ICB actually co-occurred simultaneously. Effects of DRD3 risk variant on dopamine synthesis analysis only reached trend significance in this nonparametric permutation-based approach and should therefore be interpreted with caution due to the high rate of Type I errors. In our group comparisons of DRD3 wild type and risk type carriers did not reach significance for ICB severity in a Mann-Whitney-U-Test. However, in the ANOVA a significant independent effect of DRD3 polymorphism on ICB severity was found. Overall, apathy seems to play a stronger role for ICB severity than DRD3 type. Only fifteen healthy controls were tested for ICB and apathy. Unfortunately, DRD3 polymorphism was not assessed in controls. Here, we focused on dopaminergic systems. However, other neurotransmitters, such as serotonin, may play a critical role in developing apathy or ICB35. Further, within the dopaminergic system, other genes may also be associated with the development of ICB, e.g., DRD1 (rs4680), DAT (rs28363170), or COMT (rs4680)36.
In sum, our findings suggest that apathy and the DRD3 risk variant interact to confer a synergistic risk for the severity of ICB. Both of these factors were associated with impaired striatal function, which may contribute to the development and maintenance of ICB. Our results have important clinical implications, as they highlight the need for personalised treatment approaches that take into account both genetic and behavioural factors in the management of ICB.
Furthermore, our study adds to a growing body of literature demonstrating the complex interplay between genetic and environmental factors in the development of psychiatric disorders. Future research should explore the potential mechanisms underlying the interaction between apathy and the DRD3 risk variant, as well as the role of other genetic and environmental factors in the pathogenesis of ICB.
Finally, our findings also have broader implications for our understanding of the neurobiology of motivation and reward processing. The striatum plays a key role in these processes, and its dysfunction has been implicated in a range of psychiatric disorders. Our study highlights the importance of considering the complex interactions between different factors that contribute to striatal function and provides further evidence for the need to develop personalised treatment approaches that target the underlying neurobiology of neuro-psychiatric disorders.
Methods
Study participants
Participants were recruited as part of the German KFO-219 cohort6,37,38. We included only those patients who underwent genotyping and MRI imaging, resulting in fifty-four patients with early Parkinson’s disease and fifteen age-matched healthy controls. A subgroup of 40 patients also underwent [18F]DOPA-PET imaging. Patients were recruited from the Department of Neurology of the University Hospital of Cologne, with the diagnosis of idiopathic PD according to the UK Brain Bank Criteria39. Exclusion criteria were any other diseases that may affect brain function (e.g., stroke, tumour), dementia, and any issues regarding MRI safety. Dopamine replacement therapy was discontinued for 12 hours (levodopa) and 72 hours (for dopamine agonists) prior to PET scanning. The study was carried out following the International Ethical Guidelines and the Declaration of Helsinki. The ethics committee of the medical faculty of the University of Cologne (Germany) approved the study (application number: 12-265). Written informed consent was obtained from all participants.
Clinical and neuropsychological data
We used the validated German version of QUIP-RS to assess ICB severity40. The Apathy Evaluation Scale (AES) was used to capture apathy severity41. These self-reported scores were obtained simultaneously. The Mini-Mental State Examination (MMSE) and Parkinson neuropsychometric dementia assessment (PANDA) evaluated cognitive functions. Furthermore, we assessed the Beck Depression Inventory (BDI-II) and the Barratt Impulsiveness Scale (BIS). The severity of motor symptoms in the unmedicated condition was assessed using the Unified Parkinson’s Disease Rating Scale Part III (UPDRS III). Motor complications were assessed with the Unified Parkinson’s Disease Rating Scale Part IV (UPDRS IV). Dopamine replacement therapy dosages were converted to levodopa equivalent daily doses and dopamine agonist equivalent doses, respectively42. The number of patients taking dopamine agonists was assessed.
Genetic analyses
QIAamp DNA Blood Mini Kit (#51106, QIAGEN) was used to isolate DNA from a peripheral blood sample. For the assessment of DNA quality and concentration, an ND-1000 UV/Vis-Spectrophotometer (Peqlab) was used. For SNP genotyping of rs6280, 20 ng of DNA was analysed in triplicates using allelic discrimination assays (TaqMan SNP Genotyping Assays, Applied Biosystems by Invitrogen). Genotyping PCR was performed with a 7900HT Fast Real-Time PCR System (Applied Biosystems). Sequence Detection Software version 2.3 (Applied Biosystems) was used to process fluorescence data37. Groups were defined according to carriers of the DRD3 [DRD3− (wild type): T/T; DRD3+ (risk type): C/T, C/C].
Image acquisition and preprocessing—MRI
54 PD patients underwent MRI imaging. Scanning was done on a 3 T Magnetom Prisma (Siemens, Erlangen). For the anatomical T1-weighted MPRAGE acquisition parameters were as follows: repetition time = 2300 ms, echo time = 2.32 ms, flip angle = 8, field of view = 230 mm, slice thickness = 0.9 mm, voxel size = 0.9 × 0.9 × 0.9 mm, number of slices = 192. In order to detect anatomical differences, we calculated structural volume based on voxel-based morphometry with the CAT12 toolbox (https://neuro-jena.github.io/cat/). The T1-weighted images were segmented automatically into grey and white matter. The grey matter volumes were smoothed by an 8 × 8 × 8 FWHM Gaussian Kernel and the total intracranial volume (TIV) was calculated.
Image acquisition and preprocessing—[18F]DOPA-PET
Dynamic PET images were acquired in 3D mode with a 24-detector ring scanner ECAT EXACT HRRT (Siemens, Erlangen) over 90 minutes. The subjects were lying in a quiet and dim room during image acquisition. All patients received 100 mg of Carbidopa 1 hour before intravenous injection of [18F]DOPA, with an average activity of 185 MBq. A 10-min transmission scan with a retractable [68Ga]/[68Ge] ring source was performed to correct tissue attenuation. PET images were corrected for random coincidences, attenuation, and scatter. We normalised the images using the [18F]DOPA-PET template in SPM43. The voxel-wise net influx rate constant of [18F]DOPA (DOPA-Ki), indicating dopamine synthesis capacity, was determined by a Patlak plot6.
Statistical analyses—behavioural and genetic data
The distribution of normality for QUIP-RS and AES was tested using the Kolmogorov-Smirnow-Test in addition to visual inspection. In a first step, we examined the relationship between the severity of apathy and ICB. Therefore, we performed a Spearman correlation analysis with apathy and ICB as quantitative variables, i.e., QUIP-RS and AES. We performed this analysis in PD patients and healthy controls, separately. Additionally, we investigated how far the severity of depression had an effect on ICB that was independent of apathy. To do this, we performed a partial rank correlation with QUIP-RS and BDI-II controlling for AES.
In a second step, we examined how many patients of our cohort reached the established cut-offs for ICB and apathy. Positivity for ICB was defined according to previously established QUIP-RS cut-offs for the different ICB categories40. Positivity for apathy was defined by using a previously defined cut-off of the AES in PD (AES > 33)41.
In a third step, a two-way ANOVA was used to examine the interactive effect of apathy status (categorical variable) and DRD3 receptor polymorphism (categorical variable) on ICB severity (QUIP-RS, quantitative variable). Age and sex were included as covariates of no interest in this model. Using 1000 samples and the bias corrected and accelerated method (BCa), bootstrapped confidence intervals were calculated due to non-normal distribution of QUIP-RS.
In a final step, group comparisons of neuropsychological and demographic data between DRD3− (wild type) and DRD3+ (risk type) as well as apathetic and non-apathetic were calculated based on Mann-Whitney-U-Tests. For differences in the distribution of biological sex, handedness and intake of dopamine agonists, we applied Fisher’s exact test between the groups. Furthermore, we added a column summarising the demographical information of the entire group.
Statistical analyses—imaging data
Apathy status and DRD3 polymorphism significantly predicted the severity of ICB. We performed a post-hoc analysis examining the independent effect of these two categorical predictors on striatal grey matter volume and dopamine synthesis capacity as neural correlates of the propensity to develop ICB. We tested how far grey matter volume and dopamine synthesis capacity were driven by the apathy status or DRD3 polymorphism and thereby led to more severe ICB as shown by the behavioural interaction. Therefore, we used multiple linear regression models. Due to the small sample size, different group sizes and nonparametric distribution of behavioural data, we performed voxel-based statistical nonparametric mapping using the SnPM13 package (http://www.nisox.org/Software/SnPM13/). Analyses were performed based on 5000 random permutations, and we applied variance smoothing with a 10 × 10 × 10 kernel. The Striatal Connectivity Atlas was used as an explicit mask (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases/striatumconn). A statistical threshold of p < 0.05 family-wise error (FWE)-corrected was considered significant. P values below p < 0.001 uncorrected were considered as trend significant. For image display, we used MRIcroGL (https://www.nitrc.org/projects/mricrogl).
For VBM, we specified a multiple linear regression model with apathy status (categorical variable), DRD3 carrier status (categorical variable) and TIV as covariates. There was no relevant collinearity with TIV and any other parameter of interest. In this model, the negative contrast of each factor was calculated. The other factors mentioned above were then set as covariates of no interest.
For [18F]DOPA-PET, a multiple linear regression model with DRD3 carrier status (categorical variable) and apathy status (categorical variable) as covariates was specified. In this model, the negative contrast of each factor was calculated. The other factors mentioned above were then set as covariates of no interest
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was supported by grants of the Deutsche Forschungsgemeinschaft (DFG): EI 892/3–1, KFO-219, SFB 1451 (project 431549029) and was supported by the DFG (German Research Foundation)—Project-ID C03—431549029. H.T. was supported by the programme for rotation positions/Faculty of Medicine/University of Cologne and by the Cologne Clinician Scientist Programme (CCSP)/Faculty of Medicine/University of Cologne. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (Project no. 413543196). S.P. was supported by a stipend from the Alexander von Humboldt Foundation.
Author contributions
All authors made substantial contributions. Conceptualisation: Tv.E., H.T.; methodology: Tv.E., H.T., S.P., G.N.B.; formal analysis and investigation: H.T., S.P., G.N.B.; writing—original draught preparation: H.T.; writing—review and editing: H.T., S.P., G.N.B., M.H., M.T., G.R.F., C.E., L.T., A.D., Tv.E.; acquisition of funding and data: M.T., C.E., L.T.; supervision: Tv.E. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity but are available from the corresponding author upon request.
Code availability
No code was generated during the study.
Competing interests
A.D. reports the following competing interests: Research support: Siemens Healthineers, Life Molecular Imaging, GE Healthcare, AVID Radiopharmaceuticals, Sofie, Eisai, Novartis/AAA, Ariceum Therapeutics Speaker Honorary/Advisory Boards: Siemens Healthineers, Sanofi, GE Healthcare, Biogen, Novo Nordisk, Invicro, Novartis/AAA, Bayer Vital Stock: Siemens Healthineers, Lantheus Holding, Structured therapeutics, ImmunoGen Patents: Patent for 18F-JK-PSMA- 7 (2-Alkoxy-6-[18F]Fluoronicotinoyl substituted Lys-C(O)-Glu derivatives as efficient probes for imaging of PSMA expressing tissues (Patent No.: EP3765097A1; Date of patent: Jan. 20, 2021)). The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-023-00596-9.
References
- 1.Weintraub D, et al. Impulse control disorders in parkinson disease: a cross-sectional study of 3090 patients. Arch. Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 2.Cilia R, van Eimeren T. Impulse control disorders in Parkinson’s disease: seeking a roadmap toward a better understanding. Brain Struct. Funct. 2011;216:289–299. doi: 10.1007/s00429-011-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theis H, Probst C, Fernagut P-O, van Eimeren T. Unlucky punches: the vulnerability-stress model for the development of impulse control disorders in Parkinson’s disease. NPJ Parkinsons Dis. 2021;7:112. doi: 10.1038/s41531-021-00253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundstrom K, Turpin MP. Proposed schizophrenia-related gene polymorphism: expression of the Ser9Gly mutant human dopamine D3 receptor with the Semliki Forest virus system. Biochem. Biophys. Res Commun. 1996;225:1068–1072. doi: 10.1006/bbrc.1996.1296. [DOI] [PubMed] [Google Scholar]
- 5.Savitz J, et al. The functional DRD3 Ser9Gly polymorphism (rs6280) is pleiotropic, affecting reward as well as movement. PLoS One. 2013;8:e54108. doi: 10.1371/journal.pone.0054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammes J, et al. Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. Brain. 2019;142:733–743. doi: 10.1093/brain/awz007. [DOI] [PubMed] [Google Scholar]
- 7.Krishnamoorthy S, et al. Dopamine D3 receptor Ser9Gly variant is associated with impulse control disorders in Parkinson’s disease patients. Parkinsonism Relat. Disord. 2016;30:13–17. doi: 10.1016/j.parkreldis.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Rajan R, Krishnan S, Sarma G, Sarma SP, Kishore A. Dopamine receptor D3 rs6280 is associated with aberrant decision‐making in Parkinson’s disease. Mov. Disord. Clin. Pract. 2018;5:413–416. doi: 10.1002/mdc3.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarsland D, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 1999;67:492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroi I, et al. Apathy and impulse control disorders in Parkinson’s disease: a direct comparison. Parkinsonism Relat. Disord. 2012;18:198–203. doi: 10.1016/j.parkreldis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Scott BM, et al. Co-occurrence of apathy and impulse control disorders in Parkinson disease. Neurology. 2020;95:e2769–e2780. doi: 10.1212/WNL.0000000000010965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch. Neurol. 2010;67:58–63. doi: 10.1001/archneurol.2009.294. [DOI] [PubMed] [Google Scholar]
- 13.Santin MdesN, et al. Impact of subthalamic deep brain stimulation on impulse control disorders in parkinson’s disease: a prospective study. Mov. Disord. 2021;36:750–757. doi: 10.1002/mds.28320. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Horta S, et al. Non-demented Parkinson’s disease patients with apathy show decreased grey matter volume in key executive and reward-related nodes. Brain Imaging Behav. 2017;11:1334–1342. doi: 10.1007/s11682-016-9607-5. [DOI] [PubMed] [Google Scholar]
- 15.Santangelo G, et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinsonism Relat. Disord. 2015;21:489–493. doi: 10.1016/j.parkreldis.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Cilia R, et al. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson’s disease and pathological gambling. Neurobiol. Dis. 2010;39:98–104. doi: 10.1016/j.nbd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Voon V, et al. Impulse control disorders in Parkinson’s disease: decreased striatal dopamine transporter levels. J. Neurol., Neurosurg. Psychiatry. 2014;85:148–152. doi: 10.1136/jnnp-2013-305395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Martín P, et al. Parkinson’s disease severity levels and MDS-unified parkinson’s disease rating Scale. Parkinsonism Relat. Disord. 2015;21:50–54. doi: 10.1016/j.parkreldis.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Petitet P, et al. The relationship between apathy and impulsivity in large population samples. Sci. Rep. 2021;11:4830. doi: 10.1038/s41598-021-84364-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marín-Lahoz J, et al. Parkinson’s disease: impulsivity does not cause impulse control disorders but boosts their severity. Front. Psychiatry. 2018;9:465. doi: 10.3389/fpsyt.2018.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drew DS, et al. Dopamine and reward hypersensitivity in Parkinson’s disease with impulse control disorder. Brain. 2020;143:2502–2518. doi: 10.1093/brain/awaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinkle JT, Perepezko K, Gonzalez LL, Mills KA, Pontone GM. Apathy and anxiety in de novo Parkinson’s disease predict the severity of motor complications. Mov. Disord. Clin. Pract. 2021;8:76–84. doi: 10.1002/mdc3.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carriere N, et al. Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Mov. Disord. 2014;29:897–903. doi: 10.1002/mds.25904. [DOI] [PubMed] [Google Scholar]
- 24.Yan H, Onoda K, Yamaguchi S. Gray matter volume changes in the apathetic elderly. Front. Hum. Neurosci. 2015;9:318. doi: 10.3389/fnhum.2015.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prange S, et al. Early limbic microstructural alterations in apathy and depression in de novo Parkinson’s disease. Mov. Disord. 2019;34:1644–1654. doi: 10.1002/mds.27793. [DOI] [PubMed] [Google Scholar]
- 26.Thobois S, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010;133:1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 27.Maillet A, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain. 2016;139:2486–2502. doi: 10.1093/brain/aww162. [DOI] [PubMed] [Google Scholar]
- 28.Maillet A, et al. Serotonergic and dopaminergic lesions underlying parkinsonian neuropsychiatric signs. Mov. Disord. 2021;36:2888–2900. doi: 10.1002/mds.28722. [DOI] [PubMed] [Google Scholar]
- 29.Robert GH, et al. Preoperative factors of apathy in subthalamic stimulated Parkinson disease: a PET study. Neurology. 2014;83:1620–1626. doi: 10.1212/WNL.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 30.Banwinkler M, Theis H, Prange S, van Eimeren T. Imaging the limbic system in parkinson’s disease-a review of limbic pathology and clinical symptoms. Brain Sci. 2022;12:1248. doi: 10.3390/brainsci12091248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeanneteau F, et al. A functional variant of the dopamine D3 receptor is associated with risk and age-at-onset of essential tremor. Proc. Natl. Acad. Sci. USA. 2006;103:10753–10758. doi: 10.1073/pnas.0508189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaulieu J-M, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharm. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 33.van Eimeren T, et al. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson’s disease? Neuropsychopharmacology. 2009;34:2758–2766. doi: 10.1038/npp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbosa P, et al. Lower nucleus accumbens α-synuclein load and D3 receptor levels in Parkinson’s disease with impulsive compulsive behaviours. Brain. 2019;142:3580–3591. doi: 10.1093/brain/awz298. [DOI] [PubMed] [Google Scholar]
- 35.Prange, S. et al. Limbic serotonergic plasticity contributes to the compensation of apathy in early Parkinson’s disease. Mov. Disord.37, 1211–1221 (2022). [DOI] [PubMed]
- 36.Hall A, et al. Dopamine genetic risk score predicts impulse control behaviors in Parkinson’s disease. Clin. Park. Relat. Disord. 2021;5:100113. doi: 10.1016/j.prdoa.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelzer EA, et al. Hypomania and saccadic changes in Parkinson’s disease: influence of D2 and D3 dopaminergic signalling. NPJ Parkinsons Dis. 2020;6:5. doi: 10.1038/s41531-019-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruppert MC, et al. Network degeneration in Parkinson’s disease: multimodal imaging of nigro-striato-cortical dysfunction. Brain. 2020;143:944–959. doi: 10.1093/brain/awaa019. [DOI] [PubMed] [Google Scholar]
- 39.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Probst CC, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease (QUIP) and the QUIP-rating scale in a German speaking sample. J. Neurol. 2014;261:936–942. doi: 10.1007/s00415-014-7299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santangelo G, et al. Apathy in untreated, de novo patients with Parkinson’s disease: validation study of Apathy Evaluation Scale. J. Neurol. 2014;261:2319–2328. doi: 10.1007/s00415-014-7498-1. [DOI] [PubMed] [Google Scholar]
- 42.Tomlinson CL, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 43.García-Gómez FJ, et al. [Elaboration of the SPM template for the standardization of SPECT images with 123I-Ioflupane] Rev. Esp. Med. Nucl. Imagen Mol. 2013;32:350–356. doi: 10.1016/j.remn.2013.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity but are available from the corresponding author upon request.
No code was generated during the study.