Abstract
The role of small mammals as reservoir hosts for Borrelia burgdorferi was investigated in several areas where Lyme disease is endemic in northern Spain. A low rate of infestation by Ixodes ricinus nymphs was found in the small mammal populations studied that correlated with the near-absence of B. burgdorferi sensu lato in 184 animals tested and with the lack of transmission of B. burgdorferi sensu lato to I. ricinus larvae that fed on them. In contrast, questing ticks collected at the same time and in the same areas were found to carry a highly variable B. burgdorferi sensu lato repertoire (B. burgdorferi sensu stricto, Borrelia garinii, Borrelia valaisiana, and Borrelia afzelii). Interestingly, the only isolate obtained from small mammals (R57, isolated from a bank vole) grouped by phylogenetic analyses with other Borrelia species but in a separate clade from the Lyme disease and relapsing fever organisms, suggesting that it is a new species. This new agent was widely distributed among small mammals, with infection rates of 8.5 to 12% by PCR. Moreover, a high seroprevalence to B. burgdorferi sensu lato was found in the animal sera, suggesting cross-reactivity between B. burgdorferi sensu lato and R57. Although small mammals do not seem to play an important role as reservoirs for B. burgdorferi sensu lato in the study area, they seem to be implicated in the maintenance of spirochetes similar to R57.
Lyme disease (LD) is a multisystemic zoonotic disorder caused by Borrelia burgdorferi sensu lato and transmitted by hard ticks (family Ixodidae) (11, 15, 42). There are currently 11 different genospecies of B. burgdorferi sensu lato (55, 81). In Europe, the major vector of B. burgdorferi sensu lato is the tick Ixodes ricinus and five genospecies, B. burgdorferi sensu stricto (42), Borrelia garinii (6), Borrelia afzelii (16), Borrelia valaisiana (80) and Borrelia lusitaniae (51), are present in this continent. The first three produce disease in humans (81), and B. valaisiana and B. lusitaniae have shown to infect laboratory mice (18, 23, 82). Moreover, B. lusitaniae has been isolated recently from a skin biopsy of a patient with a chronic skin lesion (18). In different European studies, small mammals (rodents and shrews) are the most important reservoir hosts for the Lyme disease agent (21, 30, 41, 48, 58, 59, 78), but birds can also play this role (39, 46, 61, 68).
The genus Borrelia has been classified using 16S rRNA and flaB (27, 53, 64) into two major groups: the LD and the relapsing fever (RF) groups. The latter group includes the species responsible for human RF in America (Borrelia hermsii, Borrelia parkeri, and Borrelia turicatae), and in southern Europe and Africa (Borrelia duttonii, Borrelia hispanica, and Borrelia crocidurae). The main vectors of the species of the RF group are the soft ticks (family Argasidae), while the human body louse transmits Borrelia recurrentis (77). However, new species of Borrelia transmitted by hard ticks (family Ixodidae) have been classified closer to the RF group: “Borrelia lonestari” (proposed name), transmitted in the United States by Amblyomma americanum (8) and Borrelia miyamotoi, transmitted in Asia by Ixodes persulcatus (28). This latter genospecies seems to have a larger distribution area, since related species have been found in Europe in I. ricinus (26, 67) and in America in Ixodes scapularis (74). The number of species of the RF group is increasing, as new species have been identified in Hyalomma aegyptium feeding in tortoises in Turkey (34) and in patients and soft ticks in Tanzania (44).
In southern Spain, a new Borrelia species has been isolated from patients and soft ticks (3) in areas where RF is endemic (5, 14, 72). Moreover, in the north of Spain there are areas where LD is endemic that coincide with the distribution of I. ricinus (19). In these areas, several series of LD cases have been described (2, 33, 63), and epidemiological studies of B. burgdorferi sensu lato in questing ticks (9), in ticks collected from animals (24), and in ticks collected from humans (25) have been performed. Since the first isolation of B. burgdorferi sensu lato in Spain (29), only a few isolates have been obtained (9, 62) and their characterization has shown a wide genospecies diversity and virulence in a mouse model (23). In the Basque country, our study region, cases of Lyme disease in humans have been reported; a serological survey showed 25% prevalence in outdoors workers, with antecedents compatible with LD in 15% of those who were seropositive (4). Moreover, our previous data confirmed the wide distribution of the vector I. ricinus and B. burgdorferi sensu lato in several areas of the Basque country (9).
This study considers the biological cycle of B. burgdorferi sensu lato in previously identified areas where of Lyme disease endemicity in the Basque country (9), with a special interest in the role of the small mammals as reservoir hosts for B. burgdorferi sensu lato, showing that they do not play an important role in our area. However, a new spirochete has been identified and is prevalent in our small mammals. The role of organisms similar to this new spirochete in the ecology of B. burgdorferi sensu lato is discussed.
MATERIALS AND METHODS
Small mammal and tick sampling.
Small mammals were captured between October 1998 and September 2000 in six different areas of the Basque country, where B. burgdorferi sensu lato was previously detected in I. ricinus (9). The features and localization of the study areas have been previously described (9). Fifty Sherman traps (Sherman Traps, Tallahasee, Fla.) and 150 INRA traps (BTS Mecanique, Besançon, France) were placed overnight, and trapped animals were brought to the laboratory for tick collection and classification (31, 54, 60). Questing ticks were also collected (by flagging in the same places where the traps were placed) and classified (31, 54, 60).
Processing of small mammals.
Live animals were maintained in the laboratory for 24 to 72 h to complete the repletion of the ticks that were feeding naturally. The engorged ticks obtained were kept at 18°C, 98% humidity, and a 12-h light cycle until molted.
Animals were anesthetized with ketamine hydrochloride (Imalgene; Merial) at a dose of 10 mg/kg intramuscularly and euthanized in a CO2 chamber. Samples from different tissues were collected (ear, urinary bladder, spleen, liver, brain, kidney, heart, mesenteric and popliteal ganglia, and blood) for culture and PCR. The animals were classified by external morphological data and skull features (1, 12).
Isolation of B. burgdorferi sensu lato
Organs were cultured in 4 ml of BSK (Barbour-Stoener-Kelly) II medium prepared as previously described (7), supplemented with 6% of rabbit serum (BSK-RS) (Sigma-Aldrich Quimica S.A., Alcobendas, Madrid, Spain), 50 μg of rifampin/ml, 50 μg of phosphomycin/ml, and 2.5 μg of amphotericin B/ml (52, 76). Before the preparation of the BSK medium, five batches of bovine serum albumin (Sigma) were tested to judge the medium's performance with low-passage autochthonous strains; the best was used to make 12-liter batches of medium. Urinary bladder and ear punch biopsies (EPB) were cultured individually for comparison with previous studies (23, 73, 76). For this, EPB were first disinfected by successive immersion in iodine, 70% ethanol, and phosphate-buffered saline (PBS), and both bladder samples and EPB were ground in BSK. Half of the homogenates were used for culture. The rest of the organs were homogenized individually in 100 μl of BSK-RS, and a 30-μl aliquot was pooled and inoculated in the same medium with antibiotics.
In some experiments, rabbit serum was replaced by sera from different animal species (horse and fetal calf sera from Biological Industries, Beit Haemek, Israel; and mouse serum from Centro Nacional de Microbiología, Majadahonda, Spain) to improve the culture of fastidiously growing isolates.
For the isolation of B. burgdorferi sensu lato from ticks, the specimens were disinfected by successive immersion in 95, 70, and 35% ethanol and PBS and homogenized in 100 μl of BSK-RS. A 30-μl aliquot was inoculated in the same medium supplemented with antibiotics. Ticks that molted under the conditions described above were cultured in pools of up to 10 of the same species, stage, and animal in which they were feeding. Ticks collected from the vegetation were cultured in pools of up to 30 nymphs or three adults.
Cultures were maintained at 33°C for 3 months and examined by dark-field microscopy to monitor the presence of spirochetes. After 3 months, the cultures were centrifuged for 5 min at 7,000 × g, and the sediments were washed twice with sterile PBS and reserved for PCR.
Animal model.
The LD animal model with C3H mice (10) was used for the recovery of isolates that grew poorly in BSK-RS medium, as previously described (23). Briefly, 3- to 4-week-old mice were inoculated intradermally with 105 spirochetes. At 2 and 4 weeks postinoculation, an EPB was cultured and subjected to PCR. Thirty days after inoculation, mice were euthanized, and internal organs and blood were processed as described above. A blood sample was taken daily from the tail vein during the first 2 weeks and examined by dark-field microscopy as previously described (3). All animals were processed according to protocols approved by the accredited animal care and use committees at Instituto de Salud Carlos III, following international regulations.
PCR analysis.
DNA from the culture sediments and mammal organs was extracted by the guanidine thiocyanate method (17). A negative control for the extraction (distilled water) was included with every 10 samples. DNA concentration was determined for each sample by spectrophotometry; 100 to 300 ng was used in the PCR. An initial screening of the samples was done by a Borrelia generic PCR targeting 16S rRNA (16S-PCR) with the oligonucleotides BORF and 16S3B (Table 1). Positive samples were analyzed by a second PCR targeting the 5S (rrf)-23S (rrl) intergenic spacer (5S-23S-PCR) specific for B. burgdorferi sensu lato, with primers previously described (Table 1) (69). PCRs were performed in a 30-μl volume with 10 mM Tris-HCl, 50 mM KCl, 1.67 mM MgCl2, 333 μM each deoxynucleoside triphosphate (Promega, Madison, Wis.), and 2.15 U of Taq DNA polymerase (Applied Biosystems, Branchburg, N.J.). Primers were used at a concentration of 30 pmol/reaction mixture for 16S-PCR and at 5 pmol/reaction mixture for 5S-23S-PCR. Cycling conditions for the 16S-PCR were 3 min at 94°C; 50 cycles each of 10 s at 94°C, 1 min at 45°C, and 1.5 min at 72°C; and a final elongation of 7 min at 72°C. For 5S-23S-PCR, the cycles consisted of 1 min at 94°C; 50 cycles each of 30 s at 94°C, 30 s at 54°C, and 1 min at 72°C; and a final elongation at 72°C for 5 min. All the amplifications were done with a PTC-200 thermocycler (MJ Research, Waltham, Mass.). DNA from Borrelia japonica (strain HO14T) was used as a positive control, and one negative PCR control (distilled water) was included with every 10 PCRs. Serial dilutions of DNA of B. garinii (PBi strain) were tested to check the sensitivity of the 16S-PCR.
TABLE 1.
Primers and probes used in this study
| Primer or probe | Sequence | Gene(s) | Source or reference |
|---|---|---|---|
| Primers | |||
| BORF | 5′-CGC TGG CAG TGC GTC TTA A | rrs | This study |
| 16S3B | 5′-biotin-GCG GCT GCT GGC ACG TAA TTA GC | rrs | 3 |
| 16S1 | 5′-CGA AGA GTT TGA TCC TGG CTTAG | rrs | 3 |
| 16S2 | 5′-GCT AAT TAC GTG CCA GCA GCC GC | rrs | 3 |
| 16S5 | 5′-GTG CGG GCC CCC GTC AAT TCC | rrs | 3 |
| 16S7 | 5′-CCA TGA TGA TTT GAC GTC ATC | rrs | 3 |
| 16S9 | 5′-CCT TGT TAC GAC TTC ACC CC | rrs | 3 |
| 23SN2 | 5′-ACC ATA GAC TCT TAT TAC TTT GAC CA | rrf-rrl | 69 |
| 5SCB | 5′-biotin-GAG AGT AGG TTA TTG CCA GGG | rrf-rrl | 69 |
| N1 | 5′-GAG CTT AAA GGA ACT TCT GAT AA | ospA | 35 |
| C1 | 5′-GTA TTG TTG TAC TGT AAT TGT | ospA | 35 |
| N2 | 5′-ATG GAT CTG GAG TAC TTG AA | ospA | 35 |
| C2 | 5′-CTT AAA GTA ACA GTT CCT TCT | ospA | 35 |
| FlaBorF1 | 5′-GCW TCT GAT GAT GCT GCT GG | flaB | This study |
| FlaBorF2 | 5′-GCA AMY CAR GAY GAR GCD ATT GC | flaB | This study |
| FlaBorR1 | 5′-GCY ACA AYH TCA TCK GTC | flaB | This study |
| FlaBorR2 | 5′-GCA ATH GCY TCR TCY TGR KTT GC | flaB | This study |
| C | 5′-GCA GTT CAA TCA GGT AAC GG | flaB | 27 |
| D | 5′-AGG TTT TCA ATA GCA TAC TC | flaB | 27 |
| 66F1 | 5′-GAA KTA GGC AAR GAY GAY CC | p66 | This study |
| 66F2 | 5′-GAR GRA CAA TTC TTG CAA GAG G | p66 | This study |
| 66F3 | 5′-CCA ACT TTA TCA AAT KCW KC | p66 | This study |
| 66F4 | 5′-GGT GAA AAA GAA TCH TGG | p66 | This study |
| 66R1 | 5′-TTT GAR TCC CAK CCA AG | p66 | This study |
| 66R2 | 5′-CCA DGA TTC TTT TTC ACC | p66 | This study |
| 66R3 | 5′-GMW GMA TTT GAT AAA GTT GG | p66 | This study |
| 66R4 | 5′-CCT CTT GCA AGA ATT GTY CYT C | p66 | This study |
| 1730F | 5′-CTT GGI CCI GGI GGA CTT TC | rpoB | 65 |
| 2900R | 5′-AGA AAT IAA IAT IGC ATC CTC | rpoB | 65 |
| GroEL-F | 5′-TAC GAT TTC TTA TGT TGA GGG | groEL | This study |
| GroEL-R | 5′-CGY CTA TCA CCA AAA CCR GGM G | groEL | This study |
| Probesa | |||
| 16S | 5′-a-GAG GAA TAA GCT TTG TAG GAA ATG ACA Ab | rrs | This study |
| R57 | 5′-a-AGT CAT TAA AGA TGT TTA ATG | rrs | This study |
| SL | 5′-a-CTT TGA CCA TAT TTT TAT CTT CCA | rrf-rrl | 69 |
| SS | 5′-a-AAC ACC AAT ATT TAA AAA ACA TAA | rrf-rrl | 69 |
| GA | 5′-a-AAC ATG AAC ATC TAA AAA CAT AAA | rrf-rrl | 69 |
| AF | 5′-a-AAC ATT TAA AAA ATA AAT TCA AGG | rrf-rrl | 69 |
| VA | 5′-a-CAT TAA AAA AAT ATA AAA AAT AAA TTT AAG G | rrf-rrl | 69 |
16S, generic probe to 16S rRNA of Borrelia spp. R57, specific probe for 16S rRNA of spirochetes similar to R57; SL, generic probe for 5S-23S rRNA of B. burgdorferi sensu lato, SS, specific probe for B. burgdorferi sensu stricto; GA, specific probe for B. garinii; AF, specific probe for B. afzelii; VA, specific probe for B. valaisiana.
a, aminolink modification of the oligonucleotides in 5′.
For further characterization of unusual isolates, several PCRs with primers based in ospA (35), flagellin (flaB) (reference 27 and this study), rpoB (65), and p66 and groEL (this study; Table 1) were performed. DNA from B. burgdorferi sensu stricto (strain B31T) and B. hermsii (strain HS1T) were used as positive PCR controls.
Reverse line blotting (RLB).
Samples amplified by 16S-PCR were subjected to RLB with probes designed in this study (Table 1). The positive samples obtained were analyzed by 5S-23S-PCR/RLB with probes described previously (69). The RLB method was performed as previously described (69), with few modifications. In brief, 56 pmol of each probe was attached to the membrane by a 10-min incubation, and the hybridization of the PCR products was performed at 48°C for 1 h.
Western blotting (WB).
Blood samples from mice were taken by intracardiac puncture. Serum samples were analyzed by WB as previously described (75) with strain Esp1 of B. burgdorferi sensu stricto (29) and strain Rio4 of B. garinii (23) as antigens, based on previous data about the frequency of these two genospecies in the study areas (9, 23). Sera were tested at a 1:50 dilution. For each strip, the grade of reactivity of p93, p58, p56, p41, p30, OspC, p21, p19, and p17 was recorded (22), and a value was assigned to each band depending on the intensity (strong reactivity, 1; medium reactivity, 0.5; weak reactivity, 0.25; no reactivity, 0). A serum was considered positive when the sum of the values for each of the selected proteins was ≥4, inconclusive when the values were between 4 and 3, and negative when the values were <3.
Sequencing and analysis.
PCR products were run in 0.8% low-melt agarose gels (Roche Diagnostics GmbH, Mannheim, Germany), and the bands of interest were purified by using the QIAquick gel extraction kit (QIAGEN GmbH, Hilden, Germany) and sequenced with the Big-Dye Terminator Cycle Sequencing kit (Applied Biosystems) on an ABI 377 DNA sequencer.
16S rRNA sequences were obtained by amplifying overlapping fragments with primers 16S1 to 16S9 (3) and groEL was obtained with primers designed in this study (Table 1). Sequences generated in this study were aligned with sequences from databases (Table 2) by using ClustalX (79). Pairwise distance matrices for the aligned sequences were determined by the Kimura two-parameter method (43) with MEGA2 software (45) and phylogenetic trees constructed by neighbor joining (71) with bootstrap analysis with 1,000 replications for evaluation of their topology (13).
TABLE 2.
Different bacteria from the order Spirochaetales used in phylogenetic analyses
| Species | Strain | Source | Country of origin | GenBank accession no.
|
|
|---|---|---|---|---|---|
| 16S rRNA | groEL | ||||
| B. afzelii | DK4 | Erythema migrans; skin | Denmark | X85194 | |
| VS461T | I. ricinus | Switzerland | AF517954 | ||
| Pko-85 | Erythema migrans; skin | Germany | AF517956 | ||
| B. andersonii | 21038T | Ixodes dentatus | United States | L46701 | |
| 21123 | I. dentatus | United States | AF517975 | ||
| B. anserina | ES-1 | Argas persicus | Unknown | U42284 | |
| B. bissettii | DN127T | I. pacificus | United States | AJ224141 | AF517974 |
| B. burgdorferi sensu stricto | B31T | I. dammini | United States | U03396 | |
| Sh-2-82 | I. dammini | United States | AF517948 | ||
| 20004 | I. ricinus | France | AF517951 | ||
| B. coriaceae | Co53T | O. coriaceus | United States | U42286 | |
| B. crocidurae | UESV/523SIS | Blood of patient | Mali | U42301 | |
| B. duttonii | UESV/117DUTT | Ornithodoros moubata | Zaire | U42288 | |
| B. garinii | PBi | CSFa of patient | Germany | X85199 | |
| G25 | I. ricinus | Sweden | AF517962 | ||
| Sika2 | I. persulcatus | Japan | AF517964 | ||
| B. hermsii | HS1T | O. hermsi | United States | U42292 | AF518000 |
| B. hispanica | UESV/246 | O. erraticus | Morocco | U42294 | |
| B. japonica | HO14T | I. ovatus | Japan | L46696 | AF517970 |
| B. lonestari | Texas 20 | A. americanum | United States | U23211 | |
| 010298 | Unknown | United States | AY552786 | ||
| B. lusitaniae | PotiB2T | I. ricinus | Portugal | X98228 | AF517971 |
| B. miyamotoi | HT31T | I. persulcatus | Japan | D45192 | |
| B. parkeri | M3001 | O. parkeri | United States | U42296 | |
| B. persica | UESV/340 | O. tholozani | Iran | U42297 | |
| B. recurrentis | A1 | Blood of patient | Ethiopia | U42300 | |
| B. sinica | CNM3T | Niviventer confucianus | China | AB022101 | |
| B. tanukii | Hk501T | I. tanuki | Japan | D67023 | AF517973 |
| B. turdi | Ac502 | I. turdus | Japan | D67024 | |
| Ya501T | I. turdus | Japan | AF517972 | ||
| B. turicatae | M2007 | O. turicata | United States | U42299 | |
| B. valaisiana | CKA4a | Apodemus agrarius | China | AB022143 | |
| VS116T | I. ricinus | Switzerland | AF517976 | ||
| Borrelia sp. | HN7 | I. granulatus | Korea | AF517987 | |
| Borrelia sp. | Antequera | Blood of patient and O. erraticus | Spain | U28502 | |
| Borrelia sp. | R57 | C. glareolus | Spain | AY626138 | AY707888 |
| Borrelia sp. | TXW-1 | Dermacentor variabilis | United States | AF467976 | |
| Brachyspira hyodysenteriae | B204 | Pig feces | Unknown | M57741 | |
| Brevinema andersonii | L31544 | Blarina brevicauda | United States | MV116 | |
| Cristispira sp. | CP1 | Oyster | United States | U42638 | |
| Leptonema illini | 3055 | Unknown | Unknown | M88719 | |
| Leptospira interrogans serovar canicola | Moulton | Blood of dog | Unknown | X17547 | |
| L. interrogans serovar Lai | 56601 | Unknown | Unknown | NC_004342 | |
| Spirochaeta aurantia | J1 | Pond mud | Unknown | M57740 | |
| Treponema denticola | ATCC 35405 | Human periodontal pocket | Canada | NC_002967 | |
| Treponema pallidum | Nichols | Patient | Unknown | M88726 | NC_000919 |
CSF, cerebrospinal fluid.
Nucleotide sequence accession numbers.
The 16S rRNA and groEL sequences from R57 have been deposited in GenBank with accession numbers AY626138 and AY707888, respectively.
RESULTS
The ratio of infestation of I. ricinus nymphs to larvae was low in small mammals.
A total of 184 animals were captured, belonging to seven different species (Table 3). The most frequently found species in all areas was the wood mouse, Apodemus sylvaticus (Table 3). A total of 901 I. ricinus larvae and only two nymphs were collected from the small mammals. No adults were found feeding on them. Other tick species (Dermacentor reticulatus, Haemaphysalis inermis, and Ixodes trianguliceps) were collected infrequently. The ratio of infestation of I. ricinus nymphs to larvae in the small mammals was 1:450.
TABLE 3.
Small mammals captured and PCR/RLB results
| Species | No. (%) of animals captureda | No. (%) positive by PCR/RLBb
|
||
|---|---|---|---|---|
| 16S generic | 16S specific R57 | 5S-23S rRNA | ||
| A. sylvaticus (wood mouse) | 130 (70.7) | 16 (12.3) | 10 (7.7) | 1 (0.8) |
| C. glareolus (bank vole) | 18 (9.8) | 4 (22.2) | 4 (22.2) | |
| Sorex coronatus (millet shrew) | 15 (8.2) | 1 (6.7) | ||
| Apodemus flavicollis (yellow-necked mouse) | 9 (4.9) | 1 (11.1) | ||
| Crocidura russula (common shrew) | 9 (4.9) | 1 (11.1) | 1 (11.1) | |
| Microtus lusitanicus (Lusitanian pine vole) | 2 (1.1) | |||
| Sorex minutus (pygmy shrew) | 1 (0.5) | 0 (0.00) | ||
| Total | 184 | 23 (12.5) | 15 (8.2) | 1 (0.5) |
Values are percentages of captured animals from the total number of captured animals.
Values are percentages of positive animals from the total number of each animal species.
Borrelia spp. were widespread in tissues of small mammals.
Animal samples were analyzed by a generic 16S-PCR/RLB designed in this study for the detection of Borrelia spp. (Table 1; Fig. 1A). This method was able to detect 8.5 fg (eight spirochetes) and was used for initial screening. Cultures from 108 animals, as well as tissue samples from 76 specimens found dead, were analyzed. Twenty-three (12.5%) of the 184 animals were positive by this technique (Table 3; Fig. 1B). EPB was the positive tissue in 20 animals; the other three positive tissues were two brain samples and one urinary bladder sample. Five of the seven species of small mammals captured yielded positive results (Table 3).
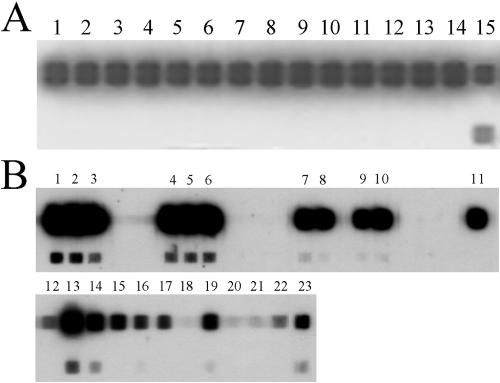
FIG. 1.
(A) RLB based on the 16S rRNA of different species of the genus Borrelia and R57. Specific probes for the genus Borrelia and R57 were used in this assay. Lanes: 1, B. japonica; 2, B. burgdorferi sensu stricto; 3, B. garinii; 4, B. afzelii; 5, B. valaisiana; 6, B. lusitaniae; 7, Spanish relapsing fever spirochete; 8, B. turicatae; 9, B. duttonii; 10, Borrelia anserina; 11, B. hermsii; 12, B. crocidurae; 13, B. recurrentis; 14, B. hispanica; 15, R57. (B) Hybridization with samples from small mammal tissues. The upper spots in each panel correspond to the 16S genetic probe for Borrelia spp.; the lower spots correspond to the 16S-specific probe for R57 spirochetes.
The B. burgdorferi sensu lato genospecies composition of the PCR-positive samples was determined by 5S-23S-PCR/RLB. From the 23 samples of small mammals that were positive by the generic 16S-PCR/RLB, only one organ sample, a brain from a wood mouse (A. sylvaticus) (Fig. 1B, line 11), was positive by this second method, and was identified as B. burgdorferi sensu stricto (Table 3). Consequently, the prevalence of the infection for B. burgdorferi sensu lato in small mammals was 0.5% (1 of 184 mammals) and 0.8% (1 of 130 mammals), when only the A. sylvaticus specimens studied were considered (Table 3).
B. burgdorferi sensu lato was isolated and detected from questing ticks but not from ticks feeding on small mammals.
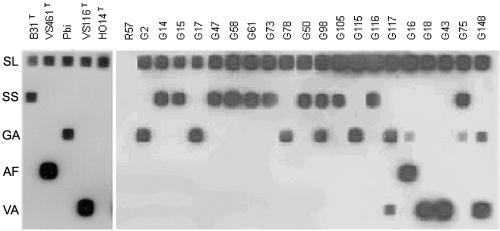
I. ricinus was the most abundant questing species found in all the areas studied. A total of 233 adults and 2,540 nymphs of this species were collected from vegetation; approximately half (98 adults in 36 pools and 1,298 nymphs in 49 pools) were cultured in BSK-RS with antibiotics. Twenty isolates from four different genospecies were obtained (Fig. 2). In addition, all the cultures were analyzed by 16S-PCR/RLB; eight additional positive samples were found (Table 4). Afterward, all positive samples from questing ticks cultures were analyzed by 5S-23S-PCR/RLB to determine the genospecies presented. B. burgdorferi sensu stricto was the most frequent genospecies identified (14 pools), followed by B. valaisiana (10 pools) and B. garinii (9 pools). B. afzelii was detected in only one pool (Table 4). In six of the nymph pools, mixed infections were detected: one pool with B. afzelii and B. garinii, one pool with B. burgdorferi sensu stricto and B. valaisiana, two pools with B. garinii and B. valaisiana, and two pools with B. garinii and B. burgdorferi sensu stricto.
FIG. 2.
5S-23S-PCR/RLB of the different isolates obtained in this study. The left panel shows the positive controls belonging to the different genospecies; the right panel shows the results with different samples. The name of the probes used is shown on the left. SL, generic probe for B. burgdorferi sensu lato; SS, specific probe for B. burgdorferi sensu stricto; GA, specific probe for B. garinii; AF, specific probe for B. afzelii; VA, specific probe for B. valaisiana. The names of the strains used as controls and the names of the isolates obtained in this study are on the top of the figure.
TABLE 4.
PCR/RLB analysis of the cultures of questing ticksa
| Stage | No.
|
16S PCR/RLBb
|
5S-23S PCR/RLBb
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ticks | Pools | 16S | R57 | SL | SS | GA | AF | VA | |
| Nymph | 1,298 | 49 | 1.85% (24) | 0.00% | 1.85% (24) | 0.93% (12) | 0.61% (8) | 0.08% (1) | 0.61% (8) |
| Adult | 98 | 36 | 4.08% (4) | 0.00% | 4.08% (4) | 2.04% (2) | 2.04% (2) | ||
16S, generic probe for 16S rRNA of Borrelia sp.; R57, specific probe for 16S rRNA of spirochetes similar to R57; SL, generic probe for 5S-23S rRNA of B. burgdorferi sensu lato; SS, specific probe for B. burgdorferi sensu stricto; GA, specific probe for B. garinii; AF, specific probe for B. afzelii; VA, specific probe for B. valaisiana.
Percentages indicate the minimum infection, considering one positive tick per pool. Values in parentheses are the number of positive pools per number of ticks processed (1,298 nymphs or 98 adults).
Engorged ticks, which were feeding naturally on the captured mammals, were allowed to molt and cultured to test for the potential of the small mammals in the transmission of B. burgdorferi sensu lato. A total of 284 molted ticks (275 I. ricinus larvae, seven H. inermis larvae, one I. ricinus nymph, and one D. reticulatus nymph) belonging to 48 small mammals were processed in 96 cultures; no spirochetes were isolated.
In addition, all these cultures were analyzed by 16S-PCR/RLB to detect the presence of Borrelia spp.; all of them were negative.
A new organism was isolated from a bank vole.
Necropsy material from 108 small mammals was cultured, and one isolate (R57) was obtained from an EPB of a bank vole (Clethrionomys glareolus) that showed the typical Borrelia morphology and movement by dark-field microscopy. The organism grew well during the first passage, but subsequent attempts to grow it in culture were unsuccessful. Attempts to recover viable organisms from infected C3H mice were unsuccessful; no spirochetemia was detected by dark-field visualization of blood smears.
The whole 16S rRNA gene from R57 (1,440 bp) was sequenced by using overlapping primers (Table 1). Also, a fragment of groEL (243 bp) was amplified and sequenced with degenerate primers (Table 1), based on aligned sequences of other members of the order Spirochaetales (Table 2). However, attempts to amplify 5S-23S-PCR/RLB (Fig. 2) and ospA, flaB, rpoB, and p66 from R57 were unsuccessful, while B. burgdorferi sensu stricto and B. hermsii yielded amplicons of the expected size (data not shown).
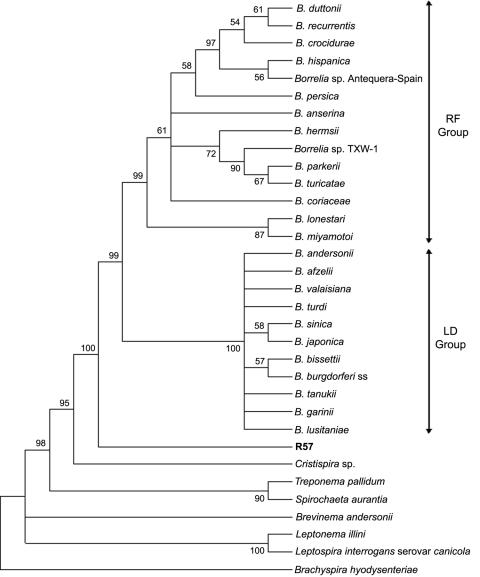
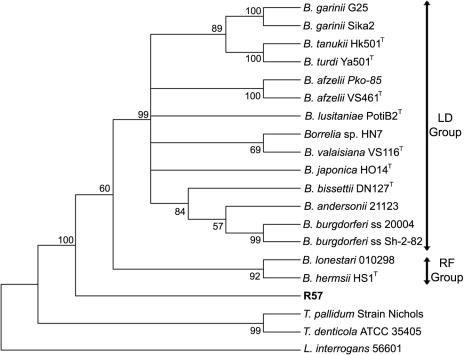
16S rRNA and groEL sequences were used to determine the taxonomic position of this isolate. Sequences from representative species of the order Spirochaetales (Table 2), including members of the B. burgdorferi sensu lato and RF groups, were used to build trees (Fig. 3 and 4). The relative position of R57 was stable in both trees and grouped this organism closer to the genus Borrelia but in a clade different from that of the other Borrelia species tested.
FIG. 3.
Neighbor-joining phylogenetic tree based in 16S rRNA sequences of the different species of the order Spirochetales and R57. ss, sensu stricto.
FIG. 4.
Neighbor-joining phylogenetic tree based in groEL sequences of the different species of the order Spirochetales and R57. ss, sensu stricto.
Spirochetes similar to R57 are widely distributed among small mammals.
A 16S probe specific for R57 was designed to investigate the presence of this organism in the samples (Fig. 1A). When the 22 positive samples from small mammals that were negative by 5S-23S-PCR/RLB were tested, 15 samples (all of them EPB) were positive (Fig. 1B). In six of them, we were able to sequence the 16S-PCR product (491 bp), which was identical to the R57 isolate. The seven remaining samples showed a weak hybridization signal with the generic Borrelia probe and a negative result for the R57 probe (Fig. 1B). The hybridization efficiency of the R57 probe was lower than that of the generic probe, as seen by the weaker signal (Fig. 1A). Considering this, all samples positive by 16S-PCR/RLB and negative by 5S-23S-PCR, from which we were unable to amplify a 16S fragment visible in agarose gels, were considered probable R57 homologs (7 of 184 samples). In summary, the prevalence of the infection by spirochetes similar to R57 among the small mammals ranged from 8.5% (15 of 184 samples) to 12% (22 of 184 samples).
In contrast, spirochetes similar to R57 were not detected in ticks. All of the 96 cultures of the molted ticks that fed on the captured mammals were negative by 16S-PCR/RLB, even the ones that fed on positive animals. Moreover, samples from questing tick cultures were also negative for the R57 probe (Table 4).
A high seroreactivity to B. burgdorferi sensu lato was found among the small mammals.
Sera samples from 80 animals were analyzed by WB. The antibody response detected in the animals was highly variable; in 8.75% (seven animals), reactivity against OspA was observed. In summary, the seroprevalence found was 13.75% (11 of 80 animals), and with 25% of the animals studied (20 of 80 animals), the results were inconclusive.
DISCUSSION
The aim of this study was to identify potential reservoir hosts for B. burgdorferi sensu lato in areas previously described as being endemic for LD in northern Spain (9). Small mammals have been described as important reservoir hosts for B. burgdorferi sensu lato (21, 30, 41, 48, 58, 59, 78). However, in this study only one isolate (R57) was obtained from a C. glareolus EPB among the 108 animals processed by culture. This isolate had the same morphology by dark-field microscopy as other B. burgdorferi sensu lato species, and phylogenetic analyses based on 16S rRNA and groEL placed it in a clade separate from that of LD and RF spirochetes. The position of R57 in both trees was identical, as an ancestor of both LD and RF Borrelia. Moreover, PCRs for 5S-23S rRNA, ospA, flaB, rpoB, and p66 yielded negative results, suggesting abundant sequence differences between this agent and other Borrelia species.
Although the isolate grew well during the first passage, attempts for subculturing the organism with the same batch of medium were unsuccessful, as the spirochetes remained viable for about 5 days but without signs of multiplication. Changing the source for the serum in the BSK formulation did not improve the culture of R57. This is not surprising, considering that several Borrelia species are noncultivable, such as the relapsing fever Borrelia isolated from patients and Ornithodoros erraticus in southern Spain (3). Also, attempts to recover the agent by passages through C3H mice were done. For this, internal organs, EPB, and blood were cultured at different times, always with negative results by both culture and PCR. Also, spirochetemia was not detected in blood smears.
A probe for the 16S-PCR/RLB specific for this new spirochete (R57) was designed in this study and confirmed the presence of spirochetes similar to R57 in all the study areas and in three of the seven species of small mammals studied. Interestingly, this new agent was highly prevalent among the small mammals (8.5 to 12%), in the absence of B. burgdorferi sensu lato.
All the 16S rRNA sequences obtained from samples positive to the R57 probe were identical. Although the samples belong to different areas, all of them are geographically close to each other. The 16S rRNA sequence identity observed in each R57-positive animal from every region would indicate that organisms similar to R57 have a highly conserved 16S rRNA sequence in the 491-bp fragments analyzed.
All the species of Borrelia are transmitted by different species of ticks, with the exception of B. recurrentis, which is transmitted by the human body louse (77). Organisms similar to R57 do not seem to be transmitted by hard ticks (family Ixodidae) or at least not by I. ricinus. Spirochetes similar to R57 were not detected in questing ticks from the same areas where R57 was prevalent in small mammals. Moreover, the 284 molted ticks analyzed, which previously fed on the small mammals, were negative by culture and PCR, even those that fed on positive animals. Also, R57 is not species specific for small mammals, since different animal species were found infected. Taking into account that a wide repertoire of ectoparasites other than ticks were found infesting the small mammals studied, including fleas, chiggers, mites, and lice (data not shown), their possible role in the transmission of this new agent should be investigated. The method for detection of R57 developed in this study will enable us to complete the study of the ecology of this agent.
Small mammals have been described as important B. burgdorferi sensu lato reservoir hosts in Europe (30), but in contrast to the situation described for other European studies (21, 41, 48, 58, 59, 78), small mammals of different species were found to be infected just occasionally by B. burgdorferi sensu lato in this study. The prevalence of the infection by B. burgdorferi sensu lato was very low (0.5%; 1 of 184), even considering prevalence only in A. sylvaticus (0.8%; 1 of 130). Prevalence was also lower than the data obtained in other studies: 30 to 40% in Germany (47, 58), 25% in the United Kingdom (49), 17.5% in Switzerland (40), 47 to 0% in The Netherlands (21), or 60% of mice and 23% of shrews in Sweden (78). Moreover, no transmission of B. burgdorferi sensu lato from small mammals to ticks that were feeding on them was demonstrated, which confirmed that A. sylvaticus at least is not acting as an important reservoir for LD Borrelia species in our area. This could be explained by the fact that I. ricinus nymphs scarcely infested them. A similar situation has been described in Ireland, where small mammals do not play an important role as reservoir hosts of B. burgdorferi sensu lato (32). The major reservoir hosts for B. burgdorferi sensu lato in the Basque country are still to be identified. The suitable candidates, with demonstrated roles as reservoirs elsewhere, are birds (39, 46, 68), dormice (56, 57), and squirrels (20, 38).
Four different genospecies have been found infecting questing ticks, the most abundant being B. burgdorferi sensu stricto, followed by B. valaisiana and B. garinii. B. afzelii was infrequently founding infecting questing ticks. This is different from other European studies (37, 70), especially those from Central Europe, where B. afzelii is highly prevalent. B. afzelii seems to be maintained in nature mainly by a tick-small mammal cycle that does not include the involvement of birds (36, 40, 66). The fact that small mammals are not playing a relevant role as reservoir hosts for B. burgdorferi sensu lato in the study areas may explain the low prevalence of B. afzelii found, similar to that in Ireland (32), where B. valaisiana was highly prevalent and birds seemed to play a relevant role as reservoir hosts for this genospecies (39, 50). B. valaisiana was also prevalent in our region, suggesting that birds may also play a role in maintaining this genospecies in our area.
Given the high prevalence of antibodies to B. burgdorferi sensu lato detected by WB in animals and the infrequent contact between these animals and B. burgdorferi sensu lato, we suggest that this seroprevalence is related to a cross-reactivity of R57 with B. burgdorferi sensu lato. An organism close to Borrelia, like R57, could induce cross-reacting antibodies, which would explain this high level of seroreactivity against B. burgdorferi sensu lato. Whether these antibodies play an immunoprophylactic role that could additionally account for the lack of circulation of LD Borrelia in small mammals from the Basque country deserves further study. A previous study in the same region showed a high level of seroprevalence against B. burgdorferi sensu lato in humans with a low prevalence of LD among the individuals investigated (4). This situation matches our data from the small mammals; spirochetes similar to R57 could be responsible for part of the high seroreactivity found among the population of the Basque country.
In conclusion, we have shown that although small mammals are not important reservoir hosts for B. burgdorferi sensu lato in areas of LD endemicity in the Basque country, they are maintaining a new spirochete species. This new species is widely distributed among small mammals within the study area; produces a disseminated infection that involves the skin, brain, and urinary bladder; and is thought to induce antibodies that cross-react with B. burgdorferi sensu lato. Signature nucleotides in the 16S rRNA sequence enabled us to design a diagnostic method that will be useful in determining the actual distribution of this agent in the area.
Acknowledgments
This work was supported by the Network on Tick-Borne Diseases (Red Temática de Investigación Cooperativa EBATRAG [G03/057]), from the Fondo de Investigación Sanitaria (FIS), Instituto de Salud Carlos III. H.G. was the recipient of fellowships from the Departamento de Agricultura y Pesca del Gobierno Vasco and from the Instituto de Salud Carlos III.
We thank J. L. Benach for his valuable comments and P. Delattre for the suggestions in the capture of small mammals. We also thank Escolástica Chaparro Tercero, Isabel Rodríguez Moreno, Manuela Rodríguez Vargas, and Inés Povedano for their excellent technical assistance.
REFERENCES
- 1.Amori, G., and L. Contoli. 1994. Morphotic, craneometric and genotypic diversification in Apodemus flavicollis and Apodemus sylvaticus. Boll. Zool. 61:353-357. [Google Scholar]
- 2.Anda, P., I. Rodríguez, A. de la Loma, M. V. Fernández, and A. Lozano. 1993. A serological survey and review of clinical Lyme borreliosis in Spain. Clin. Infect. Dis. 16:310-319. [DOI] [PubMed] [Google Scholar]
- 3.Anda, P., W. Sánchez-Yebra, M. M. Vitutia, E. Pérez, I. Rodríguez, N. Miller, P. B. Backenson, and J. L. Benach. 1996. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet 384:162-165. [DOI] [PubMed] [Google Scholar]
- 4.Arteaga, F., M. G. Golightly, A. García-Pérez, M. Barral, P. Anda, and J. C. García-Moncó. 1998. Disparity between serological reactivity to Borrelia burgdorferi and evidence of past disease in a high-risk group. Clin. Infect. Dis. 27:1210-1213. [DOI] [PubMed] [Google Scholar]
- 5.Aznar, P. 1926. Algunas investigaciones clínicas y experimentales sobre la fiebre recurrente española. Arch. Inst. Nac. Hig. Alfonso XIII 4:121-127. [Google Scholar]
- 6.Baranton, G., D. Postic, G. Saint, I., P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 7.Barbour, A. G. 1984. Isolation and cultivation of lyme disease spirochetes. Yale J. Biol. Med. 57:521-524. [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour, A. G., G. O. Maupin, G. J. Teltow, C. J. Carter, and J. Piesman. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173:403-409. [DOI] [PubMed] [Google Scholar]
- 9.Barral, M., A. García-Pérez, R. A. Juste, A. Hurtado, R. Escudero, R. E. Sellek, and P. Anda. 2002. Distribution of Borrelia burgdorferi sensu lato in Ixodes ricinus (Acari: Ixodidae) ticks from the Basque Country, Spain. J. Med. Entomol. 39:177-184. [DOI] [PubMed] [Google Scholar]
- 10.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 11.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 12.Blanco, J. C., M. Alcántara, C. Ibáñez, A. Aguilar, E. Grau, S. Moreno, J. Balbotín, G. Jordán, and R. Villafuerte. 1998. Mamíferos de España. Planeta, Barcelona, Spain.
- 13.Brown, J. K. 1994. Bootstrap hypothesis tests for evolutionary trees and other dendrograms. Proc. Natl. Acad. Sci. USA 91:12293-12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buen, E., and P. De la Cámara. 1931. Notas sobre 59 casos de fiebre recurrente española. Bol. Tecn. Dir. San. 6:193-207. [Google Scholar]
- 15.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 16.Canica, M. M., F. Nato, L. Merle, J. C. Mazie, G. Baranton, and D. Postic. 1993. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 25:441-448. [DOI] [PubMed] [Google Scholar]
- 17.Casas, I., L. Powell, P. E. Klapper, and G. M. Cleator. 1995. New method for extraction of viral RNA and DNA from cerebrospinal fluid for use in the polimerase chain reaction assay. J. Virol. Methods 53:25-36. [DOI] [PubMed] [Google Scholar]
- 18.Collares-Pereira, M., S. Couceiro, I. Franca, K. Kurtenbach, S. M. Schafer, L. Vitorino, L. Goncalves, S. Baptista, M. L. Vieira, and C. Cunha. 2004. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 42:1316-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordero del Campillo, M. L., L. Castañón-Ordóñez, and A. Reguera-Feo. 1994. Índice-catálogo de zooparásitos ibéricos. Secretariado de Publicaciones de la Universidad, León, Spain.
- 20.Craine, N. G., S. E. Randolph, and P. A. Nuttall. 1995. Seasonal variation in the role of grey squirrels as hosts of Ixodes ricinus, the tick vector of the Lyme disease spirochaete, in a British woodland. Folia Parasitol. (Prague) 42:73-80. [PubMed] [Google Scholar]
- 21.De Boer, R., K. E. Hovius, M. K. Nohlmans, and J. S. Gray. 1993. The woodmouse (Apodemus sylvaticus) as a reservoir of tick-transmitted spirochetes (Borrelia burgdorferi) in The Netherlands. Zentralbl. Bakteriol. 279:404-416. [DOI] [PubMed] [Google Scholar]
- 22.Dressler, F., R. Ackermann, and A. C. Steere. 1994. Antibody responses to the three genomic groups of Borrelia burgdorferi in European Lyme borreliosis. J. Infect. Dis. 169:313-318. [DOI] [PubMed] [Google Scholar]
- 23.Escudero, R., M. Barral, A. Pérez, M. M. Vitutia, A. L. García-Pérez, S. Jiménez, R. E. Sellek, and P. Anda. 2000. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J. Clin. Microbiol. 38:4026-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrada-Peña, A., J. A. Oteo, R. Estrada-Peña, C. Gortázar, J. J. Osácar, J. A. Moreno, and J. Castellá. 1995. Borrelia burgdorferi sensu lato in ticks (Acari: Ixodidae) from two different foci in Spain. Exp. Appl. Acarol. 19:173-180. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Soto, P. 2002. Ph.D. thesis. Garrapatas que parasitan a las personas en Castilla y León, determinación por serología de su parasitismo y detección molecular. Universidad de Salamanca, Salamanca, Spain.
- 26.Fraenkel, C. J., U. Garpmo, and J. Berglund. 2002. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J. Clin. Microbiol. 40:3308-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukunaga, M., K. Okada, M. Nakao, T. Konishi, and Y. Sato. 1996. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int. J. Syst. Bacteriol. 46:898-905. [DOI] [PubMed] [Google Scholar]
- 28.Fukunaga, M., Y. Takahashi, Y. Tsuruta, O. Matsushita, D. Ralph, M. McClelland, and M. Nakao. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 45:804-810. [DOI] [PubMed] [Google Scholar]
- 29.García-Moncó, J. C., J. L. Benach, J. L. Coleman, J. L. Galbe, A. Szczepanski, B. Fernández-Villar, H. C. Norton, and R. C. Johnson. 1992. Caracterización de una cepa española de Borrelia burgdorferi. Med. Clin. (Barcelona) 98:89-93. [PubMed] [Google Scholar]
- 30.Gern, L., A. Estrada-Peña, F. Frandsen, J. S. Gray, T. G. Jaenson, F. Jongejan, O. Kahl, E. Korenberg, R. Mehl, and P. A. Nuttall. 1998. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentralbl. Bakteriol. 287:196-204. [DOI] [PubMed] [Google Scholar]
- 31.Gil-Collado, J., J. L. Guillén-Llera, and L. M. Zapatero-Ramos. 1979. Claves para la identificación de los Ixodoidea españoles (adultos). Rev. Iber. Parasitol. 39:107-111. [Google Scholar]
- 32.Gray, J. S., F. Kirstein, J. N. Robertson, J. Stein, and O. Kahl. 1999. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in a recreational park in south-western Ireland. Exp. Appl. Acarol. 23:717-729. [DOI] [PubMed] [Google Scholar]
- 33.Guerrero, A., C. Quereda, P. Martí-Belda, and R. Escudero. 1993. Borreliosis de Lyme:¿Cómo se manifiesta en España? Med. Clin. (Barcelona) 101:5-7. [PubMed] [Google Scholar]
- 34.Guner, E. S., N. Hashimoto, T. Kadosaka, Y. Imai, and T. Masuzawa. 2003. A novel, fast-growing Borrelia sp. isolated from the hard tick Hyalomma aegyptium in Turkey. Microbiology 149:2539-2544. [DOI] [PubMed] [Google Scholar]
- 35.Guy, E. C., and G. Stanek. 1991. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J. Clin. Pathol. 44:610-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanincova, K., S. M. Schafer, S. Etti, H. S. Sewell, V. Taragelova, D. Ziak, M. Labuda, and K. Kurtenbach. 2003. Association of Borrelia afzelii with rodents in Europe. Parasitology 126:11-20. [DOI] [PubMed] [Google Scholar]
- 37.Hubálek, Z., and J. Halouzka. 1997. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur. J. Epidemiol. 13:951-957. [DOI] [PubMed] [Google Scholar]
- 38.Humair, P. F., and L. Gern. 1998. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 69:213-227. [DOI] [PubMed] [Google Scholar]
- 39.Humair, P. F., D. Postic, R. Wallich, and L. Gern. 1998. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentralbl. Bakteriol. 287:521-538. [PubMed] [Google Scholar]
- 40.Humair, P. F., O. Rais, and L. Gern. 1999. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology 118:33-42. [DOI] [PubMed] [Google Scholar]
- 41.Humair, P. F., N. Turrian, A. Aeschilimann, and L. Gern. 1993. Borrelia burgdorferi in a focus of Lyme borreliosis: epizootiologic contribution of small mammals. Folia Parasitol. (Prague) 40:65-70. [PubMed] [Google Scholar]
- 42.Johnson, R. C., G. P. Schmid, F. W. Hyde, A. G. Steigerwalt, and D. J. Brenner. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int. J. Syst. Bacteriol. 34:496-497. [Google Scholar]
- 43.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 44.Kisinza, W. N., P. J. McCall, H. Mitani, A. Talbert, and M. Fukunaga. 2003. A newly identified tick-borne Borrelia species and relapsing fever in Tanzania. Lancet 362:1283-1284. [DOI] [PubMed] [Google Scholar]
- 45.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 46.Kurtenbach, K., D. Carey, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 1998. Competence of pheasants as reservoirs for Lyme disease spirochetes. J. Med. Entomol. 35:77-81. [DOI] [PubMed] [Google Scholar]
- 47.Kurtenbach, K., A. Dizij, H. M. Seitz, G. Margos, S. E. Moter, M. D. Kramer, R. Wallich, U. E. Schaible, and M. M. Simon. 1994. Differential immune responses to Borrelia burgdorferi in European wild rodent species influence spirochete transmission to Ixodes ricinus L. (Acari: Ixodidae). Infect. Immun. 62:5344-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurtenbach, K., H. Kampen, A. Dizij, S. Arndt, H. M. Seitz, U. E. Schaible, and M. M. Simon. 1995. Infestation of rodents with larval Ixodes ricinus (Acari: Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s.l. in German woodlands. J. Med. Entomol. 32:807-817. [DOI] [PubMed] [Google Scholar]
- 49.Kurtenbach, K., M. Peacey, S. G. Rijpkema, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 1998. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurtenbach, K., S. M. Schafer, H. S. Sewell, M. Peacey, A. Hoodless, P. A. Nuttall, and S. E. Randolph. 2002. Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect. Immun. 70:5893-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Fleche, A., D. Postic, K. Girardet, O. Peter, and G. Baranton. 1997. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47:921-925. [DOI] [PubMed] [Google Scholar]
- 52.Leuba-García, S., M. D. Kramer, R. Wallich, and L. Gern. 1994. Characterization of Borrelia burgdorferi isolated from different organs of Ixodes ricinus ticks collected in nature. Zentralbl. Bakteriol. 280:468-475. [DOI] [PubMed] [Google Scholar]
- 53.Lin, T., J. H. Oliver, Jr., and L. Gao. 2003. Comparative analysis of Borrelia isolates from southeastern USA based on randomly amplified polymorphic DNA fingerprint and 16S ribosomal gene sequence analyses. FEMS Microbiol. Lett. 228:249-257. [DOI] [PubMed] [Google Scholar]
- 54.Manilla, G. 1998. Acari Ixodida. Fauna d'Italia. Edizioni Calderini, Bologna, Italy.
- 55.Masuzawa, T., N. Takada, M. Kudeken, T. Fukui, Y. Yano, F. Ishiguro, Y. Kawamura, Y. Imai, and T. Ezaki. 2001. Borrelia sinica sp. nov., a Lyme disease-related Borrelia species isolated in China. Int. J. Syst. Evol. Microbiol. 51:1817-1824. [DOI] [PubMed] [Google Scholar]
- 56.Matuschka, F. R., R. Allgower, A. Spielman, and D. Richter. 1999. Characteristics of garden dormice that contribute to their capacity as reservoirs for Lyme disease spirochetes. Appl. Environ. Microbiol. 65:707-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matuschka, F. R., H. Eiffert, A. Ohlenbusch, and A. Spielman. 1994. Amplifying role of edible dormice in Lyme disease transmission in Central Europe. J. Infect. Dis. 170:122-127. [DOI] [PubMed] [Google Scholar]
- 58.Matuschka, F. R., P. Fischer, M. Heiler, D. Richter, and A. Spielman. 1992. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J. Infect. Dis. 165:479-483. [DOI] [PubMed] [Google Scholar]
- 59.Michalik, J., T. Hofman, A. Buczek, M. Skoracki, and B. Sikora. 2003. Borrelia burgdorferi s.l. in Ixodes ricinus (Acari: Ixodidae) ticks collected from vegetation and small rodents in recreational areas of the city of Poznan. J. Med. Entomol. 40:690-697. [DOI] [PubMed] [Google Scholar]
- 60.Nosek, J., and W. Sixl. 1972. Central European ticks (Ixodoidea). Key for determination. Mitt. Abt. Zool. Landesmuseum Joanneum Graz 1:61-92. [Google Scholar]
- 61.Olsen, B., T. G. Jaenson, and S. Bergstrom. 1995. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 61:3082-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oteo, J. A., P. B. Backenson, V. Del Mar, J. C. García-Moncó, I. Rodríguez, R. Escudero, and P. Anda. 1998. Use of the C3H/He Lyme disease mouse model for the recovery of a Spanish isolate of Borrelia garinii from erythema migrans lesions. Res. Microbiol. 149:39-46. [DOI] [PubMed] [Google Scholar]
- 63.Oteo, J. A., V. Martínez de Artola, R. Gómez-Cadiñanos, J. M. Casas, and R. Grandival. 1993. Erythema chronicum migrans (Lyme's disease). Clinico-epidemiologic study of 10 cases. Rev. Clin. Esp. 193:20-23. [PubMed] [Google Scholar]
- 64.Ras, N. M., B. Lascola, D. Postic, S. J. Cutler, F. Rodhain, G. Baranton, and D. Raoult. 1996. Phylogenesis of relapsing fever Borrelia spp. Int. J. Syst. Bacteriol. 46:859-865. [DOI] [PubMed] [Google Scholar]
- 65.Renesto, P., K. Lorvellec-Guillon, M. Drancourt, and D. Raoult. 2000. rpoB gene analysis as a novel strategy for identification of spirochetes from the genera Borrelia, Treponema, and Leptospira. J. Clin. Microbiol. 38:2200-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richter, D., B. Klug, A. Spielman, and F. R. Matuschka. 2004. Adaptation of diverse Lyme disease spirochetes in a natural rodent reservoir host. Infect. Immun. 72:2442-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richter, D., D. B. Schlee, and F. R. Matuschka. 2003. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 9:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richter, D., A. Spielman, N. Komar, and F. R. Matuschka. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rijpkema, S. G., M. J. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saint-Girons, I., L. Gern, J. S. Gray, E. C. Guy, E. Korenberg, P. A. Nuttall, S. G. Rijpkema, A. Schonberg, G. Stanek, and D. Postic. 1998. Identification of Borrelia burgdorferi sensu lato species in Europe. Zentralbl. Bakteriol. 287:190-195. [DOI] [PubMed] [Google Scholar]
- 71.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 72.Sánchez-Yebra, W., Y. Díaz, P. Molina, Sedeno, P. Giner, M. M. Vitutia, and P. Anda. 1997. Tick-borne recurrent fever. Description of 5 cases. Enferm. Infecc. Microbiol. Clin. 15:77-81. (In Spanish.) [PubMed] [Google Scholar]
- 73.Schwan, T. G., W. Burgdorfer, M. E. Schrumpf, and R. H. Karstens. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J. Clin. Microbiol. 26:893-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scoles, G. A., M. Papero, L. Beati, and D. Fish. 2000. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1:21-34. [DOI] [PubMed] [Google Scholar]
- 75.Sellek, R. E., R. Escudero, H. Gil, I. Rodríguez, E. Chaparro, E. Pérez-Pastrana, A. Vivo, and P. Anda. 2002. In vitro culture of Borrelia garinii results in loss of flagella and decreased invasiveness. Infect. Immun. 70:4851-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sinsky, R. J., and J. Piesman. 1989. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 27:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sonenshine, D. E. 1993. Biology of ticks. Oxford University Press, New York, N.Y.
- 78.Talleklint, L., and T. G. Jaenson. 1993. Maintenance by hares of European Borrelia burgdorferi in ecosystems without rodents. J. Med. Entomol. 30:273-276. [DOI] [PubMed] [Google Scholar]
- 79.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, G., A. P. van Dam, A. Le Fleche, D. Postic, O. Peter, G. Baranton, R. De Boer, L. Spanjaard, and J. Dankert. 1997. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19). Int. J. Syst. Bacteriol. 47:926-932. [DOI] [PubMed] [Google Scholar]
- 81.Wang, G., A. P. van Dam, I. Schwartz, and J. Dankert. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 12:633-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeidner, N. S., M. S. Nuncio, B. S. Schneider, L. Gern, J. Piesman, O. Brandao, and A. R. Filipe. 2001. A Portuguese isolate of Borrelia lusitaniae induces disease in C3H/HeN mice. J. Med. Microbiol. 50:1055-1060. [DOI] [PubMed] [Google Scholar]