Abstract
Prescribing appropriate exercise is an important means to improve the safety and efficacy of cardiac rehabilitation. Improper exercise may induce an increased cardiovascular risk in older persons with coronary heart disease. Cardiopulmonary exercise testing (CPET)-guided cardiac rehabilitation could be helpful for providing clinical evidence for cardiac rehabilitation therapy in older persons after percutaneous coronary intervention (PCI). We retrospectively included older persons who underwent PCI and cardiac rehabilitation based on CPET at the Cardiac Rehabilitation Center of Peking University Third Hospital from January 2014 to December 2019. Patients’ baseline and follow-up clinical data were collected. A total of 403 older persons after PCI were included in the study. The mean age was 80.5 ± 4.3. The mean follow-up time was 12 ± 2 months. During the follow-up period, no significant exercise-related adverse events occurred, and the peak oxygen uptake (VO2peak) increased compared with baseline (15.5 ± 3.8 ml/min/kg vs. 17.3 ± 4.1 ml/min/kg). Among the 90 patients (22.2%) without exercise habits at baseline who started regular exercise during follow-up, the improvement in VO2peak was most significant, at 3.2 ± 0.4 ml/min/kg. Cardiac rehabilitation based on CPET improved exercise habits and exercise tolerance in older persons with coronary heart disease after PCI.
Subject terms: Cardiology, Diseases
Introduction
Cardiac rehabilitation, as an important intervention measure, is of great significance for improving the clinical outcomes of patients with coronary heart disease (CHD)1–3. For older persons who undergo revascularization, the cardiovascular risk of heart failure, arrhythmia, and sudden death remain. The cardiovascular risk during exercise may increase due to the added effects of age-related physiological changes. Therefore, prescribing appropriate exercise is an important means to improve the safety and efficacy of exercise rehabilitation.
Cardiopulmonary exercise testing (CPET) could provide essential guidance for determining the aerobic exercise intensity for CHD patients. Additional CPET testing is already being used for exercise prescription even for the elderly. However, there has been insufficient research on its influence in older CHD persons. The purpose of this study was to observe the impact of cardiac rehabilitation guidance based on CPET on cardiopulmonary exercise tolerance and exercise adherence in older persons with CHD after percutaneous coronary intervention (PCI) in the context of standardized drug treatment for CHD. This work will be helpful for providing clinical evidence for cardiac rehabilitation therapy in older persons with CHD after PCI.
Materials and methods
General information
We retrospectively included CHD patients aged ≥ 75 years who underwent PCI and exercise rehabilitation guided by CPET at the Cardiac Rehabilitation Center of Peking University Third Hospital from January 2014 to December 2019 (Fig. 1).The inclusion criteria were as follows: age ≥ 75 years; successful PCI; CPET-guided cardiac rehabilitation with a clinical follow-up evaluation after one year; and complete clinical data, medication history, biochemical data, and echocardiographic data.
Figure 1.
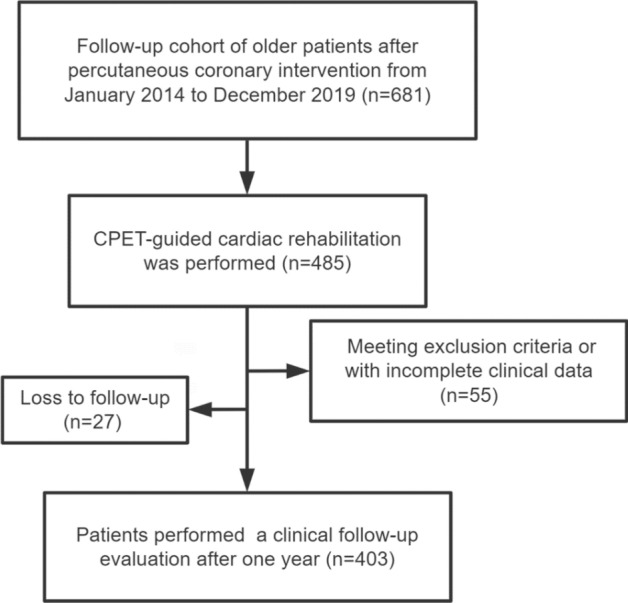
Flowchart of the study.
The exclusion criteria were as follows: positive exercise electrocardiogram (ECG); NYHA class III-IV heart function; malignant arrhythmia; valvular heart disease; history of coronary artery bypass graft surgery; and concomitant malignant tumours, haematologic diseases, rheumatic immune diseases, or severe liver or kidney dysfunction.
The study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of Peking University Third Hospital waived the need for informed consent for the present retrospective study.
A positive exercise ECG was defined as follows: R-wave dominant leads, horizontal or downsloping ST-segment depression ≥ 0.1 mV at 60–80 ms after the J-point, lasting ≥ 2 min; and nonpathological Q-wave leads, upsloping ST-segment elevation ≥ 0.1 mV, lasting ≥ 1 min.
We collected and organized the following patient data: (1) general information, including sex, age, disease diagnosis, height, weight, etc.; (2) past medical history and family history, including history of cardiovascular risk factors such as hypertension, diabetes, hyperlipidaemia, respiratory diseases such as chronic obstructive pulmonary disease, musculoskeletal and neurological disease history, and family history of early-onset CHD, etc.; (3) personal history, including smoking history, exercise habits, etc.; and (4) laboratory test, echocardiography, and CPET results.
Cardiopulmonary exercise testing
The testing equipment used was the Medgraphics (USA) ULTIMA CardiO2 Cardiopulmonary Exercise System. All patients performed exercise testing using a cycle ergometer, adopting a bicycle exercise protocol. Symptom-limited exercise was encouraged. Patients’ electrocardiogram (ECG), blood pressure, and symptoms were monitored. The entire process was carried out under the supervision of a professional physician.
During the test, continuous recording of patients' ECG, blood pressure, and gas exchange information was performed, including the following parameters: oxygen uptake at anaerobic threshold (VO2@AT); heart rate at anaerobic threshold (HR@AT); oxygen pulse at anaerobic threshold; peak oxygen uptake (VO2peak); peak heart rate (peak HR); ventilation per carbon dioxide output slope (VE/VCO2 slope); oxygen uptake efficiency slope (OUES); and 1-min heart rate recovery.
Exercise prescription
Individualized exercise rehabilitation prescriptions based on the cardiopulmonary exercise testing system were implemented. Exercise intensity was prescribed using the anaerobic threshold heart rate ± 5 bpm4, along with prescriptions for strength training and balance and coordination training. Aerobic exercise modalities such as walking, cycling, and swimming were recommended, with moderate-intensity continuous exercise performed 3–5 days per week for 30–60 min per day, including warm-up, training, and recovery sections.
Study endpoints
The primary endpoint of this study was the change in patients' exercise tolerance, represented by VO2peak and other CPET parameters during the follow-up period. Secondary endpoints included differences in exercise habits. According to the American College of Sports Medicine (ACSM) 10th Edition “ACSM’s Guidelines for Exercise Testing and Prescription”5, exercising ≥ 3 times per week, ≥ 30 min per session, and for a duration of ≥ 3 months indicated the presence of exercise habits; those who did not meet this standard were considered to have no exercise habits. Other secondary endpoints included patients' blood test results, echocardiographic parameters, and adverse events during the follow-up period6.
Statistical methods
SPSS 22.0 was used for data analysis and statistics. The Shapiro‒Wilk normality test was performed on all quantitative data in the analysed samples. Normally distributed quantitative data are expressed as the mean ± standard deviation (s), and nonnormally distributed quantitative data are expressed as the median (Q1, Q3). Group comparisons were made using paired t tests and Mann‒Whitney U tests, with paired t tests applied for normally distributed data and Mann‒Whitney U tests for nonnormally distributed data. Categorical variables were analysed using chi-square tests. A two-sided P < 0.05 was considered statistically significant.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Peking University Third Hospital (Approval Number 2020113).
Informed consent
Patient consent was waived due to its retrospective nature of observation.
Results
General Patient Information
A total of 403 older persons with CHD after PCI were analysed (Table 1). The average age of the patients was 80.5 ± 4.3 years, and there were 305 (75.6%) males. A total of 336 (83.4%) patients had one or more CHD risk factors (including hypertension, diabetes, hyperlipidaemia, and smoking). A total of 134 (16.6%) patients had a history of myocardial infarction. Complete revascularization was achieved in 316 (78.4%) patients. A total of 185 (45.9%) patients were classified as NYHA functional class II.
Table 1.
Clinical information of older CHD patients after PCI.
| Parameters | Total (%), mean ± SD |
|---|---|
| Age (years) | 80.5 ± 4.3 |
| Male, N (%) | 305 (75.6) |
| BMI (Kg/m2) | 25.1 ± 3.5 |
| History of myocardial infarction, N (%) | 134 (16.6) |
| Complete revascularization, N (%) | 316 (78.4) |
| NYHA class II, N (%) | 185 (49.5) |
| Hypertension, N (%) | 271 (67.2) |
| Diabetes, N (%) | 116 (28.7) |
| Hyperlipidemia, N (%) | 262 (65.0) |
| Smoking history, N (%) | 143 (35.4) |
| Family history of CHD, N (%) | 93 (23.0) |
| Exercise habits, N (%) | 256 (63.5) |
CHD coronary heart disease, PCI percutaneous coronary intervention, BMI body mass index.
Comparison of baseline and follow-up patient indicators
General patient data, laboratory indicators, echocardiographic parameters, and CPET indicators were analysed at baseline and during the follow-up period (Table 2). No deaths occurred during the follow-up period. In terms of laboratory indicators, patients had lower low-density lipoprotein (LDL) levels (2.0 ± 0.6 mmol/L vs. 1.4 ± 0.5 mmol/L, P = 0.032) and lower N-terminal pro-brain natriuretic peptide (NT-proBNP) levels (305.5 ± 19.4 pg/ml vs. 225.6 ± 11.1 pg/ml, P = 0.013) during the follow-up period.
Table 2.
Characteristics of older persons after PCI during the baseline and follow-up period.
| Characteristics | Baseline | Follow-up | P value |
|---|---|---|---|
| BMI (kg/m2) | 25.1 ± 2.9 | 25.0 ± 2.8 | 0.726 |
| Laboratory indicators | |||
| Cr (μmol/L) | 88.0 ± 11.5 | 87.4 ± 21.8 | 0.544 |
| LDL (mmol/L) | 2.0 ± 0.6 | 1.4 ± 0.5 | 0.032 |
| Hb (g/L) | 132.4 ± 16.5 | 145.1 ± 14.9 | 0.653 |
| NT-proBNP (pg/ml) | 305.5 ± 19.4 | 225.6 ± 11.1 | 0.013 |
| Echocardiography indicators | |||
| LVEDD (mm) | 47.1 ± 5.6 | 48.1 ± 3.7 | 0.565 |
| LVEF (%) | 65.7 ± 8.3 | 66.8 ± 7.6 | 0.317 |
| Sm (cm/s) | 9.3 ± 1.9 | 9.0 ± 1.5 | 0.191 |
| E/Em | 6.9 ± 2.2 | 7.5 ± 2.3 | 0.028 |
| LAP (mmHg) | 10.8 ± 2.8 | 10.1 ± 2.8 | 0.017 |
| CPET indicators | |||
| VO2@AT (ml/min/kg) | 12.9 ± 3.7 | 13.8 ± 4.2 | < 0.001 |
| HR@AT (bpm) | 98 ± 13 | 97 ± 11 | 0.809 |
| VO2peak (ml/min/kg) | 15.5 ± 3.8 | 17.3 ± 4.1 | 0.013 |
| Peak HR (bpm) | 118 ± 17 | 122 ± 16 | 0.012 |
| Peak SBP (mmHg) | 169 ± 24 | 171 ± 25 | 0.089 |
| VE/VCO2 slope | 31.9 ± 5.1 | 30.9 ± 6.1 | < 0.001 |
| OUES | 1488.8 ± 395.2 | 1498.1 ± 328.1 | 0.453 |
| HRR | 17 ± 7 | 19 ± 8 | < 0.001 |
PCI percutaneous coronary intervention, BMI body mass index, Cr creatinine, LDL low density lipoprotein, Hb hemoglobin, NT-proBNP N-terminal pro-brain natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVEF left ventricular ejection fraction, Sm systolic velocity of mitral annulus, E/Em the ratio of early diastolic transmitral flow velocity to early diastolic tissue velocity, LAP left atrium pressure, VO2@AT oxygen uptake at anaerobic threshold, HR@AT heart rate at anaerobic threshold, VO2peak peak oxygen uptake, Peak HR peak heart rate, Peak SBP peak systolic blood pressure, VE/VCO2 slope ventilation per carbon dioxide output slope, OUES oxygen uptake efficiency slope, HRR heart rate recovery.
In terms of echocardiographic parameters, there was no significant change in the left ventricular ejection fraction (LVEF) during the follow-up period (65.7 ± 8.3% vs. 66.8 ± 7.6%, P = 0.317), but indicators of left ventricular diastolic dysfunction, i.e., the ratio of early diastolic transmitral flow velocity to early diastolic tissue velocity (6.9 ± 2.2 vs. 7.5 ± 2.3, P = 0.028) and left atrium pressure (10.8 ± 2.8 mmHg vs. 10.1 ± 2.8 mmHg, P = 0.028), improved.
In terms of cardiopulmonary exercise parameters, patients had higher VO2@AT (12.9 ± 3.7 ml/min/kg vs. 13.8 ± 4.2 mmHg, P < 0.001), VO2peak (15.5 ± 3.8 ml/min/kg vs. 17.3 ± 4.1 mmHg, P = 0.013), and peak HR (118 ± 17 bpm vs. 122 ± 16 bpm, P = 0.012) values during the follow-up period than at baseline. The VE/VCO2 slope, reflecting respiratory efficiency, improved (31.9 ± 5.1 vs. 30.9 ± 6.1, P < 0.001), and the 1 min heart rate recovery increased (17 ± 7 bpm vs. 19 ± 8 bpm, P < 0.001).
In terms of exercise-related adverse effects, 33 patients (8.3%) had muscle soreness or strain. 17 injurious falls (4.2%) occurred.2 falls (0.05%) resulting in medical care. There were no severe injurious falls or falls resulting in fractures.
Patients were grouped based on whether they had exercise habits during the follow-up period. At the follow-up, patients with exercise habits were younger (80.7 ± 4.3 ml/kg/min vs. 83.9 ± 3.9 ml/kg/min, P = 0.007) and had higher ΔVO2peak (1.62 ± 0.31 ml/kg/min vs. − 0.02 ± 0.01 ml/kg/min, P < 0.001) and VO2@AT (14.2 ± 4.1 ml/kg/min vs. 12.9 ± 3.4 ml/kg/min, P < 0.001) values than patients without exercise habits. No significant differences were observed between the two groups in terms of laboratory or echocardiographic parameters (Table 3).
Table 3.
CPET indicators of older persons after PCI during the follow-up period.
| Characteristics | With exercise habits (n = 315) | Without exercise habits (n = 88) | P value |
|---|---|---|---|
| Age (years) | 80.7 ± 4.3 | 83.9 ± 3.9 | 0.007 |
| Male (%) | 251 (79.6) | 54 (61.3) | < 0.001 |
| BMI (kg/m2) | 25.0 ± 2.9 | 25.4 ± 3.0 | 0.736 |
| Laboratory indicators | |||
| Cr (μmol/L) | 88.2 ± 13.9 | 89.1 ± 17.3 | 0.057 |
| LDL (mmol/L) | 1.9 ± 0.6 | 1.8 ± 0.5 | 0.609 |
| Hb (g/L) | 137.1 ± 15.4 | 140.3 ± 15.3 | 0.671 |
| NT-proBNP (pg/ml) | 274.7 ± 14.5 | 251.5 ± 13.8 | 0.217 |
| Echocardiography indicators | |||
| LVEDD (mm) | 47.5 ± 4.9 | 48.1 ± 4.3 | 0.513 |
| LVEF (%) | 66.0 ± 8.0 | 67.6 ± 7.9 | 0.387 |
| Sm (cm/s) | 9.2 ± 1.7 | 8.9 ± 1.7 | 0.981 |
| E/Em | 7.2 ± 2.2 | 7.8 ± 2.4 | 0.384 |
| LAP (mmHg) | 10.3 ± 2.8 | 11.1 ± 3.0 | 0.540 |
| CPET indicators | |||
| VO2@AT (ml/min/kg) | 14.2 ± 4.1 | 12.9 ± 3.4 | 0.002 |
| HR@AT (bpm) | 97 ± 12 | 98 ± 13 | 0.426 |
| VO2peak (ml/min/kg) | 17.6 ± 4.0 | 13.9 ± 4.3 | < 0.001 |
| ΔVO2peak (ml/min/kg) | 1.62 ± 0.31 | − 0.02 ± 0.01 | < 0.001 |
| Peak HR (bpm) | 123 ± 16 | 119 ± 17 | 0.026 |
| Peak SBP (mmHg) | 170 ± 25 | 165 ± 27 | 0.834 |
| VE/VCO2 slope | 31.2 ± 5.0 | 31.2 ± 6.7 | 0.989 |
| OUES | 1505.9 ± 350.5 | 1460.3 ± 362.4 | 0.289 |
| HRR | 20 ± 8 | 17 ± 9 | 0.034 |
PCI percutaneous coronary intervention, BMI body mass index, Cr creatinine, LDL low density lipoprotein, Hb hemoglobin, NT-proBNP N-terminal pro-brain natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVEF left ventricular ejection fraction, Sm systolic velocity of mitral annulus, E/Em the ratio of early diastolic transmitral flow velocity to early diastolic tissue velocity, LAP left atrium pressure, VO2@AT oxygen uptake at anaerobic threshold, HR@AT heart rate at anaerobic threshold, VO2peak peak oxygen uptake, Peak HR peak heart rate, Peak SBP peak systolic blood pressure, VE/VCO2 slope ventilation per carbon dioxide output slope, OUES oxygen uptake efficiency slope, HRR heart rate recovery.
Improvement in patients’ exercise habits
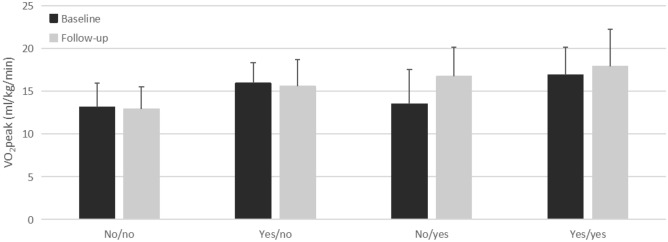
Changes in patients’ exercise habits and exercise tolerance from baseline to follow-up were as follows: 57 patients (14.1%) had no exercise habits at baseline or during the follow-up period; 31 patients (7.6%) had exercise habits at baseline but did not maintain them during the follow-up period; 90 patients (22.2%) had no exercise habits at baseline but started regular exercise during the follow-up period; and 225 patients (55.8%) maintained exercise habits at baseline and during the follow-up period. The baseline and follow-up oxygen uptake in each group is shown in Fig. 2, with ΔVO2peak values of − 0.2 ± 0.1 ml/kg/min, − 0.3 ± 0.1 ml/kg/min, 3.2 ± 0.4 ml/kg/min, and 1.0 ± 0.2 ml/kg/min, respectively. The improvement in VO2peak was most pronounced in patients who had no exercise habits at baseline but developed them during the follow-up period. VO2peak did not improve in patients without exercise habits during the follow-up period, regardless of whether they had exercise habits at baseline.
Figure 2.
Patients’ exercise habits and VO2peak before and after intervention.
Spearman correlation analysis was performed on patients’ clinical data with ΔVO2peak. Exercise habits during the follow-up period (rs = − 0.601, P < 0.001), age (rs = − 0.353, P < 0.001), and history of myocardial infarction (rs = − 0.293, P = 0.029) were significantly correlated with ΔVO2peak, while other clinical data and combined medication use were not significantly correlated with ΔVO2peak. Multivariate linear regression analysis was performed on ΔVO2peak with the parameters that were significantly correlated with ΔVO2peak in the univariate analysis, with the main factors such as exercise habits during the follow-up period as independent variables. The results showed that exercise habits during the follow-up period (B = − 0.406, SE = 0.063, t = − 6.650, P = 0.001, 95% CI − 0.561 to − 0.284), age, and history of myocardial infarction were independent influencing factors of ΔVO2peak (Table 4).
Table 4.
Multiple factor analysis of ΔVO2peak.
| B | SE | t | P | 95% CI | |
|---|---|---|---|---|---|
| Age | − 0.114 | 0.087 | − 6.940 | 0.023 | (− 0.158, − 0.061) |
| Myocardial infarction history | − 1.054 | 0.610 | − 2.074 | 0.017 | (− 1.391, − 0.772) |
| With exercise habits during follow-up period | − 0.406 | 0.063 | − 2.650 | 0.001 | (− 0.561, − 0.284) |
Discussion
Significant clinical benefits of exercise rehabilitation for older persons with CHD
PCI has become the most important means of revascularization for CHD patients, not only effectively improving patients’ clinical symptoms but also significantly reducing the mortality of acute myocardial infarction and high-risk angina patients. However, for older persons, the cardiovascular risk of heart failure, arrhythmia, and sudden death remain after PCI due to the added effects of age-related physiological changes. Compared to non-CHD patients, exercise tolerance is significantly reduced in CHD patients. The decline in exercise tolerance is even more pronounced in older persons with CHD, who may also have mental issues such as depression and dementia, severely affecting their quality of life7–9.
Cardiac rehabilitation was an important part of secondary prevention of cardiovascular diseases10. There was certain particularity in the implementation of cardiac rehabilitation on different population11,12. Previous studies found that increased daily physical activity was associated with a reduced mortality rate in CHD patients, particularly in those who were sedentary, as they experienced greater cardiovascular benefits13. Cardiac rehabilitation could promote the physical and mental health of older persons, improving their quality of life14–17, and offer benefits for older CHD patients, such as reduced mortality and rehospitalization rates and further revascularization18,19. Many studies have reported that aerobic exercise training improves exercise capacity, frailty, and quality of life in older CHD patients20–23. However, the patients included in these previous studies were relatively young compared with those in the current study. In particular, in CHD patients aged 75 and older, muscle degeneration and atrophy are more severe; additionally, there is a lack of ligament toughness and elasticity and a greatly reduced capacity for stress responses in the nervous system. Consequently, the risk of exercise-related injuries may increase. Providing an appropriate exercise intensity is a crucial aspect of cardiac rehabilitation, enhancing the efficacy and safety of the treatment.
Advantages of CPET-guided exercise rehabilitation
CPET is an objective and accurate method for assessing cardiopulmonary function in patients after PCI and is relatively safe24,25. For older CHD patients, CPET not only reflects the level of cardiopulmonary function and disease severity but could also be used to assess patients’ balance ability and quality of life26. By providing reasonable exercise recommendations based on the collected data, CPET could be used to prescribe exercise rehabilitation programs for older CHD patients, helping to mitigate risk during exercise training.
In the past, the maximum heart rate has often been used as the standard for designing exercise programs in cardiac rehabilitation. It is usually difficult for older persons to achieve their maximum heart rate, and there are unpredictable exercise risks. Moreover, heart rate could be affected by medications such as beta-blockers. A more ideal standard is to individually evaluate patients’ exercise capacity; as exercise capacity improves, CPET could provide guidance for different stages of exercise recommendations.
Impact of CPET-guided exercise rehabilitation on exercise capacity and exercise habits
In this study, the average age of the patients was 80.5 ± 4.3 years. Exercise intensity was guided based on patients’ anaerobic threshold oxygen uptake during exercise tests, encouraging them to engage in regular, continuous, moderate-intensity exercise. The average follow-up period was 12 ± 2 months, after which patients' exercise habits were reassessed and CPET was repeated. The results showed a significant increase in VO2peak and other indicators in the regular exercise group, suggesting that CPET-guided exercise rehabilitation can significantly improve patients’ exercise capacity.
This study found that some patients’ exercise habits changed during the follow-up period after receiving cardiac rehabilitation exercise guidance. At enrolment, 7.6% of patients had exercise habits but did not maintain them during the follow-up period. In contrast, more patients (22%) without exercise habits at enrolment began to develop regular exercise habits during the follow-up period.
In previous research, cardiac rehabilitation increased exercise participation in CHD patients27. CPET-based exercise rehabilitation guidance may help older CHD patients gain more confidence in the safety of exercise, thus increasing their enthusiasm for physical activity. Additionally, this study found that patients with exercise habits during the follow-up period had a significantly higher exercise capacity than those without exercise habits. Patients who did not maintain exercise during the follow-up period experienced a decline in exercise capacity due to age or disease progression, which may counteract the benefits gained from their previous regular exercise.
Many medical institutions conduct insufficient exercise assessments and cardiac exercise rehabilitation for older CHD patients due to concerns about exercise-related adverse events or potential risks, which limits the development of cardiac exercise rehabilitation programs. However, this study’s results suggest that even older CHD patients after PCI can safely and effectively improve their exercise capacity through CPET-guided exercise rehabilitation, with the improvement of exercise habits being a crucial aspect. Current research has also explored the use of remote medical devices to improve patients' adherence to cardiac exercise rehabilitation28,29. However, due to potential barriers in using remote medical devices and the greater social and economic challenges faced by older persons, further exploration of simpler and more effective methods is needed to enhance the therapeutic effects of exercise rehabilitation in this population.
This study had certain limitations, as it was a single-centre study. The results may have limited generalizability, necessitating further validation in more medical institutions. Additionally, this study was retrospective and did not involve prospective interventional research. The older persons included in the study had few comorbidities and were able to cooperate with cardiopulmonary exercise tests and exercise rehabilitation. As such, the study's conclusions may not apply to older CHD patients with multiple comorbidities, a bedridden status, or a poor exercise capacity. Furthermore, due to a high amount of missing data regarding quality-of-life scores, such as the SF-36 questionnaire scores, these were not included in the statistical analysis.
Conclusions
Individualized exercise prescription based on CPET is a safe and effective way to guide cardiac rehabilitation in older CHD patients after PCI, improving their exercise capacity and exercise habits.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: T.S., Z.W., P.W., W.Z.; data collection: C.R., Y.S., W.G., F.L., G.L.; analysis and interpretation of results: T.S., Y.W., W.Z.; draft manuscript preparation: T.S., W.Z. All authors reviewed the manuscript.
Data availability
The study data are available from the corresponding author upon reasonable request and with the permission of all contributing authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tao Shen and Yuwei Wang.
Contributor Information
Zhimin Wei, Email: 51807124@qq.com.
Peng Wang, Email: bysywp@126.com.
Wei Zhao, Email: beate_vv@bjmu.edu.cn.
References
- 1.Dibben GO, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: A meta-analysis. Eur Heart J. 2023;44(6):452–469. doi: 10.1093/eurheartj/ehac747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols S, McGregor G, Breckon J, et al. Current Insights into exercise-based cardiac rehabilitation in patients with coronary heart disease and chronic heart failure. Int. J. Sports Med. 2021;42(1):19–26. doi: 10.1055/a-1198-5573. [DOI] [PubMed] [Google Scholar]
- 3.Sandercock GR, Cardoso F, Almodhy M, et al. Cardiorespiratory fitness changes in patients receiving comprehensive outpatient cardiac rehabilitation in the UK: A multicentre study. Heart (British Cardiac Society) 2013;99(11):785–790. doi: 10.1136/heartjnl-2012-303055. [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riebe, D. E. J., Liguori, G., et al. ACSM’s guidelines for exercise testing and prescription. Am. Coll. Sports Med. 147–151 (2018).
- 6.El-Khoury F, Cassou B, Charles MA, et al. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: Systematic review and meta-analysis of randomised controlled trials. BMJ (Clinical Research ed) 2013;347:f6234. doi: 10.1136/bmj.f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen SJ, Schirmer H, Wilsgaard T, et al. Cardiac rehabilitation and symptoms of anxiety and depression after percutaneous coronary intervention. Eur. J. Prev. Cardiol. 2018;25(10):1017–1025. doi: 10.1177/2047487318778088. [DOI] [PubMed] [Google Scholar]
- 8.Lutz AH, Delligatti A, Allsup K, et al. Cardiac rehabilitation is associated with improved physical function in frail older adults with cardiovascular disease. J. Cardiopul. Rehabil. Prev. 2020;40(5):310–318. doi: 10.1097/HCR.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 9.Jepma P, Jorstad HT, Snaterse M, et al. Lifestyle modification in older versus younger patients with coronary artery disease. Heart (British Cardiac Society) 2020;106(14):1066–1072. doi: 10.1136/heartjnl-2019-316056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J. Am. Coll. Cardiol. 2016;67(1):1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Ghisi GLM, Kim WS, Cha S, et al. Women’s cardiac rehabilitation barriers: Results of the international council of cardiovascular prevention and rehabilitation’s first global assessment. Can. J. Cardiol. 2023 doi: 10.1016/j.cjca.2023.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Lin MT, Chen WC, Wu CH, et al. The effectiveness of cardiac rehabilitation in non-ischemic dilated cardiomyopathy patients: A pilot study. J. Formos. Med. Assoc. 2020;119(2):627–634. doi: 10.1016/j.jfma.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Stewart RAH, Held C, Hadziosmanovic N, et al. Physical activity and mortality in patients with stable coronary heart disease. J. Am. Coll. Cardiol. 2017;70(14):1689–1700. doi: 10.1016/j.jacc.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Lo YP, Chiang SL, Lin CH, et al. Effects of individualized aerobic exercise training on physical activity and health-related physical fitness among middle-aged and older adults with multimorbidity: A randomized controlled trial. Int. J. Environ. Res. Public Health. 2020;18(1):101. doi: 10.3390/ijerph18010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smart TFF, Doleman B, Hatt J, et al. The role of resistance exercise training for improving cardiorespiratory fitness in healthy older adults: A systematic review and meta-analysis. Age Ageing. 2022;51(6):143. doi: 10.1093/ageing/afac143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepera G, Christina M, Katerina K, et al. Effects of multicomponent exercise training intervention on hemodynamic and physical function in older residents of long-term care facilities: A multicenter randomized clinical controlled trial. J. Bodyw. Mov. Ther. 2021;28:231–237. doi: 10.1016/j.jbmt.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Lavie CJ, Milani RV, Littman AB. Benefits of cardiac rehabilitation and exercise training in secondary coronary prevention in the elderly. J. Am. Coll. Cardiol. 1993;22(3):678–683. doi: 10.1016/0735-1097(93)90176-2. [DOI] [PubMed] [Google Scholar]
- 18.Bruning RS, Sturek M. Benefits of exercise training on coronary blood flow in coronary arter y disease patients. Prog Cardiovasc Dis. 2015;57(5):443–453. doi: 10.1016/j.pcad.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenger NK. Exercise testing and training of the elderly coronary patient. Chest. 1992;101(5 Suppl):309s–s311. doi: 10.1378/chest.101.5_Supplement.309S. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Chen YJ, Tu HP, et al. Benefits of exercise training and the correlation between aerobic capacity and functional outcomes and quality of life in elderly patients with coronary artery disease. Kaohsiung J Med Sci. 2014;30(10):521–530. doi: 10.1016/j.kjms.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigienė A, Petruševičienė D, Barasaitė V, et al. Cardiac rehabilitation and complementary physical training in elderly patients after acute coronary syndrome: A pilot study. Medicina (Kaunas, Lithuania) 2021;57(6):529. doi: 10.3390/medicina57060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigienė A, Petruševičienė D, Barasaitė V, et al. Frailty and different exercise interventions to improve gait speed in older adults after acute coronary syndrome. Medicina (Kaunas, Lithuania) 2021;57(12):1344. doi: 10.3390/medicina57121344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deka P, Pathak D, Klompstra L, et al. High-intensity interval and resistance training improve health outcomes in older adults with coronary disease. J. Am. Med. Dir. Assoc. 2022;23(1):60–65. doi: 10.1016/j.jamda.2021.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Shen T, Ren C, Zhao W, et al. Development and validation of a prediction model for cardiovascular events in exercise assessment of coronary heart disease patients after percutaneous coronary intervention. Front. Cardiovasc. Med. 2022;9:798446. doi: 10.3389/fcvm.2022.798446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen T, Liu D, Lin Z, et al. A machine learning model to predict cardiovascular events during exercise evaluation in patients with coronary heart disease. J. Clin. Med. 2022;11(20):6061. doi: 10.3390/jcm11206061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guazzi M, Arena R, Halle M, et al. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2016;39(14):1144–1161. doi: 10.1093/eurheartj/ehw180. [DOI] [PubMed] [Google Scholar]
- 27.Dibben GO, Dalal HM, Taylor RS, et al. Cardiac rehabilitation and physical activity: Systematic review and meta-analysis. Heart (British Cardiac Society) 2018;104(17):1394–1402. doi: 10.1136/heartjnl-2017-312832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Ren C, Liu P, et al. Effect of smartphone-based telemonitored exercise rehabilitation among patients with coronary heart disease. J. Cardiovasc. Transl. Res. 2020;13(4):659–667. doi: 10.1007/s12265-019-09938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherrenberg M, Zeymer U, Schneider S, et al. EU-CaRE study: Could exercise-based cardiac telerehabilitation also be cost-effective in elderly? Int. J. Cardiol. 2021;340:1–6. doi: 10.1016/j.ijcard.2021.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data are available from the corresponding author upon reasonable request and with the permission of all contributing authors.