Abstract
A total of 119 Bacillus thuringiensis strains (83 type strains and 26 native isolates), as well as five B. cereus group species, were analyzed by repetitive extragenic palindromic sequence-based PCR analysis (Rep-PCR) fingerprinting. Primers Bc-REP-1 and Bc-REP-2 were specifically designed according to an extragenic 26-bp repeated sequence found in the six B. cereus group genomes reported. A total of 47 polymorphic bands were detected, and the patterns varied from 5 to 13 bands in number and from 0.2 to 3.8 kb in size. Virtually each type strain showed a distinctive B. cereus (Bc)-Rep-PCR pattern, except for B. thuringiensis serovars dakota (H serotype 15 [H15]) and sotto (H4a,4b), as well as serovars amagiensis (H29) and seoulensis (H35), which shared the same patterns. As expected, serovar entomocidus (H6) and its biovar subtoxicus showed an identical pattern; similarly, serovars sumiyoshiensis (H3a,3d) and fukuokaensis (H3a,3d,3e), which share two antigenic determinants, also showed identical Bc-Rep-PCR patterns. Interestingly, serovars israelensis (H14) and malaysiensis (H36), which share several phenotypic attributes, also showed identical Bc-Rep-PCR patterns. Native, coleopteran-active strains, including the self-agglutinated LBIT-74 strain, showed Bc-Rep-PCR patterns identical or very similar to that of the tenebrionis strain. Likewise, native mosquitocidal strains (including some self-agglutinated strains) also showed patterns identical or very similar to that of the serovar israelensis IPS-82 strain. Additionally, native β-exotoxin-producing strains from serovar thuringiensis showed patterns identical to that of the B. thuringiensis type strain. The B. cereus group-specific Bc-Rep-PCR fingerprinting technique was shown to be highly discriminative, fast, easy, and able to identify B. thuringiensis serotypes, including nonflagellar and self-agglutinated strains.
Bacillus thuringiensis is a gram-positive, flagellar, entomopathogenic bacterium that produces parasporal crystals constituted of insecticidal Cry proteins during the sporulation process (23, 52). Some strains also produce a thermostable adenine nucleotide analogue called β-exotoxin or thuringiensin (34). For decades, B. thuringiensis was developed and used as a control agent for lepidopteran pests, until the discovery of the mosquitocidal B. thuringiensis serovar israelensis in 1977 by Goldberg and Margalit (11) and the discovery of the coleopteran-active strain tenebrionis in 1983 by A. Krieg (31).
B. thuringiensis, Bacillus cereus, Bacillus anthracis, and Bacillus mycoides, as well as the recently described Bacillus pseudomycoides (41) and Bacillus weihenstephanensis (33), constitute the so-called B. cereus group. Several authors (4, 8, 19) have suggested that these species should constitute only one species, due to their high genetic similarity. From these species, B. thuringiensis is the most diverse, and its strains have been classified in 84 serovars (serovarieties) (32), including the recently described serovar jordanica (H serotype 71 [H71]) (29). Serotyping is still the most widely accepted subspecific classification technique for varieties of B. thuringiensis, even if strains from the same serovar do not necessarily share the biochemical, genetic, or toxicological attributes (3).
While some serovars, such as serovar israelensis (H14), include strains with practically the same attributes (2), other serovars include strains with a wide diversity of features. This is the case of serovar morrisoni (H8a,8a), which includes some strains with toxicity toward mosquito larvae (44), others toward coleopteran larvae (22), and some others toward lepidopteran larvae (13). On the other hand, strains from different serovars may show high biochemical, genetic, and toxicological similarity, such as strains IMR 81-1 (serovar malaysiensis), 11S2-1 (serovar canadensis), B 175 (serovar thompsoni), K6 (self agglutinated), and B 51(self agglutinated), highly similar to serovar israelensis (50). Additionally, serotyping is useless for nonmotile strains as well as the so-called self-agglutinated strains, besides the agglutination found in some B. cereus strains with H antigens (32, 42).
Alternative typing methods for B. thuringiensis strains have been tested, mostly based on molecular techniques, such as arbitrary primer-PCR technology (7, 18), ribosomal DNA restriction fragment length polymorphism (RFLP) (1, 48), and amplified fragment length polymorphism (AFLP) (45), among others (39, 58), most of them using a limited number of strains. Diversity of rRNA intergenic spacer sequences of 31 strains proved insufficient to discriminate between isolates (97 to 99% similarity) (6). On the other hand, ribotyping (16S, 23S, and 5S rRNA gene RFLP) of 80 serovars of B. thuringiensis showed a great diversity of patterns (27, 28), similar to the diversity found with fluorescent AFLP, when 34 B. thuringiensis serovars were analyzed along with strains of B. cereus and B. anthracis (21).
Repetitive extragenic palindromic sequence-based PCR analysis (Rep-PCR) is a DNA fingerprinting technique originally based on the design of PCR primers from Rep sequences found in the Escherichia coli and Salmonella typhimurium genomes (56). Amplicons obtained from contiguous Rep sequences generate distinctive electrophoretic patterns among different strains. Similar approaches use other repetitive sequences, such as the so-called ERIC and BOX sequences, developed for E. coli and S. typhimurium (24) and for Streptococcus pneumoniae, respectively (37). Rep-PCR fingerprint analysis of strains has proved to be simple, fast, and reproducible in a great variety of organisms (14, 36). However, this technique has been applied to organisms with little (if any) relationship with enterobacteria, that is, organisms with no homology whatsoever with the Rep sequences of E. coli, including some eukaryotic organisms (15, 38), which may indicate that these Rep-PCR analyses are arbitrary primer-PCR analyses, in those cases. This is the case of the Rep-PCR analysis of 28 B. thuringiensis serovars, using primers from the E. coli Rep sequence (46). We know now that this sequence is not found in the B. cereus group genomes.
This report presents the B. cereus (Bc)-Rep-PCR analysis of 125 B. thuringiensis strains, including 83 serovars, two biovars, and 26 native isolates, with primers specifically designed from a 26-bp Rep sequence found in the B. cereus group genomes.
MATERIALS AND METHODS
Bacterial strains.
Type and biotype strains of B. thuringiensis were kindly donated by the International Entomopathogenic Bacillus Center (IEBC), Pasteur Institute, France (Table 1), as well as other non-type strains, such as serovar Morrisoni strain tenebrionis (T08 017), serovar morrisoni PG14 (T08 018), the standard serovar israelensis (IPS-82), serovar canadensis 11S2.1 (T05A030), serovar thompsoni B175 (T12007), the autoagglutinated K6 (AAT028), and B51 (AATO21). B. cereus DSM31 (species type strain), B. cereus subsp. moritai (CER 081), B. cereus CER 183, B. mycoides IP-M 001 (species type strain), B. anthracis 7702, and the type strain of B. subtilis (IP-S 001) were donated by the Pasteur Institute. The B. thuringiensis serovar morrisoni strain san diego was directly isolated from the commercial product M-One (Mycogen Corp). Native strains (LBIT series) are part of the native B. thuringiensis stock collection at CINVESTAV-Irapuato, Mexico (Table 2).
TABLE 1.
B. thuringiensis type strains from the IEBC, Institut Pasteur, Paris, France subjected to Bc-Rep-PCR fingerprinting
| Serovar or biovar | H serotype | IEBC no. |
|---|---|---|
| Serovar | ||
| thuringiensis | 1 | T01 001 |
| finitimus | 2 | T02 001 |
| alesti | 3a,3c | T03 001 |
| kurstaki | 3a,3b,3c | T03A 001 |
| sumiyoshiensis | 3a,3d | T03B 001 |
| fukuokaensis | 3a,3d,3e | T03C 001 |
| sotto | 4a,4b | T04 001 |
| kenyae | 4a,4c | T04B 001 |
| galleriae | 5a,5b | T05 001 |
| canadensis | 5a,5c | T05A 001 |
| entomocidus | 6 | T06 001 |
| aizawai | 7 | T07 001 |
| morrisoni | 8a,8b | T08 001 |
| ostriniae | 8a,8c | T08A 001 |
| nigeriensis | 8b,8d | T08B 001 |
| tolworthi | 9 | T09 001 |
| darmstadiensis | 10a,10b | T10 001 |
| londrina | 10a,10c | T10A 001 |
| toumanoffi | 11a,11b | T11 001 |
| kyushuensis | 11a,11c | T11A 001 |
| thompsoni | 12 | T12 001 |
| pakistani | 13 | T13 001 |
| israelensis | 14 | T14 001 |
| dakota | 15 | T15 001 |
| indiana | 16 | T16 001 |
| tohokuensis | 17 | T17 001 |
| kumamotoensis | 18a,18b | T18 001 |
| yosso | 18a,18c | T18A 001 |
| tochigiensis | 19 | T19 001 |
| yunnanensis | 20a,20b | T20 001 |
| pondicheriensis | 20a,20c | T20A 001 |
| colmeri | 21 | T21 001 |
| shandongiensis | 22 | T22 001 |
| japonensis | 23 | T23 001 |
| neoleonensis | 24a,24b | T24 001 |
| novosibirsk | 24a,24c | T24A 001 |
| coreanensis | 25 | T25 001 |
| silo | 26 | T26 001 |
| mexicanensis | 27 | T27 001 |
| monterrey | 28a,28b | T28 001 |
| jegathesan | 28a,28c | T28A 001 |
| amagiensis | 29 | T29 001 |
| medellin | 30 | T30 001 |
| toguchini | 31 | T31 001 |
| cameroun | 32 | T32 001 |
| leesis | 33 | T33 001 |
| konkukian | 34 | T34 001 |
| seoulensis | 35 | T35 001 |
| malaysiensis | 36 | T36 001 |
| andaluciensis | 37 | T37 001 |
| oswaldocruzi | 38 | T38 001 |
| brasiliensis | 39 | T39 001 |
| huazhongensis | 40 | T40 001 |
| sooncheon | 41 | T41 001 |
| jinghongiensis | 42 | T42 001 |
| guiyangiensis | 43 | T43 001 |
| higo | 44 | T44 001 |
| roskildiensis | 45 | T45 001 |
| chanpaisis | 46 | T46 001 |
| wratislaviensis | 47 | T47 001 |
| balearica | 48 | T48 001 |
| muju | 49 | T49 001 |
| navarrensis | 50 | T50 001 |
| xiaguangiensis | 51 | T51 001 |
| kim | 52 | T52 001 |
| asturiensis | 53 | T53 001 |
| poloniensis | 54 | T54 001 |
| palmanyolensis | 55 | T55 001 |
| rongseni | 56 | T56 001 |
| pirenaica | 57 | T57 001 |
| argentinensis | 58 | T58 001 |
| iberica | 59 | T59 001 |
| pingluonsis | 60 | T60 001 |
| sylvestriensis | 61 | T61 001 |
| zhaodongensis | 62 | T62 001 |
| bolivia | 63 | T63 001 |
| azorensis | 64 | T64 001 |
| pulsiensis | 65 | T65 001 |
| graciosensis | 66 | T66 001 |
| vazensis | 67 | T67 001 |
| thailandensis | 68 | T68 001 |
| pahangi | 69 | T69 001 |
| sinensis | 70 | T70 001 |
| Biovars | ||
| dendrolimus | 4a,4b | T04A 001 |
| subtoxicus | 6 | T06A 001 |
TABLE 2.
Three groups of native B. thuringiensis isolates from the CINVESTAV-Irapuato stock collection subjected to Bc-Rep-PCR fingerprinting
| Group and isolate | H serotypea | Attribute |
|---|---|---|
| Group 1 | ||
| LBIT-13 | NM | Toxic to lepidopterans |
| LBIT-18 | 8a,8b | Toxic to coleopterans |
| LBIT-24 | 8a,8b | Toxic to coleopterans |
| LBIT-73 | 8a,8b | Toxic to coleopterans |
| LBIT-74 | SA | Toxic to coleopterans |
| LBIT-196 | NSP | Toxic to coleopterans |
| LBIT-358 | 8a,8b | Toxic to coleopterans |
| LBIT-419 | 8a,8b | Toxic to coleopterans |
| Group 2 | ||
| LBIT-52 | 4a,4b | Toxic to dipterans |
| LBIT-58 | 6 | Toxic to dipterans |
| LBIT-62 | NST | Toxic to dipterans |
| LBIT-93 | 8a,8b | Toxic to dipterans |
| LBIT-94 | 6 | Toxic to dipterans |
| LBIT-153 | 14 | Toxic to dipterans |
| LBIT-163 | NST | Toxic to dipterans |
| LBIT-201 | NST | Toxic to dipterans |
| LBIT-388 | SA | Toxic to dipterans |
| LBIT-393 | 14 | Toxic to dipterans |
| LBIT-396 | SA | Toxic to dipterans |
| LBIT-426 | NST | Toxic to dipterans |
| LBIT-432 | NST | Toxic to dipterans |
| Group 3 | ||
| LBIT-63 | 1 | β-Exotoxin |
| LBIT-279 | 1 | β-Exotoxin |
| LBIT-299 | 1 | β-Exotoxin |
| LBIT-301 | 1 | β-Exotoxin |
| LBIT-398 | SA | β-Exotoxin |
NM, nonmotile; NSP, nonserotypable; SA, self agglutinated; NST, not serotyped.
DNA extraction.
DNA was extracted from each strain, following a modified protocol reported previously (51). Fresh 30-ml Luria-Bertani broth cultures (optical density at 600 nm, 1) were centrifuged at 3,000 × g for 5 min at 4°C, and the pellets were washed again in 10 ml of J buffer (1.0 M Tris-HCl, 0.1 M EDTA, 0.15 M NaCl [pH 8]). Pellets were resuspended in 4 ml of J buffer, and lysozyme was added to a final concentration of 4 mg/ml, followed by incubation at 37°C for 30 min. Then, 50 μl of RNase (10 mg/ml) was added, and suspensions were incubated for 15 min at 50°C. Next, 200 μl of 20% sodium dodecyl sulfate was added and incubated for 20 min at 70°C, followed by the addition of 120 μl of proteinase K (10 mg/ml) and incubation overnight at 55°C. A total of 1.15 ml of NaCl 6 M was then added, gently mixed in ice for 15 min, and centrifuged at 3,900 × g for 20 min at 4°C. The supernatant was mixed with an equal volume of isopropanol and centrifuged at 17,000 × g for 20 min at 4°C. The pellet was washed with 70% ethanol, air dried, and dissolved in 200 μl of Tris-EDTA buffer (pH 8). DNA was quantified by spectrophotometry, and samples were stored at −20°C until further use.
Search for REPs in the B. cereus genome and primer design.
Due to the availability of the first B. cereus genome in 2003 (http://ergo.integratedgenomics.com/B_cereus.html), REP sequences were searched in this genome to design specific REP primers for the B. cereus group. All the extragenic sequences in the genome were analyzed with scripts written in Perl (http://www.perl.org/). Short REP sequences were combined to obtain larger ones until a highly conserved 26-bp sequence was found, showing the highest repeatability, in terms of both the number of repeats within the genome and the homology between the repeats. Its presence within the recently reported B. cereus group genomes was corroborated by searching the sequence in the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) (http://www.ebi.ac.uk/fasta33/) and the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) data banks. Direct and reverse primers were designed according to this sequence to amplify inter-REP regions.
Rep-PCR amplification conditions.
PCR mixtures were prepared as follows: 100 ng of template DNA, 300 ng of each primer, 5 mM MgCl2, 200 μM deoxynucleoside triphosphate mixture, and 2.5 U of Taq DNA polymerase (Invitrogen) to a 25-μl final volume. PCR amplifications were performed under the following conditions: an initial denaturation of 5 min at 94°C, followed by 34 cycles each of denaturation at 94°C for 1 min, annealing at 42°C for 1 min, and polymerization at 72°C for 1.5 min. Amplification was finished with an extension step at 72°C for 7 min. All PCR amplifications were performed with a Perkin-Elmer GeneAmp PCR System 2400. Amplified samples were kept at −20°C until electrophoretic analysis was performed.
Electrophoretic analysis.
Bc-Rep-PCR patterns were visualized by agarose gel electrophoresis. Aliquots of 10 μl each of the amplification products were loaded onto 1.2% agarose slabs (11 by 14 cm) and run in TAE (40 mM Tris-acetate, 1 mM EDTA) buffer at 2 V/cm during 5 h. Slabs were stained with 0.4 μg of ethidium bromide/ml and documented with a Gel Doc 2000 gel system (Bio-Rad). Molecular weight analysis of patterns was performed with the Quantity One version 4.2.1 software (Bio-Rad), with the 1-kb DNA ladder (Invitrogen) as a molecular weight marker.
Analysis of Bc-Rep-PCR patterns.
Polymorphic bands from all the Rep-PCR patterns were individually identified by their specific migration rates in the electrophoretic analyses. Once bands were properly and distinctively identified, binary (0/1) matrices were constructed to compare the patterns. Jaccard's similarity coefficients were generated by the SIMQUAL subroutine from the NTSYS-pc 2.02j (Applied Biostatistics, Inc.) package. Cluster analyses along with their corresponding dendrograms were generated by the unweighted-pair group method using average linkages (UPGMA), with the SAHN and TREE subroutines from the NTSYS-pc package.
RESULTS
REP sequence in the B. cereus genome.
The following 26-bp Bc-REP sequence was found in the B. cereus genome: CCCCACTGATTAAAGTTTCACTTTAT. Bases 11 to 16 paired with bases 20 to 25, forming a palindromic sequence and a potential hairpin with a 6-bp stem and an estimated total secondary structure energy of −2.2 Kcal/mol. This section of the Bc-REP is highly conserved in the analyzed genomes (see below). The Bc-REP sequence was analyzed by the fasta3 program (http://www.ebi.ac.uk/fasta33) and BLAST (http://www.ncbi.nlm.nih.gov/), finding the sequence in both directions of the B. cereus, B. anthracis, and B. thuringiensis reported genomes (Table 3). No further significant matches were found in all the genomes and nucleotide sequences available at the EMBL and NCBI gene banks, including the poorly sequenced B. mycoides (see below).
TABLE 3.
Frequency of the Bc-REP and designed primer sequences in five reported genomes of the B. cereus groupa
| Frequency | Genome
|
|||||
|---|---|---|---|---|---|---|
| Bc1 | Bc2 | Bc3 | Ba1 | Ba2 | Bt | |
| Bc-REP 100% | 28 | 58 | 15 | 14 | 14 | 15 |
| Bc-REP 96% | 24 | 18 | 6 | 6 | 6 | 8 |
| Direct | 89 | 94 | 32 | 28 | 28 | 33 |
| Reverse | 37 | 71 | 20 | 18 | 18 | 19 |
Bc1, B. cereus ATCC 14579 genome (NC 004722.1); Bc2, B. cereus ATCC 10987 genome (NC 003909.8); Bc3, B. cereus ZK genome (NC 006274); Ba1, B. anthracis strain Ames genome (NC 003997.3); Ba2, B. anthracis strain Sterne genome (NC 005945.1); Bt, B. thuringiensis strain 97.27 genome (NC 005957.1); Bc-REP 100%, frequency of sequences showing 100% homology with Bc-REP; Bc-REP 96%, frequency of sequences showing 96% homology with Bc-REP; Direct, frequency of sequences showing 100% homology with the designed direct primer; Reverse, frequency of sequences showing 100% homology with the designed reverse primer.
Primer design and PCR amplification.
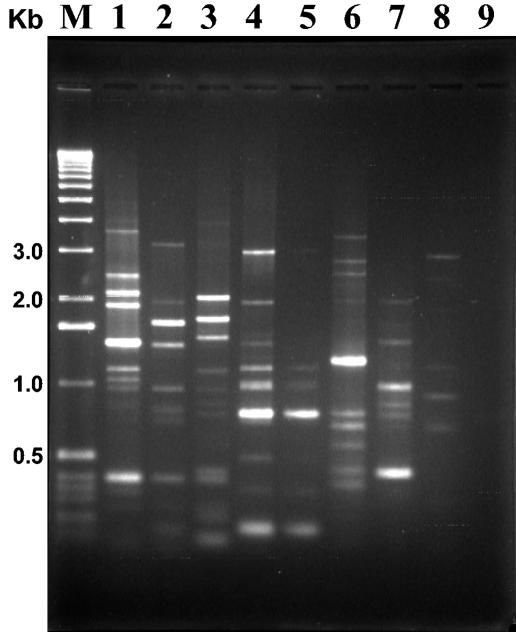
Two different primers were designed from the Bc-REP sequence, a direct 18-mer primer called Bc-REP-1 (5′-ATTAAAGTTTCACTTTAT-3′) and a reverse 14-mer primer called Bc-REP-2 (5′-TTTAATCAGTGGGG-3′), both with an estimated Tm of 42°C. These primers were frequently found in the B. cereus group reported genomes (Table 3). Primers were tested in combination and separately and under a series of Mg2+ and template DNA concentrations. Best amplification and defined patterns were obtained with the combination of primers, used with 5 mM Mg2+ concentration. No difference was detected when template DNA varied from 0.1 to 1 μg in the PCR mixture. Preliminary PCR tests with DNA from six different B. cereus group strains indicated the usefulness of those primers, as specific and reproducible patterns were obtained from B. thuringiensis serovar israelensis (mosquitocidal type strain), B. thuringiensis LBIT-13 (nonserotypable strain due to the lack of flagella), B. cereus DSM31 (species type strain), B. cereus subsp. moritai (CER 081), B. cereus CER 183, B. mycoides IP-M 001 (species type strain), and B. anthracis 7702. The type strain of B. subtilis (IP-S 001) was also included in the comparison, but only four faint (probably unspecific) bands were amplified (Fig. 1).
FIG. 1.
Bc-Rep-PCR fingerprint patterns of the B. cereus group strains. Lane 1, B. thuringiensis serovar israelensis; lane 2, B. thuringiensis LBIT-13; lane 3, B. cereus DSM31; lane 4, B. cereus CER81; lane 5, B. cereus CER 183; lane 6, B. mycoides IP-M 001; lane 7, B. anthracis 7702; lane 8, B. subtilis IP-S 001; lane 9, negative control; lane M, molecular weight marker (1-kb DNA ladder; Invitrogen).
Bc-Rep-PCR fingerprinting of B. thuringiensis type strains.
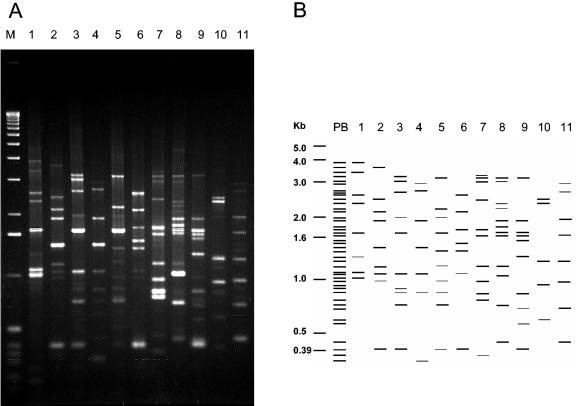
Once PCR conditions were established and primers were tested, 83 B. thuringiensis type strains, the biovars subtoxicus and dendrolimus, and strains tenebrionis and morrisoni PG14 were analyzed. Practically all the Bc-Rep-PCR patterns obtained from the type strains varied from 5 to 13 bands in number and from 0.2 to 3.8 kb in band size. Only serovars graciosensis (H66) and muju (H49) showed three bands in their patterns. In all, 47 polymorphic bands were identified from all the Bc-Rep-PCR patterns; no common bands were detected for all of them. Figure 2 shows the Bc-Rep-PCR patterns of 11 type serovar strains of B. thuringiensis, showing all the polymorphic bands, as well as a schematic representation of them.
FIG. 2.
Bc-Rep-PCR fingerprinting (A) and schematic representation (B) of 11 B. thuringiensis type strains. Lane 1, serovar amagiensis; lane 2, serovar israelensis; lane 3, serovar jinghongiensis; lane 4, serovar sumoyoshiensis; lane 5, serovar coreanensis; lane 6, serovar pakistani; lane 7, serovar konkukian; lane 8, serovar guiyangiensis; lane 9, serovar kurstaki; lane 10, serovar vazensis; lane 11, serovar brasiliensis; lane PB, polymorphic banding; lane M, molecular weight marker (1-kb DNA ladder; Invitrogen).
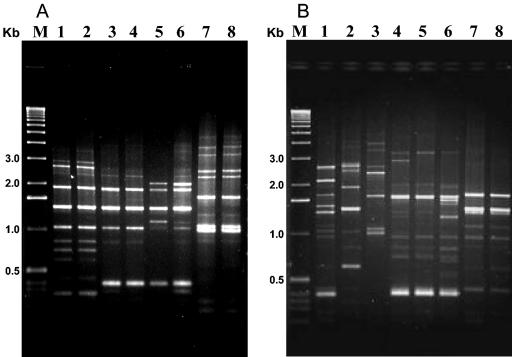
The overwhelming majority of the type strains showed distinctive Bc-Rep-PCR patterns. However, a few serovars shared the same pattern, such as the apparently unrelated serovars sotto (H4a,4b) and dakota (H15); the mosquitocidal (and highly related) serovars israelensis (H14) and malaysiensis (H36); also serovars sumiyoshiensis (H3a,3d) and fukuokaensis (H3a,3d,3e), which share two H-antigenic determinants; and the apparently unrelated serovars amagiensis (H29) and seoulensis (H35). The serovar entomocidus (H6) and its biovar subtoxicus (H6) also showed the same pattern, but the serovar sotto (H4a,4b) and its biovar dendrolimus (H4a,4b) showed a similar but not identical pattern, with the pattern of serovar leesis (H33) more similar to that of biovar dendrolimus (Fig. 3).
FIG. 3.
Bc-Rep-PCR fingerprinting of 17 different type strains of B. thuringiensis. (A) Lane 1, serovar sumiyoshiensis; lane 2, serovar fukuokaensis; lane 3, serovar dakota; lane 4, serovar sotto; lane 5, biovar dendrolimus; lane 6, serovar leesis; lane 7, serovar amagiensis; lane 8, serovar seoulensis. (B) Lane 1, serovar pakistani; lane 2, serovar alesti; lane 3, serovar kyushuensis; lane 4, serovar galleriae; lane 5, serovar aizawai; lane 6, serovar kurstaki; lane 7, serovar entomocidus; lane 8, biovar subtoxicus. Lanes M, molecular weight marker (1-kb DNA ladder; Invitrogen).
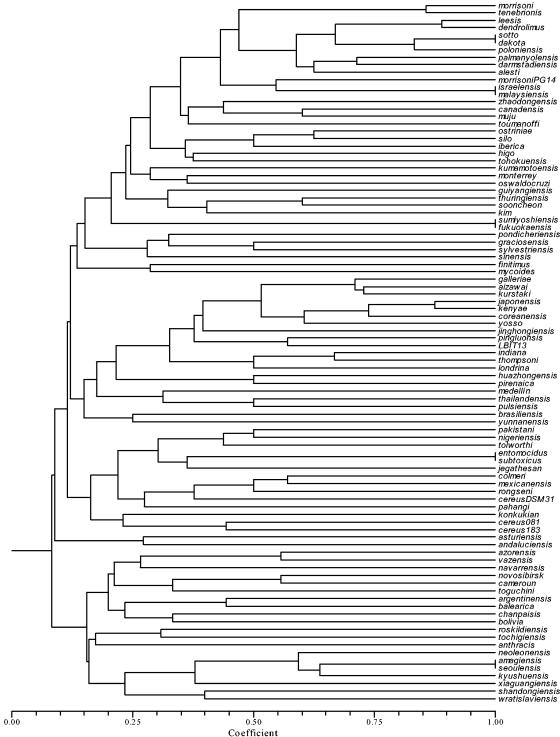
These results were corroborated once the binary matrix was analyzed and a dendrogram was generated by UPGMA (Fig. 4). It shows, for example, that the mosquitocidal strain serovar morrisoni PG14 (H8a,8b) was more related to other mosquitocidal strains, such as serovars israelensis (H14) and malaysiensis (H36), than to the other two serovar morrisoni (H8a,8b) strains (the type strain and tenebrionis). Interestingly, it also shows that all the strains that share H-antigenic determinants, such as serovars sotto (H4a,4b) and kenyae (H4a,4c), are widely separated in the dendrogram, just as happens with serovar alesti (H3a,3c) and serovar kurstaki (H3a,3b,3c), which are also separated in the dendrogram, and these from the serovar sumiyoshiensis-fukuokaensis complex. However, serovars kurstaki (H3a,3b,3c), galleriae (H5a,5b), and aizawai (H7) form a very tight group.
FIG. 4.
Dendrogram estimated from the Bc-Rep-PCR patterns obtained from 83 type serovars, two biovars, three isolates of B. thuringiensis, and five B. cereus group strains, using the Jaccard coefficient and UPGMA.
Bc-Rep-PCR fingerprinting of B. thuringiensis native isolates.
A total of 26 native strains, from the CINVESTAV-Irapuato B. thuringiensis stock collection (LBIT series), were analyzed by Bc-Rep-PCR (Table 2). They included the LBIT-13 nonmotile strain, 7 native strains with coleopteran activity, 13 mosquitocidal strains, and 5 β-exotoxin-producing strains. Among these strains, four (LBIT-74, LBIT-388, LBIT-396, and LBIT-398) are self agglutinated (nonserotypable).
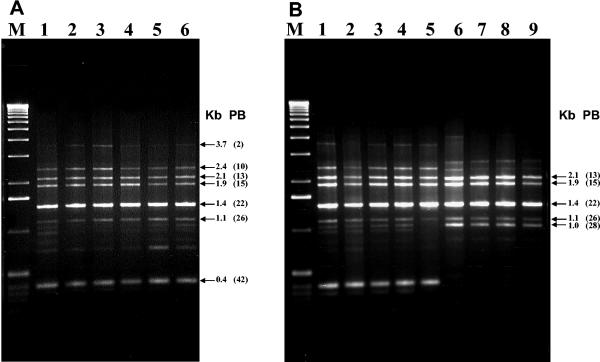
The mosquitocidal strains showed a Bc-Rep-PCR pattern identical to that of the mosquitocidal IPS-82 standard, including not only the serovar israelensis (H14) strains, but also one serovar kenyae strain (H4a,4c; strain LBIT-52) and one serovar entomocidus strain (H6; strain LBIT-58), as well as two self-agglutinated strains (LBIT-396 and LBIT-388) (Fig. 5). Additionally, another group of mosquitocidal strains, highly related to the first one, was represented by the serovar morrisoni PG-14 strain, along with strains LBIT-93 (serovar morrisoni; H8a,8b), LBIT-94 (entomocidus; H6), and LBIT-426 (not serotyped) (Fig. 5). Accordingly, other nonmosquitocidal strains, tested and characterized earlier (50), showed Bc-Rep-PCR patterns identical to that of israelensis (Fig. 6).
FIG. 5.
Bc-Rep-PCR fingerprint patterns of native mosquitocidal B. thuringiensis isolates. (A) Lane 1, serovar israelensis IPS-82; lane 2, LBIT-52 (H4a,4b); lane 3 LBIT-58 (H6); lane 4, LBIT-153 (H14); lane 5, LBIT-163 (not serotyped); lane 6, LBIT-201 (not serotyped). (B) Lane 1, LBIT-62 (not serotyped); lane 2, LBIT-388 (self agglutinated); lane 3, LBIT-393 (H 14); lane 4, LBIT-396 (self agglutinated); lane 5, LBIT-432 (not serotyped); lane 6, morrisoni PG14 (H8a,8b); lane 7, LBIT-93 (H8a,8b); lane 8, LBIT-94 (H6), lane 9, LBIT-426 (not serotyped). Lanes M, molecular weight marker (1-kb DNA ladder; Invitrogen); Kb, band size; PB, polymorphic band.
FIG. 6.
Bc-Rep-PCR fingerprint patterns of mosquitocidal B. thuringiensis strains. Lane 1, serovar morrisoni PG14 (H8a,8b); lane 2, serovar israelensis (H14); lane 3, serovar malaysiensis (H 36); lane 4, serovar canadensis 11S2.1 (H5a,5c); lane 5, serovar thompsoni B175 (H12); lane 6, K6 (self agglutinated); lane 7, B51 (self agglutinated); lane 8, serovar medellin 163.131 (H30); lane M, molecular weight marker (1-kb DNA ladder; Invitrogen).
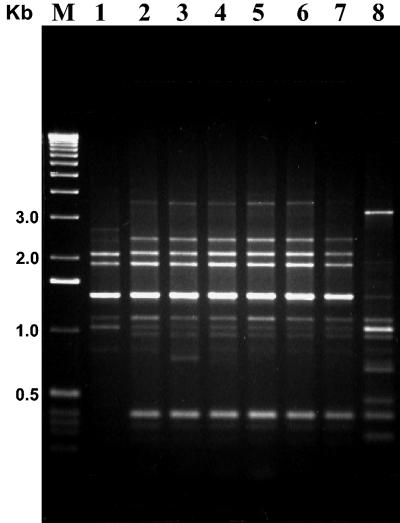
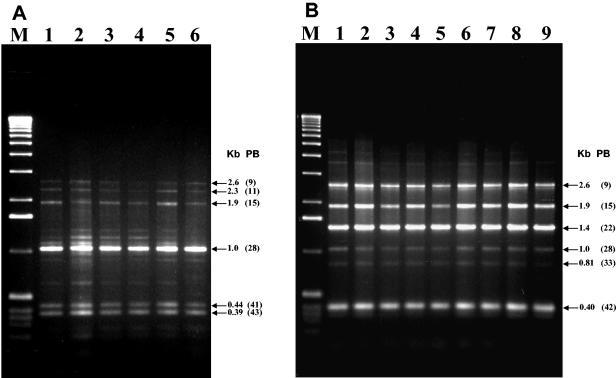
On the other hand, the seven strains with coleopteran activity showed Bc-Rep-PCR patterns identical to those of the reference serovar morrisoni tenebrionis strain and the san diego strain. They included not only serovar morrisoni (H8a,8b) strains but also one self-agglutinated strain (LIBT-74) and one nonserotypable strain (LBIT-196) (Fig. 7). Also, the β-exotoxin-producing strains, which belong to the serovar thuringiensis (H1) (except for the self-agglutinated LBIT-398 strain), showed Bc-Rep-PCR patterns identical to that of the type strain T01 001 (serovar thuringiensis; H1) (Fig. 7).
FIG. 7.
Bc-Rep-PCR fingerprint patterns of β-exotoxin-producing (A) and coleopteran-active (B) B. thuringiensis isolates. (A) Lane 1, serovar thuringiensis (T01 001); lane 2, LBIT-63 (H1); lane 3, LBIT-279 (H1); lane 4, LBIT-299 (H1); lane 5, LBIT-301 (H); lane 6, LBIT-398 (self agglutinated). (B) Lane 1, strain tenebrionis (T08 017); lane 2, strain san diego (H8a,8b); lane 3 LBIT-18 (H8a,8b); lane 4, LBIT-24 (H a,8b); lane 5, LBIT-73 (H8a,8b); lane 6, LBIT-74 (self agglutinated); lane 7, LBIT-196 (nonserotypable); lane 8, LBIT-358 (H8a,8b); lane 9, LBIT-419 (H8a,8b). Lanes M, molecular weight marker (1-kb DNA ladder; Invitrogen); Kb, band size; PB, polymorphic band.
DISCUSSION
Serotyping is the best-known technique for identifying and characterizing B. thuringiensis strains. So far, 84 serotypes and two biovars are known (29, 32); however, serotyping shows some constraints, such as its inability to process the so-called self-agglutinated and immobile (nonflagellar) strains. Also, this technique is unable to differentiate between B. thuringiensis and some B. cereus strains; it cannot show a phylogenetic relationship between the serotypes (32). Although serotyping is a reliable and straightforward technique, it is performed only in a few laboratories around the world, in particular, the Pasteur Institute in France, where the B. thuringiensis type collection is held. Therefore, alternative techniques (47), especially molecular techniques, are being developed to try to overcome those constrains.
Rep-PCR has been widely used on a variety of bacterial (and nonbacterial) species (5, 9, 15, 17, 38, 54) to characterize and identify strains. It has also been used for strains within the genus Bacillus (including B. thuringiensis), but based on the use of REPs found in other unrelated bacteria, such as the streptococcal BOX (10), the enterobacterial REP (35, 46), and the enterobacterial ERIC (35). In fact, a quick search for all these sequences in the six B. cereus group genomes reported showed no significant matches, indicating that actual BOX, REP, or ERIC analyses of these strains may be uncertain and should be reviewed. However, partial homology of enterobacterial REPs was found in Bacillus sporothermodurans, which allowed a real Rep-PCR analysis of the strains (20).
The presence of REP sequences in prokaryotes is common (36) and has been used for the design of species- or group-specific primers. That is the case of a 26-bp REP found in Neisseria spp., which allowed the design of specific primers for the analysis of N. gonorrhoeae and N. meningitides strains (57). Based on this approach, we looked for and found a 26-base REP common in the six B. cereus group reported genomes, which also include B. anthracis and B. thuringiensis. This REP (Bc-REP) allowed the design of two specific primers for the B. cereus group and proved their applicability by amplifying discrete and reproducible patterns in B. cereus, B. thuringiensis, B. anthracis, and B. mycoides strains. Their specificity was corroborated when a B. subtilis strain showed only faint bands and an undefined pattern, which may be caused to a partial homology with the Bc-REP.
The strong relationship between these species has been corroborated before, either by DNA hybridization (53), ribotyping (48), AFLP (21, 49, 55), or BOX-PCR (10) analyses of a number of strains. In all cases, strains from all these species intermingle within the same dendrogram, with, in general, the B. anthracis strains being the most homogeneous, the B. cereus and B. thuringiensis strains being the most diverse, and the B. mycoides strains being the least related to the rest. In our study, only three B. cereus, one B. anthracis, and one B. mycoides strains were analyzed; although all five strains intermingled in the same dendrogram with the B. thuringiensis strains, more strains from the other species were required to corroborate the same trend when Bc-REP-PCR analysis was used.
The main purpose of this report is the Rep-PCR characterization of the B. thuringiensis type strains, using specific primers for the B. cereus group. All the type strains were included, except for the most recent serotype described last year (29). Bc-REP-PCR fingerprinting of the type strains showed that practically all the serotypes displayed a distinct pattern. It also shows the putative phylogenetic relationship between the 83 serotypes and two biovars included in the analysis. Only a few strains showed identical patterns, such as serovars entomocidus and its biovar subtoxicus, both isolated by Heimpel (26) in Canada. Similar results were obtained by Phucharoen et al. (46) and Brousseau et al. (7), but they differ from the results obtained by Priest et al. (48) and Joung and Côté (27). On the other hand, Bc-REP-PCR patterns from the serovar sotto and its biovar dendrolimus slightly differ from each other, with the serovar sotto pattern identical to that of serovar dakota and the serovar leesis pattern the closest to that of biovar dendrolimus. Interestingly, ribotyping of these strains (27) indicated that while biovar dendrolimus and serovars leesis and dakota were phylogenetically related, serovar sotto was located in a separate group. Other serovars that shared the same Bc-REP-PCR pattern were serovars amagiensis and seoulensis. This is in agreement with a previous random amplified polymorphic DNA analysis of these strains (16); ribotyping also connects both strains in the same group (27).
Other serovars highly related by their Bc-REP-PCR pattern were serovars galleriae, aizawai, and kurstaki, which agrees with previously reported DNA hybridization and RFLP analyses (40, 48). These results may indicate that these associated and highly common serovars form a tight phylogenetically related group, whose segregation should be reviewed. Likewise, such segregation should be reviewed for the highly related serovars sumiyoshiensis and fukuokaensis, indiana and thompsoni; amagiensis, seoulensis, and kyushuensis; thuringiensis and sooncheon; azorensis and vazensis; and monterrey and oswaldocruzi. All these serovars appear closely related among each other in the phylogenetic dendrogram generated by the Bc-REP-PCR fingerprinting and by previously reported ribotyping (27). Other serovars highly related by their Bc-REP-PCR pattern, such as serovars silo and ostriniae, palmanyolensis and darmstadiensis, japonensis and kenyae, and colmeri and mexicanensis show less of a relationship by ribotyping analysis (27).
Serovars israelensis and malaysiensis also share the same Bc-REP-PCR pattern, which is in agreement with their high relationship (mosquitocidal specificity, cry gene content, and crystal morphology) (50); however, ribotyping is unable to recognize such a relationship and locate both strains in separate groups (27). Interestingly, other mosquitocidal strains with attributes practically identical to those of serovar israelensis such as K6 (AAT028), B51 (AATO21), canadensis 11S2.1 (T05A030), and serovar thompsoni B175 (T12007) (50) also display Bc-REP-PCR patterns identical to those of serovars israelensis and malaysiensis. On the other hand, the mosquitocidal serovar medellin, which has attributes different from those of serovar israelensis (43, 50), also shows a very different Bc-REP-PCR pattern. These results may indicate that the genomic relationship between the B. thuringiensis strains is not necessarily defined only by their toxic specificity, but by a series of attributes, such as cry gene content, crystal morphology, and plasmid pattern. This was also corroborated when native mosquitocidal isolates, highly related to serovar israelensis, showed identical Bc-REP-PCR patterns, even when some of these isolates were self agglutinated or belonged to a serotype different from that of israelensis. These results not only corroborate the reported genomic homogeneity of the serovar israelensis strains (2) but also imply that the same homogeneity occurs in other non-israelensis strains, as long as they share other attributes.
Another mosquitocidal strain, serovar morrisoni PG14, is known to share only some of the serovar israelensis characteristics (toxic specificity, some cry gene content, and crystal morphology) (25, 50); accordingly, its Bc-REP-PCR pattern is similar but not identical to that of serovar israelensis. Interestingly, its pattern is significantly different from that of the serovar morrisoni type strain. This serovar also includes the coleopteran-active strains tenebrionis and san diego, previously reported to be the same strain (30); however, contrary to the PG14 strain, the identical Bc-Rep-PCR pattern of both strains is very similar to that of the serovar morrisoni type strain. Also, similar to the results obtained with the mosquitocidal strains, the native coleopteran-active strains share the same Bc-Rep-PCR pattern as the serovar tenebrionis strain, most of them serotyped as serovar morrisoni but also including two nonserotypable strains. Genomic homogeneity may also occur in this group, similar to that observed with the serovar israelensis group. Interestingly, the same homogeneity was found in the group of native strains that produce β-exotoxin and belong to serovar thuringiensis (H1) (except for the self-agglutinated LBIT-398 strain). These results may indicate that Bc-Rep-PCR fingerprinting of B. thuringiensis strains is useful not only to differentiate between serovars, but also to properly identify the nonserotypable strains and, most of all, to recognize more accurately the evolutionary relationship between strains, to whichever serovar they belong.
B. thuringiensis constitutes a genetically diverse species; the great number of strains known today may form distinctive groups, according to their phenetic and genetic traits. Serotyping has been a useful tool to try to discriminate those groups since its establishment in 1962 (12); however, as strains mounted up, shortcomings started to appear in the technique. Molecular tools have been developed in recent years, trying to offer a new typing alternative for B. thuringiensis strains and to recognize the actual phylogenetic relationships between subspecific groups. Bc-Rep-PCR offers a new tool to identify these groups, based on the use of specific primers designed from a REP sequence found in the B. cereus group. The potential of this technique was tested in this work and proved to be sensitive, specific, reproducible, and fast; it may become a standardized characterization procedure. It may also help in the establishment of a new subspecies-level classification of B. thuringiensis.
Acknowledgments
We thank Regina Basurto, Guadalupe Mireles, Juan Caballero, and Javier Luévano for their excellent technical support.
This work was partially supported by grant 35320-B, CONACYT, Mexico.
REFERENCES
- 1.Akhurst, R. J., E. W. Lyness, Q. Y. Zhang, D. J. Cooper, and D. E. Pinnock. 1997. A 16S rRNA gene oligonucleotide probe for identification of Bacillus thuringiensis isolates from sheep fleece. J. Invertebr. Pathol. 69:24-30. [DOI] [PubMed] [Google Scholar]
- 2.Ankarloo, J., D. A. Caugant, B. M. Hansen, A. Berg, A. B. Kolstø, and A. Lövgren. 2000. Genome stability of Bacillus thuringiensis subsp. israelensis isolates. Curr. Microbiol. 40:51-56. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, A. I., W. Beckman, and P. Dunn. 1986. Bacillus thuringiensis and related insect pathogens. Microbiol. Rev. 50:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash, C., J. A. E. Farrow, M. Dorsch, E. Stackebrandt, and M. D. Collins. 1991. Comparative analysis of Bacillus anthracis, Bacillus cereus, and related species on the basis of reverse transcriptase sequencing of 16S rRNA. Int. J. Syst. Bacteriol. 41:343-346. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya, D., P. M. Sarma, S. Krishnan, S. Mishra, and B. Lal. 2003. Evaluation of genetic diversity among Pseudomonas citronellolis strains isolated from oily sludge-contaminated sites. Appl. Environ. Microbiol. 69:1435-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourque, S. N., J. R. Valero, M. C. Lavoie, and R. C. Levesque. 1995. Comparative analysis of the 16S to 23S ribosomal intergenic spacer sequences of Bacillus thuringiensis strains and subspecies and of closely related species. Appl. Environ. Microbiol. 61:1623-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brousseau, R., A. Saint-Onge, G. Préfontaine, L. Masson, and J. Cabana. 1993. Arbritary primer polymerase chain reaction, a powerful method to identify Bacillus thuringiensis serovars and strains. Appl. Environ. Microbiol. 59:114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson, C. R., D. Caugant, and A. B. Kolstø. 1994. Genotypic diversity among Bacillus cereus and Bacillus thuringiensis strains. Appl. Environ. Microbiol. 60:1719-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L. S., A. Figueredo, F. O. Pedrosa, and M. Hungria. 2000. Genetic characterization of soybean rhizobia in Paraguay. Appl. Environ. Microbiol. 66:5099-5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherif, A., L. Brusetti, S. Borin, A. Rizzi, A. Boudabous, H. Khyami-Horani, and D. Daffonchio. 2003. Genetic relationship in the ‘Bacillus cereus group’ by rep-PCR fingerprinting and sequencing of a Bacillus anthracis-specific rep-PCR fragment. J. Appl. Microbiol. 94:1108-1119. [DOI] [PubMed] [Google Scholar]
- 11.de Barjac, H. 1978. Une nouvelle varietè de Bacillus thuringiensis très toxique pour les moustiques: B. thuringiensis var. israelensis serotype 14. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 286:797-800. [PubMed] [Google Scholar]
- 12.de Barjac, H., and A. Bonnefoi. 1962. Essai de classification biochimique et sérologique de 24 souches de Bacillus du type B. thuringiensis. Entomophaga 7:5-31. [Google Scholar]
- 13.de Barjac, H., and E. Frachon. 1990. Classification of Bacillus thuringiensis strains. Entomophaga 35:233-240. [Google Scholar]
- 14.de-Brujin, F. 1992. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergenic consensus) sequences and polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl. Environ. Microbiol. 58:2180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edel, V., C. Steinberg, I. Avelange, G. Laguerre, and C. Alabouvette. 1995. Comparison of three molecular methods for the characterization of Fusarium oxysporum strains. Phytopathology 85:579-585. [Google Scholar]
- 16.Gaviria-Rivera, A. M., and F. G. Priest. 2003. Molecular typing of Bacillus thuringiensis serovars by RAPD-PCR. Syst. Appl. Microbiol. 26:254-261. [DOI] [PubMed] [Google Scholar]
- 17.Gonzaléz, Á., N. Hierro, M. Poblet, N. Rozes, A. Mas, and J. M. Guillamón. 2004. Application of molecular methods for the differentiation of acetic acid bacteria in a red wine fermentation. J. Appl. Microbiol. 96:853-860. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, B. M., P. H. Damgaard, J. Eilenberg, and J. C. Pederson. 1998. Molecular and phenotypic characterization of Bacillus thuringiensis isolated from leaves and insects. J. Invertebr. Pathol. 71:106-144. [DOI] [PubMed] [Google Scholar]
- 19.Helgason, E., O. A. Økstad, D. A. Caugant, H. A. Johansen, A. Fouet, M. Mock, I. Hegna, and A.-B. Kolstø. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman, L., and M. Heyndrickx. 2000. The presence of intragenically REP-like elements in Bacillus sporothermodurans is sufficient for REP-PCR typing. Res. Microbiol. 151:255-261. [DOI] [PubMed] [Google Scholar]
- 21.Hill, K. K., L. O. Ticknor, R. T. Okinaka, M. Asay, H. Blair, K. A. Bliss, M. Laker, P. E. Pardington, A. P. Richardson, M. Tonks, D. J. Beecher, J. D. Kemp, A. B. Kolstø, A. C. L. Wong, P. Keim, and P. J. Jackson. 2004. Fluorescent amplified fragment length polymorphism analysis of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis isolates. Appl. Environ. Microbiol. 70:1068-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höfte, H., J. Seurink, A. Van Houtven, and M. Vaeck. 1987. Nucleotide sequence of a gene encoding an insecticidal protein of Bacillus thuringiensis var. tenebrionis toxic against coleoptera. Nucleic Acids Res. 15:7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höfte, H., and H. R. Whiteley. 1989. Insecticidal crystal proteins of Bacillus thuringiensis. MIcrobiol. Rev. 53:242-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulton, C. S. J., C. F. Higgins, and P. M. Sharp. 1991. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol. Microbiol. 5:825-834. [DOI] [PubMed] [Google Scholar]
- 25.Ibarra, J. E., and B. A. Federici. 1986. Parasporal bodies of Bacillus thruringiensis subsp. morrisoni (PG-14) and Bacillus thuringiensis subsp. israelensis are similar in protein composition and toxicity FEMS Microbiol. Lett. 39:79-84. [Google Scholar]
- 26.International Entomopathogenic Bacillus Centre. 1998. International Entomopathogenic Bacillus Centre catalogue no. 1. Unité des Bactéries Entomopathogènes. Institut Pasteur, Paris, France.
- 27.Joung, K. B., and J. C. Côté. 2001. Phylogenetic analysis of Bacillus thuringiensis serovars based on 16S rRNA gene restriction fragment length polymorphisms. J. Appl. Microbiol. 90:115-122. [DOI] [PubMed] [Google Scholar]
- 28.Joung, K. B., and J. C. Côté. 2001. A phylogenetic analysis of Bacillus thuringiensis serovars by RFLP-based ribotyping. J. Appl. Microbiol. 91:279-289. [DOI] [PubMed] [Google Scholar]
- 29.Khyami-Horani, H., M. Hajaij, and J. F. Charles. 2003. Characterization of Bacillus thuringiensis ser. jordanica (serotype H 71), a novel serovariety isolated in Jordan. Curr. Microbiol. 47:26-31. [DOI] [PubMed] [Google Scholar]
- 30.Krieg, V. A., A. M. Huger, and W. Schnetter. 1987. “Bacillus thuringiensis var. san diego” Stamm M-7 ist identisch mit dem zuvor in Deutschland isolierten käferwirsamen B. thuringiensis subsp. tenebrionis Stamm BI 256-82. J. Appl. Entomol. 104:417-424. [Google Scholar]
- 31.Lambert, B., and M. Peferoen. 1992. Insecticidal promise of Bacillus thuringiensis. Facts and mysteries about a successful biopesticide. BioScience 42:112-122. [Google Scholar]
- 32.Lecadet, M.-M., E. Frachon, V. C. Dumanoir, H. Ripouteau, S. Hamon, P. Laurent, and I. Thiéry. 1999. Updating the H-antigen classification of Bacillus thuringiensis. J. Appl. Microbiol. 86:660-672. [DOI] [PubMed] [Google Scholar]
- 33.Lechner, S., R. Mayer, K. P. Francis, B. M. Pruss, T. Kaplan, E. Wiessner-Gunkel, G. S. Stewart, and S. Scherer. 1998. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Bacteriol. 48:1373-1382. [DOI] [PubMed] [Google Scholar]
- 34.Levinson, B. L., K. J. Kasyan, S. S. Chiu, T. C. Currier, and J. M. González, Jr. 1990. Identification of β-exotoxin production, plasmids encoding β-exotoxin, and a new exotoxin in Bacillus thuringiensis by using high-performance liquid chromatography. J. Bacteriol. 172:3172-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima, A. S. G., A. M. Guidelli, I. L. Abreu, and M. V. F. Lemos. 2002. Identification of new isolates of Bacillus thuringiensis using rep-PCR products and δ-endotoxin electron microscopy. Gen. Mol. Biol. 25:225-229. [Google Scholar]
- 36.Lupski, J. R., and G. M. Weinstock. 1992. Short, interspersed repetitive DNA sequences in prokaryotic genomes. J. Bacteriol. 174:4525-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin, B., O. Humbert, M. Camara, E. Guenci, J. Walker, T. Mitchell, P. Andrew, M. Prudhomme, G. Alloing, R. Hukenbeck, D. A. Morrison, G. J. Boulnois, and J. P. Claverys. 1992. A highly conserved repeated DNA element located in the chromosome of Streptococcus pneumoniae. Nucleic Acids Res. 20:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald, J. G., E. Wong, and G. P. White. 2000. Differentiation of Tilletia species by rep-PCR genomic fingerprinting. Plant. Dis. 84:1121-1125. [DOI] [PubMed] [Google Scholar]
- 39.Miteva, V., A. Abadjieva., and R. Grigorova. 1991. Differentiation among strains and serotypes of Bacillus thuringiensis by M13 fingerprinting. J. Gen. Microbiol. 137:593-600. [Google Scholar]
- 40.Nakamura, L. K. 1994. DNA relatedness among Bacillus thuringiensis serovars. Int. J. Syst. Bacteriol. 44:125-129. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura, L. K. 1998. Bacillus pseudomycoides sp. nov. Int. J. Syst. Bacteriol. 48:1031-1034. [DOI] [PubMed] [Google Scholar]
- 42.Ohba, H., and K. Aizawa. 1986. Frequency of acrystalliferous spore-forming bacteria possessing flagellar antigens of Bacillus thuringiensis. J. Basic Microbiol. 26:185-188. [Google Scholar]
- 43.Orduz, S., W. Rojas, M. M. Correa, A. E. Montoya, and H. de Barjac. 1992. A new serotype of Bacillus thuringiensis from Colombia toxic to mosquito larvae. J. Invertebr. Pathol. 59:99-103. [DOI] [PubMed] [Google Scholar]
- 44.Padua, L. E., M. Ohba, and K. Aizawa. 1984. Isolation of a Bacillus thuringiensis strain (serotype 8a:8b) highly and selectively toxic against mosquito larvae. J. Invertebr. Pathol. 44:12-17. [Google Scholar]
- 45.Pattanayak, D., K. Srinivasan, A. D. Mandaokar, A. Shukla, R. Bhalla, and P. A. Kumar. 2000. AFLP fingerprinting and genotypic characterization of some serovares of Bacillus thuringiensis. World J. Microbial. Biotechnol. 16:667-672. [Google Scholar]
- 46.Phucharoen, K., W. Chugjatupornchai, and S. Panyim. 1999. Differentiation of Bacillus thuringiensis subspecies using repetitive extragenic palindromic PCR (REP-PCR) genomic fingerprinting. Asia Pac. J. Mol. Biol. Biotechnol. 7:79-83. [Google Scholar]
- 47.Priest, F. G., M. Goodfellow, and C. Todd. 1988. A numerical classification of the genus Bacillus. J. Gen. Microbiol. 134:1847-1882. [DOI] [PubMed] [Google Scholar]
- 48.Priest, F. G., D. A. Kaji, Y. B. Rosato, and V. P. Canhos. 1994. Characterization of Bacillus thuringiensis and related bacteria by ribosomal RNA gene restriction fragment length polymorphisms. Microbiology 140:1015-1022. [DOI] [PubMed] [Google Scholar]
- 49.Radnedge, L., P. G. Agron, K. K. Hill, P. J. Jackson, L. O. Ticknor, P. Keim, and. G. L. Anderson. 2003. Genome differences that distinguish Bacillus anthracis from Bacillus cereus and Bacillus thuringiensis. Appl. Envirol. Microbiol. 69:2755-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ragni, A., I. Thiéry, and A. Delécluse. 1996. Characterization of six highly mosquitocidal Bacillus thuringiensis strains that do not belong to H-14 serotype. Curr. Microbiol. 32:48-54. [DOI] [PubMed] [Google Scholar]
- 51.Rosso, M. L., and A. Delécluse. 1997. Distribution of the insertion element IS240 among Bacillus thuringiensis strains. Curr. Microbiol. 34:348-353. [DOI] [PubMed] [Google Scholar]
- 52.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Somerville, H. J., and M. L. Jones. 1972. DNA competition studies within the Bacillus cereus group of bacilli. J. Gen. Microbiol. 73:257-265. [DOI] [PubMed] [Google Scholar]
- 54.Spigaglia, P., and P. Mastrantonio. 2003. Evaluation of repetitive element sequence-based PCR as a molecular typing method for Clostridium difficile. J. Clin. Microbiol. 41:2454-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ticknor, L. O., A.-B. Kolstø, K. K. Hill, P. Keim, M. Laker, M. Tonks, and P. J. Jackson. 2001. Fluorescent amplified fragment length polymorphism analysis of Norwegian Bacillus cereus and Bacillus thuringiensis soil isolates. Appl. Environ. Microbiol. 67:4863-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Versalovic, J., M. Schneider, F. J. De Bruijn, and J. R. Lupski. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5:25-40. [Google Scholar]
- 57.Versalovic, J., and J. R. Lupski. 1995. DNA Fingerprinting of Neisseria strains by rep-PCR. Methods Mol. Cell. Biol. 5:96-104. [Google Scholar]
- 58.Yu, J., L. Tan, Y. Liu, and Y. Pang. 2002. Phylogenetic analysis of Bacillus thuringiensis based on PCR amplified fragment polymorphisms of flagellin genes. Curr. Microbiol. 45:139-143. [DOI] [PubMed] [Google Scholar]