Abstract
The virulent cos-type Streptococcus thermophilus phage DT1 was previously isolated from a mozzarella whey sample, and its complete genomic sequence is available. The putative ori of phage DT1 is characterized by three inverted and two direct repeats located in a noncoding region between orf36 and orf37. As the replication ability of the putative ori and flanking genes could not be established, its ability to confer phage resistance was tested. When ori is cloned on a high-copy-number plasmid, it provides protection to S. thermophilus strains against phage infection during milk fermentation. This protection is phage specific and strain dependent. Then, a detailed transcriptional map was established for the region located between the cro-like gene (orf29) and the ori. The results of the Northern blots indicated that the transcription of this region started 5 min after the onset of phage infection. Comparative analysis of the expression of the cro-ori region in the three S. thermophilus cos-type phages DT1, Sfi19 (virulent), and Sfi21 (temperate) reveals significant differences in the number and size of transcripts. The promoter upstream of orf29 was further investigated by primer extension analysis, and its activity was confirmed by a chloramphenicol acetyltransferase assay, which showed that the phage promoter is more efficient than the constitutive bacterial promoter of the S. thermophilus operon encoding the general proteins of the phosphoenolpyruvate:sugar phosphotransferase system. However, the phage promoter is less efficient than the pts promoter in Lactococcus lactis and in Escherichia coli.
Thermophilic lactic acid bacteria, such as Streptococcus thermophilus, are widely used as starter cultures in many yogurt- and cheese-manufacturing processes. Bacteriophage infection of these lactic acid bacteria is the main factor impairing lactic acid fermentations, which ultimately decreases the efficiency of the production process and the value of the final product.
S. thermophilus phages are currently classified into two general groups based on their mode of DNA packaging and their major structural proteins (27). The first group includes phages with cohesive genome extremities (cos type), and the second group contains phages with a DNA packaging scheme that proceeds via a headful mechanism (pac type) (27). To date, the complete nucleotide sequence of six S. thermophilus phage genomes is available through public databases: four cos-type phages, Sfi21 (temperate) (6), DT1 (virulent) (52), Sfi19 (virulent) (33), and 7201 (virulent) (49), and two pac-type phages, O1205 (temperate) (48) and Sfi11 (virulent) (31). Comparative analysis indicated a close genetic relationship between temperate and virulent S. thermophilus phages. It was even proposed that virulent S. thermophilus phages were derived from temperate phages by a combination of rearrangements and deletion events within the lysogeny module (4, 33). Despite these genetic modifications in the lysogeny module, a gene coding for a cro-like repressor is still found among all the virulent S. thermophilus phages analyzed so far (32), and it could play a role in the replication of these phages.
Our knowledge of phage DNA replication and gene expression is still limited for S. thermophilus phages. The availability of such information will likely point towards novel means of controlling phage infections. For instance, phage resistance mechanisms have been engineered with phage genetic elements, such as the origin of replication (ori) and antisense RNA genes (for a review, see reference 10). Phage oris are mainly characterized by a noncoding region containing several inverted and direct repeats (20, 36). An antiphage system named PER (phage-encoded resistance) was previously associated with the presence of the bacteriophage ori provided on a plasmid (20). It is presumed that phage replication factors are titrated by the plasmid harboring the phage ori. This effect was first described in Lactococcus lactis (20) and later in S. thermophilus with the ori from phages Sfi19, Sfi21, O1205, 7201, and κ3 (15, 49, 50).
The gene organization in the vicinity of ori is relatively conserved among S. thermophilus phages. This region is essentially composed of genes expressed early after the onset of infection, such as those encoding the helicase and primase. Interestingly, antiphage systems based on the expression of an antisense RNA, complementary to the helicase (50) as well as the primase (51) mRNAs of a cos-type S. thermophilus bacteriophage, were recently designed. The antisense RNA strategy is effective against cos-type and pac-type bacteriophages that have homologous genes. The gene silencing technology is even more effective if combined with the PER system. It is noteworthy that these phage-based antiviral strategies were developed without prior analysis of the phage genome expression. Only recently, the complete transcriptional maps of the S. thermophilus cos-type phages Sfi19 (54) and Sfi21 (55, 56) were reported. Transcriptional maps of some lactococcal phages are also available (30, 34, 40). Usually, three classes of transcripts are observed: early, middle, and late. Most of them are not preceded by a consensus promoter sequence (55, 56).
The cos-type phage DT1 was previously isolated from a failed milk fermentation, and its complete genomic sequence was the first available for a virulent S. thermophilus phage (52). In this study, we report the genetic analysis of the cro-ori region of phage DT1.
MATERIALS AND METHODS
Bacterial strains, plasmids, and bacteriophages.
The bacteria, phages, and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown aerobically at 37°C in Luria-Bertani (LB) medium. S. thermophilus strains were grown at 42°C without agitation in M17 broth (Quélab) supplemented with 0.5% (wt/vol) lactose (LM17). L. lactis strains were grown at 30°C without agitation in M17 broth supplemented with 0.5% (wt/vol) glucose (GM17). When needed, antibiotics were added as follows: 20 μg of chloramphenicol per ml or 250 μg of erythromycin per ml for E. coli, and 5 μg of chloramphenicol per ml or 5 μg of erythromycin per ml for L. lactis and S. thermophilus. The culture media used in phage experiments were supplemented with 10 mM CaCl2.
TABLE 1.
Bacterial strains, phages, and plasmids
| Strain, phage, or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Cloning host strain | Invitrogen |
| SMQ-538 | DH5α(pFB1); Emr Cmr | This work |
| SMQ-636 | DH5α(pFB4); Apr Cmr | This work |
| SMQ-822 | DH5α(pBV5030); Emr | This work |
| SMQ-823 | DH5α(pGA1); Emr Cmr | This work |
| S. thermophilus | ||
| ATCC 19258 | Source of pts promoter | American Type Culture Collection |
| SMQ-119 | Host for phages Q1 and Q3 | 39 |
| SMQ-301 | Host for phage DT1 | 52 |
| SMQ-495 | Host for phages DT1 and MD2 | 14 |
| SMQ-533 | SMQ-301(pFB3); Cmr | This work |
| SMQ-535 | SMQ-495(pFB3); Cmr | This work |
| SMQ-536 | SMQ-495(pNZ123); Cmr | This work |
| SMQ-629 | SMQ-301(pFB1); Emr Cmr | This work |
| SMQ-630 | SMQ-301(pBV5030); Emr | This work |
| SMQ-633 | SMQ-301(pNZ123); Cmr | This work |
| SMQ-634 | SMQ-119(pNZ123); Cmr | This work |
| SMQ-635 | SMQ-119(pFB3); Cmr | This work |
| SMQ-817 | SMQ-301(pGA1); Emr Cmr | This work |
| L. lactis | ||
| MG1363 | Plasmid-free strain | 16 |
| SMQ-537 | MG1363(pFB1); Emr Cmr | This work |
| SMQ-819 | MG1363(pBV5030); Emr | This work |
| SMQ-820 | MG1363(pGA1); Emr Cmr | This work |
| Bacteriophages | ||
| DT1 | cos type | 52 |
| MD2 | cos type | 14 |
| Q1 | pac type | 39 |
| Q3 | pac type | 39 |
| Plasmids | ||
| pBV5030 | Promoter probe vector; Emr | 2 |
| pFB1 | 268-bp amplified fragment of P1 promoter cloned into pBV5030 | This work |
| pFB3 | 750-bp EcoRI restriction fragment from DT1 cloned into pNZ123; PER+ | This work |
| pFB4 | 5-kb HindIII restriction fragment from DT1 cloned into pTRK333 | This work |
| pGA1 | 273-bp amplified fragment of pts promoter cloned into pBV5030 | This work |
| pNZ123 | Cloning vector; Cmr | 13 |
| pTRK333 | Origin-screening vector; Apr Tetr Cmr | 38 |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Emr, erythromycin resistance; PER+, phage-encoded resistance; Tetr, tetracycline resistance.
Phages were propagated according to Jarvis (22) and purified on a discontinuous-step CsCl gradient followed by a one-step continuous gradient with a CsCl solution density of 1.4 g/ml instead of 1.5 g/ml. For plaque enumeration, 100 μl of phage suspension and 500 μl of a S. thermophilus culture (optical density at 600 nm [OD600] of 1.0) were added to 3 ml of LM17 top agar and poured on LM17 plates supplemented with 0.1% (vol/vol) milk and 0.25% (wt/vol) glycine to increase plaque size. Growth curves, burst size, and the efficiency at which center of infection formed (ECOI) were performed as reported earlier (37). The efficiency of plaque formation (EOP) was calculated as described previously (42).
DNA techniques.
Routine DNA manipulations were performed according to Sambrook and Russell (41). The Maxi Lambda DNA purification kit (Qiagen) was used for phage DNA purification as specified previously (25). To determine the ability of the phage DT1 ori to drive plasmid replication, DNA restriction fragments obtained from digestion of the phage genome with XbaI or HindIII were cloned into the vector pTRK333 (38), which can replicate in E. coli but not in S. thermophilus. This vector also contains a chloramphenicol acetyltransferase gene functional in S. thermophilus. Plasmid DNA transformation from the clones obtained in E. coli was then attempted in S. thermophilus SMQ-301. Ligation mixtures were also electroporated directly into S. thermophilus. Growth of S. thermophilus transformants on chloramphenicol-containing media can only occur in the presence of a functional replicon.
To verify the PER effect of phage DT1 ori on various S. thermophilus phages, a 750-bp EcoRI fragment (coordinates 29805 to 30555) containing the ori was cloned into the high-copy vector pNZ123 (13). The resulting plasmid, pFB3, was then transformed into S. thermophilus with the usual procedures (7, 21).
The P1 promoter region of phage DT1 was amplified with the primers ORIPRO (5′-GAATTCGTCGACCGGTCGCAAAAACCGACTTGAGTGGA-3′) and FB2 (5′-CTGCAGCTGCAGTCATTTTGTTGTTCCTCCTTCCT-3′) containing restriction sites for EcoRI and PstI. The amplicon was then cloned into the promoter-screening vector pBV5030 (2) to generate pFB1. The promoter region of the pts operon of S. thermophilus ATCC 19258 was amplified with the primers PTSP1 (5′-GAATTCGAATTCTATATGATTGTTTTTGCACAAAAATG-3′) and PTSP2 (5′-CTGCAGCTGCAGGCCATAATGGAGTCTCCTTCT-3′). The amplicon was cloned into pBV5030 to generate pGA1. Clones were confirmed by DNA sequencing performed by the DNA sequencing service of the Université Laval with an ABI Prism 3100 apparatus. Sequence analysis was performed with the Wisconsin Package software (version 10.3) of the Genetics Computer Group (12). The sequences used in this study were from accession numbers AF085222 (complete genome of phage DT1), AF115102 (complete genome of phage Sfi19), and NC_000872 (complete genome of phage Sfif21).
Replication of DT1 in the presence of its ori in trans.
The experiment was conducted with S. thermophilus SMQ-301(pNZ123) and S. thermophilus SMQ-301(pFB3). Phage DT1 replication in infected cells was monitored according to Hill et al. (19, 20) with the following modifications: LM17 broth was inoculated at 1% (vol/vol) with an overnight culture and incubated at 42°C until an OD600 of 1.0 was reached. Two samples of 1 ml each were then taken. Total DNA of the first sample was extracted, while the second sample was incubated at 42°C and DNA extraction was carried out at the end of the experiment. CaCl2 (10 mM) was then added to the culture before infection with phage DT1 at a multiplicity of infection (MOI) of 1. One-milliliter samples were taken immediately after DT1 inoculation and every 5 min thereafter for 30 min.
To extract the total DNA from the samples, cells were immediately centrifuged and washed with 1 ml of a cold washing solution containing 50 mM Tris-HCl (pH 8.0), 5 mM EDTA (pH 8.0), and 100 mM NaCl. Cell pellets were resuspended in a lysis solution containing 20 mM Tris-HCl (pH 8.0), 10 mM EDTA (pH 8.0), 150 mM NaCl, and 30 mg/ml lysozyme and left on ice for 30 min. Fifty microliters of 10% (wt/vol) sodium dodecyl sulfate and 20 μl of proteinase K (20 mg/ml) were added, and the mixture was incubated at 65°C for 20 min. Phenol-chloroform extractions were performed, and the DNA was precipitated with 0.1 volume of 3 M potassium acetate (pH 4.8) and 1 volume of isopropanol. The DNA pellet was resuspended in 20 μl of Tris-HCl (pH 8.0) and digested with XbaI. The resulting fragments were separated by electrophoresis on a 0.8% (wt/vol) agarose gel.
The antiviral efficacy of pFB3 was also evaluated by milk fermentation assays. Both phage-sensitive and -resistant strains were inoculated at 5% (vol/vol) from an overnight culture (LM17) in 10 ml of pasteurized milk (Natrel). Dilutions of a given concentration of phages were added, and an aliquot was immediately taken for pH reading at time zero. The tubes were incubated at 42°C in a water bath for 4 h. Finally, a second pH reading was taken, and the ΔpH was obtained by subtracting both values.
Northern blots.
A 100-ml LM17 broth was inoculated with 1% (vol/vol) of an overnight culture of S. thermophilus SMQ-301 and incubated at 42°C until the OD600 reached 0.5. CaCl2 was added to the culture at a final concentration of 10 mM. The cells were then challenged with the virulent phage DT1 at an MOI of 10. Samples of 1.5 ml were taken at 0, 2, 5, 7, 12, 17, and 22 min after the beginning of infection. The transcription was stopped by adding rifampicin at 150 μg per ml. The cells were collected by centrifugation and kept at −80°C until RNA extraction. Total RNA was extracted with the RNeasy Mini kit (Qiagen) according to the manufacturer's protocol. Then, the RNA samples were treated with 1 μl of RNase-free DNase I (10 U/μl) in the presence of 2 μl of RNase inhibitor (40 U/μl) for 30 min at 37°C. Five micrograms of each RNA sample and ladder (Invitrogen) were separated by electrophoresis on a 1% (wt/vol) agarose-formaldehyde gel and transferred to a nylon membrane as described by Sambrook and Russell (41).
RNA was stained with methylene blue after transfer from the agarose-formaldehyde gels to nylon membranes as described elsewhere (18, 41). Immobilized RNA was hybridized with DNA probes labeled with digoxigenin-dUTP with the PCR digoxigenin labeling mix (Roche) as specified by the manufacturer. The various probes were obtained following PCR amplification with phage DT1 genomic DNA, and the primers presented in Table 2. Hybridization and detection conditions were carried out according to the manufacturer's protocol (Roche). Transcripts were detected with the chemiluminescent substrate CDP-STAR (Roche) and Biomax films (Kodak).
TABLE 2.
Probes and primers used in the transcriptional analysis
| Probe | Region | Primers | Sequence (5′ to 3′) | Probe length (bp) |
|---|---|---|---|---|
| A | orf29 | DT1-29F | GTTAGTTAGGAAGGAGGAAC | 226 |
| DT1-29R | TCAAAAGCCATACAAATCAGAC | |||
| B | orf30 to orf32 | DT95 | CCAAACGCACAAACTGGTTT | 1,426 |
| DT83 | TACATCTTCCCACGCAGTC | |||
| C | orf30 | DT1-30F | ACTAGTCACTTAGTGCGGT | 222 |
| DT1-30R | GTTGGCTACGGATTGAAACA | |||
| C1 | orf31 | DT1-31F | TTTTAGCACCAGAAGTTTTTG | 259 |
| DT1-31R | TCACTCATCGCTGCTAAAC | |||
| C2 | End of orf32 | DT84 | GACTGCGTGGGAAGATGTA | 227 |
| DT1-32R | GGTCTTCAATCTTGCAAGCTG | |||
| C3 | orf33 | DT76 | CAGACGACATTGTTCTCGGAA | 771 |
| DT61 | TTACTAGCAACCTCTTGCCG | |||
| D | orf33 to orf36 | DT62 | CGGCAAGAGGTTGCTAGTAA | 1,484 |
| DT20 | GCTCGGTTTACTTGGTGAGA | |||
| E | orf36 | DT19 | TCACCAAGTAAACCGAGC | 1,440 |
| DT12 | CTACTGCTGCTTTCTTCGCC | |||
| F | orf37 | DT1-37F | TTCTGAAGCAGCAACAGTTA | 294 |
| DT1-37R | TGCTTGTTGGAATAAGCAG | |||
| G | orf38 | DT1-38F | TCACTAATTCAAAATCAAATTCG | 291 |
| DT1-38R | CAACTATCCTCATAGCATCT |
Primer extension.
The primer Cat86Rev (5′-ATCACCAAGCTATATGAGCATCGGG-3′) was 5′ end labeled with T4 polynucleotide kinase and [γ-32P]dATP. Primer extension was performed with 20 μg of total RNA, 0.036 μM of the labeled primer, 2X PCR buffer (20 mM Tris-HCl [pH 8.3], 3.0 mM MgCl2, 100 mM KCl), and 5.6 mM dithiothreitol. The deoxynucleoside triphosphates were added to a final concentration of 5 mM. The reaction mixture was incubated at 80°C for 10 min and at 55°C for 50 min. The extension was performed with 200 U of Expand reverse transcriptase (Roche) for 1 h at 42°C. DNase-free RNase was added at a final concentration of 20 μg per ml, and the mixture was incubated at 37°C for 15 min. The extended product was ethanol precipitated and dissolved in 5 μl of stop solution (T7 sequencing kit, Amersham Biosciences). The product was separated on a 6% (wt/vol) polyacrylamide gel containing 7 M urea. The DNA sequence of the relevant portion was sequenced with the same primer and pFB1 as the template. The sequence reaction was performed with the T7 sequencing kit.
Chloramphenicol acetyltransferase assays.
E. coli cells were grown overnight at 37°C with agitation in 20 ml of YT medium (41). Cells were harvested by centrifugation, washed, and resuspended in 2 ml of Tris-HCl, 1 mM (pH 7.6). Cells were disrupted by sonication, and the cell debris was removed by centrifugation. The supernatants containing the total protein extracts were used for the assays. For S. thermophilus and L. lactis, cells from a 500-ml culture (OD600 of 0.4) were harvested by centrifugation and washed with sodium phosphate buffer (8 mM NaH2PO4, 42 mM Na2HPO4 [pH 7.5]) containing 5 mM β-mercaptoethanol. Cell extracts were prepared by grinding with alumina (53) and kept at −80°C until used. Chloramphenicol acetyltransferase assays were performed at 37°C for 10 and 60 min as reported elsewhere (28), except that 250 ng of protein from the total cell extracts was used for each assay.
RESULTS
Analysis of a putative phage ori.
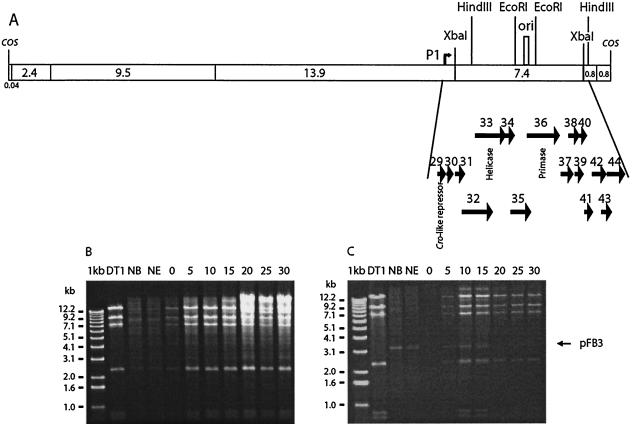
It has been suggested that the ori of phage DT1 was located between orf36 and orf37 (52). A noncoding region containing direct and indirect repeats, characteristics of phage oris, was found between these two open reading frames. Cloning of different restriction fragments containing this putative ori into the ori-probe vector pTRK333 were undertaken in E. coli and S. thermophilus in order to find a DNA fragment from the DT1 genome capable of sustaining plasmid replication. Clones containing selected DNA fragments of various length covering the ori region could not be obtained or were unstable in E. coli. Only one stable clone (pFB4) was obtained in E. coli and it contained a 5-kb HindIII fragment covering orf36 to orf44 (Fig. 1A).
FIG. 1.
Schematic illustration of the phage DT1 genome analyzed in this study and its replication in S. thermophilus SMQ-301 infected cells. (A) XbaI restriction map of the phage DT1 genome. The bent arrow marks the position of promoter P1. (B) Agarose gel electrophoresis of DNA from SMQ-301(pNZ123). (C) Agarose gel electrophoresis of DNA from SMQ-301(pFB3). NB, uninfected cells before addition of DT1; NE, uninfected cells at the end of the experiment. Lane numbers correspond to the time (in minutes) after the beginning of the infection. Plasmid pFB3 is indicated by an arrow.
Despite several attempts, S. thermophilus could not be transformed with pFB4. As the replication ability of the putative ori and flanking genes could not be established in S. thermophilus, its ability to confer phage resistance (PER effect) was tested. A 750-bp EcoRI fragment containing the ori (coordinates 29805 to 30555) (Fig. 1A) was cloned into the high-copy vector pNZ123, generating plasmid pFB3, and then transformed directly into S. thermophilus SMQ-301. The strains S. thermophilus SMQ-301(pNZ123) and SMQ-301(pFB3) were then infected with DT1, and the phage DNA replication was monitored over time. Large amounts of phage DNA were observed in the infected S. thermophilus SMQ-301(pNZ123) strain after 5 min (Fig. 1B). However, only small amounts of phage DNA were detected in the cells harboring the ori plasmid, and the amount decreased 10 min after infection (Fig. 1C). The copy number of pFB3 increased slightly after phage infection.
Effect of pFB3 on infectivity of various S. thermophilus phages.
Following the demonstration that the ori provided a PER effect, the EOP, ECOI, and burst size were measured with different phage-host systems. Although pFB3 conferred on S. thermophilus SMQ-301 a phage resistance phenotype (EOP 10−5, ECOI 3%) against the virulent cos-type phage DT1, its efficiency was somewhat limited (EOP 10−2, ECOI 57%) in strain S. thermophilus SMQ-495 (Table 3). The plasmid pFB3 provided only slight resistance (EOP 10−1) against the virulent phage MD2, which is also a member of the cos group, and had no significant effect against the virulent pac-type phages Q1 and Q3.
TABLE 3.
Effect of the ori-containing plasmid pFB3 on various S. thermophilus phagesa
| Strain | Phage | Type | EOP | ECOI (%) | Burst size
|
|
|---|---|---|---|---|---|---|
| pNZ123 | pFB3 | |||||
| SMQ-119 | Q1 | pac | 0.5 ± 0.03 | ND | ND | ND |
| Q3 | pac | 0.8 ± 0.10 | ND | ND | ND | |
| SMQ-301 | DT1 | cos | 3 × 10−5 ± 2 × 10−5 | 3 ± 2 | 142 ± 47 | 1.3 ± 0.2 |
| SMQ-495 | DT1 | cos | 9 × 10−2 ± 2 × 10−2 | 57 ± 1 | 143 ± 23 | 31 ± 10 |
| MD2 | cos | 0.1 ± 0.01 | ND | ND | ND | |
Results are the means of two independent experiments. ND, not determined.
To determine the efficiency of the antiviral system in dairy fermentation, the strains were grown in milk with various phage concentrations. In the absence of phage, the milk assay showed a small decrease in the acidification (ΔpH = 0.2) with strains carrying the pFB3 plasmid (Table 4), indicating that plasmid maintenance had a slight negative effect on cellular metabolic activity. For S. thermophilus SMQ-301, in the presence of pFB3 the decreased pH was the same with or without phages even at a high phage concentration (107 PFU/ml). Interestingly, even S. thermophilus SMQ-495 showed a marked increase in phage resistance, as 106 phages were needed to reduce milk acidification, compared to 102 phages for the sensitive strain. Overall, the milk fermentation assay confirmed the efficiency of pFB3 in protecting S. thermophilus strains against phage DT1 infection.
TABLE 4.
Efficiency of S. thermophilus PER system in milk fermentation
| Phage DT1 titer (PFU/ml) | ΔpHa
|
|||
|---|---|---|---|---|
| SMQ-301(pNZ123) | SMQ-301Z(pFB3) | SMQ-495Z(pNZ123) | SMQ-495Z(pFB3) | |
| 0 | 2.09 ± 0.04 | 1.83 ± 0.11 | 1.94 ± 0.06 | 1.75 ± 0.13 |
| 2 × 101 | 2.04 ± 0.13 | 1.82 ± 0.09 | 1.71 ± 0.11 | 1.76 ± 0.02 |
| 2 × 102 | 1.98 ± 0.07 | 1.85 ± 0.11 | 1.18 ± 0.19 | 1.83 ± 0.12 |
| 2 × 103 | 1.39 ± 0.36 | 1.83 ± 0.13 | 0.77 ± 0.14 | 1.81 ± 0.14 |
| 2 × 104 | 0.77 ± 0.13 | 1.83 ± 0.10 | 0.37 ± 0.11 | 1.79 ± 0.07 |
| 2 × 105 | 0.34 ± 0.10 | 1.78 ± 0.12 | 0.23 ± 0.07 | 1.77 ± 0.06 |
| 2 × 106 | 0.11 ± 0.04 | 1.91 ± 0.01 | 0.16 ± 0.11 | 1.35 ± 0.35 |
| 2 × 107 | 0.03 ± 0.03 | 1.83 ± 0.13 | 0.08 ± 0.05 | 0.50 ± 0.26 |
The ΔpH was the difference in pH at time zero and after 4 h of incubation at 42°C. Results are the mean of three independent experiments.
Transcriptional analysis of cro-ori region.
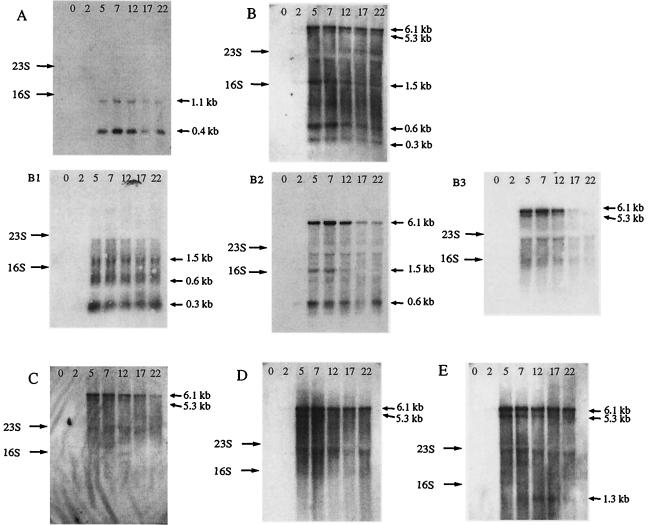
Comparative genome analyses previously showed the presence of a gene coding for a Cro-like repressor in virulent and temperate S. thermophilus phages as well as significant nucleotide differences in this region. In order to investigate the putative involvement of this regulator in the replication of S. thermophilus phages, a detailed transcriptional map was established for the region located between the cro-like gene (orf29) and the ori of DT1. Northern blot experiments were performed to study the transcription of the eight DT1 genes located between orf29 and the ori (Fig. 2). Ten probes were used to analyze this region and to identify the genes associated with the transcripts (Table 2).
FIG. 2.
Northern blots of total RNA isolated from S. thermophilus SMQ-301 at the indicated time (in minutes) after infection with phage DT1. The letters A to G on the left side represent the probes used (see Table 2 and Fig. 3 for details). The estimated sizes of the observed transcripts are indicated on the right.
Eight transcripts of various sizes were detected in this region with these probes (Fig. 3) and they were all detected 5 min after the beginning of the phage infection (Fig. 2). The amounts of some transcripts decreased over time, but the signals were detected up to 22 min, the last time point examined. Interestingly, at least two mRNAs covered each open reading frame, thereby securing an efficient transcription of this genomic area. Two large transcripts (5.3 and 6.1 kb) covered seven open reading frames (orf32 to orf38) and the ori, while several small transcripts spanned three open reading frames (orf29 to orf31). The 6.1-kb mRNA was more abundant throughout the period tested, while the 5.3-kb mRNA was the least abundant. Two other probes covering orf28 and orf39 were also used to confirm the start and the end of some transcripts (data not shown). Putative promoters were identified by in silico sequence analysis upstream of orf29 (TTGACA-N17-TATAAT), orf30 (TTGACA-N17-TATATA), and orf32 (TTGATG-N17-GATAAT).
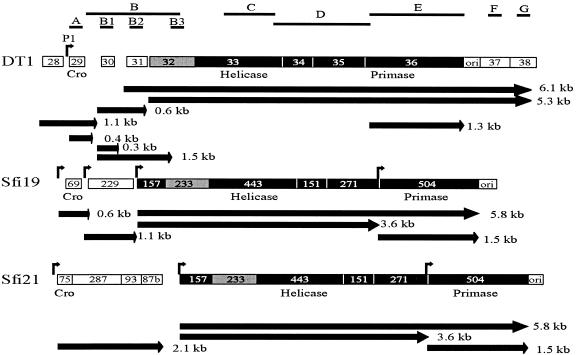
FIG. 3.
Comparison of the transcriptional maps of the cro-ori regions of S. thermophilus phages DT1, Sfi19, and Sfi21. The DNA probes used in the Northern blot experiments are indicated by the letters A to G and black lines above the transcripts. The arrows indicate the direction of transcription. The estimated sizes of the observed transcripts are also indicated. Bent arrows designate promoter sequences. Deduced proteins showing between 70% and 80% amino acid identity are in grey, while those sharing more than 90% amino acid identity are in black.
Comparison of the cro-ori region of phages DT1, Sfi19, and Sfi21.
To compare the transcriptional map of DT1 with the S. thermophilus phages Sfi19 (virulent) and Sfi21 (temperate), the cro-ori region of DT1 was aligned with the homologous DNA region of Sfi19 (orf69-ori) and Sfi21 (orf75-ori) (Fig. 3). The sequence analysis revealed that the cro-like repressor gene (orf29) of DT1 did not have homology with the cro-like repressors of Sfi19 (orf69) and Sfi21 (orf75). Nevertheless, a promoter was identified upstream of these genes. In phage Sfi21, the promoter upstream of orf75 generated a single 2.1-kb transcript covering four open reading frames, while a smaller transcript was observed for DT1 (0.4 kb) and Sfi19 (0.6 kb) as it covered only the cro-like gene. Contrary to phages Sfi19 and Sfi21, several small transcripts were detected in the region spanning orf29 and orf31 of phage DT1 (Fig. 3). orf30 and orf31 are unique to phage DT1, as they had no counterpart in the genomes of phages Sfi19 and Sfi21.
Downstream of orf31 of DT1, a region of approximately 5 kb is almost identical between the three phages and covers orf32 up to the ori in DT1 and from orf233 to the ori in Sfi19 and Sfi21. Two large transcripts (5.3 and 6.1 kb) spanned this region in DT1, while two smaller transcripts (3.6 and 5.8 kb) were found in phages Sfi19 and Sfi21. For the three phages, a shorter mRNA (1.3 or 1.5 kb) corresponding to the transcription of the putative primase gene was also observed. The ori of phage DT1, characterized by the presence of many secondary structures, may serve as terminator of the 1.3-kb transcript covering the putative primase gene. However, it appears to be leaky, as the two larger transcripts bypassed the ori. On the contrary, the ori appears to terminate two transcripts (1.5 and 5.8 kb) in phages Sfi19 and Sfi21.
Characterization of P1 promoter.
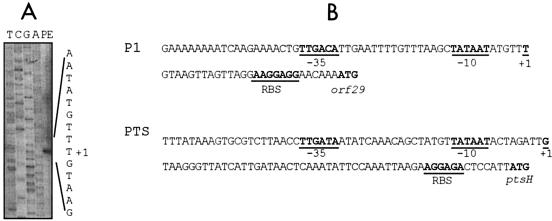
Since a gene coding for a cro-like repressor is present in all S. thermophilus phage genomes analyzed thus far, we further analyzed the putative promoter (P1) identified upstream of orf29. As indicated above, P1 possesses the −35 and −10 consensus sequence (TTGACA-N17-TATAAT) (17). This region was amplified from the DT1 genome and the amplicon was cloned upstream of a promoterless chloramphenicol acetyltransferase (cat) gene into the pBV5030 vector, and the resulting plasmid was designated pFB1. A primer extension experiment with a primer specific to the cat gene was performed with total RNA isolated from S. thermophilus SMQ-301(pFB1). Primer extension analysis located the transcriptional start point 6 nucleotides downstream from the last nucleotide of the inferred −10 box (Fig. 4).
FIG. 4.
(A) Mapping of the transcription start site from the P1 promoter by primer extension (PE). Nucleotide position +1 is indicated. The nucleotide sequence of pFB1 was determined with the same primer (lanes T, C, G, and A). (B) Partial nucleotide sequence of the P1 and PTS promoter regions. Putative −35 and −10 boxes and the putative ribosome binding sites (RBS) are indicated.
The relative strength of the P1 promoter was then compared to the strong bacterial promoter (TTGATA-N17-TATAAT) of the operon coding for the general proteins of the phosphoenolpyruvate:sugar phosphotransferase system pts of S. thermophilus ATCC 19258 (9). The promoter of the pts operon was amplified from S. thermophilus ATCC 19258 and cloned in pBV5030, and the resulting plasmid (pGA1) was introduced by electroporation into E. coli DH5-α, L. lactis MG1363, and S. thermophilus SMQ-301. The activity of the P1 and PTS promoters was measured by determining the CAT activity in these three strains (Table 5). While no CAT activity was detected in cells carrying only the promoter-probe vector pBV5030, it was detected in strains bearing pFB1 and pGA1, confirming the functionality of these two promoters. The CAT activity was approximately twofold higher in S. thermophilus when the cat gene was under the control of P1 compared with the promoter of the pts operon. Conversely, in E. coli and L. lactis, control of cat expression by the pts promoter resulted in higher levels of CAT activity than with promoter P1.
TABLE 5.
Chloramphenicol acetylation in various strainsa
| Bacteria | Acetylated chloramphenicol (%/min)
|
||
|---|---|---|---|
| pBV5030 | pFB1 (P1) | pGA1 (pts) | |
| E. coli DH5α | 0.0 ± 0.0 | 1.0 ± 0.3 | 1.5 ± 0.2 |
| L. lactis MG1363 | 0.0 ± 0.0 | 0.3 ± 0.1 | 2.5 ± 0.3 |
| S. thermophilus SMQ-301 | 0.0 ± 0.0 | 1.5 ± 0.4 | 0.8 ± 0.0 |
The activity is expressed as the percentage of chloramphenicol acetylated per minute at 37°C by 250 ng of protein from the total cell extracts. The means of three independent experiments are presented.
DISCUSSION
Analysis of the phage ori.
Despite several attempts, we were unable to obtain a stable plasmid containing DNA from phage DT1 that could replicate autonomously in S. thermophilus. This may be explained by the presence of early genes in the vicinity of the ori, as early-transcribed phage genes are often toxic to the bacterial cell (29). Other groups were also unsuccessful in cloning a functional ori from an S. thermophilus phage (15, 49). The fact that similar results were obtained in this study is not surprising, as the ori region appears to be very well conserved among S. thermophilus phages. Since no self-replicating plasmids were obtained, the PER phenotype usually conferred by phage ori was verified to assess the functionality of the putative DT1 ori identified by in silico analysis. A strong inhibition of phage DT1 DNA replication was observed in cells carrying the ori cloned on a high-copy plasmid. This is presumably the result of the titration of phage replication factors by the ori. The copy number of the ori-containing plasmid increased but only slightly, which is likely due to the fact that the ori was already cloned on a high-copy plasmid.
The assays conducted with other phage-host systems showed that the resistance conferred by plasmid pFB3 was phage specific, which is consistent with observations made by Foley et al. (15). As noticed by Stanley et al. (49), the resistance was also strain dependent in S. thermophilus. Indeed, the presence of pFB3 conferred stronger protection against phage DT1 in S. thermophilus SMQ-301 than in SMQ-495. Plasmid-strain interactions probably account for this difference, as the burst size of DT1 was similar in the wild-type strains SMQ-301 and SMQ-495. These data demonstrate that the ori of phage DT1 confers a PER phenotype during milk fermentation but the protection is limited to certain phages and thus is likely to be used only for specific applications.
Transcriptional analysis.
The transcriptional map of the cro-ori region of DT1 was compared with that of Sfi19 (54) and Sfi21 (56). In contrast to the virulent phages DT1 and Sfi19, which contain only a cro-like repressor gene, the temperate phage Sfi21 has a complete and functional lysogeny module. These three phages have a consensus promoter upstream of the cro-like repressor gene. For the virulent phages DT1 and Sfi19, the promoter enabled the transcription of a short mRNA that covered only the cro-like gene. For Sfi21, the corresponding promoter generated a 2.1-kb mRNA covering part of the lysogeny module (orf75 to orf87b). Additional transcriptional start points are necessary for the transcription of the conserved downstream genes of these three phages.
Interestingly, the oris of phages Sfi19 and Sfi21 appear to act as stronger terminators than the ori of phage DT1. A transcript covering only the putative primase gene was also detected for the three phages. This small transcript suggests that the primase has to be produced at higher levels for efficient phage genome replication. It was shown recently that antisense RNA targeting an S. thermophilus phage primase was found to be a more effective phage defense strategy than antisense RNA targeting helicase (51), strengthening the observation that the primase is essential for phage replication and is required in substantial amounts.
It was previously hypothesized that the ancestor of DT1 (likely a temperate phage) may have lost part of a lysogenic module to become a lytic variant (52). Bruttin et al. (4-6) showed the emergence of lytic phages after several passages of the temperate phage Sfi21 on an indicator strain. These phage mutants had two types of deletions. The first deletion eliminated the genes involved in immunity control, and the second deletion resulted in the loss of genes coding for the integrase, immunity, and part of the cI repressor. Similar genetic rearrangements may have occurred in the ancestor of phage DT1. As a consequence, in the lytic phage DT1, the transcription profile was altered, resulting in several short transcripts. Expression analysis of the cro-ori region of these three phages clearly showed that a given transcriptional map should not be assumed for all phages of the same group. Additional analysis should be performed with other phages to have a comprehensive view of phage gene expression. Such analyses should lead to identification of a suitable target for the development of effective phage-resistant S. thermophilus strains based on antisense RNA (50, 51).
Characterization of the P1 promoter.
To our knowledge, this is the first study on the characterization of an S. thermophilus phage promoter. By contrast, several promoters have been studied in various S. thermophilus strains (43-47) and other lactic acid bacteria phages (1, 3, 11, 24, 35, 36, 58, 59). The P1 promoter possesses the bacterial consensus promoter sequence, while the promoter of the pts operon differs from the consensus by one nucleotide (TTGATA-N17-TATAAT). The transcriptional start point of the P1 promoter is located 6 nucleotides downstream from the last nucleotide of the −10 region, while that of the pts promoter is initiated at 9 nucleotides downstream from the last nucleotide of the −10 box (9). The P1 promoter is more active than the promoter of the pts operon in S. thermophilus, while the opposite is found in E. coli and L. lactis. The divergence of activity for these two promoters suggests that the phage P1 promoter has an optimal sequence for the RNA polymerase of S. thermophilus to ensure efficient transcription of the early genes, while the pts promoter is subjected to regulatory expression mechanisms according to cell requirements. Similarly, the streptococcal phage promoter may not possess the optimal sequence for its recognition by the RNA polymerases from E. coli and L. lactis.
The analysis of several promoters of Bacillus spp. and other gram-positive bacteria indicated that several conserved regions are necessary for the recognition of the promoter by the RNA polymerase (8, 57). The most significant region (named −16) consists of a TRTG motif (57). The P1 promoter has internal and external sequences similar to those identified in Bacillus subtilis promoters. The P1 promoter has a partial −16 box (TAAG) as well as An stretches from positions −54 to −51 and from −44 to −38. It also possesses an A downstream from the −10 box and a CTG located just upstream of the −35 box. Several T's are also present between the −35 and −10 consensus sequences. The pts promoter also has some sequences present among promoters of B. subtilis and other gram-positive bacteria, such as the −16 box (TATG) and An stretches (Fig. 4). With synthetic promoters introduced in L. lactis, Jensen and Hammer (23) also demonstrated that the sequences internal and external to the −35 and −10 boxes are also critical for promoter strength. The dinucleotide TG is often located one nucleotide upstream of the −10 box. A 4-bp consensus sequence (ATTC) located just upstream from the −35 sequence is also frequently found among lactococcal promoters. These differences, and possibly others, may be responsible for promoter strength in the three bacteria analyzed in this study.
Acknowledgments
We are grateful to L. Topisirovic for providing the pBV5030 vector.
This study was funded by a grant (to S.M.) from the Natural Sciences and Engineering Research Council of Canada. F.B., A.C., M.D., and G. L. are recipients of graduate student scholarships from the Fonds Québécois de Recherche sur la Nature et les Technologies.
REFERENCES
- 1.Binishofer, B., I. Moll, B. Henrich, and U. Bläsi. 2002. Inducible promoter-repressor system from the Lactobacillus casei phage φFSW. Appl. Environ. Microbiol. 68:4132-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bojovic, B., G. Djordjevic, and L. Topisirovic. 1991. Improved vector for promoter screening in lactococci. Appl. Environ. Microbiol. 57:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brondsted, L., M. Pedersen, and K. Hammer. 2001. An activator of transcription regulates phage TP901-1 late gene expression. Appl. Environ. Microbiol. 67:5626-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruttin, A., and H. Brüssow. 1996. Site-specific spontaneous deletions in three genome regions of a temperate Streptococcus thermophilus phage. Virology 219:96-104. [DOI] [PubMed] [Google Scholar]
- 5.Bruttin, A., F. Desiere, S. Lucchini, S. Foley, and H. Brüssow. 1997. Characterization of the lysogeny DNA module from the temperate Streptococcus thermophilus bacteriophage φSfi21. Virology 233:136-148. [DOI] [PubMed] [Google Scholar]
- 6.Bruttin, A., S. Foley, and H. Brüssow. 1997. The site-specific integration system of the temperate Streptococcus thermophilus bacteriophage φSfi21. Virology 237:148-158. [DOI] [PubMed] [Google Scholar]
- 7.Buckley, N. D., C. Vadeboncoeur, D. J. Leblanc, L. N. Lee, and M. Frenette. 1999. An effective strategy, applicable to Streptococcus salivarius and related bacteria, to enhance or confer electroporation competence. Appl. Environ. Microbiol. 65:3800-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, Q., Z. Qu, Y. Wan, H. Zhang, and D. Shen. 2000. Cloning, molecular characterization, and application of rice epiphytic Bacillus pumilus promoter fragments. Curr. Microbiol. 43:244-248. [DOI] [PubMed] [Google Scholar]
- 9.Cochu, A., C. Vadeboncoeur, S. Moineau, and M. Frenette. 2003. Genetic and biochemical characterization of the phosphoenolpyruvate: glucose/mannose phosphotransferase system of Streptococcus thermophilus. Appl. Environ. Microbiol. 69:5423-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffey, A., and R. P. Ross. 2002. Bacteriophage-resistance systems in dairy industry starter strains: molecular analysis to application. Antonie van Leeuwenhoek 82:303-321. [PubMed] [Google Scholar]
- 11.Constable, A., and B. Mollet. 1994. Isolation and characterization of promoter regions from Streptococcus thermophilus. FEMS Microbiol. Lett. 122:85-90. [DOI] [PubMed] [Google Scholar]
- 12.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vos, W. M. 1987. Gene cloning and expression in lactic streptococci. FEMS Microbiol. Lett. 46:281-295. [Google Scholar]
- 14.Duplessis, M., and S. Moineau. 2001. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol. Microbiol. 41:325-336. [DOI] [PubMed] [Google Scholar]
- 15.Foley, S., S. Lucchini, M.-C. Zwahlen, and H. Brüssow. 1998. A short noncoding viral DNA element showing characteristics of a replication origin confers bacteriophage resistance to Streptococcus thermophilus. Virology 250:377-387. [DOI] [PubMed] [Google Scholar]
- 16.Gasson, M. J. 1983. Plasmid complement of Streptococcus cremoris NCDO712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harley, C. B., and R. P. Reynold. 1987. Analysis of E. coli promoter sequences. Nucleic Acids Res. 15:2343-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrin, D. L., and G. W. Schmidt. 1988. Rapid, reversible staining of Northern blots prior to hybridization. BioTechniques 6:196. [PubMed] [Google Scholar]
- 19.Hill, C., J. Massey, and T. R. Klaenhammer. 1991. Rapid method to characterize lactococcal bacteriophage genome. Appl. Environ. Microbiol. 57:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, C., L. A. Miller, and T. R. Klaenhammer. 1990. Cloning, expression, and sequence determination of bacteriophage fragment encoding bacteriophage resistance in Lactococcus lactis. J. Bacteriol. 172:6419-6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis, A. W. 1978. Serological studies of a host range mutant of a lactic streptococcal bacteriophage. Appl. Environ. Microbiol. 36:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen, P. R., and K. Hammer. 1998. The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl. Environ. Microbiol. 64:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakikawa, M., W. Nobukatsu, T. Funawatashi, M. Oki, H. Yasukawa, A. Taketo, and K.-I. Kodaira. 1998. Promoter/repressor system of Lactobacillus plantarum phage φgle: characterization of the promoters pR49-pR-pL and overproduction of the Cro-like protein Cng in Escherichia coli. Gene 215:371-379. [DOI] [PubMed] [Google Scholar]
- 25.Labrie, S., and S. Moineau. 2000. Multiplex PCR for detection and identification of lactococcal bacteriophages. Appl. Environ. Microbiol. 66:987-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakshmidevi, G., B. E. Davidson, and A. J. Hillier. 1990. Molecular characterization of promoters of the Lactococcus lactis subsp. cremoris temperate bacteriophage BK5-T and identification of a phage gene implicated in the regulation of promoter activity. Appl. Environ. Microbiol. 56:934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Marrec, C., D. van Sinderen, L. Walsh, E. Stanley, E. Vlegels, S. Moineau, P. Heinze, G. F. Fitzgerald, and B. Fayard. 1997. Two groups of bacteriophages infecting Streptococcus thermophilus can be distinguished on the basis of mode of packaging and genetic determinants for major structural proteins. Appl. Environ. Microbiol. 63:3246-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lévesque, C., S. Brassard, J. Lapointe, and P. H. Roy. 1994. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene 142:49-54. [DOI] [PubMed] [Google Scholar]
- 29.Liu, J., M. Dehbi, G. Moeck, F. Arhin, P. Bauda, D. Bergeron, M. Callejo, V. Ferretti, N. Ha, T. Kwan, J. McCarty, R. Srikumar, D. Williams, J. J. Wu, P. Gros, J. Pelletier, and M. DuBow. 2004. Antimicrobial drug discovery through bacteriophage genomics. Nat. Biotechnol. 22:185-191. [DOI] [PubMed] [Google Scholar]
- 30.Lubbers, M. W., K. Schofield, N. R. Waterfield, and K. M. Polzin. 1998. Transcription analysis of the prolate-headed lactococcal bacteriophage c2. J. Bacteriol. 180:4487-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucchini, S., F. Desiere, and H. Brüssow. 1998. The structural gene module in Streptococcus thermophilus bacteriophage φSfi11 shows a hierarchy of relatedness to Siphoviridae from a wide range of bacterial hosts. Virology 246:63-73. [DOI] [PubMed] [Google Scholar]
- 32.Lucchini, S., F. Desiere, and H. Brüssow. 1999. Comparative genomics of Streptococcus thermophilus phage species supports a modular evolution theory. J. Virol. 73:8647-8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucchini, S., F. Desiere, and H. Brüssow. 1999. The genetic relationship between virulent and temperate Streptococcus thermophilus bacteriophages: whole genome comparison of cos-site phages Sfi19 and Sfi21. Virology 260:232-243. [DOI] [PubMed] [Google Scholar]
- 34.Madsen, P. L., and K. Hammer. 1998. Temporal transcription of the lactococcal temperate phage TP901-1 and DNA sequence of the early promoter region. Microbiology 144:2203-2215. [DOI] [PubMed] [Google Scholar]
- 35.Madsen, S. M., D. Mills, G. Djordjevic, H. Israelsen, and T. R. Klaenhammer. 2001. Analysis of the genetic switch and replication region of a P335-type bacteriophage with an obligate lytic lifestyle on Lactococcus lactis. Appl. Environ. Microbiol. 67:1128-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath, S., J. F. M. Seegers, G. F. Fitzgerald, and D. van Sinderen. 1999. Molecular characterization of a phage-encoded resistance system in Lactococcus lactis. Appl. Environ. Microbiol. 65:1891-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moineau, S., E. Durmaz, S. Pandian, and T. R. Klaenhammer. 1993. Differentiation of two abortive mechanisms by using monoclonal antibodies directed toward lactococcal bacteriophage capsid proteins. Appl. Environ. Microbiol. 59:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moineau, S., S. Pandian, and T. R. Klaenhammer. 1994. Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl. Environ. Microbiol. 60:1832-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moineau, S., S. A. Walker, B. J. Holler, E. R. Vedamuthu, and P. A. Vandenbergh. 1995. Expression of a Lactococcus lactis phage resistance mechanism by Streptococcus thermophilus. Appl. Environ. Microbiol. 61:2461-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parreira, R., R. Valyasevi, A. L. Lerayer, S. D. Ehrlich, and M.-C.Chopin. 1996. Gene organization and transcription of a late-expressed region of a Lactococcus lactis phage. J. Bacteriol. 178:6158-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Sanders, M. E., and T. R. Klaenhammer. 1980. Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl. Environ. Microbiol. 40:500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slos, P., J.-C. Bourquin, Y. Lemoine, and A. Mercenier. 1991. Isolation and characterization of chromosomal promoters of Streptococcus salivarius subsp. thermophilus. Appl. Environ. Microbiol. 57:1333-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solaiman, D. K. Y., and G. A. Somkuti. 1995. Expression of cho and melC operons by a Streptococcus thermophilus synthetic promoter in Escherichia coli. Appl. Microbiol. Biotechnol. 43:285-290. [DOI] [PubMed] [Google Scholar]
- 45.Solaiman, D. K. Y., and G. A. Somkuti. 1995. Expression of Streptomyces melC and choA genes by a cloned Streptococcus thermophilus promoter STP2201. J. Ind. Microbiol. 15:39-44. [DOI] [PubMed] [Google Scholar]
- 46.Somkuti, G. A., and D. K. Y. Solaiman. 1997. STP2201, a chromosomal promoter sequence of Streptococcus thermophilus. Curr. Microbiol. 35:180-185. [DOI] [PubMed] [Google Scholar]
- 47.Somkuti, G. A., and D. H. Steinberg. 1999. Promoter activity of the pER341-borne STPhsp in heterologous gene expression in Escherichia coli and Streptococcus thermophilus. FEMS Microbiol. Lett. 179:431-436. [DOI] [PubMed] [Google Scholar]
- 48.Stanley, E., G. F. Fitzgerald, C. Le Marrec, B. Fayard, and D. van Sinderen. 1997. Sequence analysis and characterization of φO1205, a temperate bacteriophage infecting Streptococcus thermophilus CNRZ 1205. Microbiology 143:3417-3429. [DOI] [PubMed] [Google Scholar]
- 49.Stanley, E., L. Walsh, A. van der Zwet, G. F. Fitzgerald, and D. van Sinderen. 2000. Identification of four loci isolated from two Streptococcus thermophilus phage genomes responsible for mediating bacteriophage resistance. FEMS Microbiol. Lett. 182:271-277. [DOI] [PubMed] [Google Scholar]
- 50.Sturino, J. M., and T. R. Klaenhammer. 2002. Expression of antisense RNA targeted against Streptococcus thermophilus bacteriophages. Appl. Environ. Microbiol. 68:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sturino, J. M., and T. R. Klaenhammer. 2004. Antisense RNA targeting of primase interferes with bacteriophage replication in Streptococcus thermophilus. Appl. Environ. Microbiol. 70:1735-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay, D., and S. Moineau. 1999. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology 255:63-76. [DOI] [PubMed] [Google Scholar]
- 53.Vadeboncoeur, C. 1984. Structure and properties of the phosphoenolpyruvate: glucose phosphotransferase system of oral streptococci. Can. J. Microbiol. 30:495-502. [DOI] [PubMed] [Google Scholar]
- 54.Ventura, M., and H. Brüssow. 2004. Temporal transcription map of the virulent Streptococcus thermophilus bacteriophage Sfi19. Appl. Environ. Microbiol. 70:5041-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ventura, M., A. Bruttin, C. Canchaya, and H. Brüssow. 2002. Transcription analysis of Streptococcus thermophilus phages in the lysogenic state. Virology 302:21-32. [DOI] [PubMed] [Google Scholar]
- 56.Ventura, M., S. Foley, A. Bruttin, S. Chennoufi Chibani, C. Canchaya, and H. Brüssow. 2002. Transcription mapping as a tool in phage genomics: the case of the temperate Streptococcus thermophilus phage Sfi21. Virology 296:62-76. [DOI] [PubMed] [Google Scholar]
- 57.Voskuil, M. I., and G. H. Chambliss. 1998. The −16 region of Bacillus subtilis and other Gram-positive bacterial promoters. Nucleic Acids Res. 26:3584-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker, S. A., C. S. Dombroski, and T. R. Klaenhammer. 1998. Common element regulating gene expression in temperate and lytic bacteriophage of Lactococcus species. Appl. Environ. Microbiol. 64:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker, S. A., and T. R. Klaenhammer. 1998. Molecular characterization of a phage-inducible middle promoter and its transcriptional activator from the lactococcal bacteriophage φ31. J. Bacteriol. 180:921-931. [DOI] [PMC free article] [PubMed] [Google Scholar]