Abstract
Background
Breast cancer‐related lymphoedema can be a debilitating long‐term sequela of breast cancer treatment. Several studies have investigated the effectiveness of different treatment strategies to reduce the risk of breast cancer‐related lymphoedema.
Objectives
To assess the effects of conservative (non‐surgical and non‐pharmacological) interventions for preventing clinically‐detectable upper‐limb lymphoedema after breast cancer treatment.
Search methods
We searched the Cochrane Breast Cancer Group's (CBCG) Specialised Register, CENTRAL, MEDLINE, EMBASE, CINAHL, PEDro, PsycINFO, and the World Health Organization (WHO) International Clinical Trials Registry Platform in May 2013. Reference lists of included trials and other systematic reviews were searched.
Selection criteria
Randomised controlled trials that reported lymphoedema as the primary outcome and compared any conservative intervention to either no intervention or to another conservative intervention.
Data collection and analysis
Three authors independently assessed the risk of bias and extracted data. Outcome measures included lymphoedema, infection, range of motion of the shoulder, pain, psychosocial morbidity, level of functioning in activities of daily life (ADL), and health‐related quality of life (HRQoL). Where possible, meta‐analyses were performed. Risk ratio (RRs) or hazard ratio (HRs) were reported for dichotomous outcomes or lymphoedema incidence, and mean differences (MDs) for range of motion and patient‐reported outcomes.
Main results
Ten trials involving 1205 participants were included. The duration of patient follow‐up ranged from 2 days to 2 years after the intervention. Overall, the quality of the evidence generated by these trials was low, due to risk of bias in the included trials and inconsistency in the results.
Manual lymph drainage
In total, four studies used manual lymph drainage (MLD) in combination with usual care or other interventions. In one study, lymphoedema incidence was lower in patients receiving MLD and usual care (consisting of standard education or exercise, or both) compared to usual care alone. A second study reported no difference in lymphoedema incidence when MLD was combined with physiotherapy and education compared to physiotherapy alone. Two other studies combining MLD with compression and scar massage or exercise observed a reduction in lymphoedema incidence compared to education only, although this was not significant in one of the studies. Two out of the four studies reported on shoulder mobility where MLD combined with exercise gave better shoulder mobility for lateral arm movement (shoulder abduction) and forward flexion in the first weeks after breast cancer surgery, compared to education only (mean difference for abduction 22°; 95% confidence interval (CI) 14 to 30; mean difference for forward flexion 14°; 95% CI 7 to 22). Two of the studies on MLD reported on pain, with inconsistent results. Results on HRQoL in two studies on MLD were also contradictory.
Exercise: early versus delayed start of shoulder mobilising exercises
Three studies examined early versus late start of postoperative shoulder exercises. The pooled relative risk of lymphoedema after an early start of exercises was 1.69 (95% CI 0.94 to 3.01, 3 studies, 378 participants). Shoulder forward flexion was better at one and six months follow‐up for participants who started early with mobilisation exercises compared to a delayed start (two studies), but no meta‐analysis could be performed due to statistical heterogeneity. There was no difference in shoulder mobility or self‐reported shoulder disability at 12 months follow‐up (one study). One study evaluated HRQoL and reported difference at one year follow‐up (mean difference 1.6 points, 95% CI ‐2.14 to 5.34, on the Trial Outcome Index of the FACT‐B). Two studies collected data on wound drainage volumes and only one study reported higher wound drainage volumes in the early exercise group.
Exercise: resistance training
Two studies compared progressive resistance training to restricted activity. Resistance training after breast cancer treatment did not increase the risk of developing lymphoedema (RR 0.58; 95% CI 0.30 to 1.13, two studies, 358 participants) provided that symptoms are monitored and treated immediately if they occur. One out of the two studies measured pain where participants in the resistance training group reported pain more often at three months and six months compared to the control group. One study reported HRQoL and found no significant difference between the groups.
Patient education, monitoring and early intervention
One study investigated the effects of a comprehensive outpatient follow‐up programme, consisting of patient education, exercise, monitoring of lymphoedema symptoms and early intervention for lymphoedema, compared to education alone. Lymphoedema incidence was lower in the comprehensive outpatient follow‐up programme (at any time point) compared to education alone (65 people). Participants in the outpatient follow‐up programme had a significantly faster recovery of shoulder abduction compared to the education alone group.
Authors' conclusions
Based on the current available evidence, we cannot draw firm conclusions about the effectiveness of interventions containing MLD. The evidence does not indicate a higher risk of lymphoedema when starting shoulder‐mobilising exercises early after surgery compared to a delayed start (i.e. seven days after surgery). Shoulder mobility (that is, lateral arm movements and forward flexion) is better in the short term when starting shoulder exercises earlier compared to later. The evidence suggests that progressive resistance exercise therapy does not increase the risk of developing lymphoedema, provided that symptoms are closely monitored and adequately treated if they occur.
Given the degree of heterogeneity encountered, limited precision, and the risk of bias across the included studies, the results of this review should be interpreted with caution.
Keywords: Female; Humans; Male; Patient Education as Topic; Breast Neoplasms; Breast Neoplasms/therapy; Drainage; Drainage/methods; Exercise Therapy; Exercise Therapy/methods; Lymphedema; Lymphedema/prevention & control; Quality of Life; Randomized Controlled Trials as Topic; Range of Motion, Articular; Resistance Training; Resistance Training/methods; Shoulder Joint; Shoulder Joint/physiopathology
Plain language summary
Interventions for preventing lymphoedema (swelling of the arm) after breast cancer treatment
Review question
We reviewed the evidence about the effect of interventions on preventing lymphoedema in women after breast cancer surgery.
Background
About one in five people treated for breast cancer develop lymphoedema later on. We reviewed the available evidence to determine whether some methods, such as manual lymph drainage (a massage therapy), compression, exercise or only education could help prevent lymphoedema.
Study characteristics The evidence is current to May 2013. Ten studies were included: four studies used manual lymph drainage with usual care, or combined with exercise or compression versus usual care or education alone (395 participants); three studies examined early versus late start of postoperative shoulder exercises (378 people); two studies used either progressive resistance exercise or restricted activity (358 people); and one study investigated a physiotherapy care plan versus no physiotherapy (65 people). The duration of patient follow‐up ranged from two days to two years after the intervention.
Key results
No firm conclusion can be drawn about the effect of manual lymph drainage in addition to exercise and education on preventing the incidence of lymphoedema. This is because the two included studies found contradicting results. In addition, no firm conclusion can be drawn about manual lymph drainage in combination with other interventions, because only two studies were found that each tested different combinations. One of these studies found that manual lymph drainage combined with exercise lowered the risk of lymphoedema. The other study combined manual lymph drainage with compression, but this study was too small to draw conclusions.
Arm mobility (i.e. reaching upwards over the head) was better after manual lymph drainage than without it, but this improvement lasted only for the first few weeks after breast cancer surgery.
When assessing whether early or late shoulder exercises reduced the likelihood of developing lymphoedema, the studies did not provide a clear result. The likely incidence of lymphoedema ranged from 5% to 27% (early start) compared to 4% to 20% (for delayed start) during the first 6 to 12 months after surgery. Starting shoulder exercises immediately after surgery may improve shoulder mobility in the first month, compared to starting after the first week but no firm conclusions can be drawn and mobility is comparable later on.
Progressive resistance training did not increase the risk of developing lymphoedema compared to restricted activity, on the basis that symptoms were monitored and treated immediately if they occurred.
For all investigated interventions, no firm conclusion can be drawn about their effectiveness in reducing pain or improving quality of life.
Quality of the evidence
The evidence was considered to be low quality, except for the evidence on resistance training, which was of moderate quality. This was because many studies had shortcomings in how they were conducted; there were only a small number of studies for each intervention; the results differed between comparable studies; and the groups studied were relatively small.
Summary of findings
Summary of findings for the main comparison. Early physiotherapy including MLD for patients at risk for secondary upper limb lymphoedema after breast cancer treatment.
| Early physiotherapy including MLD for patients at risk for secondary upper limb lymphoedema after breast cancer treatment | ||||||
| Patient or population: patients at risk for secondary upper limb lymphoedema after breast cancer treatment Settings: Hospital/outpatient clinic Intervention: early physiotherapy including MLD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early physiotherapy including MLD | |||||
| Time to event (Lymphoedema) volumetry Follow‐up: 12 months | Low | HR ranged from 0.26 to 1.31 | 270 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | ||
| Not estimable | Not estimable | |||||
| High | ||||||
| Not estimable | Not estimable | |||||
| Lymphoedema ‐ short term follow up Volumetry Follow‐up: mean 3 months | Low | RR ranged from 0.14 to 1.4 | 226 (2 studies) | ⊕⊝⊝⊝ very low5,6,7 | ||
| Not estimable | Not estimable | |||||
| High | ||||||
| Not estimable | Not estimable | |||||
| Lymphoedema ‐ medium term follow up Volumetry Follow‐up: 6 to 12 months | Low | RR ranged from 0.02 to 1.26 | 385 (4 studies) | ⊕⊝⊝⊝ very low8,9,10 | ||
| Not estimable | Not estimable | |||||
| High | ||||||
| Not estimable | Not estimable | |||||
| Shoulder range of motion for abduction ‐ short term follow up goniometer. Scale from: 0° to 180°. Follow‐up: 2 to 4 weeks | The mean shoulder range of motion for abduction ‐ short term follow up in the control groups was 121° 11 | The mean shoulder range of motion for abduction ‐ short term follow up in the intervention groups was 22°higher (14° to 30° higher) | 183 (2 studies) | ⊕⊕⊝⊝ low12 | ||
| Shoulder range of motion for forward flexion ‐ short term follow up goniometer. Scale from: 0° to 180°. Follow‐up: 2 to 4 weeks | The mean shoulder range of motion for forward flexion ‐ short term follow up in the control groups was 126° 11 | The mean shoulder range of motion for forward flexion ‐ short term follow up in the intervention groups was 14°higher (7° to 22° higher) | 183 (3 studies) | ⊕⊕⊝⊝ low12 | ||
| Shoulder range of motion for abduction ‐ medium term follow up goniometer. Scale from: 0° to 180°. Follow‐up: 6 to 12 months | Not estimable11 | The mean shoulder range of motion for abduction ‐ medium term follow up in the intervention group ranged from 3.1° lower to 16.9° higher | 183 (3 studies) | ⊕⊝⊝⊝ very low13,14,15 | ||
| Shoulder range of motion for forward flexion ‐ medium term follow up goniometer. Scale from: 0° to 180°. Follow‐up: 6 to 12 months | Not estimable | The mean shoulder range of motion for forward flexion ‐ medium term follow up in the intervention group ranged from 0° to 14° higher | 183 (3 studies) | ⊕⊝⊝⊝ very low13,14,15 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Range reflects observed risk measures in two studies 2 A higher percentage in the intervention group had level III dissection (43% vs 33%) and a higher percentage had radiotherapy on the axilla (10 vs 6) in one study (Devoogdt 2011). Radiotherapy was more often used in control group in one study (Torres 2010). No blinding of participants and personnel both studies. Per protocol analysis in one study (Torres 2010). 3 No evidence of effect in one study (Devoogdt 2011), large effect in one study (Torres 2010). Contradicting point estimates. 4 No meta‐analysis was possible due to statistical heterogeneity, 95%CI includes clinically relevant values in both directions in one study (Devoogdt, 2011) 5 No allocation concealment in one study (Zimmermann 2012), no blinding of outcome assessment in one study (Zimmermann 2011). 6 No evidence of effect in one study (Devoogdt 2011), large effect in the second study (Zimmermann 2012). 7 No meta‐analysis was possible, one study with a very large confidence interval (Zimmermann 2012) one study with small confidence interval (Devoogdt 2011) 8 No allocation concealment in one study (Zimmermann 2012), selective outcome reporting in one study (Castro‐Sanchez 2011). No blinding of outcome assessment in three studies (Castro‐Sanchez 2011; Torres 2010; Zimmermann 2011). No intention‐to‐treat analysis in one study (Torres 2010). Groups not comparable at baseline in two studies (Castro‐Sanchez 2011, Torres 2010). Treatment of groups differed apart from assigned intervention (Castro‐Sanchez 2011, Torres 2010). 9 Strong statistical heterogeneity. 10 Broad 95% CIs including clinically‐relevant effects in both directions in three studies (Castro‐Sanchez 2011; Torres 2011; Devoogdt 2011). 11 Final scores were available for one study only 12 No allocation concealment in one study (Zimmermann 2012). No blinding of outcome assessment in both studies. No intention‐to‐treat analysis in one study (Torres 2010). Treatment of groups differed apart from assigned intervention (Torres 2010). 13 No allocation concealment in one study (Zimmermann 2012). No blinding of outcome assessment in both studies. No intention‐to‐treat analysis in one study (Torres 2010). Groups not comparable at baseline in one study for radiotherapy treatment (Torres 2010). Treatment of groups differed apart from assigned intervention (Torres 2010). 14 Large effect in favour of intervention in one study (Zimmermann 2012), small non‐significant effect favouring the control group in another study (Torres 2010) 15 Broad 95% CI in one none‐significant study includes potentially clinically‐relevant effects in both directions (Torres 2010).
Summary of findings 2. Early shoulder mobilising exercises compared to delayed shoulder mobilising exercises for patient surgically treated for breast cancer.
| Early shoulder mobilising exercises compared to Delayed shoulder mobilising exercises for patient surgically treated for breast cancer | ||||||
| Patient or population: patients at risk for secondary upper limb lymphoedema after breast cancer treatment Settings: hospital Intervention: early shoulder mobilising exercises Comparison: delayed shoulder mobilising exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed shoulder mobilising exercises | Early shoulder mobilising exercises | |||||
| Lymphoedema ‐ medium term follow up Volumetry/ Circumference Follow‐up: 6‐12 months | Low1 | RR 1.69 (0.94 to 3.01) | 378 (3 studies) | ⊕⊝⊝⊝ very low2,3,4 | ||
| 5 per 100 | 8 per 100 (5 to 15) | |||||
| High1 | ||||||
| 27 per 100 | 46 per 100 (25 to 81) | |||||
| Shoulder range of motion for abduction ‐ short term follow up goniometer. Scale from: 0 to 180. Follow‐up: 1 month | Not estimable | The mean shoulder range of motion for abduction ‐ short term follow up in the intervention group ranged from 6° to 43° higher | 262 (2 studies) | ⊕⊝⊝⊝ very low2,5 | ||
| Shoulder range of motion for abduction ‐ medium term follow up goniometer. Scale from: 0 to 180. Follow‐up: 6 to 12 months | Not estimable | The mean shoulder range of motion for abduction ‐ medium term follow up in the intervention group ranged from 8.3° lower to 21.3° higher | 378 (3 studies) | ⊕⊝⊝⊝ very low6,7,8 | ||
| Shoulder range of motion for forward flexion ‐ short term follow up goniometer. Scale from: 0 to 180. Follow‐up: 1 month | Not estimable | The mean shoulder range of motion for forward flexion ‐ short term follow up in the intervention group ranged from 7° to 35.7° higher | 262 (2 studies) | ⊕⊕⊝⊝ low2,9 | ||
| Shoulder range of motion for forward flexion ‐ medium term follow up goniometer. Scale from: 0 to 180. Follow‐up: 6 to 12 months | Not estimable | The mean shoulder range of motion for forward flexion ‐ medium term follow up in the intervention group ranged from 0.6° lower to 5° higher | 321 (3 studies) | ⊕⊝⊝⊝ very low6,7,10 | ||
| Shoulder range of motion for external rotation ‐ medium term follow up11 goniometer. Scale from: 0 to 90. Follow‐up: 6 to 12 months | Not estimable | The mean shoulder range of motion for external rotation ‐ medium term follow up in the intervention group ranged from 1° lower to 8° higher | 378 (3 studies) | ⊕⊝⊝⊝ very low6,12 | ||
| Shoulder range of motion for internal rotation ‐ medium term follow up goniometer. Scale from: 0 to 90. Follow‐up: 6 to 12 months | The mean shoulder range of motion for internal rotation ‐ medium term follow up in the control groups was 76° | The mean shoulder range of motion for internal rotation ‐ medium term follow up in the intervention groups was 2.4°higher (0.14° lower to 4.9° higher) | 378 (3 studies) | ⊕⊕⊝⊝ low6 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Assumed range of background risk taken from observed control‐group incidence in the included studies 2 No allocation concealment in one study (Bendz 2002). No blinding of outcome assessment in one study (Bendz 2002). No explicit statistical consideration for cluster randomisation (Bendz 2002). Unclear risk of bias for allocation procedure and concealment and attrition in one study (Cinar 2008). Unequal treatment of groups besides intervention in one study (Cinar). 3 Large and statistically‐significant effect in favour of intervention in one study (Todd 2008). Statistically non‐significant effects in favour of control group in another study (Bendz 2002). 4 Broad 95% confidence interval including clinically‐relevant effect in non‐significant meta‐analysis. 5 Small and non‐significant effect in one study (Bendz 2002). Large statistically‐significant effect in another study (Cinar 2008). Data pooling could not be performed due to significant statistical heterogeneity. 6 No allocation concealment in one study (Bendz 2002). No blinding of outcome assessment in one study (Bendz 2002). High risk of attrition bias in one study (Todd 2008). No explicit statistical consideration for cluster randomisation (Bendz 2002). Unclear risk of bias for allocation procedure and concealment and attrition in one study (Cinar 2008). Unequal treatment of groups besides intervention in one study (Cinar). 7 No meta‐analysis could be performed due to significant statistical heterogeneity, with contradicting effect estimates in three studies: (Bendz 2002; Cinar 2008; Todd 2008) 8 Very broad 95% CIs including both neutral values and large clinically‐relevant effects in two studies (Bendz 2002, Todd 2008). Data pooling was not possible due to significant statistical heterogeneity. 9 No data pooling was possible due to significant statistical heterogeneity, but point estimates are in favour of early mobilisation and statistically significant in both studies (Bendz 2002; Cinar 2008). 10 95% confidence interval includes both neutral and potentially clinically relevant values in one study (Todd 2008), and a small clinically‐irrelevant effect in the lower boundary of the CI in a second study (Bendz 2002). 11 Pooled data are from 6 month follow‐up (Bendz 2002) and 12 month follow‐up (Todd 2008). 12 Two studies with non‐significant effect with point estimate favouring delayed exercise (Bendz 2002, Todd 2008), one study with a large statistically‐significant effect favouring early exercise (Cinar 2008).
Summary of findings 3. Progressive resistance exercise for patients at risk for secondary upper limb lymphoedema after breast cancer treatment.
| Progressive resistance exercise for patients at risk for secondary upper limb lymphoedema after breast cancer treatment | ||||||
| Patient or population: patients at risk for secondary upper limb lymphoedema after breast cancer treatment Settings: Intervention: progressive resistance exercise | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Progressive resistance exercise | |||||
| Lymphoedema Volumetry Follow‐up: 12 to 24 months | Study population1 | RR 0.58 (0.3 to 1.13) | 351 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 12 per 100 | 7 per 100 (4 to 14) | |||||
| Low1 | ||||||
| 8 per 100 | 5 per 100 (2 to 9) | |||||
| High1 | ||||||
| 17 per 100 | 10 per 100 (5 to 20) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Assumed risks are based on observed control group risks in the included trials 2 Both studies did not blind participants for the intervention. In one study, activity levels over time increased in both experimental and control group, despite requests to the control group not to increase activity levels during study period. One study (Sagen 2009) had more patients lost to follow up in the experimental group; data were imputed using last observation carried forward. Contact with a physiotherapist was more frequent in the experimental group in one study, which may reinforce self‐care/risk‐reducing behaviour (Sagen 2009).
Background
Breast cancer is the most common type of cancer among women. Worldwide, it has been estimated that 1.38 million new cases were diagnosed in 2008. The incidence is especially high in the developed countries of the world, with an estimated age‐standardised incidence in 2008 of 76 cases per 100,000 women in the United States, 83.2 per 100,000 in Canada, 84.8 per 100,000 in Australia and 89.7 per 100,000 women in Western Europe (Globocan 2008). Advances in breast cancer treatment have resulted in better survival prospects after diagnosis. As a consequence, an increasing number of people are confronted with early and late side effects of breast cancer treatment. One of the most important side effects of breast cancer treatment is secondary lymphoedema. The reported incidence of lymphoedema following breast cancer treatment varies from 6% to 54% (Clark 2005; Kwan 2010; Norman 2010; Park 2008; Paskett 2007; Petrek 1998; Shih 2009). A recent systematic review and meta‐analysis estimated the risk of developing arm lymphoedema to be 16.6%, taking all studies into account, and 21% based on meta‐analysis of cohort studies (DiSipio 2013). Lymphoedema incidence increases with the time since treatment (Cormier 2010; DiSipio 2013; Petrek 1998). The variability in reported incidence is due, in part, to differences in the criteria used to define lymphoedema (Cormier 2010; Petrek 1998). Lymphoedema can be a debilitating condition that negatively affects health‐related quality of life, body image, finances, social participation and activity level (Engel 2003; Paskett 2007; Vassard 2010). The economic burden of breast cancer‐related lymphoedema was studied in a two‐year follow‐up study after breast cancer treatment in which insurance claims data were used. The estimated difference in the two‐year costs between women who were diagnosed with breast cancer‐related lymphoedema and those without lymphoedema ranged from USD 14,877 to USD 23,167. The true costs may be underestimated in that study because of the use of claims data and the limited duration of follow‐up (Shih 2009).
Description of the condition
Pathophysiology of lymphoedema
Lymphoedema is the accumulation of interstitial fluid as a result of insufficient lymph drainage (Brennan 1992). After breast cancer treatment, secondary lymphoedema may occur as a result of insufficient lymph drainage from the upper limb. This is due to partial or total destruction of the lymphatic system with surgery or radiotherapy. Additionally, cancer treatment may induce qualitative changes in the structure of the skin and subcutaneous tissues of the arm or trunk, such as scarring or subcutaneous fibrosis. Insufficient lymph drainage due to these changes can also lead to the development of lymphoedema.
Diagnosis of lymphoedema
Several different diagnostic criteria for the presence of lymphoedema are used. Lymphoedema may be defined as a certain amount of absolute or relative change in limb circumference. Circumference can be measured using a tape measure or perimetry. Other criteria are absolute or relative changes in total limb volume. Volume can be estimated from circumference measurements, water displacement or laser scanning. Bioimpedance spectrometry can be used to estimate the amount of extracellular fluid. The diagnosis of lymphoedema is sometimes made by self‐reporting of symptoms. The wide variety of ways to define and diagnose lymphoedema complicates the interpretation of research on its incidence, prevalence, risk factors, treatment and prevention (Paskett 2007).
Risk factors
Findings in the literature on treatment‐related and patient‐related risk factors are inconsistent. The treatment factor most consistently associated with lymphoedema is the extent of surgery. Besides the extent of local surgery, this specifically includes axillary lymph node dissection and the number of lymph nodes removed (DiSipio 2013; Hayes 2008; Meeske 2008; Norman 2010; Park 2008; Ridner 2011; Tsai 2009a; Yen 2009). Radiotherapy has been associated with an elevated risk of lymphoedema in some studies (Kwan 2010; Park 2008; Tsai 2009a) but not in others (Goldberg 2010; Helyer 2010; Meeske 2008; Norman 2010; Paskett 2007; Yen 2009). This inconsistency may be due, in part, to the heterogeneity of radiotherapy treatment protocols. Of the clinical characteristics associated with an increased risk of developing lymphoedema, higher body mass index (BMI) and higher body weight are the most consistent (DiSipio 2013; Goldberg 2010; Helyer 2010; Meeske 2008; Norman 2010; Park 2008; Ridner 2011). Other clinical risk factors include positive lymph nodes and advanced disease (Kwan 2010; Meeske 2008; Park 2008; Tsai 2009a; Yen 2009). Coming from a black race has also been suggested as a risk factor in some studies (Kwan 2010; Norman 2010), although other studies found no such association (Meeske 2008; Paskett 2007; Yen 2009). Higher age has been identified both as a risk factor (Hayes 2008) and as a protective factor (Kwan 2010; Meeske 2008; Norman 2010). Higher education or socioeconomic status has also been identified both as a risk factor (Norman 2010) and as a protective factor (Hayes 2008; Kwan 2010).
Description of the intervention
Various preventive interventions are employed to minimise the risk of developing lymphoedema after treatment for breast cancer. For this review, we considered conservative interventions: non‐surgical and non‐pharmacological interventions. These include, but may not be limited to, the interventions as described below.
Exercise
Performing exercise has been debated to be both a risk factor and a risk‐reducing factor (Ewertz 2011). Exercise increases blood flow and the blood pressure in the upper limb, and consequently increases lymph production. On the other hand, muscle activity in the limb stimulates lymph flow (often referred to as the 'muscle pump'), improving lymph drainage. Interindividual physiological variation seems to exist with regard to changes in lymphatic drainage during exercise (Lane 2007). Exercises that specifically aim to stimulate lymph flow from the extremity towards the thorax may, if effective, lower the risk of developing lymphoedema. Exercises that improve the range of motion and strength of the upper limb may also improve daily use of the arm thus improving lymph drainage through muscle activity (Box 2002).
Patient education
Participant education can be provided verbally, or through written materials. Education is intended to help participants understand the changes in fluid regulation in the affected limb and the influence of external factors on fluid regulation. Risk minimisation strategies may additionally be discussed as part of the education, including lifestyle advice, such as maintaining activity levels and a healthy BMI, information on early self‐detection of lymphoedema, and measures that can be taken in case swelling occurs (Box 2002; Park 2008). Although education may be effective in encouraging preventive self‐care measures, it may also unintentionally reduce other desirable forms of behaviour, such as activities involving the arm on the affected side (Lee 2009).
Monitoring and early intervention
Monitoring involves regular follow‐up appointments to objectively judge the status of the affected limb and to reinforce behaviour that is thought to be beneficial for preventing lymphoedema. Subclinical lymphoedema may be diagnosed with the help of techniques such as bioimpedance spectrometry or whole limb perimetry. The rationale for monitoring is that the sooner lymphoedema is diagnosed then the sooner it can be adequately addressed, thus limiting morbidity (Stout 2008), although it is unclear whether or not subclinical lymphoedema will ultimately result in clinically‐detectable lymphoedema in individual patients.
Compression therapy
Compression therapy may consist of wearing compression garments in various compression classes, and using bindings or pneumatic compression devices. The rationale for compression therapy is based on providing resistance to swelling, as well as to improving the ‘muscle pump’ function. Compression therapy has been recommended for the treatment and control of manifest lymphoedema of the limbs (Preston 2008), but is also sometimes used for prevention of lymphoedema.
Manual lymph drainage
Manual lymph drainage (MLD) is a massage technique that involves gentle compression of the skin to stimulate lymph flow and manual stimulation of lymph nodes to increase their activity. MLD generally aims at improving consistency of the oedema and reducing or stabilising lymphoedema. Reducing lymphoedema is achieved by stimulating the formation of physiological lymphatic shunts or alternative pathways for lymph drainage. Some evidence suggests that MLD could be effective in reducing upper limb volume in people with existing lymphoedema although it is usually combined with other treatment modalities (Devoogdt 2010; Preston 2008; Torres 2010). Some advocate the use of MLD to prevent lymphoedema by activating alternative drainage pathways (Torres 2010). Techniques of manual lymph drainage may also be used to improve tissue consistency and tissue compliance of the surgical scar, with the objective to improve lymphatic flow through the tissue and range of motion.
Lymph taping (Kinesio tape)
The concept of lymph taping is relatively novel. This therapy involves the application of elastic, thermo‐adhesive tape in such a way that lymph drainage towards the lymph nodes is facilitated. Kinesio tape has been suggested as a replacement for bandaging in the treatment of lymphoedema (Tsai 2009b).
Why it is important to do this review
Considering the impact of lymphoedema on the quality of life of people after breast cancer therapy and the associated societal costs, efforts should be made to prevent its occurrence. Unfortunately, there is no conclusive evidence to date on the optimal strategy to prevent lymphoedema. Preventive treatments carry with them direct and indirect costs that should be balanced against possible gains. A research recommendation for a systematic review addressing this subject was made in the NHS Database of Uncertainties about the Effects of Treatments (DUETS) at http://www.library.nhs.uk/DUETs/ViewResource.aspx?resID=302437 (Duets). The review presented here aims to summarise current evidence in such a way that it can be used to guide clinical decisions, and support the development of evidence‐based guidelines for the prevention of lymphoedema in people with breast cancer.
Objectives
To assess the effects of conservative (non‐surgical and non‐pharmacological) interventions for preventing clinically‐detectable upper‐limb lymphoedema after breast cancer treatment.
Methods
Criteria for considering studies for this review
Types of studies
We considered eligible for inclusion all types of randomised controlled trials (RCTs) that had reported secondary lymphoedema as the primary outcome, and had compared a conservative intervention to either usual care, placebo intervention, or some other intervention.
Types of participants
Trials with participants of both sexes and all ages at risk of developing lymphoedema in the upper limb after treatment for breast cancer were eligible for inclusion. Treatments for breast cancer could include: surgical treatment for breast cancer with axillary lymph node dissection, sentinel lymph node biopsy or axillary sampling, with or without radiotherapy to the axilla or the supraclavicular fossa or both; or radiotherapy alone. Trials in people who had been diagnosed with lymphoedema or cancer recurrence were not eligible for inclusion.
Types of interventions
We considered trials of exercise therapy, patient education, monitoring and early intervention, manual lymph drainage (MLD), compression therapy (bandages, a compression sleeve, pneumatic compression), and lymph taping; or any combination of these interventions. We also considered trials with other non‐pharmacological and non‐surgical interventions eligible for inclusion if they were identified in the search, provided that the studies met the other inclusion criteria.
Types of outcome measures
Primary outcomes
The primary outcome in our review is the occurrence of lymphoedema. This could be reported as either a dichotomous outcome or as a continuous outcome (volume or percentage volume change). Time‐to‐event data, with lymphoedema as the event, was also used, if reported. Because of the variety of ways in which lymphoedema can be defined and diagnosed, studies were only considered eligible if they had used a predefined criterion for establishing lymphoedema that was based, at least in part, on an objective assessment. This included circumference measurements, water displacement methods, bioimpedance measurements, laser scanning, perimetry and dual energy X‐ray absorptiometry (DEXA) scanning. This means we did not include studies that had evaluated an intervention based solely on a diagnosis of lymphoedema made by a healthcare professional or on self‐reported swelling or complaints of oedema.
Secondary outcomes
Secondary outcome measures of interest were:
infection, defined as any inflammation (redness, pain, heat and swelling) for which antibiotics were prescribed;
active range of motion (AROM) of the upper limb; and
level of functioning in activities of daily living (ADL), as a self‐reported measure or as rated by an assessor using a validated measurement instrument.
The following self‐reported measures were also included as secondary outcomes, whenever assessed with a validated measurement instrument:
pain;
health‐related quality of life (including both physical and mental well‐being); and
psychosocial morbidity (emotional or psychosocial distress).
Any reported adverse effects of the preventive treatments were documented.
Search methods for identification of studies
See: Breast Cancer Group methods used in reviews.
No language or publication date restrictions were imposed. We only considered research that has been published in peer‐reviewed scientific journals.
Electronic searches
We searched the following databases.
(a) The Cochrane Breast Cancer Group's (CBCG) Specialised Register. Details of the search strategies used by the Group for the identification of studies and the procedure used to code references are outlined in the Group's module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). Trials with the key words 'lymphoedema/ lymphedema', 'upper‐limb lymphoedema/ lymphedema', 'exercise', 'education', 'patient monitoring', 'manual lymph drainage', 'compression therapy', 'compression bandages', 'compression sleeve', 'pneumatic compression', 'lymph taping' and 'kinesiotape' were extracted and considered for inclusion in the review.
(b) MEDLINE via PubMed. See Appendix 1 for the search strategy.
(c) EMBASE via Ovid (1980 to May 2013). See Appendix 2 for the search strategy.
(d) The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials. See Appendix 3 for the search strategy.
(e) The Cumulative Index to Nursing and Allied Health Literature (CINAHL) through EBSCO (1980 to May 2013). See Appendix 4 for the search strategy.
(f) The Physiotherapy Evidence Database (PEDro) via http://www.pedro.org.au/ (1980 to May 2013). See Appendix 5 for the search strategy.
(g) PsycINFO through Ovid (1980 to May 2013). See Appendix 6 for the search strategy.
(h) The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 4, April 2013). See Appendix 7.
Searching other resources
References of included articles and relevant identified reviews were handsearched for previously unidentified studies.
Data collection and analysis
Selection of studies
All studies identified through the electronic searching were screened for eligibility by two authors independently (MS and MT or CA). An initial selection was carried out based on the title of the study. Studies were classified as potentially eligible if the title of the study report indicated a randomised controlled trial (RCT) on the prevention of lymphoedema using a conservative therapy. If no judgement could be made about the eligibility of a study based on the title, the judgement was based on title and abstract. Any disagreements about eligibility were resolved in consensus meetings. The same procedure was applied to references found in articles reporting on included studies. Review articles identified in the search were screened for relevance and reference lists were checked to identify additional potentially eligible studies. Final decisions about inclusion for all studies judged potentially eligible were based on the full text of the study report.
Data extraction and management
Two authors (MS and MT) performed data extraction independently, using data collection forms that were developed and pre‐tested for the purpose of this study. In the case of disagreement, agreement was reached in a consensus meeting. If no consensus could be reached, the decision was made by a third author (CA).
For each included study, the following characteristics were collected:
1. study information (year, country, setting, sample size, method of randomisation, blinding and method of outcome assessment including the definition of lymphoedema in the case of a dichotomous outcome, duration of follow‐up);
2. baseline characteristics of study participants (age, sex, disease stage);
3. intervention used for the prevention of lymphoedema (type of treatment, dosage of treatment, description of usual care condition);
4. comparator (alternative intervention or follow‐up only);
5. aggregated outcomes (proportions of incident cases, or relative risks for dichotomous data, or means and standard deviations for continuous data);
6. adverse effects reported; and
7. loss to follow‐up (number and reasons).
If the data and methods reported were insufficient for data extraction or risk of bias assessment, the authors of included studies were contacted for additional information.
Assessment of risk of bias in included studies
Risk of bias was assessed using the Cochrane risk of bias tool for the appraisal of RCTs, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (Higgins 2011). The tool contains six domains and each domain was assigned a judgement related to the risk of bias. The judgement could be 'low risk', 'high risk', or 'unclear risk'. The latter judgement was assigned if the risk of bias of a characteristic in an included study was judged to be unclear, or if there was insufficient information on which to base the judgement.
The six domains are:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessors;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
Other sources of bias specifically addressed were comparability of the groups at baseline, intention to treat analysis, and equal treatment of groups except for the allocated intervention. Specifically, additional contact with a healthcare professional due to the nature of the intervention may also reinforce risk‐reduction behaviour, such as self‐care; this may result in an overestimation of the effect. Since the effectiveness of self‐care and other risk‐reduction behaviour is unclear, risk of bias from other sources was set to 'unclear' if this was the only potential source of bias, or 'high' if there were additional concerns related to risk of bias from other sources. Judgements on comparability of groups at baseline were based on magnitude of the differences rather than statistical significance.
Two authors (MS and MT) independently assessed each included trial. Results were compared and discussed in a consensus meeting. If no consensus could be reached, a third author (CA) made the decision. In cases where no clear judgement could be reached based on the trial report, the trial authors were contacted to obtain additional details. The risk of bias is reported with a 'Risk of bias' table and graph for each outcome measure.
Measures of treatment effect
Statistics to express treatment effects are reported for each outcome separately. We used the measure of effect as estimated in the intention‐to‐treat analysis. The method of assessment is reported for each outcome.
Dichotomous outcomes
For dichotomous outcomes, such as a diagnosis of lymphoedema, the treatment effect was expressed as a risk ratio with 95% confidence intervals (CIs).
Continuous outcomes
For continuous outcomes, such as limb volume, and self‐reported measures, such as health‐related quality of life, psychosocial morbidity, level of ADL functioning and active range of motion of the upper limb, the treatment effect was expressed as the mean difference or the standardised mean difference if different scales had been used. If no mean differences and CIs were reported, they were calculated from the available summary data using Review Manager software (RevMan 5).
For outcome variables measured with the same instrument, final scores and change scores (the difference between baseline scores and final scores) were reported in the included trials. In future updates of this review, we will preferably extract the adjusted final scores for the meta‐analysis if these are reported.
If final scores and change scores could be pooled together, they were presented for subgroups in the corresponding forest plot. If it was not possible to extract standard deviations (SDs) for a particular outcome, attempts were made to obtain the SDs from the study authors. If no further details could be obtained, missing SDs were imputed using the square root of the average of the variances (standard deviation squared) from all other included studies for that measure, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions.
Time‐to‐event outcomes
For time‐to‐event outcomes such as time to diagnosis of lymphoedema, the treatment effect was expressed as a hazard ratio (HR). Only unadjusted HRs were available and extracted from the identified studies. In future updates of the review, if the HR and associated variances cannot be obtained directly from the trial publication, we will obtain these data indirectly using the methods described by Parmar 1998, by employing other available summary statistics or data extracted from published Kaplan‐Meier curves.
Unit of analysis issues
Unit of analysis issues were not encountered in this review. However for future updates of this review that include studies with multiple intervention groups, comparable groups within a study will be combined to create a single pair‐wise comparison, if this is possible. If necessary we will reduce the sample size for the control group when making multiple comparisons. If this is not possible, only one comparison will be made per meta‐analysis.
Given the nature of the primary outcome, no cross‐over trials or cluster‐randomised trials were expected to be identified in the search.
Dealing with missing data
For studies that were listed in trial registers, reported outcomes were compared with those specified in the protocol. If outcomes as described in the methods section of the publication or the trial registration file were not presented in the available publications, the authors were contacted for additional details.
Assessment of heterogeneity
Three authors (MS, MT and CA) jointly judged the extent of clinical heterogeneity for studies that had comparable goals and type of intervention, but differences with respect to treatment protocols or population. Outcomes that were judged potentially eligible for meta‐analyses were used to generate summary measures of treatment effect. Subsequently, statistical heterogeneity was assessed by visual inspection of the forest plots and quantified using the Chi² statistic and the I² statistic, as provided by Review Manager software (RevMan 5).
For the Chi² statistic, a P value of 0.10 was set to indicate statistically‐significant heterogeneity, rather than the conventional value of 0.05. The I² statistic indicates the percentage of the variability in effect estimates that is due to heterogeneity. We considered an I² statistic greater than 50% as large. The value of the I² statistic was evaluated alongside the magnitude and direction of effect and the P value for the Chi² statistic for heterogeneity (Higgins 2011).
Because of the small number of studies available per outcome, we used a fixed‐effect model in all cases. For future updates of this review, if there is no statistical evidence of heterogeneity we will use the fixed‐effect model (Mantel 1959); while if significant heterogeneity exists we will use the random‐effects model (DerSimonian 1986) and sources of heterogeneity will be explored.
Assessment of reporting biases
Given the small number of studies per outcome, no funnel plots were generated to test for reporting bias. Future updates of this review will include funnel plots if sufficient studies are available (Higgins 2011).
Data synthesis
Treatment effects from studies with comparable interventions and outcomes were visualized in forest plots. Summary estimates were calculated only if statistical heterogeneity was within the prespecified limits of acceptability.
Since the reported numbers of events in the majority of studies reflected point‐prevalence rather than cumulative incidence, we used the risk ratio (RR) for dichotomous outcomes.
If time‐to‐event analyses were presented, hazard ratios were extracted. RRs and HRs were combined in separate plots. The results were stratified according to the duration of follow‐up, combining studies with short follow‐up (less than six months) and medium length follow‐up (6 months up to two years) in separate plots. Published data on long‐term follow‐up (more than 2 years) were not available, but may be examined in future updates of the review.
For continuous outcomes, mean differences (MD) were used for limb volume and standardised mean differences (SMD) for self‐reported measures.
For dichotomous outcomes, fixed‐effect analyses using the Mantel‐Haenszel method were conducted on all occasions considering the small number of studies (Mantel 1959). If the results were judged too heterogeneous, forest plots were generated, but no total summary statistics were presented.
All analyses were performed using Review Manager software (RevMan 5) in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and R3.0.1 (R Statistical Package).
Subgroup analysis and investigation of heterogeneity
Due to the small number of studies identified no subgroup analyses were possible. In future updates of this review, if a large amount of heterogeneity is found, subgroup analyses will be performed for people with and without axillary clearance and people with and without radiotherapy treatment, if sufficient data are available. If there are sufficient studies, subgroup analyses will also be performed to examine the impact of study quality on outcome measures.
Sensitivity analysis
Due to the small number of identified studies per intervention, and the fact that meta‐analysis was not possible in most cases, no sensitivity analyses were performed. In future updates of this review, if adequate data are available, we will perform sensitivity analyses to assess the robustness of our results by repeating the analysis with the following adjustments:
repeating the analysis excluding studies with high risk of bias;
repeating the analysis each time excluding unpublished results.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
See: Figure 1.
1.
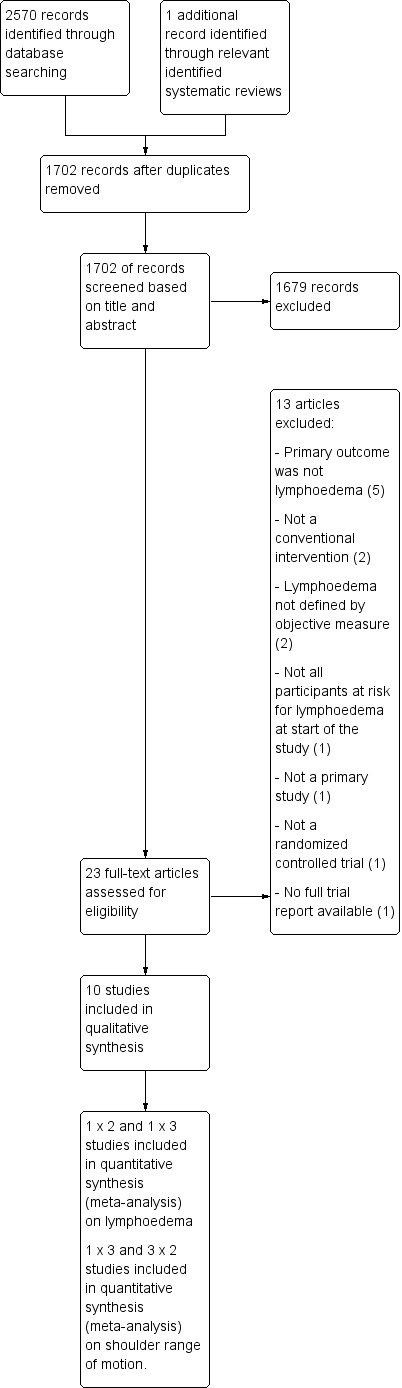
Study flow diagram.
A total of 2570 records were identified in the initial search, of which six were relevant reviews. In the reference lists of these reviews, one additional potentially eligible study was identified.
After removing duplicates, 1702 unique titles remained. Of these, 1679 were excluded based on title and abstract screening.
Included studies
Twenty‐three of the 1702 unique records were retrieved for full text evaluation. Of these 10 fulfilled all inclusion criteria (Bendz 2002; Box 2002; Castro‐Sanchez 2011; Cinar 2008; Devoogdt 2011; Sagen 2009; Schmitz 2010; Todd 2008; Torres 2010; Zimmermann 2012).
For three of the included studies, additional publications were available (Box 2002; Zimmermann 2012; Schmitz 2010). These publications concerned reports on additional outcome measures (Zimmermann 2009 for Zimmermann 2012; Box 2002 for Box 2002; Speck 2010 for Schmitz 2010), a publication on the trial protocol (Schmitz 2009 for Schmitz 2010) and a paper on adverse events (Brown 2012 for Schmitz 2010).
Although studies including both men and women were eligible for inclusion in the review, all studies concerned women only. All included studies had evaluated the occurrence of lymphoedema, but different study questions and interventions had been addressed:
Four trials in five publications investigated the effectiveness of manual lymph drainage, alone or in combination with other interventions, for the prevention of lymphoedema after breast cancer surgery (Castro‐Sanchez 2011; Devoogdt 2011; Torres 2010; Zimmermann 2012; Zimmermann 2009).
Two studies in four publications were non‐inferiority trials investigating the safety of progressive resistance exercise after breast cancer surgery, with regard to lymphoedema risk (Sagen 2009; Schmitz 2010).
Three studies, (Bendz 2002; Cinar 2008; Todd 2008), investigated the influence of different postoperative rehabilitation protocols (early versus late start of shoulder‐mobilisation exercises after surgery for breast cancer) on the risk of subsequent secondary lymphoedema.
One study, (Box 2002), investigated the effects of a comprehensive out‐patient physiotherapy program for women surgically treated for breast cancer, that included education, monitoring, exercise and early intervention for prevention of lymphoedema.
Six studies (Bendz 2002; Box 2002; Cinar 2008; Todd 2008; Torres 2010; Zimmermann 2012) included shoulder range of motion as a secondary outcome measure.
Four studies (Bendz 2002; Castro‐Sanchez 2011; Sagen 2009; Torres 2010) reported pain as a secondary outcome measure.
Four studies included HRQoL as a secondary outcome measure (Castro‐Sanchez 2011; Devoogdt 2011; Schmitz 2010; Todd 2008).
We did not identify any studies evaluating the effectiveness of lymph taping for prevention of lymphoedema.
Full details on trial characteristics and outcomes are provided in the 'Characteristics of included studies' table.
Excluded studies
Thirteen full‐text publications were excluded (Ahmed 2006; Anderson 2012; Boccardo 2009; Box 2009; Campisi 2002; Chandrakaladharan 2009; de Rezende 2006; Hayes 2012; Le‐Vu 1997; Oliveira 2009; Sarri 2010; Sisman 2012; Wang 2005).
Reasons for exclusion were the use of (partly) non‐conservative interventions (Boccardo 2009; Campisi 2002); a primary outcome other than lymphoedema (Anderson 2012; de Rezende 2006; Hayes 2012; Le‐Vu 1997; Oliveira 2009; Sarri 2010); and the use of subjective measures or unclear criterion for defining lymphoedema, or both (Hayes 2012; Le‐Vu 1997; Wang 2005). One manuscript was not a primary study, but a synopsis of another study (Box 2009). One record of a potentially eligible study was a conference abstract (Chandrakaladharan 2009). No corresponding article could be identified and no additional information could be obtained from the institution where the work was done. Since the available data from the conference abstract was insufficient for the purposes of this review, the study was excluded. One study was not a randomised controlled trial (Sisman 2012). Another study was excluded because it also included people with lymphoedema at baseline (Ahmed 2006).
See the 'Characteristics of excluded studies' table for further details.
Risk of bias in included studies
Information on one or more items related to risk of bias was unclear or not reported in seven studies (Bendz 2002; Box 2002; Castro‐Sanchez 2011; Cinar 2008, Devoogdt 2011; Torres 2010; Zimmermann 2012) The authors of these studies were contacted for further clarification and the missing information was obtained in all but one case (Cinar 2008).
Detailed information on risk of bias for all studies is described in the 'Risk of bias' tables under Characteristics of included studies and in the risk of bias graph in Figure 2.
2.
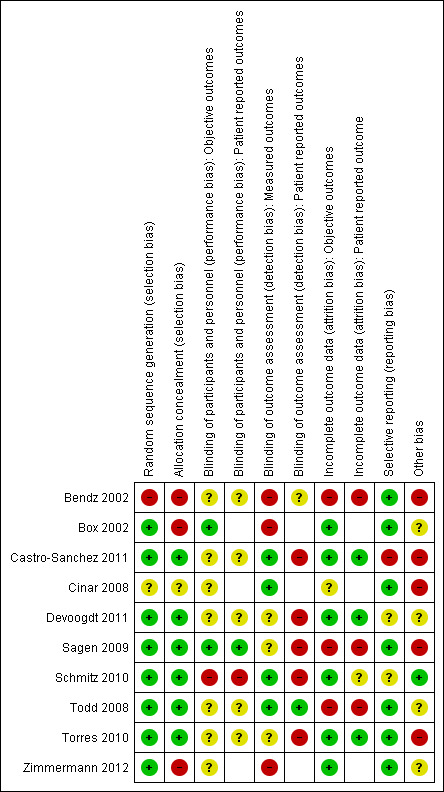
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All studies used randomisation for treatment allocation. In one study, a cluster randomised study, the method of treatment allocation per time period was not described in detail and it cannot be excluded that this may have been quasi randomised (Bendz 2002).
Allocation concealment
Allocation concealment was explicitly ensured in six studies (Castro‐Sanchez 2011; Devoogdt 2011; Sagen 2009; Schmitz 2010; Todd 2008; Torres 2010). One study did not mention allocation concealment (Bendz 2002). Since cluster randomisation was applied over periods of four weeks, allocation was to a certain extent predictable (see also random sequence generation). In two studies, allocation was not sufficiently blinded (Box 2002; Zimmermann 2012).
Blinding
Blinding of participants and personnel
None of the studies relied on blinding of study participants for the intervention. Blinding of personnel was applied in only one study (Box 2002).
Lack of blinding of participants and personnel may lead to different impact on risk of bias across different types of interventions and outcome. For objective outcomes, the risk of bias was judged as low in two studies (Box 2002; Sagen 2009), high in one study (Schmitz 2010), and unclear in seven studies (Bendz 2002; Castro‐Sanchez 2011; Cinar 2008; Devoogdt 2011; Todd 2008; Torres 2010; Zimmermann 2012). Although compliance with the experimental intervention was measured and reported in some studies, this was not the case for compliance with the control condition in all but two studies (Sagen 2009; Schmitz 2010).
Risk of bias due to lack of blinding for patient‐reported outcomes was classified as high in one study (Schmitz 2010), low in one study (Sagen 2009) and unclear in five studies ( Bendz 2002; Castro‐Sanchez 2011; Devoogdt 2011; Todd 2008; Torres 2010).The remaining studies (Box 2002; Cinar 2008; Zimmermann 2012) did not use patient‐reported outcomes.
Blinding of outcome assessors
In three studies, there was no blinding of outcome measurement or blinding was insufficiently assured for the primary outcome (Bendz 2002; Box 2002; Zimmermann 2012). In three studies (Devoogdt 2011; Sagen 2009; Torres 2010), risk of bias was unclear for the primary outcome, lymphoedema. Although outcome assessors were blinded, participants would be examined if they reported symptoms in between regular follow‐up points. Since participants were not blinded to the intervention, the inclination for participants to report symptoms may have been different between the intervention and the control groups. Participants diagnosed with lymphoedema received treatment, so bias would affect cumulative incidence as well as point prevalence estimates. This bias applies to lymphoedema only, but not to other objective outcomes.
In the remaining four studies (Castro‐Sanchez 2011; Cinar 2008; Schmitz 2010; Todd 2008) outcome assessment was sufficiently blinded.
Seven studies assessed patient‐reported outcomes. Of these, risk of bias due to lack of blinding of outcome assessors was considered high in five studies (Castro‐Sanchez 2011; Devoogdt 2011; Sagen 2009; Schmitz 2010; Torres 2010), unclear in one study (Bendz 2002), and low in one study (Todd 2008).
Incomplete outcome data
Loss to follow‐up was limited in most studies. Risk of bias for objective outcomes due to differential loss to follow‐up was considered high in three studies (Bendz 2002; Sagen 2009; Todd 2008), unclear in one study (Cinar 2008), and low in all other studies (Box 2002; Castro‐Sanchez 2011; Devoogdt 2011; Schmitz 2010; Torres 2010; Zimmermann 2012).
For patient‐reported outcomes, risk of bias due to incomplete outcome data was high in three studies (Bendz 2002; Sagen 2009; Todd 2008), unclear in one study (Schmitz 2010), and low in the remaining three studies that included patient‐reported outcomes (Castro‐Sanchez 2011; Devoogdt 2011; Torres 2010).
Selective reporting
Risk of reporting bias was low in most studies. For six trials (Box 2002; Devoogdt 2011; Sagen 2009; Schmitz 2010; Todd 2008; Torres 2010; ), enquiries were made about unreported data by contacting the authors of the studies. These results were obtained in all but two cases (Devoogdt 2011; Schmitz 2010). Consequently risk of bias was set to 'unclear' for these studies. In one study (Castro‐Sanchez 2011), the measures as reported for lymphoedema differed from the measure as defined in the methods section, and therefore the risk of bias was judged to be high.
Other potential sources of bias
In four studies, assignment to the treatment group also implied that participants had more contact with a healthcare professional compared to the control group (Castro‐Sanchez 2011; Cinar 2008; Sagen 2009; Zimmermann 2012).
One study had statistically‐significant differences in HRQoL at baseline that were not controlled for in the analysis (Castro‐Sanchez 2011), and one study had differences in number of participants with pre‐existing shoulder problems between groups at baseline, as well as differences in the number of people receiving radiotherapy (Cinar 2008).
One study used a cluster randomised design, which was not accounted for explicitly in the analysis (Bendz 2002).
Effects of interventions
See: Table 1; Table 2; Table 3
Manual lymph drainage (MLD)
Incidence of treatment failure (occurrence of lymphoedema)
Four trials, involving 395 participants, tested MLD alone or in combination with other interventions. In two of these studies, manual lymph drainage as an added intervention to usual care was investigated, allowing for the evaluation of the unique effect of MLD (Devoogdt 2011; Zimmermann 2012). Two other studies investigated the effect of MLD in combination with another intervention compared to education alone (Castro‐Sanchez 2011; Torres 2010).
In Devoogdt 2011, both cumulative incidence up to each follow‐up point and point prevalence at each follow‐up point were reported. In Castro‐Sanchez 2011, Torres 2010 and Zimmermann 2012 no explicit distinction was made and reported numbers were treated as cumulative incidence.
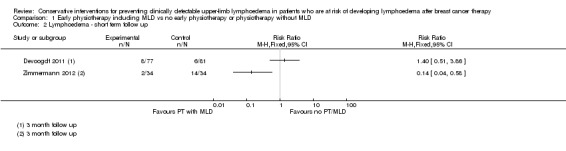
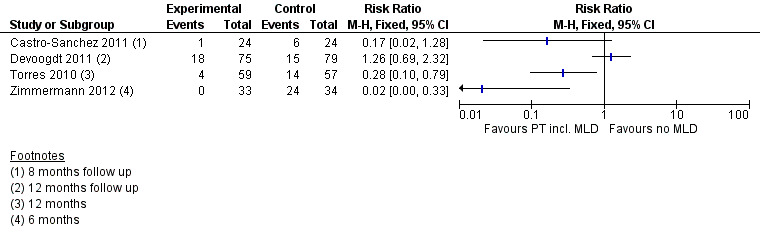
Due to substantial clinical and statistical heterogeneity both for short‐term (less than 6 months) and medium‐term (more than 6 months, less than 24 months) follow‐up, (I² = 86%, P = 0.008; and I² = 84%, P < 0.001 respectively for RR; and I² = 84%, P = 0.01 for the HR), no meta‐analyses were performed. The results of all studies comparing physiotherapy with MLD to any other intervention are summarized in a single forest plot without totals (see: Figure 3 (Analysis 1.1); Analysis 1.2; Figure 4 (Analysis 1.3)), and a narrative summary of the results is provided below. A summary of the main outcomes of these studies is also provided in 'Summary of findings' table 1.
3.

Forest plot of comparison: 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, outcome: 1.1 Time to event for lymphoedema.
1.1. Analysis.

Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 1 Time to event for lymphoedema.
1.2. Analysis.
Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 2 Lymphoedema ‐ short term follow up.
4.
Forest plot of comparison: 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, outcome: 1.3 Lymphoedema ‐ medium term follow up.
1.3. Analysis.
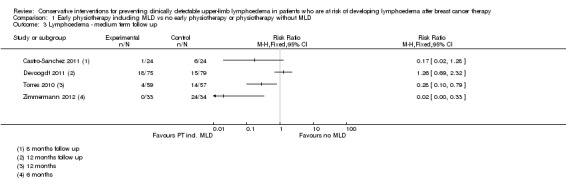
Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 3 Lymphoedema ‐ medium term follow up.
Physiotherapy including MLD versus physiotherapy without MLD
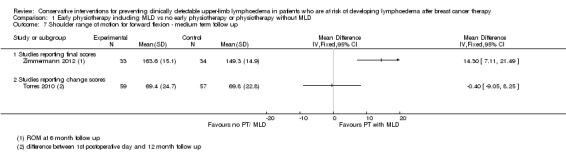
One study (Zimmermann 2012) that investigated MLD in addition to routine physiotherapy consisting of exercises of the upper limb and chest, compared to a control group that had routine physiotherapy only, found a large lymphoedema risk‐reducing effect of MLD (RR 0.14, 95% CI 0.04 to 0.58, P < 0.003 at 3 month follow‐up; RR 0.02, 95% CI 0.00 to 0.33, P < 0.001 at 6 months follow‐up). Risk of bias in this study was high.
Another study (Devoogdt 2011), with moderate risk of bias, found no added value of MLD in combination with routine physiotherapy consisting of exercises and education in comparison to routine physiotherapy only (RR 1.40, 95% CI 0.51 to 3.86, P = 0.51 at 3 month follow‐up; RR 0.96, 95% CI 0.45 to 2.05, P = 0.92 at 6 month follow‐up; RR 1.26, 95% CI 0.69 to 2.32, P = 0.45 at 12 month follow‐up). In this study, comparisons were also made for time‐to‐event for the occurrence of lymphoedema. There was no statistically‐significant difference between the groups (HR 1.30, 95% CI 0.67 to 2.53, P = 0.44). Results on lymphoedema risk as defined by a different criterion (an increase of 2 cm or more in the difference in arm circumference between the affected and healthy side at two or more adjacent measurement points compared with the difference before surgery), which was included as a secondary outcome measure, were qualitatively similar.
MLD in combination with other interventions versus education only
Castro‐Sanchez 2011 reported a statistically‐significant reduction in lymphoedema risk for people receiving a combined intervention of MLD, compression, scar massage and education, compared to those receiving education alone, although the 95% confidence interval as calculated from the available data included the null value of the RR (RR 0.17, 95% CI 0.02 to 1.28, calculated P = 0.097; reported P = 0.042 at 8‐month follow‐up). Risk of bias in this study was moderate.
A second study compared MLD combined with exercise therapy and education to education only (Torres 2010). In this study, there was a statistically‐significant reduction in lymphoedema risk at the 12‐month follow‐up in favour of the intervention group (RR 0.28, 95% CI 0.10 to 0.79, P = 0.01 ). Time‐to‐event analysis in this study suggested a statistically‐significant difference in favour of the intervention group (HR 0.26, 95% CI 0.09 to 0.79, P = 0.01). Risk of bias in this study was high.
Infection
No data on this outcome.
Active range of motion (AROM) of the upper limb
Two studies, examined the effect of early physiotherapy consisting of MLD plus exercise on shoulder range of motion (Torres 2010; Zimmermann 2012). Both studies had high risk of bias. P‐values were not available from Torres 2010 and these values were calculated from the reported mean changes and standard deviations.
Pooling the results of the early postoperative phase (equal to or less than three weeks) resulted in a mean difference for abduction of 22° (95% CI 14 to 30, P < 0.00001, Analysis 1.4) and a mean difference for forward flexion of 14° (95% CI 7 to 22, P = 0.0001, Analysis 1.5) in favour of the intervention group.
1.4. Analysis.
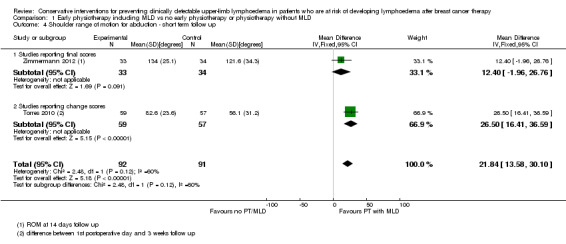
Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 4 Shoulder range of motion for abduction ‐ short term follow up.
1.5. Analysis.
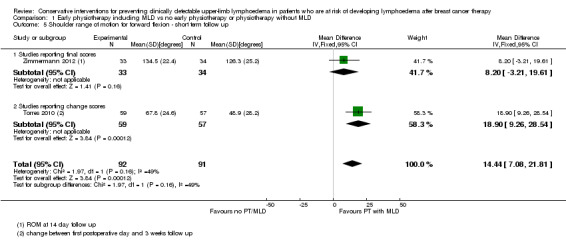
Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 5 Shoulder range of motion for forward flexion ‐ short term follow up.
At medium term follow‐up (equal to or greater than six months), Torres 2010 reported a small and statistically non‐significant difference in favour of the control group, in improvement of shoulder range of motion from first postoperative day to 12 month follow‐up: intervention group ‐3° (95% CI ‐11 to 4, P = 0.42) for abduction, and of ‐0.4° (95% CI ‐9 to 8, P = 0.93) for forward flexion. Zimmermann 2012 reported a statistically‐significant mean difference of 17° (95% CI 10 to 24, P < 0.001) for abduction and 14° (95% CI 7 to 21; P < 0.001) for forward flexion, in favour of the intervention group.
No meta‐analyses could be performed due to considerable statistical heterogeneity (I² = 93%, P < 0.01 and I² = 85%, P = 0.01, for abduction and forward flexion, respectively), and a forest plot is provided without totals (Analysis 1.6; Analysis 1.7).
1.6. Analysis.
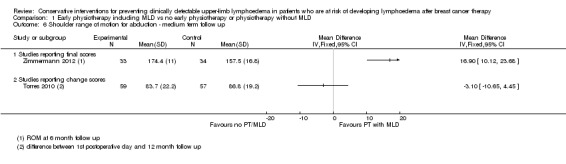
Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 6 Shoulder range of motion for abduction ‐ medium term follow up.
1.7. Analysis.
Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 7 Shoulder range of motion for forward flexion ‐ medium term follow up.
Only one of the studies (Torres 2010) included range of motion for rotations and found a small and statistically non‐significant difference in recovery of medial rotation (15° versus 10° improvement, calculated P for the mean difference in change = 0.09, reported 95% CI for the difference in observed means: 4 to 11) and lateral rotation (8° versus 7°, calculated P for the mean difference in change = 0.69, reported 95% CI for the difference in observed means: 1 to 6) in favour of the early physical therapy group at 3 weeks and 12 months respectively after the first postoperative day. Refer to Analysis 1.6 and Analysis 1.7.
ADL function
No data on this outcome.
Pain
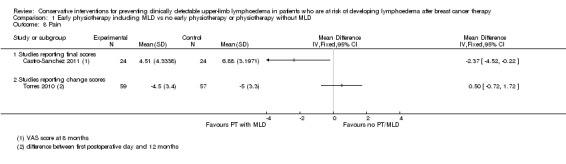
Two studies (Castro‐Sanchez 2011; Torres 2010), both with high risk of bias, addressed pain as a secondary outcome and both evaluated combined interventions including manual lymph drainage versus education alone. No meta‐analysis was performed due to statistical heterogeneity (I² = 81%, P = 0.02, Analysis 1.8).
1.8. Analysis.
Comparison 1 Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD, Outcome 8 Pain.
In Torres 2010, people who received manual lymph drainage, exercise and education reported greater improvement in pain score from baseline at three weeks (‐4.2 points versus ‐3.8 points change on a 0 to 10 scale; reported 95% CI of the difference in means ‐0.7 to 1.7; calculated P for the difference in change = 0.46) but less improvement at 12 months (‐4.5 versus ‐5.0, 95% CI ‐0.72 to 1.72, P = 0.42) compared to participants who received education alone.
In Castro‐Sanchez 2011, participants receiving MLD and using a compression sleeve for 8 months reported lower pain scores on a 0 to 10 rating scale, compared to participants who received education only. Mean difference and 95% CI were calculated from provided means and 95% CIs: mean difference ‐2.4 points, 95% CI ‐4.5 to ‐0.2, reported P = 0.056, calculated P = 0.03.
Health‐related Quality of Life (HRQoL)
MLD in combination with other interventions versus education only
Two studies on MLD in combination with other interventions assessed HRQoL as a secondary outcome measure (Castro‐Sanchez 2011; Devoogdt 2011). Due to clinical heterogeneity meta‐analysis was deemed inappropriate and a narrative synthesis has been provided.
In Devoogdt 2011, no statistically‐significant differences were found in the mental and physical summary component scores of the 36‐item Medical Outcomes Study Short‐Form (SF‐36) between participants who received MLD in combination with exercise and education, and participants who received education only. This study had moderate risk of bias.
In Castro‐Sanchez 2011, participants receiving MLD plus compression had statistically‐significantly better mean scores than participants receiving education only, for physical functioning (144 versus 109, P = 0.02), social functioning (144 versus 124, P = 0.02), fatigue (47 versus 71, P = 0.03) and financial difficulties (6 versus 14, P = 0.04) as measured with the EORTC QLQ‐C30 questionnaire. Risk of bias in this study was high. In particular, there were baseline differences in several domains of the QLQ‐C30 (see 'other types of bias' in the Risk of bias in included studies for Castro‐Sanchez 2011).
Psychosocial morbidity
No data on this outcome.
Adverse Events
No data on this outcome.
Exercise
Incidence of treatment failure (occurrence of lymphoedema)
Early versus delayed onset of mobilising shoulder exercises after breast cancer treatment
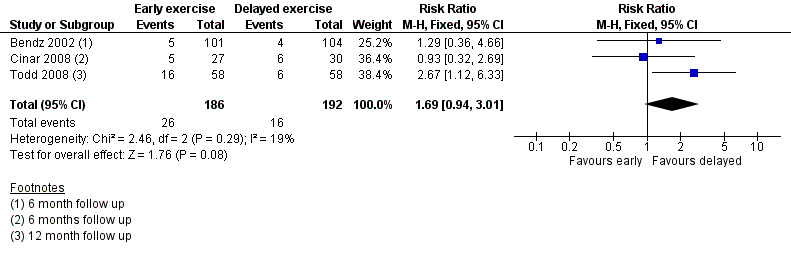
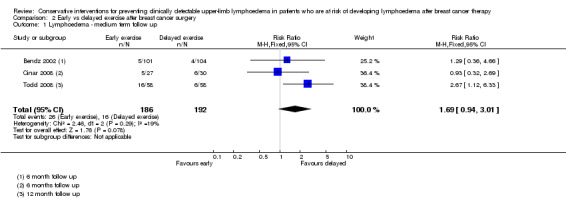
Three trials, involving 378 participants, all with high risk of bias for the primary outcome, investigated the influence of early versus delayed onset of full range mobilising shoulder exercises after breast cancer surgery (Bendz 2002; Cinar 2008; Todd 2008).
Meta‐analysis resulted in a summary estimate of the relative risk of lymphoedema at medium‐term follow‐up (6 to 12 months) between early and late start of full range exercises of 1.69 (95% CI 0.94 to 3.01, P = 0.08) (Figure 5 (Analysis 2.1)).
5.
Forest plot of comparison: 2 Early vs delayed exercise after breast cancer surgery, outcome: 2.1 Lymphoedema ‐ medium term follow up.
2.1. Analysis.
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 1 Lymphoedema ‐ medium term follow up.
Progressive resistance exercise after breast cancer treatment
The meta‐analysis of two non‐inferiority studies (Sagen 2009; Schmitz 2010) indicated that weight training after breast cancer treatment did not increase lymphoedema risk (RR 0.58, 95% CI 0.30 to 1.13, P = 0.11; Figure 6 (Analysis 3.1)).
6.
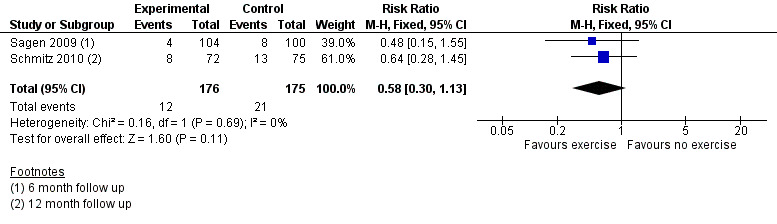
Forest plot of comparison: 3 Progressive resistance exercise vs no exercise, outcome: 3.1 Lymphoedema in studies with ≥6 month follow up < 24 months.
3.1. Analysis.
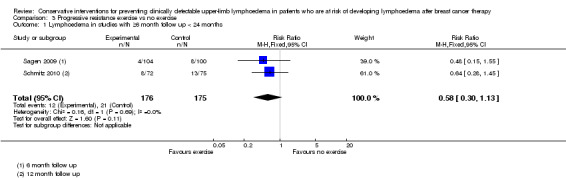
Comparison 3 Progressive resistance exercise vs no exercise, Outcome 1 Lymphoedema in studies with ≥6 month follow up < 24 months.
One study, (Sagen 2009), compared a supervised physiotherapy programme of moderate progressive resistance exercises (starting at 0.5 kilograms) two to three times a week, with a regimen of activity restriction (i.e. avoiding heavy or strenuous physical activities, including aerobic or other types of exercise classes involving heavy upper limb physical activity, and lifting and carrying objects over 3 kilograms) and physiotherapy (passive mobilisation and massage) once a week, for 6 months. In both groups, lymphoedema treatment was started if participants reported symptoms.
RRs calculated from reported point prevalences were 0.69 (95% CI 0.23 to 2.09, P = 0.56), 0.48 (95% CI 0.15 to 1.55, P = 0.24) and 1.04 (95% CI 0.51 to 2.09, P = 0.92) at three months, six months and 24 months, respectively. This study had high risk of bias.
The second study, Schmitz 2010, with moderate risk of bias, compared progressive resistance exercise (starting with the lowest weight and using the smallest possible increments) plus immediate treatment of lymphoedema at first symptoms versus no exercise, and accepted the equivalence hypothesis on lymphoedema risk (RR = 0.64, 95% CI 0.28 to 1.45, P for equivalence = 0.003; (contrary to superiority trials, in equivalence trials or non‐inferiority trials, the null hypothesis states that there is a difference between the groups. Thus a P‐value smaller than 0.05 is considered statistically‐significant evidence of non‐inferiority or equivalence).
Infection
Early versus delayed onset of mobilising shoulder exercises after breast cancer treatment
Infection rates were reported in one study (Cinar 2008). No statistically‐significant differences in wound infection rates were observed between early supervised start of mobilising shoulder exercises compared to a delayed start (RR 0.83, 95% CI 0.20 to 3.39, P = 0.80). Risk of bias for this outcome was unclear.
Active range of motion (AROM) of the upper limb
Early versus delayed onset of mobilising shoulder exercises after breast cancer treatment
Three studies reported on early versus delayed full range shoulder mobilisation after breast cancer surgery (Bendz 2002; Cinar 2008; Todd 2008). Two of these studies reported on short‐term results at one month follow‐up (Bendz 2002; Cinar 2008). Meta‐analysis could be performed for internal rotation only, due to statistical heterogeneity for forward flexion (I² = 97%, P < 0.001), abduction (I² = 97%, P < 0.001) and external rotation (I² = 89%, P = 0.003).
Forward flexion at short‐term follow‐up
In both Bendz 2002 and Cinar 2008, people with an early start of exercises had better forward flexion at one month; 7° (95% CI 3 to 11, P < 0.001) and 36° (95% CI 27 to 45, P < 0.001), respectively (Analysis 2.2).
2.2. Analysis.
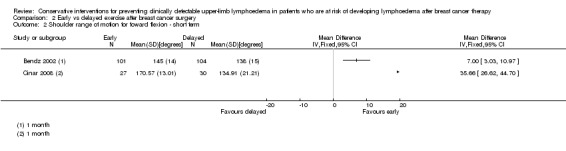
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 2 Shoulder range of motion for foward flexion ‐ short term.
Abduction at short‐term follow‐up
Abduction did not differ significantly at one month in Bendz 2002. In Cinar 2008, the early exercise group had better shoulder function at one month: mean difference for abduction 43°, 95% CI 32 to 55, P < 0.001) (Analysis 2.3).
2.3. Analysis.
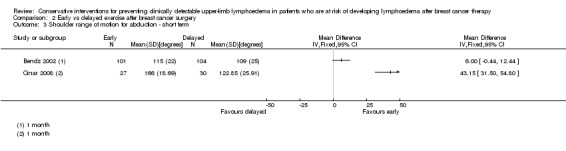
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 3 Shoulder range of motion for abduction ‐ short term.
External rotation at short‐term follow‐up
External rotation did not differ significantly at one month in Bendz 2002, but in Cinar 2008 the early exercise group had better function at one month (mean difference 15°, 95% CI 7 to 23, P < 0.001) (Analysis 2.4).
2.4. Analysis.
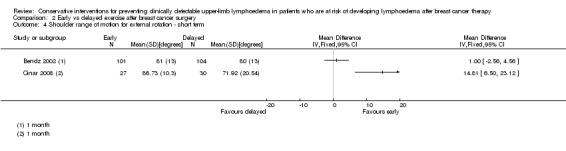
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 4 Shoulder range of motion for external rotation ‐ short term.
Internal rotation at short‐term follow‐up
All three studies included medium‐term follow‐up measurements at six months (Bendz 2002; Cinar 2008) or 12 months (Todd 2008). Data‐pooling was possible for shoulder internal rotations only, due to statistical heterogeneity for forward flexion (I² = 90%, P < 0.001), abduction (I² = 92%, P < 0.001) and external rotation (I² = 58%, P = 0.09). The pooled estimate for internal rotation showed no statistically‐significant or potentially clinically‐relevant difference as indicated by the 95% confidence interval, between early and delayed shoulder exercises (Analysis 2.5).
2.5. Analysis.
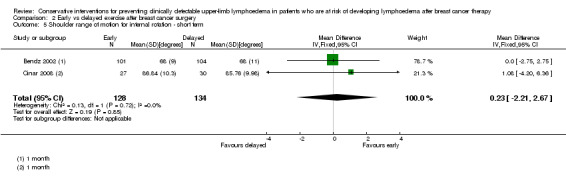
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 5 Shoulder range of motion for internal rotation ‐ short term.
Forward flexion at medium‐term follow‐up
In Bendz 2002, participants who started early with full range shoulder exercises had a small but statistically‐significant better range of motion for forward flexion up to two years post‐treatment (at 6 months: 5°, 95% CI 2 to 8, P < 0.01; at 2 years: 3°, 95% CI 0 to 6, P < 0.05). In Cinar 2008, people who started early with shoulder exercises had statistically significantly better function for forward flexion (mean difference 15°, 95% CI 11 to 20, P < 0.001). In Todd 2008, there was no statistically‐significant difference in forward flexion at 12 months follow‐up (Analysis 2.6).
2.6. Analysis.
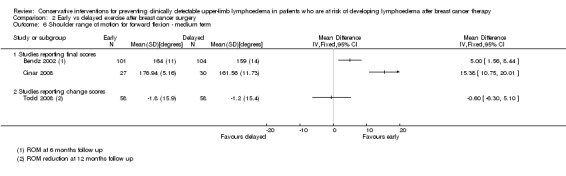
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 6 Shoulder range of motion for forward flexion ‐ medium term.
Abduction at medium‐term follow‐up
In Bendz 2002, people who started early with shoulder exercises had no statistically significant better abduction at six months (6°, 95% CI ‐1 to 13, P = 0.11), but did so at 2 years follow‐up (mean difference 9°, 95% CI 1 to 17, P = 0.03). In Cinar 2008, participants in the early mobilisation group also had statistically significantly better abduction at six months (mean difference 21°, 95% CI 13 to 30, P < 0.001). In Todd 2008, participants in both groups had poorer range of motion for abduction at 12 months compared to baseline. Although the difference in abduction between the groups was not statistically significant, it was observed that the early mobilisation group had worse shoulder function than the delayed mobilisation group, and the 95% CI included a clinically‐relevant difference (mean difference ‐8, 95% CI ‐17 to 0.4, P = 0.06). (Analysis 2.7)
2.7. Analysis.
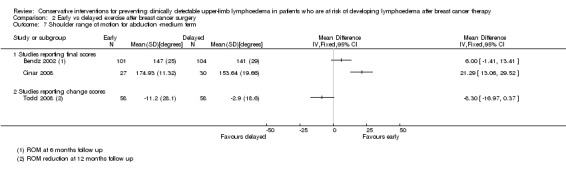
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 7 Shoulder range of motion for abduction ‐medium term.
External rotation at medium‐term follow up
In Bendz 2002, people who started early with shoulder exercises had no statistically‐significant different external rotation at 6 months or at 2 years follow‐up (at 6 months: mean difference ‐1°, 95% CI ‐4 to 2, P = 0.53; at 2 years: mean difference 0°, 95% CI ‐3 to 3, P = 1.00). In Cinar 2008, participants in the early mobilisation group also had statistically significantly better external rotation at six months (mean difference 8°, 95% CI 0.5 to 16, P = 0.04). In Todd 2008, external rotation in participants in both groups recovered almost completely at 12 months compared to baseline, with no statistically‐significant differences between the groups (mean difference ‐1°, 95% CI ‐6 to 4, P = 0.82; Analysis 2.8).
2.8. Analysis.
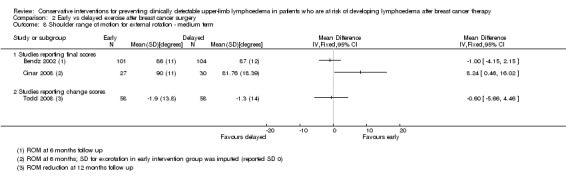
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 8 Shoulder range of motion for external rotation ‐ medium term.
Internal rotation at medium‐term follow‐up
The summary estimate for internal rotation at six‐month follow‐up showed no statistically‐significant or potentially clinically‐relevant difference for internal rotation (Analysis 2.9).
2.9. Analysis.
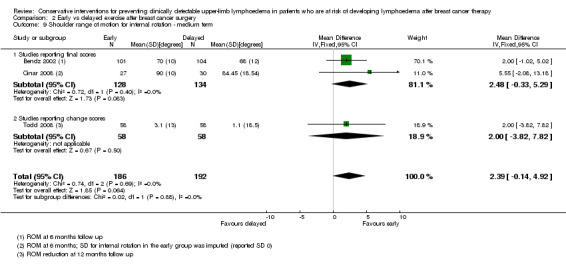
Comparison 2 Early vs delayed exercise after breast cancer surgery, Outcome 9 Shoulder range of motion for internal rotation ‐ medium term.
ADL Function
Early versus delayed onset of mobilising shoulder exercises after breast cancer treatment
Only one of the studies reported on ADL function (Todd 2008). In this study, there were no significant differences between early mobilisation and delayed mobilisation in Shoulder Disability Questionnaire score at one‐year follow‐up (a mean of 1.7 positively scored items versus 1.9 for early and delayed start respectively, P = 0.64). Risk of bias for this outcome was classified as high due to attrition, but it seems unlikely that such bias would have changed the conclusion with regard to differences in ADL functioning as measured with the SDQ.
Pain
Early versus delayed onset of mobilising shoulder exercises after breast cancer treatment
Only one study, with high risk of bias, examined the effects of early versus delayed exercise on pain. No statistically‐significant differences were found for pain scores at any follow‐up point up to two years in people who started early with mobilisation exercises and people who had a delayed start of exercises (Bendz 2002).
Progressive resistance exercise after breast cancer treatment
One study, with moderate risk of bias for this outcome, examined the effect of progressive resistance exercise on pain (Sagen 2009). People who were engaged in moderate progressive resistance exercise reported pain significantly more often at 3 months and 6 months (P < 0.001), but not at 24 months, compared to a control group with 6 months of activity restrictions, massage and passive mobilisation: 78% versus 45% at 3 months; 60% versus 36% at 6 months; and 39% versus 34% at 24 months.
Health‐related Quality of Life (HRQoL)
Due to clinical heterogeneity, meta‐analysis was deemed inappropriate and a narrative synthesis has been provided.
Early versus delayed onset of mobilising shoulder exercises after breast cancer treatment
One study that compared early versus delayed start of exercises reported on HRQoL (Todd 2008). There were no statistically‐significant differences in HRQoL as measured by the Functional Assessment of Cancer Therapy‐Breast Trial Outcome Index (TOI) at 12 months follow‐up, between people who started early with mobilisation exercises and people who had a delayed start (32.5 versus 30.9, P = 0.10). This difference was smaller than the 5 points difference that was considered to be clinically important by the authors. Risk of bias for this outcome was classified as high due to attrition, but it seems unlikely that such bias would have changed the conclusion with regard to differences in Quality of Life as measured with the TOI.
Progressive resistance exercise after breast cancer treatment
In Schmitz 2010, no statistically‐significant differences were found in the mental and physical summary component scores of the SF‐36 between participants who engaged in progressive resistance exercise and those who did not increase their activity level, at 12 months follow‐up (mean percent change mental component score: 3.3 versus 3.1, P = 0.92; mean percent change physical component score: 6.6 versus 4.1, P = 0.10, for exercise and control group, respectively). Risk of bias for this outcome in this study was unclear.
Psychosocial morbidity
No data on this outcome.
Adverse Events
Adverse events were reported for four of the included studies (Cinar 2008; Sagen 2009; Schmitz 2010; Todd 2008), all of which investigated exercise interventions.
Early versus delayed onset of mobilising shoulder exercises after breast cancer treatment
Although this was not specifically described as an adverse event, statistically higher wound drainage volume (P = 0.004) was reported in the early mobilisation group compared to the delayed mobilisation group in one study (Todd 2008), with low risk of bias for this outcome, but not in another study with unclear risk of bias (Cinar 2008). Absolute values of drainage volume were not reported.
Progressive resistance exercise after breast cancer treatment
Self‐reported (musculoskeletal) injury was assessed with a 1‐year recall, using a survey. The OR for musculoskeletal injury in the weightlifting group compared to the control group was 5.6 (95% CI 0.31 to 26.1, P = 0.44 (Schmitz 2010)). Another study noted a 1.5% incidence of musculoskeletal adverse events (two people with frozen shoulder, one with a supraspinatus tendinopathy), but did not specify in which of the groups these occurred (Sagen 2009).
Patient education, monitoring and early intervention
Incidence of treatment failure (occurrence of lymphoedema)
There were no studies that evaluated either patient education, or monitoring and early intervention alone.
There was one study with high risk of bias (Box 2002), involving 65 participants, that employed an extensive program ('PMCP') of patient education, supervision of exercises and adjustment of self‐directed shoulder exercises, and monitoring of lymphoedema symptoms and early intervention for lymphoedema or shoulder problems if deemed necessary. The control group received an instruction booklet only.
Absolute numbers of people at risk in each group at each time point were not available from the published reports. From a survival curve obtained from the authors of the study, the number of participants in each group was obtained by subtracting the number of censored people up to that time point. 2x2 tables were then constructed for each follow‐up point and risk estimates were calculated. No statistically‐significant difference in prevalence of lymphoedema, as defined by a greater than 200 mL or 10% change from preoperative volume, was found between the control group and the intervention group at 1 month (RR 1.03, 95% CI 0.07 to 15.8, P = 1.00), 3 months (RR 0.40, 95% CI 0.12 to 1.37, P = 0.18), 6 months (RR 0.22, 95% CI 0.03 to 1.78, P = 0.20), 12 months (RR 0.52, 95% CI 0.10 to 2.60, P = 0.67) and 24 months (RR 0.34, 95% CI 0.10 to 1.15, P = 0.10). There also were no statistically‐significant differences at each follow‐up point for any of the other criteria for lymphoedema.
Infection
No data on this outcome
Active range of motion (AROM) of the upper limb
The recovery pattern for range of motion of shoulder abduction was more favourable for the participants who received the PMCP compared to the control group (P = 0.001), with the intervention group returning to preoperative levels at 3 months, compared to 6 months in the control group. No statistically‐significant differences between groups were observed for recovery pattern of the other shoulder movements (forward flexion, extension, and rotations).
ADL Function
No data on this outcome.
Pain
No data on this outcome.
Health‐related Quality of Life (HRQoL)
No data on this outcome.
Psychosocial morbidity
No data on this outcome.
Adverse Events
No data on this outcome.
Compression therapy
Incidence of treatment failure (occurrence of lymphoedema)
Compression therapy was studied in a single study (Castro‐Sanchez 2011), involving 48 participants, in which it was combined with MLD and education (see 'Incidence of treatment failure ‐ MLD in combination with other interventions versus education only'). A separate evaluation of compression therapy is therefore not possible, but the combined intervention was not statistically significantly more effective than education only in preventing lymphoedema (RR 0.17, 95% CI 0.02 to 1.28, reported P = 0.042, calculated P = 0.097 at 8 month follow‐up).
Infection
No data on this outcome.
Active range of motion (AROM) of the upper limb
No data on this outcome.
ADL Function
No data on this outcome.
Pain
See Castro‐Sanchez 2011 in the MLD section.
Health‐related Quality of Life (HRQoL)
See Castro‐Sanchez 2011 in the MLD section.
Psychosocial morbidity
No data on this outcome.
Adverse Events
No data on this outcome.
Discussion
Summary of main results
In this systematic review we included ten randomised controlled trials investigating different types of interventions to reduce the risk of secondary lymphoedema after breast cancer treatment.
MLD
Four studies with a total of 385 participants studied the effectiveness of MLD. The evidence of the effectiveness of MLD on lymphoedema risk is conflicting. Differences in dosage and administration of the MLD intervention in the two studies that allowed for evaluation of the effectiveness of MLD (Devoogdt 2011; Zimmermann 2012) may in part account for the observed differences in effect. It should also be noted, however, that overall risk of bias in Zimmermann 2012 was higher than in Devoogdt 2011. In particular, allocation concealment and blinding of outcome assessment were lacking in Zimmermann 2012, both of which are typically associated with larger effect estimates (Wood 2008).
The results of two other studies on MLD suggest that a combined physiotherapy intervention containing MLD may reduce the risk of developing lymphoedema compared to education only. The extent to which MLD accounts for the observed effect cannot be estimated from these studies. It is unclear whether the observed positive effects resulted from the concurrent compression therapy (Castro‐Sanchez 2011) or exercise therapy (Torres 2010) rather than MLD or vice versa. The results should also be interpreted with caution, since both trials suffered from risk of bias at several points.
No conclusions can be drawn from the available studies with regard to effects of MLD, with or without additional intervention, on pain.
The observed effects on shoulder function suggest that combined MLD and exercise may lead to better shoulder intervention in the first few weeks after surgery compared to education only. Results on long‐term effects were inconsistent. These findings, too, should be interpreted with caution owing to the overall low quality of the evidence.
Early versus delayed shoulder mobilisation
Three of the included trials compared early versus delayed full‐range shoulder exercises after axillary dissection in a total of 378 breast cancer patients at risk for lymphoedema (Bendz 2002; Cinar 2008; Todd 2008). The meta‐analysis did not yield a statistically‐significant elevated risk of lymphoedema after early start of exercises. However, the point estimate favoured a delayed start. A delayed start of exercises does not seem to have a negative influence on recovery of shoulder range of motion in the medium term, but immediate postoperative start of exercise leads to better shoulder function in the short term (up to 6 months).
Progressive resistance exercise
Two studies evaluated the safety of progressive resistance exercises after breast cancer surgery including axillary lymph node dissection, in a total of 351 participants. The results of these studies support the hypothesis that resistance training does not increase lymphoedema risk, and may even reduce the risk, provided that lymphoedema symptoms are closely monitored and adequate treatment is initiated as soon as symptoms become apparent (Sagen 2009; Schmitz 2010).
Overall completeness and applicability of evidence
The number of studies that investigated the effectiveness of a conservative intervention for prevention of lymphoedema after breast cancer surgery was small, and the types of intervention studied were limited. None of the included studies investigated the effect of compression therapy only (either by bandaging, compression sleeves or pneumatic compression), or of lymph taping. There were no studies evaluating the effect of education or risk‐reduction advice compared to no education, or surveillance and early intervention.
Not all relevant outcome measures were used in the identified studies. ADL functioning in relation to the affected arm was measured with a validated self‐report measure in only one study (Todd 2008). Infection was reported in Cinar 2008, but none of the other studies included it as an outcome measure or adverse effect. None of the included studies addressed psychosocial morbidity (depression or anxiety).
Quality of the evidence
The overall quality of the evidence ranged from very low to low, with the exception of the comparison of progressive resistance training with no exercise, which was graded as moderate (Table 1; Table 2; Table 3)
Lack of blinding accounted for an important part of the reasons for downgrading the quality of the evidence, as it was judged to be unclear or insufficient in the majority of studies. The type of interventions under investigation made it very difficult, if not impossible, to adequately blind participants. The impact of this on the observed outcomes is difficult to estimate and may differ between types of interventions. Since adherence to the assigned intervention was not explicitly addressed in eight of the studies, this may have introduced bias towards the null hypothesis in superiority trials, and towards the alternative hypothesis in non‐inferiority trials.
The definitions used for lymphoedema among the included studies differed, with some studies reporting on lymphoedema based on several different criteria. Since all of the studies included a volume criterion to define incident cases, we extracted the results based on this criterion for studies reporting on several definitions but failing to specify the definition used as the primary outcome. In Castro‐Sanchez 2011 the primary outcome measure for lymphoedema as defined in the methods section was not reported. Since incident cases of lymphoedema were reported and the authors provided a sufficiently objective criterion, we used this outcome for our analyses. Even though all studies included a volume criterion, these too differed between studies. Also, different ways of measuring limb volume were used. These variations added to the observed heterogeneity.
Most studies reported cumulative incidence of lymphoedema, but a number of the studies did this by reporting the prevalent cases at a certain follow‐up point. Since limb volume is variable over time, and transient episodes of lymphoedema may occur, the reported number of cases observed at a particular follow‐up measurement could be considered point prevalence rather than cumulative incidence.
The use of a priori power calculation was not included in the risk of bias assessment. It should be noted, however, that sample size calculations were not performed in five studies (Bendz 2002; Castro‐Sanchez 2011; Cinar 2008; Torres 2010; Zimmermann 2012). Power calculations were performed based on volume differences rather than incidence of lymphoedema in two studies (Sagen 2009; Todd 2008). All studies reporting a priori power calculations recruited the targeted number of participants.
Potential biases in the review process
We performed a comprehensive search in the most relevant databases. We refrained from using a methodological filter, to make sure that no relevant studies would be missed due to misclassification in the databases. Neither did we impose a language restriction.
The studies identified included both studies with positive findings and studies with negative findings. Although the number of studies per outcome and intervention was too small to make a formal analysis, we have found no clues that indicate possible publication bias.
We corresponded with the authors of six studies to obtain additional information on risk of bias related to study characteristics, and additional outcome data. These data were obtained in most cases, which makes our review more complete. On the other hand, it also means that some of the details on study methodology and study results have not yet gone through a peer‐review process.
An important limitation of this review was that we included only studies that used lymphoedema as the primary outcome. As a result of this restriction, studies may have been missed that reported on lymphoedema as a secondary outcome in trials on exercise, postoperative rehabilitation protocols or other interventions.
Agreements and disagreements with other studies or reviews
A Cochrane systematic review (McNeely 2010) studied the effect of exercise interventions on upper limb dysfunction due to breast cancer treatment. This review included a number of studies that reported lymphoedema as a secondary outcome. The reported results with regard to the effects of early versus late start of exercise on lymphoedema incidence are congruent with our results.
Chan 2010 also performed a systematic review on the effectiveness of exercise programmes on shoulder mobility and lymphoedema. While that review included some studies that did not meet the inclusion criteria of the current review, the authors also conclude that exercise is safe with regard to lymphoedema risk.
Some of the results that we calculated, based on the available data and using Review Manager software (RevMan 5), were inconsistent with the results as reported in the source publications.
Castro‐Sanchez 2011 reported a statistically‐significant difference in lymphoedema incidence at eight‐month follow‐up. Using the data as reported, our analysis did not show a statistically‐significant reduction in lymphoedema risk for people receiving a combined intervention of MLD, exercise, scar massage and education, compared to those receiving education alone (RR = 0.13, 95% CI 0.014 to 1.18, P = 0.097), which is probably due to the use of a Chi² test without Yates' correction, instead of a (more appropriate) Fisher's exact test.
Conversely, Castro‐Sanchez 2011 reported a clinically‐relevant, but statistically non‐significant, difference in pain scores (reported P = 0.056) whereas in our analysis based on the reported mean scores and 95% CIs, this difference was statistically significant (calculated P = 0.03). We do not have an explanation for this difference; it seems unlikely that differences occurred due to rounding, since confidence intervals were reported precisely (up to 2 decimals).
The results as reported by Box 2002 were also not entirely consistent with our calculations based on the available data, but this did not result in a qualitatively different conclusion.
Authors' conclusions
Implications for practice.
The aim of this review was to summarise current evidence and thus provide information that can be used to guide clinical decisions and guideline development. Unfortunately, the overall low quality of the evidence does not allow for firm conclusions on the effect of MLD, compression, exercise or a combination of these interventions for prevention of upper limb lymphoedema in people at risk after breast cancer treatment.
Although the comparison of early versus delayed start of shoulder exercises showed no significant influence on lymphoedema incidence, the point estimate suggested a lower risk of lymphoedema after delayed start. An early start may result in better range of motion in the short term compared to a late start, but this difference disappears from six months onward. Other studies have shown that delaying postoperative shoulder rehabilitation reduces postoperative wound drainage volumes and wound drainage time, although it does not reduce incidence of seroma formation (McNeely 2010). Clinicians who consider early recovery of shoulder function as very important may want to consider early onset of exercise. Otherwise, delaying exercise for a week after the operation could be considered.
Current evidence supports that progressive resistance exercise is safe, and potentially beneficial for reducing lymphoedema risk in people treated for breast cancer. The beneficial effects of resistance training on physical functioning, fatigue and quality of life are well established. Breast cancer survivors can therefore be actively encouraged to engage in such exercise and can be informed that this will not increase their risk of developing chronic upper limb lymphoedema, provided that they monitor their symptoms and see to it that lymphoedema is treated in a timely manner should it occur.
Implications for research.
Considering the low number of studies identified, the heterogeneity of interventions applied in these studies, and the overall low quality of the evidence available to date, future studies are needed. Many of the included studies in this review did not report on important methodological characteristics related to risk of bias. Therefore, we would stress the importance of adhering to the CONSORT guidelines for reporting future clinical trials (Altman 1996; Schulz 2010).
Using a commonly agreed upon criterion for clinically detectable lymphoedema would greatly facilitate the interpretation of future studies, but unfortunately no such single criterion currently exists. Alternatively, future studies could choose to incorporate a number of methods to assess lymphoedema and report results based on each of those, while clearly specifying the criterion used as the primary outcome variable.
Future studies should preferably use survival analysis to assess the effectiveness of interventions, as this takes into consideration that even if lymphoedema is not prevented, its onset may be postponed by the intervention.
Including infection, pain, limitations in ADL functioning, quality of life and mood, and adverse events as secondary outcomes is recommended.
Further research is needed to provide more robust evidence on the (combined) interventions as described in this review, as well as to examine the effectiveness of preventive compression and MLD as a single intervention, kinesio taping, and early intervention for subclinical lymphoedema. Although results from an observational study suggest that early detection by self‐examination and subsequent treatment with conservative interventions may reduce the severity of lymphoedema (Stout 2008), randomised controlled trials are needed to confirm these findings.
The effect of patient education also needs further study in randomised controlled trials. While it is generally agreed upon that providing risk‐reduction advice should be part of routine care after breast cancer treatment, it is currently unclear whether the benefits outweigh potential harms (Fu 2010; Lee 2009; Round 2006).
In addition, the cost‐effectiveness from a societal perspective should be evaluated for all interventions.
Acknowledgements
The authors would like to thank Faridi van Jetten, Academic Medical Center, University of Amsterdam for her help with developing the search strategy; Chen Xiao Chen, BM, School of Nursing, University of Wisconsin‐Madison; and Anna Miquel Cases, The Netherlands Cancer Institute for their help with translation of the non‐English language studies; all authors of included studies who were very helpful by clarifying details about the study and providing additional data; the Cochrane Breast Cancer Group and especially Dr. Melina Willson, editor, for her support with developing this review; and Fergus Tai for his help with the searches.
Appendices
Appendix 1. MEDLINE via Pubmed (1980 to present)
("breast neoplasms"[MeSH] OR breast cancer*[tiab] OR breastcancer*[tiab] OR breast tumor*[tiab] OR breast tumour*[tiab] OR mammary neoplasm*[tiab] OR mammary carcinoma*[tiab] OR breast neoplasm*[tiab] OR breast carcinoma*[tiab] OR breast malignan*[tiab] OR breast metastas*[tiab] OR mammary malignan*[tiab] OR mammary metastas*[tiab]) AND (lymphoedema[tiab] OR "lymphedema"[MeSH Terms] OR lymphedema[tiab] OR lymphatic edema[tiab] OR oedema[tiab] OR "edema"[MeSH Terms] OR edema[tiab] OR swelling[tiab] OR elephantias*[tiab]) AND (prevent*[tiab] OR "prevention and control"[Subheading] OR "Preventive Health Services"[Mesh] OR "Early Diagnosis"[Mesh] OR "Risk"[Mesh] OR risk*[tiab] OR "Risk Reduction Behavior"[MAJR] OR reducing[tiab] OR "Probability"[Mesh] OR restrict*[tiab] OR prevalence*[tiab] OR "Prevalence"[Mesh])
Appendix 2. EMBASE via Ovid (1980 to present)
| 1 | exp breast cancer/ or (breast cancer* or breastcancer* or breast tumor* or breast tumour* or mammary neoplasm* or mammary carcinoma* or breast neoplasm* or breast carcinoma*).ti,ab. |
| 2 | lymphedema/ or elephantiasis/ or (lymphoedema or lymphedema or lymph edema or lymphatic edema or oedema or edema).ti,ab. |
| 3 | prevention/ or early diagnosis/ or risk/ or *risk reduction/ or probability/ or prevalence/ or prediction/ or (prevent* or risk* or reducing or restrict* or prevalence*).ti,ab. |
| 4 | 1 and 2 and 3 |
| 5 | limit 4 to embase |
Appendix 3. WHO ICTRP Search Portal
Basic Searches:
1. Conventional interventions for preventing clinically detectable upper‐limb lymphoedema in patients who are at risk of developing lymphoedema after breast cancer therapy
2. Lymphoedema AND prevent*
3. Lymphedema AND prevent*
Advanced Searches:
1. Title: Conventional interventions for precenting clinically detectable upper‐limb lymphoedema in patients who are at risk of developing lymphoedema after breast cancer therapy
Recruitment Status: ALL
2. Condition: breast cancer AND (lymphoedema OR lymphedema OR lymphatic oedema OR lymphatic edema OR oedema OR edema)
Intervention: prevention OR control OR early diagnosis OR risk reduction behavior OR exercise OR patient education OR early intervention OR monitoring OR compression therapy OR manual lymph drainage OR lymph taping OR kinesiotape
Recruitment Status: ALL
Appendix 4. The Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO (1980 to present)
| S4 | S1 and S2 and S3 |
| S3 | ( ( (MH "Early Diagnosis+") or (MH "Relative Risk") or (MH "Probability") or (MH "Prevalence") ) or ( ( ( TI restrict* OR AB restrict* ) or ( TI prevalence* OR AB prevalence* ) ) or ( ( TI prevent* OR AB prevent* ) or ( TI risk* OR AB risk* ) or ( TI reducing OR AB reducing ) ) ) ) or ( TI predict* or AB predict* ) or (MH "Risk Factors+") |
| S2 | ( (MH "Lymphedema+") or ( ( TI lymphoedema or AB lymphoedema ) or ( TI lymphedema or AB lymphedema ) or ( TI lymph edema or AB lymph edema ) ) or ( ( TI lymphatic edema or AB lymphatic edema ) or ( TI oedema or AB oedema ) or ( TI edema or AB edema ) ) ) or ( ( TI swelling or AB swelling ) or ( TI elephantias* or AB elephantias* ) ) |
| S1 | (MH "Breast Neoplasms+") or ( ( TI breast cancer* or AB breast cancer* ) or ( TI breastcancer* or AB breastcancer* ) or ( TI breast tumor* or AB breast tumor* ) ) or ( ( TI breast tumour* or AB breast tumour* ) or ( TI mammary neoplasm* or AB mammary neoplasm* ) or ( TI mammary carcinoma* or AB mammary carcinoma* ) or ( TI breast neoplasm* or AB breast neoplasm* ) or ( TI breast carcinoma* or AB breast carcinoma* ) ) |
Appendix 5. Physiotherapy Evidence Database (PEDro) via http://pedro.org.au (1980 to present)
1. Abstract and title: cancer
2. Problem: oedema
Appendix 6. PsycINFO via Ovid (1980 to present)
| 1 | lymphoedema.id. or (lymphoedema or lymphedema or lymphatic edema or oedema or edema or swelling or elephantias*).ti,ab. |
| 2 | risk factors/ or risk factors.id. or (prevent* or risk* or reducing or restrict* or prevalence*).ti,ab. |
| 3 | breast neoplasms/ or breast cancer.id. or (breast cancer* or breastcancer* or breast tumor* or breast tumour* or mammary neoplasm* or mammary carcinoma* or breast neoplasm* or breast carcinoma* or breast malignan* or breast metastas* or mammary malignan* or mammary metastas*).ti,ab. |
| 4 | 1 and 2 and 3 |
Appendix 7. CENTRAL
#1 MeSH descriptor: [Lymphedema] explode all trees
#2 lymphoedema* or lymphedema* or lymphatic oedema* or lymphatic edema* or oedema* or edema* or swelling
#3 MeSH descriptor: [Elephantiasis] explode all trees
#4 #1 or #2 or #3
#5 MeSH descriptor: [Breast Neoplasms] explode all trees
#6 breast neoplasm or breast cancer or breast tumour or breast tumor or breast carcinoma
#7 #5 or #6
#8 #4 and #7
#9 'prevention and control' or prevent* or control or early diagnosis or risk reduction behavior or exercise or patient education or early intervention or monitoring or compression therapy or manual lymph drainage or lymph taping or kinesiotape
#10 #8 and #9
Data and analyses
Comparison 1. Early physiotherapy including MLD vs no early physiotherapy or physiotherapy without MLD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to event for lymphoedema | 2 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2 Lymphoedema ‐ short term follow up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Lymphoedema ‐ medium term follow up | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Shoulder range of motion for abduction ‐ short term follow up | 2 | 183 | Mean Difference (IV, Fixed, 95% CI) | 21.84 [13.58, 30.10] |
| 4.1 Studies reporting final scores | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [‐1.96, 26.76] |
| 4.2 Studies reporting change scores | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 26.50 [16.41, 36.59] |
| 5 Shoulder range of motion for forward flexion ‐ short term follow up | 2 | 183 | Mean Difference (IV, Fixed, 95% CI) | 14.44 [7.08, 21.81] |
| 5.1 Studies reporting final scores | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 8.20 [‐3.21, 19.61] |
| 5.2 Studies reporting change scores | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 18.9 [9.26, 28.54] |
| 6 Shoulder range of motion for abduction ‐ medium term follow up | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Studies reporting final scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Studies reporting change scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Shoulder range of motion for forward flexion ‐ medium term follow up | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Studies reporting final scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Studies reporting change scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pain | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Studies reporting final scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Studies reporting change scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Early vs delayed exercise after breast cancer surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lymphoedema ‐ medium term follow up | 3 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.94, 3.01] |
| 2 Shoulder range of motion for foward flexion ‐ short term | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Shoulder range of motion for abduction ‐ short term | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Shoulder range of motion for external rotation ‐ short term | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Shoulder range of motion for internal rotation ‐ short term | 2 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐2.21, 2.67] |
| 6 Shoulder range of motion for forward flexion ‐ medium term | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Studies reporting final scores | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Studies reporting change scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Shoulder range of motion for abduction ‐medium term | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Studies reporting final scores | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Studies reporting change scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Shoulder range of motion for external rotation ‐ medium term | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Studies reporting final scores | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Studies reporting change scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Shoulder range of motion for internal rotation ‐ medium term | 3 | 378 | Mean Difference (IV, Fixed, 95% CI) | 2.39 [‐0.14, 4.92] |
| 9.1 Studies reporting final scores | 2 | 262 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [‐0.33, 5.29] |
| 9.2 Studies reporting change scores | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐3.82, 7.82] |
Comparison 3. Progressive resistance exercise vs no exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lymphoedema in studies with ≥6 month follow up < 24 months | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bendz 2002.
| Methods | Cluster randomised controlled trial (clusters formed by 4 week time periods of treatment). | |
| Participants | Women treated for breast cancer with radical mastectomy or quadrantectomy, including ALND, with or without radiotherapy to the chest wall. Mean age 58 (SD 11). | |
| Interventions |
Intervention group (available n = 101) Immediate full‐range exercise supervised by a physical therapist: Preoperative instructions to use the arm as much as comfortable, avoiding lifting and carrying heavier items and avoid forced movements for 14 days. From day 14 forward, full‐range mobilising exercises were given to both groups, to be performed 3 times a day. Control group (available n = 104) Delayed full‐range exercise: preoperative instruction on shoulder/arm exercise programme, to be started on the first postoperative day. No abduction or elevation for 3 days, slowly increasing to elevation and abduction to 90º during 14 days. |
|
| Outcomes |
Primary outcome:
Lymphoedema, defined as 10% or greater change in volume of the operated arm, corrected for preoperative differences, using the formula: (volume difference between operated and non‐operated arm at baseline ‐ volume difference at follow‐up)/ postoperative volume of the operated arm * 100. Secondary outcomes: Range of motion (goniometer) for shoulder flexion, abduction and rotation; Pain (4 point ordinal scale based on visual analogue scale); Hand grip strength (vigorimeter); Subjective estimation of heaviness and tension (VAS). |
|
| Follow up | 1 month, 6 months, 24 months follow‐up | |
| Country, setting | Sweden, University Hospital | |
| Year of conduct | 1994 to 1996 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Cluster randomisation was used to alternate periods of 4 weeks", which is not a truly random sequence. |
| Allocation concealment (selection bias) | High risk | There is no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Neither participants nor personnel were blinded for the intervention, but due to the nature and duration of the intervention and the use of cluster randomisation, performance bias seems unlikely. |
| Blinding of participants and personnel (performance bias) Patient reported outcomes | Unclear risk | Neither participants nor personnel were blinded for the intervention, but due to the nature and duration of the intervention and the use of cluster randomisation performance bias seems unlikely. |
| Blinding of outcome assessment (detection bias) Measured outcomes | High risk | Outcome assessors were not blinded. |
| Blinding of outcome assessment (detection bias) Patient reported outcomes | Unclear risk | Participants were not blinded. Due to cluster randomisation, and due to the fact that the duration of the intervention was 2 weeks, with self‐reported pain assessed at 1 month, 6 months, and 2 years postoperatively, it seems unlikely that self‐reported pain was strongly affected by participants' knowledge of group allocation. |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Drop‐out rates at 2 years follow‐up were twice as high in the early exercise group compared to the delayed exercise group (16 vs 8) and reasons for drop out differed between groups. Also 25 participants dropped out before the first assessment and were not included in any of the subsequent analyses. |
| Incomplete outcome data (attrition bias) Patient reported outcome | High risk | Drop‐out rates at 2 years follow‐up were twice as high in the early exercise group compared to the delayed exercise group (16 vs 8) and reasons for drop out differed between groups. Also 25 participants dropped out before the first assessment and were not included in any of the subsequent analyses or reported upon in the tables. |
| Selective reporting (reporting bias) | Low risk | Outcomes for all variables are reported. |
| Other bias | High risk | It is unclear whether the groups were comparable on all relevant risk factors such as number of removed nodes and BMI. There is no explicit statistical consideration for the cluster randomisation. |
Box 2002.
| Methods | Parallel group randomised controlled trial, stratification by surgical procedure (complete local excision or modified radical mastectomy). | |
| Participants | Patients treated surgically for breast cancer (all stages except advanced disease), complete local excision or modified radical mastectomy, including ALND. Mean age (SD) 56 (10.6) |
|
| Interventions |
Intervention Group (n = 32) Physiotherapy Management Care Plan (PMCP). PMCP includes preoperative individual risk assessment, identification of possible risk factors, education on the lymphatic system, education about early signs of lymphoedema and introduction of risk‐minimisation strategies for identified precipitating factors in the preoperative phase. Postoperatively, outpatient reviews are scheduled (monitoring of shoulder ROM, progression of exercise, provision of LO awareness, individualised intervention if required). Control Group (n = 33) No physiotherapy. |
|
| Outcomes |
Primary outcomes: Lymphoedema defined by three criteria (each was evaluated separately): 1. Increase of 5 cm or more from preoperative sum of circumferences of the arm, operated arm vs non‐operated side; 2. Increase of 200 mL or more from preoperative total arm volume difference between the operated and non‐operated side. 3. Multifrequency Bioelectrical Impedance Measurement: A MFBIA ratio of the arm operated side and non‐operated side lower than 95% confidence interval from preoperative data; or a 10% change from baseline in the ratio of operated arm to unoperated arm. Secondary outcomes: Range of motion (goniometer) for shoulder flexion, abduction, extension and rotations; Non‐validated functional tasks questionnaire. |
|
| Follow up | 1, 3, 6, 12 months | |
| Country, setting | Australia, University Hospital | |
| Year of conduct | 1996 to 1999 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | High risk | Chronological recruitment with allocation from random number table, without attempts at blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Medical and nursing staff were blinded for group allocation, participants were not. Contamination seems unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Measured outcomes | High risk | Outcome measurements were taken by a blinded PT for “as many women as possible”; it is unclear in how many cases this was actually the case. |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | There is a 9% loss to follow‐up, for reasons unrelated to the outcome. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods sections are reported. |
| Other bias | Unclear risk | Women in the treatment group on average had more lymph nodes removed (16 vs 13 nodes), more often had level 2 (81% vs 64%) or 3 (16% vs 9%) axillary dissection, and more often had radiotherapy (66% vs 49%). No sensitivity analysis or adjusted analysis were performed due to the low number of events. Analyses on shoulder function measurements were adjusted for age, number of removed lymph nodes, level of ALND, history of shoulder problems, radiotherapy, chemotherapy and wound infection. |
Castro‐Sanchez 2011.
| Methods | Parallel group randomised controlled trial. | |
| Participants | Women aged 30 to 60, treated for breast cancer (stages not specified) including partial axillary dissection and adjuvant radiotherapy. | |
| Interventions |
Intervention group (n = 24) Elastic compression sleeve + manual lymph drainage 5 times a week for 6 months; Leduc method transthoracical and thoraco‐abdominal and manual lymph drainage of the arm. Control group (n = 24) Patient education |
|
| Outcomes |
Primary outcome:
Lymphoedema, defined as: between group mean difference in percentage upper arm volume difference (from circumference measurements) between affected vs non‐affected side (not reported). Secondary outcomes: Incident cases of lymphoedema, defined as > 2 cm increase in the circumferential measurements at 2 adjacent marked points in comparison with the corresponding contralateral arm. Volume of the arm. Body composition: fat‐free mass (g/kg/d), fat mass (kg), amount of extracellular water (l) as measured with bioimpedance measurements. Temperature of the back of the hand, anterior forearm and elbow. Health‐related quality of life (EORTC QLQ‐C30). Pain (10 point visual analogue scale). Functional Shoulder rating scale UCLA (composite score of self‐reported complaints and limitations, ROM measurements and strength measurements). |
|
| Follow up | 8 months | |
| Country, setting | Spain, 2 university hospitals | |
| Year of conduct | 2008 to 2009 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number table was used. |
| Allocation concealment (selection bias) | Low risk | Randomisation cards were placed in opaque envelopes that were opened by a therapist who was not involved in baseline assessments. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants and personnel were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of participants and personnel (performance bias) Patient reported outcomes | Unclear risk | Participants and therapist were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of outcome assessment (detection bias) Measured outcomes | Low risk | Outcome assessor was blinded for group allocation. |
| Blinding of outcome assessment (detection bias) Patient reported outcomes | High risk | Self report for pain and HRQoL may be affected by participants' knowledge of group allocation. |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | There is no loss to follow‐up. |
| Incomplete outcome data (attrition bias) Patient reported outcome | Low risk | There is no loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Reported incident cases with lymphoedema are based on a different criterion than defined in the methods section. |
| Other bias | High risk | At baseline, limb volume on the operated side was lower in the intervention group compared to the control group (307 mL vs 378 mL), no corrections were made to take this difference into account in the between‐group comparison of volume at follow‐up. At baseline, the intervention group had lower scores than the control group for the EORTC QLQ‐C30 domains of: Physical functioning (114 vs 123) Role functioning (88 vs 96) Social functioning (120 vs 126) Global health (73 vs 87) Constipation (4 vs 11) Diarrhoea (44 vs 53) Financial difficulties (5 vs 14) No corrections were made to take these differences into account. The intervention group had more contacts with a therapist, which may reinforce other behaviour such as compliance to exercises and self‐care measures. |
Cinar 2008.
| Methods | Parallel groups randomised controlled trial. | |
| Participants | Women (mean age 53, range 29 to 72), surgically treated for breast cancer with radical modified mastectomy. | |
| Interventions |
Intervention group (n = 27) Early postoperative shoulder mobilisation: Shoulder immobilisation on first day, PT‐supervised active exercises hand and elbow. Gradually increasing shoulder‐mobilising exercises from day 2 onwards, with passive stretching from day 5 forward. After removal of wound drain 15 sessions of individual PT outpatients setting, mobilising and strengthening exercises for the shoulder upper limb. Home‐based exercise in following 8 weeks, and education on risk reducing behavior. Control group (n = 30) Delayed approach to shoulder exercises, starting after removal of the wound drain. Home‐based after initial physiotherapist‐delivered exercise instruction, and education on risk reducing behavior. |
|
| Outcomes |
Primary outcome: Lymphoedema defined as 1.5 cm to 3cm difference in circumference of the treated vs the non‐treated upper limb (mild oedema); 3 cm to 5 cm difference (moderate); > 5 cm difference (severe). Secondary outcome: Non‐validated questionnaire on functional activities involving the shoulder. |
|
| Follow up | 5 days, 1, 3, 6 months | |
| Country, setting | Turkey, hospital | |
| Year of conduct | < 2007 (no exact time provided) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is mentioned that treatment allocation was randomised, the method is not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants and personnel were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of outcome assessment (detection bias) Measured outcomes | Low risk | Outcome assessor was blinded to group allocation |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | No attrition was reported, but the number of participants at follow‐up is not reported and there is no consort diagram. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported. |
| Other bias | High risk | Women in the treatment group on average less often had radiotherapy treatment (10; 37% vs 14; 47% ). The intervention group had more contacts with a therapist, which may reinforce other behaviour such as compliance to exercises and self‐care measures. |
Devoogdt 2011.
| Methods | Parallel groups randomised controlled trial, stratification for Body Mass Index and adjuvant radiotherapy. | |
| Participants | People treated for breast cancer (all stages except advanced disease) including ALND. | |
| Interventions |
Intervention group (n = 79) Provision of guidelines about prevention of lymphoedema, passive shoulder mobilisation, active shoulder exercises, scar massage and manual lymph drainage (40 one‐hour sessions, 3 times/week). Control group (n = 81) Provision of guidelines about prevention of lymphoedema, passive shoulder mobilisation, active shoulder exercises, scar massage |
|
| Outcomes |
Primary outcome: Cumulative incidence of lymphoedema defined as: 1. 200 mL or more increase in arm volume difference between healthy and operated side compared to the difference before surgery. 2. Time to develop lymphoedema, by same criterion Secondary outcomes: Cumulative incidence of lymphoedema defined as 2 cm or more increase in arm circumference difference at any two adjacent points between healthy and operated side. Time to develop lymphoedema by the same criterion. Point prevalence of lymphoedema using both criteria. Point prevalence of subjective lymphoedema. Increase of arm volume. Health‐related quality of life (MOS Short Form 36 component scores for physical and mental health). Range of motion of the upper limb (not reported). Lymphoscintigraphic examination (not reported). Lymph‐SBP questionnaire (not reported). |
|
| Follow up | 12 months | |
| Country, setting | Belgium, University Hospital | |
| Year of conduct | 2007 to 2009 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using permuted blocks, stratification for Body Mass Index and adjuvant radiotherapy. |
| Allocation concealment (selection bias) | Low risk | Allocation to treatment groups was concealed. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants and personnel were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of participants and personnel (performance bias) Patient reported outcomes | Unclear risk | Participants and personnel were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of outcome assessment (detection bias) Measured outcomes | Unclear risk | Outcome assessors were blinded. However, lymphoedema was assessed at scheduled follow‐up measurements or in case of self‐reported symptoms. Participants were not blinded for the intervention which may have induced differences in propensity towards reporting symptoms based on knowledge of group allocation. |
| Blinding of outcome assessment (detection bias) Patient reported outcomes | High risk | Self report for pain and HRQoL may be affected by participants' knowledge of group allocation. |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | There was a very low dropout rate. A sensitivity analysis by the review authors supported the conclusions. |
| Incomplete outcome data (attrition bias) Patient reported outcome | Low risk | There was a very low dropout rate. |
| Selective reporting (reporting bias) | Unclear risk | Range of motion was measured according to the trial protocol, but not reported. Data were not yet available at the time of writing for this review. |
| Other bias | Unclear risk | A higher percentage in the intervention group had level III dissection (43% vs 33%) and a higher percentage had radiotherapy on the axilla (10 vs 6), which may lead to increased risk for the intervention group. |
Sagen 2009.
| Methods | Parallel group randomised controlled trial. | |
| Participants | Women aged 32 to 75, treated for early stage breast cancer with mastectomy or breast‐conserving therapy with ALND (level I and II), with or without radiotherapy, chemotherapy or hormone treatment. | |
| Interventions |
Intervention (n = 104) Supervised physiotherapy consisting of moderate progressive resistance exercise training 2 to 3 times a week, without restriction in activities. Control (n = 100) Restricted activity for the affected limb for 6 months (avoidance of heavy or strenuous activities, carrying or lifting over 3 kg). Supervised physiotherapy consisting of passive manual mobilisation, light massage, once a week |
|
| Outcomes |
Primary outcome: Lymphoedema defined as 10% or more increase in Voldiff = (volume of the affected ‐ volume of the heterolateral arm)/volume of the heterolateral arm *100, measured by water displacement volumetry. Secondary outcomes: Pain (ordinal scale with 3 categories, based on visual analogue scale). Sensation of heaviness (VAS). |
|
| Follow up | 24 months | |
| Country, setting | Norway, 2 University Hospitals | |
| Year of conduct | 1999 to 2003 | |
| Notes | The study question was based on an equivalence hypothesis, but the study was analysed as a superiority trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by computer program. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants and personnel were not blinded. Self‐reported physical activity scores were lower in the control group than in the intervention group during the intervention (3 months and 6 months) which suggests there was no contamination. |
| Blinding of participants and personnel (performance bias) Patient reported outcomes | Low risk | Participants and personnel were not blinded. Self‐reported physical activity scores were lower in the control group than in the intervention group during the intervention (3 months and 6 months). |
| Blinding of outcome assessment (detection bias) Measured outcomes | Unclear risk | The blinded outcome assessor was not involved in the interventions performed at the outpatient clinics. However, ALE treatment was given whenever necessary during the 6 month intervention and whenever requested between the 6 month and 2 year follow‐up. Since participants were not blinded, there may have been differences in reporting symptoms of LO between experimental and control group. |
| Blinding of outcome assessment (detection bias) Patient reported outcomes | High risk | Participants were not blinded. Self‐reported pain may be affected by participants knowledge of group allocation. |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | More participants were lost to follow‐up in group 1 (no activity restriction) compared to group 2: 13 vs 10, 14 vs 3 and 36 vs 16 at 3, 6 and 24 months respectively. A last observation carried forward procedure was employed. Since lymphoedema incidence increases over time, this approach is questionable. |
| Incomplete outcome data (attrition bias) Patient reported outcome | High risk | More participants were lost to follow‐up in group 1 (no activity restriction) compared to group 2: 13 vs 10, 14 vs 3 and 36 vs 16 at 3, 6 and 24 months respectively. A last observation carried forward procedure was employed. Data on 17 participants in group 1 and 15 participants in group 2 were not reported at 3‐months follow‐up and apparently imputed at six months and two years. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section are reported. |
| Other bias | High risk | People in the activity restriction group also received usual care physiotherapy treatment once a week, which included massage, while people in the exercise group did not receive massage. The intervention group had more contacts with a therapist, which may reinforce other behaviour such as compliance to exercises and self‐care measures. Arm lymphoedema was treated in both groups, both during the intervention period and during follow‐up. The figures as reported are based on point‐prevalence at follow‐up points, not as cumulative incidence. It is therefore unclear how many people in each group developed lymphoedema at some point during the follow‐up that resolved as a result of therapy. This may lead to a biased interpretation of equivalence. |
Schmitz 2010.
| Methods | Parallel groups equivalence trial. | |
| Participants | Females, unilateral BRCA, non‐metastatic 1 to 5 years post treatment, BMI < 50, currently cancer free, no medical conditions limiting exercise, weight stable, no weight lifting in the year before study entry, no plans for surgery or leave > 1 month during study period, not actively trying to lose weight, > 1 LN removed, no current lymphoedema. | |
| Interventions |
Intervention group (n = 77) 1 year membership to community fitness centre, progressive resistance exercises in groups of 2 to 6, supervised for 13 weeks. Unsupervised for the rest of the study period. Progressive resistance exercises with dumbbells or machines, in 3 sets of 10 reps, increasing weight with the smallest possible increment after completing 2 sessions of 3x10 reps without symptoms of lymphoedema. Control group (n = 77) Controls were asked not to change baseline level of exercise during study period. |
|
| Outcomes |
Primary lymphoedema outcome: Lymphoedema defined as: interlimb difference of > 5%, determined by water displacement volumetry: (affected arm volume – unaffected arm volume)/unaffected arm volume Secondary lymphoedema outcomes: Lymphoedema defined as: greatest circumferential difference of > 5% and clinician‐based diagnosis based on CTCAE v3.0. Health‐related quality of life (SF36). Body image (Body Image and Relationships Scale). Pain (not reported). Musculoskeletal adverse events. |
|
| Follow up | 12 months | |
| Country, setting | USA, University Medical Center | |
| Year of conduct | 2005 to 2008 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation balancing for age, NRN, obesity and RT |
| Allocation concealment (selection bias) | Low risk | Computerised sequence generation (minimisation) |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Participants and personnel were not blinded for the intervention. Although participants in the control group were asked not to change their baseline physical activity level, average self‐reported physical activity in MET‐min/week increased with 370 MET‐min/week and 360MET‐min/week in the control group. It is unclear whether this involved strength training as well, although there was no significant increase in strength in the control group. |
| Blinding of participants and personnel (performance bias) Patient reported outcomes | High risk | Participants and personnel were not blinded for the intervention. |
| Blinding of outcome assessment (detection bias) Measured outcomes | Low risk | Outcome observers lymphoedema were blinded to group allocation. Participants were asked not to reveal group assignment before measurement sessions. |
| Blinding of outcome assessment (detection bias) Patient reported outcomes | High risk | Participants were not blinded. Self‐reported HRQoL may be affected by participants' knowledge of group allocation |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | The drop‐out rate and reasons for drop out were comparable between groups for the primary outcome; sensitivity analysis (best case/worst case scenario) was performed and findings were robust. |
| Incomplete outcome data (attrition bias) Patient reported outcome | Unclear risk | For participant‐reported outcomes, the attrition rate was 23.3% in the intervention group and 20.8% in the control group at the 12 month follow‐up. Time since diagnosis of the evaluable participants in the control group was on average 5 months more than in the intervention group. |
| Selective reporting (reporting bias) | Unclear risk | There were no results reported on pain. |
| Other bias | Low risk | No other potential sources of bias were identified. |
Todd 2008.
| Methods | RCT, parallel groups, single blind. | |
| Participants | Women with early breast cancer admitted for surgery including ALND. | |
| Interventions |
Intervention group (n = 58) Delayed (1 week) full‐range shoulder mobilisation exercises. During the first week, exercise was limited to below 90° in all planes of movement. Exercises were to be performed 4 times per day, until full shoulder movement was restored. Control group (n = 58) Immediate (within 2 days after surgery) vigorous, full‐range, shoulder mobilisation exercises, following the same regimen as the intervention group. |
|
| Outcomes |
Primary outcome: Lymphoedema defined as: 200 mL or more volume difference between the arms on the operated side and the non‐operated side. Secondary outcomes: Range of motion of the shoulder for flexion, abduction, medial rotation and lateral rotation as measured with a goniometer. HRQoL using the Trial Outcome Index of the Functional Assessment of Cancer Therapy ‐ Breast (FACT‐B). Grip strength (JAMAR). Shoulder disability (Shoulder disability questionnaire). |
|
| Follow up | 12 months | |
| Country, setting | UK, 2 secondary care National Health Service trusts. | |
| Year of conduct | 2003 to 2006 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using random number table and sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Women were randomised by an objective third person after completion of baseline measures. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants and personnel were not blinded for group allocation, but only one participant in the delayed mobilisation group did not receive the allocated intervention, so bias due to contamination is unlikely. |
| Blinding of participants and personnel (performance bias) Patient reported outcomes | Unclear risk | Participants and personnel were not blinded for group allocation, but only one patient in the delayed mobilisation group did not receive the allocated intervention, so bias due to contamination is unlikely. |
| Blinding of outcome assessment (detection bias) Measured outcomes | Low risk | Outcome observer was blinded, and participants were instructed not to reveal group allocation during follow‐up visits. |
| Blinding of outcome assessment (detection bias) Patient reported outcomes | Low risk | Participants were not blinded, but HRQoL was assessed at one year follow‐up. Given the nature and the duration of the intervention, it seems unlikely that knowledge of group allocation would have influenced participants' self‐reported HRQoL. |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Results were imputed using last observation carried forward; sensitivity analysis yields the possibility of a non‐significant RR (whereas a significant RR is reported). |
| Incomplete outcome data (attrition bias) Patient reported outcome | High risk | Sensitivity analysis of the primary outcome allowed for a different conclusion with regard to lymphoedema risk, and consequently risk of bias in participant‐reported outcome measures cannot be excluded. |
| Selective reporting (reporting bias) | Low risk | All outcome measures mentioned in the methods section are reported. |
| Other bias | Unclear risk | It is unclear how many participants in each group were treated for lymphoedema in the period between baseline and follow‐up measurements (this was dependent on self‐reported lymphoedema complaints and subsequent clinical evaluation). |
Torres 2010.
| Methods | Parallel groups randomised controlled trial. | |
| Participants | Women after unilateral breast cancer surgery including ALND, mean age 52.9 (SD 11.6), (N = 120). Eighty percent of the women received radiotherapy treatment, 82% chemotherapy. | |
| Interventions |
Intervention group (n = 60) Manual Lymph Drainage (thorax, breast, axilla and upper arm), scar massage and exercise therapy (stretching, functional activities, active and assisted exercises of the shoulder) for 3 weeks (3 visits/week), and education. Control group (n = 60) Education only. |
|
| Outcomes |
Primary outcome: Lymphoedema, defined as a 2 cm or greater increase in the circumference of any two adjacent points compared with measurements in the other arm. Secondary outcomes: Pain (VAS); Range of motion of the shoulder; Lymphoedema by other criteria (not reported); Time to event for lymphoedema |
|
| Follow up | 1, 3, 6 and 12 months (event rates for lymphoedema only available for 12 months). | |
| Country, setting | Spain, University hospital | |
| Year of conduct | 2005 to 2007 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done based on a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Participants were enrolled in order of arrival. Randomisation was performed by a different person from the recruiter. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants and personnel were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of participants and personnel (performance bias) Patient reported outcomes | Unclear risk | Participants and therapist were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of outcome assessment (detection bias) Measured outcomes | Unclear risk | An independent observer performed all follow‐up measurements; however participants were not blinded for the intervention which may have induced differences in propensity towards reporting symptoms based on knowledge of group allocation. This may have biased the estimation of lymphoedema incidence, but not measurements of range of motion of the shoulder. |
| Blinding of outcome assessment (detection bias) Patient reported outcomes | High risk | Self report for pain and HRQoL may be affected by participants' knowledge of group allocation. |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Data are available for all patients who were not excluded from the study (however, analysis was per protocol, see 'other bias'). |
| Incomplete outcome data (attrition bias) Patient reported outcome | Low risk | Data are available for all participants who were not excluded from the study (however, analysis was per protocol, see 'other bias'). |
| Selective reporting (reporting bias) | Low risk | Data on secondary outcomes are not reported in the publication, but were made available by the researchers. |
| Other bias | High risk | Radiotherapy was more often given to participants in the control group (+11%). Trial analysis was per protocol. 3 people in the control group and 1 patient in the intervention group who did not receive the allocated intervention were excluded. |
Zimmermann 2012.
| Methods | Parallel groups randomised controlled trial. | |
| Participants | Women after breast cancer surgery, mean age 67 (range 34 to 81) | |
| Interventions |
Intervention group (n = 33): manual lymph drainage (modified Földi and Strößenreuther method), 5 times a week during first 2 weeks, then twice a week from day 14 until 6 months, in addition to standardised physiotherapy (exercises of upper limb and chest). Control group (n = 34): self drainage and standardized physiotherapy |
|
| Outcomes |
Primary outcome:
Lymphoedema, measured through the water displacement method. Volume of lymphoedema is expressed as the ratio of the difference between arm volume on the operated and nonoperated sides divided by arm volume, nonoperated side. Cutoff points used for lymphoedema: < 5% absence; 5% to 10% mild; 10% to 20% moderate; > 20% substantial. Secondary outcome: Range of motion (goniometer) for shoulder flexion, abduction, extension. |
|
| Follow up | 2, 7, 14 days, 3 months, 6 months | |
| Country, setting | Germany, teaching hospital | |
| Year of conduct | 2003 to 2004 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using computerised list. |
| Allocation concealment (selection bias) | High risk | Fixed block length, no mention of blinding of allocation. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants and personnel were not blinded for the intervention. The risk of contamination is unclear. |
| Blinding of outcome assessment (detection bias) Measured outcomes | High risk | No attempts at blinding were made. |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Outcome is complete for all participants. |
| Selective reporting (reporting bias) | Low risk | All outcome data is available. |
| Other bias | Unclear risk | The intervention group had more contacts with a therapist, which may reinforce other behaviour such as compliance to exercises and self‐care measures. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2006 | In both groups people were included who already had lymphoedema. Allocation was not stratified for presence of lymphedema, hence no subgroups could be examined. |
| Anderson 2012 | Lymphoedema was not the primary outcome in this study on the effect and safety of a structured exercise programme with lymphoedema prevention module on quality of life. |
| Boccardo 2009 | The intervention was in part non‐conservative (microsurgical operation in case of appearance of lymphoedema, as established by lymphoscintigraphy). |
| Box 2009 | Not a primary study, but a synopsis of Todd 2008 |
| Campisi 2002 | The intervention was in part non‐conservative (microsurgical lymphatic‐venous anastomoses in people non responsive to early physical therapy for lymphoedema, as established by lymphoscintigrapy). |
| Chandrakaladharan 2009 | Full text could not be obtained from the author, study was published as an abstract only. |
| de Rezende 2006 | The study evaluated shoulder function and wound drainage volumes. Lymphoedema was not an outcome. |
| Hayes 2012 | Lymphoedema was not a primary outcome in this study on effect of exercise on quality of life, and the outcome measure used was not sufficiently objective. |
| Le‐Vu 1997 | The primary outcome was seroma formation. Lymphoedema was assessed at some point between 8 and 24 months, but only by self report questionnaire or clinician‐based diagnosis. |
| Oliveira 2009 | The primary outcome was range of motion of the shoulder. Arm circumferences were included as secondary outcome measure. No results on lymphoedema are reported except that there was no statistically‐significant difference between the groups at all follow‐up points. |
| Sarri 2010 | The primary outcome was lymphatic flow as measured by lymphscintigrapy, as a surrogate endpoint for lymphoedema. |
| Sisman 2012 | Not a randomised controlled trial. |
| Wang 2005 | No clear and sufficiently objective measure for lymphoedema was defined. |
Characteristics of ongoing studies [ordered by study ID]
Ben Selvan 2008.
| Trial name or title | The influence of prophylactic application of the class 2 upper limb stockings in carcinoma breast patients in reducing the incidence of Breast cancer related lymph edema |
| Methods | Parallel group single blinded randomised controlled trial, 36 month follow‐up, N = 178. |
| Participants | Parallel group single blinded randomised controlled trial, 36 month follow‐up, N = 178. |
| Interventions | Class 2 elastic compression stockings: for a period of 3 months from the first post operative period, vs no stocking. |
| Outcomes | Primary outcome: percentage of reduction in arm volume (circumference measurements) in the study group. Secondary outcome: incidence of lymphedema on the 10th post‐operative day and at three months follow up. |
| Starting date | Registered on 27‐11‐2008 |
| Contact information | Ben Selvan, C.K. Christian Medical College, Department of Surgery, 632004, Vellore, Tamil Nadu, India. drckben@yahoo.com |
| Notes | CTRI/2008/091/000249 |
Pain 2012.
| Trial name or title | Prevention of breast cancer‐related lymphoedema following axillary lymph node clearance. |
| Methods | Parallel group randomised controlled trial, 36 month follow‐up, N = 178. |
| Participants | People who have had axillary node clearance for breast cancer. |
| Interventions | Manual lymph drainage, in addition to skin care, compression garments and exercise vs skin care, compression garments and exercise only. |
| Outcomes | Limb volume using circumference measurements, validation of bio‐impedance technology. |
| Starting date | 1‐10‐2011 |
| Contact information | Pain, S, Norfolk & Norwich University Hospital, Colney Lane, Norwich, Norfolk NR4 7UY, United Kingdom, simon.pain@nnuh.nhs.uk |
| Notes |
Differences between protocol and review
The term 'conventional interventions' in the protocol was changed to 'conservative interventions'.
Contributions of authors
Draft the protocol: MS, MT, CA, CL, PB
Study selection: MS, MT, CA
Extract data from studies: MS, MT, CA
Enter data into RevMan: MS, MT
Carry out the analysis: MS, MT
Interpret the analysis: MS, MT, CL
Draft the final review: MS, MT, CA, CL, NA, PB
Disagreement resolution:CA
Update the review: CA
Sources of support
Internal sources
Department of Physiotherapy, The Netherlands Cancer Institute ‐ Antoni van Leeuwenhoek Hospital (NKI‐AVL), Netherlands.
External sources
Cochrane Incentive Scheme grant (NIHR), UK.
Declarations of interest
The authors have no competing interests.
New
References
References to studies included in this review
Bendz 2002 {published data only}
- Bendz I, Fagevik Olsén M. Evaluation of immediate versus delayed shoulder exercises after breast cancer surgery including lymph node dissection ‐ A randomised controlled trial. The Breast 2002;11(3):241‐8. [DOI] [PubMed] [Google Scholar]
Box 2002 {published data only}
- Box RC, Reul‐Hirche HM, Bullock‐Saxton JE, Furnival CM. Physiotherapy after breast cancer surgery: results of a randomised controlled study to minimise lymphoedema. Breast Cancer Research and Treatment 2002;75(1):51‐64. [DOI] [PubMed] [Google Scholar]
- Box RC, Reul‐Hirche HM, Bullock‐Saxton JE, Furnival CM. Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Research and Treatment 2002;75(1):35‐50. [DOI] [PubMed] [Google Scholar]
Castro‐Sanchez 2011 {published data only}
- Castro‐Sánchez AM, Moreno‐Lorenzo C, Matarán‐Peñarrocha GA, Aguilar‐Ferrándiz ME, Almagro‐Céspedes I, Anaya‐Ojeda J. Preventing lymphoedema after breast cancer surgery by elastic restraint orthotic and manual lymphatic drainage: a randomized clinical trial. Medicina Clinica 2010;137(5):204‐7. [DOI] [PubMed] [Google Scholar]
Cinar 2008 {published data only}
- Cinar N, Seckin U, Keskin D, Bodur H, Bozkurt B, Cengiz O. The effectiveness of early rehabilitation in patients with modified radical mastectomy. Cancer nursing 2008;31(2):160‐5. [PUBMED: 18490892] [DOI] [PubMed] [Google Scholar]
Devoogdt 2011 {published data only}
- Devoogdt N, Christiaens MR, Geraerts I, Truijen S, Smeetsbreast A, Leunen K, et al. Effect of manual lymph drainage in addition to guidelines and exercise therapy on arm lymphoedema related to breast cancer: randomised controlled trial. British Medical Journal 2011;343:d5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagen 2009 {published data only}
- Sagen A, Kåresen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow‐up. Acta Oncologica 2009;48:1102‐10. [DOI] [PubMed] [Google Scholar]
Schmitz 2010 {published data only}
- Brown CJ, Troxel AB, Schmitz KH. Safety of weightlifting among women with or at risk for breast cancer‐related lymphedema: musculoskeletal injuries and health care use in a weightlifting rehabilitation trial. The Oncologist 2012;17(8):1120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis‐Grant L, Smith R, et al. Weight lifting for women at risk for breast cancer‐related lymphedema: a randomized trial. JAMA 2010;304(24):2699‐705. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Troxel AB, Cheville A, Grant L, Bryan CJ, Gross CR, et al. Physical activity and lymphedema (the PAL trial): Assessing the safety of progressive strength training in breast cancer survivors. Contemporary Clinical Trials 2009;30(3):233‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, et al. Changes in the Body Image and Relationship Scale following a one‐year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Research and Treatment 2010;121(2):421‐30. [DOI] [PubMed] [Google Scholar]
Todd 2008 {published data only}
- Todd J, Scally A, Dodwell D, Horgan K, Topping A. A randomised controlled trial of two programmes of shoulder exercise following axillary node dissection for invasive breast cancer. Physiotherapy 2008;94:265‐73. [Google Scholar]
Torres 2010 {published data only}
- Torres Lacomba M, Yuste Sánchez MJ, Zapico Goni A, Prieto Merino D, Mayoral del Moral O, Cerezo Téllez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ 2010;340:b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmermann 2012 {published data only}
- Zimmermann A, Szklarska A, Lipowicz A, Woźniewski M. Influence of manual lymph drainage on shoulder range of motion after breast cancer surgery; a randomized controlled trial [Einfluss der manuellen Lymphdrainage auf die Schulterbeweglichkeit nach Brustkrebsoperation Eine randomisierte kontrollierte klinische Studie]. PT_Zeitschrift für Physiotherapeuten 2009;7(61):602‐610. [Google Scholar]
- Zimmermann A, Woźniewski M, Szklarska A, Lipowicz A, Szuba A. Efficacy of manual lymphatic drainage in preventing secondary lymphedema after breast cancer surgery. Lymphology 2012;45(3):103‐12. [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2006 {published data only}
- Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. Journal of Clinical Oncology 2006;24(18):2765‐72. [DOI] [PubMed] [Google Scholar]
Anderson 2012 {published data only}
- Anderson RT, Kimmick GG, McCoy TP, Hopkins J, Levine E, Miller G, et al. A randomized trial of exercise on well‐being and function following breast cancer surgery: the RESTORE trial. Journal of cancer survivorship : research and practice 2012;6(2):172‐81. [PUBMED: 22160629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boccardo 2009 {published data only}
- Boccardo FM, Ansaldi F, Bellini C, Accogli S, Taddei G, Murdaca G, et al. Prospective evaluation of a prevention protocol for lymphedema following surgery for breast cancer. Lymphology 2009;1(42):1‐9. [PubMed] [Google Scholar]
Box 2009 {published data only}
- Box R. Restriction of the range of arm elevation exercises for one week after surgery for breast cancer can reduce the incidence of lymphoedema. Australian Journal of Physiotherapy 2009;55(1):64‐6. [DOI] [PubMed] [Google Scholar]
Campisi 2002 {published data only}
- Campisi C, Boccardo F, Zilli A, Maccio A, Napoli F, Ferreira Azevedo W Jr, et al. Lymphedema secondary to breast cancer treatment: possibility of diagnostic and therapeutic prevention. Annali Italiani di Chirurgia 2002;73(5):493‐8. [PubMed] [Google Scholar]
Chandrakaladharan 2009 {published data only}
- Chandrakaladharan BS, Paul MJ, Nair A. Randomized control trial to evaluate the influence of class II compression stockings in preventing the development of lymphoedema in breast carcinoma patients. Annals of Oncology. 2009; Vol. Conference: IMPAKT Breast Cancer Conference Brussels Belgium 2009, issue conference publication ii69.
de Rezende 2006 {published data only}
- Rezende LF, Franco RL, Rezende MF, Beletti PO, Morais SS, Gurgel MS. Two exercise schemes in postoperative breast cancer: comparison of effects on shoulder movement and lymphatic disturbance. Tumori 2006;92(1):55‐61. [DOI] [PubMed] [Google Scholar]
Hayes 2012 {published data only}
- Hayes SC, Rye S, Disipio T, Yates P, Bashford J, Pyke C, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment‐related side effects following breast cancer. Breast Cancer Research and Treatment 2012;137(1):175‐86. [DOI] [PubMed] [Google Scholar]
Le‐Vu 1997 {published data only}
- Le‐Vu B, Guillaume MHB. Efficacy of massage and mobilization of the upper limb after surgical treatment of breast cancer. Bulletin du Cancer 1997;84(10):957‐61. [PubMed] [Google Scholar]
Oliveira 2009 {published data only}
- Oliveira MMF, Gurgel MSC, Miranda MS, Okubo MA, Feijó LFA, Souza GA. Efficacy of shoulder exercises on locoregional complications in women undergoing radiotherapy for breast cancer: clinical trial. Revista Brasileira de Fisioterapia [Brazilian Journal of Physical Therapy] 2009;13(2):136‐43. [Google Scholar]
Sarri 2010 {published data only}
- Sarri AJ, Moriguchi MS, Dias R, Peres SV, Silva ET, Koga KH, et al. Physiotherapeutic stimulation: Early prevention of lymphedema following axillary lymph node dissection for breast cancer treatment. Experimental and Therapeutic Medicine 2010;1(1):147‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sisman 2012 {published data only}
- Sisman H, Sahin B, Duman BB, Tanriverdi G. Nurse‐assisted education and exercise decrease the prevalence and morbidity of lymphedema following breast cancer surgery. Journal of B.U.ON. 2012;17(3):565‐9. [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang BG, Yuan XY, Wang QT, Luang XD, Wang CP, Jia AL, et al. (2005) [Functional rehabilitation gymnastics for the edema of upper limbs and the activity of shoulder joint in postoperative patients with breast cancer]. " Zhongguo Linchuang Kangfu [Chinese Journal of Clinical Rehabilitation] 2005;9(30):16‐9. [Google Scholar]
References to ongoing studies
Ben Selvan 2008 {published data only}
- The influence of prophylactic application of the class 2 upper limb stockings in carcinoma breast patients in reducing the incidence of Breast cancer related lymph edema. Ongoing study Registered on 27‐11‐2008.
Pain 2012 {published data only}
- Prevention of breast cancer‐related lymphoedema following axillary lymph node clearance.. Ongoing study 1‐10‐2011.
Additional references
Altman 1996
- Altman D G. Better reporting of randomised controlled trials: the CONSORT statement. British Medical Journal 1996;313:570‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan 1992
- Brennan MJ. Lymphedema following the surgical treatment of breast cancer: a review of pathophysiology and treatment. Journal of Pain and Symptom Management 1992;7:110‐6. [DOI] [PubMed] [Google Scholar]
Chan 2010
- Chan DN, Lui LY, So WK. Effectiveness of exercise programmes on shoulder mobility and lymphoedema after axillary lymph node dissection for breast cancer: systematic review. Journal of advanced nursing 2010;66(9):1902‐14. [PUBMED: 20626480] [DOI] [PubMed] [Google Scholar]
Clark 2005
- Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three‐year follow‐up study. QJM: Monthly Journal of the Association of Physicians 2005;98(5):343‐8. [DOI] [PubMed] [Google Scholar]
Cormier 2010
- Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta‐analysis of cancer‐related secondary lymphedema. Cancer 2010;116:5138‐49. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Devoogdt 2010
- Devoogdt N, Kampen M, Geraerts I, Coremans T, Christiaens MR. Different physical treatment modalities for lymphoedema developing after axillary lymph node dissection for breast cancer: a review. European journal of obstetrics, gynecology, and reproductive biology 2010;149(1):3‐9. [PUBMED: 20018422] [DOI] [PubMed] [Google Scholar]
DiSipio 2013
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta‐analysis. The lancet oncology 2013;14(6):500‐15. [PUBMED: 23540561] [DOI] [PubMed] [Google Scholar]
Duets
- NHS. NHS Database of Uncertainties about the Effects of Treatments (DUETS):. http://www.library.nhs.uk/DUETs/ViewResource.aspx?resID=302437 accessed June 2011.
Engel 2003
- Engel J, Kerr J, Schlesinger‐Raab A, Eckel R, Sauer H, Holzel D. Predictors of quality of life of breast cancer patients. Acta Oncologica 2003;42:710‐8. [DOI] [PubMed] [Google Scholar]
Ewertz 2011
- Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncologica 2011;50(2):187‐93. [DOI] [PubMed] [Google Scholar]
Fu 2010
- Fu MR, Chen CM, Haber J, Guth AA, Axelrod D. The effect of providing information about lymphedema on the cognitive and symptom outcomes of breast cancer survivors. Annals of surgical oncology 2010;17(7):1847‐53. [PUBMED: 20140528] [DOI] [PubMed] [Google Scholar]
Globocan 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Available from: http://globocan.iarc.fr 2010.
Goldberg 2010
- Goldberg JI, Wiechmann LI, Riedel ER, Morrow M, Zee KJ. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Annals of Surgical Oncology 2010;17:3278‐86. [DOI] [PubMed] [Google Scholar]
Hayes 2008
- Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. Journal of Clinical Oncology 2008;26:3536‐42. [DOI] [PubMed] [Google Scholar]
Helyer 2010
- Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast Journal 2010;16:48‐54. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kwan 2010
- Kwan ML, Darbinian J, Schmitz KH, Citron R, Partee P, Kutner SE, et al. Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Archives of Surgery 2010;145:1055‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lane 2007
- Lane KN, Dolan LB, Worsley D, McKenzie DC. Upper extremity lymphatic function at rest and during exercise in breast cancer survivors with and without lymphedema compared with healthy controls. Journal of Applied Physiology 2007;103:917‐25. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee TS, Kilbreath SL, Sullivan G, Refshauge KM, Beith JM, Harris LM. Factors that affect intention to avoid strenuous arm activity after breast cancer surgery. Oncology Nursing Forum 2009;36:454‐62. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
McNeely 2010
- McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, et al. Exercise interventions for upper‐limb dysfunction due to breast cancer treatment. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD005211.pub2] [DOI] [PubMed] [Google Scholar]
Meeske 2008
- Meeske KA, Sullivan‐Halley J, Smith AW, McTiernan A, Baumgartner KB, Harlan LC, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Research and Treatment 2008;113:383‐91. [DOI] [PubMed] [Google Scholar]
Norman 2010
- Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiology, Biomarkers and Prevention 2010;19(11):2734‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2008
- Park JH, Lee WH, Chung HS. Incidence and risk factors of breast cancer lymphoedema. Journal of Clinical Nursing 2008;17(11):1450‐9. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Paskett 2007
- Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiology, Biomarkers and Prevention 2007;16:775‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrek 1998
- Petrek JA, Heelan MC. Incidence of breast carcinoma‐related lymphedema. American Cancer Society Lymphedema Workshop. Cancer 1998;83 Suppl(12):2776‐81. [DOI] [PubMed] [Google Scholar]
Preston 2008
- Preston N, Seers K. Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003141] [DOI] [PubMed] [Google Scholar]
R Statistical Package [Computer program]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Version 3.0.0. http://www.r‐project.org/, 2013.
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ridner 2011
- Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment‐related lymphedema. Supportive Care in Cancer 2011;19:853‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Round 2006
- Round T, Hayes SC, Newman B. How do recovery advice and behavioural characteristics influence upper‐body function and quality of life among women 6 months after breast cancer diagnosis?. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer 2006;14(1):22‐9. [PUBMED: 16012817] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shih 2009
- Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2‐year follow‐up study. Journal of Clinical Oncology 2009;27:2007‐14. [DOI] [PubMed] [Google Scholar]
Stout 2008
- Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008;112:2809‐19. [DOI] [PubMed] [Google Scholar]
Tsai 2009a
- Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott‐Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta‐analysis of treatment factors. Annals of Surgical Oncology 2009;16:1959‐72. [DOI] [PubMed] [Google Scholar]
Tsai 2009b
- Tsai HJ, Hung HC, Yang JL, Huang CS, Tsauo JY. Could Kinesio tape replace the bandage in decongestive lymphatic therapy for breast‐cancer‐related lymphedema? A pilot study. Annals of Surgical Oncology 2009;16:1959‐72. [DOI] [PubMed] [Google Scholar]
Vassard 2010
- Vassard D, Olsen MH, Zinckernagel L, Vibe‐Petersen J, Dalton SO, Johansen C. Psychological consequences of lymphoedema associated with breast cancer: A prospective cohort study. European Journal of Cancer 2010;46:3211‐8. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical research ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yen 2009
- Yen TW, Fan X, Sparapani R, Laud PW, Walker AP, Nattinger AB. A contemporary, population‐based study of lymphedema risk factors in older women with breast cancer. Annals of Surgical Oncology 2009;16:979‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]


