Abstract
A simple and cost-effective diagnostic tool (TB Screen Test) for the screening of patients with pulmonary and extrapulmonary tuberculosis and for differentiation of those individuals from individuals without tuberculosis, other common infections, and healthy controls has been developed. The serological responses of purified mycobacterial glycolipid antigens were examined by a liposome agglutination assay. The assay was able to detect very low antiglycolipid antibody concentrations in the infected individuals. The sera from the tuberculosis patient group had significantly higher concentrations of antiglycolipid antibody than the sera from uninfected control subjects, with 94% sensitivity and 98.3% specificity. Glycolipids of Mycobacterium tuberculosis H37Rv antigens were isolated, purified, and characterized. After interchelation with liposome particles, these purified antigens specifically bound to the antiglycolipid antibodies present in the sera of patients with tuberculosis, resulting in the formation of a blue agglutination. This protocol clearly differentiates healthy controls and M. bovis BCG-vaccinated subjects from those with active tuberculosis. The resultant diagnostic tool, the TB Screen Test, is more economical and rapid (4 min) than other currently available products and can be used for the mass screening of a heavily afflicted population.
Millions of people have died from tuberculosis (TB), a leading chronic infectious killer of youth and adults and the second most common infectious disease worldwide (44). Advances in rapid diagnostic techniques are urgently required both for the early management of the 8 million to 12 million new cases of TB that lead to 2 million deaths each year and for the 2 billion individuals already infected with Mycobacterium tuberculosis who are at risk of developing disease (44). In addition, about 4.6 million people worldwide are coinfected with human immunodeficiency virus (HIV) and M. tuberculosis (45). In India, about 0.5 million people die annually due to TB. A delayed or missed diagnosis of TB is also one of the leading causes of M. tuberculosis transmission and mortality (23).
Although TB can be fully cured with the use of appropriate antibiotics, the major hurdle to treatment for TB lies in the late diagnosis of the disease due to the lack of simple and cost-effective diagnostic products. Mycobacteria are complex unicellular organisms with a resilient cell wall structure and can suppress the host immune response by the immunomodulatory action mediated by their cell wall constituents. It is well known that after infection survival in phagocytic macrophage cells is regulated by cell surface glycolipids. These glycolipids have a role in providing the intracellular pathogens with pathogenic (38) and virulence (7) properties and possess a high discriminatory quality for serodiagnosis (in terms of both sensitivity and specificity). The cell envelope of M. tuberculosis contains an additional layer beyond the peptidoglycan that is exceptionally rich in unusual lipids, glycolipids, and polysaccharides (10). This layer protects the M. tuberculosis cell from the hydrolytic enzymes and toxic radicals produced by macrophages.
The present study explores the potential utility of glycolipid antigens (in a multiple-antigen cocktail) for the serodiagnosis of active TB in humans. A combination of antigen cocktails isolated from M. tuberculosis strain H37Rv (ATCC 27294) was analyzed on thin-layer chromatography (TLC) plates (immunostaining) against pooled sera from patients confirmed to have TB on the basis of clinical symptoms and with the BACTEC 460 system. The antigenic cocktail was interchelated with liposome particles and titrated with sera from individuals with clinically confirmed TB.
The methodology proposed here offers the possibility for the development of a rapid and cost-effective diagnostic test that could be marketed and commercialized for the screening and detection of M. tuberculosis infection in humans. This diagnostic tool can be used in settings where a modern infrastructure and laboratory facilities are not available and can be used for the routine screening of large numbers of patients for TB.
MATERIALS AND METHODS
Bacterial culture and growth conditions.
A lyophilized culture of M. tuberculosis H37Rv (ATCC 27294) on a Lowenstein-Jansen agar slant (2 × 109 CFU/ml) was obtained from the Central Japanese Leprosy Mission in Asia Research Institute for Leprosy, Agra, India. The bacterial culture was harvested by centrifugation (10,000 × g for 20 min) at 4°C, and the pellet was washed by resuspension in 100 ml of phosphate-buffered saline (PBS; pH 7.2). Finally, the bacterial pellet was resuspended in 10 ml of TEN buffer (pH 8.0; 10 mM Tris HCl, 1 mM EDTA, 100 mM NaCl), heat inactivated at 80°C (water bath) for 45 min, and then sonicated (15% pulse, 150 W) and lyophilized.
Extraction and isolation of antigen(s).
Glycolipid antigens were extracted and isolated by a previously described protocol (29), with slight modification. In brief, the lyophilized mycobacterial powder (5 g) was placed into a glass reagent bottle, and 100 ml of a chloroform-methanol mixture (2:1) was added to it. This mixture was stirred at room temperature for 60 min and filtered through Whatman no. 1 filter paper. A 1/5 volume of 0.7% KCl (20.0 ml) was added to the filtrate, and the mixture was shaken five to six times. The suspension was transferred to a separation funnel and kept at 2 to 8°C for overnight until two distinct layers were separated. The lower organic phase was washed with 1/5 volume of washing solvent (chloroform-methanol-water; 3:48:47) as described above by keeping it at 2 to 8°C for overnight. The upper aqueous phase was removed, and the lower organic phase was retained after filtration. The organic phase was dried by evaporating the solvent in a rotary solvent evaporator at 40°C. The moisture was removed by flushing the dried mixture with nitrogen gas. Neutral lipids were removed from the dried mixture by adding 50 ml of chilled acetone while the mixture was vortexed for 10 min and then filtered through Whatman no. 1 filter paper. This step was repeated until the lipids in the flask became whitish or colorless. The contents of the flask were filtered through Whatman no. 1 filter paper, and the filtrate was discarded. The lipids present on the filter paper were dissolved with chloroform-methanol (2:1) and transferred to a round-bottom flask. The solvent was evaporated on a rotary evaporator under reduced pressure at 40°C. The weight difference (WB − WA = 155.5 − 153.0 = 2.5 g of crude lipids, where WA is the weight of the empty round-bottom flask and WB is the weight of the empty flask with lipid) signifies the amount of crude antigens present in the flask. The crude preparation was reconstituted in 10 ml of chloroform-methanol (2:1) and stored at −20°C for further use.
Purification of antigen(s).
Silica gel H (S.D. Fine Chemicals, New Delhi, India), which was activated at 110°C for 1 h (in a hot-air oven) was packed into a glass column (2.5 by 30 cm) with manual tapping, and a known quantity of crude material (1.0 g/5 ml of stock) was loaded on either side of the column. The column was run in an ascending direction in a chromatographic jar (4.5 by 25 cm) with purification solvent (150 ml; mobile phase) at a ratio of 65:25:4 (chloroform-methanol-water) (22) and room temperature until the solvent reached the other end of the column. The column was removed from the chromatographic jar and placed in a fume hood to evaporate the solvent from the column. A 1-cm length of each fraction was carefully scrapped with a clean rod to separate the individual molecules that were adsorbed with the silica gel, depending on the mobility and retardation factor (Rf) value (percent mobilities of the five fractions, 46.6, 53.4, 58.3, 67.2, and 72.4%) of the individual molecule. The individual fractions were collected and placed into clean dry glass test tubes, which were labeled with the respective fraction number. Ten milliliters of extraction solvent (mixture of chloroform-methanol [2:1]) was added to each test tube, and the test tubes were kept at room temperature for 30 min. The purity of the eluted material was analyzed by TLC, and the selected fractions were further filtered through Whatman no. 1 filter paper to remove the silica gel from the samples. The pure fractions were pooled and run on preparative TLC plates to reconfirm the Rf value. After extensive study, the individual bands were scratched from the TLC plate (Fig. 1), the silica gel was removed, and the samples were analyzed by liquid chromatography with a mass spectrometer. Further biochemical characterization (43) and immunological characterization (17, 27) of the glycolipid antigen fractions were done as described previously. The pure fractions were pooled and used for further study. The Rf value was calculated by using the following formula: (distance traveled by the solvent/distance traveled by solute) × 100.
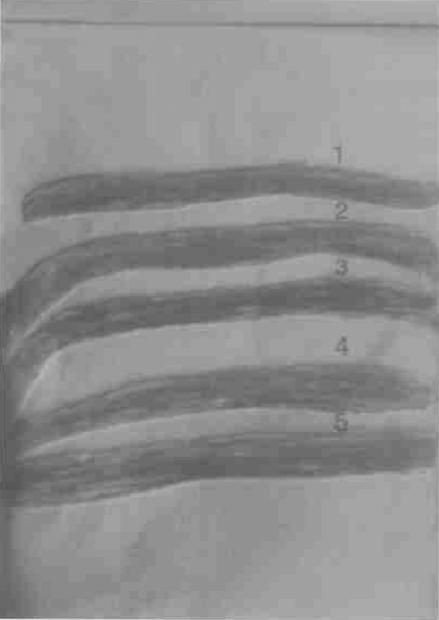
FIG. 1.
Extraction of immunologically significant purified antigenic glycolipids from a preparative TLC plate. Bands 1 to 5 were scratched from the TLC plate and were used for further study.
Estimation of amounts of glycolipids.
The glycolipid moieties present in the samples were quantified by a previously described procedure (43), followed by staining with the periodate-Schiff reagent for the detection of phosphatidates and glycolipids. In addition, the purification solvent (chloroform-methanol-water [65:25:4) method for the detection of glycolipids was used as described previously (22). The presence of glycolipids in these samples was further confirmed by α-naphthol staining for glycolipids (34). These samples were analyzed in detail by TLC and stained with vapor from iodine crystals in a dry, humidity-free chromatography staining chamber. Various methods were used to determine the concentration of glycolipid present in the appropriate samples. These included biochemical and immunological methods (17, 43), followed by estimation of the amounts of sugar residues present in the samples.
The glycolipid sample (0.1 ml) was dried in a clean glass test tube, and 2 ml of orcinol solution (5-methylresorcinol; 2 mg/ml of 70% [vol/vol] sulfuric acid) was added to the sample. The reaction mixture was heated at 80°C for 20 min, and after it cooled, the absorbance at 505 nm of the color that developed was measured. The amounts of sugar moieties present in the glycolipids were calculated by using a glucose calibration curve.
Characterization of antigen(s). (i) Immunostaining on TLC plates.
The antiglycolipid antibodies were detected by immunostaining on TLC plates, as described previously (36), with minor modifications. The TLC plates (silica gel H-50 on an aluminum sheet; Merck, Darmstadt, Germany) were cut to 2.5 by 6.5 cm and activated at 110°C for 10 min. The plates were taken out and kept at room temperature (with the avoidance of moisture), a pencil dot was made 1.0 cm from either end, and 20-μl samples were gradually loaded. The samples were run in the descending direction in a chromatographic jar containing 5 ml of purification solvent (for glycolipids), which consisted of a mixture of chloroform-methanol-water (65:25:4), and the solvent was run to the other end. The TLC plates were carefully removed from the jar with forceps and were kept over a blotting sheet at room temperature so that they could dry. Serum samples from patients with clinically confirmed TB were diluted 1:20 (0.2 ml of serum and 3.8 ml of PBS [pH 7.2]), and the diluted serum sample was poured into a clean petri plate. The TLC plate was dipped in the serum sample without shaking and was kept at 37°C for 1 h. The TLC plate was rapidly washed twice (without shaking) with wash buffer (PBS and 0.1% Tween 20), followed by incubation at 37°C for 30 min with rabbit anti-human immunoglobulin G (IgG) conjugated with peroxidase enzymes (Calbiochem, La Jolla, Calif.) diluted to a final concentration of 1:5,000. The TLC plate was again washed and developed with 3,3′,6′6-diaminobenzidine (DAB; Sigma, St. Louis, Mo.) solution (0.1% DAB in 100 mM Tris (hydroxymethyl) aminomethane hydrochloride [pH 8.0], 100 mM NiCl2, 0.006% H2O2). The reaction was stopped by washing the TLC plates with distilled water. The compound reacted with Rf values (46.6, 53.4, 58.3, 67.2, and 72.4) similar to those described above (Fig. 2).
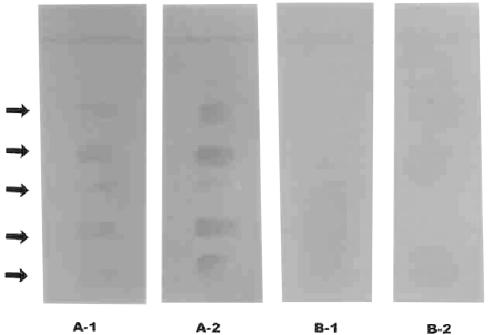
FIG. 2.
Immunoreactivity of antigenic glycolipid characterized on a TLC plate with a serum sample from a patient confirmed to have an active case of TB with the BACTEC 460 system. Serum samples: A-1, immunoreactivity of antiglycolipid antibodies with serologically positive sera of patients with active pulmonary TB on a TLC plate; A-2, immunoreactivity of antiglycolipid antibodies with serologically positive sera of patients with extrapulmonary TB on a TLC plate; B-1, immunoreactivity of serologically significant antigens with sera from healthy BCG-vaccinated humans for cross-reactivity study on a TLC plate; B-2, immunoreactivity of serologically significant antigens with sera from BCG-unvaccinated healthy humans for cross-reactivity study on a TLC plate. Arrows indicate reactive bands.
(ii) ELISA.
Polystyrene 96-well microtiter enzyme-linked immunosorbent assay (ELISA) plates (F.8; Maxisorp; Nunc, Roskilde, Denmark) coated with 100 μl of antigen solution (1.0 μg/ml in n-hexane) per well were dried overnight at 37°C and blocked with 1.0% polyvinylpyrrolidone (PVP; Himedia, Mumbai, India) in PBS (pH 7.5) at 37°C for 3 h. The plates were washed with wash solution (PBS and 0.1% Tween 20) for 1 min at room temperature and dried under vacuum in a desiccator. Dilution buffer (100 μl; PBS, 1.0% PVP, 0.1% Tween 20 [pH 7.5]) and 10 μl of a serum sample from a patient with clinically confirmed TB were added to each well, and the plates were incubated at 37°C for 30 min. The plates were washed five times for 5 min each time with wash buffer (PBS, 0.5 M NaCl, 0.1% Tween 20). Goat anti-human IgG conjugated to peroxidase (Calbiochem) was diluted at ratio of 1:50,000 in dilution buffer (PBS, 0.1% Tween 20, 1.0% PVP), 100 μl of this mixture was added to each well, and the plates were incubated at 37°C for 30 min. After the plate was washed as described above, 100 μl of 3,3′5,5′-tetramethylbenzidene (1.0% in dimethyl sulfoxide [Sigma]) was prepared in substrate buffer (citrate phosphate buffer, 0.006% H2O2 [pH 5.0]) and added to each well. The plates were incubated at room temperature for 15 min in the dark. The reaction was stopped with 50 μl of stop solution (2.5 N H2SO4), and the absorbance at 450 nm was measured with a microtiter plate reader (Dynatech, Chantilly, Va.). The cutoff value was determined by using the mean optical density of the negative control ±0.2. The gray area for the samples was calculated as a value of ±10% of the cutoff value. Since the chloroform and methanol solutions are corrosive for the microtiter ELISA plates, the samples were dried (by the evaporation method) and dissolved with n-hexane. After characterization of the antigens, the antigenic cocktails were interchelated with liposome vesicles, which were found to be the best model for such a study.
(iii) Liposomes.
Liposomes were prepared as described previously (3), with minor modification. Phosphatidylcholine (100 mg), cholesterol (500 mg; Sigma), antigenic suspension (20 mg; antigenic cocktail), and dye (50 μl; 1.0% Sudan black B in chloroform) were placed in a predried round-bottom flask. The solvent was evaporated with a rotary vacuum evaporator under reduced pressure. The dried contents were dissolved in 40 ml of absolute alcohol (99.9%; Hyman) and were kept at 4°C for 1 h. Sucrose solution (4 ml; 150 mM) was placed in a polypropylene centrifuge tube (capacity, 35 ml), and 4 ml of a preprepared alcoholic antigen suspension was added to the tube while the tube was gently vortexed. The tubes were kept overnight at 4°C for liposome swelling, and then the tubes were individually vortexed with 10 ml of PBS (pH 6.5) buffer and centrifuged (Beckman, Fullerton, Calif.) at 15,000 × g for 10 min. The supernatant was discarded, and the pellet was resuspended in 20 ml of B2 buffer (pH 7.2; NaH2PO4 · 0.2 H2O, 10 mM; KH2PO4, 10 mM; EDTA, 10 mM; choline chloride, 10%; and thimerosal, 0.1%). This mixture was stored at 4°C for further use and was used as the liposomal antigen reagent with the other components provided with the kit.
Method of testing.
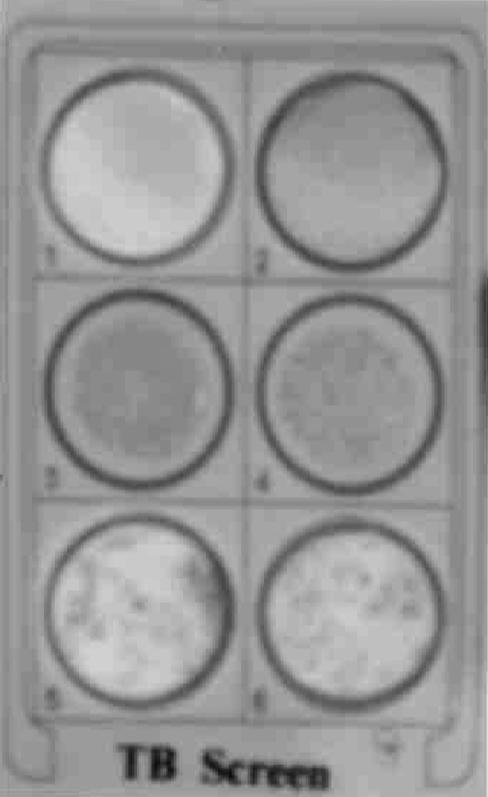
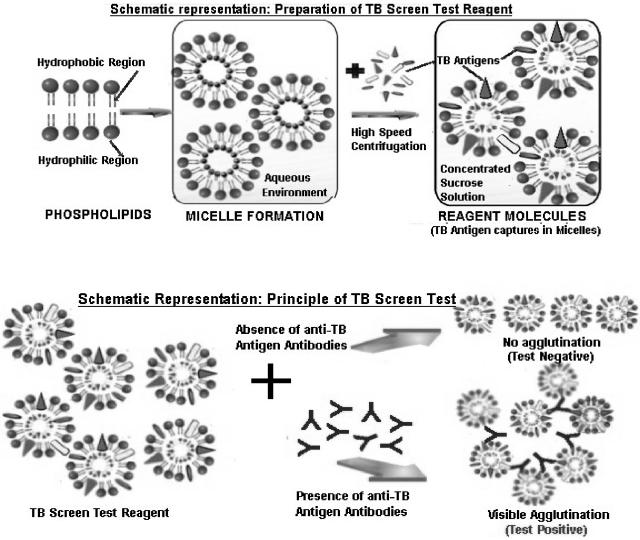
All the components (the positive controls, the negative controls, the antigen suspensions, and the samples to be tested) were brought to room temperature before the experiments were performed (Fig. 3). The positive controls, negative controls, and freshly procured or frozen test serum samples (25 μl each) were added and spread evenly inside the circular zone of the hydrophobic material-coated plastic slide, as demonstrated on the test card. For convenience, zone 1 and zone 2 were spread with the positive control (antirabbit serum) and the negative control (normal rabbit serum), respectively. Thereafter, 25 μl of the liposome antigenic suspension was added to each zone and the card was gently swirled for 4 min. The clumping of the specific antigen and antibody as a dark blue agglutination was observed for the positive controls as well as those samples which contained antibodies against mycobacterial glycolipid in samples from patients with active M. tuberculosis infection. No clumping on the card indicated a negative result. The peripheral drying on the circular zone indicated indiscriminate results, which required further confirmation within 15 to 30 days, as these samples contained undetectable levels of antigen (Fig. 4 and 5). Schematic representations of the preparation of the TB Screen Test reagent and the principle of the TB Screen Test are shown in Fig. 6.
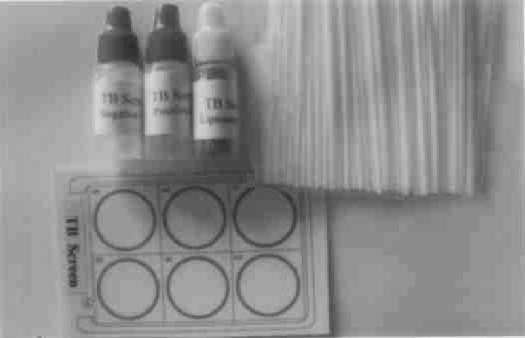
FIG. 3.
Accessories for TB Screen Test: TB Screen Test card; negative control, positive control, and liposomal antigen suspension (bottles from left to right, respectively); and mixing sticks.
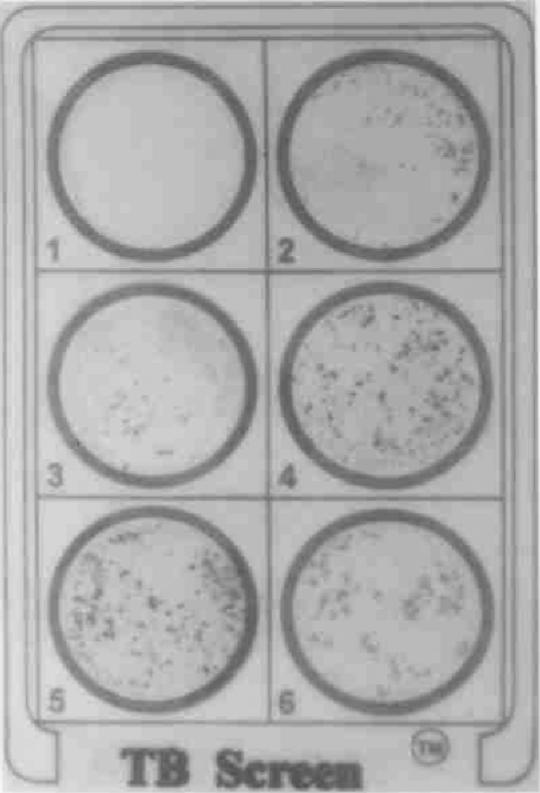
FIG. 4.
TB Screen Test results. Zones: 1, negative control (pooled, normal human sera); 2, positive control (pooled sera from patients with active TB); 3 and 4, serum samples from patients with confirmed cases of active TB; 5 and 6, serum samples from patients with confirmed cases of extrapulmonary TB.
FIG. 5.
TB Screen Test results. Zones: 1, negative control; 2, serum sample with peripheral drying; 3 to 6, strengths of agglutination 1+ to 4+, respectively. Compare the results presented here with those in Fig. 4.
FIG. 6.
Schematic representations of the preparation of the TB Screen Test reagent and the principle of the TB Screen Test.
Statistical analysis.
The sensitivity and specificity of the assay were calculated as described by Toman (41) in comparison with the results of culture and smear analyses, including the patient's history, signs and symptoms, confirmation of M. tuberculosis infection by pathological analysis, and drug treatment status. Sensitivity was calculated as [number specimens confirmed to be positive/(number of specimens confirmed to be positive/number of specimens with false-negative results)] × 100. Specificity was calculated as [number of specimens confirmed to be negative/(number of specimens with false-positive results/number of specimens confirmed to be negative)] × 100.
RESULTS
Sera collected from the outpatient departments from different hospitals and pathology centers in Bhopal and Gwalior, India, were enrolled in the present study to cover the maximum population diversity possible. The patients were diagnosed with TB on the basis of clinical and radiological evaluations as well as smear staining and sputum culture. A total of 1,813 serum samples were included in this study. Sera from healthy individuals without any clinical symptoms of TB were included as negative controls to evaluate the specificity of the test. Most of these serum samples were obtained from BCG-vaccinated healthy subjects. The non-TB sera were generally from either healthy individuals or patients with a variety of diseases other than TB. The sera were stored frozen and were used within 1 year from the time of collection. Details about the 1,813 serum samples selected are described in the following sections.
Active pulmonary TB group (n = 285).
A total of 285 serum samples were obtained from individuals confirmed to have active pulmonary TB. According to the clinical and laboratory diagnoses, the specimens were divided into the following four subgroups: (i) the smear-positive and culture-negative subgroup, which consisted of 180 specimens collected from patients admitted into a hospital but prior to commencement of their treatment; (ii) the smear-negative and culture-positive subgroup, which consisted of 52 samples collected from a group of patients with chronic M. tuberculosis infection, of whom 45 were receiving chemotherapy; (iii) the smear-positive and culture-negative subgroup, which consisted of 35 specimens from patients who were found to be negative by culture but who were found to have symptoms typical of TB with bacteria in their sputum samples; and (iv) the smear-negative and culture-negative subgroup, which consisted of 18 specimens that were negative by culture or smear analysis but that were from patients who were found to have symptoms typical of TB, some of whom were found to have latent M. tuberculosis infection.
Extrapulmonary TB group (n = 79).
A total of 79 samples were from patients with extrapulmonary TB, such as tuberculous lymphadenitis (n = 12), bone joint tuberculosis (n = 26), abdominal tuberculosis (n = 18), tuberculous pericarditis (n = 16), genitourinary tuberculosis (n = 3), and pleurisy (n = 4). These conditions were confirmed according to the clinical symptoms as well as the results of culture and smear analyses of tissue biopsy specimens.
Non-TB group (n = 511).
The non-TB group incorporated serum samples from subjects with other infections, such as patients with drug-treated, clinically negative TB (n = 60), healthy household contacts of TB patients (n = 50), BCG-vaccinated children (n = 15), hepatitis B virus-positive patients (n = 15), patients with common infections (n = 27), and healthy humans (n = 344).
Other common infection group (n = 156).
Serum samples were obtained from individuals with respiratory and nonrespiratory diseases, such as pneumonia (n = 16), bronchitis and bronchial asthma (n = 12), malaria (n = 22), typhoid (n = 18), syphilis (n = 12), gonorrhea (n = 18), rheumatoid arthritis (n = 16), the common cold (n = 14), pulmonary aspergillosis (n = 15), and lung abscesses (n = 13). None of these subjects were found to have clinical features of TB.
It is evident from the data in Table 1 that of 285 samples from patients with pulmonary TB studied, 270 were found to be positive and 7 were found to be negative. In addition, 8 samples were found to have indeterminate results. Of 79 samples from patients with extrapulmonary TB (Table 2), 67 were found to be positive, whereas 12 were found to be negative by the TB Screen Test. Finally, a total number of 364 samples from various groups of patients with TB (Table 1, n = 285; Table 2, n = 79) were included in our internal studies and were considered for the calculation of sensitivity. However, a total 667 samples, as described in Table 3 (n = 511) and Table 4 (n = 156), were considered for in-house studies of the specificity of our TB Screen Test. In particular, among 511 specimens from the non-TB group (Table 3), 488 were found to be negative, 6 were found to be false positive, and 17 were found to be indeterminate. Again, of 156 samples from patients with common infections tested (Table 4), 143 were found to be negative, 5 were found to be false positive, and 8 were found to have indeterminate results. In addition, an external study was also conducted by incorporating the serum samples from groups with various disease conditions. In this context, a total of 782 samples from various groups, such as patients with active TB (pulmonary and extrapulmonary) and M. tuberculosis-HIV coinfection (n = 242) and patients with other respiratory diseases, nonrespiratory diseases, and BCG-vaccinated healthy subjects (n = 540), were tested by external agencies; and these results were also considered for sensitivity and specificity calculations (Table 5).
TABLE 1.
Detailed analysis of pulmonary TB patients by internal studies
| AFB smear result/ culture resulta | No. of serum samples tested | No. of serum samples with the following TB Screen Test results
|
Sensitivity (%) | ||
|---|---|---|---|---|---|
| Positive | Negative | Indeterminate | |||
| +/+ | 180 | 175 | 4 | 5 | 97.2 |
| −/+ | 52 | 48 | 2 | 2 | 92.8 |
| +/− | 35 | 33 | 2 | 94.5 | |
| −/− | 18 | 14 | 3 | 1 | 81.8 |
| Total | 285 | 270 | 7 | 8 | 95.0 |
AFB, acid-fast bacillus; +, positive result; −, negative result.
TABLE 2.
Detailed analysis of extrapulmonary TB group by internal studies
| Extrapulmonary TB group | No. of serum samples tested | No. of serum samples with the following TB Screen Test results:
|
Sensitivity (%) | |
|---|---|---|---|---|
| Positive | Negative | |||
| Tuberculous lymphadenitis | 12 | 10 | 2 | 85.7 |
| Bone joint TB | 26 | 23 | 3 | 89.6 |
| Abdominal TB | 18 | 16 | 2 | 90.0 |
| Tuberculous pericarditis | 16 | 13 | 3 | 84.2 |
| Genitourinary TB | 3 | 2 | 1 | 75.0 |
| Pleurisy | 4 | 3 | 1 | 80.0 |
| Total | 79 | 67 | 12 | 86.8 |
TABLE 3.
Detailed analysis of non-TB group by internal studies
| Sample source | No. of samples tested | Outcome | No. of serum samples with the following TB Screen Test result:
|
Specificity (%) | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Indeterminate | ||||
| Drug-treated, clinically negative patients | 60 | Cured of TB | 51 | 9 | 100 | |
| Healthy household contacts | 50 | Clinically positive | 50 | 100 | ||
| BCG-vaccinated children | 15 | Clinically healthy | 15 | 100 | ||
| Hepatitis B virus-positive individuals | 15 | M. tuberculosis negative | 15 | 100 | ||
| Patients with common infections (not TB) | 27 | Clinically negative for TB | 25 | 2 | 100 | |
| Healthy subjects human serum | 344 | Clinically healthy | 6 | 332 | 6 | 98.2 |
| Total | 511 | 6 | 488 | 17 | 98.8 | |
TABLE 4.
Detailed analysis of patients with other common infection by internal studies
| Infection | No. of serum samples tested | No. of serum samples with the following TB Test Screen result:
|
Specificity (%) | ||
|---|---|---|---|---|---|
| Positive | Negative | Indeterminate | |||
| Pneumonia | 16 | 15 | 1 | 100 | |
| Bronchitis or bronchial asthma | 12 | 2 | 9 | 1 | 85.7 |
| Malaria | 22 | 22 | 100 | ||
| Typhoid | 18 | 18 | 100 | ||
| Syphilis | 12 | 12 | 100 | ||
| Gonorrhea | 18 | 18 | 100 | ||
| Rheumatoid arthritis | 16 | 15 | 1 | 100 | |
| Common cold | 14 | 2 | 10 | 2 | 87.5 |
| Pulmonary aspergillosis | 15 | 14 | 1 | 100 | |
| Lung abscess | 13 | 1 | 10 | 2 | 92.8 |
| Total | 156 | 5 | 143 | 8 | 96.8 |
TABLE 5.
Validation of sensitivity and specificity of TB Screen Test by studies by external agencies
| Study and group | No. of samples tested | Clinical history | No. of samples with the following TB Screen Test result:
|
Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Indeterminate | |||||
| Sensitivity study | |||||||
| Pulmonary TB tuberculosis | 204 | Clinically confirmed | 198 | 4 | 2 | 97.1 | |
| Extra-pulmonary TB | 28 | Clinically confirmed | 24 | 4 | 87.5 | ||
| TB-HIV coinfected | 8 | HIV positive and M. tuberculosis positive by smear and culture | 7 | 1 | 88.8 | ||
| Subtotal | 240 | 229 | 8 | 3 | 95.41 | ||
| Specificity study | |||||||
| Leprosy | 28 | Clinically confirmed | 2 | 25 | 1 | 93.3 | |
| HIV and M. tuberculosis positive | 10 | Clinically confirmed | 10 | 100 | |||
| Other common infections | 63 | Clinically confirmed | 62 | 1 | 100 | ||
| Pneumonia | 15 | Clinically confirmed | 15 | 100 | |||
| Bronchitis | 10 | Clinically confirmed | 09 | 1 | 100 | ||
| Malaria | 18 | Clinically confirmed | 18 | 100 | |||
| Typhoid | 12 | Clinically confirmed | 12 | 100 | |||
| Hepatitis | 8 | Clinically confirmed | 8 | 100 | |||
| Rheumatoid arthritis | 6 | Clinically confirmed | 1 | 5 | 85.7 | ||
| BCG vaccinated | 435 | Histopathologically and physiologically confirmed | 8 | 419 | 8 | 94.5 | |
| Subtotal | 605 | 11 | 583 | 11 | 96.36 | ||
An overall sensitivity of 94.0% was obtained with the collection of 604 TB serum samples (Tables 1, 2, and 5). Of these, 27 serum samples showed false-negative results, whereas 11 serum samples gave indeterminate results. An overall specificity of 98.3% was obtained with 1,209 serum samples from non-TB patients and patients with other common infections (Tables 2, 3, and 5). Of these, only 22 samples were found to be false positive, whereas 36 samples gave indeterminate results. However, all samples from both groups with indeterminate results were considered negative. Sera from 15 children recently immunized with BCG were tested for any cross-reactivity by the test. None of these serum samples showed positive results, thereby indicating the suitability of the test for use with BCG-vaccinated populations, such as those in India and other developing countries. Fifteen samples from hepatitis B virus-positive individuals were also evaluated for cross-reactivity; however, no reaction was observed with any of the serum samples from hepatitis B virus-positive individuals tested.
Furthermore, the performance of the TB Screen Test was determined by using in-house as well as coded serum samples from leading hospitals, research institutions, and a pathology center in India: the Hopkins Research Institute, Mumbai; the Department of Chest Medicine, KEM Hospital, Mumbai; Centre JALMA, Central Research Institute of Leprosy, Agra; Department of Pathology Gandhi Medical College (GMC), Bhopal; and Madhav Institute of Technology and Science (MITS), Pathology Centre, Gwalior. The results of these evaluations are furnished in Table 6. All the samples received from these centers were tested in our laboratory to determine the performance of our test kit and in order to see whether the kit performs differentially with samples from different populations. The results presented here suggest that regardless of the variations in the geographical locations of the patients, the kit responded in the same manner with all the samples obtained from different regions of India.
TABLE 6.
Multicenter evaluation results of TB Screen Test
| Center | Total no. of samples tested | No. of samples confirmed to be positive | No. of positive samples with the following TB Screen Test result:
|
Sensitivity (%) | No. of samples confirmed to be negative | No. of negative samples with the following TB Screen Test result:
|
Specificity (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||||
| KEM Hospital, Mumbai | 126 | 83 | 72 | 11 | 88.2 | 43 | 4 | 39 | 91.4 |
| Hopkins Research Institute, Mumbai | 37 | 25 | 21 | 4 | 88.2 | 12 | 4 | 8 | 75.0 |
| Centre JALMA, Central Research Institute of Leprosy, Agara | 60 | 40 | 37 | 3 | 93.0 | 20 | 3 | 17 | 86.9 |
| GMC, Bhopal | 100 | 66 | 59 | 7 | 90.4 | 34 | 7 | 27 | 82.9 |
| MITS, Pathology Centre, Gwalior | 128 | 46 | 44 | 2 | 95.8 | 82 | 9 | 73 | 90.1 |
| Total | 451 | 260 | 233 | 27 | 191 | 27 | 164 | ||
A comparative evaluation of the TB Screen Test was also done by using a commercially available ELISA (KP-90) and the MycoDot (lipoarrabinomannan [LAM]) test with coded sera from patients with clinically confirmed TB received from the research institutions, medical colleges, and pathology center in India mentioned above. A total of 152 samples from patients with confirmed cases of active TB (patients with pulmonary and extrapulmonary TB and patients with HIV-M. tuberculosis coinfection) were separately tested by the TB Screen Test, with the KP-90 kit, and by the MycoDot test (for sensitivity). Of 152 samples tested, 143 were found to be positive by our test, whereas 108 and 117 samples were found to be positive with the KP-90 ELISA kit and by the MycoDot test, respectively. A total of 446 serum samples, including sera from humans (n = 33), BCG-vaccinated children (n = 15), patients with leprosy (n = 60), and patients with other common infections (n = 27), were also incorporated in comparative studies in order to check the specificities. It is evident from the results that the TB Screen Test is highly sensitive and specific compared with the KP-90 ELISA kit and the MycoDot test (Table 7). Furthermore, to check the reproducibility of the test, five different batches were assigned to other investigators while keeping the formulations constant. We as well as other technical investigators tested the same specimens described above and obtained almost similar results (variations, ±2 to 5%).
TABLE 7.
Comparative evaluation of TB Screen Test by internal studiesa
| Serum sample source | No. of serum samples with the indicated result by the following test:
|
Sensitivity (%) of TB Screen Test % | Specificity (%) of TB Screen Test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Results of KP-90 ELISA kit
|
Results of MycoDot LAM Test
|
Results of TB Screen Test
|
||||||
| Positive | Negative | Positive | Negative | Positive | Negative | ||||
| Patients with confirmed cases of TB | 152 | 108 | 44 | 117 | 35 | 143 | 9 | 94.4 | 100 |
| Healthy humans (age, 15-45 yr) | 344 | 13 | 331 | 10 | 334 | 8 | 336 | 97.7 | |
| BCG-vaccinated children (age, 5-12 yr) | 15 | 3 | 12 | 4 | 11 | 2 | 13 | 88.2 | |
| Patients with leprosy (JALMA) | 60 | 14 | 46 | 12 | 48 | 8 | 52 | 88.2 | |
| Patients with common infections | 27 | 4 | 23 | 4 | 23 | 2 | 25 | 93.1 | |
| Total | 598 | 126 | 472 | 147 | 451 | 163 | 435 | 94.4 | 95.7 |
Sensitivity and specificity were calculated by consideration of samples with indeterminate results as negative. The samples with indeterminate results were retested within a month (41).
DISCUSSION
Evidence from various studies strongly suggests that the unique disaccharide on M. leprae, a phenolic glycolipid, is an immunologically specific determinant of M. leprae (5). It was found to be serologically active if the antigenic sites were adequately exposed in aqueous media, either by incorporating it into a liposome in an immunodiffusion test (28) or by using sodium deoxycholate in the coating solution in an ELISA (6). An ELISA for detection of the glycolipid has also been described (46, 47).
Several antigens of M. tuberculosis have been found to be useful for the serodiagnosis of clinical disease (14, 15); among these, some glycolipids have been shown to be immunogenic (20, 27) by ELISA. The difficulty in developing suitable tests has resulted from the fact that M. tuberculosis and M. leprae produce large numbers of immune response-producing proteins, some of which appear to be common to other microorganisms that may or may not be pathogenic. Hence, positive test results produced by known antigens are generally unreliable (false positive), and other tests must therefore be carried out to confirm the presence of TB or leprosy. Recently, M. tuberculosis species-specific trehalose-glycolipid (sulfolipid SL-IV) (27, 31) and serologically active glycolipids extracted from M. bovis BCG (30) have been described. Nonpeptidic antigens from the M. tuberculosis cell wall have been the focus of extensive studies to determine their potential role as protective antigens or serological markers of TB (21).
Traditional methods (smear and culture) are simpler and less expensive than the new molecular biology-based diagnostic tests, such as PCR, which are based on the amplification of nucleic acid. Serological methods seem to be the ideal choice; and thus, many mycobacterial antigens have been evaluated, such as cellular extracts and proteins (1); polysaccharides, DNA, and RNA (14, 15); glycolipids (21, 27); and other biomolecules. In addition to LAM (33) and 2,3,6,6-diacyltrehalose (42), 2,3,6-triacyltrehalose, 2,3,6,6-tetraacyltrehalose-2-sulfate (sulfolipid SL-I), and trehalose-6,6-dimycolate (cord factor) (2, 12, 21) have been used in ELISA techniques.
The clinically isolated strains (26) were purified by column chromatography and were analyzed by ELISA (17). It has been clearly accepted that TB patients produce antibodies against more than one antigen (24), and consequently, a wide spectrum of humoral responses exists in these patients. Thus, use of a combination of specific purified antigens (in multiantigen cocktails) may increase the sensitivity of serodiagnosis (21, 25).
A colloidal gold-based serological assay (immunochromatographic test) was developed by using five purified M. tuberculosis antigens immobilized on a nitrocellulose test strip for the qualitative detection of IgG antibodies (16). Consequently, the mycobacterial cell wall component LAM, a highly immunogenic cell wall-associated glycolipid, has been used (in the MycoDot LAM test) for the detection of antimycobacterial antibodies (33). However, it has not been found to be useful for the diagnosis of TB in sub-Saharan African countries, especially where HIV infection is prevalent (35). Testing of sera for reactions against a combination of antigen 5, antigen 60, and another mycobacterial antigen, KP-90, does not appear to increase the accuracy over testing for reactions against each individual antigen (9). Serological studies have been done with either a mixture of purified extracted glycolipids (13); adsorbed mycobacterial sonicates (32); and more specific mycobacterial antigens, such as antigen 5, antigen 60a, the 30-kDa antigen, the p32 antigen (derived from the purified protein derivative), and LAM (8). Moreover, several glycolipids have been reported to be highly immunogenic in patients with TB and leprosy (18, 19, 37). Sera from some of these patients have been found to be reactive with M. tuberculosis and M. africanum (27), whereas sera from some of them were found to be only weakly reactive (30). Further studies have focused on chemical characterization of the mycobacterial cell wall (2, 11) to obtain species-specific antigens for the detection of antibodies in the sera of TB patients. An enzyme immunoassay in which the glycolipid antigen trehalose-6,6-dimycolate purified from M. tuberculosis H37Rv was used as an antigen for the detection of anti-M. tuberculosis Ig. This glycolipid has been shown to be an effective antigen for serodiagnosis (2, 12, 21). The tuberculin skin test (Mantoux test), based on the delayed-type hypersensitivity phenomenon by using purified protein derivative, has also been developed. However, this test cannot differentiate between active and inactive M. tuberculosis infections and has been found to be neither a sensitive nor a specific method, particularly with sera from BCG-vaccinated immunized individuals (25). Consequently, the BACTEC 460 system, a radiometric system considered to be the “gold standard,” was developed. The BACTEC 460 system is based on the detection of the CO2 liberated by bacteria during the metabolism and decarboxylation of 14C. However, this is a very costly and time-consuming method and has the accompanying problem of the need for the disposal of radioactive waste, which therefore requires technical expertise along with established laboratory facilities.
Recently, with the advent of recombinant DNA technology, scientists and researchers around the world have been trying to develop kits based on PCR. However, the application of PCR-based diagnosis is beset with the need for expertise and is also very costly (14). Many workers have attempted to isolate the species-specific antigen for use in diagnostic tests, but this task has proved to be very difficult for two reasons; first, specific antigenic determinants often occur on the same protein molecule as the shared antigen, therefore making it impossible for purification even by affinity chromatography based on binding with a specific antibody; second, given determinants may be present on a range of molecules with different physiochemical properties. Thus, preparative techniques based on such differences (gel filtration and ion-exchange chromatography) have not proved very useful. Furthermore, due to antigenic diversity in patients with mycobacterial disease, especially in those with TB, no single antigen can cover all positive cases.
Developing countries require a cost-effective test for the serodiagnosis of TB which can be easily incorporated into the clinical laboratory routine as well as easily used in remote areas. We have previously developed a shotgun gene library-based ELISA (recombinant DNA technology) (4), the current laboratory method for the rapid genomic characterization of M. avium-M. intracellulare complex (39), and an antiglycolipid antibody-based liposome agglutination test (Rapid TB/M card test) for the diagnosis of TB (40). These tests can be used as supplemental tests for the mass screening of M. tuberculosis-infected individuals in developing countries without the need for laboratory facilities with modern instrumentation. However, the TB Screen Test would be a more advantageous tool for the clinical confirmation of TB in specimens from those individuals with active M. tuberculosis infections.
A number of serum samples from patients with common diseases, such as bacterial pneumonia, viral pneumonia, nonspecific bronchitis, lung cancer, pleural cancer, the common cold, leprosy, malaria, typhoid, and hepatitis B, were included to study the specificity of the TB Screen Test. Confirmation of the diagnosis of TB for the sensitivity study was done on the basis of a combination of acid-fast bacillus-positive, smear-positive, and culture-positive results; the findings on chest X ray; and the clinical symptoms. The liposomal agglutination TB Screen Test is a rapid in vitro test for the diagnosis and screening of subjects with TB, in which a suspension of mycobacterial glycolipids (cocktail of purified antigens) interchelated into the bilayer membrane of the blue liposome reacts with antibodies in the specimen. This results in agglutination of the liposome, which appears as blue clumps against the white background of the test card (Fig. 4 and 5). The reaction is depicted diagrammatically in Fig. 6 (schematic representation of principal of TB Screen Test). A clear dark blue agglutination can be observed for positive serum samples. A positive test result can be given a gradation ranging from 1+ to 4+, depending on the agglutination strength of M. tuberculosis-positive serum. A sample with a negative result does not show any agglutination (Fig. 4 and 5). It is recommended that sera with peripheral drying be retested after 15 to 30 days. This test, the TB Screen Test, is designed in such a way as to detect antiglycolipid antibodies in patients with active disease with high degrees of sensitivity and specificity.
The data obtained in this study indicate that the TB Screen Test can be used for the routine diagnosis of pulmonary and extrapulmonary TB and can clearly differentiate healthy subjects and BCG-vaccinated individuals from those with active TB. However, the test is a rapid, single-step, agglutination-based assay for the detection of antibodies against M. tuberculosis in freshly procured human serum or human serum that has been stored at −20°C within 4 min of the time of collection. The overall sensitivity and specificity have been found to be 98.5 and 85.5%, respectively.
Because of the incorporation of multiple antigens into the assay, it should be considered the world's first liposome-based rapid assay for the diagnosis of TB. However, these results clearly pertain to the development of a specific test for TB, which has the desired sensitivity and specificity compared with those of other commercially available tests (Table 7). The clear signal for a positive result by the test, the economy of the test, the suitability for the test to be performed even in remote areas where no laboratory facilities with modern instrumentation are available, and its handiness make it useful as a rapid assay for the diagnosis of TB.
Acknowledgments
We thank V. M. Katoch, Director, JALMA Research Institute for Leprosy, Agra, India, for providing M. tuberculosis H37RV. We gratefully acknowledge V. A. Ramnani, GMC, Bhopal, India; and we thank Shweta Sahai, MITS, Gwalior, India, for the evaluation studies conducted in her facilities.
REFERENCES
- 1.Arikan, S., S. Tuncer, D. Us, S. Unal, and S. Ustacelebi. 1998. Anti-Kp90 IgA antibodies in the diagnosis of active tuberculosis. Chest 114:1253-1257. [DOI] [PubMed] [Google Scholar]
- 2.Baer, H. H., and X. Wu. 1993. Synthesis of α, α-trehalose 2,3- and 2,3′-diesters with palmitic and stearic acid: potential immunoreactants for the serodiagnosis of tuberculosis. Carbohydr. Res. 238:215-230. [DOI] [PubMed] [Google Scholar]
- 3.Bangham, A. D., M. M. Standish, and J. C. Watkins. 1965. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 13:238-252. [DOI] [PubMed] [Google Scholar]
- 4.Bisen, P. S., S. K. Garg, R. P. Tiwari, P. R. Tagore, D. Tiwari, R. Chandra, R. Karnik, N. Thaker, N. Desai, P. K. Ghosh, M. Fraziano, and V. Colizzi. 2003. Analysis of shotgun expression library of Mycobacterium tuberculosis genome for immunodominant polypeptide: potential use in serodiagnosis. Clin. Diagn. Lab. Immunol. 6:1051-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brett, S. J., S. N. Payne, P. Draper, and R. Gigg. 1984. Analysis of the major antigenic determinants of the characteristic phenolic glycolipid from Mycobacterium leprae. Clin. Exp. Immunol. 56:89-96. [PMC free article] [PubMed] [Google Scholar]
- 6.Brett, S. J., P. Draper, S. N. Payne, and R. J. Rees. 1983. Serological activity of a characteristic phenol glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52:271-279. [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, J., T. Fujiwara, P. Brannan, M. McNeil, S. J. Turco, J. C. Sibille, M. Snapper, P. Aisen, and B. R. Bloom. 1989. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc. Natl. Acad. Sci. USA 86:2453-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, S. L., Z. Reggiardo, T. M. Danial, D. J. Girling, and D. A. Mitchison. 1990. Serodiagnosis of tuberculosis using an ELISA with antigen 5 and a hemagglutination assay with glycolipid antigens. Am. Rev. Respir. Dis. 142:385-390. [DOI] [PubMed] [Google Scholar]
- 9.Chiang, I. H., J. Suo, K. J. Bai, T. P. Lin, K. T. Luh, C. J. Yu, and P. C. Yang. 1997. Serodiagnosis of tuberculosis. A study comparing three specific mycobacterial antigens. Am. J. Respir. Crit. Care Med. 156:906-911. [DOI] [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Gamier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. I. Barry, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osbome, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Daffe, M., F. Papa, A. Laszlo, and H. L. David. 1989. Glycolipids of recent isolates of Mycobacterium tuberculosis: chemical characterization and immunoreactivity. J. Gen. Microbiol. 135:2759-2766. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto, K., S. Oka, N. Fujiwara, T. Okamoto, Y. Okuda, R. Maekura, T. Kuroki, and I. Yano. 1998. Rapid serodiagnosis of Mycobacterium avium-intracellulare complex infection by ELISA with cord factor (trehalose 6,6-dimycolate), and serotyping using the glycopeptidolipid antigen. Microbiol. Immunol. 42:689-696. [DOI] [PubMed] [Google Scholar]
- 13.Escamilla, L., R. Mancilla, W. Glender, and L. M. Lopez-Marin. 1996. Mycobacterium fortuitum glycolipids for the serodiagnosis of pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 154:1864-1867. [DOI] [PubMed] [Google Scholar]
- 14.Garg, S. K., R. P. Tiwari, D. Tiwari, R. Singh, D. Malhotra, V. K. Ramnani, G. B. K. S. Prasad, R. Chandra, M. Fraziano, V. Colizzi, and P. S. Bisen. 2003. Diagnosis of tuberculosis: available technologies, limitations and possibilities. J. Clin. Lab Anal. 16:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg, S. K., M. B. Santucci, S. Fouad, C. Saltini, P. S. Bisen, V. Colizzi, and M. Fraziano. 2004. Tuberculosis therapeutics: past achievements, present road-blocks and future perspectives. Lett. Drug Design Discovery 1:35-44. [Google Scholar]
- 16.Gounder, C., F. C. De Queiroz Mello, M. B. Conde, W. R. Bishai, A. L. Kritski, R. E. Chaisson, and S. E. Dorman. 2002. Field evaluation of a rapid immunochromatographic test for tuberculosis. J. Clin. Microbiol. 40:1989-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hines, M. E., II, J. M. Jaynes, S. A. Barker, J. C. Newton, F. M. Enright, and T. G. Snider III. 1993. Isolation and partial characterization of glycolipid fractions from Mycobacterium avium serovar 2 (Mycobacterium paratuberculosis 18) that inhibit activated macrophages. Infect. Immun. 61:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter, S. W., T. Fujiwara, and P. J. Brennan. 1982. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257:15072-15078. [PubMed] [Google Scholar]
- 19.Hunter, S. W., H. Gaylord, and P. J. Brennan. 1986. Structure and antigenicity of phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 261:12345-12351. [PubMed] [Google Scholar]
- 20.Julian, E., M. Cama, P. Martinez, and M. Luquin. 2001. An ELISA for five glycolipids from the cell wall of Mycobacterium tuberculosis: Tween-20 interference in the assay. J. Immunol. Methods 251:21-30. [DOI] [PubMed] [Google Scholar]
- 21.Julian, E., L. Matas, A. Perez, J. Alcaide, M. A. Laneelle, and M. Luquin. 2002. Serodiagnosis of tuberculosis: comparison of immunoglobulin A (IgA) responses to sulfolipid I with IgG and IgM responses to 2,3-diacyltrehalose,2,3,6-Triacyltrehalose, and Cord Factor antigens. J. Clin. Microbiol. 40:3782-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepage, M. 1964. Isolation and characterization of an etherified form of styryl glucoside. J. Lipid Res. 53:587-592. [PubMed] [Google Scholar]
- 23.Lienhardt, C., J. Rowley, K. Manneh, G. Lahai, D. Needham, P. Milligan, and K. P. McAdam. 2001. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int. J. Tuberc. Lung Dis. 5:233-239. [PubMed] [Google Scholar]
- 24.Lyashchenko, K., R. Colangeli, M. Houde, H. Al Jahdali, D. Menzies, and M. L. Gennaro. 1998. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyashchenko, K., M. Manca, R. Colangeli, A. Heijbel, A. Williams, and M. L. Gennaro. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz, M., M. Luquin, M. Garcia-Barcelo, E. Julian, V. Ausina, and M. A. Laneelle. 1997. Distribution of surface-exposed antigenic glycolipids in recent clinical isolates of Mycobacterium tuberculosis. Res. Microbiol. 148:405-412. [DOI] [PubMed] [Google Scholar]
- 27.Papa, F., P. Cruaud, and H. L. David. 1989. Antigenicity and specificity of selected glycolipid fractions from Mycobacterium tuberculosis. Res. Microbiol. 140:569-578. [DOI] [PubMed] [Google Scholar]
- 28.Payne, S. N., P. Draper, and R. J. Rees. 1982. Serological activity of purified glycolipid from Mycobacterium leprae. Int. J. Lepr. 50:220-221. [PubMed] [Google Scholar]
- 29.Reggiardo, Z., and G. Middlebrook. 1974. Serologically active glycolipid families from Mycobacterium bovis BCG. I. Extraction, purification and immunologic studies. Am. J. Epidemiol. 100:469-476. [DOI] [PubMed] [Google Scholar]
- 30.Reggiardo, Z., and G. Middlebrook. 1974. Serologically active glycolipid families from Mycobacterium bovis BCG. II. Serologic studies on human sera. Am. J. Epidemiol. 100:477-486. [DOI] [PubMed] [Google Scholar]
- 31.Ridell, M., G. Wallerstrom, D. E. Minnikin, R. C. Bolton, and M. A. Magnusson. 1992. A comparative serological study of antigenic glycolipids from Mycobacterium tuberculosis. Tubercle Lung Dis. 73:101-105. [DOI] [PubMed] [Google Scholar]
- 32.Rosen, E. U. 1990. The diagnostic value of an enzyme-linked immune sorbent assay using absorbed mycobacterial sonicates in children. Tubercle 71:127-130. [DOI] [PubMed] [Google Scholar]
- 33.Sada, E., P. J. Brennan, T. Herrera, and M. Torres. 1990. Evaluation of lipoarrabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siakotos, A. N. 1965. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J. Am. Oil Chem. Soc. 42:913-919. [DOI] [PubMed] [Google Scholar]
- 35.Somi, G. R., R. J. O'Brien, G. S. Mfinanga, and Y. A. Ipuge. 1999. Evaluation of the Mycodot test in patients with suspected tuberculosis in a field setting in Tanzania. Int. J. Tuberc. Lung Dis. 3:231-238. [PubMed] [Google Scholar]
- 36.Sorice, M., T. Griggi, A. Circella, T. Garofalo, F. d'Agostino, V. Pittoni, G. M. Pontieri, L. Lenti, and G. Valesini. 1994. Detection of antiphospholipid antibodies by immunostaining on thin layer chromatography plates. J. Immunol. Methods 173:49-54. [DOI] [PubMed] [Google Scholar]
- 37.Sugden, E. A., B. S. Samagh, D. R. Bundle, and J. R. Duncan. 1987. Lipoarabinomannan and lipid-free arabinomannan antigens of Mycobacterium paratuberculosis. Infect. Immun. 55:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sut, A., S. Sirugue, S. Sixou, F. Lakhdar-Ghazal, J. F. Tocanne, and G. Laneelle. 1990. Mycobacteria glycolipids as potential pathogenicity effectors: alteration of model and natural membranes. Biochemistry 29:8498-8502. [DOI] [PubMed] [Google Scholar]
- 39.Tiwari, R. P., D. Tiwari, R. Chandra, and P. S. Bisen. Submitted for publication.
- 40.Tiwari, R. P., S. K. Garg, D. Tiwari, M. Fraziano, V. Collizzi, R. Chandra, and P. S. Bisen. Submitted for publication.
- 41.Toman, K. 1981. Sensitivity, specificity and predictive value of diagnostic tests. Bull. Int. Tuberc. 56:19-30. [PubMed] [Google Scholar]
- 42.Tortola, M. T., M. A. Laneelle, and N. Martin-Casabona. 1996. Comparison of two 2,3-diacyl trehalose antigens from Mycobacterium tuberculosis and Mycobacterium fortuitum for serology in tuberculosis patients. Clin. Diagn. Lab. Immunol. 3:563-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Work, T. S., and E. Work. 1978. Laboratory techniques in biochemistry and molecular biology. Amsterdam. Elsevier/North-Holland.
- 44.World Health Organization. 2003. Global tuberculosis control: surveillance, planning, financing. WHO report 2003 WHO/CDS/TB/2003.316. World Health Organization, Geneva, Switzerland.
- 45.World Health Organization 2002. World Health Organization strategic framework to decrease the burden of TB/HIV. World Health Organization, Geneva, Switzerland.
- 46.Yanagihara, D. L., V. L. Barr, C. V. Knisley, A. Y. Tsang, J. K. McClatchy, and P. J. Brennan. 1985. Enzyme-linked immunosorbent assay of glycolipid antigens for identification of mycobacteria. J. Clin. Microbiol. 21:569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young, D. B., and T. M. Buchanan. 1983. A serological test for leprosy with a glycolipid specific for M. leprae. Science 221:1057-1059. [DOI] [PubMed] [Google Scholar]