Abstract
The causal roles of muscle weakness in cardiometabolic diseases and osteoporosis remain elusive. This two-sample Mendelian randomization (MR) study aims to explore the causal roles of muscle weakness in the risk of cardiometabolic diseases and osteoporosis. 15 single nucleotide polymorphisms (SNPs, P < 5 × 10−8) associated with muscle weakness were used as instrumental variables. Genetic predisposition to muscle weakness led to increased risk of coronary artery disease (inverse variance weighted [IVW] analysis, beta-estimate: 0.095, 95% confidence interval [CI]: 0.023 to 0.166, standard error [SE]:0.036, P-value = 0.009) and reduced risk of heart failure (weight median analysis, beta-estimate: − 0.137, 95% CI − 0.264 to − 0.009, SE:0.065, P-value = 0.036). In addition, muscle weakness may reduce the estimated bone mineral density (eBMD, weight median analysis, beta-estimate: − 0.059, 95% CI − 0.110 to − 0.008, SE:0.026, P-value = 0.023). We found no MR associations between muscle weakness and atrial fibrillation, type 2 diabetes or fracture. This study provides robust evidence that muscle weakness is causally associated with the incidence of coronary artery disease and heart failure, which may provide new insight to prevent and treat these two cardiometabolic diseases.
Subject terms: Diseases, Endocrinology, Medical research
Introduction
Muscle weakness commonly occurs as the advancing age and it is a fundamental component of frailty and sarcopenia1–3. Compared to individuals in twenties, population with over 70 years are estimated to suffer from up to 20% lost muscle mass4. Loss of muscle mass (sarcopenia) is closely associated with muscle weakness which may affect health outcomes5,6. Patients with muscle weakness commonly have some difficulties in daily activity and low muscle strength as measured by hand grip strength, which has become a predictive factor of morbidity and mortality4,7. Muscle weakness is heritable and can be used for genetic studies8.
Several observational studies reported that muscle weakness had some association with the incidence of cardiometabolic diseases and osteoporosis, but these results are conflicting9–15. Potential confounding factors and reverse causality in these studies may affect the association between muscle weakness and cardiometabolic diseases/osteoporosis. Cardiometabolic diseases and osteoporosis are also highly polygenic traits based on the results of genome-wide association studies (GWASs)16–22.
Mendelian randomization (MR) study is widely used to establish the causal relationship between exposure phenotype and outcome phenotype, with the advantages of preventing reverse causation and potential confounding factors23–27. Furthermore, the two-sample MR study is able to increase the scope and statistical power of MR25,28–31. Due to the high heritability of muscle weakness, cardiometabolic diseases and osteoporosis, this two-sample MR study aims to explore the causal influence of muscle weakness on the incidence of cardiometabolic diseases and osteoporosis.
Methods
Genetic instrument for muscle weakness
The largest available GWAS meta-analysis included 22 independent cohorts with maximum hand grip strength recorded (i.e. the UK Biobank, the US Health and Retirement Study, the Framingham Heart Study, and others) and total 256,523 individuals of European descent aged 60 years or older. Among them, 46,596 participants was diagnosed with muscle weakness based on hand grip strength and EWGSOP definition: grip strength < 30 kg for male individuals and < 20 kg for female individuals32.
Initially, 15 single nucleotide polymorphisms (SNPs) showed robust association with muscle weakness (P < 5 × 10−8). Linkage disequilibrium (LD) between selected SNPs was calculated using European samples from the 1000 Genomes project. No SNPs were excluded due to high LD (r2 ≥ 0.001). Finally, 15 SNPs were used as instrumental variables (Supplementary Table 1). The proxy SNPs in linkage disequilibrium (LD, r2 > 0.9) were used if original SNPs were unavailable in the outcome database. Thus, rs6488725 was used as the proxy for rs34464763 among all outcomes (Supplementary Table 2).
Outcome data sources
The genetic associations of each outcome from GWASs were presented in Table 1. Briefly, we included the GWAS summary data of cardiometabolic diseases including coronary artery disease (547,261 individuals) from UK Biobank and CARDIoGRAMplusC4D33, heart failure (977,323 individuals) from UK Biobank34, atrial fibrillation (587,446 individuals) from one large meta-analysis35 and type 2 diabetes (898,130 individuals) from DIAGRAM36. In terms of osteoporosis and fracture, the outcome measures included bone mineral density (BMD) as estimated by heel quantitative ultrasound (eBMD) and fracture among 426,824 people. Fracture cases were defined as any fracture apart from the fracture of skull, face, hands, feet, pathological fractures due to malignancy, atypical femoral fractures, periprosthetic and healed fracture37. Most GWASs were adjusted for sex, body mass index (BMI) and genetic principal components. All participants were all from European descent except for those with atrial fibrillation from predominant European descent (mixed descents). Supplementary Table 2 showed the summary statistics for the SNPs related to muscle weakness and corresponding statistics of outcomes.
Table 1.
Details of studies and datasets used for analyses.
| Traits | Samples size | Population | Consortium or cohort study (link URL) | |
|---|---|---|---|---|
| Exposure | Muscle weakness | 2,56,523 | European | Meta-analysis of 22 cohorts |
| Cardiometabolic diseases | Coronary artery disease | 5,47,261 | European | UK Biobank and CARDIoGRAMplusC4D (https://cvd.hugeamp.org/) |
| Heart failure | 9,77,323 | European | UK Biobank (http://www.broadcvdi.org/) | |
| Atrial fibrillation | 5,87,446 | Mixed | Meta analysis of more than 50 studies (http://www.broadcvdi.org/) | |
| Type 2 diabetes | 8,98,130 | European | DIAGRAM (http://diagram-consortium.org) | |
| Osteoporosis | eBMD | 4,26,824 | European | GEFOS (http://www.gefos.org) |
| Fracutre | 4,26,824 | European |
Statistical analyses
To determine causal influence of muscle weakness on each outcome, we conducted the inverse variance weighted (IVW) analysis because more than 2 SNPs were available. IVW method used a meta-analysis approach to combine Wald estimates for each SNP in order to get the overall estimates of the effect of muscle weakness on each outcome23. The weighted median and MR-Egger regression methods were also applied to estimate the effects. Cochrane’s Q-statistic was used to assess the heterogeneity of SNP effects and P < 0.05 indicated significant heterogeneity38. MR pleiotropy residual sum and outlier test (MR-PRESSO) aimed to assess the presence of pleiotropy and the effect estimates were recalculated after outlying SNPs were excluded39.
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved and the ethical approval for each study can be found in the original publications (including informed consent from each participant). P < 0.05 indicated statistical difference. All of these analyses were conducted in R V.4.0.4 by using the R packages of ‘MendelianRandomization’40, ‘TwoSampleMR’41 and ‘MR-PRESSO’42.
Ethical approval
The ethical approval for each study included in this investigation can be found in the original publications.
Results
Cardiometabolic diseases
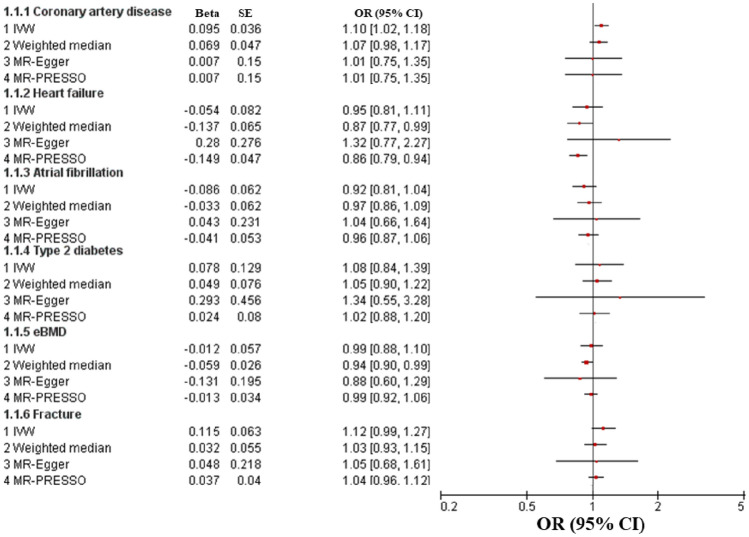
We evaluated the causal effect of muscle weakness on coronary artery disease, heart failure, atrial fibrillation and type 2 diabetes in this MR analysis (Table 2). IVW analysis demonstrated that genetically muscle weakness played a significant causal role in the increased risk of coronary artery disease (beta-estimate: 0.095, 95% CI 0.023 to 0.166, SE:0.036, P-value = 0.009), but it was not supported by the weighted-median analysis (beta-estimate: 0.069, 95% CI − 0.023 to 0.161, SE:0.047, P-value = 0.141, Fig. 1).
Table 2.
Mendelian randomization estimates of muscle weakness on outcomes.
| Variables | IVW | Weighted median | MR-Egger | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | 95% CI | P-value | Q value | I2 | Heterogeneity P value | Estimate | SE | 95% CI | P-value | Estimate | SE | 95% CI | P-value | Intercept | SE | 95% CI | Pleiotropy P value | |
| Cardiometabolic disease | |||||||||||||||||||
| Coronary artery disease | 0.095 | 0.036 | 0.023, 0.166 | 0.009 | 15.716 | 17.30% | 0.265 | 0.069 | 0.047 | − 0.023, 0.161 | 0.141 | 0.007 | 0.150 | − 0.287, 0.300 | 0.965 | 0.005 | 0.008 | − 0.011, 0.020 | 0.545 |
| Heart failure | − 0.054 | 0.082 | − 0.214, 0.106 | 0.506 | 47.308 | 70.40% | 0.000 | − 0.137 | 0.065 | − 0.264, − 0.009 | 0.036 | 0.280 | 0.276 | − 0.261, 0.821 | 0.310 | − 0.019 | 0.015 | − 0.048, 0.010 | 0.206 |
| Atrial fibrillation | − 0.086 | 0.062 | − 0.207, 0.035 | 0.162 | 30.186 | 53.60% | 0.007 | − 0.033 | 0.062 | − 0.154, 0.088 | 0.595 | 0.043 | 0.231 | − 0.411, 0.496 | 0.853 | − 0.007 | 0.012 | − 0.031, 0.017 | 0.562 |
| Type 2 diabetes | 0.078 | 0.129 | − 0.175, 0.331 | 0.547 | 130.963 | 89.30% | 0.000 | 0.049 | 0.076 | − 0.101, 0.198 | 0.523 | 0.293 | 0.456 | −0. 600, 1.186 | 0.520 | − 0.012 | 0.025 | −0. 060, 0.036 | 0.621 |
| Osteoporosis | |||||||||||||||||||
| eBMD | − 0.012 | 0.057 | − 0.123, 0.099 | 0.837 | 435.276 | 96.80% | 0.000 | −0. 059 | 0.026 | − 0.110, − 0.008 | 0.023 | − 0.131 | 0.195 | − 0.513, 0.251 | 0.502 | 0.007 | 0.011 | − 0.014, 0.028 | 0.522 |
| Fracture | 0.115 | 0.063 | −0. 008, 0.238 | 0.066 | 42.444 | 67.00% | 0.000 | 0.032 | 0.055 | − 0.075, 0.139 | 0.561 | 0.048 | 0.218 | − 0.380, 0.476 | 0.827 | 0.004 | 0.012 | −0. 020, 0.027 | 0.745 |
Figure 1.
OR (95% CI) for causal association between muscle weakness and each outcome through multiple analyses.
According to weighted-median analysis, muscle weakness showed substantially causal effect on the reduced incidence of heart failure (beta-estimate: − 0.137, 95% CI − 0.264 to − 0.009, SE:0.065, P-value = 0.036), but it was not confirmed in the IVW analysis (beta-estimate: − 0.054, 95% CI − 0.214 to 0.106, SE:0.082, P-value = 0.506, Fig. 1). In addition, IVW analyses found that muscle weakness demonstrated no remarkable MR association with atrial fibrillation (beta-estimate: − 0.086, 95% CI − 0.207 to 0.035, SE:0.062, P-value = 0.162) or type 2 diabetes (beta-estimate: 0.078, 95% CI − 0.175 to 0.331, SE:0.129, P-value = 0.547), which were also confirmed by weighted-median analyses (Fig. 1).
Osteoporosis
This MR analysis also included outcome measures of eBMD and fracture (Table 2). According to weighted-median analysis, muscle weakness was casually associated with decreased eBMD (beta-estimate: − 0.059, 95% CI − 0.110 to − 0.008, SE:0.026, P-value = 0.023), but it was not supported by the IVW analysis (beta-estimate: − 0.012, 95% CI − 0.123 to 0.099, SE:0.057, P-value = 0.827, Fig. 1). Muscle weakness revealed no causal influence on fracture by IVW analysis (beta-estimate: 0.115, 95% CI − 0.008 to 0.238, SE:0.063, P-value = 0.066) or weighted-median analysis (beta-estimate: 0.032, 95% CI − 0.075 to 0.139, SE:0.055, P-value = 0.561, Fig. 1).
Evaluation of assumptions and sensitivity analyses
Little evidence of directional pleiotropy was found for all models (MR-Egger intercept P-value > 0.05, Table 2). There was significant heterogeneity for heart failure, atrial fibrillation, type 2 diabetes, eBMD and fracture. Thus, among the 15 SNP instrumental variables associated with muscle weakness, MR-PRESSO method identified 2 outliers (rs13107325, rs10952289) for heart failure, one outlier (rs143384) for atrial fibrillation, four outliers (rs7624084, rs34415150, rs10952289, rs62102286) for type 2 diabetes, 11 outliers (rs12140813, rs958685, rs7624084, rs13107325, rs34415150, rs10952289, rs11236213, rs34464763, rs3118903, rs8061064, rs62102286) for eBMD and 2 outliers (rs10952289, rs34464763) fracture (Table 3).
Table 3.
Mendelian randomization estimates between muscle weakness and outcomes after excluding outliers detected by MR-PRESSO.
| Outcomes | Estimate | SE | 95% CI | P-value |
|---|---|---|---|---|
| Heart failure excluding 2 outliers (rs13107325, rs10952289) | − 0.149 | 0.047 | −0. 241, −0. 056 | 0.002 |
| Atrial fibrillation excluding one outlier (rs143384) | − 0.041 | 0.053 | − 0.144, 0.063 | 0.441 |
| Type 2 diabetes excluding four outliers (rs7624084, rs34415150, rs10952289, rs62102286) | 0.024 | 0.080 | − 0.131, 0.180 | 0.759 |
| eBMD excluding 11 outliers (rs12140813, rs958685, rs7624084, rs13107325, rs34415150, rs10952289, rs11236213, rs34464763, rs3118903, rs8061064, rs62102286) | − 0.013 | 0.034 | − 0.079, 0.053 | 0.704 |
| Fracture excluding 2 outliers (rs10952289, rs34464763) | 0.037 | 0.040 | − 0.042, 0.117 | 0.355 |
After excluding these outlying SNP variants, these remarkable MR associations were confirmed between muscle weakness and increased risk of coronary artery disease (Fig. 1 and Table 3). In addition, muscle weakness was confirmed to have a causal effect on low risk of heart failure (beta-estimate: − 0.149, 95% CI − 0.241 to − 0.056, SE:0.047, P-value = 0.002, Fig. 1 and Table 3). The MR association between muscle weakness with other outcomes were not changed after excluding the outlying SNP variants (Table 3).
Discussion
Our two-sample MR study found the robustly causal effect of muscle weakness on increased risk of coronary artery disease and decreased risk of heart failure, and these strong MR associations were confirmed by the sensitivity analyses. These positive findings indicated that the regulatory mechanisms of muscle weakness may provide new insight to prevent and treat coronary artery disease and heart failure. In addition, muscle weakness may have a causal role in reduced eBMD. We found no causal effect of muscle weakness on atrial fibrillation, type 2 diabetes or fracture.
Several observational studies and meta-analysis explored the association between muscle results and cardiometabolic diseases, but no conclusive results were found9,13,43. One meta-analysis revealed that handgrip strength was an independent predictor of cardiometabolic diseases in community-dwelling populations, but this this association was not significant after adjusting for baseline risk factors9,43. One recent MR analysis found no causality in the association between handgrip strength (European population) and coronary artery disease (mixed population). The large-scale genetic discovery analysis identified 16 loci associated with grip strength (P < 5 × 10−8) among 195,180 individuals as instrumental variables10, and that MR study included GWAS summary data related to coronary heart disease among 184,305 individuals44.
Our large-scale MR study was performed in larger populations including 256,523 individuals of European descent for muscle weakness and 547,261 individuals of European descent for coronary artery disease. Totally, 15 loci associated with grip strength (P < 5 × 10−8) were used as instrumental variables. The results provided the robust evidence for the causal association between muscle weakness and increased risk of coronary artery disease, which was confirmed by multiple sensitivity analyses. Muscle weakness and low muscle mass reduces total energy expenditure, which may result in high fat mass. Accumulated body fat mass triggers chronic inflammation, and is thought to be a risk factor for the development and progression of coronary artery disease45–47.
One leading cause of heart failure is coronary artery disease, but heart failure can be also caused by arrhythmias, hypertension, type 2 diabetes mellitus, obesity, and lifestyle factors (such as smoking). A large-scale observational study found that higher hand grip strength was independently associated with lower incidence of heart failure48. On the contrary, our MR study revealed that muscle weakness was causally associated with lower incidence of heart failure, which was confirm by the IVW analysis after excluding the outlying SNPs (beta-estimate: − 0.149, 95% CI − 0.241 to − 0.056, SE:0.047, P-value = 0.002, Fig. 1 and Table 3). This positive finding was very interesting, and may be attributed by the atrophy of the muscle fibers and reduced requirement of cardiac output due to low muscle mass49.
Patients with osteoporosis typically have the features of low bone mass, BMD and bone strength, which can increase the risk of fracture50–54. Several observational studies revealed the significant correlation between low grip strength and low BMD of the bones adjacent to the muscles related to grip55–57. In 1,168 menopausal women, Osei-Hyiaman et al. found the significant relationship between grip strength and BMD of metacarpal index55. Hasegawa et al. revealed that BMD of the distal radius was more associated with hand grip strength than with cross-sectional muscle area57. In contrast, Zimmermann et al. documented that hand grip strength in postmenopausal women showed no impact on vertebral BMD, but only affected femur BMD58, while Foley et al. documented no correlation between hand grip strength and femoral BMD59.
Considering these insistent results, our MR analyses revealed that muscle weakness may have a causal role in reduced eBMD. It is postulated that muscle contraction force provides a mechanical stress on the bones, which is accepted as an important osteogenic stimulus. There is bi-directional bone-muscle crosstalk, which is probably mediated by cytokines, osteokines, myokines, and other growth factors60. In addition, low BMD associated with muscle weakness may be associated with systemic inflammation and oxidative stress61,62.
Our results demonstrated that genetically muscle weakness was unlikely to be causally associated with atrial fibrillation, type 2 diabetes or fracture. The potential causal effect of muscle weakness to reduce eBMD was not translated to affect the risk of fracture. This two-sample MR study aims to investigate the causal effect of muscle weakness on the risk of cardiometabolic diseases and osteoporosis, and has the advantage of preventing reverse causation and confounding factors. The intercepts for the MR-Egger analysis suggest no directional pleiotropy for all outcomes. However, several limitations should be taken into consideration. Firstly, all the included participants are of predominantly European, and we can not directly apply our findings for other populations. Secondly, GWAS summary statistics can not be used to conduct MR analysis based on different age stratums. Thirdly, the contribution of muscle weakness to low eBMD is not translated to increased incidence of fracture, but the detail mechanisms are unclear.
Conclusion
This two-sample MR study provides strong evidence to confirm that muscle weakness is a significantly causal factor for increased risk of coronary artery disease and reduced risk of heart failure, and the related mechanisms may help prevent and treat these two diseases.
Supplementary Information
Acknowledgements
The authors acknowledged the GEnetic Factors for OSteoporosis Consortium, the UK Biobank and Breast Cancer Association Consortium for contributing the data used in this work.
Author contributions
X.Q.M., B.H., M.Z.Z., Y.Z. and X.J.C. conducted literature search, data collection and statistical analysis. X.Q.M., B.H., Y.S.O. and X.J.C. conducted study design, data interpretation and manuscript preparation.
Funding
This study was funded by Medical Research Project of Luzhou-Southwest Medical University (2019LZXNYDJ37), Scientific Research Cultivation Project of The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University (2022-CXTD-08), China Postdoctoral Science Foundation (2022M720602), Chongqing Special Postdoctoral Science Foundation (XmT2022072), Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0836).
Data availability
Data supporting the findings of this study were available within the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bin He, Email: binheing@163.com.
Xiaojun Chen, Email: chenxj2012@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-46837-y.
References
- 1.Owen AM, Patel SP, Smith JD, Balasuriya BK, Mori SF, Hawk GS, Stromberg AJ, Kuriyama N, Kaneki M, Rabchevsky AG, Butterfield TA, Esser KA, Peterson CA, Starr ME, Saito H. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. Elife. 2019 doi: 10.7554/eLife.49920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28(5):495–503. doi: 10.1016/j.nut.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manini TM, Clark BC. Dynapenia and aging: An update. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 6.Cawthon PM, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, Magaziner JM, Newman AB, Brown T, Kiel DP, Cummings SR, Shardell M, Guralnik JM, Woodhouse LJ, Pahor M, Binder E, D'Agostino RB, Quian-Li X, Orwoll E, Landi F, Orwig D, Schaap L, Latham NK, Hirani V, Kwok T, Pereira SL, Rooks D, Kashiwa M, Torres-Gonzalez M, Menetski JP, Correa-De-Araujo R, Bhasin S. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: Proceedings of the sarcopenia definition and outcomes consortium conference. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020;75(7):1317–1323. doi: 10.1093/gerona/glz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Bautmans I, Bertière MC, Brandi ML, Al-Daghri NM, Burlet N, Cavalier E, Cerreta F, Cherubini A, Fielding R, Gielen E, Landi F, Petermans J, Reginster JY, Visser M, Kanis J, Cooper C. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016;16(1):170. doi: 10.1186/s12877-016-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K. Hand grip strength: A phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet. Epidemiol. 2002;23(2):110–122. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Wang W, Liu T, Zhang D. Association of grip strength with risk of all-cause mortality, cardiovascular diseases, and cancer in community-dwelling populations: A meta-analysis of prospective cohort studies. J. Am. Med. Dir. Assoc. 2017;18(6):551.e17–551.e35. doi: 10.1016/j.jamda.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Willems SM, Wright DJ, Day FR, Trajanoska K, Joshi PK, Morris JA, Matteini AM, Garton FC, Grarup N, Oskolkov N, Thalamuthu A, Mangino M, Liu J, Demirkan A, Lek M, Xu L, Wang G, Oldmeadow C, Gaulton KJ, Lotta LA, Miyamoto-Mikami E, Rivas MA, White T, Loh PR, Aadahl M, Amin N, Attia JR, Austin K, Benyamin B, Brage S, Cheng YC, Cięszczyk P, Derave W, Eriksson KF, Eynon N, Linneberg A, Lucia A, Massidda M, Mitchell BD, Miyachi M, Murakami H, Padmanabhan S, Pandey A, Papadimitriou I, Rajpal DK, Sale C, Schnurr TM, Sessa F, Shrine N, Tobin MD, Varley I, Wain LV, Wray NR, Lindgren CM, MacArthur DG, Waterworth DM, McCarthy MI, Pedersen O, Khaw KT, Kiel DP, Pitsiladis Y, Fuku N, Franks PW, North KN, van Duijn CM, Mather KA, Hansen T, Hansson O, Spector T, Murabito JM, Richards JB, Rivadeneira F, Langenberg C, Perry JRB, Wareham NJ, Scott RA. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat. Commun. 2017;8:16015. doi: 10.1038/ncomms16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, Galizia G, Della-Morte D, Gargiulo G, Cacciatore F, Bonaduce D, Landi F, Abete P. Sarcopenia and heart failure. Nutrients. 2020;12(1):211. doi: 10.3390/nu12010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canteri AL, Gusmon LB, Zanini AC, Nagano FE, Rabito EI, Petterle RR, Jonasson TH, Boguszewski CL, Borba VZC. Sarcopenia in heart failure with reduced ejection fraction. Am. J. Cardiovasc. Dis. 2019;9(6):116–126. [PMC free article] [PubMed] [Google Scholar]
- 13.Ko BJ, Chang Y, Jung HS, Yun KE, Kim CW, Park HS, Chung EC, Shin H, Ryu S. Relationship between low relative muscle mass and coronary artery calcification in healthy adults. Arterioscler. Thromb. Vasc. Biol. 2016;36(5):1016–1021. doi: 10.1161/ATVBAHA.116.307156. [DOI] [PubMed] [Google Scholar]
- 14.Grill B, Levangie PK, Cole M, Rosenberg D, Jensen L. Bone mineral density among individuals with residual lower limb weakness after polio. PM & R. 2019;11(5):470–475. doi: 10.1016/j.pmrj.2018.08.387. [DOI] [PubMed] [Google Scholar]
- 15.Eguchi Y, Toyoguchi T, Orita S, Shimazu K, Inage K, Fujimoto K, Suzuki M, Norimoto M, Umimura T, Shiga Y, Inoue M, Koda M, Furuya T, Maki S, Hirosawa N, Aoki Y, Nakamura J, Hagiwara S, Akazawa T, Takahashi H, Takahashi K, Shiko Y, Kawasaki Y, Ohtori S. Reduced leg muscle mass and lower grip strength in women are associated with osteoporotic vertebral compression fractures. Arch. Osteoporos. 2019;14(1):112. doi: 10.1007/s11657-019-0668-0. [DOI] [PubMed] [Google Scholar]
- 16.Warrington NM, Beaumont RN, Horikoshi M, Day FR, Helgeland Ø, Laurin C, Bacelis J, Peng S, Hao K, Feenstra B, Wood AR, Mahajan A, Tyrrell J, Robertson NR, Rayner NW, Qiao Z, Moen GH, Vaudel M, Marsit CJ, Chen J, Nodzenski M, Schnurr TM, Zafarmand MH, Bradfield JP, Grarup N, Kooijman MN, Li-Gao R, Geller F, Ahluwalia TS, Paternoster L, Rueedi R, Huikari V, Hottenga JJ, Lyytikäinen LP, Cavadino A, Metrustry S, Cousminer DL, Wu Y, Thiering E, Wang CA, Have CT, Vilor-Tejedor N, Joshi PK, Painter JN, Ntalla I, Myhre R, Pitkänen N, van Leeuwen EM, Joro R, Lagou V, Richmond RC, Espinosa A, Barton SJ, Inskip HM, Holloway JW, Santa-Marina L, Estivill X, Ang W, Marsh JA, Reichetzeder C, Marullo L, Hocher B, Lunetta KL, Murabito JM, Relton CL, Kogevinas M, Chatzi L, Allard C, Bouchard L, Hivert MF, Zhang G, Muglia LJ, Heikkinen J, Morgen CS, van Kampen AHC, van Schaik BDC, Mentch FD, Langenberg C, Luan J, Scott RA, Zhao JH, Hemani G, Ring SM, Bennett AJ, Gaulton KJ, Fernandez-Tajes J, van Zuydam NR, Medina-Gomez C, de Haan HG, Rosendaal FR, Kutalik Z, Marques-Vidal P, Das S, Willemsen G, Mbarek H, Müller-Nurasyid M, Standl M, Appel EVR, Fonvig CE, Trier C, van Beijsterveldt CEM, Murcia M, Bustamante M, Bonas-Guarch S, Hougaard DM, Mercader JM, Linneberg A, Schraut KE, Lind PA, Medland SE, Shields BM, Knight BA, Chai JF, Panoutsopoulou K, Bartels M, Sánchez F, Stokholm J, Torrents D, Vinding RK, Willems SM, Atalay M, Chawes BL, Kovacs P, Prokopenko I, Tuke MA, Yaghootkar H, Ruth KS, Jones SE, Loh PR, Murray A, Weedon MN, Tönjes A, Stumvoll M, Michaelsen KF, Eloranta AM, Lakka TA, van Duijn CM, Kiess W, Körner A, Niinikoski H, Pahkala K, Raitakari OT, Jacobsson B, Zeggini E, Dedoussis GV, Teo YY, Saw SM, Montgomery GW, Campbell H, Wilson JF, Vrijkotte TGM, Vrijheid M, de Geus E, Hayes MG, Kadarmideen HN, Holm JC, Beilin LJ, Pennell CE, Heinrich J, Adair LS, Borja JB, Mohlke KL, Eriksson JG, Widén EE, Hattersley AT, Spector TD, Kähönen M, Viikari JS, Lehtimäki T, Boomsma DI, Sebert S, Vollenweider P, Sørensen TIA, Bisgaard H, Bønnelykke K, Murray JC, Melbye M, Nohr EA, Mook-Kanamori DO, Rivadeneira F, Hofman A, Felix JF, Jaddoe VWV, Hansen T, Pisinger C, Vaag AA, Pedersen O, Uitterlinden AG, Järvelin MR, Power C, Hyppönen E, Scholtens DM, Lowe WL, Jr, Davey Smith G, Timpson NJ, Morris AP, Wareham NJ, Hakonarson H, Grant SFA, Frayling TM, Lawlor DA, Njølstad PR, Johansson S, Ong KK, McCarthy MI, Perry JRB, Evans DM, Freathy RM. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet. 2019;51(5):804–814. doi: 10.1038/s41588-019-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, Zeng L, Ntalla I, Lai FY, Hopewell JC, Giannakopoulou O, Jiang T, Hamby SE, Di Angelantonio E, Assimes TL, Bottinger EP, Chambers JC, Clarke R, Palmer CNA, Cubbon RM, Ellinor P, Ermel R, Evangelou E, Franks PW, Grace C, Gu D, Hingorani AD, Howson JMM, Ingelsson E, Kastrati A, Kessler T, Kyriakou T, Lehtimäki T, Lu X, Lu Y, März W, McPherson R, Metspalu A, Pujades-Rodriguez M, Ruusalepp A, Schadt EE, Schmidt AF, Sweeting MJ, Zalloua PA, AlGhalayini K, Keavney BD, Kooner JS, Loos RJF, Patel RS, Rutter MK, Tomaszewski M, Tzoulaki I, Zeggini E, Erdmann J, Dedoussis G, Björkegren JLM, Schunkert H, Farrall M, Danesh J, Samani NJ, Watkins H, Deloukas P. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat. Genet. 2017;49(9):1385–1391. doi: 10.1038/ng.3913. [DOI] [PubMed] [Google Scholar]
- 18.Iotchkova V, Huang J, Morris JA, Jain D, Barbieri C, Walter K, Min JL, Chen L, Astle W, Cocca M, Deelen P, Elding H, Farmaki AE, Franklin CS, Franberg M, Gaunt TR, Hofman A, Jiang T, Kleber ME, Lachance G, Luan J, Malerba G, Matchan A, Mead D, Memari Y, Ntalla I, Panoutsopoulou K, Pazoki R, Perry JRB, Rivadeneira F, Sabater-Lleal M, Sennblad B, Shin SY, Southam L, Traglia M, van Dijk F, van Leeuwen EM, Zaza G, Zhang W, Amin N, Butterworth A, Chambers JC, Dedoussis G, Dehghan A, Franco OH, Franke L, Frontini M, Gambaro G, Gasparini P, Hamsten A, Issacs A, Kooner JS, Kooperberg C, Langenberg C, Marz W, Scott RA, Swertz MA, Toniolo D, Uitterlinden AG, van Duijn CM, Watkins H, Zeggini E, Maurano MT, Timpson NJ, Reiner AP, Auer PL, Soranzo N. Discovery and refinement of genetic loci associated with cardiometabolic risk using dense imputation maps. Nat. Genet. 2016;48(11):1303–1312. doi: 10.1038/ng.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, LeFaive J, VandeHaar P, Gagliano SA, Gifford A, Bastarache LA, Wei WQ, Denny JC, Lin M, Hveem K, Kang HM, Abecasis GR, Willer CJ, Lee S. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 2018;50(9):1335–1341. doi: 10.1038/s41588-018-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: Advances and challenges. Nat. Rev. Genet. 2012;13(8):576–588. doi: 10.1038/nrg3228. [DOI] [PubMed] [Google Scholar]
- 21.Trajanoska K, Rivadeneira F. The genetic architecture of osteoporosis and fracture risk. Bone. 2019;126:2–10. doi: 10.1016/j.bone.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Yang TL, Shen H, Liu A, Dong SS, Zhang L, Deng FY, Zhao Q, Deng HW. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2020;16(2):91–103. doi: 10.1038/s41574-019-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016;35(11):1880–1906. doi: 10.1002/sim.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalbeth N, Topless R, Flynn T, Cadzow M, Bolland MJ, Merriman TR. Mendelian randomization analysis to examine for a causal effect of urate on bone mineral density. J. Bone Miner. Res. 2015;30(6):985–991. doi: 10.1002/jbmr.2434. [DOI] [PubMed] [Google Scholar]
- 25.He B, Lyu Q, Yin L, Zhang M, Quan Z, Ou Y. Depression and osteoporosis: A Mendelian randomization study. Calcif. Tissue Int. 2021;109(6):675–684. doi: 10.1007/s00223-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson SC, Traylor M, Markus HS. Circulating vitamin K1 levels in relation to ischemic stroke and its subtypes: A Mendelian randomization study. Nutrients. 2018;10(11):1575. doi: 10.3390/nu10111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, Zhang M, Quan Z, Deng L, Li Y, He B. Systematic influence of circulating bilirubin levels on osteoporosis. Front. Endocrinol. 2021 doi: 10.3389/fendo.2021.719920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: Subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 2013;178(7):1177–1184. doi: 10.1093/aje/kwt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015;30(7):543–552. doi: 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He B, Zhao J, Zhang M, Yin L, Quan Z, Ou Y, Huang W. Causal roles of circulating adiponectin in osteoporosis and cancers. Bone. 2022;155:116266. doi: 10.1016/j.bone.2021.116266. [DOI] [PubMed] [Google Scholar]
- 31.He B, Chen X, Liu H, Zhang M, Yin B, Yin L, Quan Z, Ou Y, Sun M, Zhu Y, Cui H. Causal roles of sleep duration in osteoporosis and cardiometabolic diseases: A Mendelian randomization study. BioMed Res. Int. 2022;2022:6819644. doi: 10.1155/2022/6819644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones G, Trajanoska K, Santanasto AJ, Stringa N, Kuo CL, Atkins JL, Lewis JR, Duong T, Hong S, Biggs ML, Luan J, Sarnowski C, Lunetta KL, Tanaka T, Wojczynski MK, Cvejkus R, Nethander M, Ghasemi S, Yang J, Zillikens MC, Walter S, Sicinski K, Kague E, Ackert-Bicknell CL, Arking DE, Windham BG, Boerwinkle E, Grove ML, Graff M, Spira D, Demuth I, van der Velde N, de Groot L, Psaty BM, Odden MC, Fohner AE, Langenberg C, Wareham NJ, Bandinelli S, van Schoor NM, Huisman M, Tan Q, Zmuda J, Mellström D, Karlsson M, Bennett DA, Buchman AS, De Jager PL, Uitterlinden AG, Völker U, Kocher T, Teumer A, Rodriguéz-Mañas L, García FJ, Carnicero JA, Herd P, Bertram L, Ohlsson C, Murabito JM, Melzer D, Kuchel GA, Ferrucci L, Karasik D, Rivadeneira F, Kiel DP, Pilling LC. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat. Commun. 2021;12(1):654. doi: 10.1038/s41467-021-20918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ. Res. 2018;122(3):433–443. doi: 10.1161/CIRCRESAHA.117.312086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, Hedman Å K, Wilk JB, Morley MP, Chaffin MD, Helgadottir A, Verweij N, Dehghan A, Almgren P, Andersson C, Aragam KG, Ärnlöv J, Backman JD, Biggs ML, Bloom HL, Brandimarto J, Brown MR, Buckbinder L, Carey DJ, Chasman DI, Chen X, Chen X, Chung J, Chutkow W, Cook JP, Delgado GE, Denaxas S, Doney AS, Dörr M, Dudley SC, Dunn ME, Engström G, Esko T, Felix SB, Finan C, Ford I, Ghanbari M, Ghasemi S, Giedraitis V, Giulianini F, Gottdiener JS, Gross S, Guðbjartsson DF, Gutmann R, Haggerty CM, van der Harst P, Hyde CL, Ingelsson E, Jukema JW, Kavousi M, Khaw KT, Kleber ME, Køber L, Koekemoer A, Langenberg C, Lind L, Lindgren CM, London B, Lotta LA, Lovering RC, Luan J, Magnusson P, Mahajan A, Margulies KB, März W, Melander O, Mordi IR, Morgan T, Morris AD, Morris AP, Morrison AC, Nagle MW, Nelson CP, Niessner A, Niiranen T, O’Donoghue ML, Owens AT, Palmer CNA, Parry HM, Perola M, Portilla-Fernandez E, Psaty BM, Rice KM, Ridker PM, Romaine SPR, Rotter JI, Salo P, Salomaa V, van Setten J, Shalaby AA, Smelser DT, Smith NL, Stender S, Stott DJ, Svensson P, Tammesoo ML, Taylor KD, Teder-Laving M, Teumer A, Thorgeirsson G, Thorsteinsdottir U, Torp-Pedersen C, Trompet S, Tyl B, Uitterlinden AG, Veluchamy A, Völker U, Voors AA, Wang X, Wareham NJ, Waterworth D, Weeke PE, Weiss R, Wiggins KL, Xing H, Yerges-Armstrong LM, Yu B, Zannad F, Zhao JH, Hemingway H, Samani NJ, McMurray JJV, Yang J, Visscher PM, Newton-Cheh C, Malarstig A, Holm H, Lubitz SA, Sattar N, Holmes MV, Cappola TP, Asselbergs FW, Hingorani AD, Kuchenbaecker K, Ellinor PT, Lang CC, Stefansson K, Smith JG, Vasan RS, Swerdlow DI, Lumbers RT. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat. Commun. 2020;11(1):163. doi: 10.1038/s41467-019-13690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, Arking DE, Barnard J, Bartz TM, Benjamin EJ, Bihlmeyer NA, Bis JC, Bloom HL, Boerwinkle E, Bottinger EB, Brody JA, Calkins H, Campbell A, Cappola TP, Carlquist J, Chasman DI, Chen LY, Chen YI, Choi EK, Choi SH, Christophersen IE, Chung MK, Cole JW, Conen D, Cook J, Crijns HJ, Cutler MJ, Damrauer SM, Daniels BR, Darbar D, Delgado G, Denny JC, Dichgans M, Dörr M, Dudink EA, Dudley SC, Esa N, Esko T, Eskola M, Fatkin D, Felix SB, Ford I, Franco OH, Geelhoed B, Grewal RP, Gudnason V, Guo X, Gupta N, Gustafsson S, Gutmann R, Hamsten A, Harris TB, Hayward C, Heckbert SR, Hernesniemi J, Hocking LJ, Hofman A, Horimoto A, Huang J, Huang PL, Huffman J, Ingelsson E, Ipek EG, Ito K, Jimenez-Conde J, Johnson R, Jukema JW, Kääb S, Kähönen M, Kamatani Y, Kane JP, Kastrati A, Kathiresan S, Katschnig-Winter P, Kavousi M, Kessler T, Kietselaer BL, Kirchhof P, Kleber ME, Knight S, Krieger JE, Kubo M, Launer LJ, Laurikka J, Lehtimäki T, Leineweber K, Lemaitre RN, Li M, Lim HE, Lin HJ, Lin H, Lind L, Lindgren CM, Lokki ML, London B, Loos RJF, Low SK, Lu Y, Lyytikäinen LP, Macfarlane PW, Magnusson PK, Mahajan A, Malik R, Mansur AJ, Marcus GM, Margolin L, Margulies KB, März W, McManus DD, Melander O, Mohanty S, Montgomery JA, Morley MP, Morris AP, Müller-Nurasyid M, Natale A, Nazarian S, Neumann B, Newton-Cheh C, Niemeijer MN, Nikus K, Nilsson P, Noordam R, Oellers H, Olesen MS, Orho-Melander M, Padmanabhan S, Pak HN, Paré G, Pedersen NL, Pera J, Pereira A, Porteous D, Psaty BM, Pulit SL, Pullinger CR, Rader DJ, Refsgaard L, Ribasés M, Ridker PM, Rienstra M, Risch L, Roden DM, Rosand J, Rosenberg MA, Rost N, Rotter JI, Saba S, Sandhu RK, Schnabel RB, Schramm K, Schunkert H, Schurman C, Scott SA, Seppälä I, Shaffer C, Shah S, Shalaby AA, Shim J, Shoemaker MB, Siland JE, Sinisalo J, Sinner MF, Slowik A, Smith AV, Smith BH, Smith JG, Smith JD, Smith NL, Soliman EZ, Sotoodehnia N, Stricker BH, Sun A, Sun H, Svendsen JH, Tanaka T, Tanriverdi K, Taylor KD, Teder-Laving M, Teumer A, Thériault S, Trompet S, Tucker NR, Tveit A, Uitterlinden AG, Van Der Harst P, Van Gelder IC, Van Wagoner DR, Verweij N, Vlachopoulou E, Völker U, Wang B, Weeke PE, Weijs B, Weiss R, Weiss S, Wells QS, Wiggins KL, Wong JA, Woo D, Worrall BB, Yang PS, Yao J, Yoneda ZT, Zeller T, Zeng L, Lubitz SA, Lunetta KL, Ellinor PT. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 2018;50(9):1225–1233. doi: 10.1038/s41588-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, Payne AJ, Steinthorsdottir V, Scott RA, Grarup N, Cook JP, Schmidt EM, Wuttke M, Sarnowski C, Mägi R, Nano J, Gieger C, Trompet S, Lecoeur C, Preuss MH, Prins BP, Guo X, Bielak LF, Below JE, Bowden DW, Chambers JC, Kim YJ, Ng MCY, Petty LE, Sim X, Zhang W, Bennett AJ, Bork-Jensen J, Brummett CM, Canouil M, Ec Kardt KU, Fischer K, Kardia SLR, Kronenberg F, Läll K, Liu CT, Locke AE, Luan J, Ntalla I, Nylander V, Schönherr S, Schurmann C, Yengo L, Bottinger EP, Brandslund I, Christensen C, Dedoussis G, Florez JC, Ford I, Franco OH, Frayling TM, Giedraitis V, Hackinger S, Hattersley AT, Herder C, Ikram MA, Ingelsson M, Jørgensen ME, Jørgensen T, Kriebel J, Kuusisto J, Ligthart S, Lindgren CM, Linneberg A, Lyssenko V, Mamakou V, Meitinger T, Mohlke KL, Morris AD, Nadkarni G, Pankow JS, Peters A, Sattar N, Stančáková A, Strauch K, Taylor KD, Thorand B, Thorleifsson G, Thorsteinsdottir U, Tuomilehto J, Witte DR, Dupuis J, Peyser PA, Zeggini E, Loos RJF, Froguel P, Ingelsson E, Lind L, Groop L, Laakso M, Collins FS, Jukema JW, Palmer CNA, Grallert H, Metspalu A, Dehghan A, Köttgen A, Abecasis GR, Meigs JB, Rotter JI, Marchini J, Pedersen O, Hansen T, Langenberg C, Wareham NJ, Stefansson K, Gloyn AL, Morris AP, Boehnke M, McCarthy MI. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018;50(11):1505–1513. doi: 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, Vulpescu NA, Forgetta V, Kleinman A, Mohanty ST, Sergio CM, Quinn J, Nguyen-Yamamoto L, Luco AL, Vijay J, Simon MM, Pramatarova A, Medina-Gomez C, Trajanoska K, Ghirardello EJ, Butterfield NC, Curry KF, Leitch VD, Sparkes PC, Adoum AT, Mannan NS, Komla-Ebri DSK, Pollard AS, Dewhurst HF, Hassall TAD, Beltejar MG, Adams DJ, Vaillancourt SM, Kaptoge S, Baldock P, Cooper C, Reeve J, Ntzani EE, Evangelou E, Ohlsson C, Karasik D, Rivadeneira F, Kiel DP, Tobias JH, Gregson CL, Harvey NC, Grundberg E, Goltzman D, Adams DJ, Lelliott CJ, Hinds DA, Ackert-Bicknell CL, Hsu YH, Maurano MT, Croucher PI, Williams GR, Bassett JHD, Evans DM, Richards JB. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019;51(2):258–266. doi: 10.1038/s41588-018-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowden J, Hemani G, Davey Smith G. Invited commentary: Detecting individual and global horizontal pleiotropy in mendelian randomization—A job for the humble heterogeneity statistic? Am. J. Epidemiol. 2018;187(12):2681–2685. doi: 10.1093/aje/kwy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess S, Thompson SG. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015;181(4):251–260. doi: 10.1093/aje/kwu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yavorska OO, Burgess S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017;46(6):1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gubelmann C, Vollenweider P, Marques-Vidal P. No association between grip strength and cardiovascular risk: The CoLaus population-based study. Int. J. Cardiol. 2017;236:478–482. doi: 10.1016/j.ijcard.2017.01.110. [DOI] [PubMed] [Google Scholar]
- 44.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikäinen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, König IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimäki T, Loos RJF, Melander O, Metspalu A, März W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O'Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015;47(10):1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee K. Muscle mass and body fat in relation to cardiovascular risk estimation and lipid-lowering eligibility. J. Clin. Densitom. 2017;20(2):247–255. doi: 10.1016/j.jocd.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J. Cell. Biochem. 2015;116(7):1171–1178. doi: 10.1002/jcb.25077. [DOI] [PubMed] [Google Scholar]
- 47.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 48.Sillars A, Celis-Morales CA, Ho FK, Petermann F, Welsh P, Iliodromiti S, Ferguson LD, Lyall DM, Anderson J, Mackay DF, Pellicori P, Cleland J, Pell JP, Gill JMR, Gray SR, Sattar N. Association of fitness and grip strength with heart failure: Findings from the UK Biobank population-based study. Mayo Clin. Proc. 2019;94(11):2230–2240. doi: 10.1016/j.mayocp.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 49.Beyer SE, Sanghvi MM, Aung N, Hosking A, Cooper JA, Paiva JM, Lee AM, Fung K, Lukaschuk E, Carapella V, Mittleman MA, Brage S, Piechnik SK, Neubauer S, Petersen SE. Prospective association between handgrip strength and cardiac structure and function in UK adults. PLoS ONE. 2018;13(3):e0193124. doi: 10.1371/journal.pone.0193124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ensrud KE, Crandall CJ. Osteoporosis. Ann. Intern. Med. 2017;167(3):ITC17–ITC32. doi: 10.7326/AITC201708010. [DOI] [PubMed] [Google Scholar]
- 51.Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Chen X, Zhu Y, Yin L, Quan Z, Ou Y, He B. Causal associations of circulating adiponectin with cardiometabolic diseases and osteoporotic fracture. Sci. Rep. 2022;12(1):6689. doi: 10.1038/s41598-022-10586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He B, Zhu Y, Cui H, Sun B, Su T, Wen P. Comparison of necroptosis with apoptosis for OVX-induced osteoporosis. Front. Mol. Biosci. 2021;8(1262):790613. doi: 10.3389/fmolb.2021.790613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He B, Yin L, Zhang M, Lyu Q, Quan Z, Ou Y. Causal effect of blood pressure on bone mineral density and fracture: A Mendelian randomization study. Front. Endocrinol. 2021 doi: 10.3389/fendo.2021.716681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osei-Hyiaman D, Ueji M, Toyokawa S, Takahashi H, Kano K. Influence of grip strength on metacarpal bone mineral density in postmenopausal Japanese women: A cross-sectional study. Calcif. Tissue Int. 1999;64(3):263–266. doi: 10.1007/s002239900615. [DOI] [PubMed] [Google Scholar]
- 56.Di Monaco M, Di Monaco R, Manca M, Cavanna A. Handgrip strength is an independent predictor of distal radius bone mineral density in postmenopausal women. Clin. Rheumatol. 2000;19(6):473–476. doi: 10.1007/s100670070009. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa Y, Schneider P, Reiners C. Age, sex, and grip strength determine architectural bone parameters assessed by peripheral quantitative computed tomography (pQCT) at the human radius. J. Biomech. 2001;34(4):497–503. doi: 10.1016/S0021-9290(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann CL, Smidt GL, Brooks JS, Kinsey WJ, Eekhoff TL. Relationship of extremity muscle torque and bone mineral density in postmenopausal women. Phys. Ther. 1990;70(5):302–309. doi: 10.1093/ptj/70.5.302. [DOI] [PubMed] [Google Scholar]
- 59.Foley KT, Owings TM, Pavol MJ, Grabiner MD. Maximum grip strength is not related to bone mineral density of the proximal femur in older adults. Calcif. Tissue Int. 1999;64(4):291–294. doi: 10.1007/s002239900621. [DOI] [PubMed] [Google Scholar]
- 60.Picca A, Calvani R, Manes-Gravina E, Spaziani L, Landi F, Bernabei R, Marzetti E. Bone-muscle crosstalk: Unraveling new therapeutic targets for osteoporosis. Curr. Pharm. Des. 2017;23(41):6256–6263. doi: 10.2174/1381612823666170526112300. [DOI] [PubMed] [Google Scholar]
- 61.Sakuma K, Yamaguchi A. Sarcopenia and cachexia: The adaptations of negative regulators of skeletal muscle mass. J. Cachexia Sarcopenia Muscle. 2012;3(2):77–94. doi: 10.1007/s13539-011-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Leung KS, Chow SK, Cheung WH. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia) J. Orthop. Transl. 2017;10:94–101. doi: 10.1016/j.jot.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study were available within the paper.