CASE
An 84-year-old Caucasian woman with a past medical history significant for coronary artery disease status post 3 vessel coronary artery bypass grafting, mitral valve repair with a mitral ring, chronic kidney disease stage IV, chronic obstructive pulmonary disease not on home oxygen, and insulin-dependent diabetes presents to the Structural Heart and Valve clinic with a chief complaint of worsening shortness of breath. An echocardiogram reveals severe mitral regurgitation (regurgitant fraction, 45%, effective regurgitant orifice [ERO], 0.46 mm2), mean gradient, 3 mm Hg). Her Society of Thoracic Surgery predicted operative mortality risk score calculates to 9.4%. The heart team deems her to be a candidate for a valve-in-ring transcatheter mitral valve replacement (TMVR); however, her preprocedural planning computed tomography (CT) scan demonstrates a thickened interventricular septum and concern for left ventricular outflow tract (LVOT) obstruction.
INTRODUCTION
TMVR is becoming a viable option for patients with severe mitral valve disease and no surgical options.1 LVOT obstruction is a known complication of TMVR that portends a poor prognosis.2 This review discusses the current state of TMVR and reviews strategies to prevent LVOT obstruction, with an emphasis on Heart Team decision-making and promising prophylactic, electrosurgical techniques to modify the mitral valve leaflets before valve implantation.
TRANSCATHETER MITRAL VALVE REPLACEMENT
The advent of transcatheter aortic valve replacement (TAVR) began a revolution in the treatment of valvular heart disease. Over the past decade, skills and technology previously used in the aortic space have been modified for the treatment of mitral valve disorders. Transcatheter mitral valve replacement (TMVR) has become a possible treatment for patients with mitral stenosis or regurgitation and no surgical options.
Currently, there are no commercially dedicated TMVR systems.1 Available options are limited to clinical trials or the off-label use of balloon expandable aortic valves modified for the mitral position. All current transcatheter mitral valves available under research protocols require transapical placement, whereas the Edwards Sapien Valve (Edwards Life Science, Irvine, CA) (Fig. 1) can be delivered either transapically or transseptally. Given the risk profile of this patient cohort and that most devices are either first-generation or off-label, postprocedure mortality remains high.2
Fig. 1.
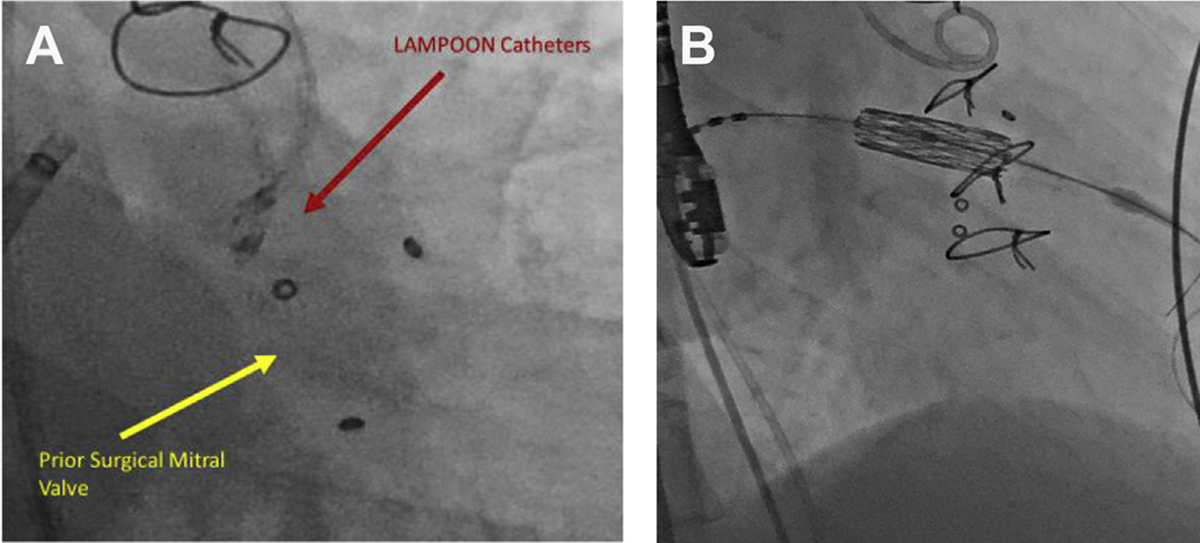
(A, B) S3 valve positioning for V-i-V TMVR post LAMPOON. (Courtesy of Edwards Lifesciences, Irvine, CA.)
TMVR is feasible for valve-in-surgical valve (V-i-V) procedures,3 valve in prior surgically placed mitral rings, and valve-in-mitral annular calcification (MAC).4 Of the 3 interventions, V-i-V is the least technically challenging, given the support of the prior valve frame and the known inner diameter of the prosthetic valve, which is useful for sizing purposes. The annuloplasty ring during mitral valve-in-ring therapy also provides an anchor for TMVR deployment but procedural outcomes are limited by potential ring dehiscence and limitations in valve deployment. TMVR in MAC is the most technically challenging replacement to perform and requires a circumferential ring of calcium. Valve deployment may be limited by the uneven nature of MAC and leads to paravalvular leak.5
As TMVR is a relatively new approach to the treatment of mitral valve disease, no large-scale randomized trials have been completed to date. In a large multicenter registry of valve-in-MAC, initial success was reported in 76.6% of cases and 46.3% of patients were alive at 1 year. Notably, 11.2% of patients experienced LVOT obstruction with hemodynamic compromise. Of these 13 patients, only 2 were alive at 1-year follow-up. When outcomes were stratified by operator experience, there was no statistically difference in the rate of LVOT between early and late experience.2
LEFT VENTRICULAR OUTFLOW TRACT OBSTRUCTION
LVOT obstruction from permanent displacement of the anterior mitral valve leaflet is more common in valve-in-ring and valve-in-MAC procedures. In addition, approximately 50% of patients are excluded from clinical trials given the risk of LVOT obstruction. Given the significant nature of this problem, much work has focused on the prediction, prevention, and treatment of LVOT obstruction.6
Defining the Left Ventricular Outflow Tract
The anterior mitral valve leaflet (AML) physically separates the inflow and outflow zones of the left ventricle. Before TMVR, the AML moves into the LVOT during diastole and retracts out of the LVOT during systole. During TMVR, valve struts can be either circumferentially covered or the anterior mitral leaflet will be placed in a permanently “open” position creating a neo-LVOT.7 In certain at-risk patients, the AML may encroach into the LVOT, causing obstruction and a functional subaortic stenosis. Given the significant mortality associated with LVOT obstruction, detailed preprocedural CT assessment is essential to comprehensively evaluate risk. Threatened LVOT obstruction is an exclusion criteria for approximately 50% of patients in contemporary clinical trials.6
Preprocedural 3-dimensional CT allows for the accurate modeling of cardiac anatomy and deployment of a virtual transcatheter heart valve. The mitral valve plane is defined as the basal-most insertion point of the mitral valve leaflets. In most cases, using a gated cardiac CT, this plane should be measured at 40% of the cardiac cycle to determine the narrowest possible neo-LVOT. Using the double-oblique method, the plane can be measured in both sagittal and coronal cross section. Using these dimensions, a valve size can be determined and a virtual valve can be placed.8 The valve is typically placed 80% ventricular and 20% atrial; however, it should be modeled at various positions in the event of valve migration during deployment.9 LVOT surface areas can be obtained after virtual valve placement, and a neo-LVOT area can be determined. Retrospective analysis of 38 patients undergoing TMVR determined that a neo-LVOT area ≤189 mm2 portended a higher risk of LVOT obstruction10 (Fig. 2).
Fig. 2.
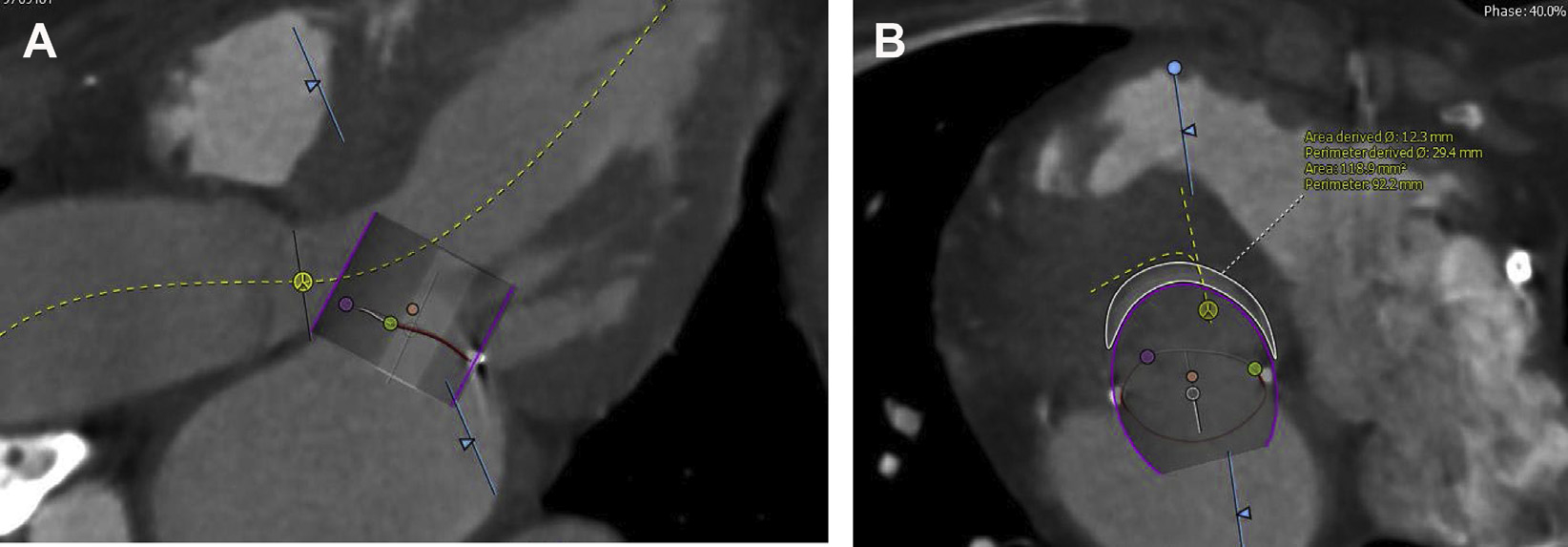
CT assessment for LVOT obstruction. Detailed preprocedure CT analysis allows for assessment of LVOT obstruction risk. Note, measurements are performed in the 40% phase of the cardiac cycle with the valve placed so that 20% is atrial and 80% ventricular (A). In this patient, the neo-LVOT area of 118.9 mm2 (B) predicts a high risk of LVOT obstruction after TMVR.
Aorto-mitral angulation, left ventricular (LV) size, and interventricular septal size are also essential for a comprehensive assessment of LVOT obstruction risk. In general, as the angle between the aortic and mitral angle approaches 90° and LV size decreases, the risk of LVOT obstruction increases.11
Prevention of Left Ventricular Outflow Tract Obstruction
Surgical splitting of the anterior mitral valve leaflet
David’s12 technique allows for splitting of the anterior mitral leaflet during open cardiac surgery. In patients at risk of LVOT obstruction, a hybrid approach to valve replacement that involves surgical laceration followed by TMVR is feasible, especially given advancements in minimally invasive and robotic mitral surgery. However, the cardiac risk profile of most patients undergoing TMVR precludes them from any form of cardiac surgery. Because of this, the remainder of this review will focus on solely catheter-based solutions to LVOT obstruction.
Alcohol septal ablation
Alcohol septal ablation, a noninvasive technique to reduce septal musculature, is used as a therapeutic strategy in patients with hypertrophic obstructive cardiomyopathy. Case reports have also described alcohol septal ablation to reduce LVOT obstruction post-TMVR.13,14 However, this procedure is limited in patients with a relatively thin interventricular septum. The septal pathway of the His-Purkinje system also increases the risk of postablation complete heart block necessitating permeant pacemaker implantation.
To date, the only systematic study of patients who have undergone this technique as a “bail-out” strategy is limited to a report of 6 patients treated at 6 different centers. In this cohort, 5 patients had immediate reduction in LVOT gradient and 4 were alive at 30 days.15 There have been no prospective studies of alcohol septal ablation before TMVR. Applying this strategy to selected patients may prove to be a viable alternative to preemptive splitting of the anterior leaflet; however, the need for myocardial remodeling necessitates approximately a 6-week waiting period. Also, there is a mortality risk from this procedure as a stand-alone prevention strategy.
Radiofrequency ablation
Alcohol septal ablation is not a viable strategy to prevent LVOT obstruction in patients with an anatomically unsuitable septal perforator or a thin interventricular septum. Although still investigational in nature, radiofrequency ablation of the interventricular septum may be a viable strategy for these patients. This procedure does not require preprocedure CT and can be guided by transesophageal echocardiography (TEE). Using techniques originally designed for arrhythmia ablation, radiofrequency catheters can be placed near the predicted area of LVOT obstruction (Fig. 3). After ablating the myocardium, it becomes dyskinetic or akinetic, thereby creating a larger neo-LVOT. This procedure has been used twice in our institution and its long-term outcomes are unknown. The limitations of this technique relegate its use to a compassionate basis.
Fig. 3.
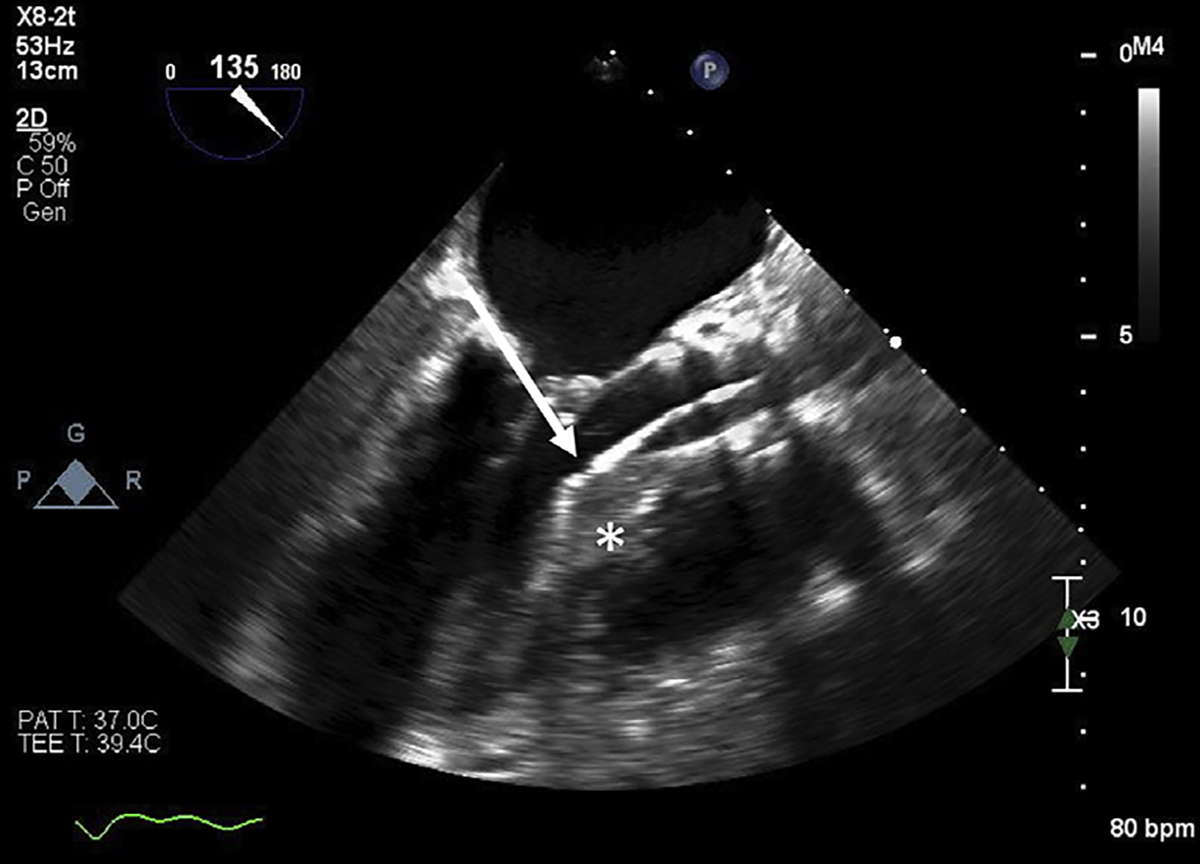
Radiofrequency ablation of the interventricular septum using TEE guidance. Radiofrequency ablation catheters (arrow) can be used to ablate the interventricular septum (star) when there are no anatomically suitable septal perforators for alcohol septal ablation.
Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction
The LAMPOON (Intentional Percutaneous Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction) technique was developed to mitigate the risk of LVOT obstruction before TMVR with a Sapien valve in the mitral position. LAMPOON uses transcatheter electrosurgery to mimic David’s12 surgical anterior resection with chordal sparing (Fig. 4). As there are no commercially available catheters for LAMPOON, the technique was developed using modified commercially available devices.7
Fig. 4.
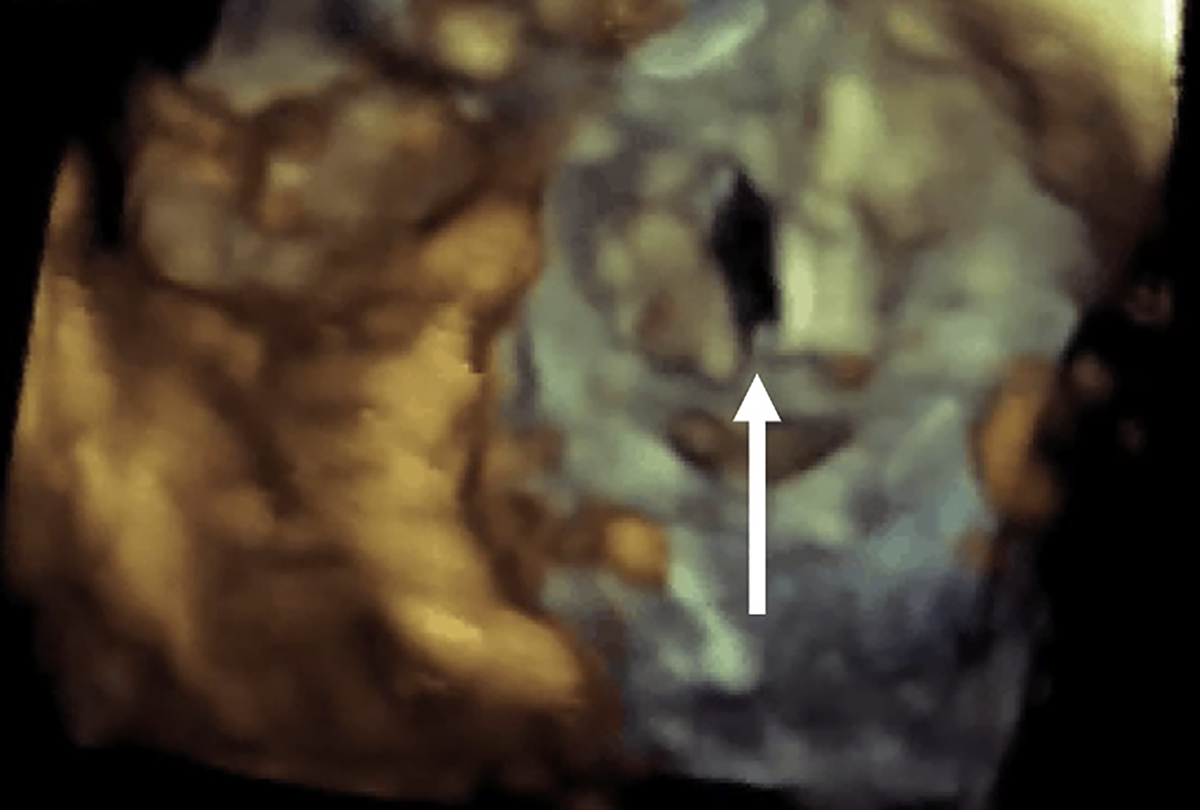
Anterior mitral leaflet post LAMPOON. Three-dimensional TEE of a lacerated AML. This technique decreases the risk of LVOT obstruction by allowing for blood flow through open transcatheter heart valve cells that otherwise would be covered by the anterior mitral leaflet. The arrow is pointing to the “split” anterior mitral valve leaflet post LAMPOON.
In the first step of the procedure, two 6-French coronary guiding catheters are advanced across the aortic valve. One catheter is positioned in the LVOT at the base of the A2 scallop and the other catheter is placed retrograde into the left atrium. Electrosurgical crossing is achieved by modification of techniques used for transcaval TAVR access. First an insulating polymer jacket wire converter (Piggyback, Teleflex, Morrisville, NC) is placed inside of the LVOT catheter. For electrosurgical crossing, a 0.014-inch by 300 cm guide wire is placed through this jacket and electrified at 50W (pure cut). Once the wire passes through the base of the A2 scallop it is captured by a snare placed in the left atrium and externalized (Fig. 5).
Fig. 5.
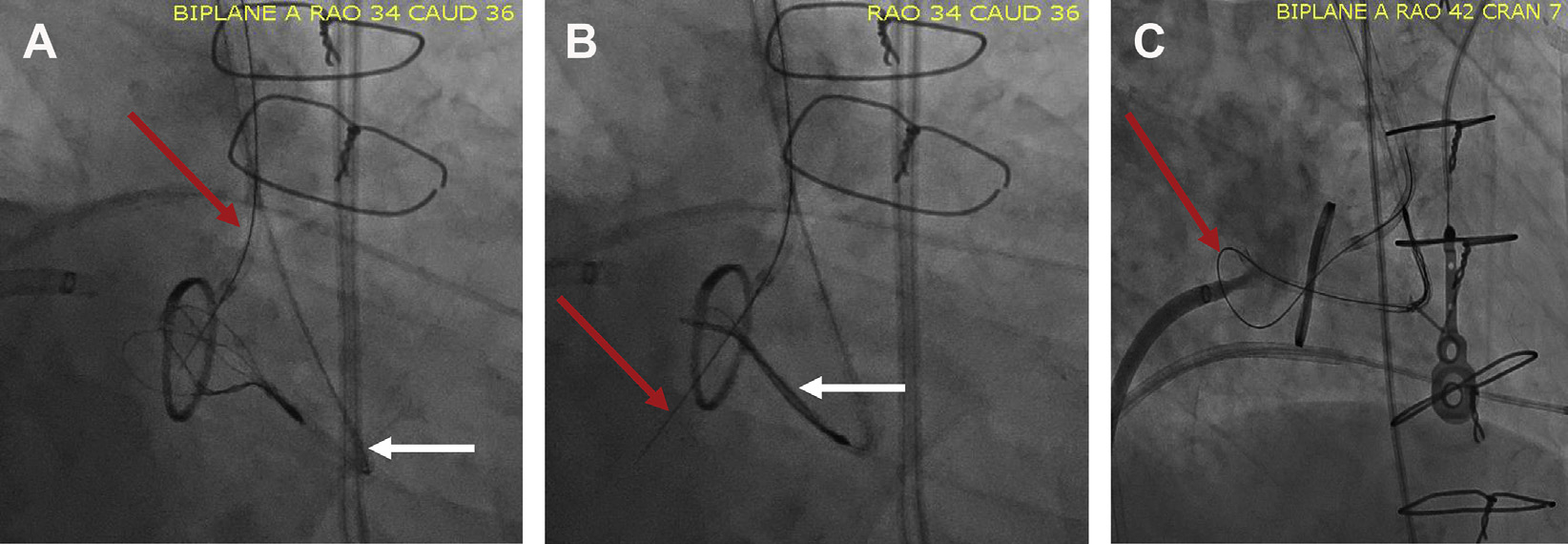
LAMPOON technique. The LAMPOON technique was developed using modifications of commercially available devices. (A) A snare is placed in the left atrium (white arrow) and a guidewire is placed in the LVOT catheter (red arrow). The guidewire is electrified, passed through the base of the A2 scallop, and snared in the left atrium (B). The wire is then externalized, kinked, and positioned at the base of the anterior leaflet. Once electrified, the wire can traverse and lacerate the anterior leaflet decreasing obstruction risk post-TMVR (C).
Once externalized, a segment of the guidewire is denuded and kinked. By kinking the wire, energy can be focused on the inner curvature, which is positioned in the anterior mitral leaflet. Again, the guidewire is electrified and pulled back to fully split the anterior mitral leaflet. During this electrification, dextrose is injected to avoid the dispersion of charge from the blood’s iron content.6
The first-in-human experience was systematically studied in 5 patients with prohibitive risk of LVOT obstruction before TMVR. Of these 5 patients, 3 had a prior mitral ring, 1 had a prior mitral band, and 1 had MAC. Results of this original work were promising with 80% survival at 30 days and no significant paravalvular leak, major bleeding, or vascular complications. No patients in the original study had a critical LVOT gradient after TMVR and there were no impediments to function of the transcatheter heart valve.6
Following the results of this experience, an early feasibility trial was conducted to study LAMPOON in a larger population. This multicenter center included 30 subjects who were ineligible for isolated TMVR and were poor candidates for other clinical trials. This cohort had a risk of severe LVOT obstruction from either a skirt neo-LVOT of less than 150 mm2 or a long redundant anterior mitral leaflet. In this cohort, 100% of patients had successful traversal of the AML, laceration of the AML, and TMVR implantation. In-hospital survival in this study was 93%, which is significantly higher than reported in other clinical trials. Seventy percent of patients met criteria for technical success and did not require emergency surgery, bail-out alcohol septal ablation, unplanned paravalvular leak device closure, or need for a second valve, consistent with the limitations of using an off-label Sapien valve in the mitral position.
Four patients in the LAMPOON IDE had an LVOT gradient greater than 30 mm Hg after TMVR deployment. Review of postprocedure CT images suggested that insufficient laceration (tip laceration, n 5 1) and the valve skirt of the S3 (n 5 3) can create LVOT obstruction (the concept of a “skirt” neo-LVOT measurement). Patients with a “skirt neo-LVOT” of less than 150 mm216 will likely require adjunctive procedures, such as alcohol or radiofrequency septal ablation (Fig. 6). Although further validation is still needed, measuring skirt neo-LVOT appears to be a necessary additional measurement before TMVR.
Fig. 6.
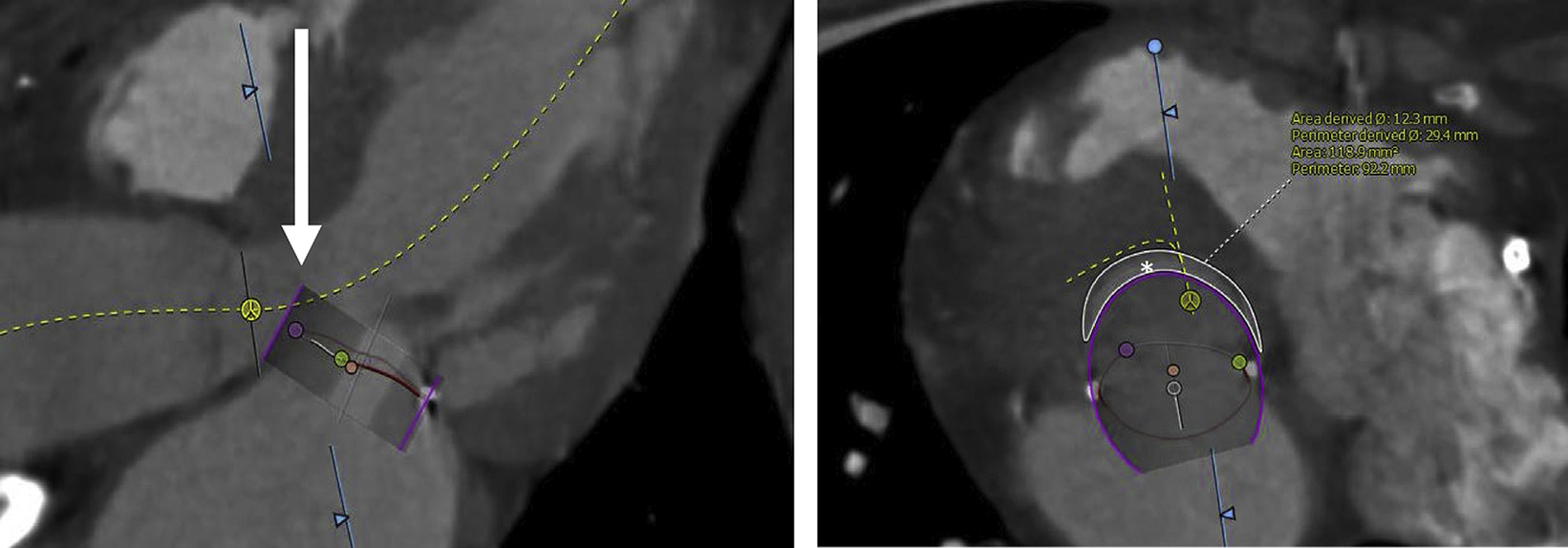
CT assessment for “skirt” obstruction. After TMVR, the valve skirt can protrude in the LVOT. As this is a fixed structure, LVOT obstruction can occur despite successful laceration of the anterior mitral leaflet. After assessment of the neo-LVOT, the “skirt” neo-LVOT should be modeled by simulating a virtual valve skirt (arrow) and measuring the predicted area (asterisk). Patients with a “skirt” neo-LVOT of less than 150 mm2 will often require an adjunctive procedure to decrease the risk of obstruction.
Our center has also applied the LAMPOON technique to patients with bioprosthetic mitral valve replacement before transcatheter valve-in-valve procedures. Using standard LAMPOON techniques, the anterior leaflet of the bioprosthetic valve was lacerated and the Sapien valve was deployed without significant LVOT obstruction. Although the technique has not been systematically studied in this population, it appears that LAMPOON is a viable strategy for patients with prior bioprosthetic valves who are at high risk of LVOT obstruction.
In addition to the standard assessments of the neo-LVOT, anterior mitral leaflet length (>24 mm) should be carefully assessed before TMVR. A case series by Greenbaum and colleagues17 describes 3 patients with long or redundant anterior mitral leaflets undergoing TMVR. In this cohort, the anterior mitral leaflet interfered with transcatheter heart valve function by either prolapsing through the valve, preventing valve pressurization, or causing a dynamic LVOT obstruction. LAMPOON is a viable preventive strategy preventing these complications in these patients.
Limitations of intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction
Although LAMPOON is a promising strategy, it requires a high degree of operator skill and a heart team with expertise in electrosurgical techniques and interventional echocardiography. The technique is also limited in patients with a heavily calcified anterior mitral leaflet, although traversal is often possible with careful preprocedural planning, catheter placement, and increased voltage. An essential component of LAMPOON is to ensure the laceration is electrosurgical rather than mechanical. Patients with mechanical splitting of the leaflet are at greatest risk of hemodynamic instability compared with electrical cutting. Given these limitations, the use of LAMPOON is currently relegated to centers of expertise that have an interest/expertise in TMVR.
Mechanical splitting of the anterior leaflet
Although LAMPOON relies on electrosurgical principles to split the AML, work by Lee and colleagues18 described mechanical splitting of the leaflet. After obtaining transapical access, the anterior leaflet is pierced with a needle, a guidewire is inserted into the right superior pulmonary artery, and balloon inflation is performed to detach the AML from the mitral annulus. Similar to most techniques for AML modification, this strategy is described only in a case report. Notable limitations are the need for cardiopulmonary bypass and the potential for an inexact splitting of the AML.
Preparation of a U-stitch to correct lateral deflection for endovascular mitral replacement in short landing zone technique
Transcatheter valve-in-valve therapy allows for coaxial deployment of the transcatheter heart valve in the prior surgical valve frame. The lack of such a frame in Valve-in-Ring or Valve-in-MAC therapy make coaxial valve deployment significantly more challenging. The Preparation of a U-stitch to correct Lateral deflection for Endovascular mitral replacement in short landing zone (POULEZ) technique relies on a modification of the Edwards Commander (Edwards Life Science, Irvine, CA) delivery system. A 135-cm-long 3 to 0 polypropylene suture is passed through the catheter nosecone beyond the balloon and through a single petal of the valve pusher. After aligning both along the inner curvature of the delivery system, the distal suture is secured with a knot and the proximal suture is held alongside the delivery handle. TMVR workflow continues in the usual fashion until the valve delivery system crosses the atrial septum. At this stage, the pusher is retracted, creating a fulcrum, and all deflection is removed from the commander delivery system. By placing tension on the free suture end, the valve deflects both medially and anteriorly.19 This technique may decrease the risk of LVOT obstruction by optimizing coaxiality and appropriate valve placement.
Kissing-balloon technique
A kissing-balloon technique has been used to secure the neo-LVOT. Described in a case report, transesophageal echocardiography was used to guide a 20-mm valvuloplasty balloon into the LVOT. By simultaneously inflating this balloon with transcatheter heart valve deployment, the LVOT was sized and the transcatheter heart valve was optimally oriented.20 Although this technique is less invasive than others described, it does have significant limitations. Namely, the LVOT balloon does not permanently alter the anterior mitral leaflet increasing the potential risk for LVOT obstruction, and it may increase the likelihood that the transcatheter heart valve is not deployed coaxially.
SUMMARY
TMVR is a promising strategy for patients without options for surgical mitral valve replacement; however, the technique remains limited by a lack of commercially available systems and the risk of LVOT obstruction. This risk can be significantly reduced by detailed CT analysis, procedural planning, and the use of techniques to modify either the anterior mitral leaflet or the interventricular septum. In the future, dedicated devices may make these approaches more efficient, safer, and reproducible.
KEY POINTS.
Transcatheter mitral valve replacement is a promising strategy for patients with severe mitral valve disease and no surgical options.
Left ventricular outflow tract obstruction is a life-threatening complication of transcatheter mitral valve replacement. There are currently no commercially available devices to prevent left ventricular outflow tract obstruction.
Multiple techniques to prevent left ventricular outflow tract obstruction have been developed by modifying current devices.
The LAMPOON technique is a promising strategy using electrosurgical techniques to reduce the risk of left ventricular outflow tract obstruction.
Disclosure Statement:
Dr V. Babaliaros is a consultant for both Edwards and Abbott. Dr A. Greenbaum is a consultant for both Edwards and Abbott. Dr T. Rogers is a consultant/proctor for Edwards Lifesciences and Medtronic.
REFERENCES
- 1.Regueiro A, Granada JF, Dagenais F, et al. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol 2017;69(17):2175–92. [DOI] [PubMed] [Google Scholar]
- 2.Guerrero M, Urena M, Himbert D, et al. 1-year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol 2018;71(17):1841–53. [DOI] [PubMed] [Google Scholar]
- 3.Kamioka N, Babaliaros V, Morse MA, et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve-in-valve therapy. JACC Cardiovasc Interv 2018;11(12):1131–8. [DOI] [PubMed] [Google Scholar]
- 4.Eleid MF, Whisenant BK, Cabalka AK, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv 2016;9(11):1161–74. [DOI] [PubMed] [Google Scholar]
- 5.Blanke P, Dvir D, Cheung A, et al. Mitral annular evaluation with CT in the context of transcatheter mitral valve replacement. JACC Cardiovasc Imaging 2015;8(5):612–5. [DOI] [PubMed] [Google Scholar]
- 6.Babaliaros VC, Greenbaum AB, Khan JM, et al. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement: first-in-human experience. JACC Cardiovasc Interv 2017;10(8):798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan JM, Greenbaum AB, Khan JM, et al. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: pre-clinical findings. JACC Cardiovasc Interv 2016;9(17):1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanke P, Naoum C, Dvir D, et al. Predicting LVOT obstruction in transcatheter mitral valve implantation: concept of the neo-LVOT. JACC Cardiovasc Imaging 2017;10(4):482–5. [DOI] [PubMed] [Google Scholar]
- 9.Wang DD, Eng M, Greenbaum A, et al. Predicting LVOT obstruction after TMVR. JACC Cardiovasc Imaging 2016;9(11):1349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang DD, Eng MH, Greenbaum AB, et al. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv 2018;92(2):379–87. [DOI] [PubMed] [Google Scholar]
- 11.Blanke P, Naoum C, Webb J, et al. Multimodality imaging in the context of transcatheter mitral valve replacement: establishing consensus among modalities and disciplines. JACC Cardiovasc Imaging 2015;8(10):1191–208. [DOI] [PubMed] [Google Scholar]
- 12.David T Mitral valve replacement with preservation of chorae tendinae: rationale and technical considerations. Ann Thorac Surg 1986;4:680–2. [DOI] [PubMed] [Google Scholar]
- 13.Deharo P, Urena M, Himbert D, et al. Bail-out alcohol septal ablation for left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv 2016;9(8):e73–6. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero M, Wang DD, O’Neill W. Percutaneous alcohol septal ablation to acutely reduce left ventricular outflow tract obstruction induced by transcatheter mitral valve replacement. Catheter Cardiovasc Interv 2016;88(6):E191–7. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero M, Wang DD, Himbert D, et al. Short-term results of alcohol septal ablation as a bail-out strategy to treat severe left ventricular outflow tract obstruction after transcatheter mitral valve replacement in patients with severe mitral annular calcification. Catheter Cardiovasc Interv 2017;90(7):1220–6. [DOI] [PubMed] [Google Scholar]
- 16.Khan JM, Rogers T, Babaliaros VC, et al. Predicting left ventricular outflow tract obstruction despite anterior mitral leaflet resection: the “Skirt NeoLVOT”. JACC Cardiovasc Imaging 2018;11(9):1356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenbaum AB, Condado JF, Eng M, et al. Long or redundant leaflet complicating transcatheter mitral valve replacement: Case vignettes that advocate for removal or reduction of the anterior mitral leaflet. Catheter Cardiovasc Interv 2018;92(3):627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee R, Hui DS, Helmy TA, et al. Transapical mitral replacement with anterior leaflet splitting: a novel technique to avoid left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg 2018;155(3):e95–8. [DOI] [PubMed] [Google Scholar]
- 19.Babaliaros V, Greenbaum AB, Kamioka N, et al. Bedside modification of delivery system for transcatheter transseptal mitral replacement with POULEZ system and SAPIEN-3 valve. JACC Cardiovasc Interv 2018;11(12):1207–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahhab Z, Ren B, de Jaegere PPT, et al. Kissing balloon technique to secure the neo-left ventricular outflow tract in transcatheter mitral valve implantation. Eur Heart J 2018;39(23):2220. [DOI] [PubMed] [Google Scholar]


