Abstract
Toxic shock syndrome toxin 1 (TSST-1), produced by Staphylococcus aureus (including methicillin-resistant S. aureus), is a superantigenic toxin responsible for toxic shock syndrome as well as neonatal TSS-like exanthematous disease. TSST-1 exhibits its deleterious effects by leading to the abnormal proliferation of, e.g., Vβ2+ T cells and overproduction of proinflammatory cytokines. In the present study we examined the inhibitory effect of a Chinese herbal extract, anisodamine, on TSST-1 using human peripheral blood mononuclear cells (PBMCs). Anisodamine inhibited the production of proinflammatory cytokines better than interleukin-10 (an anti-inflammatory cytokine). The inhibitory effect of anisodamine was greater than that of any tropane alkaloid examined. Anisodamine acted directly on both monocytes and T cells in human PBMCs, and the effect was confirmed at the transcriptional level. Inhibition of NF-κB activation was also demonstrated. In contrast, no significant inhibition of Vβ2+ T-cell proliferation was observed. In mice injected with TSST-1, anisodamine treatment significantly decreased serum proinflammatory cytokine levels and prevented TSST-1-induced death. These results suggest that anisodamine specifically acts against the production of cytokines (inflammatory cytokines in particular) and not against Vβ2+ T-cell proliferation and that anisodamine may have a beneficial effect on TSST-1-associated disease.
Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA; a major agent in nosocomial infections), produces a number of superantigenic toxins, such as toxic shock syndrome toxin 1 (TSST-1) (21, 33). TSST-1 is the major causative toxin of toxic shock syndrome (TSS), which is a life-threatening staphylococcal infection (33), and neonatal TSS-like exanthematous disease (NTED), which was identified as a staphylococcal infection in the neonatal period (30, 31).
TSST-1 binds directly to the major histocompatibility complex (MHC) class II molecules on antigen-presenting cells (APCs) and to the Vβ elements of antigen receptors of T cells. This trimolecular interaction leads to the activation of T cells, i.e., the abnormal proliferation of Vβ2+ T cells (19) and the production of massive amounts of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-2, and gamma interferon (IFN-γ) (12, 13, 19, 21).
Anisodamine (raceanisodamine hydrochloride) is the active ingredient of Chinese herbal extracts that possesses the chemical structure of tropane alkaloids. It is a vasoactive drug and has been used to treat acute disseminated intravascular coagulation in patients in bacteremic shock (29, 37, 38, 41). In previous studies, we demonstrated that anisodamine has an inhibitory effect on the production of TNF-α, IL-1β, and IL-8 from human peripheral blood monocytes stimulated with Shiga toxin (Stx) (39, 40). Stx is the major toxin responsible for hemolytic-uremic syndrome (HUS) caused by enterohemorrhagic Escherichia coli (14). Anisodamine also prolonged the survival of mice injected with Stx (39).
It therefore seemed reasonable to expect that anisodamine would inhibit the abnormal activation of T cells by TSST-1 and be useful for the treatment of patients with these diseases. In this study, we investigated the inhibitory effect of anisodamine (and related compounds) on the release of proinflammatory and anti-inflammatory cytokines from TSST-1-stimulated human peripheral blood mononuclear cells (PBMCs). We also investigated whether anisodamine interferes with Vβ2+ T-cell proliferation following the injection of TSST-1 and also the protective effect of anisodamine on the lethality of TSST-1 in mice.
MATERIALS AND METHODS
Reagents.
Purified TSST-1 was purchased from Toxin Technology (Sarasota, Fla.). Anisodamine (raceanisodamine hydrochloride; C17H24NO4) was obtained from Hangzhou Drugs (Hangzhou, People's Republic of China). Atropine {α-(hydroxymethyl) benzeneacetic acid (3-end)-8-methyl-8-azabicyclo [3.2.1] oct-3-yl ester}, scopolamine {(αS)-α-(hydroxymethyl)-benzeneaceic acid (1α,2β,4β,5α,7β)-9-methyl-3-oxa-9-azatricyclo [3,3,1,02,4] non-7-yl ester}, and aspirin [2-(acetyloxy) benzoic acid] were purchased from Wako Pure Chemicals (Osaka, Japan). d-Galactosamine was from Sigma Chemical (St. Louis, Mo.). The mouse monoclonal antibody I2C3 (anti-HLA-DR/DP), which binds to the MHC class II molecules on APCs, and anti-CD3 and anti-CD28 monoclonal antibodies were described previously (9). Fluorescein isothiocyanate (FITC)-Vβ2 and phycoerythrin (PE)-CD3 antibodies were purchased from Immunotech (Marseill, France) and Becton Dickinson Labware (Oxnard, Calif.), respectively. Recombinant IL-2 (rIL-2) was from Shionogi (Osaka, Japan).
Cells and culture media.
The human monocytic cell line THP-1 (35) was described previously (39, 40). RPMI 1640 medium (Gibco BRL, Grand Island, N.Y.) and the supplements (100 μg of streptomycin per ml, 100 U of penicillin per ml, 10% fetal bovine serum, and 5 × 10−5 M 2-mercaptoethanol) were also described previously (9, 17, 39).
Preparation of human PBMCs.
Peripheral blood samples from six healthy adults were used in this study. Human PBMCs were isolated as described previously (9, 17, 39). PBMCs were isolated by Ficoll-Conray (specific gravity, 1.077) density gradient centrifugation and washed twice in phosphate-buffered saline. The cells were finally suspended in RPMI 1640 medium at a concentration of 106 to 107 cells/ml.
Preparation of human peripheral blood monocytes.
For the preparation of monocytes, human PBMCs suspended in RPMI 1640 medium at 107 cells/ml were incubated for 3 h at 37°C with 5% CO2. Nonadherent cells were removed after the incubation, and adherent monocytes were collected (39, 40). The concentration of monocytes (suspended in RPMI 1640 medium) was 106 cells/ml.
Preparation of human peripheral blood T cells.
Whole T-cell preparations were obtained from human PBMCs by either the nylon wool method (11) or the sheep erythrocyte (SRBC) rosette method (9). By the former method, the nonadherent cells (described above) were incubated in a column filled with nylon wool (Wako Pure Chemicals, Osako, Japan) for 1 h at 37°C with 5% CO2. The nonadherent T cells (but not the adherent B cells) were eluted with RPMI 1640 medium. The T-cell fraction contained 106 cells/ml. The purity of the T cells (number of CD3+ cells/total number of cells) was 92%; to determine the purity, the T-cell fraction was stained with PE-CD3 and examined by flow cytometry with an EPICS XL flow cytometer (Beckman Coulter, Fullerton, Calif.).
T-cell preparations were also obtained from human PBMCs by the SRBC rosette method. A purification step with antibody-coupled magnetic beads (Dynabeads; Dynal, Oslo, Norway) was repeated once, and the T cells were suspended in RPMI 1640 medium at a concentration of 106 cells/ml. The purity of the T cells, determined as described above, was 98%.
Stimulation of human PBMCs with TSST-1 and inhibition by anisodamine.
Human PBMCs (5 × 105 cells) in RPMI 1640 medium were added to each well of a 48-well plate (Becton Dickinson). TSST-1 was then added at 10 ng/ml, as described previously (9). In parallel experiments, anisodamine was added at concentrations ranging from 3 to 50 μg/ml. The plates were incubated at 37°C for 3 to 72 h with 5% CO2. The levels of immunoreactive TNF-α, IL-2, IFN-γ, and IL-10 in the cell-free culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA; Genzyme TECHNE, Minneapolis, Minn.), following the instructions of the manufacturer. The data are presented as the means ± standard deviations of experiments with PBMCs from six healthy adults.
Stimulation of peripheral blood T cells with anti-CD3 and anti-CD28 antibodies.
The peripheral blood T cells (5 × 105 cells) in RPMI 1640 medium prepared by the SRBC rosette method were added to each well of a 48-well plate, which was precoated with anti-CD3 and anti-CD28 antibodies (9). In parallel experiments, anisodamine was added at concentrations ranging from 3 to 50 μg/ml. The plates were incubated at 37°C for 12 to 48 h with 5% CO2. The levels of cytokines in the cell-free culture supernatants were measured as described above.
Reconstruction of a mixture of monocytes and T cells separately treated with anisodamine.
The monocytes and T cells prepared by the nylon wool method were separately treated with anisodamine (50 μg/ml) for 12 h at 37°C in RPMI 1640 medium. The cells were washed three times with RPMI 1640 medium. The monocytes and T cells, which were treated or not treated with anisodamine, were combined at a ratio of 1:9 in RPMI 1640 medium (a ratio of 1:9 was used, because monocytes were present at ca. 10% in PBMCs [5]). The concentrations were 106 cells/ml. The reconstructed cells were then stimulated with TSST-1 (10 ng/ml) for 48 h at 37°C with 5% CO2, and the amount of cytokines in the cell-free culture supernatants was measured.
Assay for cell viability.
Cell viability was measured by the trypan blue method (8). Cell samples were mixed with an equal volume of a 0.5% trypan blue solution, and the cells that excluded the dye were enumerated under a light microscope.
Quantitative assay of TSST-1-induced cytokine gene transcription and inhibition by anisodamine.
Human PBMCs (2.5 × 106 cells/ml) were stimulated with TSST-1 (10 ng/ml) for 3 h in the presence or the absence of anisodamine (50 μg/ml). The cells were suspended in Isogen (a mixture containing phenol and guanidine thiocyanate; Wako Pure Chemicals, Osaka, Japan) (39, 40) and stored at −30°C. Total cellular RNA was extracted with acid guanidinium thiocyanate-phenol-chloroform. The amount of RNA was assessed with a RiboGreen RNA quantitation kit (Molecular Probes, Breda, The Netherlands). Reverse transcription-PCR (RT-PCR) was performed as described previously (39, 40). The primers specific for GAPDH (glyceroaldehyde-3-phosphate dehydrogenase; a housekeeping gene) are described elsewhere (39, 40). The primers specific for TNF-α, IL-2, and IFN-γ have also been described previously (16). The primers specific for IL-10 were purchased from Continental Laboratory Products (San Diego, Calif.). The amplified PCR products were analyzed by gel electrophoresis with 1.5% agarose and stained with ethidium bromide. HaeIII-digested fragments of φX174 replicative-form DNA (Life Technologies, Gaithersburg, Md.) were used as molecular size standards. The specificities of the RT-PCR products were confirmed by restriction enzyme digestion (40). Quantitative measurements were made by a real-time PCR assay with SYBR Green I and an ABI Prism 7900HT sequence detector (Applied Biosystems, Foster City, Calif.) (28). Copy numbers (CNs) were calculated as follows: corrected cytokine mRNA CN = (cytokine mRNA CN/GAPDH mRNA CN) × GAPDH mRNA CN in unstimulated cells. Then, the relative CN (in percent) was obtained from the formula (corrected cytokine mRNA CN/corrected cytokine mRNA CN of unstimulated cells) × 100.
The percent inhibition of cytokine (e.g., TNF-α) mRNA expression by anisodamine was calculated as follows: (relative CN of TNF-α mRNA in cells stimulated with TSST-1 in the presence of anisodamine/relative CN of TNF-α mRNA in cells stimulated with TSST-1) × 100.
Assay for anti-NF-κB activities of anisodamine.
THP-1 cells (107 cells/ml) were stimulated with TSST-1 (10 μg/ml) for 1 h in the presence or the absence of anisodamine (50 μg/ml). The cells were then washed with phosphate-buffered saline; the cell nucleus was extracted; and the amount of the active form of NF-κB (p65) in the nucleus, which promotes gene transcription, was measured with an ELISA kit (NF-κB/p65 ActivELISA; Imgenex, San Diego, Calif.), according to the instructions of the manufacturer.
Assay for TSST-1-induced proliferative responses and inhibition by anisodamine.
The following two methods were used to evaluate the proliferative responses. In one experiment, flow cytometric analysis was performed as described previously (9). Briefly, PBMCs (2 × 106 cells/ml) were stimulated with TSST-1 (10 ng/ml) in the presence or the absence of anisodamine (50 μg/ml) for 3 days. This was followed by a further incubation with rIL-2 to allow reexpression of the T-cell receptor (3, 9). The cells were then stained with FITC-Vβ2 and PE-CD3 and examined by two-color flow cytometry with an EPICS XL flow cytometer (Beckman Coulter).
In another experiment, the incorporation of [3H]thymidine into cells was examined. Human PBMCs (106 cells) were stimulated with TSST-1 (10 ng/ml) in the presence or the absence of anisodamine (1 to 200 μg/ml) in 0.2-ml volumes in flat-bottom 96-well microplates (Corning Glass Works, Corning, N.Y.) for 72 h. The cultures were pulsed with 0.5 μCi (18.5 kBq) of [3H]thymidine for the last 16 h of the culture period, as described previously (9, 20).
TSST-1 challenge experiment with mice.
Specific-pathogen-free male C57BL/6 mice (age, 8 weeks; weight, 18 to 22 g) were used for the TSST-1 challenge experiment. They were purchased from Charles River Japan Inc. (Yokohama, Japan) and were fed a standard diet and water. Mice were sensitized by intraperitoneal (i.p.) injection of 20 mg of d-galactosamine 30 min before challenge with TSST-1, as described previously (22, 23). d-Galactosamine-sensitized mice have been described previously (4, 7, 23). To determine the 50% lethal dose (LD50) of TSST-1, d-galactosamine-sensitized mice (six mice in each group) were injected i.p. with 1, 2.5, 5, 10, and 15 μg of TSST-1; and the survival of the mice was monitored over 5 days; survival rates were 100, 100, 67, 50, and 17%, respectively, and the LD50 was determined to be 10 μg per mouse. d-Galactosamine-sensitized mice were then randomly divided into four groups (15 mice each): three groups injected with TSST-1 and one group injected with saline. TSST-1 at 10 μg, a dose that corresponded to the LD50, was injected i.p. into the mice. Of the three groups of mice that received TSST-1 injections, two groups were treated by i.p. injection of anisodamine (10 or 50 mg/kg of body weight) 10 min after the TSST-1 injection. The survival of the mice was monitored every 6 h after the TSST-1 injection, and deaths were recorded over 5 days.
Measurement of serum cytokine levels in mice.
Sera were periodically obtained by cardiac tapping from the mice sensitized with d-galactosamine as described above, and the levels of cytokines in sera were determined by ELISA.
Statistical analysis.
Data from the in vitro study were evaluated by Mann-Whitney's U test. Data on the survival rates of the treated and the untreated groups of mice were evaluated by Fisher's exact probability test. The level of significance was a P value less than 0.05.
RESULTS
Comparison of the inhibitory effects of tropane alkaloids and aspirin on TSST-1-induced TNF-α production in PBMCs.
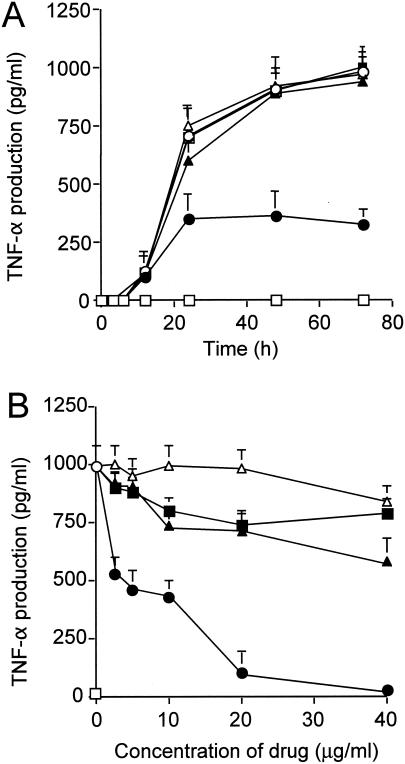
A marked induction of TNF-α was observed when human PBMCs were stimulated with TSST-1 for 3 to 72 h (Fig. 1A). The simultaneous addition of anisodamine (at 20 μg/ml) resulted in lower levels of TNF-α induction. The levels of inhibition caused by other tropane alkaloids (atropine and scopolamine) and aspirin (at 20 μg/ml) were not significant. When human PBMCs were stimulated with TSST-1 for 48 h in the presence of various concentrations of the drugs, anisodamine again manifested a much greater inhibitory effect at any concentration tested compared with those of other tropane alkaloids and aspirin (Fig. 1B).
FIG. 1.
Time course of TNF-α production in TSST-1-stimulated human PBMCs in the presence or the absence of anisodamine and related compounds (A) and the effects of various concentrations of the drugs (B). (A) PBMCs (5 × 105 cells) were stimulated with TSST-1 (10 ng/ml) for the indicated periods of time. Addition of the drugs (at 20 μg/ml) was as follows: open circles, none; closed circles, anisodamine; closed triangles, atropine (a tropane alkaloid related to anisodamine); closed squares, scopolamine (a tropane alkaloid related to anisodamine); open triangles, aspirin; open squares, TNF-α levels in control samples without TSST-1 stimulation. (B) PBMCs (5 × 105 cells) were stimulated with TSST-1 (10 ng/ml) for 48 h in the presence or the absence of the drugs. Open circles and open squares, TNF-α levels in control samples with and without TSST-1 stimulation, respectively. Addition of the drugs (at the indicated concentrations) was as follows: closed circles, anisodamine; closed triangles, atropine; closed squares, scopolamine; open triangles, aspirin. The levels of immunoreactive TNF-α in the cell-free culture supernatants (A and B) were measured by ELISA.
Proinflammatory and anti-inflammatory cytokine production in TSST-1-stimulated PBMCs and inhibition by anisodamine.
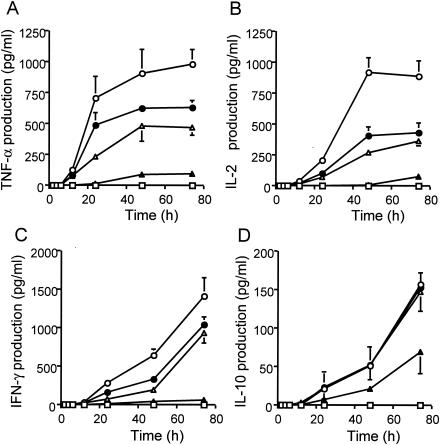
When human PBMCs were stimulated with TSST-1 for 3 to 72 h, TSST-1 induced the production of proinflammatory cytokines better than IL-10 (an anti-inflammatory cytokine) did (Fig. 2). The production of proinflammatory cytokines was inhibited by anisodamine in a dose-dependent manner (Fig. 2A to C). The production of IL-10 was inhibited to a much lesser extent (Fig. 2D). For instance, the rates of inhibition by anisodamine at 25 μg/ml (or at 50 μg/ml) after 72 h of incubation were 52.0% (90.3%) for TNF-α, 59.0% (91.8%) for IL-2, and 33.8% (95.5%) for IFN-γ; but the rate was only 6.7% (56.4%) for IL-10 (P < 0.05). Significant inhibition of the production of IL-10 by anisodamine was observed only at concentrations greater than 50 μg/ml (Fig. 2D).
FIG. 2.
Time course of proinflammatory and anti-inflammatory cytokine production in TSST-1-stimulated human PBMCs and effect of anisodamine addition. PBMCs (5 × 105 cells) were stimulated with TSST-1 (10 ng/ml) in the presence or the absence of anisodamine. Cell-free culture supernatants were periodically taken, and the levels of immunoreactive cytokines in the supernatants were measured by ELISA. Concentrations of anisodamine added: open circles, none; closed circles, 12.5 μg/ml; open triangles, 25 μg/ml; closed triangles, 50 μg/ml. Open squares, cytokine levels in control samples without TSST-1 treatment. (A) TNF-α; (B) IL-2; (C) IFN-γ; (D) IL-10.
Direct inhibitory effect of anisodamine on T cells and monocytes.
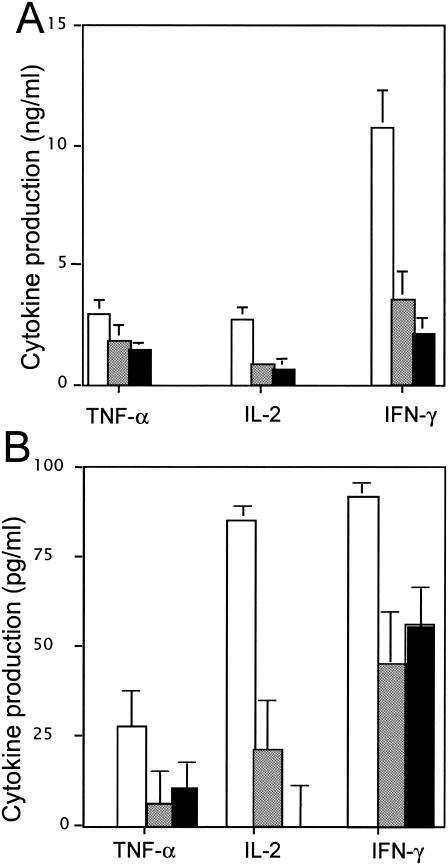
To investigate whether the action of anisodamine is directed at T cells, human peripheral blood T cells were isolated and stimulated with anti-CD3 and anti-CD28 antibodies for 48 h in the presence or the absence of anisodamine. Stimulation with the antibodies in the absence of anisodamine caused a marked induction of TNF-α, IL-2, and IFN-γ; and the simultaneous addition of anisodamine significantly decreased the responses (Fig. 3A). The rates of inhibition by anisodamine at 25 μg/ml (or at 50 μg/ml) were 37.9% (51.7%) for TNF-α, 67.3% (80.4%) for IL-2, and 70.0% (77.8%) for IFN-γ. The inhibitory levels observed were comparable to the TSST-1-stimulated responses in human PBMCs.
FIG. 3.
Direct action of anisodamine on T cells or monocytes prepared from human PBMCs. (A) Human peripheral blood T cells (5 × 105 cells) were stimulated with anti-CD3 and anti-CD28 antibodies in the presence or the absence of anisodamine for 48 h. The culture supernatants were assayed for cytokine levels by ELISA. The concentrations of anisodamine added were as follows: open bars, none; shaded bars, 25 μg/ml; solid bars, 50 μg/ml. (B) Reconstruction experiments were performed with isolated human peripheral blood T cells and monocytes. The compositions of the cells were as follows: open bars, T cells plus monocytes; shaded bars, T cells plus monocytes pretreated with anisodamine; solid bars, T cells pretreated with anisodamine plus monocytes. The mixed cells were stimulated with TSST-1 (10 ng/ml) for 48 h, and the culture supernatants were assayed for cytokines by ELISA.
Next, reconstruction experiments were performed. For this, isolated T cells or isolated monocytes were separately pretreated with anisodamine (50 μg/ml), washed, and then mixed together for experiments with TSST-1 (Fig. 3B). When only T cells were pretreated with anisodamine and then mixed, the rates of inhibition were 59% for TNF-α, 98% for IL-2, and 39% for IFN-γ, while when only monocytes were pretreated with anisodamine and then mixed, the rates were 77.2% for TNF-α, 75% for IL-2, and 51.3% for IFN-γ.
Effects of anisodamine on post-TSST-1-stimulation in PBMC.
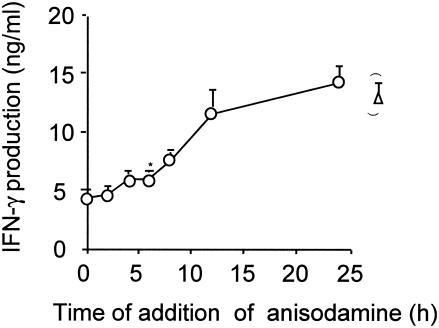
Human PBMCs was stimulated with TSST-1, anisodamine was periodically added, and then the cytokine (IFN-γ) levels were examined after 48 h of incubation (Fig. 4). The inhibitory effect of anisodamine was significant up to 6 h poststimulation with TSST-1 (P < 0.05). The inhibitory effect was no longer significant from 9 h poststimulation. Inhibitory effects similar to those shown in Fig. 4 were observed with TNF-α and IL-2 (data not shown).
FIG. 4.
Effects of anisodamine on TSST-1-induced production of IFN-γ by human PBMCs. Human PBMCs were stimulated with TSST-1 (10 ng/ml). Anisodamine was then added at the indicated times to the stimulated cell cultures at a concentration of 50 μg/ml (open circles). Cytokine levels in the cell-free culture supernatants were measured 48 h after TSST-1 stimulation. The data on the right (open triangle) represent cytokine levels in the absence of anisodamine. The results were obtained from triplicate experiments. *, P < 0.05 compared with the results for the TSST-1-stimulated cases in the absence of anisodamine. Similar inhibitory effects were also obtained with TNF-α and IL-2.
Inhibition of TSST-1-induced cytokine gene transcription by anisodamine.
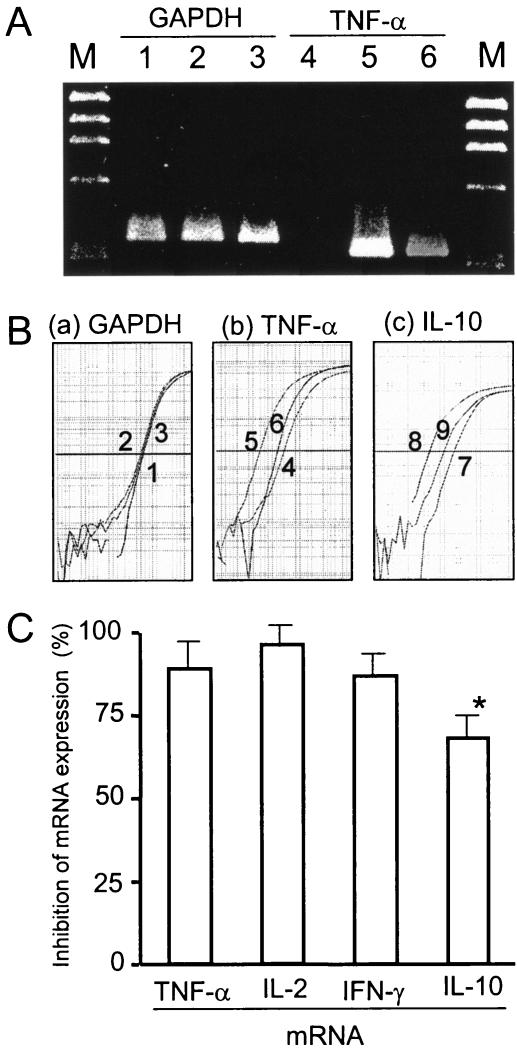
Human PBMCs were stimulated with TSST-1 for 3 h in the presence or the absence of anisodamine. Total cellular RNA was extracted, and cytokine (TNF-α) mRNA levels were determined on gels (Fig. 5A). Human PBMCs not stimulated with TSST-1 gave a very faint band of TNF-α mRNA, while TSST-1-stimulated human PBMCs gave an obvious band of TNF-α mRNA, the intensity of which diminished markedly on the simultaneous addition of anisodamine (Fig. 5A, lanes 4 to 6). In contrast, the transcription of the GAPDH housekeeping gene was not affected by these treatments (Fig. 5A, lanes 1 to 3), indicating the equivalent loading of the samples and also the fact that anisodamine did not cause a general degradation of the mRNA.
FIG. 5.
Suppression of TSST-1-induced cytokine mRNA expression by anisodamine. (A) Effect of anisodamine on TNF-α mRNA expression was examined by RT-PCR. Human PBMCs (2.5 × 106 cells) were stimulated with TSST-1 (10 ng/ml) for 3 h in the presence or the absence of anisodamine (50 μg/ml). After incubation, the PBMCs were procured and total cellular RNA was extracted. mRNA was reverse transcribed and amplified by PCR with cytokine-specific primers. RT-PCR products: lanes 1 to 3, the housekeeping gene GAPDH; lanes 4 to 6, the TNF-α gene; lanes 1 and 4, no TSST-1 stimulation; lanes 2 and 5, TSST-1 stimulation; lanes 3 and 6, TSST-1 stimulation in the presence of anisodamine; lanes M, molecular size marker (HaeIII-digested φX174 replicative-form DNA fragments). (B, parts a and b) Experiments similar to those for TNF-α gene expression whose results are shown in panel A were performed by a real-time PCR assay. The relative copy numbers of the gene sequences for GAPDH (a) and TNF-α (b) were estimated as described in Materials and Methods. Samples 1 to 6 correspond to those in lanes 1 to 6 in panel A. (B, part c) IL-10 gene expression examined by a real-time PCR assay, as described above. Sample 7, no TSST-1 stimulation; sample 8, TSST-1 stimulation; sample 9, TSST-1 stimulation in the presence of anisodamine. (C) On the basis of the relative copy numbers obtained in the experiment whose results are shown in panel B, the percent inhibition of TNF-α and IL-10 mRNA expression by anisodamine was calculated and is shown. Experiments similar to the experiments whose results are shown in panel B were performed with IL-2 and IFN-γ, and the percent inhibition by anisodamine was evaluated and is also shown. *, P < 0.05 compared with the data for the proinflammatory cytokines (TNF-α, IL-2, and IFN-γ).
The TNF-α mRNA levels were also quantitatively measured by a real-time PCR assay (Fig. 5B, parts a and b). On the basis of the results of the real-time PCR assay (Fig. 5B, part b), the inhibition by anisodamine was calculated to be 88.8% (for TNF-α) (Fig. 5C). Anisodamine also interfered with the transcription of IL-2 and IFN-γ by 96.0 and 86.8%, respectively (Fig. 5C). Consistent with the cytokine production levels, inhibition of transcription of IL-10 by anisodamine was observed to a lesser extent (67.5%) (Fig. 5B, part c, and C).
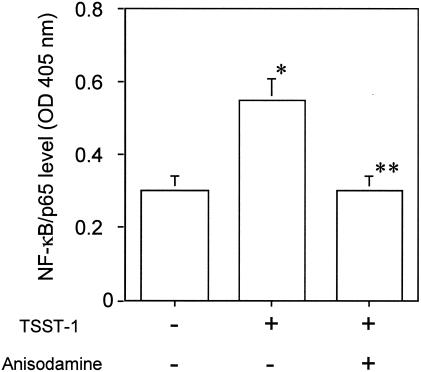
Inhibition of TSST-1-induced NF-κB activation in THP-1 cells by anisodamine.
THP-1 cells were stimulated with TSST-1 for 1 h in the presence or the absence of anisodamine. The stimulation caused the activation of NF-κB, as demonstrated by detection of the active form in a complex with p65 in the nucleus (Fig. 6). However, this effect was diminished by the simultaneous addition of anisodamine (Fig. 6).
FIG. 6.
Inhibition of TSST-1-induced NF-κB activation in THP-1 cells by anisodamine. THP-1 cells were stimulated with TSST-1 (10 μg/ml) for 1 h in the presence or the absence of anisodamine (50 μg/ml). Nuclear extracts were prepared, and the amount of activated NF-κB (a complex with p65) was measured with an ELISA kit with anti-65 antibodies. *, P < 0.05 compared with the NF-κB level without TSST-1 stimulation; **, P < 0.05 compared with the NF-κB level with TSST-1 stimulation in the absence of anisodamine.
Effect of anisodamine on cell viability.
The addition of anisodamine (up to 50 μg/ml) to human PBMCs, monocytes, T cells, or THP-1 cells resulted in no significant loss of viability, as evidenced by the results of the trypan blue exclusion assay. More than 98% of the cells survived.
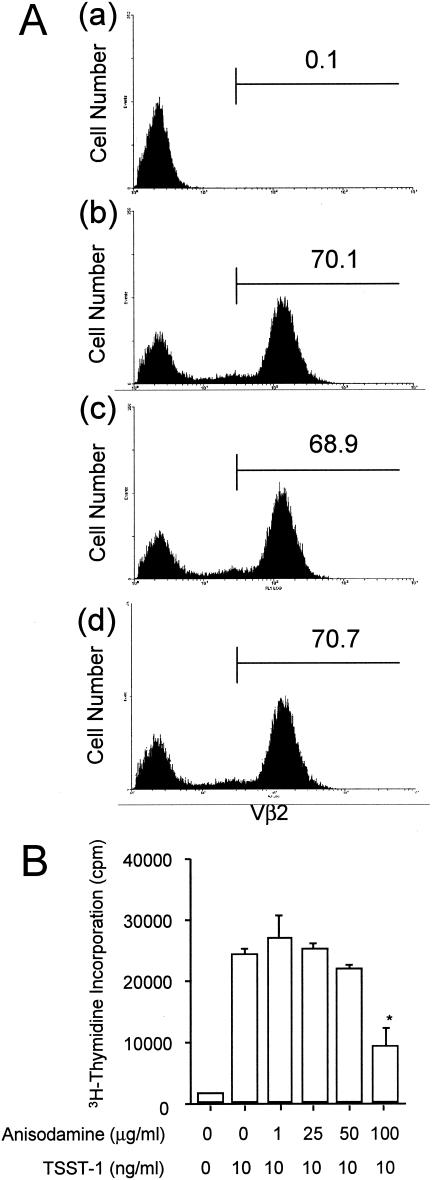
Inhibition of TSST-1-induced proliferative responses of PBMCs by anisodamine.
Two methods were used to determine the levels of inhibition of the TSST-1-induced proliferative responses of PBMCs by anisodamine. In one experiment, the change of the Vβ2+ T-cell population in human PBMCs was assessed by flow cytometric analysis (Fig. 7A). Stimulation of human PBMCs with TSST-1 caused a drastic expansion of the Vβ2+ T-cell population; 0.1% for unstimulated PBMCs (control) versus 70.1% for stimulated PBMCs (Fig. 7A, parts a and b). The simultaneous addition of anisodamine at 50 μg/ml (Fig. 7A, part c) or 100 μg/ml (Fig. 7A, part d), however, had no significant inhibitory effect on the proliferation of the Vβ2+ T cells (70.1% for stimulated PBMCs versus 68.9 to 70.7% for stimulated PBMCs in the presence of anisodamine) (Fig. 7A, parts b to d), in contrast to the effect on the production of cytokines (as described above).
FIG. 7.
Inhibition of the TSST-1-stimulated proliferative responses of human PBMCs by anisodamine. (A) PBMCs (2 × 106 cells/ml) were stimulated with TSST-1 (10 ng/ml) for 72 h in the presence or the absence of anisodamine (50 or 100 μg/ml). To allow reexpression of the T-cell receptor, the cells were further treated with rIL-2. The cells were stained with FITC-Vβ2 and PE-CD3 antibodies, and then the labeled cells were examined by flow cytometric analysis. (Part a) CD3+ cells (T cells) in PBMCs without TSST-1 treatment; (part b) CD3+ cells (T cells) in PBMCs after stimulation with TSST-1; (part c) CD3+ cells (T cells) in PBMCs after stimulation with TSST-1 in the presence of 50 μg of anisodamine per ml; (part d) CD3+ cells (T cells) in PBMCs after stimulation with TSST-1 in the presence of 100 μg of anisodamine per ml. The numbers within each panel represent the percentage of Vβ2+ T cells. (B) PBMCs were stimulated with TSST-1 (10 ng/ml) for 72 h in the presence or the absence of various concentrations of anisodamine. The cultures were then pulsed with [3H]thymidine for 16 h, and the amount of [3H]thymidine (in counts per minute) incorporated into PBMCs was measured. *, P < 0.05 compared with the levels of [3H]thymidine incorporated in the TSST-1-stimulated cells in the absence of anisodamine.
In the other experiment, the incorporation of [3H]thymidine into TSST-1-stimulated human PBMCs was examined. Again, stimulation of PBMCs with TSST-1 caused a drastic increase in [3H]thymidine levels, and the simultaneous addition of anisodamine at 50 μg/ml resulted in no significant inhibitory effect (Fig. 7B), although in this experiment anisodamine at 100 μg/ml exhibited an inhibitory effect to some extent (63.4%) (Fig. 7B). This inhibition was not due to cell death, because in the presence of 100 μg of anisodamine per ml, more than 96% of the PBMCs survived, as evidenced by the trypan blue exclusion assay.
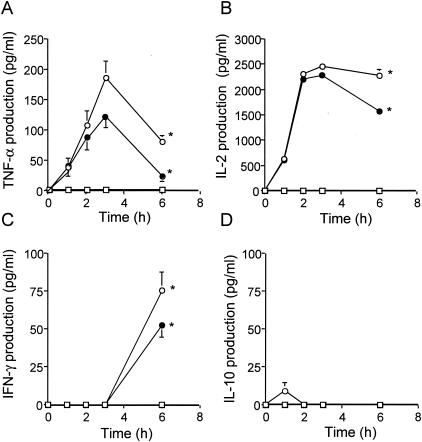
Inhibitory effect of anisodamine on serum cytokine levels in mice.
d-Galactosamine-sensitized mice were treated with TSST-1 (10 μg), and the cytokine levels in serum were determined periodically (Fig. 8). The TNF-α and IL-2 levels peaked at about 3 h and 2 to 3 h, respectively (Fig. 8A and B), and IFN-γ appeared later than 3 h (at about 6 h; Fig. 8C) after the TSST-1 injection. The degree of enhancement of IL-10 production in mice injected with TSST-1 was extremely low (Fig. 8D).
FIG. 8.
Effects of anisodamine on proinflammatory and anti-inflammatory cytokine levels in serum after TSST-1 injection in mice. Groups of 12 mice were injected i.p. with 20 mg of d-galactosamine 1 h before an i.p. challenge with 10 μg of TSST-1. Treatment (i.p. injection) with anisodamine at 50 mg/kg of body weight was carried out 10 min after the TSST-1 injection. Sera were obtained periodically by cardiac tapping, and the cytokine levels in sera were determined by ELISA. (A) TNF-α; (B) IL-2; (C) IFN-γ; (D) IL-10. Anisodamine treatment of mice injected with TSST-1 was as follows: open circles, none; closed circles, 50 mg/kg. Open squares, no TSST-1 injection (control). *, P < 0.05.
Anisodamine treatment (dose, 50 mg/kg of body weight) of the mice injected with TSST-1 significantly reduced the concentrations of TNF-α, IL-2, and IFN-γ in serum compared with those in saline-treated (control) mice (P < 0.05).
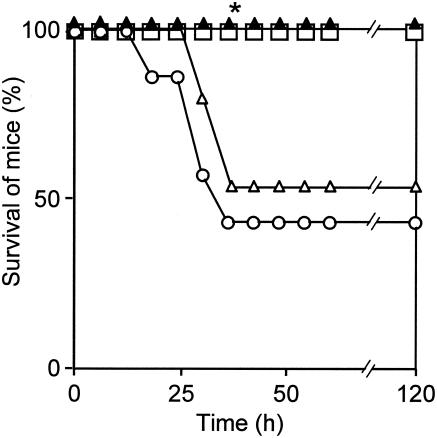
Protective effect of anisodamine against TSST-1 in mice.
The protective effect of anisodamine against TSST-1 was examined in d-galactosamine-sensitized mice (Fig. 9). The injection of TSST-1 (10 μg) into mice resulted in 60.0% mortality (40.0% survival). In contrast, anisodamine treatment (dose, 50 mg/kg of body weight) of the mice injected with TSST-1 resulted in a significantly higher rate of survival (100%) over 5 days (P < 0.05).
FIG. 9.
Protection of mice from the lethality of TSST-1 by anisodamine. Injection of TSST-1 and treatment of d-galactosamine-sensitized mice with anisodamine (50 mg/kg) were carried out as described in the legend to Fig. 8. d-Galactosamine-sensitized mice injected only with saline (with no TSST-1) were used as controls (open squares). Treatment with anisodamine of mice injected with TSST-1 was as follows: open circles, none; open triangles, 10 mg/kg of body weight; closed triangles, 50 mg/kg of body weight. *, P < 0.05 compared with the TSST-1-injected, untreated mice (open circles).
DISCUSSION
S. aureus produces a number of superantigenic toxins, such as TSST-1 (21, 33). TSST-1 is a potent T-cell activator and a major cause of TSS (33). TSST-1 directly binds to the MHC class II molecules on APCs and to the T-cell receptor-bearing specific Vβ elements of T cells. In adults, this trimolecular interaction leads to the massive proliferation of TSST-1-reactive, specific Vβ region-expressing CD4+ T cells such as Vβ2+ and Vβ4+ T cells. In addition, TSST-1-stimulated adult peripheral blood CD4+ T cells show strong responses to restimulation with TSST-1 and stimulation with IL-2 in vitro (12, 13, 19, 21). This abnormal activation of CD4+ T cells leads to the overproduction of proinflammatory cytokines such as TNF-α, IL-1β, IL-2, and IFN-γ, resulting in an abnormal reaction in the whole body (12, 33).
MRSA infection in neonatal intensive care units (NICUs) has become a serious problem in Japan. In the early neonatal period, especially in newborns in NICUs, TSST-1 produced from MRSA induces NTED (30-32). Vβ2+ T cells selectively expanded in NTED patients in the acute phase were anergic to stimulation with TSST-1, and this was thought to be the cause of the spontaneous reduction of the symptoms (15). Also, in the acute phase, the levels of cytokines such as TNF-α in serum are high (27).
Anisodamine, an alkaloid extracted from a Chinese herb, is a vasoactive drug that appears to be efficacious for the treatment of clinical and experimental bacteremic shock and has been known for its therapeutic effect on acute disseminated intravascular coagulation since 1965 (29, 37, 38, 41). It has also been demonstrated to improve the mortality rate from meningococcemia by from 67 to 12.4% (37).
In previous studies, we demonstrated that anisodamine has an inhibitory effect on the production of TNF-α, IL-1β, and IL-8 from Stx-stimulated human monocytes; prolongs the survival of mice injected with Stx; and decreases the lethality of Stx (39, 40). Stx is the major toxin responsible for HUS caused by enterohemorrhagic E. coli (14); and the host's inflammatory responses, including the TNF-α, IL-1β, IL-6, and IL-8 responses, are involved in the development of HUS (10).
Anisodamine inhibits lipopolysaccharide (LPS)-induced TNF-α gene expression and has an antishock effect (36). It also inhibits LPS-induced activation of the NF-κB pathway (26). However, the inhibitory effects of anisodamine on the actions of superantigenic toxins from gram-positive bacteria have not been investigated.
In this study, we demonstrated that anisodamine remarkably inhibits TSST-1-stimulated proinflammatory cytokine (TNF-α, IL-2, and IFN-γ) production in human PBMCs. This inhibitory effect of anisodamine was greater than that of any other tropane alkaloid examined. Aspirin, which has been shown to inhibit IL-4 production (2), had no effect on the TSST-1-stimulated production of proinflammatory cytokines. In addition, we demonstrated some specificity to the inhibitory action of anisodamine. Anisodamine inhibited proinflammatory cytokine production better than IL-10 (an anti-inflammatory cytokine) production in TSST-1-stimulated PBMCs, although only one anti-inflammatory cytokine has been studied.
We also examined the mechanisms of action of anisodamine. Anisodamine inhibited the production of TNF-α, IFN-γ, and IL-2 in anti-CD3 and anti-CD28 antibody-stimulated peripheral blood T cells; and in combination experiments, in which isolated T cells were pretreated with anisodamine and then washed and added back to the monocytes, the inhibition of TSST-1-induced cytokine production was again obvious. These results suggested that anisodamine acts directly on T cells (responding to TSST-1).
Anisodamine exerted its inhibitory effect via down-regulation at the mRNA level. Again, the inhibition of TSST-1-induced mRNA synthesis by anisodamine was significantly greater for proinflammatory cytokines than for IL-10 (an anti-inflammatory cytokine).
Trede et al. (34) reported that TSST-1 can stimulate the human monocytic cell line THP-1 to activate NF-κB. In our experiment, in which isolated monocytes were pretreated with anisodamine and then washed and added back to the T cells, the inhibition of TSST-1-induced cytokine production was remarkable, indicating that anisodamine acts directly even on monocytes (which are crucial in presenting surperantigens). So, using THP-1 cells, we examined whether or not TSST-1-induced NF-κB activation is inhibited by anisodamine. Such inhibition was demonstrated in this study. It is therefore conceivable that the inhibition of TSST-1-induced cytokine production in human PBMCs by anisodamine takes place via the inhibition of the NF-κB activation pathway.
The inhibition of cytokine production by anisodamine was obvious with anisodamine at a concentration of 50 μg/ml or less. However, the inhibition of TSST-1-induced cell proliferation by anisodamine was observed at concentrations greater than 100 μg/ml and was not significant at 50 μg/ml or less. This was true in the experiment in which the incorporation of [3H]thymidine into TSST-1-stimulated human PBMCs was examined. In particular, in the experiment in which flow cytometry was used to assess the Vβ2+ T-cell population in human PBMCs, no significant inhibition of Vβ2+ T-cell proliferation was observed. Thus, it was concluded that anisodamine at concentrations less than 50 μg/ml preferentially inhibits TSST-1-stimulated cytokine production but not TSST-1-induced cell proliferation in human PBMCs. This, together with the finding that anisodamine inhibits TSST-1-induced proinflammatory cytokine production better than IL-10 (an anti-inflammatory cytokine) production in human PBMCs, clearly shows the specific nature of the action of anisodamine in human PBMCs.
It has been shown that TSST-1 stimulates PBMCs to produce cytokines such as IL-2, which in turn causes an abnormal proliferation of T cells, which leads to the overproduction of proinflammatory cytokines (12, 13, 19, 21). Although anisodamine inhibits cytokine (including IL-2) production by TSST-1-stimulated PBMCs, T-cell proliferation proceeds even in the presence of anisodamine. A most likely explanation for this is that the inhibitory action of anisodamine against IL-2 production is incomplete and there is IL-2 leakage, and thus, T-cell proliferation is still achieved by stimulation with the IL-2 which was produced in the presence of anisodamine. It has been reported that T-cell proliferation is induced even by a low concentration of IL-2 (6, 24).
T-cell proliferation and cytokine production are not always observed in parallel, and in some cases, cytokine production is induced in the situation in which T-cell numbers remain constant (or decreased) (H. Watanabe, personal communication). It would be conceivable that there is a specific step(s) for cytokine induction and that anisodamine preferentially inhibits such a specific step(s) for cytokine induction (rather than steps for T-cell proliferation).
Several agents have been shown to inhibit TSST-1-stimulated cytokine production. IL-10 inhibits the TSST-1-stimulated production of TNF-α, IL-1, IL-6, and IFN-γ by human PBMCs; but it has less of an effect on TSST-1-induced T-cell proliferation (18). Interestingly, this inhibition is very similar to that observed in this study.
In contrast, dexamethasone, an anti-inflammatory agent, inhibits both TSST-1-induced cytokine production and T-cell proliferation via the suppression of TSST-1-enhanced expression of the IL-2 receptor, CD25 (18). Pentoxifylline, a methylxanthine derivative that inhibits endotoxemia and the LPS-induced release of TNF-α, inhibits TSST-1-induced TNF-α, IL-1β, and IFN-γ production by human PBMCs and T-cell proliferation (20). In addition, pentoxifylline protects mice from the lethality of TSST-1 (20). Wortmannin, a phosphatidylinositol 3-kinase inhibitor, inhibits TSST-1-induced TNF-α production by human PBMCs (25).
Mice are generally resistant to the effects of bacterial toxins (including staphylococcal superantigens), but pretreatment of mice with d-galactosamine increases their sensitivity (7, 23). d-Galactosamine is a hepatotoxic agent that causes depletion of UTP and changes in uracyl nucleotides, resulting in impaired biosynthesis of the macromolecular cell constituents in hepatocytes (4), and this in turn increases the sensitivity of mice to the lethal effects of endotoxin more than 100,000-fold (7). Just like the case of endotoxin, lethal shock by a staphylococcal superantigen is also induced in d-galactosamine-sensitized mice (23).
In this study with d-galactosamine-sensitized mice, we demonstrated that the levels of cytokines induced by TSST-1 in serum were significantly reduced by anisodamine and that anisodamine indeed protected the mice against the lethal effect of TSST-1. The concentration of anisodamine used in the in vitro study may appear to be high. In clinical use, doses of anisodamine as high as 30 to 50 mg/kg may be given intravenously several times a day. Thus, despite a rapid half-life (estimated to be 40 min) in plasma, peak levels in plasma that approach high concentrations can be seen (1). It is thought that the concentrations that we used are consistent with those that are used in the clinical situation. The Chinese medicine anisodamine, which is used as a vasoactive agent in children in China, may be a potential drug for the treatment of TSST-1-mediated diseases.
In addition, anisodamine possesses beneficial effects against various infections (infections caused by gram-negative bacteria [26, 29, 36-41]) as well. Thus, the pharmacological action of anisodamine is not superantigen specific. Perhaps anisodamine inhibits a key cytokine expression signal cascade shared by T cells and monocytes (and macrophages).
Acknowledgments
We thank Hisami Watanabe for discussion.
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Culture and Sports of Japan and a grant from the Kampou Science Foundation of Japan.
REFERENCES
- 1.Anonymous, from the Chinese Academy of Medical Sciences and Beijing Friendship Hospital. 1973. Absorption, distribution and excretion of anisodamine. Zhonghua Yi Xue Za Zhi 5:274-278. [PubMed] [Google Scholar]
- 2.Cianferoni, A., J. T. Schroeder, J. Kim, J. W. Schmidt, L. M. Lichtenstein, S. N. Georas, and V. Casolaro. 2001. Selective inhibition of interleukin-4 gene expression in human T cells by aspirin. Blood 97:1742-1749. [DOI] [PubMed] [Google Scholar]
- 3.Davison, S., M. Allen, R. Vaughan, and J. Barker. 2000. Staphylococcal toxin-induced T cell proliferation in atopic eczema correlates with increased use of superantigen-reactive Vbeta-chains in cutaneous lymphocyte-associated antigen (CLA)-positive lymphocytes. Clin. Exp. Immunol. 121:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decker, K., and D. Keppler. 1974. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev. Physiol. Biochem. Pharmacol. 71:77-106. [DOI] [PubMed] [Google Scholar]
- 5.Delles, A. M., K. Rittenhouse-Olson, J. Morgan, and A. R. Oseroff. 2002. A simple method for the purification of human peripheral blood antigen presenting cells (dendritic cells, monocytes/macrophages, and B lymphocytes). Immunol. Investig. 31:233-245. [DOI] [PubMed] [Google Scholar]
- 6.Fathman, C. G., and J. G. Frelinger. 1983. T-lymphocyte clones. Annu. Rev. Immunol. 1:633-655. [DOI] [PubMed] [Google Scholar]
- 7.Galanos, C., M. A. Freudenberg, and W. Reutter. 1979. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 76:5939-5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatti, R., S. Belletti, J. Uggeri, M. V. Vettori, A. Mutti, R. Scandroglio, and G. Orlandini. 2004. Methylmercury cytotoxicity in PC12 cells is mediated by primary glutathione depletion independent of excess reactive oxygen species generation. Toxicology 204:175-185. [DOI] [PubMed] [Google Scholar]
- 9.Imanishi, K., K. Seo, H. Kato, T. Miyoshi-Akiyama, R. H. Zhang, Y. Takanashi, Y. Imai, and T. Uchiyama. 1998. Post-thymic maturation of migrating human thymic single-positive T cells: thymic CD1a− CD4+ T cells are more susceptible to anergy induction by toxic shock syndrome toxin-1 than cord blood CD4+ T cells. J. Immunol. 160:112-119. [PubMed] [Google Scholar]
- 10.Inward, C. D., M. Varagunam, D. Adu, D. V. Milford, and C. M. Taylor. 1997. Cytokines in haemolytic uraemic syndrome associated with verocytotoxin-producing Escherichia coli infection. Arch. Dis. Child. 77:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julius, M. H., E. Simpson, and L. A. Herzenberg. 1973. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur. J. Immunol. 3:645-649. [DOI] [PubMed] [Google Scholar]
- 12.Kalyan, S., and A. W. Chow. 2004. Human peripheral gammadelta T cells potentiate the early proinflammatory cytokine response to staphylococcal toxic shock syndrome toxin-1. J. Infect. Dis. 189:1892-1896. [DOI] [PubMed] [Google Scholar]
- 13.Kappler, J., B. Kotzin, L. Herron, E. W. Gelfand, R. D. Bigler, A. Boylston, S. Carrel, D. N. Posnett, Y. Choi, and P. Marrack. 1989. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science 244:811-813. [DOI] [PubMed] [Google Scholar]
- 14.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. Infect. Dis. 151:775-782. [DOI] [PubMed] [Google Scholar]
- 15.Kato, H., N. Takahashi, Y. Arimura, K. Imanishi, H. Nishida, and T. Uchiyama. 2002. The percentage of superantigen-reactive T cells in peripheral blood significantly decreases before massively increasing in patients with neonatal TSS-like exanthematous disease in the early acute phase. J. Infect. Chemother. 8:111-114. [DOI] [PubMed] [Google Scholar]
- 16.Kim, Y. S., S. Zheng, S. H. Yang, H. L. Kim, C. S. Lim, D. W. Chae, R. Chun, J. S. Lee, and S. Kim. 2001. Differential expression of various cytokine and chemokine genes between proliferative and non-proliferative glomerulonephritides. Clin. Nephrol. 56:199-206. [PubMed] [Google Scholar]
- 17.Kobayashi, H., Y. Tanaka, J. Yagi, H. Toma, and T. Uchiyama. 2001. Gamma/delta T cells provide innate immunity against renal cell carcinoma. Cancer Immunol. Immunother. 50:115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krakauer, T. 1995. Inhibition of toxic shock syndrome toxin-1-induced cytokine production and T cell activation by interleukin-10, interleukin-4, and dexamethasone. J. Infect. Dis. 172:988-992. [DOI] [PubMed] [Google Scholar]
- 19.Krakauer, T. 1999. Immune response to staphylococcal superantigens. Immunol. Res. 20:163-173. [DOI] [PubMed] [Google Scholar]
- 20.Krakauer, T., and B. G. Stiles. 1999. Pentoxifylline inhibits superantigen-induced toxic shock and cytokine release. Clin. Diagn. Lab. Immunol. 6:594-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, H., A. Llera, and R. A. Mariuzza. 1998. Structure-function studies of T-cell receptor-superantigen interactions. Immunol. Rev. 163:177-186. [DOI] [PubMed] [Google Scholar]
- 22.Miethke, T., K. Duschek, C. Wahl, K. Heeg, and H. Wagner. 1993. Pathogenesis of the toxic shock syndrome: T cell mediated lethal shock caused by the superantigen TSST-1. Eur. J. Immunol. 23:1494-1500. [DOI] [PubMed] [Google Scholar]
- 23.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J. Exp. Med. 175:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabholz, M., and H. R. MacDonald. 1983. Cytolytic T lymphocytes. Annu. Rev. Immunol. 1:273-306. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez, M., N. Fernandez-Troy, M. Buxade, R. P. Casaroli-Marano, D. Benitez, C. Perez-Maldonado, and E. Espel. 1999. Wortmannin inhibits translation of tumor necrosis factor-alpha in superantigen-activated T cells. Int. Immunol. 11:1479-1489. [DOI] [PubMed] [Google Scholar]
- 26.Ruan, Q. R., W. J. Zhang, P. Hufnagl, C. Kaun, B. R. Binder, and J. Wojta. 2001. Anisodamine counteracts lipopolysaccharide-induced tissue factor and plasminogen activator inhibitor-1 expression in human endothelial cells: contribution of the NF-kappa B pathway. J. Vasc. Res. 38:13-19. [DOI] [PubMed] [Google Scholar]
- 27.Soderquist, B., K. Kanclerski, K. G. Sundqvist, P. Colque-Navarro, H. Holmberg, T. Vikerfors, and R. Mollby. 1998. Cytokine response to staphylococcal exotoxins in Staphylococcus aureus septicemia. Clin. Microbiol. Infect. 4:366-372. [DOI] [PubMed] [Google Scholar]
- 28.Stordeur, P., L. F. Poulin, L. Craciun, L. Zhou, L. Schandene, de A. Lavareille, S. Goriely, and M. Goldman. 2002. Cytokine mRNA quantification by real-time PCR. J. Immunol. Methods 259:55-64. [DOI] [PubMed] [Google Scholar]
- 29.Su, J. Y., L. Wu, and C. Tang. 1983. Experimental study in rabbits of the antishock effect of anisodamine (654-2), and its mechanism of action. Resuscitation 10:173-184. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi, N., H. Kato, K. Imanishi, K. Miwa, S. Yamanami, H. Nishida, and T. Uchiyama. 2000. Immunopathophysiological aspects of an emerging neonatal infectious disease induced by a bacterial superantigen. J. Clin. Investig. 106:1409-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi, N., H. Nishida, H. Kato, K. Imanishi, Y. Sakata, and T. Uchiyama. 1998. Exanthematous disease induced by toxic shock syndrome toxin 1 in the early neonatal period. Lancet 351:1614-1619. [DOI] [PubMed] [Google Scholar]
- 32.Takizawa, Y., I. Taneike, S. Nakagawa, S. Ishii, T. Kurabayashi, K. Tanaka, F. Gejyo, and T. Yamamoto. 2003. Molecular DNA analysis of methicillin-resistant Staphylococcus aureus (MRSA) infection in a neonatal intensive care unit. Acta Med. Biol. 51:103-109. [Google Scholar]
- 33.Todd, J. K. 1998. Toxic shock syndrome. Clin. Microbiol. Rev. 1:432-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trede, N. S., E. Castigli, R. S. Geha, and T. Chatila. 1993. Microbial superantigens induce NF-kappa B in the human monocytic cell line THP-1. J. Immunol. 150:5604-5613. [PubMed] [Google Scholar]
- 35.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 36.Wang, L. Z., Y. Q. Liu, Y. H. Cui, F. H. Zhu, B. S. Wang, and N. Lun. 1999. Effects of dexamethasone, cyproheptadine, anisodamine, and dinoprostone on TNF alpha production in endotoxic shock. Zhongguo Yao Li Xue Bao 20:171-174. [PubMed] [Google Scholar]
- 37.Xiu, R. J. 1980. Studies on microcirculation in Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Microvasc. Res. 20:371-373. [DOI] [PubMed] [Google Scholar]
- 38.Xiu, R. J., D. E. Hammerschmidt, P. A. Coppo, and H. S. Jacob. 1982. Anisodamine inhibits thromboxane synthesis, granulocyte aggregation, and platelet aggregation. A possible mechanism for its efficacy in bacteremic shock. JAMA 247:1458-1460. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, H. M., Z. L. Ou, F. Gondaira, M. Ohmura, S. Kojio, and T. Yamamoto. 2001. Protective effect of anisodamine against Shiga toxin-1: inhibition of cytokine production and increase in the survival of mice. J. Lab. Clin. Med. 137:93-100. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, H. M., Z. L. Ou, and T. Yamamoto. 2001. Anisodamine inhibits Shiga toxin type 2-mediated tumor necrosis factor-alpha production in vitro and in vivo. Exp. Biol. Med. 226:597-604. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, S., A. M. Chang, C. F. Li, Z. J. Li, Z. J. Yin, X. Zhao, S. L. Liang. 1987. Mechanism of the therapeutic effect of anisodamine in disseminated intravascular coagulation: study of platelet adhesion and aggregation, malondialdehyde, thromboxane B2, 6-keto-prostaglandin F1α, and microcirculation. Exp. Hematol. 15:65-71. [PubMed] [Google Scholar]