Abstract
The management of staphylococcal diseases is increasingly difficult with present medical approaches. Preventive and therapeutic vaccination is considered to be a promising alternative; however, little is known about immune correlates of protection and disease susceptibility. To better understand the immune recognition of Staphylococcus aureus by the human host, we studied the antistaphylococcal humoral responses in healthy people in comparison to those of patients with invasive diseases. In a series of enzyme-linked immunosorbent assay analyses performed using 19 recombinant staphylococcal cell surface and secreted proteins, we measured a wide range of antibody levels, finding a pronounced heterogeneity among individuals in both donor groups. The analysis revealed marked differences in the antibody repertoires of healthy individuals with or without S. aureus carriage, as well as in those of patients in the acute phase of infection. Most importantly, we identified antigenic proteins for which specific antibodies were missing or underrepresented in infected patients. In contrast to the well-described transient nature of disease-induced antistaphylococcal immune response, it was demonstrated that high-titer antistaphylococcal antibodies are stable for years in healthy individuals. In addition, we provide evidence obtained on the basis of opsonophagocytic and neutralizing activity in vitro assays that circulating antistaphylococcal serum antibodies in healthy donors are functional. In light of these data we suggest that proper serological analysis comparing the preexisting antibody repertoires of hospitalized patients with different outcomes for nosocomial staphylococcal infections could be extremely useful for the evaluation of candidate vaccine antigens in addition to protection data generated with animal models.
Staphylococcus aureus is one of the most common bacterial causes of infections in both hospitals and communities and imposes a medical problem of increasing severity (5, 12). Coagulase-positive S. aureus is the most pathogenic staphylococcal species and an opportunistic pathogen that can cause illnesses ranging from minor infections to life-threatening diseases. The high incidence of staphylococcal infections is related to an increase in the use of catheters and prosthetic devices and in the number of immunity-compromised patients. Importantly, the emergence and the disease-causing capacity of staphylococci are strongly related to the widespread use of antibiotics combined with the enormous potential of this bacterium to develop multidrug resistance (31, 45). Moreover, the most serious staphylococcal infections are still associated with high mortality, despite the availability of effective antibiotics. As a consequence, new medical treatment regimens are needed in the management of staphylococcal diseases. Immunological approaches such as antistaphylococcal vaccination certainly have the potential for preventive and therapeutic treatment. However, despite the high prevalence of and medical need to prevent S. aureus infections in the human population, our understanding of the immune correlates of protection in general and our knowledge of the staphylococcal antigens that are recognized by the human immune system in particular are still incomplete.
Staphylococci are primarily extracellular pathogens; consequently, host defense relies mainly on innate immunological mechanisms supported by antistaphylococcal adaptive humoral responses. This notion is well supported by the increased frequency of staphylococcal infections among individuals deficient in antibodies (hypo- and agammaglobulinemias) and neutrophil function (30). In spite of the ability of the organism to produce a large number of toxins and extracellular products, the hallmarks of S. aureus infections are dissemination through the blood and multiplication that can be most efficiently controlled by phagocytosis. Thus, complement-mediated opsonization is essential for the elimination of S. aureus by the human host (9). Although it has been shown that serum immunoglobulin (Ig) preparations can neutralize toxins from S. aureus in in vitro assays (10, 18), further studies are needed to determine the importance and involvement of circulating human antibodies in protection.
Previously, several studies have investigated the human immune response to S. aureus in infected patients but only a few have investigated the response in apparently healthy individuals. In most of the serological studies, antibody levels against total bacterial lysates or few selected proteins were determined and increased levels were measured in convalescing patients (4, 7, 8, 48). Further evidence for the role of antibodies in protection comes from experimental studies conducted with animals. It has been shown that antibodies against certain bacterial components of S. aureus, such as surface adhesins (ClfA, FnBp, and collagen binding protein) (16, 21, 23, 44), lipoproteins (Pbp2a) (49), surface polysaccharides (types 5 and 8), and poly-N-succinyl beta-1-6 glucosamine (PNSG) (15, 27, 35), as well as secreted toxins (22, 26, 37) are of benefit in defense against staphylococcal infection. This is in accordance with the data from a clinical study performed using a type 5 and a type 8 polysaccharide conjugate vaccine that caused a decreased prevalence of bacteremia in hemodialysis patients (50).
As antibodies have been shown to contribute to protection against staphylococcal disease, it is reasonable to speculate that susceptibility to disease correlates with the levels of antistaphylococcal antibodies. While infected patients would be expected to display low levels of antibodies in the acute phase of the disease, healthy individuals may possess protective antibodies dependent on previous encounters with the pathogen.
In this study we therefore aimed at analyzing antibody responses against multiple S. aureus antigens of healthy individuals and acute-phase patients with documented staphylococcal infection. The comparison of antibody levels and responses might thus identify “missing” antibodies that contribute to disease susceptibility or “early antigens” that induce antibody responses at disease onset. In addition, we evaluated the relationship between levels and functionality of antibodies in vitro.
MATERIALS AND METHODS
Human serum samples.
Sera were collected from healthy individuals without evidence of S. aureus infection and from patients in the acute phase of diseases caused by S. aureus. Samples were obtained from 70 adults (between 20 and 60 years old; average age, ∼30 years) and 89 children (from 0 to 18 years old). We performed serial sampling (two to four samples from 13 donors) from the adult donor group for studies of the stability of antibody levels and nasal carriage; thus, a total of 100 serum samples were analyzed. A total of 42 samples were obtained from patients with documented S. aureus infections such as wound infection (17 subjects), bacteremia and sepsis (15 subjects), and other diseases such as pneumonia, arthritis, urinary tract infection, catheter-related infections, and peritonitis (10 subjects) within 2 to 8 days after disease onset. The patients were between 25 and 92 years of age, with the majority (23 out of 44) above 70 years of age. Sera with serial sampling (acute, early, and late convalescent phases) were collected from patients suffering from orthopedic prosthetic device-related infections. The serum samples used for the enzyme-linked immunosorbent assay (ELISA) were stored at 4°C with 0.02% sodium azide as preservative; those used for functional assays were stored at −80°C without preservative.
ELISA.
ELISA was performed according to standard protocols (using a 50-μl volume in each step except for blocking [100 μl]). Briefly, 96-well plates (Maxisorp; NUNC, Roskilde, Denmark) were coated with antigens (50 μl) diluted in 0.1 M NaHCO3 buffer (pH 9.3) to a concentration of 10 μg/ml for total lysate and culture supernatant proteins prepared from a S. aureus spa (protein A-deficient) strain or 2 to 5 μg/ml for recombinant proteins. The plates were incubated overnight at 4°C. Peptide ELISA was performed with biotin-labeled peptides and coated on streptavidin ELISA plates (EXIQON, Vedbaek, Denmark) at 10 μg/ml, according to the manufacturer's instructions. After blocking of nonspecific sites with 1% bovine serum albumin (BSA)-phosphate-buffered saline (PBS), human sera were added at various dilutions. The optimal dilutions of sera were 200-, 1,000-, and 5,000-fold for recombinant proteins and peptides and 5,000- to 50,000-fold for total lysate, supernatant proteins, lipoteichoic acid (LTA), and peptidoglycan (PG). The plates were incubated for 1.5 h at 37°C. After washing with PBS-0.1% Tween 20 (PBS-T), horseradish peroxidase-conjugated goat anti-human antibodies (Southern Biotechnology Associates, Birmingham, Ala.) were added to each well at a 1,000-fold dilution and plates were incubated for 1 h at 37°C. After the last washing step with PBS-0.1% Tween 20, incubation with the substrate 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) was performed for 30 min at 37°C, and titers were measured at 405 nm on a Sunrise ELISA reader (Tecan, Maennedorf, Switzerland). All serum samples were analyzed in duplicate, and mean values were calculated. Internal positive- and negative-control sera were included on each plate. The results were expressed as ELISA units calculated from optical density (OD) readings, serum dilutions in the linear range of response, and total IgG and IgA concentrations of individual serum samples.
Avidity measurements were performed by including Na-isothiocyanate (in 1, 2, 3, or 4 M final concentrations) during the incubation of the antigens with serum samples in the 1,000- to 25,000× dilution range according to the method of Romero-Steiner et al. (46).
Serum IgG and IgA concentrations were determined by ELISA. Affinity-purified goat anti-human IgG or anti-human IgA capture antibodies (Bethyl Laboratories, Montgomery, Tex.) were used at a 10 μg/ml concentration for coating 96-well plates (Maxisorp; NUNC). Serial dilutions of sera (1:10,000 to 1:600,000) were made in PBS-BSA, and human reference serum (Bethyl Laboratories) (in the range of 7.8 to 1,000 ng/ml) was used as a standard. Highly specific horseradish peroxidase-labeled goat anti-human IgG or anti-human IgA secondary antibodies (Bethyl Laboratories) were used as detecting reagents according to the recommendations of the manufacturers (dilution, ∼1:50,000).
Staphylococcal antigens.
Total bacterial lysate was prepared using the lysostaphin digestion method. Briefly, S. aureus was cultivated overnight at 37°C with 150-rpm shaking in brain heart infusion (BHI) medium. Approximately 1010 bacteria were harvested and washed with PBS. The pellet was resuspended in 200 μl of PBS buffer containing protease inhibitor cocktail Complete EDTA-free tablets (Roche Diagnostics, Mannheim, Germany) and 20 μg of lysostaphin (Sigma-Aldrich, Steinheim, Germany). After enzymatic digestion at 37°C for 30 min, the cells were disrupted by sonication using a microsonicator (Sonoplus HD 2200; Bandelin Electronics, Berlin, Germany) and the soluble fractions were recovered by centrifugation. Culture supernatant fraction was prepared by ethanol precipitation. Briefly, culture supernatants of overnight S. aureus cultures grown in BHI medium were incubated with 3 volumes of ice-cold ethanol at −20°C for at least 12 h and precipitated proteins were collected by centrifugation. The pellet was dried and resuspended in PBS. Protein concentrations were determined by the Bradford method (protein assay; Bio-Rad, Munich, Germany).
The staphylococcal recombinant proteins were expressed as fusion proteins with a StrepII tag (IBA, Göttingen, Germany), six-His tag, or glutathione S-transferase (GST) tag. All genes were cloned using genomic DNA from the S. aureus COL strain. DNA was amplified by gene-specific oligonucleotides with incorporated BsaI and SalI sites for StrepII- and GST-tagged proteins (IsdB and StbA), respectively. Restriction enzyme-digested PCR products were cloned into BsaI-cleaved pASK-IBA4 vector (IBA) or into SalI-cleaved pGEX-4T vector. The recombinant genes encoding the gram-positive signal peptides (according to the SignalP prediction program) and C-terminal sequences downstream from the sortase cleavage site (LPXT↑G) (where “X” represents any amino acid) were removed. Sequences corresponding to SD repeats (serine, aspartic acid) of ClfB and the Sdr proteins were also omitted. ClfA was produced as an N-terminal six-His-tagged protein, and the corresponding DNA was amplified from pCF4, a multicopy plasmid carrying a clfA gene from the S. aureus Newman strain (a gift from Tim Foster, Trinity College, Dublin, Ireland) (33), by gene-specific oligonucleotides with incorporated XhoI/NdeI sites and cloned into the pFS23 vector. The recombinant proteins were purified from bacterial extracts of Escherichia coli BL21 induced by anhydrotetracyclin (pASK-IBA4) or IPTG (isopropyl-ā-d-thiogalactopyranoside) (pGEX-4T) by affinity chromatography with StrepTactin Sepharose (IBA), glutathione-Sepharose (Amersham Biosciences, Uppsala, Sweden) or Ni-nitrilotriacetic acid agarose (QIAGEN, Hilden, Germany) (according to the manufacturer's instructions).
LTA and PG were purified from S. aureus and purchased from Sigma-Aldrich.
Peptides were synthesized in small scale by use of standard F-moc chemistry on a Rink amide resin (PepChem, Tübingen, Germany) by the use of a SyroII synthesizer (Multisyntech, Witten, Germany). After the sequence was assembled, peptides were elongated with Fmoc-epsilon-aminohexanoic acid and biotin (Sigma-Aldrich). Peptides were cleaved off the resin with 93% TFA-5% triethylsilane-2% water for 1 h, dried under vacuum, and freeze-dried three times using acetonitrile-water (1:1). The presence of the correct mass was verified by mass spectrometry on a Reflex III matrix-assisted laser desorption ionization-time of flight (Bruker, Bremen, Germany).
In vitro opsonophagocytosis assay.
S. aureus Wood strain cells were labeled with the fluorescent dye Alexa 488 (Molecular Probes, Leiden, The Netherlands), and following preopsonization with 10% human serum for 15 min at 37°C, 2% complement (guinea pig serum; Institute for Immunology, Johannes Gutenberg-University, Mainz, Germany) and 2 × 106 macrophages of the cell line P388.D1 were added to 2 × 107 S. aureus cells and incubation was continued for 15 min at 37°C. The phagocytic cells were washed three times to remove loosely attached bacteria before fixation with 2% paraformaldehyde. Opsonization was monitored as an increase in mean fluorescence intensity of the phagocytic cells measured with a fluorescence-activated cell sorter (FACS) (BD Biosciences, Bedford, Mass.).
In vitro bactericidal assay.
Phagocytic cells and bacteria were incubated in the presence of complement to measure antibody-dependent killing on the basis of the loss of viable bacteria as determined by colony counting. In brief, S. aureus 8325-4 cells were washed twice in Hanks balanced salt solution and the cell density was adjusted to 105 CFU in 50 μl of Hanks balanced salt solution. Bacteria were incubated with human serum IgGs (0.25 to 4 μg) and guinea pig complement (up to 5%) in a total volume of 100 μl for 60 min at 4°C. Preopsonized bacteria were mixed with macrophages (murine cell line RAW264.7; 2 × 106 cells per 100 μl) at a 1:20 ratio and were incubated at 37°C on a rotating shaker at 500 rpm. An aliquot of each sample was diluted in sterile water and incubated for 5 min at room temperature to lyse macrophages. Triplicates were then plated onto BHI agar plates, and colonies were counted with a Countermat flash colony counter (IUL Instruments, Königswinter, Germany).
Toxin neutralization assay.
During the assay setup it was established that 250 ng of recombinant S. aureus alpha-toxin (Toxin Technology, Sarasota, Fla.) resulted in about 90% hemolysis of rabbit red blood cells as quantitated by measuring released hemoglobin. To compare the characteristics of neutralizing activity of human sera, individual samples in the 20- to 320-fold dilution range were preincubated with 250 ng of alpha-toxin before being added to rabbit blood (30-fold diluted with PBS) for 30 min at 37°C. Hyperimmune anti-alpha-toxin rabbit serum was used in the assay as a positive control, and toxin-neutralizing activity expressed as a percentage of inhibition of hemolysis-hemoglobin release was quantified by spectophotometric measurements at 545 nm and expressed as a percentage of lysis (100% lysis was achieved by incubation of rabbit red blood cells in distilled water).
Western blotting.
Proteins were separated by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a mini-Protean electrophoresis system (Bio-Rad, Austria) and transferred to a nitrocellulose membrane (ECL; Amersham Biosciences, Buckinghamshire, United Kingdom) by a semidry transfer system (Bio-Rad, Vienna, Austria). After overnight blocking in 5% milk, human sera at 1:10,000 dilutions were added, and horseradish peroxidase-labeled goat anti-human IgGs (Southern Biotechnology Associates) were used for specific detection of immune complexes. The signal was developed using an ECL detection system (Amersham Biosciences, Buckinghamshire, United Kingdom).
Purification of IgGs.
Highly enriched preparations of IgGs were generated by protein G affinity chromatography according to the manufacturer's instructions (UltraLink immobilized protein G; Pierce, Rockford, Ill.). Antibody concentrations were measured at an OD of 280 nm (OD280) and were also estimated by SDS-PAGE analysis (using BSA as a standard).
Statistical analysis.
An unpaired Student's t test was used to compare ELISA values between two groups. Differences were considered significant when the P value was equal to or less than 0.05.
RESULTS
A wide range of antistaphylococcal antibody levels in healthy individuals.
It has been reported previously that serum levels of antibodies recognizing S. aureus components can be quite high not only in infected patients but also in clinically healthy individuals. We therefore measured antistaphylococcal antibodies in serum samples obtained from healthy adults who did not suffer from any apparent infectious diseases at the time of sampling. The sera obtained from 54 different donors showed great variability in total levels of antibodies as measured by ELISA using total bacterial lysates or culture supernatants of in vitro-grown S. aureus 8325-4 spa (lacking Protein A) as coating antigens (Fig. 1). We determined the contribution of antistaphylococcal antibodies by analyzing samples characterized with the highest and lowest antistaphylococcal titers for total and specific IgG content and found that approximately 2.5 to 3.0% of the total serum IgG antibodies reacted with the staphylococcal lysate in high-titer sera whereas the lowest-titer samples resulted in reactions with only 0.1% (data not shown). We also detected a wide range of IgA levels that were lower than those of IgG (Fig. 1). However, there was no correlation between the two antibody classes of individual samples, since we detected a significant number of sera with high IgA and low IgG titers as well as sera with low IgA and high IgG titers.
FIG. 1.
Antistaphylococcal IgG and IgA antibody levels show great variability in healthy individuals. Antibody levels were determined by ELISA using total bacterial lysate (upper panel) or culture supernatant (lower panel) from S. aureus 8325-4 (spa) (grown in BHI overnight) as a coating antigen and serum samples from 54 healthy adults. Antigen-antibody interactions were detected with highly specific anti-human IgG and IgA antibodies. Results are shown as OD405 values for serum dilutions in the linear range (1:50,000 for lysate and 1:10,000 for culture supernatant).
Induction of antistaphylococcal IgM, IgA, and IgG antibodies during childhood.
The analysis of sera obtained from 89 healthy children ranging in age from 2 months to 18 years showed that antistaphylococcal antibody levels increased with age (Fig. 2). However, the three main antibody classes were induced with different kinetics. The first antibodies to appear were of the IgM class and reached a plateau by the age of 2 years. Antistaphylococcal IgAs reached the highest level in 4- to 6-year-old children and did not show an increase in older age groups. In contrast to IgM and IgA levels, mean IgG levels increased continuously until adolescence (13 to 15 years). The pronounced heterogeneity in IgG and IgA antibody levels also seen in adults (Fig. 1) was already apparent in 7- to 9-year-old children.
FIG. 2.
Kinetics of induction of antistaphylococcal antibodies during childhood. IgM, IgA, and IgG antibodies were detected by ELISA using total bacterial lysates prepared from S. aureus 8325-4 (spa) as a coating antigen and serum samples from 89 healthy children in different age groups. Results are shown as OD405 values for serum dilution in the linear range (1:10,000 for IgG and 1:200 for IgA and IgM). For each of the eight different age categories, 9 to 16 sera were analyzed for antibody levels.
Antistaphylococcal antibodies react mainly with surface components.
We observed that levels of antistaphylococcal IgG antibodies were very similar when total bacterial lysate or whole S. aureus cells were used as coating antigens (data not shown). This result suggested that the majority of antistaphylococcal IgGs reacted with the surface of the pathogen. LTA and PG, the two most abundant cell wall components, have been known to be highly immunogenic in humans. Consistent with this notion, we found a strong correlation between IgG levels seen with these cell wall components and those seen with total lysate (Fig. 3A). In competitive ELISAs using LTA and PG as soluble antigens and total S. aureus lysate as coating antigen, we determined that LTA and PG contributed to approximately 60 to 85% and 15 to 25% of total antistaphylococcal IgG reactivity, respectively (Fig. 3B and data not shown). However, the presence of LTA and PG did not contribute to IgA level changes (Fig. 3C).
FIG. 3.
The majority of antistaphylococcal IgG antibodies are induced by lipoteichoic acid and peptidoglycan. (A) Correlation between serum antibody levels against LTA (upper panel) or PG (lower panel) and total S. aureus 8325-4 (spa) lysate. Sera from 40 healthy donors were diluted 10,000-fold for LTA and bacterial lysate measurements and 5,000-fold for PG. Results are shown as OD405 values. (B) Determination of the contribution of LTA to total antistaphylococcal reactivity with competition ELISA using soluble LTA as a competitor at the indicated amounts and total S. aureus lysate as a coating antigen. (C) IgG and IgA anti-LTA and anti-PG antibody levels were determined in standard ELISAs using five different human serum samples from healthy adults determined to have high total antistaphylococcal antibody titers.
Staphylococcal cell surface and secreted proteins induce IgG and IgA antibodies in healthy adults and acutely infected patients.
To determine the antigenic specificity of circulating antistaphylococcal antibodies present in sera of healthy individuals, a collection of recombinant S. aureus proteins was analyzed. The selection of proteins was made on the basis of the predicted surface localization and/or determined function; the selected proteins included 11 members of the LPXTG motif-containing cell wall-anchored protein family, four lipoproteins, and two surface and two secreted proteins (Table 1). All 19 recombinant proteins were expressed in E. coli and purified by affinity chromatography using affinity tags fused to the N or C terminus of each protein. The integrity and purity of the proteins was evaluated by SDS-PAGE analysis (Fig. 4A) or tag-specific immunodetection (data not shown). Antibodies against these extracellular proteins were measured in sera from 40 healthy adults and 42 patients in the early phase of staphylococcal diseases to identify antibodies that were induced by the onset of disease or were missing and underrepresented in acute-phase patients.
TABLE 1.
Recombinant S. aureus proteins used in the studya
| Protein | ORF | MW | Location | Binding partner | Reference |
|---|---|---|---|---|---|
| Clumping factor A (ClfA) | SA0856 | 102 | Cell wall | Fg | 33 |
| Clumping factor B (ClfB) | SA2652 | 97 | Cell wall | Fg | 36 |
| Fibronectin-binding protein A (FnbA) | SA2511 | 118 | Cell wall | Fn, Fg | 17 |
| Serine-asparate repeat protein C (SdrC) | SA0608 | 103 | Cell wall | ND | 24 |
| Serine-asparate repeat protein D (SdrD) | SA0609 | 149 | Cell wall | Ca2+ | 24 |
| Serine-asparate repeat protein E (SdrE) | SA0610 | 126 | Cell wall | ND | 24 |
| Mrp protein (FmtB) | SA2150 | 263 | Cell wall | ND | 25 |
| Surface anchor family protein (LPXTG) | SA2505 | 136 | Cell wall | ND | |
| Iron-regulated surface determinant (IsdB) | SA1138 | 72 | Cell wall | Hb | 32 |
| Staphylococcal tf-binding protein A (StbA/IsdA) | SA1140 | 30 | Cell wall | Tf | 52 |
| Haptoglobin-hemoglobin binding protein A (HarA) | SA1781 | 101 | Cell wall | Hp-Hb | 13 |
| ABC transporter | SA0688 | 35 | Membrane | ND | |
| Transferrin receptor (TfR) | SA0799 | 38 | Membrane | ND | |
| Elastin binding protein (EbpS) | SA1522 | 53 | Membrane | Elastin | 40 |
| Penicillin-binding protein 2 (Pbp2) | SA0033 | 76 | Membrane | β-Lactam | 11 |
| Enolase (Eno) | SA0842 | 47 | Surface | Laminin | 3 |
| MHC-II analog protein (Map) | 53 | Surface | Fn, Fg, Vn | 34 | |
| Truncated Maps (Map-w) | SA1751b | Bsp, Tbs | |||
| Coagulase (Coa) | SA0209 | 72 | Secreted | Fg, Pth | 43 |
| Extracellular fibrinogen-binding protein (Efb/Fib) | SA1168 | 19 | Secreted | Fg | 2 |
The open reading frame (ORF) numbers are from The Institute for Genomic Research (TIGR), CMR database (http://www.tigr.org/tigr-scripts/CMR2/CMRHomePage.spl) for the S. aureus COL except where indicated. MW, predicted molecular weight; Fn, fibronectin; Fg, fibrinogen; Pth, prothrombin. Hb, hemoglobin; Hp, haptoglobin; Tf, transferrin; Vn, vitronectin; Bsp, bone sialoprotein; Tbs, thrombospondin; ND, not defined.
ORF number for S. aureus strain N315.
FIG. 4.
Comparison of IgG and IgA antibody levels against staphylococcal surface proteins. (A) Purified recombinant proteins were visualized by Coomassie blue staining of SDS-12% PAGE to assess integrity and purity. Proteins are indicated by common names; predicted molecular weight values are shown in parentheses. All proteins except Stbp and IsdB (GST negative) were expressed with a six-His tag. (B) IgG and IgA antibody levels in sera from healthy and infected individuals were determined by ELISA with recombinant proteins. Antibody reactivity was expressed in ELISA units calculated from OD readings obtained from measurements with 1,000-fold serum dilutions. Representative examples of antigens showing higher, equal, or lower IgG (upper panel) and IgA (lower panel) antibody levels in healthy donors (open circle) relative to infected donors (filled circle) are shown. Median values are shown for both groups, and statistically significant differences are indicated by asterisks.
Standard ELISA measurements were performed with saturating amounts of recombinant proteins to quantitatively analyze antibody levels. We detected diverse levels of both IgG and IgA serum antibodies with all of the recombinant proteins tested (Table 2 and Fig. 4B). Some antigens, such as the Sdr proteins, seemed to be very potent at inducing both antibody classes, while the majority of antigens preferentially induced IgGs. Both donor groups showed a wide range of antibody reactivities when tested with individual recombinant proteins; however, for the majority of proteins, no significant differences in median values of ELISA units were seen. Significantly higher IgG levels (P < 0.05) were measured in the patient group compared to the results seen with the group of healthy adults for SdrD, HarA, FnbpA, Enolase, EbpS, and SA0688 (ABC iron transporter), whereas IgA levels were higher only for HarA. Most importantly, IgG antibodies against three proteins—ClfB, coagulase, and Efb—and the IgA levels for ClfB were significantly underrepresented in the sera of infected patients relative to those of healthy individuals (Table 2 and Fig. 4B). In contrast, total antistaphylococcal IgG and IgA levels for the same serum sample (as measured using total bacterial lysates) were comparable, although infected patients displayed a wider range of antibody levels than healthy adults (Fig. 4B, leftmost panels).
TABLE 2.
IgG and IgA antibody levels measured using recombinant staphylococcal proteinsa
| Protein | IgG for:
|
IgA for:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Infected patients (n = 42) | Healthy
|
Patient (n = 33) | Healthy
|
|||||
| Total (n = 40) | Noncarrier (n = 9) | Carrier (n = 15) | Total (n = 40) | Noncarrier (n = 9) | Carrier (n = 15) | |||
| SdrC | 416 (31-1,877) | 276 (92-1,796) | 278 (67-568) | 308 (9-1,796) | 433 (42-1,125) | 398 (0-1,182) | 545 (24-1,017) | 396 (35-893) |
| SdrD | 397 (48-1,119) | 98 (50-1,352) | 110 (5-195) | 127 (28-717) | 462 (66-1,472) | 428 (0-1,063) | 423 (65-998) | 433 (34-898) |
| SdrE | 378 (34-1,545) | 329 (164-2,740) | 336 (135-653) | 322 (86-2,740) | ND | ND | ND | ND |
| ClfA | 135 (38-1,032) | 141 (97-685) | 147 (56-517) | 138 (42-422) | 25 (6-108) | 34 (0-333) | 34 (0-110) | 24 (1-333) |
| ClfB | 216 (65-1,043) | 385 (186-1,359) | 466 (135-980) | 264 (115-629) | 52 (10-260) | 41 (0-235) | 34 (20-85) | 44 (12-186) |
| IsdB | 909 (105-3,351) | 979 (768-2,713) | 971 (621-1,522) | 1011 (31-2,552) | 113 (35-273) | 87 (0-319) | 120 (63-228) | 72 (0-319) |
| HarA | 462 (74-1,546) | 276 (180-815) | 414 (123-566) | 194 (0-705) | 265 (26-1,127) | 88 (19-517) | 71 (27-182) | 87 (19-517) |
| StbA | 854 (170-3,505) | 734 (409-2,809) | 888 (306-1,563) | 890 (301-1,999) | 45 (5-225) | 28 (1-245) | 31 (14-133) | 30 (4-245) |
| Mrp | 176 (45-789) | 126 (65-1,656) | 123 (26-243) | 123 (15-1,489) | 14 (2-139) | 9 (0-116) | 13 (5-31) | 10 (0-30) |
| FnbpA | 234 (0-2,364) | 183 (138-983) | 206 (73-311) | 181 (55-399) | ND | ND | ND | ND |
| Pbp2a | 118 (17-536) | 73 (49-828) | 90 (35-135) | 81 (16-682) | 37 (0-399) | 30 (0-234) | 22 (5-92) | 29 (0-105) |
| Map-w | 311 (59-2,923) | 332 (169-1,086) | 516 (115-1,086) | 270 (56-979) | 138 (28-427) | 103 (1-626) | 194 (4-365) | 75 (16-397) |
| Enolase | 96 (34-378) | 54 (38-163) | 79 (36-148) | 42 (26-67) | ND | ND | ND | ND |
| EbpS | 341 (84-1,737) | 228 (158-812) | 250 (104-737) | 225 (69-529) | 34 (8-299) | 19 (0-225) | 24 (1-50) | 18 (4-225) |
| SA2505 | 88 (7-993) | 100 (61-322) | 118 (37-225) | 108 (19-322) | 26 (0-727) | 23 (0-420) | 25 (0-420) | 23 (2-345) |
| SA0688 | 217 (0-1,267) | 90 (68-807) | 93 (41-807) | 88 (45-193) | 6 (2-329) | 9 (1-32) | 10 (2-23) | 9 (2-32) |
| SA0799 | UD | UD | UD | UD | UD | UD | UD | UD |
| Coa | 16 (5-84) | 34 (23-204) | 36 (12-187) | 35 (14-204) | 22 (0-90) | 36 (0-223) | 48 (3-155) | 49 (3-110) |
| Fib | 74 (8-597) | 152 (110-775) | 142 (72-296) | 131 (52-563) | 24 (0-128) | 32 (0-116) | 40 (6-116) | 29 (4-102) |
Median ELISA units reflecting antibody levels are shown for each recombinant protein in each donor group. Significantly increased values for infected patient versus healthy patient or noncarrier versus carrier comparisons are shown in boldface characters. Minimum and maximum ELISA units are shown in parentheses. Noncarriers and carriers tested negative and positive, respectively, at least twice. ND, not determined; UD, underdetection.
Carriage and antistaphylococcal antibody levels.
To correlate antibody levels with bacterial carriage, we determined levels of nasal and nasopharyngeal colonization of adults at the time of serum sampling with routine clinical microbiological tests. A total of 30% of individuals tested positive for at least one time point for nasal carriage; 10% of them also tested positive for nasopharyngeal colonization. Although we could not find a strong correlation between carriage and antibody levels, there was a tendency for higher IgG and lower IgA levels in permanent carriers compared to the results seen with individuals for whom carriage was not detected at any time point (Fig. 5). By analyzing the relationship between carriage and antibody levels against individual recombinant S. aureus proteins, we observed higher levels of IgG against ClfB, HarA, and Map-w and of IgAs against ClfB in healthy individuals who repeatedly tested negative for S. aureus according to the results obtained with nasal and pharyngeal swab cultures relative to results for those who were found to be intermittent or permanent carriers (Table 2).
FIG. 5.
Tendency for higher IgG and lower IgA levels in S. aureus nasal carriers. IgG and IgA levels for total S. aureus 8325-4 (spa) bacterial lysates were measured by ELISA and individual data points are shown for noncarriers (open circle) (n = 17), transient carriers (gray-shaded circle) (n = 13), and permanent carriers (filled circle) (n = 9). Median values are indicated for all groups.
Immunodominant peptide epitopes of S. aureus identified using high-titer sera from healthy individuals react with sera from seroconverted patients.
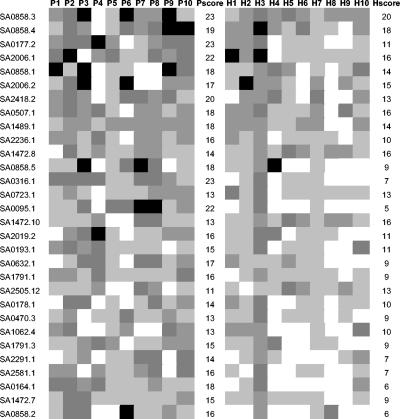
In our previous work we used serum samples with high antistaphylococcal titers to identify novel antigens by a genome-wide method (14). In these experiments S. aureus genomic peptide expression libraries were exposed to selection by antibodies derived from high-titer sera also included in this study. The majority of the identified epitopes and almost all of the most frequently selected epitopes belonged to surface located or secreted proteins of S. aureus (14). The immunogenicity of these antigens was further evaluated by ELISA using synthetic peptides corresponding to these epitopes. In a series of automated ELISA measurements 20 human serum samples—10 from infected patients and 10 from healthy individuals—determined to have high-titer antibodies against S. aureus were analyzed for their epitope-specific antibody levels (Fig. 6). Peptide epitopes inducing high levels of antibodies in many individuals belonged to surface and secreted proteins, such as the secretory extracellular matrix and plasma binding protein (Empbp), aerolysin-leukocidin family protein, exotoxin, staphylococcal IgG binding proteins protein A and Sbi, SdrH, LysM domain proteins, Cell wall associated fibronectin binding protein (Ebh), bifunctional autolysin, LPXTG protein, and membrane proteins. The majority of these peptide epitopes reacted with sera derived from infected patients as well as sera from healthy individuals. Moreover, we observed seroconversion against immunogenic epitopes when we compared serum samples obtained from the acute- and convalescent-phase patients with prosthetic-device-related S. aureus infections (Fig. 7; patients P60 and P63). In contrast, in patients suffering from chronic infections epitope-specific antibody levels did not change during convalescence from an acute exacerbation (Fig. 7; patient P66). (In total, 12 patients [5 acutely infected and 7 chronically infected] were included in this analysis, and the data shown for these three patients are representative).
FIG. 6.
Biotin-labeled peptides (e.g., SA0858.3) representing selected epitopes were used as coating antigens in ELISAs to measure specific IgG levels from human sera. Lanes P1 to P10, sera from infected patients; lanes H1 to H10, sera from healthy individuals. Black panels represent high reactivity; dark grey panels represent intermediate reactivity; grey panels represent low reactivity; light grey panels represent very low reactivity. Each score was calculated from the number of positive serum results and the extent of reactivity. SA0095, IgG binding protein A (Spa); SA0164, gramicidin S synthetase 2-related protein; SA0177, glucokinase regulator-related protein; SA0178, PTS system, IIBC components; SA0193, maltose ABC transporter, maltose binding protein; SA0316, conserved hypothetical protein; SA0470, exotoxin; SA0507, LysM domain protein; SA0632, membrane protein; SA0723, LysM domain protein; SA0858, secretory extracellular matrix and plasma binding protein (Empbp); SA1062, bifunctional autolysin; SA1472, cell wall-associated fibronectin binding protein (Ebh); SA1489, recombinant protein U; SA1791, FtsK/SpoIIIE family protein; SA2006, aerolysin/leukocidin family protein; SA2019, sdrH protein, putative; SA2291, staphyloxanthin biosynthesis protein; SA2236, ribosomal protein L2; SA2418, IgG binding protein SBI; SA2505, cell wall surface anchor family protein (LPXTG); SA2581, staphyloxanthin biosynthesis protein.
FIG. 7.
Novel peptide epitopes identified with high-titer staphylococcal sera induce antibodies in infected patients during convalescence. A total of 14 biotin-labeled peptides representing epitopes with high antibody reactivities with sera from healthy adults were used as capture antigens on streptavidin-coated ELISA plates. Two serum samples were taken from patients with osteomyelitis in the acute phase (open bar) and of the convalescent phase (filled bar) and used to measure IgG antibodies induced during disease. Patients P60 and P63 had acute osteomyelitis, while patient P66 suffered from chronic infection. Serum samples were taken 3 to 4 weeks apart.
Stability and avidity of antistaphylococcal antibody levels in healthy adults.
To investigate the stability of antistaphylococcal antibodies in high-titer healthy individuals, we collected and analyzed serum samples at different time points during a 1-year time period. By comparing the ELISA results obtained at 0, 4, 9, 11, or 12 months, we observed that IgG and IgA levels of antibody against the different staphylococcal components were stable over a year without changing profile and that no fluctuation in the abundance was detected whether single antigenic components, such as recombinant proteins (Fig. 8), LTA, or peptidoglycan, or a complex mixture of antigens (total lysate, culture supernatant) were tested (data not shown).
FIG. 8.
Stable levels of circulating antistaphylococcal serum antibodies in healthy individuals. Serial serum samples were taken from eight healthy adults (patients H1 to H8) at different time points (as indicated) and analyzed by ELISA for antigen-specific IgG antibody levels by the use of four different recombinant proteins. Results are expressed as ELISA units calculated from OD readings at 1,000-fold serum dilutions.
Because avidity is an important parameter of antibody quality, we measured the level of antibodies capable of binding to complex staphylococcal antigens (bacterial lysate) or to a highly immunogenic surface protein (IsdB) in the presence of different concentrations of the chaotropic reagent sodium isothiocyanate. The total absorbance in individual sera with similar initial titers was equally reduced, indicating that levels of antibody avidity were very comparable between the healthy and patient donor groups (data not shown).
Antistaphylococcal antibody levels correlate with functionality.
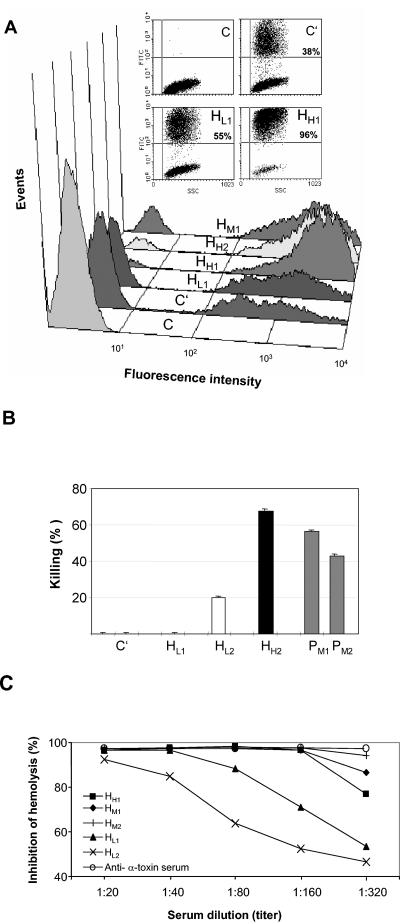
We reported previously that pools of high-titer sera from healthy individuals and from infected patients were equally effective in inducing complement-dependent opsonization (14). We now investigated whether a correlation between antistaphylococcal ELISA titers and functional activity could be established. In a FACS-based in vitro opsonophagocytosis assay using fluorescently labeled S. aureus, we compared several human serum samples characterized with low, intermediate, or high antistaphylococcal antibody levels for their activity to induce the uptake of S. aureus by cultured murine monocytic cells (p388/D1) (Fig. 9A) and freshly isolated human polymorphonuclear leukocytes (data not shown). The enhancement of opsonophagocytosis correlated well with IgG levels, but such a correlation was not observed with antistaphylococcal IgA levels.
FIG. 9.
Antistaphylococcal antibody levels correlate with functionality. (A) A FACS-based opsonophagocytosis assay was performed with fluorescently labeled S. aureus (Wood strain) and P388.D1 mouse macrophage cells in the presence of 10% human sera with different total antistaphylococcal titers. Sera were from low (HL1)-, intermediate (HM1)-, and high (HH1 and HH2)-titer sera, all from healthy (H) donors. C, control (no serum or complement added); C′, only complement (no serum added); HL1, HM1, and HH1 and HH2, 10% serum added in the presence of complement. (B) Levels of bactericidal killing activity of total serum IgG preparations were determined by a plating assay. IgG (1 μg) was preincubated with S. aureus 8325-4 cells before addition to cultured mouse RAW264.7 monocytic cells in the presenceof complement. Low (HL1 and HL2)-, intermediate (PM1 and PM2)-, and high (HH2)-titer sera were from healthy donors (H) or infected patients (P). Bactericidal activity is expressed as the percentage of decrease in CFU levels relative to the results seen with control samples (no IgG). (C) Toxin neutralization activity was measured as the level of hemolysis of rabbit red blood cells by alpha-toxin from S. aureus in the presence of human sera used at 20- to 320-fold dilutions. Low (HL1 and HL2)-, intermediate (HM1 and HM2)-, and high (HH1)-titer sera were from healthy (H) donors. Toxin-neutralizing activity was expressed as a percentage of inhibition of hemolysis by alpha-toxin at different serum dilutions. Hemolysis was measured as a release of hemoglobin that was quantified by spectrophotometric measurements at 545 nm. Rabbit anti-alpha-toxin serum was used as a positive control in the assay.
To provide further evidence about the functionality of circulating antibodies, we performed an in vitro bactericidal assay using purified IgG preparations and the cell line RAW264.7 as a source of phagocytic cells. While IgGs from high-titer sera induced bacterial killing efficiently, the same amount of IgGs from low-titer sera was much less effective (Fig. 9B). The bactericidal activities of patient sera with high levels of antistaphylococcal antibodies were comparable to those of high-titer sera from healthy individuals.
A sensitive in vitro toxin neutralization assay using recombinant alpha-toxin of S. aureus and rabbit blood was performed as a third assay to correlate antibody levels with functionality. Five selected sera from healthy donors with different levels of total antistaphylococcal antibodies were analyzed. We observed that serum samples characterized with the lowest total antistaphylococcal IgG levels displayed the lowest toxin-neutralizing activity, while a high antistaphylococcal titer correlated with efficient toxin neutralization activity (Fig. 9C).
DISCUSSION
S. aureus is an opportunistic pathogen and, as such, has multiple encounters with a large proportion of the community without causing serious diseases. Every fourth individual is a long-term nasal or anorectal carrier of S. aureus. Yet immune recognition, as measured by antibody responses, can be very different in distinct individuals of the human population. In this regard, our study showed that healthy individuals can have high levels of circulating antistaphylococcal antibodies, as indicated by the results of earlier studies (7). In addition, our data indicate that antistaphylococcal IgG levels can differ greatly, amounting to 0.1 to 3% of total serum antibodies. We show here that this great heterogeneity in antibody levels is already obvious in healthy adolescents aged 13 to 15 years, suggesting that antibody levels are already established during childhood.
There are at least three different ways that S. aureus can stimulate the adaptive immune system and induce specific antibodies: (i) exposure at mucosal surfaces that does not result in colonization; (ii) colonization at mucosal surfaces (nasopharynx and intestines) and special anatomical niches (anterior nares and anorectal locations); (iii) latent or clinically apparent infections of otherwise sterile body sites (wounds, blood, bones, etc.). We addressed the issue of why individuals show a large heterogeneity of antistaphylococcal antibody levels by comparing serum from carriers and noncarriers. We learned that carriage was associated with a tendency to have higher antistaphylococcal IgG and lower serum IgA levels, as determined with total bacterial lysates. However, certain individuals who repeatedly tested negative for nasopharyngeal carriage also displayed high IgG reactivities.
The role of serum IgA antibodies in antistaphylococcal immunity is not well characterized. A previous study obtained results that are important for our observation that both secretory and serum IgAs were shown to induce opsonophagocytosis of S. aureus by polymorphonuclear leukocytes in vitro (20).
Staphylococci cause life-threatening (mainly nosocomial) infections in only a small subset of infected patients. It is generally accepted that antibodies are crucial in protecting individuals against extracellular bacteria such as S. aureus. The great heterogeneity in antistaphylococcal antibody levels in the population may thus indicate that certain individuals are immune to S. aureus and that high levels of functional antibodies make the host less susceptible to staphylococcal diseases. Several studies have indicated that intravenous IgG preparations from human plasma of healthy blood donors contain antibodies that are effective against S. aureus. The most convincing study was performed with IgG preparations obtained from human plasma samples selected on the basis of high ClfA antibody levels that showed neutralizing and opsonophagocytic function in in vitro assays and protection in animal models (53). It is interesting in that regard that our results obtained in three independent in vitro assays demonstrated that circulating antibodies in healthy individuals are functional and that activity correlates with antibody levels.
Nevertheless, as the vast majority of antibodies are induced by lipoteichoic acid, which fails to promote opsonization (42), levels as such cannot be used to predict protection. We therefore evaluated the contribution of individual antigens as well as specific antibody levels against the individual proteins to the overall antistaphylococcal antibody response in sera from acute-phase patients and healthy individuals. Sera from both donor groups recognized multiple recombinant protein components and showed, on average, levels of IgG antibodies that were higher than those of IgA antibodies. While most of the antigens showed comparable antibody levels, we found significantly higher IgG levels in sera from infected patients for six surface proteins, namely, SdrD, HarA, FnbpA, Enolase, EbpS, and SA0688, suggesting that these antigens are expressed early during disease and recognized by the patients' immune system. Most importantly, we identified three proteins, clumping factor B, extracellular fibrinogen binding protein (Efb/Fib), and coagulase, that detected significantly lower antibody levels in acute-phase patients compared to the results seen with healthy individuals, indicative of their supportive function to prevent infection in healthy individuals. Interestingly, all three proteins belong to a group of six fibrinogen binding proteins of S. aureus (2, 6, 33, 34, 36, 39, 43). Our results are further supported by data from others showing lower antibody levels for Efb in acute-phase patients with staphylococcal sepsis whereas increased levels of IgGs for ClfA were measured (7). It is interesting in this context that Efb has been reported to inhibit complement activation that can contribute to the ability of S. aureus to evade or inactivate host immune responses (28). Judging on the basis of this function, it might be that individuals with lower levels of Efb-neutralizing antibodies are more susceptible to systemic infections caused by S. aureus.
According to clinical data, staphylococcal antibody responses evoked by disease do not seem to be protective in the long term, since many individuals encounter multiple staphylococcal infections. It has also been reported that increased antibody levels measured in convalescent patients are only transient and decrease within months postinfection (8). It was therefore surprising to detect stable levels of antibodies against staphylococcal proteins in healthy adults irrespective of their carriage status. The heterogeneity of antibody levels already established among children suggests that early exposure to S. aureus might influence subsequent immune responses and thus determine antistaphylococcal antibody levels in adulthood. Stably high levels of circulating serum antibodies (with 21 and 8 days half-life for IgG and IgA, respectively) are the result of continuous production by plasma cells. There are several mechanisms that can be implicated in the maintenance of serological memory (1) against S. aureus: the role of long-lived plasma cells and continuous antigen boosting (due to the commensal nature of this pathogens) as well as ongoing polyclonal (non-antigen-specific) activation of memory B cells already induced in childhood as the result of early interaction with S. aureus.
The immune response induced by permanent nasal carriage seems to be ineffective at prevention of systemic disease; it is common to suffer from invasive staphylococcal disease that is caused by the resident strain (41). Interestingly enough, our comparative analyses of protein-specific antibody levels revealed levels of IgG and IgA antibodies for ClfB, HarA, and Map-w in sera of nasopharyngeal carriers significantly lower than in sera of noncarriers. This observation is in agreement with our finding for acute-phase patients, who also showed significantly lower anti-ClfB IgG and IgA antibody levels, supporting the notion that high ClfB antibody levels may contribute to prevention of infection. It is noteworthy in this context that ClfB has been shown to promote adherence to epidermal cytokeratins in vitro and has been suggested to be a major determinant in S. aureus nasal colonization (38). Moreover, Map proteins have been known to possess immunomodulatory activity mediated by molecular mimicry with major histocompatibility complex class II (MHC II) molecules and as a result to interfere with the interaction of activated T cells and MHC-II-expressing antigen-presenting cells. In vivo data with map-positive and map-negative S. aureus strains suggest that Map protein plays a role in persistent staphylococcal infections (29). Judging on the basis of our previous studies, HarA—by binding to haptoglobin-hemoglobin—seems to be important for iron acquisition. In light of these virulence functions, it is attractive to speculate that lower levels of neutralizing antibodies in permanent nasal carriers play a role in S. aureus carriage.
Nasal carriage in healthy individuals is likely to be controlled by local innate and adaptive immune functions. Although there is cross-talk between local and systemic humoral immunity, further understanding of the role of specific mucosal antibodies neutralizing the function of virulence factors necessitates the analysis of antistaphylococcal Igs in nasopharyngeal secretions.
The antibodies induced by S. aureus and present in human serum constitute the molecular imprint of the in vivo expression of the corresponding antigens. Our ELISA analyses with selected staphylococcal proteins suggested that the antistaphylococcal antibody repertoires from healthy and diseased individuals overlap. Similarly, a proteomic analysis showed that sera from healthy donors and infected patients recognize a similar pattern of antigens from S. aureus (47). These data prompted us to identify staphylococcal antigens by the use of antibodies from high-titer sera from healthy people in a comprehensive genomic screening method (14). Besides proteins known to be immunogenic and/or involved in the virulence of S. aureus, a number of novel proteins were discovered by this method. Analyses of antibody levels against defined epitopes within these S. aureus antigens showed comparable immune reactivities with sera from healthy adults and infected patients, suggesting an overlapping pattern of expression of the corresponding antigens during invasive disease and during colonization and interaction without infection.
It is presently mysterious why antibodies against distinct proteins, such as the fibrinogen binding proteins, are underrepresented in infected patients and carriers. Yet it was recently reported that the S. aureus protein A, a superantigen that interacts with B-cell receptors, is capable of initiating a sequence of events by the rapid down-regulation of B-cell receptors and coreceptors which ultimately causes apoptosis of B cells. The lack or low level of antibodies reactive with certain antigens may therefore be caused by the presence of superantigens, such as protein A, which can potentially cause the elimination of distinct B cells that are concomitantly induced (19, 51).
The data presented in this study strongly indicate that larger studies that included carrier and noncarrier as well as patient donor groups would be of importance for the understanding of staphylococcal disease and its prevention. In addition, the detailed analysis of sera obtained from serial sampling before and during staphylococcal disease could provide further evidence for the importance of missing or underrepresented antibodies against distinct staphylococcal components for disease susceptibility and vaccine development.
REFERENCES
- 1.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. [DOI] [PubMed] [Google Scholar]
- 2.Bodén, M. K., and J. I. Flock. 1994. Cloning and characterization of a gene for a 19 kDa fibrinogen-binding protein from Staphylococcus aureus. Mol. Microbiol. 12:599-606. [DOI] [PubMed] [Google Scholar]
- 3.Carneiro, C. R., E. Postol, R. Nomizo, L. F. Reis, and R. R. Brentani. 2004. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect. 6:604-608. [DOI] [PubMed] [Google Scholar]
- 4.Casolini, F., L. Visai, D. Joh, P. G. Conaldi, A. Toniolo, M. Hook, and P. Speziale. 1998. Antibody response to fibronectin-binding adhesin FnbpA in patients with Staphylococcus aureus infections. Infect. Immun. 66:5433-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 to June 2002, issued August 2002. Am. J. Infect. Control 30:458-475. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, A., S. Projan, R. E. Edelstein, and V. A. Fischetti. 1995. Cloning, expression, and nucleotide sequence of a Staphylococcus aureus gene (fbpA) encoding a fibrinogen-binding protein. Infect. Immun. 63:1914-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colque-Navarro, P., M. Palma, B. Söderquist, J. I. Flock, and R. Möllby. 2000. Antibody responses in patients with staphylococcal septicemia against two Staphylococcus aureus fibrinogen binding proteins: clumping factor and an extracellular fibrinogen binding protein. Clin. Diagn. Lab. Immunol. 7:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colque-Navarro, P., B. Soderquist, H. Holmberg, L. Blomqvist, P. Olcen, and R. Möllby. 1998. Antibody response in Staphylococcus aureus septicaemia—a prospective study. J. Med. Microbiol. 47:217-225. [DOI] [PubMed] [Google Scholar]
- 9.Cunnion, K. M., H. M. Zhang, and M. M. Frank. 2003. Availability of complement bound to Staphylococcus aureus to interact with membrane complement receptors influences efficiency of phagocytosis. Infect. Immun. 71:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darenberg, J., B. Soderquist, B. H. Normark, and A. Norrby-Teglund. 2004. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: implications for therapy of toxic shock syndrome. Clin. Infect. Dis. 38:836-842. [DOI] [PubMed] [Google Scholar]
- 11.de Jonge, B. L., Y. S. Chang, D. Gage, and A. Tomasz. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 267:11248-11254. [PubMed] [Google Scholar]
- 12.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region of the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32:114-132. [DOI] [PubMed] [Google Scholar]
- 13.Dryla, A., D. Gelbmann, A. von Gabain, and E. Nagy. 2003. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 49:37-53. [DOI] [PubMed] [Google Scholar]
- 14.Etz, H., D. B. Minh, T. Henics, A. Dryla, B. Winkler, C. Triska, A. P. Boyd, J. Sollner, W. Schmidt, U. von Ahsen, M. Buschle, S. R. Gill, J. Kolonay, H. Khalak, C. M. Fraser, A. von Gabain, E. Nagy, and A. Meinke. 2002. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 99:6573-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattom, A. I., J. Sarwar, A. Ortiz, and R. Naso. 1996. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect. Immun. 64:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flock, J. I. 1999. Extracellular-matrix-binding proteins as targets for the prevention of Staphylococcus aureus infections. Mol. Med. Today 5:532-537. [DOI] [PubMed] [Google Scholar]
- 17.Flock, J. I., G. Froman, K. Jonsson, B. Guss, C. Signas, B. Nilsson, G. Raucci, M. Hook, T. Wadstrom, and M. Lindberg. 1987. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 6:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauduchon, V., G. Cozon, F. Vandenesch, A. L. Genestier, N. Eyssade, S. Peyrol, J. Etienne, and G. Lina. 2004. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J. Infect. Dis. 189:346-353. [DOI] [PubMed] [Google Scholar]
- 19.Goodyear, C. S., and G. J. Silverman. 2003. Death by a B cell superantigen: in vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J. Exp. Med. 197:1125-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorter, A., P. S. Hiemstra, P. C. Leijh, M. E. van der Sluys, M. T. van den Barselaar, L. A. van Es, and M. R. Daha. 1987. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology 61:303-309. [PMC free article] [PubMed] [Google Scholar]
- 21.Hall, A. E., P. J. Domanski, P. R. Patel, J. H. Vernachio, P. J. Syribeys, E. L. Gorovits, M. A. Johnson, J. M. Ross, J. T. Hutchins, and J. M. Patti. 2003. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect. Immun. 71:6864-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, D. L., K. Omoe, S. Sasaki, H. Sashinami, H. Sakuraba, Y. Yokomizo, K. Shinagawa, and A. Nakane. 2003. Vaccination with nontoxic mutant toxic shock syndrome toxin 1 protects against Staphylococcus aureus infection. J. Infect. Dis. 188:743-752. [DOI] [PubMed] [Google Scholar]
- 23.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 24.Josefsson, E., K. W. McCrea, D. N′Eidhin, D. O'Connell, J. Cox, M. Hook, and T. J. Foster. 1998. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology 144:3387-3395. [DOI] [PubMed] [Google Scholar]
- 25.Komatsuzawa, H., K. Ohta, M. Sugai, T. Fujiwara, P. Glanzmann, B. Berger-Bachi, and H. Suginaka. 2000. Tn551-mediated insertional inactivation of the fmtB gene encoding a cell wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 45:421-431. [DOI] [PubMed] [Google Scholar]
- 26.LeClaire, R. D., R. E. Hunt, and S. Bavari. 2002. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect. Immun. 70:2278-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, J. C., J. S. Park, S. E. Shepherd, V. Carey, and A. Fattom. 1997. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect. Immun. 65:4146-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, L. Y., M. Hook, D. Haviland, R. A. Wetsel, E. O. Yonter, P. Syribeys, J. Vernachio, and E. L. Brown. 2004. Inhibition of complement activation by a secreted Staphylococcus aureus protein. J. Infect. Dis. 190:571-579. [DOI] [PubMed] [Google Scholar]
- 29.Lee, L. Y., Y. J. Miyamoto, B. W. McIntyre, M. Hook, K. W. McCrea, D. McDevitt, and E. L. Brown. 2002. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J. Clin. Investig. 110:1461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liese, J. G., V. Jendrossek, A. Jansson, T. Petropoulou, S. Kloos, M. Gahr, and B. H. Belohradsky. 1996. Chronic granulomatous disease in adults. Lancet 347:220-223. [DOI] [PubMed] [Google Scholar]
- 31.Lowy, F. D. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J. Clin. Investig. 111:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazmanian, S. K., E. P. Skaar, A. H. Gaspar, M. Humayun, P. Gornicki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906-909. [DOI] [PubMed] [Google Scholar]
- 33.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1995. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol. Microbiol. 16:895-907. [DOI] [PubMed] [Google Scholar]
- 34.McGavin, M. H., D. Krajewska-Pietrasik, C. Rydén, and M. Höök. 1993. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect. Immun. 61:2479-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 36.Ní Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson, I. M., M. Verdrengh, R. G. Ulrich, S. Bavari, and A. Tarkowski. 1999. Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J. Infect. Dis. 180:1370-1373. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell Microbiol. 4:759-770. [DOI] [PubMed] [Google Scholar]
- 39.Palma, M., A. Haggar, and J. I. Flock. 1999. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J. Bacteriol. 181:2840-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park, P. W., J. Rosenbloom, W. R. Abrams, J. Rosenbloom, and R. P. Mecham. 1996. Molecular cloning and expression of the gene for elastin-binding protein (ebpS) in Staphylococcus aureus. J. Biol. Chem. 271:15803-15809. [DOI] [PubMed] [Google Scholar]
- 41.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9:605-610. [DOI] [PubMed] [Google Scholar]
- 42.Peterson, P. K., B. J. Wilkinson, Y. Kim, D. Schmeling, S. D. Douglas, P. G. Quie, and J. Verhoef. 1978. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J. Clin. Investig. 61:597-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phonimadaeng, P., M. O'Reilly, P. Nowlan, A. J. Bramley, and T. J. Foster. 1990. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol. Microbiol. 4:393-404. [DOI] [PubMed] [Google Scholar]
- 44.Rennermalm, A., Y. H. Li, L. Bohaufs, C. Jarstrand, A. Brauner, F. R. Brennan, and J. I. Flock. 2001. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 19:3376-3383. [DOI] [PubMed] [Google Scholar]
- 45.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 46.Romero-Steiner, S., J. Fernandez, C. Biltoft, M. E. Wohl, J. Sanchez, J. Feris, S. Balter, O. S. Levine, and G. M. Carlone. 2001. Functional antibody activity elicited by fractional doses of Haemophilus influenzae type b conjugate vaccine (polyribosylribitol phosphate-tetanus toxoid conjugate). Clin. Diagn. Lab. Immunol. 8:1115-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royan, S., L. Sharp, S. P. Nair, S. Crean, B. Henderson, S. Poole, G. L. Scott, and A. W. Evans. 2000. Identification of the secreted macromolecular immunogens of Staphylococcus aureus by analysis of serum. FEMS Immunol. Med. Microbiol. 29:315-321. [DOI] [PubMed] [Google Scholar]
- 48.Ryding, U., B. Christensson, B. Soderquist, and T. Wadstrom. 1995. Antibody response to Staphylococcus aureus collagen binding protein in patients with S. aureus septicaemia and collagen binding properties of corresponding strains. J. Med. Microbiol. 43:328-334. [DOI] [PubMed] [Google Scholar]
- 49.Senna, J. P., D. M. Roth, J. S. Oliveira, D. C. Machado, and D. S. Santos. 2003. Protective immune response against methicillin resistant Staphylococcus aureus in a murine model using a DNA vaccine approach. Vaccine 21:2661-2666. [DOI] [PubMed] [Google Scholar]
- 50.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 51.Silverman, G. J., S. P. Cary, D. C. Dwyer, L. Luo, R. Wagenknecht, and V. E. Curtiss. 2000. A B cell superantigen-induced persistent “hole” in the B-1 repertoire. J. Exp. Med. 192:87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor, J. M., and D. E. Heinrichs. 2002. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol. Microbiol. 43:1603-1614. [DOI] [PubMed] [Google Scholar]
- 53.Vernachio, J., A. S. Bayer, T Le, Y. L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 47:3400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]