Abstract
OBJECTIVE:
The study aimed to evaluate serum interleukin-28 levels in COVID-19 patients and correlate the results with disease severity.
MATERIAL AND METHODS:
This study included 90 patients who presented to the COVID-19 outpatient clinics, hospital wards, and intensive care units. Serum interleukin-28, C-reactive protein, lactate dehydrogenase, fibrinogen, d-dimer, and ferritin levels were measured. Patients were divided into 3 groups based on clinical severity to mild, moderate, and severe groups (each group consisted of 30 patients).
RESULTS:
There were significant differences in serum C-reactive protein, lactate dehydrogenase, fibrinogen, d-dimer, ferritin, and interleukin-28 levels between all groups. The mean serum interleukin-28 levels of all patients were 383.74 ± 63.58 ng/L. The mean serum interleukin-28 levels were 335.52 ± 42.12 ng/L in the mild group, 366.88 ± 41.27 ng/L in the moderate group, and 453.46 ± 36.78 ng/L in the severe group.
CONCLUSION:
There were significant differences in comparisons of all pairs (P < .05). Interleukin-28 may be a promising biomarker for detecting disease severity in COVID-19 patients.
Keywords: Health, interleukin-28, marker, Sars-Cov-2
MAIN POINTS
Interleukin (IL-28) plays an essential role in viral defense.
The role of IL-28 in defense against SARS-Cov-2 infection is not clear.
Serum IL-28 levels were higher in the moderate group and the highest in the severe group compared to the mild group, in COVID-19 patients.
Interleukin-28 levels may be used to predict mortality and mechanical ventilation need, independently of C-reactive protein and other infection markers.
Introduction
Starting in December 2019, the COVID-19 pandemic has caused more than 6 million deaths worldwide, and numbers are increasing continuously. People with documented COVID-19 infections show symptoms ranging from mild to severe, and the course of the disease is widely variable. The disease may start as a mild respiratory tract infection. It can lead to severe viral pneumonia, acute respiratory distress syndrome, multiple organ failure, and even death in the course of infection.1 Due to the capability of infecting large volumes of people and causing severe illness that requires hospitalization in inpatient clinics or intensive care units, a successful risk classification system is required to prevent unnecessary hospitalizations and lower the burden in healthcare systems worldwide. Age-, gender-, and comorbidity-based scoring system has been tested to predict mortality before.2 C-reactive protein (CRP), d-dimer, creatinine, ferritin, lactate dehydrogenase (LDH) levels, neutrophil to lymphocyte ratio, and many other biomarkers have been tested to predict mortality and severity of disease in COVID-19 patients.3-6 While some biomarkers were helpful in risk assessment, more clinical research and new biomarkers are required for a better classification system.
Interleukin 28 (IL-28) is a member of the interferon gamma (IFN-λ) family consisting of IL-28A, IL-28B, and IL-29, with their own class II receptor generally found in epithelial cells.7 Interleukin -28 cytokine family plays a vital role against viruses by inducing 2',5'-oligoadenylate synthetase which is an antiviral enzyme that counteracts viruses by degrading RNAs of viruses and interferon-stimulated gene factor-3 which is a transcriptional factor that regulates transcription of the interferon-stimulated genes.8 Interleukin-28 has been shown to enhance adaptive immunity against viruses by promoting antigen-specific interferon-gamma release and increasing the cytotoxic potential of CD8+ T cells.9 It has also been shown that mutations in the IL-28B gene may affect the viral clearance of the hepatitis C virus and response to antivirals.10-11 Though IL-28 plays an essential role in viral defense, the potency of IL-28 in infections and immune response shows biological variations due to widely determining single-nucleotide polymorphisms (SNPs) and variable expression rates.12-13 The role of IL-28 in defense against COVID-19 infection is not clear. However, due to its variable expression rates and its important role in viral immunity, we have hypothesized that blood levels of IL-28 might be an indicator of the course of the disease and be used for risk assessment in COVID-19 patients. Therefore, we have studied the relationship between IL-28 levels in the blood and COVID-19 severity in patients as a risk assessment method.
Material and Methods
A total of 90 patients who applied to the COVID-19 outpatient clinics and wards in Erzurum City Hospital that met the inclusion criteria and agreed to participate in the study were enrolled. Ethical committee approval was received from the Ethics Committee of Erzurum City Hospital (approval No: 2021/01-04). Written and verbally informed consent was obtained from all patients or legal representatives. Inclusion criteria were to have at least 1 positive COVID-19 real-time polymerase chain reaction (RT-PCR) result within 7 days, male or female gender, and age above 18 years old. Pregnant and breastfeeding women were excluded from the study.
Patients were divided into 3 groups as each group consisting of 30 patients. Patients with normal chest x-ray results, who did not need hospitalization, and who had no hypoxemia were grouped into “mild;” patients with pneumonia on computed tomography (CT) scans or chest x-rays with accompanying dyspnea or hypoxemia who needed to be hospitalized in the wards but did not need high-flow nasal oxygen (HFNO) or mechanical ventilation (invasive or non-invasive) or intensive care were grouped into “moderate;” and patients who needed intensive care due to respiratory failure or shock were grouped in “severe” groups. Venous blood samples were collected on the first day of application to the hospital in the mild group and on first day of hospitalization (ICU or ward) for moderate and severe groups. Death ratios and invasive mechanical ventilation requirement (invasive and non-invasive) ratios were recorded.
Venous blood samples from each patient were collected in a serum tube (without an anticoagulant) and centrifuged for 20 minutes at 2000-3000 rpm. The supernatants formed after centrifugation were collected in microcentrifuge tubes. Obtained serum samples were aliquoted and stored in a freezer at −20˚C until the analysis day.
Beckman Coulter AU5800 analyzer measured serum CRP, LDH, and ferritin levels. The STA R Max 3 (Stago) hemostasis and coagulation analyzer measured serum fibrinogen and d-dimer levels. A commercial ELISA kit (BT Lab, Catalog No: E3042Hu 96 T, Lot no: 202101015) measured serum IL-28 levels according to the manufacturer's instructions in an Epoch Microplate Spectrophotometer.
Statistical Analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 25.0 (IBM Corp.; Armonk, NY, USA) package program. The normality of data was determined with Kolmogorov–Smirnov test. Descriptive statistical analyses (mean ± standard deviation) and percentages were used. One-way analysis of variance (ANOVA) test and post hoc Tukey test were performed to compare biochemical values of groups. Chi-square test was used to determine the relationship between 2 variables (groups and gender/mechanical ventilation requirement/mortality) in a cross-tabulation. P values less than .05 at the 95% CI were considered statistically significant. Receiver operating characteristic (ROC) analysis was performed to define the optimal IL-28 cut-off values for detecting mortality and mechanical ventilation requirement.
Results
The mean age of all patients was 58.5 ± 13.6 years. There was no significant difference in terms of age between the 3 groups (P = .149). Chi-square test results showed that there was no significant difference in terms of gender (P = .875); and there were significant differences in terms of death, mechanical ventilation requirement (invasive and non-invasive), and invasive mechanical ventilation requirement (P < .001 for all comparisons).
All patients who died and who required mechanical ventilation were in the severe group. The demographic, clinical characteristics, and biochemical values of the patients are summarized in Table 1.
Table 1.
Demographic, Clinical Characteristics, and Biochemical Values of the Patients
| All (n = 90) | Mild (n = 30) | Moderate (n = 30) | Severe (n = 30) | |
|---|---|---|---|---|
| Age (years) | 58.5 ± 13.6 (P = .149) |
54.6 ± 12.0 | 61.1 ± 14.2 | 59.9 ± 14.1 |
| Gender, M/F (%) | 53/47 | 50/50 (X 2 = 0.268; P = .875) | 53/47 | 57/43 |
| Death, n (%) | 6 (6.6) | 0 (0 %) (X 2 = 12.8; P < .001) | 0 (0) | 6 (20 ) |
| MV req. Inv., n (%) | 9 (9.9) | 0 (0%) (X2 = 20.0; P < .001) |
0 (0) | 9 (30) |
| MV Req. All n (%) | 17 (18.7) | 0 (0%) (X2 = 32.9; P < .001) |
0 (0) | 17 (56.7) |
| CRP (mg/L) | 34.46 ± 51.33* (P = .011) |
11.21 ± 5.42a
(P = .019) |
46.57 ± 57.47 | 44.82 ± 56.70b
(P = .028) |
| LDH (U/L) | 380.43 ± 221.86* (P < .001) | 212.83 ± 67.69a
(P = .01) |
383.07 ± 168.88c
(P = .003) |
539.80 ± 249.10b (P < .001) |
| Fibrinogen (mg/dL) | 393.37 ± 165.50* (P = .018) | 357.07 ± 105.41a (P = .037) | 462.20 ± 154.60c
(P = .036) |
358.43 ± 201.21 |
| d-Dimer (ng/mL) | 1941.09 ± 1130.20* (P = .028) | 386.89 ± 136.52 | 2082.90 ± 1051.32 | 3249.87 ± 1275.34b (P = .021) |
| Ferritin (ng/mL) | 680.15 ± 282.52* (P < .001) | 166.15 ± 69.57a
(P < .001) |
836.96 ± 389.08 | 1037.33 ± 576.51b (P < .001) |
| IL-28 (ng/L) | 383.74 ± 63.58* (P < .001) |
335.52 ± 42.12a
(P = .009) |
366.88 ± 41.27c
(P < .001) |
453.46 ± 36.78b
(P < .001) |
Results are expressed as mean ± standard deviation and n (%); P: one-way analysis of variance (ANOVA) test statistics P value for group comparisons of numerical variables (age, CRP, LDH, fibrinogen, D-dimer, ferritin, and IL-28) and chi-square test statistics P value for group comparisons of categorical variables (gender, death, intubation, and mechanical ventilation); X 2: Pearson chi-square value; * P < .05 for one-way ANOVA test; a,b,c show one-way ANOVA post hoc Tukey test P values (aSignificant difference between mild and moderate patients; bSignificant difference between mild and severe patients; cSignificant difference between moderate and severe patients).
M/F, male/female; MV req Inv., invasive mechanical ventilation requirement; MV Req. all, mechanical ventilation requirement invasive and non-invasive.
One-way ANOVA test showed significant differences in serum CRP, LDH, fibrinogen, d-dimer, ferritin, and IL-28 levels between all groups (P < .05 for all comparisons). All patients' mean serum IL-28 levels were 383.74 ± 63.58 ng/L (Table 1). There were significant differences in terms of serum IL-28 levels between mild, moderate, and severe groups, being higher in the moderate group (366.88 ± 41.27 ng/L) and the highest in the severe group (453.46 ± 36.78 ng/L) compared to the mild group (335.52 ± 42.12 ng/L) (Figure 1).
Figure 1.
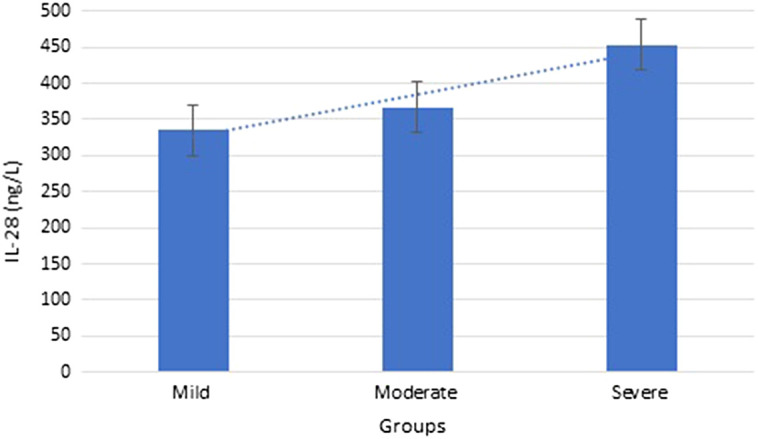
IL-28 values of groups. IL, interleukin.
Receiver operating characteristic curve analysis for predicting mechanical ventilation requirement (both invasive and non-invasive) results in the area under the ROC curve (AUC) of 0.84 (95% CI: 0.75-0.93). The cut-off value of IL-28 to predict mechanical ventilation needs with high sensitivity was 391.71 ng/L (100% sensitivity and 60.0% specificity, likelihood ratio (LR): 2.47) (Figure 2A).
Figure 2.
ROC curve for prediction of (A) mechanical ventilation (invasive and non-invasive) requirement, (B) invasive mechanical ventilation requirement, (C) non-invasive mechanical ventilation requirement, and (D) mortality with IL-28 values. ROC, receiver operating characteristic.
Receiver operating characteristic curve analysis for predicting the need for invasive mechanical ventilation resulted in AUC of 0.87 (95% CI: 0.79-0.96). The cut-off value of IL-28 to predict the need for invasive mechanical ventilation with high sensitivity was 401.23 ng/L (100% sensitivity and 70.0% specificity, LR: 3.29) (Figure 2).
Receiver operating characteristic curve analysis for predicting non-invasive mechanical ventilation requirement resulted in the AUC of 0.80 (95% CI: 0.68-0.92). The cut-off IL-28 value to predict non-invasive mechanical ventilation with moderate sensitivity was 423.95 ng/L (71.4% sensitivity and 77.8% specificity (LR: 3.21) (Figure 2).
Receiver operating characteristic curve analysis for predicting mortality resulted in AUC of 0.86 (95% CI: 0.76-0.97). The cut-off IL-28 value to predict mortality with high sensitivity was 401.23 ng/L (100% sensitivity and 67.5% specificities, LR: 3.03). (Figure 2).
Discussion
In this study, we tested whether IL-28 from the IFN-λ family can be used as a marker to show the severity of the disease in COVID-19 patients. There were significant differences in terms of serum IL-28 levels between mild, moderate, and severe groups, being higher in the moderate group (366.88 ± 41.27 ng/L) and the highest in the severe group (453.46 ± 36.78 ng/L) compared to the mild group (335.52 ± 42.12 ng/L). Various studies have shown the effect and role of the classical interferon family members in viral infections and sepsis.14 The IFN-λ family, also known as type 3 interferons, is most effective on epithelial surfaces and is known to be effective in viral defense.15
It was previously shown that there was an increase in IL-28 levels in chronic Hepatitis C Virus (HCV) and dengue fever.16 It has also been shown that HCV patients with good response to antiviral treatment used to have lower IL-28 levels.16 Although IL-28 and the IFN-λ family are generally thought to be having a positive influence in controlling viral infections, higher than normal levels of these cytokines may also be an indicator of an uncontrolled infection or viral response. The effects of the IFN-λ family and IL-28 in sepsis were demonstrated in a mice experiment.17 In this study, not only the neutralization of the IL-28 pathway decreased mortality due to sepsis in mice but it was also observed that mortality increased in mice administered recombinant IL-28 externally. In contrast, administration of IL-28 to mice without sepsis did not increase mortality. Our study showed that IL-28 levels could be used to predict mortality and mechanical ventilation requirement independent of CRP and other infection markers. Interleukin-28 levels were high in patients who needed invasive mechanical ventilation and died in our study group, which supports the idea of high IL-28 levels being associated with mortality in sepsis due to viral infection.
Contrary to the data in our study, in a study conducted on patients with Crimean Congo hemorrhagic fever, it was shown that IL-28-B gene polymorphism did not have a significant effect on mortality.18 However, there is no evaluation of IL-28 serum levels in this study. These data suggest that IFN-λ family member interleukins may have different effects on different types of viral infections.
The fact that the blockade of the IL-28 pathway in sepsis has produced promising results in terms of mortality in mice experiments is promising as a method that can be used in severe COVID-19 patients with limited treatment opportunities, and more scientific studies are needed on this subject.
An animal study has demonstrated that the upper airway microbiota of IL-28 receptor-negative mutant mice exposed to influenza virus was less affected than normal mice with IL-28 receptor, and secondary Staphylococcus aureus infection is less common in non-receptor mice.19 This study shows that the immune response induced by IL-28 in viral infections may also increase the susceptibility to secondary bacterial infections. There were no data on the frequency of secondary bacterial infections in patients who required mechanical ventilation and who died in our study. Considering these results, future studies may be helpful to show whether excessive IL-28 secretion in viral infections increases secondary bacterial infections.
This study has some limitations. First of all, the study did not have a control group without COVID-19 infection. Second, we could not determine the mRNA expression of IL-28 due to technical inadequacy. Third, COVID-19 infection may lead to some degree of renal dysfunction and concentrations of serum metabolites such as amino acids, bile acids, nucleotides and their metabolites, glycerophospholipids, fatty acids, oxidized lipids, and organic acids due to altered filtration capacity of kidneys and thus, the levels of IL28 in serum samples might be affected. Fourth, since the patients in the severe group had serious clinical conditions and were administered multiple treatments at the same time, the linearity of biochemical parameters may have been impaired.
In conclusion, IL-28 may be a candidate biomarker for determining the disease severity of COVID-19 patients. Further studies are needed to determine the optimal cut-off values.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Erzurum City Hospital (Ministry of Health) (approval No: 2021/01-04).
Informed Consent: Written and verbally informed consent was obtained from all patients or legal representatives.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.A., U.T.; Design – A.A., U.T.; Supervision – A.F.K.; Funding – A.A., U.T, A.F.K., T.T.; Materials – A.A., U.T.; Data Collection and/or Processing – A.A., U.T., T.T.; Analysis and/or Interpretation – N.K.B, U.T.; Literature Review – N.K.B, U.T.; Writing – A.A, T.T, U.T.; Critical Review – N.K.B, A.F.K.
Acknowledgments: The authors would like to thank to all Erzurum City Hospital health workers in ICU and patient wards for COVID, due to their helps during the outbreak and the duration of this research.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This study received no funding.
References
- 1. Conti P, Ronconi G, Caraffa A.et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVID-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327 331. ( 10.23812/CONTI-E) [DOI] [PubMed] [Google Scholar]
- 2. King JT, Yoon JS, Bredl ZM, et al. Accuracy of the Veterans Health Administration COVID-19 (VACO) Index for predicting short-term mortality among 1307 US academic medical centre inpatients and 427 224 US Medicare patients. J Epidemiol Community Health. 2022;76(3):254 260. ( 10.1136/jech-2021-216697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahman T, Al-Ishaq FA, Al-Mohannadi FS.et al. Mortality prediction utilizing blood biomarkers to predict the severity of COVID-19 using machine learning technique. Diagnostics (Basel). 2021;11(9):1582. ( 10.3390/diagnostics11091582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tiscia G, Favuzzi G, De Laurenzo A.et al. The prognostic value of ADAMTS-13 and von Willebrand factor in COVID-19 patients: prospective evaluation by care setting. Diagnostics (Basel). 2021;11(9):1648. ( 10.3390/diagnostics11091648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uranga A, Villanueva A, Lafuente I.et al. Risk factors for clinical deterioration in patients admitted for COVID-19: a case-control study.Rev Clinesp (Barc). 2022;222(1):22 30. ( 10.1016/j.rceng.2021.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cihakova D, Streiff MB, Menez SP.et al. High-value laboratory testing for hospitalized COVID-19 patients: a review.Future Virol. 2021;16(10):691 705. ( 10.2217/fvl-2020-0316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheppard P, Kindsvogel W, Xu W.et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4(1):63 68. ( 10.1038/ni873) [DOI] [PubMed] [Google Scholar]
- 8. Kempuraj D, Donelan J, Frydas S.et al. Interleukin-28 and 29 (IL-28 and IL-29): new cytokines with anti-viral activities.Int J Immunopathol Pharmacol. 2004;17(2):103 106. ( 10.1177/039463200401700201) [DOI] [PubMed] [Google Scholar]
- 9. Morrow MP, Pankhong P, Laddy DJ.et al. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood. 2009;113(23):5868 5877. ( 10.1182/blood-2008-11-190520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ge D, Fellay J, Thompson AJ.et al. Genetic variation in IL-28B predicts hepatitis C treatment-induced viral clearance.Nature. 2009;461(7262):399 401. ( 10.1038/nature08309) [DOI] [PubMed] [Google Scholar]
- 11. Junaid K, Rasool H, Ul Mustafa A,et al. Association of IL-28 B and IL10 polymorphism with HCV infection and direct antiviral treatment.Ann Clin Lab Sci. 2021;51(4):512 520. [PubMed] [Google Scholar]
- 12. Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL-28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41(10):1105 1109. ( 10.1038/ng.449) [DOI] [PubMed] [Google Scholar]
- 13. Rauch A, Kutalik Z, Descombes P.et al. Genetic variation in IL-28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138(4):1338 1345.e7. ( 10.1053/j.gastro.2009.12.056) [DOI] [PubMed] [Google Scholar]
- 14. Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24(8):439 454. ( 10.1089/1079990041689665) [DOI] [PubMed] [Google Scholar]
- 15. Lazear HM, Nice TJ, Diamond MS. Interferon-λ: immune functions at barrier surfaces and beyond. Immunity. 2015;43(1):15 28. ( 10.1016/j.immuni.2015.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abe H, Hayes CN, Ochi H.et al. IL-28 variation affects expression of interferon stimulated genes and peg-interferon and ribavirin therapy. J Hepatol. 2011;54(6):1094 1101. ( 10.1016/j.jhep.2010.09.019) [DOI] [PubMed] [Google Scholar]
- 17. Luo Q, Liu Y, Liu S, Yin Y, Xu B, Cao J. Interleukin 28 is a potential therapeutic target for sepsis. Clin Immunol. 2019;205:29 34. ( 10.1016/j.clim.2019.05.012) [DOI] [PubMed] [Google Scholar]
- 18. Aytekin FY, Barut HŞ, Rüstemoğlu A, Atay A, Günal Ö, Duygu F. Factors related to fatalities and clinical progression of Crimean-Congo hemorrhagic fever patients and the effects of il 28-B gene polymorphism. Arch Virol. 2019;164(2):547 557. ( 10.1007/s00705-018-4106-1) [DOI] [PubMed] [Google Scholar]
- 19. Planet PJ, Parker D, Cohen TS.et al. Lambda interferon restructures the nasal microbiome and increases susceptibility to Staphylococcus aureus superinfection. mBio. 2016;7(1):e01939 e01915. ( 10.1128/mBio.01939-15) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a 