Type 2 diabetes, a major noncommunicable disease, is typified by hyperglycemia, which stems from insulin resistance and dysfunction of pancreatic β-cells (1). Prior multi-ethnic studies hypothesized that type 2 diabetes among Caucasians was primarily driven by insulin resistance, whereas in East Asians, it was predominantly a consequence of β-cell dysfunction (2). Apart from the apparent genetic variations, obesity has been identified as the main factor responsible for the pathophysiological differences in diabetes between Caucasians and East Asians (3). Notably, China has seen a considerable increase in obesity cases in congruence with substantial changes in lifestyle and urbanization over the last four decades. In a recent population-based national cohort study, we showed that insulin resistance, rather than β-cell dysfunction, had a significant role in the pathological progression of diabetes within the Chinese population (1). This correlation was particularly pronounced among obese adults (1).
The interplay between environmental dynamics, socioeconomic status (SES), lifestyle practices, and a distinct genetic backdrop results in specific patterns of health preservation and disease onset within the Chinese population. As a result, the risk triggers and origins of diabetes and associated cardiovascular complications among this demographic may not fully align with the international expert consensus, which is primarily informed by findings from European and North American populations. This perspective collates current evidence concerning the profiles of diabetes risk factors and associated cardiovascular complications in the Chinese community, paying special attention to age-linked risk profiles and gene-environment interplays (Figure 1). It also recommends potential avenues for future research, concentrated on developing precise and effective prevention and intervention strategies to arrest the surge of diabetes and cardiovascular complications.
Figure 1.
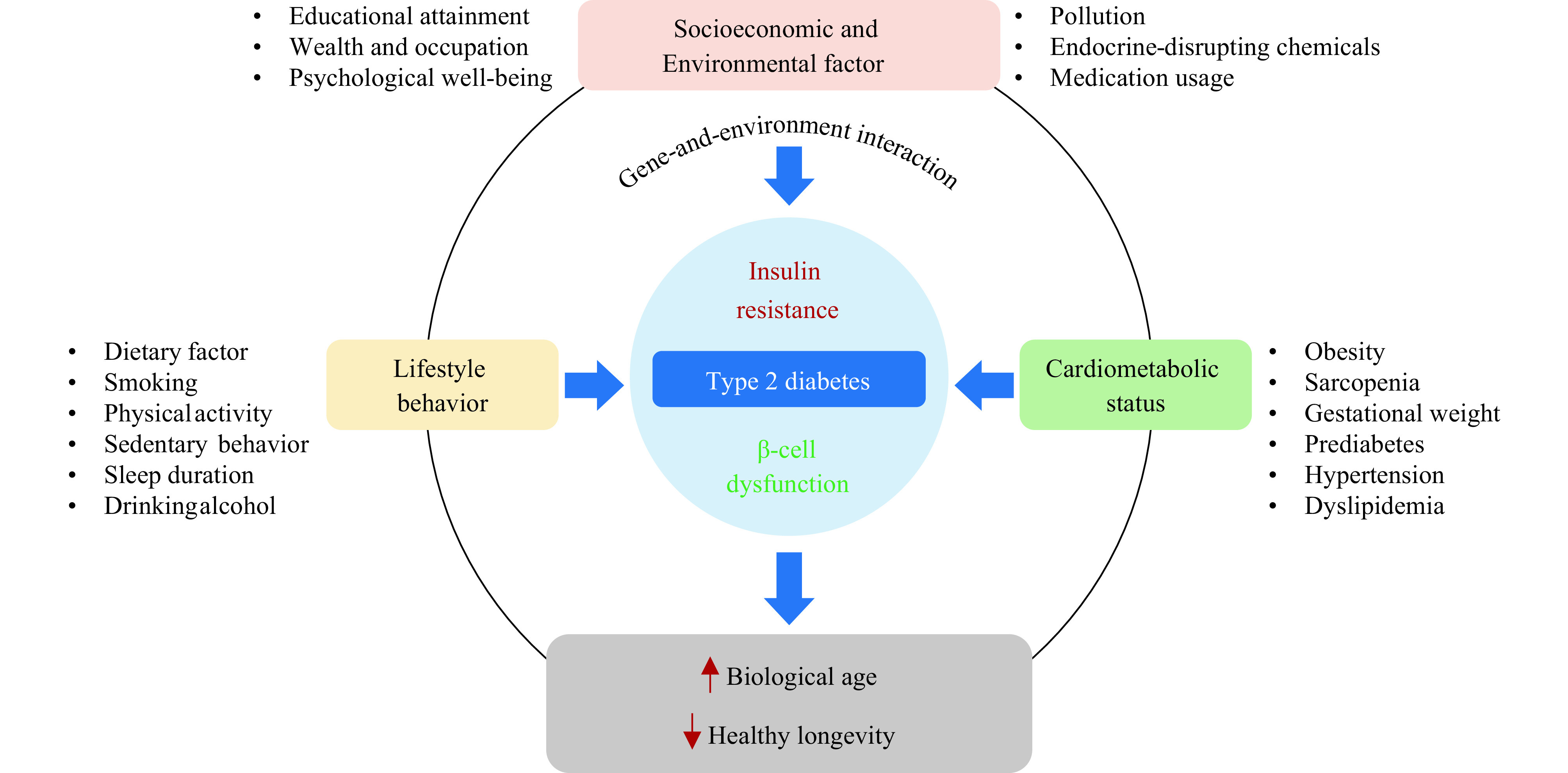
Profiles of risk factors for diabetes and the role of diabetes in healthy longevity.
Note: Type 2 diabetes is principally typified by two pathologic characteristics: insulin resistance and β-cell dysfunction. Beyond genetic predisposition, main diabetes risk factors encompass socioeconomic and environmental factors, lifestyle behaviors, and cardiometabolic status. Type 2 diabetes not only poses as a risk factor for cardiometabolic disruptions but also operates as a facilitator in the pathogenic pathway, subsequently leading to accelerated biological aging and impacting healthy longevity.
Profiles of Risk Factors for Diabetes
Large-scale epidemiological studies have demonstrated that factors such as aging, sex, SES, lifestyle choices, dietary habits, psychological health, and metabolic disturbances collectively or individually contribute to the onset of diabetes and weight gain (4–8). A prospective evaluation involving 93,781 adults within China’s Cardiometabolic Disease and Cancer Cohort (4C) Study, with a robust follow-up period equivalent to 337,932 person-years, revealed that the incidence of diabetes progressively rose, from a base rate of 5.1% in adults aged between 40–55 years to a striking 10.0% in seniors aged 75 or older (4).
Further analysis from this study exposed a series of age-specific diabetes risk profiles. Intriguingly, metabolic risk factors such as obesity, prediabetes, hypertension, and dyslipidemia, although remained accountable for most newly diagnosed diabetes cases across all age groups, showed a marginally decreasing correlation with age (4). A similar age-related decline was also observed for socioeconomic risk factors such as lower levels of education (4). In contrast, the prominence of lifestyle-induced diabetes risks, specifically below 6 hours or above 8 hours of daily sleep, amplifies distinctly with age (4). This insight underscores the need for strategizing risk management methods based on age groups, to devise more cost-effective prevention and management strategies against diabetes.
In the past ten years, there has been increasing evidence suggesting that exposure to endocrine-disrupting chemicals (EDCs), which are human-made chemicals capable of mimicking, blocking, or interfering with hormones, contributes to impaired glucose regulation and increased risk of type 2 diabetes in Chinese adults (9–12). An observational cohort study conducted in Shanghai, China, followed 2,336 participants over a 4-year period, revealing sex-specific correlations between heightened urinary bisphenol A (BPA) levels and impaired glucose homeostasis (9). This correlation includes elevated fasting plasma glucose levels and β-cell dysfunction, independent of multiple covariates and body mass index (BMI) in non-diabetic women, but not in men (9). Interestingly, within the same study group, although no significant association was found between BPA exposure and diabetes risk, high urinary BPA concentrations were positively linked to a significant rise in fasting glucose levels in adults with a greater genetic predisposition to diabetes (12).
Encouragingly, substantial evidence of gene-environment interactions suggests that a genetic predisposition to diabetes or obesity may not diminish the metabolic benefits of maintaining a healthy lifestyle (13). In fact, individuals with a higher genetic predisposition might glean even more benefits from adhering to healthier lifestyles, evident through improvements in physical activity as well as dietary patterns, and even weight loss (6–8,13). A gene-environment interaction analysis, conducted using 20-year follow-up data from the Nurses’ Health Study and the Health Professionals Follow-Up Study, showed that while genetic variations in BMI and body fat percentage were correlated with long-term weight increases, the positive impact of physical activity on weight loss was more pronounced in individuals with a high genetic susceptibility to obesity (6). Furthermore, within these same population cohorts, comprehensive evidence has underscored the interactions between healthy dietary patterns [such as the alternative healthy eating index (AHEI) 2010 and dietary approach to stop hypertension (DASH), which advocate for increased consumption of fruits, vegetables, and whole grains, as well as restricted intake of sugar-sweetened beverages, red or processed meats, and trans fats] and the genetic predilection to obesity in relation to long-term weight change (7-8), indicating that individuals with a higher genetic predisposition to obesity will likely witness greater weight loss through adherence to these healthful dietary patterns (8).
In a manner consistent with findings observed among individuals of European descent, a population-based study of 1,180 women with gestational diabetes from the Tianjin Gestational Diabetes Mellitus Prevention Program examined the interactions between the zinc transporter-8 gene (SLC30A8) genotype and gestational weight gain in relation to postpartum glycemic changes (14). The study found that optimal gestational weight control is particularly beneficial for Chinese women possessing the high-diabetes-risk SLC30A8 genotype, as it can reduce the potential of developing hyperglycemia following pregnancy (14). These findings involving gene-environment interactions suggest that healthy lifestyle behaviors such as regular physical activity, a balanced diet, and maintaining an ideal weight can help mitigate the adverse effects of genetic susceptibility to diabetes or obesity, underscoring potential tailored approaches for precision health.
Diabetes as a Risk Factor for Cardiovascular Disease and Its Impact on Longevity
Cardiovascular disease (CVD) is recognized as the most significant complication associated with diabetes and the leading cause of mortality for those who have diabetes. A prospective, nationwide analysis of 139,925 participants without CVD at baseline, drawn from the 4C Study, revealed that metabolic risk factors were largely responsible for 52.4%, 47.2%, and 37.8% of the population-attributable risk percentages for CVD event development in adult age groups: 40 to <55 years, 55 to <65 years, and 65 to <75 years, respectively (15). Within these demographic divisions, hypertension and diabetes emerged as the most prominent metabolic risk factors (15). Interestingly, among individuals aged 75 years or older, the primary risk factors for CVD transitioned to inappropriate sleep duration, lower education, hypertension, and diabetes (15). The age-specific risk factor profiles imply differential mechanisms underlying CVD development across different age groups, offering valuable insights to inform the development of age-specific preventive strategies and interventions.
Insulin resistance and its association with obesity have been identified as pivotal factors in the onset of CVD and cardiometabolic disorders in middle-aged and elderly Chinese adults (16). A prospective analysis of 111,576 participants from the 4C Study showed that, in comparison to adults with normal glucose tolerance, the hazard ratios (HRs) for CVD associated with the highest quartile of the homeostatic model assessment for insulin resistance (HOMA-IR) stood at 1.61 [95% confidence interval (CI): 1.30 to 2.00] for adults with diabetes and 1.23 (95% CI: 1.07 to 1.42) for adults with prediabetes (16). Notably, individuals with prediabetes who were both insulin-resistant and obese demonstrated increased CVD risks (16). Conversely, in adults with diabetes, the CVD risk linked to insulin resistance was consistent, irrespective of obesity (16).
Moreover, another analysis from the 4C Study indicated that adults with either prediabetes or diabetes presenting five or more ideal cardiovascular health metrics (ICVHMs), including non-smoking status or a smoking cessation period exceeding 12 months, a BMI below 23 kg/m2, the achievement of physical activity targets (equal to or exceeding 150 minutes/week of moderate intensity, 75 minutes/week of vigorous intensity, or 150 minutes/week of moderate or vigorous intensity), daily consumption of at least 4.5 cups of fruits and vegetables, total cholesterol levels below 200 mg/dL (untreated), blood pressure readings below 120 mmHg/80 mmHg (untreated), and optimal glycated hemoglobin levels (less than 5.7% for prediabetes or less than 6.5% for diabetes), exhibited no significant excess risk or lower risk of CVD when compared to individuals with normal glucose tolerance (17).
Recent Mendelian randomization (MR) studies have validated previous observational findings that diabetes and insulin resistance are risk factors for cardiometabolic diseases (18-19). Contrasting with conventional epidemiological studies, MR analysis employs genetic variants, determined at conception and randomly allocated to individuals, as instrumental variables. This enables inference of causal relationships between phenotypes and significantly reduces the possibility of reverse causality and bias arising from known or unknown confounders. A two-sample MR study, utilizing genome-wide association study (GWAS) summary statistics, proposed that diabetes diagnosed at various age ranges (<50, 50–60, 60–70, and >70 years) has equivalent causal effects on CVD and related cardiometabolic diseases (18). This suggests that genetically defined diabetes subtypes, classified by age at diagnosis, may not infer differential CVD risks (18). Another MR study revealed causal links between insulin resistance (as evidenced by fasting insulin) and six cardiometabolic diseases [type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), hypertension, coronary heart disease (CHD), myocardial infarction (MI), and small vessel stroke] (19). It further suggested that insulin resistance serves as a mediating factor in the causal relationships between sarcopenia-related traits and five aforementioned cardiometabolic diseases (type 2 diabetes, NAFLD, hypertension, CHD, and MI) (19). More specifically, insulin resistance is accountable for approximately 7% to 34% of the causal effects of grip strength or appendicular lean mass on diabetes, NAFLD, hypertension, CHD, and MI (19).
Several studies have demonstrated causal relationships between diabetes and both biological aging and longevity in humans (20-21). In a recent MR study, type 2 diabetes was conclusively identified as a causative risk factor for biological aging (20). This link was identified by two reliable DNA methylation-based indicators known as GrimAge acceleration and PhenoAge acceleration, which provide useful insights into potential interventions for reducing age-related morbidity and promoting healthy longevity (20). Importantly, type 2 diabetes also acts as a mediator in the causal pathway connecting broader environmental risk factors, such as SES, and human longevity (21). A recent comprehensive MR study, using GWAS summary statistics from over 1,000,000 European-ancestry individuals, found that genetically predicted elevated levels of SES, including higher educational attainment, household income, and occupational status, were positively associated with both a longer parental lifespan and individual longevity (21). Among these, educational attainment exhibited an independent effect. In the causal pathways, type 2 diabetes was found to mediate approximately 7.50%, 21.98%, and 47.33% of the effects of education on parental lifespan, and on the 90th and 99th percentile of individual longevity, respectively (21).
The primary socioeconomic risk factor for diabetes, according to the 4C study, was lower educational attainment. This demographic represented more than 10% of the risk factor for the disease in adults younger than 65 years (4). While an increase in ICVHMs was inversely related to the risk of CVD in populations with both high and low levels of educational attainment, there was no evidence supporting the interaction between these two factors in prediabetic or diabetic individuals (17). Insights from the Prospective Urban Rural Epidemiology study, which included 154,169 adults from 20 countries, suggest that country income levels affect socioeconomic gradients in CVD and mortality rates (22). The HRs for major CVD events, when comparing lower to higher education levels, ranged from 1.23 (95% CI: 0.96 to 1.58) for individuals in high-income countries to 2.23 (95% CI: 1.79 to 2.77) for those in low-income countries (22). Consequently, the socioeconomic context of the study population and the generalizability and reliability of existing evidence are vital considerations in interpreting the impact of risk factors on diabetes and cardiovascular complications. This will promote a nuanced comprehension of diabetes etiology and aid in building more effective prevention and intervention strategies.
Future Prospects and Challenges in Diabetes Research and Prevention
Given the escalating concerns regarding the diabetes epidemic and its impact in China, both the Diabetes Prevention and Control Action and the Healthy Diet Campaign within the Healthy China Initiative (2019–2030) underscore the importance of delivering education (e.g., health knowledge and nutritional awareness), intervention, and standard health management to the broader population and individuals with diabetes (23-24). Notably, numerous MR studies primarily rely on GWASs conducted on individuals of European descent; consequently, care must be exercised when generalizing the findings. While it is critical to collate and analyze global datasets, as they can showcase the gamut of risk factors in varied socioeconomic circumstances and offer predictions for future trajectories, it is equally crucial to formulate and implement strategies tailored specifically toward the Chinese population.
Emerging technologies are propelling diabetes research into new arenas, encompassing climate change, environmental exposure, nutrition and longevity, the human gut microbiome, multi-omics, and artificial intelligence models (25–30). There is an abundance of scientific inquiries that warrant further examination. First, understanding the intersection of climate change (e.g., global warming) and traditional risk profiles. Second, conducting in-depth assessments of EDC impacts. Third, investigating the intermediary role of the gut microbiome in metabolically healthy longevity. Fourth, establishing a medical research database specifically for the Chinese population. Fifth, initiating ground-breaking research in novel drug development and biotechnologies for metabolic diseases. Sixth, integrating multi-omics and artificial intelligence algorithms into medical services.
Conflicts of interest
No conflicts of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (82022011, 81970706), the “Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support” from Shanghai Jiao Tong University School of Medicine (20171901 Round 2), and the Innovative Research Team of High-Level Local Universities in Shanghai
References
- 1.Wang TG, Lu JL, Shi LX, Chen G, Xu M, Xu Y, et al Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. 2020;8(2):115–24. doi: 10.1016/S2213-8587(19)30425-5. [DOI] [PubMed] [Google Scholar]
- 2.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–96. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Møller JB, Pedersen M, Tanaka H, Ohsugi M, Overgaard RV, Lynge J, et al Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37(3):796–804. doi: 10.2337/dc13-0598. [DOI] [PubMed] [Google Scholar]
- 4.Wang TG, Zhao ZY, Wang GX, Li Q, Xu Y, Li M, et al Age-related disparities in diabetes risk attributable to modifiable risk factor profiles in Chinese adults: a nationwide, population-based, cohort study. Lancet Healthy Longev. 2021;2(10):e618–28. doi: 10.1016/S2666-7568(21)00177-X. [DOI] [PubMed] [Google Scholar]
- 5.Wang TG, Lu JL, Xu Y, Li M, Sun JC, Zhang J, et al Circulating prolactin associates with diabetes and impaired glucose regulation: a population-based study. Diabetes Care. 2013;36(7):1974–80. doi: 10.2337/dc12-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TG, Huang T, Heianza Y, Sun DJY, Zheng Y, Ma WJ, et al Genetic susceptibility, change in physical activity, and long-term weight gain. Diabetes. 2017;66(10):2704–12. doi: 10.2337/db17-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TG, Heianza Y, Sun DJY, Zheng Y, Huang T, Ma WJ, et al Improving fruit and vegetable intake attenuates the genetic association with long-term weight gain. Am J Clin Nutr. 2019;110(3):759–68. doi: 10.1093/ajcn/nqz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TG, Heianza Y, Sun DJY, Huang T, Ma WJ, Rimm EB, et al Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ. 2018;360:j5644. doi: 10.1136/bmj.j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Li M, Zhao ZY, Lu JL, Chen YH, Xu Y, et al Urinary bisphenol A concentration and glucose homeostasis in non-diabetic adults: a repeated-measures, longitudinal study. Diabetologia. 2019;62(9):1591–600. doi: 10.1007/s00125-019-4898-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang TG, Li M, Chen B, Xu M, Xu Y, Huang Y, et al Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(2):E223–7. doi: 10.1210/jc.2011-1989. [DOI] [PubMed] [Google Scholar]
- 11.Ning G, Bi YF, Wang TG, Xu M, Xu Y, Huang Y, et al Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann Intern Med. 2011;155(6):368–74. doi: 10.7326/0003-4819-155-6-201109200-00005. [DOI] [PubMed] [Google Scholar]
- 12.Bi YF, Wang WQ, Xu M, Wang TG, Lu JL, Xu Y, et al Diabetes genetic risk score modifies effect of Bisphenol A exposure on deterioration in glucose metabolism. J Clin Endocrinol Metab. 2016;101(1):143–50. doi: 10.1210/jc.2015-3039. [DOI] [PubMed] [Google Scholar]
- 13.Wang TG, Xu M, Bi YF, Ning G Interplay between diet and genetic susceptibility in obesity and related traits. Front Med. 2018;12(6):601–7. doi: 10.1007/s11684-018-0648-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang TG, Liu HK, Wang LS, Huang T, Li WQ, Zheng Y, et al Zinc-associated variant in SLC30A8 gene interacts with gestational weight gain on postpartum glycemic changes: a longitudinal study in women with prior gestational diabetes mellitus. Diabetes. 2016;65(12):3786–93. doi: 10.2337/db16-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TG, Zhao ZY, Yu XF, Zeng TS, Xu M, Xu Y, et al Age-specific modifiable risk factor profiles for cardiovascular disease and all-cause mortality: a nationwide, population-based, prospective cohort study. Lancet Reg Health West Pac. 2021;17:100277. doi: 10.1016/j.lanwpc.2021.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TG, Li M, Zeng TS, Hu RY, Xu Y, Xu M, et al Association between insulin resistance and cardiovascular disease risk varies according to glucose tolerance status: a nationwide prospective cohort study. Diabetes Care. 2022;45(8):1863–72. doi: 10.2337/dc22-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TG, Lu JL, Su Q, Chen YH, Bi YF, Mu YM, et al Ideal cardiovascular health metrics and major cardiovascular events in patients with prediabetes and diabetes. JAMA Cardiol. 2019;4(9):874–83. doi: 10.1001/jamacardio.2019.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye CJ, Kong LJ, Wang YY, Lin H, Wang SY, Zhao ZY, et al Causal associations between age at diagnosis of diabetes and cardiovascular outcomes: a Mendelian randomization study. J Clin Endocrinol Metab. 2023;108(5):1202–14. doi: 10.1210/clinem/dgac658. [DOI] [PubMed] [Google Scholar]
- 19.Ye CJ, Kong LJ, Wang YY, Zheng J, Xu M, Xu Y, et al Causal associations of sarcopenia-related traits with cardiometabolic disease and Alzheimer's disease and the mediating role of insulin resistance: a Mendelian randomization study. Aging Cell. 2023;22(9):e13923. doi: 10.1111/ACEL.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong LJ, Ye CJ, Wang YY, Hou TZC, Zheng J, Zhao ZY, et al Genetic evidence for causal effects of socioeconomic, lifestyle, and cardiometabolic factors on epigenetic-age acceleration. J Gerontol: Ser A. 2023;78(7):1083–91. doi: 10.1093/GERONA/GLAD078. [DOI] [PubMed] [Google Scholar]
- 21.Ye CJ, Kong LJ, Wang YY, Dou C, Zheng J, Xu M, et al Mendelian randomization evidence for the causal effects of socio-economic inequality on human longevity among Europeans. Nat Hum Behav. 2023;7(8):1357–70. doi: 10.1038/S41562-023-01646-1. [DOI] [PubMed] [Google Scholar]
- 22.Rosengren A, Smyth A, Rangarajan S, Ramasundarahettige C, Bangdiwala SI, AlHabib KF, et al Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob Health. 2019;7(6):e748–60. doi: 10.1016/S2214-109X(19)30045-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang WJ Interpretation of the diabetes prevention and control action of the healthy China initiative 2019-2030. China CDC Wkly. 2020;2(9):143–5. doi: 10.46234/ccdcw2020.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LS, Wang HJ, Wang ZH, Jiang HR, Li WY, Wang SSZ, et al Interpretation of healthy diet campaign in healthy China initiative 2019-2030. China CDC Wkly. 2021;3(16):346–9. doi: 10.46234/ccdcw2021.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogar K, Brensinger CM, Hennessy S, Flory JH, Bell ML, Shi C, et al Climate change and ambient temperature extremes: association with serious hypoglycemia, diabetic ketoacidosis, and sudden cardiac arrest/ventricular arrhythmia in people with type 2 diabetes. Diabetes Care. 2022;45(11):e171–3. doi: 10.2337/dc22-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lind PM, Lind L Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018;61(7):1495–502. doi: 10.1007/s00125-018-4621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longo VD, Anderson RM Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. 2022;185(9):1455–70. doi: 10.1016/j.cell.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou WY, Sailani MR, Contrepois K, Zhou YJ, Ahadi S, Leopold SR, et al Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature. 2019;569(7758):663–71. doi: 10.1038/s41586-019-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreitmaier P, Katsoula G, Zeggini E Insights from multi-omics integration in complex disease primary tissues. Trends Genet. 2023;39(1):46–58. doi: 10.1016/j.tig.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Singhal K, Azizi S, Tu T, Mahdavi SS, Wei J, Chung HW, et al Large language models encode clinical knowledge. Nature. 2023;620(7972):172–180. doi: 10.1038/s41586-023-06291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


