Abstract
What is already known about this topic?
The majority of Chinese patients with diabetes failed to achieve the level of physical activity recommended by clinical guidelines.
What is added by this report?
The prevalence of low-level physical activity was found to be greater in individuals diagnosed with diabetes. It was observed that patients with a protracted duration of diabetes demonstrated a propensity to participate in lower levels of physical activity compared to those with a shorter disease trajectory. The likelihood of engaging in low-level physical activity associated with diabetes was higher in rural inhabitants, those with medium-tier education, employed individuals, and individuals who had longer sleep durations.
What are the implications for public health practice?
Developing strategies and interventions to encourage greater involvement of Chinese diabetic patients in physical activity is essential. However, these strategies must take population characteristics into account.
Keywords: Diabetes Mellitus, Physical Activity, Influencing Factors
Diabetes mellitus (DM) is a chronic metabolic disorder marked by high blood glucose levels, often resulting in multi-organ and systemic insufficiency or failure. Over the last three decades, there’s been a noted increase in DM prevalence in China (1). Previous research has suggested that sufficient, appropriate physical activity (PA) can assist DM patients in regulating their blood glucose levels (2), and numerous national guidelines for comprehensive DM management advocate for regular PA (1,3). Prior studies exploring the PA level of DM patients in China typically suffered from small sample sizes and lacked comparative analysis with the general population (4-5). Thus, this study aims to examine the variations in PA between Chinese individuals with and without DM and to identify influential factors utilizing data from the third resurvey of the China Kadoorie Biobank (CKB).
The CKB constitutes one of the largest prospective cohort studies globally, surveying across 10 sites within China. The CKB obtains crucial sociodemographic data, lifestyle factors, and histories of common chronic diseases through direct, face-to-face interviews. Additionally, trained technicians perform on-site physical examinations, including height and weight measurements, adhering to a standard operating procedure.
The initial baseline data (2004–2008) of the CKB incorporated more than 512,000 participants, with the third resurvey data (2020–2021) focusing on a randomly selected subset constituting approximately 5% of the initial participant pool. This resurvey examined a total of 25,087 participants, aged between 40 and 95 years old, across the 10 original study sites and collected relevant data in a method consistent with the baseline study. Participants were selectively excluded based on certain criteria. These included missing value data related to PA, DM status, and other important covariates. Individuals with self-reported histories of tumors or asthma were also omitted from the study.
The PA level was assessed by calculating the sum of the product of the metabolic equivalent of task (MET) multiplied by the duration of each PA, measured in unit time. Within the CKB, PA levels were stratified by gender and age (either ≥65 or <65 years). Low-level PA was characterized as those falling below the lowest tertile of their corresponding gender and age group. Participants reporting a history of DM were classified as DM patients.
Continuous variables were described using mean±standard deviation, while frequencies and proportions were used for categorical variables. The chi-squared test or t-test was utilized to identify differences between groups. DM and PA level relationships were separately assessed through multiple logistic regression, adjusted for social demographic factors such as age, gender, region, educational level, and occupational status. This model also took into account body mass index (BMI), lifestyle factors (smoking, drinking, and sleep time), and history of other chronic diseases.
The effects of blood glucose control (characterized by fasting glucose: <7.0 mmol/L and ≥7.0 mmol/L; random glucose: <11.1 mmol/L and ≥11.1 mmol/L) and length of DM course (≤5, 5–10, and >10 years) on PA level were further explored. Stratified analysis ensued, in which the relationship between DM and PA level was examined at each covariate level with other covariates serving as controls. The interaction was assessed through the construction of a logistic regression model, with PA level as the dependent variable, and the product of one discrete covariate and DM as the independent variable(s). The statistical significance of the interaction between the covariate and DM was determined by the P-value from the likelihood ratio test for the product term, also adjusted for additional covariates. Linear trends of DM course impact on PA level were tested. To mitigate the risk of reverse causation, analyses were rerun while excluding DM patients diagnosed within the previous year. Data analysis for this study was executed using SPSS software (version 27.0: IBM Corp., Armonk, NY, USA), coupled with a bilateral test and a test level of P<0.05.
This study incorporated a total of 24,494 participants drawn from the CKB, the mean age being 65.5±9.1 years (Table 1), with 3,107 individuals, or 12.7%, having DM. Statistically significant differences were observed in PA levels among DM patients, with a higher prevalence of low-level PA compared to non-DM individuals (P<0.001). Additionally, variations were evident between the two groups regarding age, gender, geographic region, education level, profession, lifestyle, BMI, and history of chronic diseases.
Table 1. Basic characteristics of CKB research subjects.
| Variables | Total | DM | Non-DM | P-value |
| Abbreviation: DM=diabetes mellitus; BMI=body mass index; PA=physical activity; CKB=China Kadoorie Biobank. * Low, medium, and high level of education in CKB refers to primary school and below, secondary school and college and above. † Other chronic medical history includes cardiovascular disease, chronic obstructive pulmonary disease, and hypertension. | ||||
| n | 24,494 | 3,107 | 21,387 | |
| Age (years, 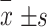 ) ) |
65.5±9.1 | 65.5±9.1 | 65.5±9.1 | <0.001 |
| Male (%) | 35.6 | 66.9 | 36.0 | 0.001 |
| Region (%) | <0.001 | |||
| Urban | 49.7 | 39.7 | 41.0 | |
| Rural | 50.3 | 60.3 | 59.0 | |
| Education level* (%) | 0.032 | |||
| Low | 54.1 | 51.6 | 51.9 | |
| Medium | 41.9 | 43.8 | 43.6 | |
| High | 4.0 | 4.6 | 4.5 | |
| Occupation (%) | <0.001 | |||
| Employed | 24.8 | 37.5 | 36.0 | |
| Retired/unemployed | 75.2 | 62.5 | 64.0 | |
| Smoking (%) | <0.001 | |||
| Never | 74.6 | 71.1 | 71.5 | |
| Previous | 8.2 | 7.3 | 7.4 | |
| Current | 17.2 | 21.6 | 21.1 | |
| Drinking (%) | <0.001 | |||
| Never | 70.6 | 65.8 | 66.4 | |
| Previous | 4.3 | 3.2 | 3.4 | |
| Current | 25.1 | 31.0 | 30.2 | |
| Sleep duration (h, %) | <0.001 | |||
| ≤6 | 37.6 | 34.1 | 34.5 | |
| 7–8 | 42.6 | 47.0 | 46.4 | |
| ≥9 | 19.8 | 18.9 | 19.1 | |
| BMI (kg/m2, %) | <0.001 | |||
| Low and normal | 38.4 | 47.6 | 46.4 | |
| Overweight | 42.9 | 38.4 | 38.9 | |
| Obesity | 18.7 | 14.0 | 14.7 | |
| Other chronic disease history† (%) |
45.0 | 67.2 | 41.8 | <0.001 |
| Low-level PA (%) | 33.3 | 39.8 | 32.3 | <0.001 |
Upon adjusting for potential confounders, we found no heightened likelihood of diminished PA in patients with DM compared to their non-DM counterparts [odds ratio (OR): 1.04, 95% confidence interval (CI): 0.95–1.13] (Table 2). Notably, individuals with a DM history surpassing a decade exhibited the highest propensity towards low-level PA, corresponding to an OR (95% CI) at 1.12 (1.00, 1.26). We also observed a significant linear trend linking the course of DM with reduced PA levels (Ptrend<0.001, Table 2). However, the contrast in the likelihood of diminished PA between DM patients exhibiting poor versus good glucose control did not reach statistical significance.
Table 2. Association of DM, DM course, and blood glucose control with low-level PA.
| DM conditions | Low-Level PA* |
| Abbreviation: DM=diabetes mellitus; PA=physical activity. *Adjusted for age, gender, region, education level, occupation, smoking, drinking, sleep duration, BMI, and other chronic medical history. | |
| DM diagnosis | |
| No | 1.00 |
| Yes | 1.04 (0.95, 1.13) |
| The course of DM | |
| Non-DM | 1.00 |
| ≤5 years | 0.97 (0.84, 1.13) |
| 5–10 years | 0.94 (0.80, 1.12) |
| >10 years | 1.12 (1.00, 1.26) |
| P trend | <0.001 |
| Blood glucose control | |
| Non-DM | 1.00 |
| Good | 1.03 (0.93, 1.14) |
| Poor | 1.04 (0.92, 1.19) |
The interaction between all population characteristics and DM was found to be statistically significant (P<0.05), with the exception of smoking (P=0.052). When conducting stratified analyses, instances of DM-associated low-level PA were found to be more prevalent among rural, medium-educated, employed individuals and those with sleep durations of ≥9 h (Table 3).
Table 3. Association of DM with low-level PA at different levels of population characteristics.
| Subgroups | Low-Level PA* | ||
| Non-DM | DM |
Interaction
P-value |
|
| Abbreviation: PA=physical activity; DM=diabetes mellitus. * All adjusted for variables other than the stratification variable in Table 3; † Low, medium, and high level of education in CKB refers to primary school and below, secondary school and college and above; § Other chronic medical history includes cardiovascular disease, chronic obstructive pulmonary disease, and hypertension. | |||
| Age (years) | <0.001 | ||
| <60 | 1.00 | 1.26 (1.00–1.57) | |
| ≥61 | 1.00 | 1.02 (0.93–1.12) | |
| Sex | <0.001 | ||
| Male | 1.00 | 1.00 (0.86–1.17) | |
| Female | 1.00 | 1.06 (0.96–1.18) | |
| Region | <0.001 | ||
| Urban | 1.00 | 0.93 (0.82–1.05) | |
| Rural | 1.00 | 1.20 (1.06–1.36) | |
| Education level† | <0.001 | ||
| Low | 1.00 | 1.00 (0.89–1.12) | |
| Medium | 1.00 | 1.15 (1.00–1.31) | |
| High | 1.00 | 0.74 (0.47–1.17) | |
| Occupation | <0.001 | ||
| Employed | 1.00 | 1.49 (1.20–1.86) | |
| Retired/unemployed | 1.00 | 0.99 (0.91–1.01) | |
| Smoking | 0.052 | ||
| Never | 1.00 | 1.02 (0.92–1.12) | |
| Previous | 1.00 | 1.26 (0.94–1.69) | |
| Current | 1.00 | 1.07 (0.85–1.33) | |
| Drinking | <0.001 | ||
| Never | 1.00 | 1.03 (0.93–1.14) | |
| Previous | 1.00 | 0.83 (0.55–1.26) | |
| Current | 1.00 | 1.09 (0.92–1.30) | |
| Sleep duration (h) | 0.011 | ||
| ≤6 | 1.00 | 0.97 (0.84–1.11) | |
| 7–8 | 1.00 | 1.01 (0.89–1.16) | |
| ≥9 | 1.00 | 1.22 (1.01–1.47) | |
| Other chronic disease history§ | <0.001 | ||
| Yes | 1.00 | 1.03 (0.93–1.14) | |
| No | 1.00 | 1.09 (0.94–1.26) | |
The sensitivity analysis, excluding DM patients diagnosed within 1 year, yielded results that were consistent with the initial findings (Supplementary Tables S1–S2, available in https://weekly.chinacdc.cn/).
DISCUSSION
In the current investigation, we analyzed the third resurvey data from CKB and found that, while no significant disparity existed in the prevalence of low-level PA between Chinese individuals with and without DM, the occurrence of low-level PA was higher in those managing DM. Previous studies have primarily reported low adherence rates to the guideline-recommended level of PA in Chinese DM patients, absent any comparison with their non-DM cohorts (4-5). By contrasting the PA levels in subjects with and without DM, we were able to somewhat control the background PA levels.
Furthermore, our findings indicated that patients with a protracted duration of DM were more likely to participate in low-intensity PA. This trend suggests that as DM progresses, patients often face complications, including coronary heart disease, chronic kidney disease, and eye disease, significantly limiting their potential for PA (6). Our findings indicated a higher likelihood of decreased PA associated with DM amongst rural-dwelling, moderately educated individuals who routinely sleep for nine or more hours. Relative to urban patients, those residing in rural areas often receive inadequate health education and exhibit lower health management awareness. Consequently, their levels of PA are more susceptible to the influence of DM. Individuals with lower educational levels typically engage in occupations involving substantial PA, making their PA less vulnerable to the impacts of DM. Conversely, highly educated persons have a higher awareness of the critical role of PA and often engage in greater levels of leisure-time PA (7), thereby rendering moderately educated persons more susceptible to lower PA associated with DM compared to other education levels. Prior research has demonstrated an increased perception of exertion during physical activity in individuals with inadequate sleep, which influences upper and lower limb performance (8). Correspondingly, our study found that those sleeping for nine or more hours also exhibited lower PA levels. This suggests that both insufficient and excessive sleep could potentially be detrimental to PA.
Our research was based upon an extensive study conducted in China. The sizeable participant group, the wealth of information regarding exposure and disease specifics, and the impeccable data quality enhanced our findings. However, our study is not without limitations.
The study was subject to some limitations. The first limitation is inherent to a cross-sectional analysis — it prevents us from determining the causal correlation between DM and PA. To validate the soundness of our results, we performed a sensitivity test, excluding DM-affected participants identified within the past year. The consistency of our results gives confidence in their robustness. The second limitation refers to the exclusion of participants due to missing variables, which potentially introduced selection bias to our study. Lastly, we relied on patient self-reporting to determine the presence of DM. This approach may inadvertently exclude individuals with undiagnosed DM. Compared to those aware of their DM status, this group may pay less attention to health management and, as a result, have lower PA levels. By utilizing self-reported data alone, the likelihood of overestimating PA amongst DM patients increases, creating the potential for false-negative conclusions.
Our results underscore the necessity of crafting actionable strategies and interventions designed to inspire a higher level of PA engagement among Chinese DM patients. It is paramount that such guidelines and interventive actions are grounded in an understanding of Chinese cultural and societal characteristics, thereby facilitating a more targeted enhancement of PA levels among this patient population.
Conflicts of interest
No conflicts of interest.
SUPPLEMENTARY MATERIAL
Table S1. Association of DM, DM course, and blood glucose control with low-level PA.
| DM conditions | Low-level PA* |
| Abbreviation: DM=diabetes mellitus; PA=physical activity; BMI=body mass index. *Adjusted for age, gender, region, education level, occupation, smoking, drinking, sleep duration, BMI, and other chronic medical history. | |
| Non-DM | 1.00 |
| DM | 1.04 (0.95, 1.13) |
| The course of DM | |
| Non-DM | 1.00 |
| ≤5 years | 0.97 (0.82, 1.14) |
| 5–10 years | 0.94 (0.79, 1.12) |
| >10 years | 1.12 (1.00, 1.26) |
| P trend | <0.001 |
| Blood glucose control | |
| Non-DM | 1.00 |
| Good | 1.03 (0.92, 1.20) |
| Poor | 1.05 (0.92, 1.20) |
Table S2. Association of DM with low-level PA at different levels of population characteristics.
| Subgroups | Low-level PA* | ||
| Non-DM | DM | Interaction P-value | |
| Abbreviation: PA=physical activity; DM=diabetes mellitus; CKB=China Kadoorie Biobank. * All adjusted for variables other than the stratification variable in Table 3; † Low, medium, and high level of education in CKB refers to primary school and below, secondary school and college and above; § Other chronic medical history includes cardiovascular disease, chronic obstructive pulmonary disease, and hypertension. | |||
| Age(years) | <0.001 | ||
| <60 | 1.00 | 1.25 (0.99–1.59) | |
| ≥61 | 1.00 | 1.03 (0.93–1.13) | |
| Sex | <0.001 | ||
| Male | 1.00 | 1.00 (0.85–1.17) | |
| Female | 1.00 | 1.07 (0.96–1.19) | |
| Region | 0.001 | ||
| Urban | 1.00 | 0.94 (0.83–1.06) | |
| Rural | 1.00 | 1.20 (1.06–1.36) | |
| Education level† | <0.001 | ||
| Low | 1.00 | 1.00 (0.90–1.13) | |
| Medium | 1.00 | 1.14 (0.99–1.31) | |
| High | 1.00 | 0.70 (0.44–1.11) | |
| Occupation | <0.001 | ||
| Employed | 1.00 | 1.43 (1.14–1.79) | |
| Retired/unemployed | 1.00 | 1.00 (0.91–1.10) | |
| Smoking | 0.094 | ||
| Never | 1.00 | 1.02 (0.93–1.13) | |
| Previous | 1.00 | 1.26 (0.93–1.70) | |
| Current | 1.00 | 1.04 (0.83–1.31) | |
| Drinking | <0.001 | ||
| Never | 1.00 | 1.03 (0.93–1.14) | |
| Previous | 1.00 | 0.88 (0.57–1.36) | |
| Current | 1.00 | 1.10 (0.92–1.31) | |
| Sleep duration (h) | 0.008 | ||
| ≤6 | 1.00 | 0.96 (0.83–1.11) | |
| 7–8 | 1.00 | 1.01 (0.88–1.16) | |
| ≥9 | 1.00 | 1.24 (1.02–1.50) | |
| Other chronic diseases history§ | <0.001 | ||
| Yes | 1.00 | 1.03 (0.93–1.15) | |
| No | 1.00 | 1.08 (0.92–1.26) | |
Funding Statement
Supported by National Natural Science Foundation of China (82192904, 82192901, 82192900); “Precision Medicine Research” Key Project, National Key Research and Development Program of China (2016YFC0900500); Kadoorie Charitable Foundation in Hong Kong of China
Contributor Information
Dianjianyi Sun, Email: dsun1@bjmu.edu.cn.
Canqing Yu, Email: yucanqing@pku.edu.cn.
References
- 1.Chinese Diabetes Society Guideline for the prevention and treatment of type 2 diabetes mellitus in China(2020 edition)(Part 1) Chin J Pract Internal Med. 2021;41(8):668–95. doi: 10.19538/j.nk2021080106. [DOI] [Google Scholar]
- 2.Francesconi C, Lackinger C, Weitgasser R, Haber P, Niebauer J. Physical activity and exercise training in the prevention and therapy of type 2 diabetes mellitus. Wien Klin Wochenschr 2016;128 Suppl 2:S141-5. http://dx.doi.org/10.1007/s00508-015-0923-3.
- 3.Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, et al American association of clinical endocrinology consensus statement: comprehensive type 2 diabetes management algorithm - 2023 update. Endocr Pract. 2023;29(5):305–40. doi: 10.1016/j.eprac.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 4.He XW, Pan J, Pan MX, Wang JW, Dong JF, Yuan HD, et al Dietary and physical activity of adult patients with type 2 diabetes in Zhejiang province of eastern China: data from a cross-sectional study. J Diabetes Investig. 2016;7(4):529–38. doi: 10.1111/jdi.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo MF, Yu QM, Ma X, Chen YC, Wang Y Analysis of physical activity status of type 2 diabetes patients in community. Chin J Prev Control Chronic Dis. 2020;28(11):866–9. doi: 10.16386/j.cjpccd.issn.1004-6194.2020.11.016. [DOI] [Google Scholar]
- 6.Wang FF, Lu M, Wang Y Analysis of influencing factors on blood glucose control and level of physical activity among patients with type 2 diabetes. Chin J Public Health Manage. 2020;36(4):541–3. doi: 10.19568/j.cnki.23-1318.2020.04.027. [DOI] [Google Scholar]
- 7.Chen Y, Fan YG, Leng RX, Mao YM, Liao T, Ye D. Influencing factors for substandard physiques among residents aged 20-69 years in Anhui Province. J Prev Med 2021;33(7):649-55. 10.19485/j.cnki.issn2096-5087.2021.07.001. (In Chinese).
- 8.Ng SW, Popkin BM Time use and physical activity: a shift away from movement across the globe. Obes Rev. 2012;13(8):659–80. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


