Abstract
Of the three immunoglobulin G (IgG) isotypes described to occur in camelids, IgG2 and IgG3 are distinct in that they do not incorporate light chains. These heavy-chain antibodies (HCAbs) constitute approximately 50% of the IgG in llama serum and as much as 75% of the IgG in camel serum. We have produced isotype-specific mouse monoclonal antibodies (MAbs) in order to investigate the roles of HCAbs in camelid immunity. Seventeen stable hybridomas were cloned, and three MAbs that were specific for epitopes on the γ chains of llama IgG1, IgG2, or IgG3 were characterized in detail. Affinity chromatography revealed that each MAb bound its isotype in solution in llama serum. The antibodies bound to the corresponding alpaca IgGs, to guanaco IgG1 and IgG2, and to camel IgG1. Interestingly, anti-IgG2 MAbs bound three heavy-chain species in llama serum, confirming the presence of three IgG2 subisotypes. Two IgG2 subisotypes were detected in alpaca and guanaco sera. The MAbs detected llama serum IgGs when they were bound to antigen in enzyme-linked immunosorbent assays and were used to discern among isotypes induced during infection with a parasitic nematode. Diseased animals, infected with Parelaphostrongylus tenuis, did not produce antigen-specific HCAbs; rather, they produced the conventional isotype, IgG1, exclusively. Our data document the utility of these MAbs in functional and physiologic investigations of the immune systems of New World camelids.
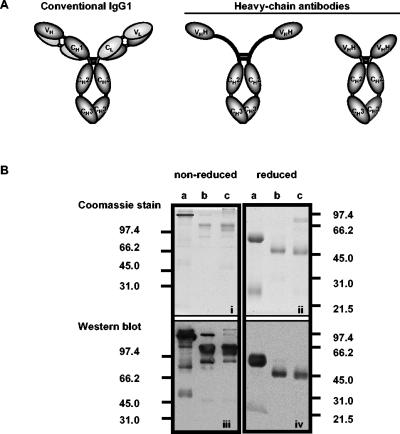
The discovery of camelid immunoglobulin Gs (IgGs) that violated established rules governing the structures of antibodies was described in 1993 (12). Unlike their conventional IgG1 counterpart, camelid IgG2 and IgG3 do not associate with light chains and are called heavy-chain antibodies (HCAbs) (Fig. 1A). Genomic and/or cDNA sequences have been obtained for six γ constant (Cγ) genes: Cγ1a and Cγ1b for IgG1; Cγ2a, Cγ2b, and Cγ2c for the IgG2 HCAb; and Cγ3 for the IgG3 HCAb (20, 22, 35, 39). The Cγ2b gene was identified in genomic clones prepared from llamas (39) and has not been described to occur in other camelids. Variation in masses among the IgG2 subisotypes is predicted from variability in the lengths of exons encoding the hinge regions. Extended hinge regions, deletion of the CH1 domain, and amino acid substitutions in the V domain that make it more hydrophilic promote the flexibility and solubility of HCAbs (5, 19, 21, 31, 35). These properties may expand the antigen-binding repertoire of the immune system by enabling HCAbs to bind otherwise inaccessible epitopes. Given their unusual physical properties, it seems likely that HCAbs play a novel role in immune defense.
FIG. 1.
(A) Structure of camelid IgGs (data from reference 12). (B, top) Coomassie blue-stained SDS-PAGE gel of purified llama IgG isotypes resolved under nonreducing and reducing conditions. (a and b) IgG1 and IgG3 (eluted from protein G); (c) IgG2 (eluted from protein A). (Bottom) Western blot of gels identical to Coomassie blue-stained gels developed with polyclonal anti-llama IgG (H+L). Molecular masses (in kilodaltons) of protein standards run in parallel are indicated.
Investigations of camelid antibodies have focused largely on manipulating or mimicking the architecture of the variable domains of the HCAbs (VHH) for application to medical therapy and biotechnology (4, 18, 24, 30). Unlike conventional antibodies, HCAbs use a single VHH to bind an epitope. The absence of the light-chain variable domain is compensated for by extended complementarity-determining regions (CDR) that provide an adequate antigen-binding surface and demonstrate affinities comparable to those of conventional antibodies (3, 5, 28). An exceptional property of HCAbs that has fueled research activity is their ability to inhibit enzymes by inserting an extended CDR into the active site (17, 32). This property has not been observed in conventional antibodies. The aggregate physical and binding characteristics of HCAbs, along with the ease of production of recombinant, active VHH in the Saccharomyces cerevisiae system (10, 34), have prompted researchers to explore their use as immunological tools.
There are three published reports of the involvement of HCAbs in camelid immunity, specifically, in the response to trypanosome infection and to immunization with bacterial proteins (12, 30, 33). The production of IgG isotypes in response to infection with parasitic nematodes has not yet been investigated. A limiting factor for investigations into camelid immunity has been a shortage of well-characterized, isotype-specific reagents.
Currently, polyclonal antibodies that react broadly with camelid IgGs are available from commercial sources. Monoclonal antibodies (MAbs) specific for dromedary (Camelus dromedarius) IgG1 and IgM have been produced and characterized (1). At this time, analysis of lamoid HCAbs can be conducted only with chromatographically separated immunoglobulin fractions. The application of isotype-specific MAbs in experimental and diagnostic assays will facilitate direct assessment of the composition and specificity of the lamoid IgG response to infection. Results of such assays will engender a better understanding of the roles of HCAbs in immune defense.
Here we describe the production and characterization of MAbs specific for conventional and heavy-chain isotypes of llama IgG. Seventeen stable hybridomas were cloned, and three MAbs that were specific for epitopes on the γ chains of llama IgG1, IgG2, or IgG3 fractions purified on protein A and protein G were characterized in detail. The antibodies bound to the corresponding alpaca IgGs, to guanaco IgG1 and IgG2, and to Bactrian IgG1. Anti-IgG2 MAbs bound three heavy-chain species in llama serum, confirming the presence of three IgG2 subisotypes. Two IgG2 subisotypes were detected in alpaca and guanaco sera. The MAbs detected llama serum IgGs when they were bound to antigen in enzyme-linked immunosorbent assays (ELISA) and were used to discern among isotypes induced during infection with a parasitic nematode.
MATERIALS AND METHODS
Animals and sera.
BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) and housed in the James A. Baker Institute vivarium according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. Blood samples were obtained by jugular venipuncture from selected camelids (one healthy and four diseased llamas and one healthy alpaca, guanaco, and Bactrian camel) that were brought into the Cornell University Hospital for Animals or examined by the ambulatory service. Serum was separated by centrifugation and stored at −20°C. Infection with Parelaphostrongylus tenuis was confirmed in diseased llamas by observation of nematodes within the central nervous system during necropsy. Sera from llamas in Pullman, Washington, an area in which P. tenuis is not endemic, were kindly provided by William Foreyt of Washington State University.
Antibodies.
Polyclonal goat anti-llama IgG (H+L) conjugated to horseradish peroxidase (HRP; Bethyl Laboratories Inc., Montgomery, Tex.) was used in ELISA and Western blotting. Monoclonal mouse antibodies were detected with HRP-conjugated goat anti-mouse antibodies (ICN/Cappel, Aurora, Ohio). Three MAbs, 27E10 (anti-IgG1), 19D8 (anti-IgG2), and 8E1 (anti-IgG3), were selected for use in the serologic assays. An ELISA to determine the isotype of the MAbs employed rat MAbs to mouse isotypes and HRP-conjugated mouse anti-rat κ-chain antibodies (BD PharMingen, San Diego, Calif.).
Purification of llama IgGs.
Llama IgG isotypes were purified using affinity chromatography as described elsewhere (12, 33). Briefly, serum was first loaded onto a protein G-Sepharose 4B column (Sigma Chemical Co., St. Louis, Mo.), and the unbound fraction was collected and loaded on a protein A-Sepharose 4B column (Sigma Chemical Co.). IgG3 was eluted from protein G with 0.15 M NaCl-0.58% acetic acid (pH 3.5), and IgG1 was eluted with 0.1 M glycine-HCl (pH 2.7). IgG2 was eluted from protein A with 0.15 M NaCl-0.58% acetic acid, pH 4.5. Fractions were neutralized immediately with 0.1 M Tris-HCl, pH 9.0.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blots.
Serum and chromatographically separated IgGs were resolved in discontinuous polyacrylamide gels (16). Minigels were used, except for the determination of apparent molecular masses; instead, proteins were resolved in standard 15-cm-long gels. Samples and molecular mass markers (Bio-Rad Laboratories, Hercules, Calif.) were boiled for 7 min in sample preparation buffer, with or without 2-mercaptoethanol, before being loaded in the gels. Gels were stained with Coomassie blue or were blotted onto nitrocellulose membranes.
Western blotting procedures were performed at room temperature. Incubation periods were 1 h unless specified otherwise. Membranes were blocked with 5% skim milk in Tris-buffered saline and washed with Tris-buffered saline containing 0.05% Tween 20 and 0.1% bovine serum albumin (Sigma). Primary antibodies were diluted in blocking solution, and conjugates were diluted in blocking solution containing 10% normal goat serum. Antibody binding was detected with a chemiluminescent substrate (ECL reagent; Amersham Pharmacia Biotech, Piscataway, N.J.) and autoradiography. Films were scanned, and images were prepared using Adobe Photoshop and Microsoft Powerpoint.
Production of monoclonal antibodies.
Mice were injected intraperitoneally with 100 to 300 μg of purified llama IgGs mixed in complete Freund's adjuvant (Sigma). One mouse per isotype was injected intravenously with purified IgG in Dulbecco's phosphate-buffered saline (DPBS) 30 days postimmunization and killed 3 days later. Spleen cells were fused with mouse myeloma SP2/0 cells (American Type Culture Collection, Manassas, Va.) according to the method of Kohler and Milstein (15).
Culture supernates were screened by ELISA for selective binding to one of the three llama IgG isotypes. Plates were coated at 4°C, and other procedures were conducted at room temperature. Well volume was 25 μl. Wells of polyvinyl 96-well microtiter plates (Costar, Cambridge, Mass.) were coated overnight with llama IgG1, IgG2, or IgG3 in 10% DPBS (1 μg/ml). Wells were blocked for 1 h with DPBS containing 0.05% Tween 20 and 5% skim milk and then incubated for 1 h with supernatants diluted 1:10 in blocking solution. Wells were washed five times with 0.05% Tween 20-DPBS and incubated for 1 h with HRP-conjugated goat anti-mouse antibody (0.6 μg/ml) in blocking solution containing 10% normal goat serum. Binding was detected with 3,3′,5,5′-tetramethylbenzidine substrate (KPL, Gaithersburg, Md.) and stopped after 30 min with 1 M H3PO4. Optical density readings were taken at 450 nm with a microplate reader (Biokinetics Reader, EL340; Bio-Tek Instruments, Winooski, Vt.).
Hybridomas that secreted MAbs specific for one llama IgG isotype were selected and cloned by limiting dilution. Hybridomas producing MAbs that were selected for further characterization were expanded by using the VectraCell system (BioVectra, Oxford, Conn.). MAbs were purified from culture supernatants by affinity chromatography using protein G-Sepharose 4B (Sigma) on an fast protein liquid chromatography system (ÄKTAFPLC; Amersham Pharmacia Biotech Inc., Piscataway, N.J.) according to the manufacturer's instructions.
Isotyping the MAbs.
A modified version of the ELISA protocol used for screening the hybridomas was developed to determine the isotypes of the MAbs. Bound MAbs were detected with rat monoclonal antibodies to mouse isotypes diluted to 1 μg/ml in blocking solution and then detected with HRP-conjugated mouse anti-rat κ chains at 2 μg/ml in blocking solution with 10% normal mouse serum.
Pepsin digestion of llama IgG1.
Purified IgG1 was dialyzed against 0.2 M sodium acetate buffer, pH 4.5, and adjusted to 2 mg/ml. Crystallized pepsin (Sigma) was dissolved in the same buffer (1 mg/ml), and digestions were performed at a ratio of 1 μg of enzyme to 20 μg of antibody. After 1, 2, 4, 6, 12, 24, and 48 h in a 37°C water bath, digestion reactions were stopped by the addition of 40 μl of 2 M Tris base. Fractions were resolved by SDS-PAGE in 8% acrylamide minigels. Gels were either stained with Coomassie blue or subjected to Western blotting.
Affinity chromatography of camelid IgGs.
Affinity columns were prepared by coupling 10 mg of a MAb (27E10, 19D8, or 8E1) to 1 g of CNBr-activated Sepharose 4B (Sigma) in the presence of 0.1 M NaHCO3, pH 8.0. Unbound active sites were blocked with 0.1 M Tris, pH 8.0, for 2 h at 4°C. The coupled gels were washed alternately with 0.1 M acetate buffer, pH 4.0, and borate-buffered saline, pH 8.5. Columns were loaded with 1 ml of llama, camel, guanaco, or alpaca serum and washed with borate-buffered saline. Bound IgGs were eluted from the columns with 0.1 M glycine-HCl buffer, pH 2.7, into tubes containing 0.1 M Tris-HCl, pH 9.0, and evaluated by Western blot analysis.
Detection of llama IgGs specific for P. tenuis antigens.
The ELISA protocol detailed above was modified to detect llama IgGs specific for the recombinant P. tenuis aspartyl protease inhibitor (rPtAPI). Preparation of the rPtAPI has been described elsewhere (7). Plates were coated with 4 μg of rPtAPI per ml. Llama sera were diluted 1:100 and added to triplicate wells. Wells were incubated with the 27E10, 19D9, or 8E1 MAbs (5 μg/ml) or HRP-goat anti-llama antibodies (1 μg/ml) diluted in blocking solution. Bound MAbs were detected with 5 μg of HRP-conjugated goat anti-mouse antibodies per ml diluted in blocking solution containing 10% normal goat serum.
Statistical analysis.
ELISA data from groups of infected and uninfected llamas were evaluated by analysis of variance. Values were considered statistically significant when P was <0.05.
RESULTS
Purification and characterization of llama IgGs.
Affinity chromatography, using protein A and protein G, was carried out in order to purify the isotypes of llama IgGs. As reported previously (33), llama IgG1 and IgG3 bound to protein G and protein A while IgG2 bound only to protein A. Comparing Coomassie blue-stained gels (Fig. 1B, panels i and ii) with Western blots (Fig. 1B, panels iii and iv) confirmed that eluted proteins were llama IgGs. Multiple bands were observed in fractions resolved under nonreducing conditions. The majority of the proteins of greater mobility were bound by polyclonal goat anti-IgG (Fig. 1B, panel iii), suggesting the occurrence of mild protein degradation during storage of samples. In each nonreduced preparation, a single band migrated between the 66.2- and 45-kDa molecular mass standards (Fig. 1B, panel iii), compatible with the masses of the corresponding monomeric heavy chains. The IgG3 fraction eluted from protein G was contaminated with IgG1 (Fig. 1B, panel iii, lane b). IgG2 coeluted with an 80-kDa protein from the protein A column (Fig. 1B, panel ii, lane c). This protein was not detected by the anti-llama IgG conjugate (Fig. 1B, panel iv, lane c), suggesting that it was not an immunoglobulin. The identity of the protein was not pursued.
Under reducing conditions, IgG1 was resolved into light chains (22.7 kDa) and γ1 heavy chains (49.4 kDa) (Fig. 1B, panel iv, lane a). The IgG3 preparation was reduced to one dominant heavy-chain species of 42.1 kDa (Fig. 1B, panel iv, lane b). The IgG2 preparation was reduced to one dominant heavy-chain species of 40.9 kDa (Fig. 1B, panel iv, lane c). Contaminants and degradation products were not evident in reducing gels. The three fractions were used to immunize BALB/c mice for isotype-specific MAb production. Because the preparations were known to be heterogeneous, the screening procedure for each fusion was designed to reduce the likelihood that cross-reactive antibodies would be selected.
Production of monoclonal antibodies.
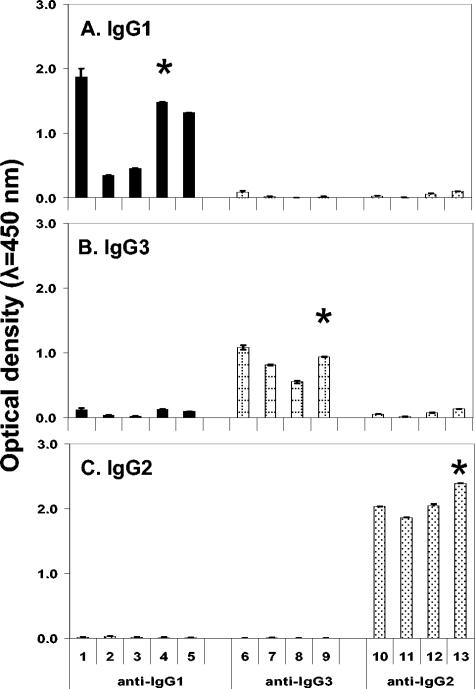
Supernatants from fusion plate wells were tested by ELISA against all three preparations of llama IgG. Thirty-four hybridomas were found to secrete antibodies reactive primarily with only one preparation. Seventeen stable cell lines were cloned to purity: five that secreted MAb that bound IgG1 and six each that bound IgG2 or IgG3. Four of the 17 MAbs were mouse IgM and were not pursued. The remaining 13 MAbs were mouse IgG1, IgG2a, or IgG2b. The levels of binding of these 13 MAbs with protein A- or G-purified llama IgGs are summarized in Fig. 2. Antibodies 27E10, 19D8, and 8E1 were selected for further characterization based on their overall high reactivities with llama IgG1, IgG2, and IgG3, respectively.
FIG. 2.
Reactivities of mouse MAbs with affinity-purified llama IgGs in ELISA. (A) IgG1; (B) IgG3; (C) IgG2. Twofold dilutions of supernatants were tested in triplicate wells against purified IgGs. Bound MAbs were detected with HRP-goat anti-mouse IgG. Columns represent mean optical densities for three replicate wells ± standard deviations of the means (I bars). Asterisks denote MAbs selected for further evaluation.
Specificities of MAbs for immunoglobulins in Western blotting.
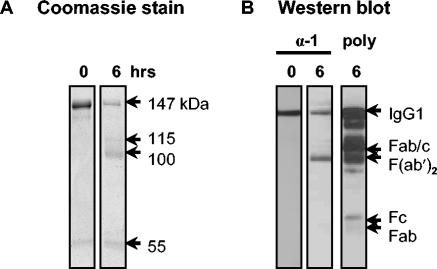
In order to confirm that the IgG1 epitope bound by 27E10 was in the heavy rather than the light chain, protein G-purified IgG1 was cleaved with pepsin, and the fragments were assayed by Western blotting. Digestion of IgG1 for 6 h yielded an array of fragments as well as residual whole IgG1 (Fig. 3A). 27E10 bound whole IgG1 as well as F(ab′)2 fragments but did not bind Fab- or Fc-like fragments (Fig. 3B). Thus, binding to IgG1 by 27E10 appeared to be dependent upon the presence of the hinge region. Further, the antibody did not bind Fab/c- like fragments (Fig. 3B) or reduced IgG1 (data not shown), compatible with a requirement for intact disulfide bridges and stable tertiary and quaternary structures in the hinge region. Our data support the conclusion that 27E10 binds within the CH1-hinge-CH2 region of IgG1.
FIG. 3.
Binding of anti-llama IgG1 MAb (27E10) to fragments of llama IgG1. (A) Coomassie blue-stained gel of llama IgG1 digested for 0 or 6 h with pepsin in acetate buffer, pH 4.5. Estimated molecular masses (in kilodaltons) are indicated. (B) Western blot showing the reactivity of 27E10 (α-1) with llama IgG1, which was digested for 0 or 6 h with pepsin and developed with HRP-goat anti-mouse antibodies. poly, HRP-goat anti-llama IgG (H+L) against pepsin-cleaved IgG1.
19D8 and 8E1 bound to IgG2 and IgG3, respectively, in Western blots when they were used at high concentrations (20 to 80 μg/ml) (data not shown). These concentrations were 20- to 80-fold those of normal working concentrations for peptide-specific antibodies, and some cross-reactivity with other IgG isotypes was evident. We concluded that the epitopes recognized by these antibodies are not well preserved under the denaturing conditions of SDS-PAGE and Western blotting.
Binding of MAbs to camelid IgGs in solution.
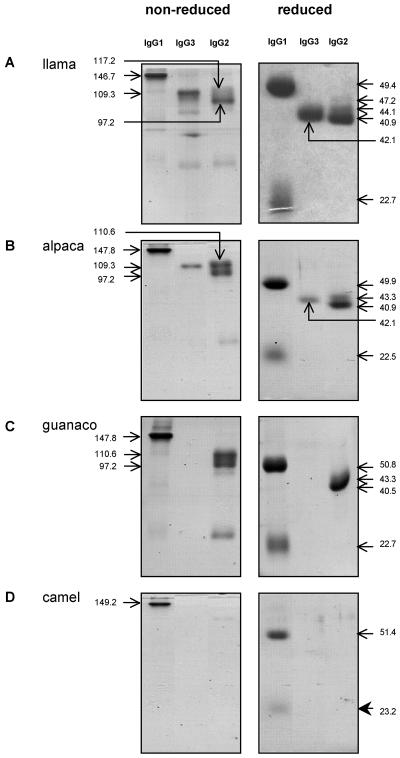
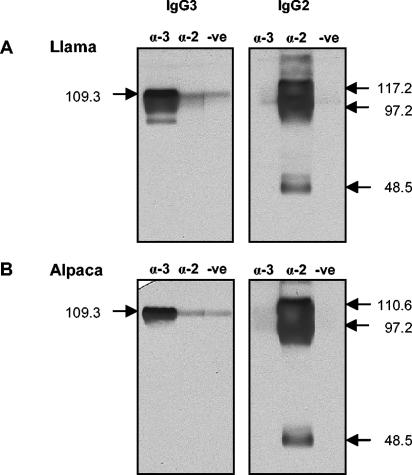
To ensure that the MAbs had been induced against epitopes on native IgGs and not to epitopes exposed during the purification process, llama serum was passed over affinity columns prepared by coupling CNBr-activated Sepharose with 27E10, 19D8, or 8E1. Each MAb bound the predicted llama IgG isotype (Fig. 4A). Unexpectedly, three protein bands were observed in the fraction eluted from the anti-IgG2 column: the 40.9-kDa species observed in protein A-purified IgG2 plus species of 44.1 and 47.2 kDa (Fig. 4A, reduced panels). The 8E1 MAb (anti-IgG3) did not bind any of the IgG2 heavy chains in Western blots, indicating that they were not IgG3 antibodies that contaminated the IgG2 preparation (Fig. 5A). These HCAbs are herein referred to as IgG2a, IgG2b and IgG2c according to their decreasing molecular weights in order to be consistent with the published reports.
FIG. 4.
Specificities of MAbs for native camelid IgGs. The anti-llama IgG1, IgG3, and IgG2 MAbs, 27E10, 8E1, and 19D8, respectively, were coupled with CN-Br activated Sepharose 4B and used to purify IgGs from camelid sera. (A) Llama serum; (B) alpaca serum; (C) guanaco serum; (D) camel serum. Eluted IgG fractions were resolved by SDS-PAGE under nonreducing and reducing conditions and stained with Coomassie blue. Estimated molecular masses (in kilodaltons) are indicated.
FIG. 5.
Reactivities of HCAb-specific MAbs with eluates from the anti-IgG3 and anti-IgG2 affinity columns in Western blots. Affinity-purified HCAbs from llama (A) and alpaca (B) sera were resolved by SDS-PAGE, blotted onto nitrocellulose, and developed with anti-IgG2 and anti-IgG3 MAbs (20 μg/ml) or normal mouse serum. α-3, anti-IgG3 (8E1) MAbs; α-2, anti-IgG2 (19D8) MAbs; -ve, negative (normal mouse serum). Estimated molecular masses (in kilodaltons) are indicated.
Binding of the anti-llama IgG MAbs to immunoglobulins in the sera of other camelid species was documented by similar experiments with MAb affinity columns. The three MAbs reacted with the homologous isotypes in alpaca serum (Fig. 4B). Similarly, the 27E10 (anti-IgG1) and 19D8 (anti-IgG2) MAbs bound to guanaco IgG1 and IgG2; however, 8E1 (anti-IgG3) did not bind a guanaco IgG3 (Fig. 4C). Unlike with llama sera, passage of alpaca and guanaco sera over the anti-IgG2 column yielded only two IgG2 species: one of 43.3 kDa and another of 40.5 kDa (guanaco) or 40.9 kDa (alpaca). Development of a Western blot of the alpaca IgG2 fraction with 8E1 (anti-IgG3) indicated that neither protein was IgG3 (Fig. 5B). The anti-IgG1 MAb (27E10) bound to camel IgG1, but neither of the HCAb-specific MAbs bound to the homologous isotypes in camel sera (Fig. 4D). In summary, the MAbs bind epitopes on native llama and alpaca IgG1, IgG2, and IgG3; guanaco IgG1 and IgG2; and camel IgG1.
Apparent molecular masses of the camelid γ and light chains isolated as described above were calculated based on their migrations in SDS-PAGE under reducing conditions (Table 1).
TABLE 1.
Molecular masses of camelid IgGs estimated by migration in SDS-PAGEa
| Animal | Molecular mass (kDa)
|
|||||
|---|---|---|---|---|---|---|
| IgG1c
|
IgG2
|
IgG3 | ||||
| Hc | Lc | a | b | c | ||
| Llama | 49.4 | 22.7 | 47.2 | 44.1 | 40.9 | 42.1 |
| Alpaca | 49.9 | 22.5 | 43.3 | 40.9 | 42.1 | |
| Guanaco | 50.8 | 22.7 | 43.3 | 40.5 | 42.1b | |
| Camel | 51.4 | 23.2 | 42.9b | 39.0b | ||
Performed with 10% acrylamide and 15-cm-wide gels.
Calculated from Western blots of sera resolved under similar conditions.
HC, heavy chain; LC, light chain.
Evaluation of llama IgGs induced during P. tenuis infection.
Sera from llamas resident in areas in which P. tenuis is endemic or not endemic were tested by ELISA for the presence of parasite-specific antibodies of each IgG isotype. Polyclonal goat anti-llama IgG conjugate was tested in parallel. All sera tested yielded high optical density readings with the polyclonal reagent. Sera from infected, diseased animals could not be distinguished from that of a healthy llama from an area of endemicity or from the sera of animals from areas where the parasite is not endemic (Fig. 6). The apparent lack of specificity may be due to binding of the conjugate to IgM as a result of its specificity for light chains. Although the recombinant protein antigen bears a His tag and was affinity purified by Ni chelation chromatography (7), it was produced in Escherichia coli and may carry trace amounts of bacterial protein. The polyclonal conjugate may bind light chains of low affinity, E. coli-specific, or other nonspecific, IgM that is likely present in the sera of all llamas. Although there are other possible explanations for the lack of discrimination by the polyclonal reagent (discussed below), our results diminish enthusiasm for its use in ELISA.
FIG. 6.
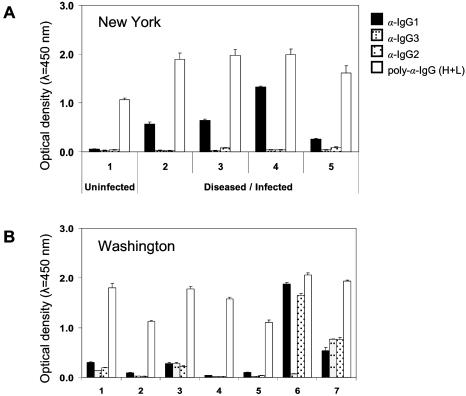
Detection of rPtAPI-specific IgG isotypes in llama sera by ELISA. (A) Llamas from a P. tenuis-endemic area (New York State). Sera from diseased llamas, confirmed to be P. tenuis infected, contained predominantly IgG1 specific for rPtAPI. No antigen-specific IgG isotypes were detected in the uninfected llama serum. Polyclonal goat anti-llama IgG (H+L) did not distinguish infected from uninfected llamas. (B) Llamas from P. tenuis-free area (Washington State). Cross-reactive IgG1 as well as IgG3 and/or IgG2 were detected in sera from uninfected llamas. The polyclonal goat antibodies yielded consistently high readings for all seven sera. Columns represent mean optical densities of sera diluted 1:100 (tested in triplicate) ± standard deviations (T bars).
In order to determine the isotype composition of the antibody response to a parasitic nematode, the ELISA was modified to incorporate the isotype-specific MAbs. Serum from the uninfected llama residing in an area in which P. tenuis is endemic lacked IgG specific for rPtAPI (Fig. 6A). In contrast, all sera collected from diseased or infected llamas contained high concentrations of IgG1 specific for the rPtAPI. Trace concentrations of IgG2 were detected in two of these animals. Because of the high prevalence of P. tenuis infection in areas of endemicity, it is impossible to assert with confidence that any llama has not been exposed to the parasite. For this reason, we tested sera from healthy llamas obtained from a region free of P. tenuis (Washington State). These sera yielded varied results. Four of seven llama serum samples had antigen-specific antibodies; however, instead of IgG1 being quantitatively dominant, IgG2 and IgG3 (or the isotypes individually) were detected at similar concentrations in the four samples. Statistical analysis of the ELISA data did not detect significant differences between P. tenuis-infected and uninfected llama sera. As described above, the antibodies may be specific for bacteria and binding contaminating E. coli proteins in the antigen preparation. Alternatively, the antibodies may be specific for rPtAPI but induced by similar antigens produced by other parasitic nematodes that are prevalent in Washington. This conclusion is supported by the high amino acid sequence similarities of APIs across nematode genera. APIs are known to be highly immunogenic (7). Overall, the results show that ELISA employing isotype-specific reagents will improve and enrich the information afforded by such assays, yet the selection of the target antigen remains critical for interpretation of the data.
DISCUSSION
We prepared MAbs specific for IgG1, IgG2, and IgG3 of llamas. Each MAb bound its soluble, native IgG and, when used to prepare affinity columns, afforded an improvement over previously described protein A and G affinity methods for separation and purification of the isotypes (12). In addition, the MAbs were effective in ELISA for detection of antigen-specific antibodies. Finally, an IgG2-specific MAb bound three discrete llama heavy chains, confirming the existence of three IgG2 isotypes in the llama, as had been inferred from DNA analyses (35, 39).
Camelids emerged in North America during the Eocene epoch some 45 to 40 million years ago; however, the Camelini and Lamini divergence did not occur until 11 to 9 million years ago (36, 38). Subsequently, the cameloids migrated across the Bering Strait, while the lamoids migrated towards South America. To date, the one-humped dromedary (Camelus dromedarius) is native to the Arabian Desert while the two-humped Bactrian (Camelus bactrianus) is found in Asia. The lamoids include guanacos (Lama guanicoe), llamas (Lama glama), alpacas (Lama pacos), and vicuñas (Vicugna vicugna) and are indigenous to South America. Domestication of llamas and alpacas is estimated to have occurred 5,500 to 6,000 years ago (37). The camelids that remained in North America became extinct, and llamas from South America were reintroduced in the late 1880s.
Microsatellite and mitochondrial DNA analyses have revealed genetic similarity between vicuña and alpaca and between guanaco and llama, suggesting that the alpaca was domesticated from the vicuña and the llama from the guanaco. It has been proposed that the alpaca should be reassigned to the genus Vicugna (Vicugna pacos) (37). Most modern day alpacas and llamas carry both guanaco- and vicuña-like DNA, reflecting a high incidence of hybridization between the domestic species. It has been estimated that as many as 80% of modern day alpacas are hybrids (29, 37). Although a likely aim of hybridization was to improve wool fiber quality in larger animals, fiber quality in domestic species has declined. Efforts are under way to improve genetic purity and fiber quality in alpaca (37).
We evaluated the binding of the MAbs to IgGs of Old and New World camelids. Vicuñas are both wild and endangered; we did not have access to sera from that species. The anti-IgG1 MAb bound its isotype in all camelids tested, including C. bactrianus. Among the New World camelids, IgG3 was not detected by the appropriate MAb in guanaco. Only two IgG2 subisotypes were detected in alpaca and guanaco, compared with three in llama. Hybridization in the New World camelids confounds any attempt to interpret these results in evolutionary terms. Although we tested sera from several animals in this study, each alpaca and llama was defined as such by phenotype rather than genotype. Expanded testing on larger numbers of llama and alpaca, including animals of defined genotypes, will be important for validation of the specificities of MAbs against IgG2 and IgG3.
The importance of immunoglobulins to the health and well-being of camelids is documented by the high mortality among neonates that fail to receive adequate colostral antibody (11). The contribution of HCAbs in the passive transfer of immunity to neonates is unknown. Furthermore, little is known of the functional contributions of camelid IgGs to immune defense. Camelids have specific pathogens, suffer from common diseases of ruminants, and, as with foot and mouth disease, are resistant to some pathogens (2, 6, 8, 25, 27). Camelid HCAbs may have distinct roles in fighting infections. Their more discrete antigen-binding domains, extended CDRs, and increased tissue accessibility enable HCAbs to bind epitopes otherwise inaccessible to conventional antibodies. In addition, HCAbs have been shown to inhibit enzymes (17, 32). The significance of these properties in immune defense and the nature of the stimuli that induce the production of HCAbs are unknown.
P. tenuis is a cause of morbidity and mortality in llamas and alpacas (9, 26). Disease results from physical trauma and cellular infiltration into the parenchymal tissues of the central nervous system induced by migrating worms. We found that infections of varied durations stimulated the production of antigen-specific IgG1 in four animals. HCAbs were not prominent in these immune responses. All four animals were ill and eventually died or were euthanized, implying that the immune response induced was not protective. However, our conclusions are based on the results obtained with a single protein antigen, and it will be important to corroborate the data obtained for PtAPI with those obtained for other antigenic proteins and glycans.
Studies of other species have shown that the specificity and sensitivity of a serologic test may be increased when it is designed to detect a particular IgG isotype. For example, detection of IgG4 antibodies specific for recombinant antigens of Fasciola hepatica, Angiostrongylus cantonensis, or Loa loa increases the sensitivity and specificity of assays for these infections in humans (13, 14, 23). Similarly, by using isotype-specific reagents, we reduced high background readings obtained using pan-immunoglobulin antibodies and demonstrated that P. tenuis-infected llamas mounted an anti-PtAPI response that was overwhelmingly IgG1. The utility of this finding was complicated by our observation that four sera from llamas in an area where the parasite is not endemic had antigen-specific IgG1, IgG2, and, in some cases, IgG3. Although these animals were dewormed every 6 months, they were reared conventionally and were likely exposed to other parasitic nematodes (William Foryet, personal communication). Ostertagia ostertagi is a common parasitic nematode of llamas and produces an API that is 74% identical in amino acid sequence to PtAPI. Infection with this or some other nematode may have induced the antibodies detected. The difference in isotype representation in the sera of diseased llamas versus the sera of llamas in areas where the parasite is not endemic may be due to the site of parasite residence (e.g., central nervous system for P. tenuis versus gastrointestinal tract for O. ostertagi) or other influences. To address the issue of cross-reactivity, we are seeking other, potentially unique, P. tenuis antigens for application in serologic tests.
In summary, we have produced and characterized MAb reagents specific for heavy-chain and conventional IgGs of llamas. The MAbs are readily applied in serologic assays and affinity chromatography and should be useful in quantitative assessments of IgG isotypes in blood, colostrum, and other body fluids. Their application in identifying IgG isotypes induced during protective and nonprotective immune responses to different types of pathogens will improve our understanding of immune defense in New World camelids and should aid in the design of effective vaccines.
Acknowledgments
We extend our appreciation to Gail Fulkerson (Spencer, N.Y.) for her assistance with this project.
This work was supported by the Collaborative Research in Preclinical and Clinical Sciences Program of the College of Veterinary Medicine, Cornell University.
REFERENCES
- 1.Azwai, S. M., S. D. Carter, and Z. Woldehiwet. 1995. Monoclonal antibodies against camel (Camelus dromedarius) IgG, IgM and light chains. Vet. Immunol. Immunopathol. 45:175-184. [DOI] [PubMed] [Google Scholar]
- 2.Bishop, J. K., and L. G. Rickard. 1987. Fecal survey of llamas (Lama glama) in Oregon: incidental recovery of Nematodirus battus. J. Am. Vet. Med. Assoc. 191:1579-1581. [PubMed] [Google Scholar]
- 3.Conrath, K. E., U. Wernery, S. Muyldermans, and V. K. Nguyen. 2003. Emergence and evolution of functional heavy-chain antibodies in Camelidae. Dev. Comp. Immunol. 27:87-103. [DOI] [PubMed] [Google Scholar]
- 4.Cortez-Retamozo, V., M. Lauwereys, G. G. Hassanzadeh, M. Gobert, K. Conrath, S. Muyldermans, P. De Baetselier, and H. Revets. 2002. Efficient tumor targeting by single-domain antibody fragments of camels. Int. J. Cancer 98:456-462. [DOI] [PubMed] [Google Scholar]
- 5.Desmyter, A., K. Decanniere, S. Muyldermans, and L. Wyns. 2001. Antigen specificity and high affinity binding provided by one single loop of a camel single-domain antibody. J. Biol. Chem. 276:26285-26290. [DOI] [PubMed] [Google Scholar]
- 6.Dirie, M. F., and O. Abdurahman. 2003. Observations on little known diseases of camels (Camelus dromedarius) in the Horn of Africa. Rev. Sci. Tech. Off. Int. Epizoot. 22:1043-1049. [DOI] [PubMed] [Google Scholar]
- 7.Duffy, M. S., N. MacAfee, M. D. B. Burt, and J. A. Appleton. 2002. An aspartyl protease inhibitor orthologue expressed by Parelaphostrongylus tenuis is immunogenic in an atypical host. Clin. Diagn. Lab. Immunol. 9:763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fondevila, N. A., F. J. Marcoveccio, J. Blanco Viera, V. K. O'Donnell, B. J. Carrillo, A. A. Schudel, M. David, A. Torres, and C. A. Mebus. 1995. Susceptibility of llamas (Lama glama) to infection with foot-and-mouth-disease virus. Zentbl. Veterinarmed. B 42:595-599. [DOI] [PubMed] [Google Scholar]
- 9.Foreyt, W. J., L. G. Rickard, S. Dowling, S. Parish, and M. Pipas. 1991. Experimental infections of two llamas with the meningeal worm (Parelaphostrongylus tenuis). J. Zoo. Wildl. Med. 22:339-344. [Google Scholar]
- 10.Frenken, L. G. J., R. H. J. van der Linden, P. W. J. J. Hermans, J. W. Bos, R. C. Ruuls, B. de Geus, and C. T. Verrips. 2000. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J. Biotechnol. 78:11-21. [DOI] [PubMed] [Google Scholar]
- 11.Garmendia, A. E., G. H. Palmer, J. C. DeMartini, and T. C. McGuire. 1987. Failure of passive immunoglobulin transfer: a major determinant of mortality in newborn alpacas (Lama pacos). Am. J. Vet. Res. 48:1472-1476. [PubMed] [Google Scholar]
- 12.Hamers-Casterman, C., T. Atarhouch, S. Muyldermans, G. Robinson, C. Hamers, E. B. Songa, N. Bendahman, and R. Hamers. 1993. Naturally occurring antibodies devoid of light chains. Nature 363:446-448. [DOI] [PubMed] [Google Scholar]
- 13.Intapan, P. M., W. Maleewong, K. Sawanyawisuth, and V. Chotmongkol. 2003. Evaluation of human IgG subclass antibodies in the serodiagnosis of angiostrongyliasis. Parasitol. Res. 89:425-429. [DOI] [PubMed] [Google Scholar]
- 14.Klion, A. D., A. Vijaykumar, T. Oei, B. Martin, and T. B. Nutman. 2003. Serum immunoglobulin G4 antibodies to the recombinant antigen, Ll-SXP-1, are highly specific for Loa loa infection. J. Infect. Dis. 187:128-133. [DOI] [PubMed] [Google Scholar]
- 15.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lauwereys, M., M. A. Ghahroudi, A. Desmyter, J. Kinne, W. Holzer, E. De Genst, L. Wyns, and S. Muyldermans. 1998. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 17:3512-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin, F., C. Volpari, C. Steinkuhler, N. Dimasi, M. Brunetti, G. Biasiol, S. Altamura, R. Cortese, R. De Francesco, and M. Sollazzo. 1997. Affinity selection of a camelized VH domain antibody inhibitor of hepatitis C virus NS3 protease. Protein Eng. 10:607-614. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen, V. K., S. Muyldermans, and R. Hamers. 1998. The specific variable domain of camel heavy-chain antibodies is encoded in the germline. J. Mol. Biol. 275:413-418. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen, V. K., R. Hamers, L. Wyns, and S. Muyldermans. 1999. Loss of splice consensus signal is responsible for the removal of the entire CH1 domain of the functional camel IGG2A heavy-chain antibodies. Mol. Immunol. 36:515-524. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen, V. K., R. Hamers, L. Wyns, and S. Muyldermans. 2000. Camel heavy-chain antibodies: diverse germline VHH and specific mechanisms enlarge the antigen-binding repertoire. EMBO J. 19:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen, V. K., A. Desmyter, and S. Muyldermans. 2001. Functional heavy-chain antibodies in Camelidae. Adv. Immunol. 79:261-296. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill, S. M., M. Parkinson, W. Strauss, R. Angles, and J. P. Dalton. 1998. Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am. J. Trop. Med. Hyg. 58:417-423. [DOI] [PubMed] [Google Scholar]
- 24.Pleschberger, M., D. Saerens, S. Weigert, U. B. Sleytr, S. Muyldermans, M. Sara, and E. M. Egelseer. 2004. An S-layer heavy chain camel antibody fusion protein for generation of a nanopatterned sensing layer to detect the prostate-specific antigen by surface plasmon resonance technology. Bioconjug. Chem. 15:664-671. [DOI] [PubMed] [Google Scholar]
- 25.Rickard, L. G. 1994. Update on llama medicine: parasites. Vet. Clin. N. Am. Food Anim. Pract. 10:239-247. [PubMed] [Google Scholar]
- 26.Rickard, L. G., B. B. Smith, E. J. Gentz, A. A. Frank, E. G. Pearson, L. L. Walker, and M. J. Pybus. 1994. Experimentally induced meningeal worm (Parelaphostrongylus tenuis) infection in the llama (Lama glama): clinical evaluation and implications for parasite translocation. J. Zoo. Wildl. Med. 25:390-402. [Google Scholar]
- 27.Rivera, H., B. R. Madewell, and E. Ameghino. 1987. Serologic survey of viral antibodies in the Peruvian alpaca (Lama pacos). Am. J. Vet. Res. 48:189-191. [PubMed] [Google Scholar]
- 28.Spinelli, S., L. G. Frenken, P. Hermans, T. Verrips, K. Brown, M. Tegoni, and C. Cambillau. 2000. Camelid heavy-chain variable domains provide efficient combining sites to haptens. Biochemistry 39:1217-1222. [DOI] [PubMed] [Google Scholar]
- 29.Stanley, H. F., M. Kadwell, and J. C. Wheeler. 1994. Molecular evolution of the family Camelidae: a mitochondrial DNA study. Proc. R. Soc. Lond. B 256:1-6. [DOI] [PubMed] [Google Scholar]
- 30.Stijlemans, B., K. Conrath, V. Cortez-Retamozo, H. Van Xong, L. Wyns, P. Senter, H. Revets, P. De Baetselier, S. Muyldermans, and S. Magez. 2004. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies: African trypanosomes as paradigm. J. Biol. Chem. 279:1256-1261. [DOI] [PubMed] [Google Scholar]
- 31.Su, C., V. K. Nguyen, and M. Nei. 2002. Adaptive evolution of variable region genes encoding an unusual type of immunoglobulin in camelids. Mol. Biol. Evol. 19:205-215. [DOI] [PubMed] [Google Scholar]
- 32.Transue, T. R., E. De Genst, M. A. Ghahroudi, L. Wyns, and S. Muyldermans. 1998. Camel single-domain antibody inhibits enzyme by mimicking carbohydrate substrate. Proteins 32:515-522. [DOI] [PubMed] [Google Scholar]
- 33.van der Linden, R., B. de Geus, W. Stok, W. Bos, D. van Wassenaar, T. Verrips, and L. Frenken. 2000. Induction of immune responses and molecular cloning of the heavy chain antibody repertoire of Lama glama. J. Immunol. Methods 240:185-195. [DOI] [PubMed] [Google Scholar]
- 34.van der Linden, R. H. J., B. de Geus, L. G. J. Frenken, H. Peters, and C. T. Verrips. 2000. Improved production and function of llama heavy chain antibody fragments by molecular evolution. J. Biotechnol. 80:261-270. [DOI] [PubMed] [Google Scholar]
- 35.Vu, K. B., M. A. Ghahroudi, L. Wyns, and S. Muyldermans. 1997. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 34:1121-1131. [DOI] [PubMed] [Google Scholar]
- 36.Webb, S. D. 1974. Pleistocene llamas of Florida, with a brief review of the lamini, p. 170-214. In S. D. Webb (ed.), Pleistocene mammals of Florida. University Presses of Florida, Gainesville.
- 37.Wheeler, J. 2003. Evolution and origin of the domestic camelids. Int. Lama Reg. Rep. 8:2. [Online.] http://www.rmla.com/origin_of_sa_camelids.htm. [Google Scholar]
- 38.Wilson, R. T. 1984. Origin and distribution, p. 1-15. In R. T. Wilson (ed.), The camel. Longman, London, United Kingdom.
- 39.Woolven, B. P., L. G. J. Frenken, P. van der Logt, and P. J. Nicholls. 1999. The structure of the llama heavy chain constant genes reveals a mechanism for heavy-chain antibody formation. Immunogenetics 50:98-101. [DOI] [PubMed] [Google Scholar]