Abstract
Objective
To assess the absolute treatment effects of intravascular imaging guided versus angiography guided percutaneous coronary intervention in patients with coronary artery disease, considering their baseline risk.
Design
Systematic review and meta-analysis.
Data sources
PubMed/Medline, Embase, and Cochrane Library databases up to 31 August 2023.
Study selection
Randomized controlled trials comparing intravascular imaging (intravascular ultrasonography or optical coherence tomography) guided versus coronary angiography guided percutaneous coronary intervention in adults with coronary artery disease.
Main outcome measures
Random effect meta-analysis and GRADE (grading of recommendations, assessment, development, and evaluation) were used to assess certainty of evidence. Data included rate ratios and absolute risks per 1000 people for cardiac death, myocardial infarction, stent thrombosis, target vessel revascularization, and target lesion revascularization. Absolute risk differences were estimated using SYNTAX risk categories for baseline risks at five years, assuming constant rate ratios across different cardiovascular risk thresholds.
Results
In 20 randomized controlled trials (n=11 698), intravascular imaging guided percutaneous coronary intervention was associated with a reduced risk of cardiac death (rate ratio 0.53, 95% confidence interval 0.39 to 0.72), myocardial infarction (0.81, 0.68 to 0.97), stent thrombosis (0.44, 0.27 to 0.72), target vessel revascularization (0.74, 0.61 to 0.89), and target lesion revascularization (0.71, 0.59 to 0.86) but not all cause death (0.81, 0.64 to 1.02). Using SYNTAX risk categories, high certainty evidence showed that from low risk to high risk, intravascular imaging was likely associated with 23 to 64 fewer cardiac deaths, 15 to 19 fewer myocardial infarctions, 9 to 13 fewer stent thrombosis events, 28 to 38 fewer target vessel revascularization events, and 35 to 48 fewer target lesion revascularization events per 1000 people.
Conclusions
Compared with coronary angiography guided percutaneous coronary intervention, intravascular imaging guided percutaneous coronary intervention was associated with significantly reduced cardiac death and cardiovascular outcomes in patients with coronary artery disease. The estimated absolute effects of intravascular imaging guided percutaneous coronary intervention showed a proportional relation with baseline risk, driven by the severity and complexity of coronary artery disease.
Systematic review registration
PROSPERO CRD42023433568.
Introduction
The advent of drug eluting stents and advances in intravascular imaging modalities, such as intravascular ultrasonography or optical coherence tomography, have improved cardiovascular outcomes in patients undergoing percutaneous coronary intervention.1 Randomized controlled trials have shown evidence supporting intravascular imaging guided percutaneous coronary intervention, primarily due to reduced rates of revascularization and stent thrombosis. For instance, the IVUS-XPL (Impact of Intravascular Ultrasound Guidance on the Outcomes of Xience Prime Stents in Long Lesions) trial, involving 1400 participants, showed a sustained reduction in major adverse cardiovascular events over five years with intravascular ultrasonography in patients with long lesions.2 However, most trials examining use of intravascular ultrasonography in complex lesions were relatively small and not sufficiently powered to assess individual clinical endpoints.
Two recent trials, OCTOBER (Optical Coherence Tomography Optimized Bifurcation Event Reduction) 3 and ILUMIEN IV: OPTIMAL PCI (Optical Coherence Tomography Guided Coronary Stent Implantation Compared with Angiography: A Multicenter Randomized Trial in Percutaneous Coronary Intervention) 4 have shown conflicting results among participants undergoing optical coherence tomography guided versus angiography guided percutaneous coronary intervention. Although OCTOBER showed a reduction in cardiovascular outcomes with optical coherence tomography guided percutaneous coronary intervention at two years in complex coronary artery bifurcation lesions, ILUMIEN IV: OPTIMAL PCI did not show differences in outcomes between optical coherence tomography and angiography guided percutaneous coronary intervention at two years.
In this context, the absolute effects of intravascular imaging seem likely to be influenced by an individual’s baseline risk, primarily determined by the complexity and severity of coronary artery disease.5 Furthermore, concerns exist about the link between the increased procedural time and potential exposure to radiation associated with intravascular imaging guided percutaneous coronary intervention. Therefore, we did a meta-analysis of contemporary randomized controlled trials to evaluate the absolute effects of therapy considering the patient’s baseline risk and to evaluate the balance between additional procedural time and cardiovascular risk reduction in intravascular imaging guided percutaneous coronary intervention.
Methods
We conducted this trial level meta-analysis according to the Cochrane Collaboration guidelines and reported it following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis).6 7
Data sources, searches, and study selection
We did a comprehensive literature search without language restriction using PubMed/Medline, Embase, and the Cochrane Library databases through 31 August 2023. We also searched websites of major cardiovascular and medicine journals (www.nejm.org; https://www.thelancet.com/; https://jamanetwork.com; https://annals.org/aim; https://academic.oup.com/eurheartj; www.onlinejacc.org; and www.ahajournals.org/journal/circ) and bibliographies of relevant studies.8 9 10 11 We used broad search terms (“angiography”, “intravascular ultrasound”, “IVUS”, “optical coherence tomography”, “OCT”, “percutaneous coronary intervention”, and “PCI”) (supplementary tables A-C).
The pre-specified inclusion criteria were randomized controlled trials comparing intravascular imaging (intravascular ultrasonography or optical coherence tomography) guided versus coronary angiography guided percutaneous coronary intervention in adults with coronary artery disease, studies using drug eluting stents, and studies reporting cardiovascular outcomes of interest. We removed duplicates and screened the remaining articles at the title and abstract level and then at the full text level (fig 1). Two authors (SUK and SA) independently conducted the study search and selection process and resolved conflicts by discussion and mutual consensus.
Fig 1.
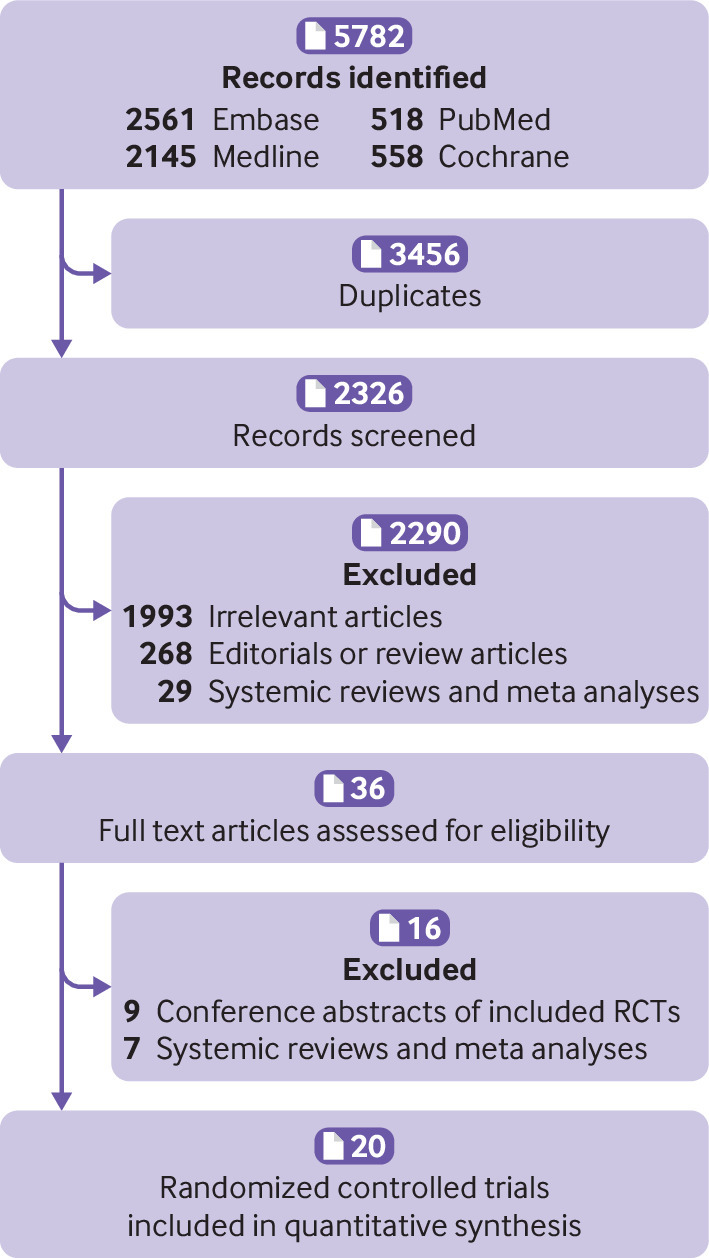
Flowchart of study selection
Data extraction
Two reviewers (SA and UAA) independently abstracted the data into the data collection sheets, appraised the accuracy of the data, did a risk of bias assessment, and resolved discrepancies by discussion or referral to the original publication. We abstracted data on characteristics of trials (supplementary table D), procedural and angiographic characteristics of patients in the trials (supplementary table E), definition of complex lesions used in trials (supplementary table F), demographic and clinical characteristics of participants (table 1), point estimates with 95% confidence intervals, number of events, and sample sizes. We abstracted data on the intention-to-treat principle.
Table 1.
Baseline demographics of trials and populations included in meta-analysis. Values are percentages unless stated otherwise
| Trial, year | No of patients | Median age, years | Men | Smoking | HTN | HLD | Diabetes | Previous MI | Previous PCI | Previous CABG | LVEF | Stable angina | ACS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVUS versus coronary angiography guided PCI | ||||||||||||||||||
| HOME DES IVUS, 200912 | 105/105 | 59/60 | 73/71 | 40/35 | 67/71 | 63/66 | 42/45 | 37/32 | 17/14 | 14/10 | NR | 38/40 | 62/60 | |||||
| RESET, 201313 | 269/274 | 63/64 | 66/55 | 22/17 | 61/66 | 61/62 | 32/30 | 1/3 | - | - | 55/54 | 53/52 | 47/48 | |||||
| AVIO, 201314 | 142/142 | 64/64 | 82/77 | 35/31 | 70/67 | 70/77 | 24/27 | - | - | - | 55/56 | 70/64 | 30/26 | |||||
| Wang et al, 201415 | 38/42 | 56/54 | 60/67 | 50/59 | 39/24 | 26/24 | 21/12 | - | - | - | 50/48 | 0/0 | 100/100 | |||||
| MOZART, 201416 | 41/42 | 67/62 | 61/57 | 42/40 | 98/100 | - | 73/81 | - | 274/12 | 15/17 | NR | 76/71 | 15/17 | |||||
| AIR CTO, 201517 | 115/115 | 67/66 | 89/80 | 39/39 | 75/70 | 22/28 | 30/27 | 21/30 | - | - | 55/56 | 71/76 | 29/24 | |||||
| CTO-IVUS, 201518 | 201/201 | 61/61 | 81/81 | 35/34 | 63/64 | - | 35/34 | 8/8 | 15/16 | 2/3 | 57/57 | 100/100 | 0/0 | |||||
| IVUS-XPL, 20202 | 700/700 | 64/64 | 69/69 | 22/26 | 65/63 | 67/65 | 36/37 | 5/4 | 11/10 | 3/2 | 63/62 | 51/51 | 49/49 | |||||
| Tan et al, 201519 | 61/62 | 77/76 | 62/69 | 44/47 | 41/47 | - | 34/30 | 16/21 | - | - | 55/53 | 30/34 | 70/66 | |||||
| Liu et al, 201920 | 167/169 | 65/65 | 64/64 | 37/36 | 70/72 | 38/38 | 34/31 | 17/14 | 20/17 | 1/1 | 56/58 | 12/11 | 86/87 | |||||
| ULTIMATE, 202121 | 724/724 | 65/66 | 74/73 | 35/32 | 71/72 | 54/55 | 30/31 | 9/12 | 17/20 | 1/1 | 61/60 | 13/13 | 79/78 | |||||
| OCT versus coronary angiography guided PCI | ||||||||||||||||||
| OCTACS, 201522 | 50/50 | 62/63 | 72/68 | 46/36 | 56/56 | 44/38 | 16/10 | 4/0 | 6/4 | 0/0 | - | 0/0 | 100/100 | |||||
| DOCTORS, 201623 | 120/120 | 61/60 | 79/76 | 42/41 | 42/49 | 47/48 | 16/19 | - | - | - | - | 0/0 | 100/100 | |||||
| ROBUST, 201824 | 105/96 | 57/59 | 83/87 | 64/59 | 50/52 | - | 17/26 | 1/6 | 4/4 | 0/0 | - | 0/0 | 100/100 | |||||
| OPTIMUM, 202025 | 56/54 | 69/69 | 79/74 | 23/18 | 77/74 | 86/85 | 52/46 | 16/15 | 4/0 | 21/35 | 61/60 | 91/91 | 9/9 | |||||
| ILUMIEN IV: OPTIMIZE PCI 20234 | 1233/1254 | 65/66 | 78/76 | 19/20 | 71/74 | 66/69 | 42/42 | 20/24 | 13/13 | 63/53 | 55/55 | 27/29 | 63/61 | |||||
| OCTOBER, 20233 | 600/601 | 66/66 | 69/69 | 50/51 | 70/75 | 76/78 | 17/16 | 28/30 | 41/43 | 1/2 | 59/58 | 55/53 | 45/47 | |||||
| IVUS/OCT versus coronary angiography guided PCI | ||||||||||||||||||
| iSIGHT, 202126 | 50/51/49 | 59/60/59 | 36/31/38 | 14/17/14 | 42/46/39 | 30/36/28 | - | - | - | - | - | 35/34/35 | 36/33/36 | |||||
| ILUMIEN III: OPTIMIZE PCI, 202127 | 136/153/142 | 66/66/67 | 74/69/73 | 13/17/23 | 78/78/75 | 75/73/77 | 36/33/28 | - | - | - | - | 35/34/35 | 36/33/36 | |||||
| RENOVATE-COMPLEX-PCI, 202328 | 1092/547 | 65/66 | 79/79 | 19/17 | 62/59 | 51/51 | 36/41 | 7/8 | 24/23 | - | 58/59 | 49/50 | 51/50 | |||||
ACS=acute coronary syndrome; AVIO=Angiography Versus IVUS Optimization; CABG=coronary artery bypass graft; CTO IVUS=Chronic Total Occlusion InterVention with drUg-eluting Stents guided by IVUS; DOCTORS=Does Optical Coherence Tomography Optimize Results of Stenting; HLD=hyperlipidemia; HTN=hypertension; ILUMIEN IV=Optical Coherence Tomography (OCT) Guided Coronary Stent Implantation Compared with Angiography: A Multicenter Randomized Trial in PCI; iSIGHT=Optical Coherence Tomography Versus Intravascular Ultrasound and Angiography to Guide Percutaneous Coronary Interventions; IVUS=intravascular ultrasonography; IVUS-XPL=Impact of Intravascular Ultrasound Guidance on the Outcomes of Xience Prime Stents in Long Lesions; LVEF=left ventricular ejection fraction; MI=myocardial infarction; MOZART=Minimizing cOntrast utiliZation With IVUS Guidance in coRonary angioplasty; OCT=optical coherence tomography; OCTACS=Optical Coherence Tomography Guided Percutaneous Coronary Intervention With Nobori Stent Implantation in Patients With Non-ST-Segment-Elevation Myocardial Infarction; OCTOBER=OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions; OPTIMUM=Online 3-Dimensional Optical Frequency Domain Imaging to Optimize Bifurcation Stenting Using UltiMaster Stent; PCI=percutaneous coronary intervention; RENOVATE COMPLEX-PCI=Randomized Controlled Trial of Intravascular Imaging Guidance versus Angiography-Guidance on Clinical Outcomes after Complex Percutaneous Coronary Intervention; RESET=Real Safety and Efficacy of a 3-Month Dual Antiplatelet Therapy Following Zotarolimus-Eluting Stents Implantation; ULTIMATE=Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions.
Risk of bias in individual studies
We used a Cochrane risk of bias assessment tool for assessing the risk of bias in randomized controlled trials (supplementary figure A).29 We assessed the risk of bias at the study level across the following domains: bias due to the randomization process; bias due to deviation from the intended intervention; bias due to missing outcome data; bias in the measurement of the outcomes; and bias in the selection of the reported results, including divergence from the registered protocol or owing to early termination for benefit. Two reviewers (SA and UAA) independently appraised the potential risks of bias, and discrepancies were resolved by discussion or adjudication by a third party.
Outcomes of interest
Our primary focus was cardiac death. Additional endpoints included myocardial infarction, stent thrombosis, target vessel revascularization, target lesion revascularization, and all cause death. We also evaluated differences in procedural characteristics—that is, duration of procedure (minutes), fluoroscopy time (minutes), and contrast volume (mL). We extracted outcomes at the maximum follow-up duration.
Data synthesis and summary measures
We did a frequentist pairwise meta-analysis for all patients and measured rate ratios for binary outcomes and mean differences for continuous outcomes with 95% confidence intervals. We measured rate ratios per person years to account for the difference in follow-up duration as it assumes a constant risk over time.30 To calculate absolute risk differences, we applied the pooled rate ratios from the meta-analysis to the baseline risk. We used the baseline risk from the angiography guided percutaneous coronary intervention arms of the trials for all outcomes. To connect the two measures, we used absolute risk difference = (rate ratio–1) × baseline risk per 1000 person years.
Baseline risk for clinical scenarios
To explore the applicability of the findings of our meta-analysis to clinical practice, we did a series of sensitivity analyses using data presented in the SYNTAX (Synergy between Percutaneous coronary intervention with Taxus and Cardiac Surgery) trials.5 31 Firstly, we determined the anticipated absolute effects within distinct risk categories defined within the SYNTAX trial. This trial delineated thirds of risk (mild 0-22, intermediate 23-32, and high ≥33) based on the compilation of angiographic features. We then did another sensitivity analysis using the SYNTAX-II trial (supplementary table G), which used a scoring system incorporating clinical and angiographic features.
We assumed that the pooled relative effects of intravascular imaging guided versus coronary angiographic guided percutaneous coronary intervention are transportable across study populations.32 33 34 35 We then took the event rates in the percutaneous coronary intervention arm reported for each SYNTAX risk category as baseline risks and calculated anticipated absolute effects by multiplying the pooled relative effects by the corresponding baseline risks. We did a parallel analysis using data from SYNTAX-II.31
Statistical analysis
We pooled outcomes by using a random effects model. We applied the DerSimonian and Laird method to estimate 𝜏.36 We used I2 statistics to measure the extent of unexplained statistical heterogeneity: we considered I2 ≥50% to be a high degree of between study statistical heterogeneity.37 We assessed small study effects and publication bias by using funnel plots and Egger’s regression tests (supplementary figures B-G).
We did subgroup analyses according to age (<65 v ≥65 years), type of intravascular imaging (intravascular ultrasonography versus optical coherence tomography), setting (acute coronary syndrome versus all comers), sample size (<500 v ≥500), and follow-up duration (<1 v ≥1 year) (supplementary table H). We did several sensitivity analyses: a leave-one-out meta-analysis (supplementary table I); complex coronary artery lesions (supplementary figures H-M); absolute risk estimates using the SYNTAX score categories (table 2); and absolute risk estimates using the SYNTAX-II (supplementary table G). Finally, we estimated the trade-off between procedural characteristics and cardiovascular outcomes; that is, to evaluate the procedural compromises associated with intravascular imaging, we calculated the absolute number of cardiovascular events increased or averted for one additional procedural or fluoroscopy minute when using intravascular imaging compared with a coronary angiogram. The supplementary methods report a detailed method for estimating absolute risk differences.
Table 2.
Anticipated absolute risk differences (ARD) per 1000 people with 95% confidence intervals (CI) of intravascular imaging on outcomes in patients undergoing percutaneous coronary intervention (PCI) across different coronary artery disease risk categories at five years
| Risk categories | Rate ratio (95% CI) | Baseline risk for coronary angiography guided PCI | ARD (95% CI) with intravascular imaging guided PCI per 1000 people | Certainty of evidence (GRADE) |
|---|---|---|---|---|
| Low risk (SYNTAX: 0-22) | ||||
| Cardiac death | 0.53 (0.39 to 0.72) | 48 per 1000 | 23 (29 to 13) fewer | High |
| Myocardial infarction | 0.81 (0.68 to 0.97) | 78 per 1000 | 15 (25 to 2) fewer | High |
| Stent thrombosis | 0.44 (0.27 to 0.72) | 16 per 1000 | 9 (12 to 4) fewer | High |
| Target vessel revascularization | 0.74 (0.61 to 0.89) | 108 per 1000 | 28 (42 to 12) fewer | High |
| Target lesion revascularization | 0.71 (0.59 to 0.86) | 121 per 1000 | 35 (50 to 17) fewer | High |
| All cause death | 0.81 (0.64 to 1.02) | 89 per 1000 | 17 fewer (32 fewer to 2 more) | Moderate |
| Intermediate risk (SYNTAX: 22-32) | ||||
| Cardiac death | 0.53 (0.39 to 0.72) | 88 per 1000 | 41 (54 to 25) fewer | High |
| Myocardial infarction | 0.81 (0.68 to 0.97) | 112 per 1000 | 21 (36 to 3) fewer | High |
| Stent thrombosis | 0.44 (0.27 to 0.72) | 19 per 1000 | 11 (14 to 5) fewer | High |
| Target vessel revascularization | 0.74 (0.61 to 0.89) | 113 per 1000 | 29 (44 to 12) fewer | High |
| Target lesion revascularization | 0.71 (0.59 to 0.86) | 128 per 1000 | 37 (52 to 18) fewer | High |
| All cause death | 0.81 (0.64 to 1.02) | 138 per 1000 | 26 fewer (50 fewer to 3 more) | Moderate |
| High risk (SYNTAX ≥33) | ||||
| Cardiac death | 0.53 (0.39 to 0.72) | 136 per 1000 | 64 (83 to 38) fewer | High |
| Myocardial infarction | 0.81 (0.68 to 0.97) | 101 per 1000 | 19 (32 to 3) fewer | High |
| Stent thrombosis | 0.44 (0.27 to 0.72) | 23 per 1000 | 13 (17 to 6) fewer | High |
| Target vessel revascularization | 0.74 (0.61 to 0.89) | 145 per 1000 | 38 (57 to 16) fewer | High |
| Target lesion revascularization | 0.71 (0.59 to 0.86) | 164 per 1000 | 48 (67 to 23) fewer | High |
| All cause death | 0.81 (0.64 to 1.02) | 192 per 1000 | 36 fewer (69 fewer to 4 more) | Moderate |
GRADE=grading of recommendations, assessment, development, and evaluation; SYNTAX=synergy between percutaneous coronary intervention with taxus and cardiac surgery.
For all analyses, we set statistical significance as P<0.05. We used RevMan V 5.4 and MAGICapp (www.magicapp.org) for all analyses.
Certainty of the evidence
Two authors (SUK and HBA) rated the certainty of evidence as high, intermediate, low, or very low by using the grading of recommendations assessment, development, and evaluation (GRADE) approach (https://gdt.gradepro.org/app/) (supplementary table J).
Patient and public involvement
No patients were involved in setting the research question, outcome measures, study design, or data interpretation. Besides lack of funding, the place where this research took place was restricted and we lacked the permission to engage patients. However, after the production of our first manuscript draft, we consulted a member of the public with established cardiovascular disease who has been advised on percutaneous coronary intervention for coronary artery disease. We received feedback that the estimate table (table 2) and certainty of evidence statements were very useful, allowing assessment of the impact of intravascular imaging guided percutaneous coronary intervention with respect to absolute risk reduction and certainty of risk reduction.
Results
Description of included trials
Of 5782 citations, we reviewed 2326 after removal of duplicates. We excluded an additional 2306 studies on the basis of the title and abstract level screening and a priori selection criteria (fig 1). Finally, we included 20 trials (n=11 698) in the analysis (table 1). Eleven trials (n=5139) exclusively focused on intravascular ultrasonography, six trials (n=4339) used optical coherence tomography, and three trials (n=2220) used both types of imaging. Twelve trials (n=6113) were conducted in patients with complex coronary lesions. The median age of participants was 64 (interquartile range 61-66) years. The median follow-up duration was 1 (1-2) years.
Our risk of bias assessment showed that 10% (2/20) of trials raised some concerns with the randomization process, 5% (1/20) had deviations from the intended intervention, 15% (3/20) had missing outcome data, and 20% (4/20) had concerns about outcome measurement. Funnel plots did not show small study effects, and Egger’s regression test did not indicate the presence of publication bias (P>0.05).
Differences in procedural characteristics
Eleven trials (n=8358) reported procedural time, five trials (n=4560) reported fluoroscopy time, and 13 trials (n=8789) reported contrast volume. Compared with coronary angiography, intravascular imaging guided percutaneous coronary intervention was associated with increased procedural time (mean difference 15.68 (95% confidence interval 13.29 to 18.07) min; P≤0.01; I2=69%), fluoroscopy time (3.23 (2.25 to 4.21) min; P≤0.01; I2 =78%), and contrast volume use (26.46 (11.14 to 41.78) mL; P≤0.01; I2=95%). Optical coherence tomography was associated with higher usage of contrast volume (mean difference 54.19 (31.43 to 76.95) mL; P<0.01; I2=96%) than intravascular ultrasonography (0.21 (−23.54 to 23.96) mL; P=0.99; I2=87%) (P for interaction <0.01).
Cardiac death
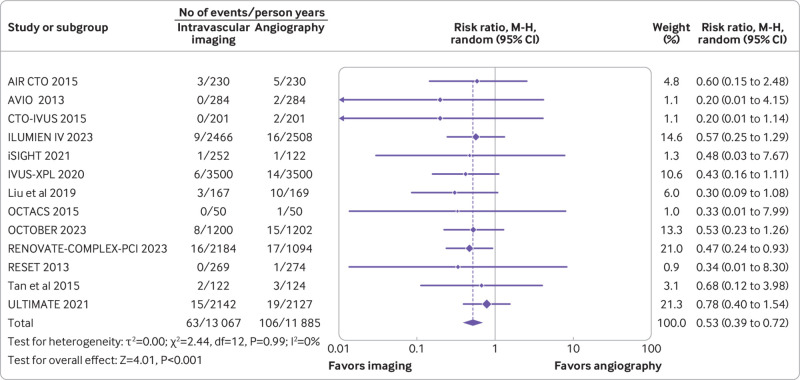
Thirteen trials (n=10 007) reported cardiac death. Compared with coronary angiography, intravascular imaging was associated with reduced risk of cardiac death (rate ratio 0.53, 95% confidence interval 0.39 to 0.72; P<0.001; I2=0%; fig 2) (absolute risk difference 10 (95% confidence interval 13 to 6) fewer per 1000 person years; high certainty).
Fig 2.
Forest plot comparing intravascular imaging guided with coronary angiography guided percutaneous intervention for cardiac death. Data obtained from randomized controlled trials using random effect meta-analysis and expressed as rate ratio. CI=confidence interval; M-H=Mantel-Haenszel
Myocardial infarction and stent thrombosis
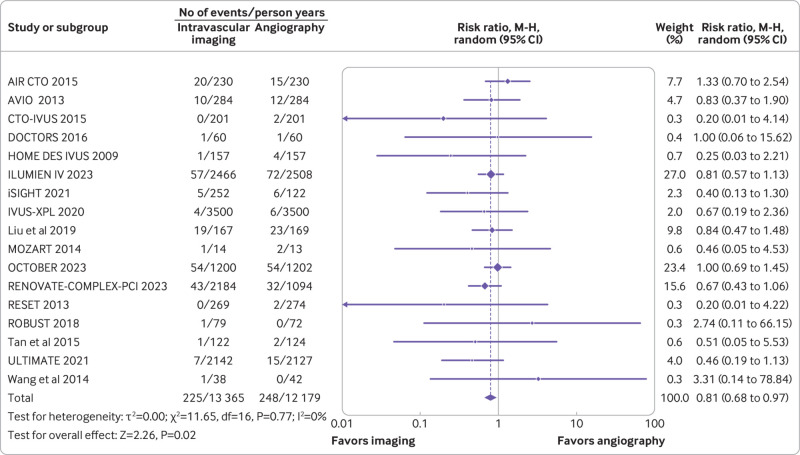
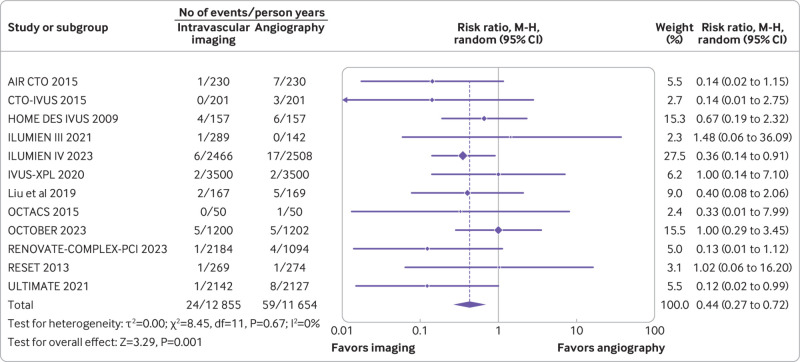
A total of 17 trials (n=11 057) reported myocardial infarction, and 12 trials (n=10 327) reported stent thrombosis. Compared with coronary angiography, intravascular imaging was associated with reduced risk of myocardial infarction (rate ratio 0.81, 0.68 to 0.97; P=0.02; I2=0%; fig 3) (absolute risk difference 9 (15 to 1) fewer per 1000 person years; high certainty) and stent thrombosis (rate ratio 0.44, 0.27 to 0.72; P=0.001; I2=0%; fig 4) (absolute risk difference 7 (9 to 3) fewer per 1000 person years; high certainty).
Fig 3.
Forest plot comparing intravascular imaging guided with coronary angiography guided percutaneous intervention for myocardial infarction (bottom). Data obtained from randomized controlled trials using random effect meta-analysis and expressed as rate ratio. CI=confidence interval; M-H=Mantel-Haenszel
Fig 4.
Forest plot comparing intravascular imaging guided with coronary angiography guided percutaneous intervention for stent thrombosis. Data obtained from randomized controlled trials using random effect meta-analysis and expressed as rate ratio. CI=confidence interval; M-H=Mantel-Haenszel
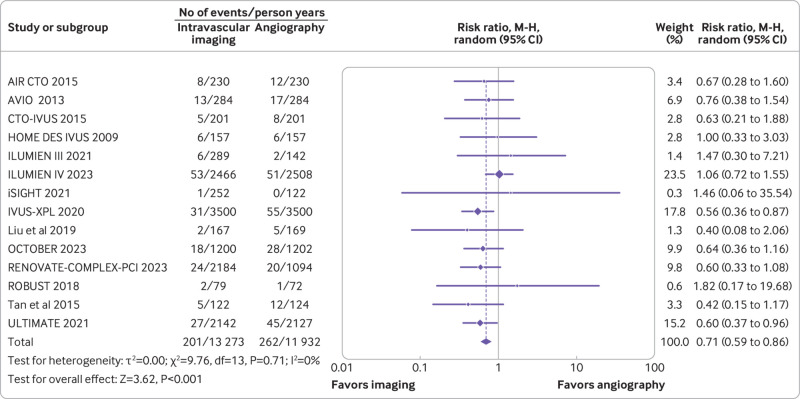
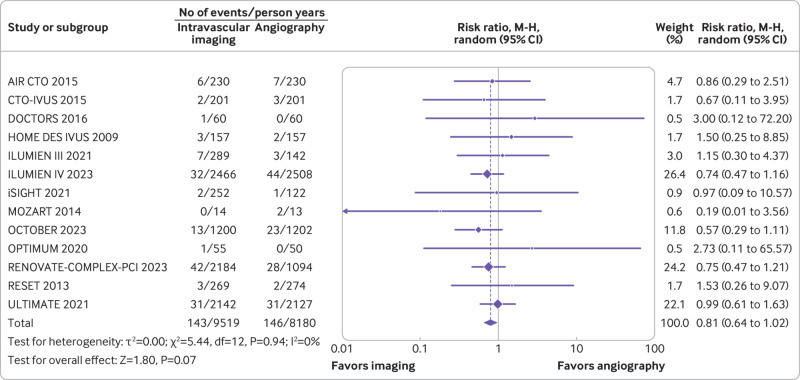
Target vessel revascularization, target lesion revascularization, and all cause death
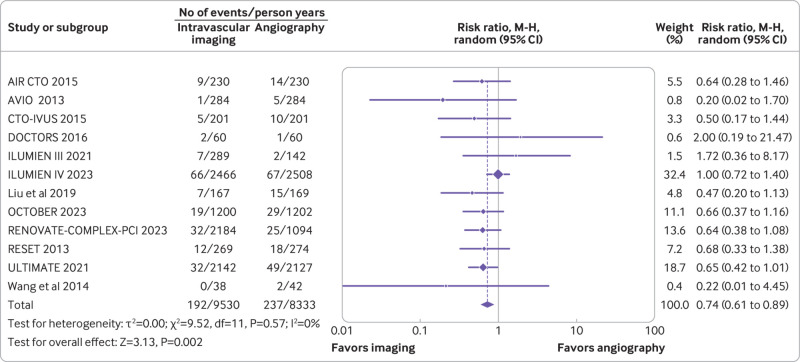
Twelve trials (n=9321) reported target vessel revascularization, 14 trials (n=10 542) reported target lesion revascularization, and 13 trials (n=9174) reported all cause deaths. Compared with coronary angiography, intravascular imaging was associated with a reduced risk of target vessel revascularization (rate ratio 0.74, 0.61 to 0.89; P=0.002; I2=0%; fig 5) (absolute risk difference 14 (21 to 6) fewer per 1000 person years; high certainty) and target lesion revascularization (rate ratio 0.71, 0.59 to 0.86; P<0.001; I2=0%; fig 6) (absolute risk difference 18 (25 to 9) fewer per 1000 person years; high certainty). However, intravascular imaging was not associated with a significant reduction in all cause deaths (rate ratio 0.81, 0.64 to 1.02; P=0.07; I2=0%; fig 7) (absolute risk difference 4 (8 to 0) fewer per 1000 person years; moderate certainty).
Fig 5.
Forest plot comparing intravascular imaging guided with coronary angiography guided percutaneous intervention for target vessel revascularization. Data obtained from randomized controlled trials using a random effect meta-analysis and expressed as rate ratio. CI=confidence interval; M-H=Mantel-Haenszel
Fig 6.
Forest plot comparing intravascular imaging guided with coronary angiography guided percutaneous coronary intervention for target lesion revascularization. Data obtained from randomized controlled trials using random effect meta-analysis and expressed as risk ratio. CI=confidence interval; M-H=Mantel-Haenszel
Fig 7.
Forest plot comparing intravascular imaging guided with coronary angiography guided percutaneous coronary intervention for all cause death. Data obtained from randomized controlled trials using random effect meta-analysis and expressed as risk ratio. CI=confidence interval; M-H=Mantel-Haenszel
Trade-off between procedural time and cardiovascular outcomes
When assessing the trade-off between the additional procedural time (~15 min) and fluoroscopy time (~3 min) and its effect on cardiovascular outcomes, we calculated that for each additional procedural minute spent using intravascular imaging we could anticipate averting approximately 1 (95% confidence interval 1 to 0) cardiac death, 1 (1 to 0) myocardial infarction, 1 (1 to 0) target vessel revascularization event, and 1 (2 to 1) target lesion revascularization event per 1000 people with use of intravascular imaging guided percutaneous coronary intervention. Furthermore, each additional minute of fluoroscopy with intravascular imaging could potentially prevent around 3 (4 to 2) cardiac deaths, 3 (5 to 0) myocardial infarctions, 2 (3 to 1) stent thrombosis events, 5 (7 to 2) target vessel revascularization events, and 6 (8 to 3) target lesion revascularization events per 1000 people.
Sensitivity analysis
Summary results were largely consistent in trials of complex coronary artery lesions (supplementary figures H-M). On the basis of the SYNTAX risk stratification, high certainty evidence showed that for low to high risk patients, intravascular imaging was likely associated with 23 (29 to 13) fewer to 64 (83 to 38) fewer cardiac deaths, 15 (25 to 2) fewer to 19 (32 to 3) fewer myocardial infarctions, 9 (12 to 4) fewer to 13 (17 to 6) fewer stent thrombosis events, 28 (42 to 12) fewer to 38 (57 to 16) fewer target vessel revascularization events, and 35 (50 to 17) fewer to 48 (67 to 23) fewer target lesion revascularization events 1000 people (table 2).
Using the SYNTAX-II score, high certainty evidence showed that intravascular imaging could lead to 13 fewer (17 fewer to 8 fewer) cardiac deaths, 5 fewer (9 fewer to 1 fewer) myocardial infarctions, 8 fewer (10 fewer to 4 fewer) stent thrombosis events, 16 fewer (23 fewer to 7 fewer) target vessel revascularization events, and16 fewer (23 fewer to 8 fewer) target lesion revascularization events per 1000 people.
Subgroup analyses
Statistical analysis showed no significant interaction effects for various subgroups (including age, type of intravascular imaging used, stent generation, setting, sample size, and follow-up duration), indicating that the relative effects of the intravascular imaging were consistent across these subgroups (supplementary table H).
Discussion
In this meta-analysis of 11 698 patients undergoing percutaneous coronary intervention with drug eluting stents, high certainty evidence suggested that intravascular imaging guided percutaneous coronary intervention was associated with reduced risk of cardiac death and cardiovascular outcomes compared with coronary angiography guided percutaneous coronary intervention. Although the duration of procedural and fluoroscopy time was extended by intravascular imaging guided percutaneous coronary intervention, the benefits of this approach outweighed the potential risks. Finally, the estimated absolute benefits of intravascular imaging guided percutaneous coronary intervention showed a proportional relation with baseline risk, driven by the severity and complexity of coronary artery disease.
Strengths and limitations of study
We focused on the absolute effects of intravascular imaging by adopting a risk based approach and evaluating the certainty of evidence with the GRADE framework, which assists clinicians in devising tailored treatment strategies rather than merely concentrating on the relative effects of the intervention. In addition, we observed a decrease in myocardial infarction, revascularization rates, and stent thrombosis accompanied by increased life expectancy due to cardiac causes. This observation becomes crucial when we consider the results from the RENOVATE-COMPLEX PCI (Guidance vs. Angiography-Guidance on Clinical Outcomes after Complex Percutaneous Coronary Intervention) trial.28 In that trial, intravascular imaging guided percutaneous coronary intervention was associated with reduced cardiac deaths compared with coronary angiography guided percutaneous coronary intervention.28 However, the secondary endpoints were not adjusted for multiple comparisons. Furthermore, specific outcomes such as myocardial infarction, revascularization, and stent thrombosis did not show significant reductions with intravascular imaging guided percutaneous coronary intervention, complicating the interpretation of the noted cardiac survival advantage with this method.28
Increased procedural time, contrast dose, and radiation exposure have been linked to periprocedural complications such as early mortality, emergency coronary artery bypass grafting, cancer, and contrast induced nephropathy.38 Concerns exist about the additional procedural time and potential higher radiation exposure associated with intravascular imaging.39 Although our study could not assess the direct association between radiation dose and outcomes, fluoroscopy time can be considered a convenient proxy for radiation exposure. Our findings indicate that the protective effect against cardiovascular events outweighs the additional fluoroscopy time or procedural duration.
Nevertheless, this meta-analysis has limitations. Firstly, the included trials have varied participant populations, outcome definitions, and follow-up periods. Secondly, we did pre-planned overall and subgroup analyses at a study level rather than an individual patient level. Therefore, we could not assess certain crucial aspects, such as the influence of stent sizing before or after intravascular imaging guided percutaneous coronary intervention, on cardiovascular outcomes. Thirdly, the criteria used for intravascular imaging guidance varied across trials. Fourthly, we did an evaluation of the five year baseline risk for various SYNTAX strata, which may potentially result in an overestimation of baseline risk. This is because the original SYNTAX study used first generation stents without incorporating contemporary antiplatelet regimens. To overcome this concern, we have also provided estimates based on the SYNTAX-II score. This allows clinicians the flexibility to choose either scoring system to estimate potential absolute risk reductions. Fifthly, we assumed similar relative risk reductions with intravascular imaging in the different SYNTAX categories, which may not necessarily be the case. Finally, while focusing on analyzing angiographic lesions through the SYNTAX scoring system, healthcare professionals must not forget to use their clinical expertise when drawing conclusions from our findings.
Comparisons with other studies
Although many meta-analyses have studied intravascular imaging guided percutaneous coronary intervention, a systematic review of 24 meta-analyses showed that only nine focused exclusively on randomized controlled trials.40 Given the potential for observational studies to introduce confounding,41 we focused exclusively on evidence obtained from randomized controlled trials. In addition, we chose cardiac death as our primary endpoint for analysis, which is more specific than the heterogeneous major adverse cardiovascular events endpoint used in previous meta-analyses. Cardiac death provides a more focused measure than all cause death, which is more likely to be influenced by competing risks, potentially diluting the effect of the intervention. Finally, to our knowledge, no previous meta-analyses have investigated the balance between extended procedural time, longer fluoroscopy duration, increased contrast volume, and the potential benefits of intravascular imaging guided percutaneous coronary intervention.8 9 10
Clinical uncertainties
Although the point estimate and the upper bound of the confidence interval hinted at a possible reduction in all cause death with intravascular imaging guided percutaneous coronary intervention, this did not reach statistical significance. The wide confidence intervals for all cause death reflect low event rates. Additionally, with a median follow-up duration of just one year for this study, detecting a statistically significant difference between the two interventions would require more extended follow-up periods and higher event rates.
Policy implications
The implications of this meta-analysis for clinical guidelines and the adoption of imaging guided strategies are multifaceted. The American College of Cardiology, American Heart Association, and Society for Cardiovascular Angiography and Interventions guidelines for coronary revascularization recommend intravascular ultrasonography for procedural guidance, particularly in cases involving left main or complex coronary artery disease (class of recommendation: 2a).1 Similarly, the European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines suggest intravascular ultrasonography or optical coherence tomography for selected patients to optimize stent implantation and intravascular ultrasonography for unprotected left main lesions (class IIa).42 Additionally, the recent European Association of Percutaneous Cardiovascular Interventions consensus statement also recommends the use of imaging guided percutaneous coronary intervention only in select groups of patients with complex lesions (including long lesions, chronic total occlusions, or left main lesions) and patients presenting with acute coronary syndrome, citing less benefit in non-complex lesions or patients with more stable clinical presentation.43 Given the observed benefits of intravascular imaging guided percutaneous coronary intervention, reassessing current guidelines and considering revisions to better reflect the evidence presented in this study are imperative.44
Despite its established benefits, the use of intravascular imaging to optimize percutaneous coronary intervention remains low in the US. A nationwide US study showed that use of intravascular imaging was below 5% between 2004 and 2014.45 A large state registry in Michigan showed that only 5.6% of all percutaneous coronary interventions in Medicare patients were done with intravascular ultrasonography.46 The results of this meta-analysis should encourage wider adoption of intravascular imaging guided percutaneous coronary intervention in clinical practice. A more systematic application of intravascular imaging as a complement to angiography would be advisable, especially for left main or proximal left anterior descending lesions, in-stent restenosis, stent thrombosis, chronic total occlusions, calcified coronary arteries, or any other situation in which angiography does not adequately show the coronary anatomy. Finally, as intravascular imaging guided percutaneous coronary intervention becomes more widespread, evaluating its cost effectiveness is crucial. A health economic assessment showed that considering 5% annual discounting, intravascular ultrasonography correlated with an increased lifetime expense of $597 per person.47 This additional cost, however, was offset by a gain of 0.04 life years and 0.05 quality adjusted life years compared with angiography, resulting in an incremental cost effectiveness ratio of $12 730 per quality adjusted life year.47 Additionally, exploratory analyses suggested that intravascular ultrasonography had greater cost effectiveness among patients with left main and complex coronary lesions, indicating its potential for both improved health outcomes and efficient resource use in these subgroups.47
Conclusion
This meta-analysis showed that intravascular imaging guided percutaneous coronary intervention was associated with reduced risk of cardiac death, driven by cardiovascular outcomes for patients undergoing percutaneous coronary intervention with drug eluting stents. The results were consistent across different imaging types and patient populations. The most significant benefits of intravascular imaging guided percutaneous coronary intervention were observed in patients with the most severe and complex coronary artery disease. Finally, the additional time invested in the procedure is outweighed by the positive outcomes and advantages associated with this approach.
What is already known on this topic
Randomized controlled trials have illustrated the potential benefits of intravascular imaging guided percutaneous coronary intervention (PCI)
Notably, lower rates of target vessel failure and stent thrombosis have been reported compared with angiography guided PCI
However, most trials were not adequately powered to evaluate individual cardiovascular endpoints, such as cardiac death or myocardial infarction
What this study adds
This meta-analysis of 20 randomized controlled trials showed that intravascular imaging guided PCI was associated with reduced risk of cardiac death and cardiovascular outcomes
These benefits were consistently observed across disease complexity, and imaging modalities
The greatest absolute benefits were observed in patients with the highest baseline risk, indicated by the severity and complexity of coronary artery disease
Web extra.
Extra material supplied by authors
Web appendix: Supplementary methods, tables, and figures
Contributors: SUK and SA are joint first authors. SUK conceived the study. SUK and SA designed search strategy, did the literature search, and screened studies for eligibility. SUK and UAA assessed the risk of bias and extracted data. SUK and HBA evaluated the certainty of evidence. SUK, SA, HBA, UAA, MAM, SA, UB, SSG, NSK, and ARS interpreted the data analysis. SUK wrote the first draft of the manuscript, and all other authors revised it. SUK and SA are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This review did not receive any funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: no support from any organization for the submitted work; NSK has received grants or contracts from Boston Scientific for work as a clinical trial investigator; SSG has received consulting fees from Medtronic, JC Medical, and WL Gore Associates and honorariums from Abbott Structural Heart; MAM has received institutional grants from Abbott Vascular and Terumo and honorariums from Terumo, Amgen, and Abbott Vascular Biosensors; UB has received honorariums from Boston Scientific and Abbott; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (the manuscript’s guarantors) affirm that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained
Dissemination to participants and related patient and public communities: We intend to engage the public in disseminating our results, including social media engagement, newsletters, and conferences.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
The statistical code and dataset are available from the corresponding author.
References
- 1. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e4-17. 10.1161/CIR.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 2. Hong SJ, Mintz GS, Ahn CM, et al. IVUS-XPL Investigators . Effect of Intravascular Ultrasound-Guided Drug-Eluting Stent Implantation: 5-Year Follow-Up of the IVUS-XPL Randomized Trial. JACC Cardiovasc Interv 2020;13:62-71. 10.1016/j.jcin.2019.09.033 [DOI] [PubMed] [Google Scholar]
- 3. Holm NR, Andreasen LN, Neghabat O, et al. OCTOBER Trial Group . OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions. N Engl J Med 2023;389:1477-87. 10.1056/NEJMoa2307770 [DOI] [PubMed] [Google Scholar]
- 4. Ali ZA, Landmesser U, Maehara A, et al. ILUMIEN IV Investigators . Optical Coherence Tomography-Guided versus Angiography-Guided PCI. N Engl J Med 2023;389:1466-76. 10.1056/NEJMoa2305861 [DOI] [PubMed] [Google Scholar]
- 5. Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629-38. 10.1016/S0140-6736(13)60141-5 [DOI] [PubMed] [Google Scholar]
- 6. van Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review Group . Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976) 2003;28:1290-9. 10.1097/01.BRS.0000065484.95996.AF [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buccheri S, Franchina G, Romano S, et al. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention With Stent Implantation: A Systematic Review and Bayesian Network Meta-Analysis of 31 Studies and 17,882 Patients. JACC Cardiovasc Interv 2017;10:2488-98. 10.1016/j.jcin.2017.08.051 [DOI] [PubMed] [Google Scholar]
- 9. Ahn JM, Kang S-J, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol 2014;113:1338-47. 10.1016/j.amjcard.2013.12.043 [DOI] [PubMed] [Google Scholar]
- 10. Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular Ultrasound Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2020;9:e013678. 10.1161/JAHA.119.013678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niu Y, Bai N, Ma Y, Zhong PY, Shang YS, Wang ZL. Efficacy of intravascular imaging-guided drug-eluting stent implantation: a systematic review and meta-analysis of randomized clinical trials. BMC Cardiovasc Disord 2022;22:327. 10.1186/s12872-022-02772-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jakabčin J, Špaček R, Bystroň M, et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv 2010;75:578-83. 10.1002/ccd.22244 [DOI] [PubMed] [Google Scholar]
- 13. Kim J-S, Kang T-S, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv 2013;6:369-76. 10.1016/j.jcin.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 14. Chieffo A, Latib A, Caussin C, et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J 2013;165:65-72. 10.1016/j.ahj.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 15. Wang H-X, Dong P-S, Li Z-J, Wang HL, Wang K, Liu XY. Application of Intravascular Ultrasound in the Emergency Diagnosis and Treatment of Patients with ST-Segment Elevation Myocardial Infarction. Echocardiography 2015;32:1003-8. 10.1111/echo.12794 [DOI] [PubMed] [Google Scholar]
- 16. Mariani J, Jr, Guedes C, Soares P, et al. Intravascular ultrasound guidance to minimize the use of iodine contrast in percutaneous coronary intervention: the MOZART (Minimizing cOntrast utiliZation With IVUS Guidance in coRonary angioplasTy) randomized controlled trial. JACC Cardiovasc Interv 2014;7:1287-93. 10.1016/j.jcin.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian N-L, Gami S-K, Ye F, et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention 2015;10:1409-17. 10.4244/EIJV10I12A245 [DOI] [PubMed] [Google Scholar]
- 18. Kim BK, Shin DH, Hong MK, et al. CTO-IVUS Study Investigators . Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ Cardiovasc Interv 2015;8:e002592. 10.1161/CIRCINTERVENTIONS.115.002592 [DOI] [PubMed] [Google Scholar]
- 19. Tan Q, Wang Q, Liu D, Zhang S, Zhang Y, Li Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med J 2015;36:549-53. 10.15537/smj.2015.5.11251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu XM, Yang ZM, Liu XK, et al. Intravascular ultrasound-guided drug-eluting stent implantation for patients with unprotected left main coronary artery lesions: A single-center randomized trial. Anatol J Cardiol 2019;21:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao XF, Ge Z, Kong XQ, et al. ULTIMATE Investigators . 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc Interv 2021;14:247-57. 10.1016/j.jcin.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 22. Antonsen L, Thayssen P, Maehara A, et al. Optical Coherence Tomography Guided Percutaneous Coronary Intervention With Nobori Stent Implantation in Patients With Non-ST-Segment-Elevation Myocardial Infarction (OCTACS) Trial: Difference in Strut Coverage and Dynamic Malapposition Patterns at 6 Months. Circ Cardiovasc Interv 2015;8:e002446. 10.1161/CIRCINTERVENTIONS.114.002446 [DOI] [PubMed] [Google Scholar]
- 23. Meneveau N, Souteyrand G, Motreff P, et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation 2016;134:906-17. 10.1161/CIRCULATIONAHA.116.024393 [DOI] [PubMed] [Google Scholar]
- 24. Kala P, Cervinka P, Jakl M, et al. OCT guidance during stent implantation in primary PCI: A randomized multicenter study with nine months of optical coherence tomography follow-up. Int J Cardiol 2018;250:98-103. 10.1016/j.ijcard.2017.10.059 [DOI] [PubMed] [Google Scholar]
- 25. Onuma Y, Kogame N, Sotomi Y, et al. OPTIMUM Investigators . A Randomized Trial Evaluating Online 3-Dimensional Optical Frequency Domain Imaging-Guided Percutaneous Coronary Intervention in Bifurcation Lesions. Circ Cardiovasc Interv 2020;13:e009183. 10.1161/CIRCINTERVENTIONS.120.009183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chamié D, Costa JR, Jr, Damiani LP, et al. Optical Coherence Tomography Versus Intravascular Ultrasound and Angiography to Guide Percutaneous Coronary Interventions: The iSIGHT Randomized Trial. Circ Cardiovasc Interv 2021;14:e009452. 10.1161/CIRCINTERVENTIONS.120.009452 [DOI] [PubMed] [Google Scholar]
- 27. Ali ZA, Karimi Galougahi K, Maehara A, et al. Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: one-year results from the ILUMIEN III: OPTIMIZE PCI trial. EuroIntervention 2021;16:1085-91. 10.4244/EIJ-D-20-00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JM, Choi KH, Song YB, et al. RENOVATE-COMPLEX-PCI Investigators . Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N Engl J Med 2023;388:1668-79. 10.1056/NEJMoa2216607 [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vandenbroucke JP, Pearce N. Incidence rates in dynamic populations. Int J Epidemiol 2012;41:1472-9. 10.1093/ije/dys142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banning AP, Serruys P, De Maria GL, et al. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. Eur Heart J 2022;43:1307-16. 10.1093/eurheartj/ehab703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmid CH, Lau J, McIntosh MW, Cappelleri JC. An empirical study of the effect of the control rate as a predictor of treatment efficacy in meta-analysis of clinical trials. Stat Med 1998;17:1923-42. [DOI] [PubMed] [Google Scholar]
- 33. Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 2002;21:1575-600. 10.1002/sim.1188 [DOI] [PubMed] [Google Scholar]
- 34. Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the ‘number needed to treat’? An empirical study of summary effect measures in meta-analyses. Int J Epidemiol 2002;31:72-6. 10.1093/ije/31.1.72 [DOI] [PubMed] [Google Scholar]
- 35. McAlister FA. Commentary: relative treatment effects are consistent across the spectrum of underlying risks...usually. Int J Epidemiol 2002;31:76-7. 10.1093/ije/31.1.76 [DOI] [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 37. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818-27. 10.1093/ije/dys041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson LW, Moore RJ, Balter S. Review of radiation safety in the cardiac catheterization laboratory. Cathet Cardiovasc Diagn 1992;25:186-94. 10.1002/ccd.1810250304 [DOI] [PubMed] [Google Scholar]
- 39. Nikolsky E, Pucelikova T, Mehran R, et al. An evaluation of fluoroscopy time and correlation with outcomes after percutaneous coronary intervention. J Invasive Cardiol 2007;19:208-13. [PubMed] [Google Scholar]
- 40. Mintz GS, Bourantas CV, Chamié D. Intravascular Imaging for Percutaneous Coronary Intervention Guidance and Optimization: The Evidence for Improved Patient Outcomes. J Soc Cardiovasc Angiogr Interv 2022;1:100413 10.1016/j.jscai.2022.100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shrier I, Boivin JF, Steele RJ, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol 2007;166:1203-9. 10.1093/aje/kwm189 [DOI] [PubMed] [Google Scholar]
- 42. Neumann F-J, Sousa-Uva M, Ahlsson A, et al. ESC Scientific Document Group . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 43. Räber L, Mintz GS, Koskinas KC, et al. ESC Scientific Document Group . Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2018;39:3281-300. 10.1093/eurheartj/ehy285 [DOI] [PubMed] [Google Scholar]
- 44. Bass TA, Abbott JD, Mahmud E, et al. 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions): A Report of the ACC Competency Management Committee. Circ Cardiovasc Interv 2023;16:e000088. 10.1161/HCV.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 45. Smilowitz NR, Mohananey D, Razzouk L, Weisz G, Slater JN. Impact and trends of intravascular imaging in diagnostic coronary angiography and percutaneous coronary intervention in inpatients in the United States. Catheter Cardiovasc Interv 2018;92:E410-5. 10.1002/ccd.27673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Madder RD, Seth M, Sukul D, et al. Rates of Intracoronary Imaging Optimization in Contemporary Percutaneous Coronary Intervention: A Report From the BMC2 Registry. Circ Cardiovasc Interv 2022;15:e012182. 10.1161/CIRCINTERVENTIONS.122.012182 [DOI] [PubMed] [Google Scholar]
- 47. Zhou J, Liew D, Duffy SJ, et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: A Health Economic Analysis. Circ Cardiovasc Qual Outcomes 2021;14:e006789. 10.1161/CIRCOUTCOMES.120.006789 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary methods, tables, and figures
Data Availability Statement
The statistical code and dataset are available from the corresponding author.