Abstract
Chronic obstructive pulmonary disease (COPD) and periodontal disease are chronic inflammatory conditions that significantly affect an individual’s overall health and well-being. Generally, the prevalence of periodontitis is higher in patients with COPD than those without COPD, which may partly be attributed to common risk factors in COPD, such as smoking, respiratory infections, and inflammation. In particular, periodontitis may exacerbate the progression of COPD and further deteriorate the respiratory system by promoting inflammatory responses and bacterial infections. Immunocytes, including neutrophils, and microorganisms such as Fusobacterium nucleatum originating from oral biofilms are believed to be crucial factors influencing to COPD. Furthermore, the potential benefits of treating periodontal disease in COPD outcomes have been investigated. Although the relationship between COPD and periodontal disease has been preliminarily studied, there is currently a lack of large-scale clinical studies to validate this association. In addition to clinical examinations, investigating biomarkers and microbiology may contribute to explore the underlying mechanisms involved in the management of these conditions. This review aims to contribute to a better understanding of the clinical and basic research aspects of COPD and periodontitis, allowing for potential therapeutic approaches and interdisciplinary management strategies.
Keywords: Periodontal disease, Chronic obstructive pulmonary disease
1. Information
Over the past few decades, increasing attention has been paid to the association between periodontal diseases and systemic conditions [1]. Many reports have indicated that periodontal disease affects systemic diseases, including diabetes [2], [3], cardiovascular diseases [4], [5], and non-alcoholic fatty liver disease [6]. The association between periodontal disease and chronic obstructive pulmonary disease (COPD) has attracted increasing attention.
Periodontal disease is an infectious disease caused by periodontal bacteria that trigger chronic inflammation and destruction of tooth-supporting structures [7], [8]. Periodontitis is one of the most common diseases in the world, as more than 40% of the world’s population suffers from different degrees of periodontitis, and this proportion continues to increase with age [9]. In Japan, at least 49.4% of the population has mild periodontitis [10]. For the clinical diagnosis of periodontitis, probing pocket depth (PPD), clinical attachment level (CAL), and bleeding on probing (BOP) are commonly used indicators [11], oral hygiene index (OHI), plaque index (PI), gingival index (GI), and other oral health examination methods can provide a more comprehensive understanding of a patient’s oral condition [12], [13].
Chronic obstructive pulmonary disease (COPD) is a group of chronic lung diseases characterized by airflow limitation, including chronic bronchitis and emphysema. According to an epidemiological study, this disease is the third leading cause of death worldwide [14]. Although COPD occurs in all regions of the world, its prevalence varies in different regions [15]. For instance, in Japan, approximately 6.8% of people over 40 years have at least one of the following airflow limitations, chronic cough/phlegm and currently treated respiratory diseases [16]. Spirometry has been the most commonly utilized diagnostic test for COPD, which assesses the impairment of forced expiratory volume (FEV1) and airflow obstruction. The gold standard for COPD diagnosis is the value of FEV1/forced vital capacity (FVC) [17].
In the early stages of research, the common risk factors between periodontal disease and COPD were investigated, such as smoking and poor oral hygiene habits, etc. [18], [19], [20]. However, as research progressed, an increasing amount of evidence suggests that periodontal disease may not only be a risk factor for COPD but also directly affect the pathology processes of COPD. Particularly, studies have suggested that bacteria in the periodontal tissues of patients suffering from periodontal disease can enter the lungs via the oral cavity or respiratory tract and potentially cause lung infections, inflammation, and exacerbate symptoms in patients [21], [22], [23]. Furthermore, periodontal disease is chronic oral infections, triggering systemic inflammatory responses associated with the development and progression of COPD [24], [25].
Therefore, this review aimed to systematically summarize and analyze previous research findings to comprehensively understand the relationship between periodontal disease and COPD, as well as to explore their possible mechanisms of association. In addition, we investigated the potential role of periodontal disease in the prevention and treatment of COPD and its significance in clinical practice.
2. Cross-sectional study
Cross-sectional studies are based on the results of investigations and examinations. The following indices were used to assess periodontal disease: the gingival index (GI), PPD, CAL, periodontal disease index (PDI), community periodontal index of treatment needs (CPITN), periodontal index for risk of infection (PIRI), and BOP. Additionally, the oral hygiene index-simplified (OHI-S) and PI were used to evaluate oral hygiene status, while the decayed, missing, and filled teeth (DMFT) and significant caries index (SiC) were used for caries assessment. Furthermore, Candida load [26], [27] and sputum tests [28] were considered. To assess COPD, researchers have utilized various tools, including the FEV1 value [29], FEV1/FVC ratio [30], Gold's criteria [31], spirometer [32], oxygen saturation (SpO2) [30], Modified Medical Research Council (MMRC) [30] and COPD Assessment Test (CAT) questionnaire [30], [31].
2.1. Asia
2.1.1. East Asia
Cross-sectional studies on the relationship between periodontitis and COPD in East Asia have been performed in Japan and China and have shown results of similarities and differences. Studies in both countries have evaluated PI, PPD, BOP, O'Leary's plaque control record (PCR), and alveolar bone level (ABL) as indicators of periodontal disease [33], [34], [35], [36], [37], [38]. For indications of COPD, the 6-minute walk test (6 MWT) and evaluation of %FEV were performed [33], [34], [35], [36], [38]. Studies in both countries have shown an association between periodontal disease and the severity of COPD, with a higher smoking ratio and prevalence of COPD in men than in women [34], [38].
Many Japanese studies have examined the association between periodontal disease and COPD with the aim of predicting the systemic health of patients with COPD by assessing their oral health status. Some studies in Japan have reported that smoking and diabetes are associated with periodontal disease and COPD [34], [36]. To examine inflammation and health status, the Body Mass Index (BMI) and blood parameters, including HbA1c, Fasting Blood Glucose (FBG), and serum albumin, were evaluated [36], [39]. Periodontal disease exacerbates systemic inflammation and COPD [34]. In another study, poor periodontal health was associated with hypoalbuminemia, suggesting poor nutritional status and inflammation in patients with COPD [39]. Furthermore, PPD, HbA1c, and FBG levels were significantly associated with occlusal force in patients with moderate/severe periodontitis. PPD was significantly associated with occlusal force among employees with moderate COPD, and atherosclerotic cardiovascular disease [36].
Studies conducted in China have aimed to prevent COPD by promoting oral health care and knowledge. Many studies have focused on the concerns about their own health status of patients with COPD [33], [34], [35], [40]. Therefore, questionnaires such as the St. George’s Respiratory Questionnaire (SGRQ), which examines the quality of life (QOL) [35], BMI, airflow obstruction, dyspnea, and exercise capacity (BODE) index, indicating the prognosis of COPD, were used. BODE showed significant associations with periodontal parameters, including the bleeding index (BI), PI, CAL, ABL, and number of teeth. In particular, PI was a major periodontal factor for predicting COPD in Chinese adults [34]. Another report showed that few remaining teeth, high plaque index, low tooth brushing times, and low regular supragingival scaling were significantly associated with exacerbations of COPD [33], suggesting improvement of periodontal health and oral hygiene might be a potential preventive strategy against COPD and that promoting dental care and oral health knowledge might help prevention and treatment for COPD. The contents of this section are summarized in Table 1.
Table 1.
Summary of cross-sectional studies in East Asia.
| Ref. No. | Study Country Year Sample Size | Parameters for Evaluated for the Diagnosis of COPD | Parameters for Evaluated for the Diagnosis of Periodontitis | Main Findings |
|---|---|---|---|---|
| [33] | Zhiqiang Liu et al. China 2012 n = 392 | Spirometer FEV1/FVC | PD CEJ BI CAL PLI | Fewer remaining teeth, high PLI scores, and low tooth brushing times are significant correlates of COPD exacerbations, indicating that improving periodontal health and oral hygiene may be a potentially preventive strategy against COPD exacerbations. |
| [34] | Yan Si et al. China 2012 n = 1019 | 6MWT, BODE index | CAL PPD BI PI ABL | A strong association between periodontitis and COPD, and PI seemed to be a major periodontal factor for predicting COPD among Chinese adults. |
| [35] | Xuan Zhou et al. China 2012 n = 306 | respiratory function and Respiratory Questionnaire(SGRQ) | missing teeth number, alveolar bone level, PD CAL BI PLI | Poor periodontal health as reflected by missing teeth and plaque index was significantly associated with lower QOL in COPD patients. Promoting dental care and health knowledge helps patients with COPD to prevent and treat COPD. |
| [36] | Shinsuke Kataoka et al. Japan 2021 n = 1022 | FEV1/FVC ratio occlusal force HbA1c FBG | PPD BOP number of teeth | PPD was significantly associated with occlusal force among employees with moderate COPD, and ASCVD, so that future health issues could be accurately predicted from the results of routine dental examinations. |
| [38] | Jane Harland et al. Japan 2018 n = 1474 | Spirometer. 1 s of forced vital capacity | PPD CPI | The association between smoking and COPD was significant for men with periodontitis but was weaker for those without periodontitis, and periodontitis is linked to systemic inflammation in smokers with COPD. |
| [39] | Takeshi Terashima et al. Japan 2017 n = 136 | serum albumin level, Spirometer | BOP PD PCR BMI | Poor periodontal health was associated with hypoalbuminemia, suggesting poor nutritional status and inflammation in COPD. |
| [40] | Zuomin Wang et al. China 2009 n = 634 | Respiratory function questionnaire | PD CAL BI PLI, number of remaining teeth, alveolar bone level | Poor periodontal health, dental care, and oral health knowledge were significantly associated with an increased risk of COPD and promoting dental care improved QOL with COPD patients. |
2.1.2. Other Asia
Twelve cross-sectional studies from Asian regions other than East Asia were conducted between 2011 and 2021. Among these, ten reports were from India [26], [27], [28], [29], [32], [41], [42], [43], [44], [45], and two were from Iran [30], [31]. While most of these reports have focused on comparing patients with COPD to healthy individuals without COPD, some have compared healthy individuals with a population that includes not only COPD but also other lung diseases, including tuberculosis and pneumonia [27], [29], [42]. Some reports have also examined the effects of the long-term use of drugs for COPD treatment, such as inhaled corticosteroids [27] and theophylline [26] on oral health.
CAL was the primary tool in 10 of the 12 reports, showing significantly higher scores in the COPD group [28], [29], [32], [43], [44], [45]. Furthermore, while CAL exhibits a significantly positive correlation with CAT [27], [31], it showed a significantly negative correlation with FEV1, FEV index [30], and SpO2 [30], and no correlation with MMRC [30] or serum cotinine [41]. The GI index showed a significant elevation in patients with COPD and/or other lung diseases compared to healthy individuals [26], [27], [41], [45]. GI also exhibited a significant negative correlation with FEV1 [45] and SpO2 [30]. Additionally, PPD was significantly greater in patients with COPD than in the control group [29], [44], [45] and displayed a significant negative correlation with FEV1 [30]. In contrast, PDI was significantly lower in the study group than in the control group. Although the study population was relatively young (20 – 45 years), inhaled corticosteroids might have limited periodontal disease progression [43].
Furthermore, the Candida load was significantly higher in patients with COPD than in controls [26], [43], particularly in those taking theophylline [26]. Meanwhile, the OHI-S score was significantly higher in the COPD group than in the control group [29], [32], [43], [44], [45]. Serum and salivary CRP levels showed significant positive correlations and were significantly higher in COPD patients than in healthy control subjects [44]. The contents of this section are summarized in Table 2.
Table 2.
Summary of cross-sectional studies in Other Asia.
| Ref. No. | Study Country Year Sample Size | Parameters for Evaluated for the Diagnosis of COPD | Parameters for Evaluated for the Diagnosis of Periodontitis | Main Findings |
|---|---|---|---|---|
| [26] | Khijmatgar et al. India 2021 n = 212 | clinical diagnosis | DMFT, SiC, CPITN, and OHI-S. Candida | The prevalence of Candida was notably elevated in the COPD patient cohort; within the realm of COPD patients, those under theophylline treatment exhibited a higher incidence of Candida colonization. |
| [27] | Parashar et al. India 2018 n = 98 | clinical diagnosis | PI, GI, AL, PPD, CPI | The results pertaining to the PI, and GI exhibited a marked elevation among patients in the test diseases group when compared to the control group. |
| [28] | Vadiraj et al. India 2013 n = 100 | Sputum | CAL, BOP | Improve oral health status may prove to lower the severity of lung infection in susceptible populations. |
| [29] | Sharma et al. India 2011 n = 200 | SES | PI, OHI, GI, PPD, CAL | Patients with respiratory conditions exhibited inferior OHI and PI scores, along with notably elevated GI, PPD, and CAL values compared to the control group. |
| [30] | Moeintaghavi et al. Iran 2018 n = 50 | FEV1/FVC ratio, CAT, MMRC, SpO2 | PPD GI PI AL | The relationship between periodontal variables and respiratory indices in the course of COPD, early treatment of periodontal diseases, might considerably reduce the severity of COPD. |
| [31] | Javaheri et al. Iran 2020 n = 71 | CAT | PPD, BOP, AL | Periodontal problems are positively associated with COPD severity as determined by GOLD criteria and negatively associated with quality of life of patients with COPD. |
| [32] | Bomble et al. India 2020 n = 117 | Spirometer | PPD, CAL, OHI | The mean scores for PPD, CAL, and OHI were higher in the COPD group compared to the non-COPD group; furthermore, the COPD group exhibited a higher proportion of smokers in comparison to the non-COPD group. |
| [41] | Kedlaya et al. India 2021 n = 80 | Serum cotinine level, smoking history | GI, CAL | Increased smoking with COPD causes a higher chance of progression of periodontal destruction but it is not statistically significant. |
| [42] | Rastogi et al. India 2019 n = 700 | clinical diagnosis | PDI, PIRI | A survey of patients with various pulmonary diseases (TB, COPD, pneumonia) led to the diagnosis of periodontitis in the majority of the study cohort, with a significant proportion being classified in the high-risk category by their PIRI scores. |
| [43] | Raj et al. India 2018 n = 340 | clinical diagnosis | DMFT, OHI, PDI, Candida | The need for regular oral health maintenance for those under COPD treatment and for greater research into the possible protective role of inhaled corticosteroids in limiting periodontal disease among patients. |
| [44] | Bhavsar et al. India 2015 n = 200 | clinical diagnosis | PI, OHI, PPD, CALs CRP level | The frequency of brushing was notably reduced in COPD patients as compared to the control group. Additionally, the GI, PI, OHI-S, PPD, and CAL exhibited elevated levels in the COPD group. |
| [45] | Peter et al. India 2013 n = 501 | FEV1 | PI, OHI, GI, PPD CAL, | A marked elevation in CAL, PD, and OHI was observed in the patients with respiratory in comparison to the control group, revealing a trend towards augmented severity of pulmonary obstruction as these periodontal parameters worsen. |
2.2. Out of Asia
Nine studies have been reported in the United States of America and Europe describing the association between COPD and indicators of periodontal disease. Among these studies, two were conducted in the USA. Particularly, a study was conducted on 58 individuals to assess the morbidity of pulmonary pathogens colonized by dental plaques. The COPD incidence was significantly higher in subjects with pathogen colonization than in non-colonized subjects in chronic care facilities [46]. The other study evaluated the association between COPD exacerbation and periodontitis in 136 patients. However, no statistically significant differences were observed between COPD exacerbation and non-exacerbation cases for any periodontitis examination parameters, and the odds ratios for periodontal examination measurements were not significantly different between the two groups [47].
In Norway, Leuckfeld et al. evaluated the association between severe COPD and periodontitis using orthopantomograms of 180 adults. They found that chronic periodontitis with a mean marginal bone level of ≥ 4 mm was significantly associated with patients with a prevalence of COPD, suggesting that periodontitis may be an independent risk factor for COPD with considering age and smoking [48]. Another association between periodontal pockets and COPD was observed in 3360 Greek outpatients. In the study, an increase in clinical attachment loss ≥ 6 mm was positively related to COPD (OR = 1.054, 95% CI = 0.244 – 1.523) after adjusting for sex, smoking, and CAL [49]. The significant association between CAL and predicted FEV1 in 1380 adults was also observed after adjusting for other confounders [50]. Moreover, patients with COPD have significantly worse periodontitis indices than healthy individuals, except for gingival inflammation. Multiple logistic regression analysis adjusting for other risk factor variables showed a significantly higher odds ratio (OR) in the COPD group for periodontitis with CAL ≥ 4 mm at ≥ 60% of sites (OR: 3.2, 95% CI: 1.0 – 9.8) [51]. Similarly, a Spanish research group reported that the prevalence of periodontitis in patients with COPD was higher compared to those without COPD (adjusted OR: 1.21, 95% CI: 1.12 – 1.30) [52]. Conversely, some studies have reported little direct association between COPD and periodontitis. An epidemiological study of 80 individuals divided into smokers with COPD, smokers without COPD, and healthy nonsmoking groups reported that periodontitis was strongly correlated with smoking, and no association was found between the risk of developing COPD and oral health problems, including periodontitis [53]. Furthermore, according to a German study of 206 adults, periodontitis was significantly correlated with age and smoking but not with pulmonary function [54].
Based on the above, although many epidemiological studies have reported a relationship between COPD and periodontitis, a variety of confounding factors involving both diseases should also be considered. The contents of this section are summarized in Table 3.
Table 3.
Summary of cross-sectional studies in other countries.
| Ref. No. | Study Country Year Sample Size | Parameters for Evaluated for the Diagnosis of COPD | Parameters for Evaluated for the Diagnosis of Periodontitis | Main Findings |
|---|---|---|---|---|
| [46] | Russel et al. USA 1999 n = 58 | Clinical diagnosis | Dental plaque | Pulmonary pathogens formed from oral plaque were associated with COPD morbidity. |
| [47] | Baldomero et al. USA 2019 n = 136 | SGRQ | PD, BOP, CAL, PI, GI | Periodontitis index was not associated with COPD exacerbations. |
| [48] | Leuckfeld et al. Norway 2008 n = 180 | Clinical diagnosis | Marginal bone level | Chronic periodontitis defined as mean marginal bone level ≥ 4 mm was significantly associated with severe COPD. |
| [49] | Chrysanthakopoulos et al. Greece 2016 n = 3360 | Self-administered questionnaire | PPD, CAL | Clinical attachment loss was significantly associated with COPD. |
| [50] | Winning et al. Northern Ireland 2019 n = 1380 | Spirometry | PPD, CAL | Chronic periodontitis was significantly associated with decreased respiratory function, after adjusting for other confounders. |
| [51] | Ledić et al. Croatia 2013 n = 136 | Spirometry | PI, PBI, RE, PPD, CAL | An association with severity of periodontitis and COPD. (OR 3.2, 95% CI 1.0–9.8) |
| [52] | MLopez-de-Andrés et al. Spain 2018 n = 51142 | Self-reported questionnaire | The prevalence of periodontitis in COPD patients was higher compared to that without COPD subjects. | |
| [53] | Bergström et al. Sweden 2013 n = 80 | Spirometry, Pulmonary X - ray, computed Tomography, and SGRQ | Remaining teeth, PPD, BOP, RE, Dental plaque | Periodontitis is strongly associated with smoking, but not with the development of COPD in smokers. |
| [54] | Henke et al. Germany 2016 n = 206 | Spirometry | PPD, Alveolar bone loss | No association was observed between periodontitis and pulmonary function, and periodontitis was associated with age and smoking habits. |
3. Cross-sectional study with biomarkers
In addition to clinical examinations, laboratory testing may offer new perspectives for assessing the association between periodontitis and COPD, as inflammatory biomarkers are another major research focus. In a Swedish study, the saliva was found to be the most suitable for the assessment of COPD biomarkers compared to serum-induced sputum and bronchoalveolar lavage fluid [55]. A study in Turkey showed that serum high-sensitive C-reactive protein (hs-CRP) levels and gingival crevicular fluid (GCF) levels of hs-CRP, interleukin-1 (IL-1b), and prostaglandin-E2 (PGE2) were significantly higher in patients with COPD compared to the controls [56]. The salivary and serum levels of matrix metalloproteinases (MMP)− 8 and − 13, and matrix metalloproteinase-1 (TIMP-1) in saliva and serum were not significantly different between the 36 participants with mild COPD and 20 non-COPD Turkish individuals through an enzyme-linked immunosorbent assay; however, serum levels of MMP-8 and MMP-8/TIMP-1 by immunofluorometric assay showed significant differences (p < 0.005) between the two groups. These findings indicated that the immunodetection of MMP-8 is dependent on the selected technique [57]. Alpha-1 antitrypsin deficiency (AATD) is a rare disease and the only robust genetic risk factor for COPD. The study assessed the severity of periodontitis indicated that COPD and AATD patients exhibited a high prevalence of periodontitis (COPD: 95%; AATD: 88%), whereas neutrophil migratory accuracy was reduced in patients with stage II–IV periodontitis with COPD or AATD [58]. Additionally, periodontitis severity was associated with lung disease severity (AATD, periodontitis vs. no periodontitis; FEV1 = 56% vs. 99% predicted; transfer factor for carbon monoxide = 59% vs. 81% predicted; p < .0001 for both) [59].
A study about the toll-like receptor 4 (TLR4) genotype showed that CP patients carrying the AG polymorphism in TLR4 rs1927907 were more susceptible to concomitant COPD than those carrying the GG genotype (p = 0.005, OR = 1.94, 95% CI: 1.22 – 3.03) after adjusting for age, sex, smoking status, and oral hygiene habits [60]. Transcriptomic analysis of chronic periodontitis (CP, GSE156993) and COPD (GSE42057, GSE94916) datasets indicated that EPB41L4A-AS1, INSR, and R3HDM1 were potential crosstalk genes between COPD and periodontitis that correlated with different infiltrating immune cells. INSR was positively correlated with hepatocytes in CP (r = 0.6714, p = 0.01679) and COPD (r = 0.5209, p < 0.001). R3HDM was positively correlated with Th1 cells in CP (r = 0.6783, p = 0.0153) and COPD (r = 0.4120, p < 0.01) [61].
Furthermore, salivary sialic acid levels were significantly higher in patients with COPD and periodontitis than in those with only periodontitis in a study that included 90 Indian participants [62]. Another case-control study from China suggested that lower serum 25-hydroxyvitamin D (25(OH)D) concentrations were significantly associated with poor periodontal health and an increased risk of COPD [63]. Corticosteroids are widely used to treat COPD and asthma [64]. Although there is no evidence to show that corticosteroid intake affected periodontal health, significantly lower levels of osteocalcin (p < 0.0001), calcium (p = 0.004), and cortisol (p = 0.03) were observed in 30 Turkish patients who were treated with corticosteroids for > 1 year [65].
Specific biomarkers and immune cells present in saliva and serum are useful in explaining the relationship between the two diseases. However, the accuracy of laboratory testing may be affected by changes in testing methods, and further research is required. The contents of this section are summarized in Table 4.
Table 4.
Summary of cross-sectional studies with biomarkers.
| Ref. No. | Study Country Year Sample Size | Samples | Methods | Main Finds |
|---|---|---|---|---|
| [55] | Ji et al. Sweden 2014 n = 80 | Saliva, induced sputum, bronchoalveolar lavage fluid and serum | ELISA, flow cytometry and RT-PCR | The mRNA and protein expression of TNF-α in smoking patients were lower than those in non-smokers. Negative correlations between lung function and saliva IL-8 and matrix metalloproteinase-9 (MMP-9) were found in smokers with COPD. |
| [56] | Öztekin et al. Turkey 2014 n = 90 | Gingival crevicular fluid and serum | ELISA and latex-enhanced immuneturbidimetric assay | COPD may be associated with periodontal disease as manifested by lower number of teeth and higher levels of inflammatory mediators especially CRP in GCF. |
| [57] | Yıldırım et al. Turkey 2013 n = 56 | Saliva and serum | Immunofluorometric assay (IFMA) and ELISA | Higher MMP-8 and MMP-8/TIMP-1 levels were detected by IFMA in COPD patients, but ELISA did not show a difference. |
| [59] | Sapey et al. United Kingdom 2020 n = 156 | Saliva and serum | ELISA and neutrophil migration | Moderate-to-severe periodontitis was associated with elevated salivary inflammatory markers and reduced accuracy of systemic neutrophil chemotaxis towards CXCL8. |
| [60] | Yu et al. China 2017 n = 712 | Serum | Real-time quantitative PCR | CP patients with TLR4 gene rs1927907 polymorphism may be more susceptible to COPD. |
| [61] | Liu et al. China 2022 n = 160 | Peripheral blood mononuclear cells | Transcriptomic analysis | EPB41L4A-AS1, INSR and R3HDM1 are potential crosstalk genes between COPD and periodontitis. |
| [62] | Rathod et al. India 2018 n = 90 | Saliva | Combined modification of the thiobarbituric acid method of Skoza and Mohos. | The mean salivary sialic acid levels were least in the healthy group followed by the periodontitis group, and it was highest in the COPD group. |
| [63] | Zhou et al. China 2012 n = 374 | Serum | ELISA | Lower serum 25(OH)D concentrations were significantly associated with poor periodontal health and an increased risk of COPD. |
| [65] | Komerik et al. Turkey 2018 n = 60 | Serum | Immulite immunometric assay and immulite chemiluminescence immunoassay | Significantly lower levels of osteocalcin, calcium and cortisol were observed in the patients on corticosteroid treatment. |
4. Cross-sectional study with pathogens
The lung microbiome was shown to be important in the etiology and progression of chronic respiratory diseases [66]. The oropharynx–lung continuum represents a potential gateway for bacterial exchange because of its close anatomical proximity, and research has shown that subclinical aspiration of oropharyngeal contents occurs universally in humans [67], [68]. A significant negative correlation was found between the abundance of Porphyromonas gingivalis and FEV1% in patients with COPD in a cross-sectional study of 80 COPD and 80 non-COPD participants in China. Additionally, an increased prevalence of P. gingivalis, Klebsiella pneumonia, Pseudomonas aeruginosa, and Streptococcus pneumonia was observed in the subgingival plaques of patients with COPD in another study [69]. However, in another case-control study with 120 participants from China, the average levels of P. gingivalis, Fusobacterium nucleatum, Treponema denticola, and Haemophilus influenzae in the subgingival plaques of patients with both COPD and periodontitis tended to be higher than those in participants with only periodontitis, but the differences were not statistically significant [70]. Owing to the limitations of these studies, the role of periodontal pathogens in the etiology and progression of COPD remains unclear.
Furthermore, a subgingival and pulmonary isolate from one patient with COPD was identified as a genetically identical Veillonella parvula strain [71]. Several studies have reported that the microbiome of the lungs more closely resembles that of the oropharynx rather than that of the nasopharynx or the lower gastrointestinal tract in healthy individuals [72], [73], [74]. Moffatt and Cookson described that the healthy lung microbiome was characterized by the presence of Actinobacteria, Bacteriodetes, Firmicutes, Fusobacteria, and Proteobacteria at the family level, whereas Prevotella, Streptococcus, and Veillonella were characterized at the species level according to studies using 16 S rRNA gene sequencing [75].
Alterations in bacterial diversity or abundance in the lung microbiome have been associated with several chronic respiratory diseases, including COPD, asthma, and bronchiectasis [76]. Disorders in the lung microbiome and abnormal inflammatory reactions are the two leading causes of acute COPD exacerbation [77]. While a significant feature of the lung microbiome in lower airway diseases was the reduced abundance of phylum Bacteroidetes, the abundance of the class Gammaproteobacteri, which contains many common lung-associated gram-negative pathogens, was increased in the lung microbiome of patients with lower airway disease [73].. Periodontitis is a major condition that disturbs the oral microbiome [78]. The exacerbation of periodontitis and increased diversity of microbiota lead to the deterioration of respiratory function [79]. Wu et al. compared the bacterial composition of subgingival plaques among COPD patients with periodontitis, COPD patients without periodontitis, periodontitis patients without COPD, and healthy individuals using 16 S rRNA gene sequencing. They reported an increase in Dysgonomonas, Desulfobulbus, and Catonella at the genus level and in Porphyromonas endodontalis, Dysgonomonas wimpennyi, Catonella morbi, and Prevotella intermedia at the species level in the COPD with periodontitis group, suggesting that an increase in the periodontitis-associated microbiota may be related to COPD. Additionally, the decrease in the genera Arcanobacterium, Oribacterium, and Streptomyces in the COPD group indicated that these three genera might be health-associated, suggesting that the decrease in these genera might exacerbate COPD [80]. Similar to the study by Wu et al. [80], Liu et al. [81] compared the microbiota of subgingival plaque and gingival crevicular fluid samples among four groups using 16 S rRNA gene sequencing. Using gingival crevicular fluid samples, the abundance of the genera Mogibacterium was increased in a COPD group without periodontitis. On the other hand, the abundance of the genera Phocaeicola and Schwartzia was higher in the COPD with periodontitis group. Based on the predicted functional analysis using gingival crevicular fluid samples, the COPD without periodontitis group showed significantly enriched protein families, such as genetic information processing, translation, replication and repair, metabolism, and glycan biosynthesis and metabolism. The COPD with periodontitis group showed significantly enriched protein families, such as genetic information processing, metabolism, translation, glycan biosynthesis and metabolism, and metabolism of cofactors and vitamins. Interestingly, subgingival plaque has the potential to reflect the differences in subgingival microbiota in patients with COPD compared to gingival crevicular fluid [81]. Tan et al. reported the homology of bacterial species between the oral cavity and lungs from the same patient with acute exacerbations of COPD using homology analysis utilizing 16 S rRNA gene sequencing. Bacteria that showed homology between the oral cavity and lungs were dental plaque pathogens such as Aggregatibacter actinomycetemcomitans, Capnocytophaga sputigena, Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola and lung pathogens such as Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Streptococcus pneumoniae [82].
These data support the hypothesis that COPD is correlated with periodontitis via these significantly altered specific bacteria, and treatment of periodontitis may reduce the exacerbation frequency of COPD. However, further studies are required to determine the role of periodontopathic bacteria in COPD. The contents of this section are summarized in Table 5.
Table 5.
Summary of the effect of periodontitis pathogens in COPD using 16 S rRNA gene sequencing.
| Ref. No. | Study Country Year Sample Size | Type | Methods | Main Finds |
|---|---|---|---|---|
| [69] | Tan et al. China 2019 n = 160 | cross-sectional study | Real-time polymerase chain reaction | Porphyromonas gingivalis, Klebsiella pneumonia, Pseudomonas aeruginosa, and Streptococcus pneumonia prevalence was increased in participants with COPD compared with control group. A significant negative association was noted between the relative content of Pg and forced expiratory volume in one second in participants with COPD. |
| [70] | Zhou et al. China 2020 n = 120 | cross-sectional study | Real-time polymerase chain reaction | The average levels of Porphyromonas gingivalis, Fusobacterium nucleatum, Treponema denticola, and Haemophilus influenzae in subgingival plaque of patients with both COPD and periodontitis were found to tend to be higher than that in the participants with only periodontitis. |
| [79] | Winning et al. Northern Ireland 2023 n = 507 | cross-sectional study | 16 S rRNA gene sequencing | Subgingival microbial diversity was associated with reduced respiratory function. |
| [80] | Wu et al. China 2017 n = 105 | cross-sectional study | 16 S rRNA gene sequencing | An increase in the genera Dysgonomonas, Desulfobulbus, and Catonella and in four species (Porphyromonas endodontalis, Dysgonomonas wimpennyi, Catonella morbi, and Prevotella intermedia) in both COPD with periodontitis patients suggests that an increase in these periodontitis-associated microbiota may be related to COPD. |
| [81] | Liu et al. China 2023 n = 112 | cross-sectional study | 16 S rRNA gene sequencing | Significant differences in the bacterial community and functional characterization of oral microbiota in COPD without periodontitis, COPD with periodontitis group. Compared to gingival crevicular fluid, subgingival plaque may be more appropriate for reflecting the difference of subgingival microbiota in periodontitis patients with COPD. |
| [82] | Tan et al. China 2014 n = 53 | cross-sectional study | 16 S rRNA gene sequencing | Dental bacteria may contribute to the pathology of acute exacerbation of COPD. |
| [110] | Sundh et al. Sweden 2021 n = 101 | longitudinal study | 16 S rRNA gene sequencing | Dental cleaning treatment is associated with a reduced frequency of COPD exacerbations. |
5. Retrospective study
A retrospective study of the association between periodontal disease and COPD was conducted in the early 2000 s. Some studies reported in the United States were based on the analysis of the National Health and Nutrition Examination Survey Ⅲ (NHANES Ⅲ), which was conducted by the National Center for Health Statistics and documented the general health and nutritional status of individuals randomly selected from sampling areas that encompassed the continental United States from 1988 to 1994 [83], [84]. Scannapieco et al. reported that participants with a history of COPD had greater periodontal attachment loss than those without a history of COPD. Especially, patients with mean attachment loss (MAL) ≥ 3.0 mm had a higher risk of COPD than those with MAL < 3.0 mm (OR: 1.45, 95% CI: 1.02 – 2.05) [83]. Hyman et al. reported a relationship between smoking, periodontal disease, and COPD [84]. They reported no significant relationship between periodontal disease and COPD in former smokers and nonsmokers. However, current smokers with ≥ 4 mm MAL had an OR of 3.71 (95% CI: 1.74 – 7.89). These results suggest that cigarette smoking may be a cofactor in the association between periodontal disease and COPD. Another study conducted in 2005 included a subset of 860 well-functioning elderly individuals [85]. This study could not provide direct inference of cause and effect, it reveals a significant association between periodontal disease and airway obstruction, an early stage of compromised pulmonary function. Chen et al. conducted research based on the latest data from the 2009–2012 NHANES in the United States, and included a total of 6313 participants aged 30 years or older [86]. Compared to those without periodontitis, the multivariate-adjusted OR of airflow obstruction for moderate and severe periodontitis were 1.38 (95% CI: 1.01 – 1.75) and 1.47 (95% CI: 1.06 – 2.01). This study suggests that moderate-to-severe periodontitis may be associated with a decline in lung function in the United States.
In Sweden, a retrospective study was conducted on older Caucasian dentate individuals from Karlskrona between the ages of 60 and 93 years, and it revealed that periodontitis was independently associated with airflow limitation. This relationship was independent of known confounders, such as smoking, BMI, exercise, systemic diseases, educational level, and living conditions [87].
In China, more than 1385 permanent residents aged over 75 years who were referred for periodontal treatment at the Shanghai Ninth People’s Hospital between 2010 and 2014 were analyzed [88]. The study showed that mortality from respiratory diseases was significantly associated with periodontal disease. After adjusting for relevant confounding factors, the hazard ratio (HR) for patients with periodontitis was 2.72 (95% CI: 1.04 – 7.11). However, the number of teeth lost was not significantly associated with total respiratory disease or mortality due to COPD. Three studies were conducted in South Korea based on the Sixth Korean National Health and Nutrition Examination Survey (KNHANES). Chung et al. revealed that periodontitis (CPI 3 or 4) was associated in males with COPD after adjusting for confounding factors (CPI 3: relative risk [RR] = 1.38, 95% CI: 1.12 – 2.05; CPI 4: RR = 1.23, 95% CI: 1.06 – 1.56) [89]. Lee et al. conducted a study based on the Sixth KNHANES 2014 [90]. The assessment of reduced pulmonary function data was classified as “normal,” “restrictive impairment,” or “obstructive impairment.” For pulmonary function assessments, obstructive pulmonary impairment, but not restrictive pulmonary impairment, was a significant predictor of periodontitis based on the univariate analysis (unadjusted OR = 1.729, 95% CI: 1.344 – 2.233). However, the associations between periodontitis and restrictive pulmonary impairment (adjusted OR = 1.059, 95% CI: 0.829 – 1.540) or obstructive pulmonary impairment (adjusted OR = 1.140, 95% CI: 0.849 – 1.530) were not statistically significant. Likewise, Jung et al. also used data from the Sixth KNHANES 2013 – 2015 (N = 7719) [91]. This report revealed that poor periodontal status exhibited a higher prevalence of COPD. Moreover, patients with COPD had more missing teeth than those without COPD. The logistic regression model, adjusted for demographic, socioeconomic, health, and oral health-related factors, showed that periodontal status was not significantly associated with COPD, whereas participants with more missing teeth had a significantly increased risk of having COPD. Recently, artificial intelligence (AI)-based statistical analysis was applied to the NHANES III dataset [92]. AI-based analysis revealed that a high CAL appeared to increase the risk of COPD; however, causal relationships could not be determined in this study.
Although some studies have reported that periodontal disease associated with COPD is a risk factor, especially in current or former smokers, others have reported no significant or limited relationship between periodontal disease and COPD. As a result, most studies have concluded that large prospective and longitudinal studies are required to examine this relationship in greater detail. The contents of this section are summarized in Table 6.
Table 6.
Summary of retrospective study.
| Ref. No. | Study Country Year Sample Size | Parameters for Evaluated for the Diagnosis of COPD | Parameters for Evaluated for the Diagnosis of Periodontitis | Main Findings |
|---|---|---|---|---|
| [83] | Scannapieco et al. America 2001 n = 13792 | FEV1/FVC ratio | Mean attachment loss (MAL), gingival bleeding, Dental health index | Patients with MAL ≥ 3.0 mm had a higher risk of COPD than those with MAL < 3.0 mm. |
| [84] | Hyman et al. America 2004 n = 7625 | FEV1/FVC ratio | Loss of Attachment | Cigarette smoking may be a cofactor in the association between periodontal disease and COPD. |
| [85] | Katancik et al. America 2005 n = 860 | FEV1/FVC ratio | GI, PPD, Loss of Attachment | While this study cannot provide direct inference of cause and effect for COPD, it revealed a significant association between periodontal disease and airway obstruction, particularly in former smokers. |
| [86] | Chen et al. America 2022 n = 6313 | FEV1/FVC ratio | PPD, AL | After adjusting for relevant confounding factors, the hazard ratio (HR) for patients with periodontitis was 2.72 (95% CI: 1.04 – 7.11), while the number of teeth lost was not significantly associated with total respiratory disease or mortality. |
| [87] | Winning et al. Sweden 2020 n = 826 | FEV1/FVC ratio | radiographical assessment, missing teeth | Periodontitis was independently associated with airflow limitation. |
| [88] | Qian et al. China 2020 n = 1385 | respiratory disease mortality | alveolar bone loss | Mortality from respiratory diseases was significantly associated with periodontal disease after adjusting for relevant confounding factors. |
| [89] | Chung et al. South Korea 2016 n = 5878 | FEV1/FVC ratio | CPI, Questionnaire for oral hygiene | Periodontitis (CPI 3 or 4) was significantly associated in males with COPD and participants with more missing teeth had a significantly increased possibility of having COPD. |
| [90] | Lee et al. South Korea 2019 n = 4004 | FEV1/FVC ratio | CPI | The association between periodontitis and restrictive impairment or obstructive impairment was not significant. |
| [91] | Jung et al. South Korea 2020 n = 7719 | FEV1/FVC ratio | CPI | There was no statistically significant association between periodontal disease and COPD among former or non-smokers. |
| [92] | Vollmer et al. America 2022 n = 15868 | Pulmonary function tests | Database extraction attachment loss | Based upon AI-based analyses, high CAL appears to increase the risk of COPD, although causal relationships cannot be concluded. |
6. Longitudinal study
There are seven papers on longitudinal studies. The oldest study on this topic was conducted in 1988. Hayes et al. reported that logistic regression analysis examined the independent contribution of bone loss measured at baseline to the subsequent risk of developing COPD over a 25-year follow-up period. Approximately 23% of the participants happened COPD, although they were healthy at baseline. Alveolar bone loss status at baseline was an independent risk factor for COPD, with patients in the worst population quintile of bone loss found to be at significantly higher risk [93].
Another report focused on the IgG antibody titer against P. gingivalis; 93 individuals were prospectively followed up for over one year to detect exacerbations. The number of exacerbations and their frequencies were significantly lower in patients with higher IgG titers than in those with normal IgG titers [94]. There has also been a large cohort study with over 20,000 subjects. The overall incidence of periodontal diseases was 1.19-fold greater in the COPD group than in the non-COPD group (32.2 vs 26.4 per 1000 person-years; CI: 1.15 – 1.24). Compared with non-COPD patients, the adjusted HRs of patients with COPD increased with the number of emergency room visits (from 1.14 [95% CI:1.10 – 1.19] to 5.09 [95% CI: 4.5 – 5.72]) and admissions (from 1.15 [95% CI: 1.10 – 1.20] to 3.17 [95% CI: 2.81 – 3.57]) [95].
Several studies in Japan have indicated that periodontitis is a risk factor for worse status in patients with COPD. Takeuchi et al. reported that there was a tendency for the adjusted risk ratio of developing rapid lung function decline (≥160 mL/3 years, the highest quartile of the distribution of FEV1 declines) to increase as mean clinical attachment levels increased (p = 0.039) [96]. In addition, the analysis was performed using a 5-year follow-up population-based cohort study. After adjustment for potential confounders, including smoking intensity, the relationship between severe periodontitis and the risk of COPD remained significant. Moreover, periodontitis severity was positively associated with the risk of developing COPD (p = 0.043) [97]. A recent study examined the relationship between the incidence of COPD and periodontitis and smoking by A Cox proportional hazard model. Both periodontitis and heavy smoking had significant effects on COPD development in a multivariable analysis [98]. In addition, a cohort study was conducted on the impact of tooth loss and periodontal disease on respiratory events in patients with COPD. After adjusting for potential confounders, the study demonstrated that the risk of COPD-related events might be attributable to both edentulism and elevated serum IL-6 levels [99]. Another study also reported that individuals with greater bone resorption exhibited more comorbidities than their counterparts. However, the study showed no significant association between periodontitis and COPD [100].
Almost all studies mentioned above reported that periodontal disease was a risk factor for COPD exacerbation. However, further studies are needed to clarify the association between periodontal disease and COPD. The contents of this section are summarized in Table 7.
Table 7.
Longitudinal studies regarding the relationship between COPD and periodontal disease.
| Ref. No. | Study Country Year Sample Size | Methods | Main Finds |
|---|---|---|---|
| [93] | Hayes et al. America 1998 n = 1231 | ABL FEV1 | ABL status at baseline was an independent risk factor for COPD, with patients in the worst population quintile of bone loss (mean ABL > 20% per site) found to be at significantly higher risk (OR = 1.8; 95% CI = 1.3 – 2.5) |
| [94] | Takahashi et al. Japan 2012 n = 109 | IgG antibody titer against Porphyromonas gingivalis | The number of exacerbations and their frequencies were significantly lower in patients with higher IgG titers than in those with normal IgG titers (0.8 vs.1.2 per year, p = 0.045, and 14.3 vs. 38.6%, p = 0.009, respectively). |
| [95] | Shen et al. China 2015 n = 22,332 | index date to the current date of periodontal diseases | Patient with COPD are at a higher risk of developing periodontal diseases than the general population. |
| [96] | Takeuchi et al. Japan 2018 n = 2557 | CAL PPD FEV1 | A positive association was observed between mean PPD levels and RR of developing rapid lung function decline (P trend = 0.047). |
| [97] | Takeuchi et al. Japan 2019 n = 1585 | PPD,CAL,FEV1/FVC | The adjusted Relative Risk of development of COPD was significantly higher in subjects with severe periodontitis than in those with no/mild periodontitis (RR = 3.51; 95% CI, 1.15–10.74). |
| [98] | Saito et al. Japan 2023 n = 50,333 | Community Periodontal Index and pulmonary function tests | Periodontitis has no interaction with smoking but has an independent effect on developing COPD. |
| [99] | Barros et al. America 2013 n = 15,792 | Respiratory and periodontal conditions | A statistically significant association was found between oral health status and COPD-related events, even adjusting for conditions such as hypertension, smoking and diabetes. |
| [100] | Zhao et al. China 2019 n = 17,400 | community periodontal index, BL, bone loss/age and number of remaining teeth | Periodontal disease experience to some extent reflects the host susceptibility to onset of common systemic comorbidities. |
7. Intervention study
There are six papers on intervention studies. Generally, COPD is exacerbated by bacterial or viral infections. Oral hygiene procedures and dental treatments also cause transient bacteremia [101], [102], [103]. Successful periodontal treatment improves systemic inflammation and positively affects other diseases, including diabetes [104], [105], [106]. Periodontal debridement performed with ultrasonic or hand instruments for chronic periodontitis has no negative effect on the quality of life or illness in patients with COPD [107]. The initial periodontal treatment group received oral hygiene instructions and full-mouth scaling and root planning using hand instruments and showed a significant reduction in the exacerbation frequency during the follow-up period (p = 0.01). Although the median exacerbations declined from three to two times in the treatment group, patients with no treatment showed an increase in exacerbations from two to three times [108]. In Taiwan, the effects of periodontal treatment on COPD were investigated in a retrospective cohort study using data from a large population. Shen et al. reported that periodontal treatment for COPD patients could reduce the risk of adverse respiratory events and mortality. During the 5-year follow-up period, the incident adverse respiratory events were lower in the treatment group than in the comparison group for ER uses and hospitalizations [109]. Periodontal treatment for COPD patients decreased COPD exacerbations and improved the ratio of FEV1 to FVC [110], [111], [112]. A Swedish study investigated whether dental cleaning was associated with improved health status, lung function, or periodontal status and whether specific components of the plaque microbiome at baseline were associated with changes in exacerbation frequency. They found that the risk of new exacerbations was significantly lower in patients both in the total population (regression coefficient 0.36 (95% CI: 0.25 – 0.52), p < 0.0001) and in the population with repeated dental cleaning (0.16 (0.10 – 0.27), p < 0.0001) compared with the no treatment group. Additionally, the FEV1 percentage of the predicted value was also decreased in periodontal treatment patients with COPD [110].
In the present, periodontal treatment may be effective in preventing COPD exacerbation. Therefore, well-designed randomized clinical trials are required. The contents of this section are summarized in Table 8.
Table 8.
Intervention studies regarding the relationship between COPD and periodontal disease.
| Ref. No. | Study Country Year Sample Size | Methods | Main Findings |
|---|---|---|---|
| [107] | Agado et al. America 2012 n = 30 | Respiratory Questionnaire | Periodontal debridement for chronic periodontitis has no effect on quality of life and illness in patients with COPD. |
| [108] | Kucukcoskun et al. Turkey 2013 n = 40 | Number of exacerbations | Initial periodontal therapy showed a significant reduction in the exacerbation frequency during the follow-up period (P = 0.01). |
| [109] | Shen et al. China 2016 n = 126,251 | Adverse respiratory event | During the 5-year follow-up period, all three types (acute exacerbation, pneumonia, and acute respiratory failure) of incident adverse respiratory events were lower in periodontal treatment group than in the comparison group for ER uses and hospitalizations. |
| [110] | Sundh et al. Sweden 2021 n = 101 | 16 S rRNA gene sequencing | Advanced dental cleaning is associated with a reduced frequency of COPD exacerbations. |
| [111] | Zhou et al. China 2014 n = 306 | Periodontal indexes, respiratory function, and COPD exacerbations | FEV1 were significantly higher in both Scaling and SRP groups compared with the control group during the follow-up (p < 0.05). |
| [112] | Sharma et al. India 2021 n = 75 | PI, GI, PPD, CAL, and BOP and spirometry (FEV1/forced vital capacity (FVC)) values | COPD patients have poorer periodontal health as compared to systemically healthy counterparts. |
8. Mechanism study in vitro and in vivo
Although the above studies have demonstrated an association between periodontal disease and COPD, the specific cause of this phenomenon remains to be elucidated for further clinical application. COPD is a disease characterized by emphysema and chronic bronchitis, long-term dyspnea, poor airflow, recurrent mucosal inflammation, and peribronchiolar fibrosis, mainly triggered by viruses, bacteria, or pollutants [113]. As periodontitis is a bacterial infectious disease with increased oral plaque, the transfer of proliferating bacteria (e.g. by aspiration) has been seen as a bridge between the two diseases [114].
8.1. Studies focusing on Fusobacterium nucleatum
Periodontal pathogens have been studied for a century, and P. gingivalis (Pg), Tannerella forsythia, and Treponema denticola have been defined as the most harmful (red complex) pathogens, while other pathogens are considered milder than them, such as Prevotella intermedia, Prevotella nigrescens and Fusobacterium nucleatum (Fn) [115]. Interestingly, the research performed by Hayata et al. showed that heat-inactivated Fn was the most inflammation-inducible bacterium among various periodontal pathogens when infecting the BEAS-2B (human bronchial cell) and human pharyngeal (Detroit 562) cell lines [116]. Although the inflammatory response of the A549 (human alveolar epithelial) cell line was questionable in their study, respiratory inoculation of heat-inactivated Fn into healthy mice demonstrated its pathogenicity, which has also been confirmed by others, accompanied by the upregulation of MMP-9 [117]. Meanwhile, a more obvious involvement of several types of immune cells and chemokines was observed when COPD was simultaneously induced by elastase injection. Compared with healthy mice, their results demonstrated a much greater deterioration of pulmonary function and upregulation of MMP-12, perforin, and mucin, indicating an exacerbation of the inflammatory response when Fn infection and COPD coexist [118]. To clinically verify these findings, Li et al. detected Fn and Pseudomonas aeruginosa (Pa) in sputum samples from patients with acute exacerbation of COPD, and the results showed a correlation between the detection of Fn and worsening of lung function [119]. With regard to the specific mechanism by which Fn exacerbates COPD, Fn was co-cultured with Pa, and it was found that the addition of Fn accelerated proliferation and plaque formation and even reduced the antibiotic susceptibility of Pa. The authors explained that enhanced proliferation and plaque formation were possibly caused by the upregulation of pelB and pslA induced by Fn-produced Fusobacterium adhesin A, and its pathogenic effect was confirmed in a subsequent study infecting the A549 cell line [120]. Furthermore, heat-inactivated Fn infection in the A549 cell line also increased the expression of angiotensin-converting enzyme 2, but the addition of the main virulence factor of Fn (butyric acid) did not affect the inflammatory response, suggesting that the pathogenic factor of Fn in COPD is something else [121].
8.2. Other studies
Only a few studies have taken a sensible approach from different perspectives, as direct evidence suggests the crucial role of Fn in this relationship. To reduce the influence of specific types of bacteria, Rosa et al. induced periodontitis by ligature placement and COPD by smoking in mice and then analyzed bronchoalveolar lavage fluid, blood, and lung samples [122]. Notably, the induction of periodontitis in mice with pre-existing COPD did not exacerbate the disease, and in fact may have relieved the progress of COPD. Given that the ligature model lacks traditional periodontal pathogens [123], and that COPD was not induced by infection, it is reliable and suggests that the key to explaining the relationship between the two diseases remains human-associated bacteria. Han et al. also performed experiments in rats to investigate this relationship but used a submucosal injection of Pg to induce periodontitis [124]. Due to the lack of COPD control group, their results can only discuss the effect of COPD on periodontitis and conclude that there was no effect; however, the evidence could be used to deny the influence of COPD on periodontitis. Moreover, since treating BEAS-2B cell lines with other heat-inactivated periodontal pathogens still stimulated the inflammation, some researchers discussed bacteria other than Fn. Since Pg and Acinetobacter baumannii (Ab) are both present in the oral cavity and pulmonary system, they discussed the differences between monoculture and co-incubation by comprehensive analysis, which resulted in several alterations in gene expression and enhanced colonization [125]. However, their conclusion was over-interpreted and needs to be confirmed by further studies due to the lack of evidence for the detection of the two bacterial species in COPD patients.
8.3. Summary
Taking all the relevant studies together, the mechanism by which periodontitis exacerbates COPD is mainly related to the transfer of bacteria in oral plaque to the respiratory system, and Fn is its strongest "candidate" so far. However, most studies have used inactivated bacteria, which do not reflect all the viral factors of the pathogen. To verify the objective link between the two diseases, further studies using live bacteria are needed to confirm the existing view and a comprehensive analysis of samples co-infected with several periodontal pathogens or whole plaques should be performed. The contents of this section are summarized in Table 9.
Table 9.
Summary of studies on mechanisms.
| Ref. No. | Study Country Year | Experimental type | Main Findings |
|---|---|---|---|
| [116] | 2019 Hayata et al. | In vivo and In vitro | Among several periodontal pathogens, exposure to elevated levels of Fn was the most potent in inducing the production of pro-inflammatory cytokines by human bronchial and pharyngeal epithelial cells, which may lead to exacerbation of COPD. |
| [117] | 2022 Suzuki et al. | In vivo and In vitro | Fn may contribute to the onset of pulmonary diseases via MMP-9 expression through extracellular-regulated kinase 1/2 and NF-ĸB activation. |
| [118] | 2022 Suzuki et al. | In vivo | The administration of Fn to elastase-treated mice enhanced inflammatory responses, production of alveolar wall destruction factors, progression of emphysema, and recruitment of mucin. |
| [119] | 2020 Li et al. | In vitro | Fn frequently coexisted with Pa in the respiratory tract of AECOPD patients, and co-culturing the two bacteria promoted bacterial proliferation and induced antibiotic tolerance by forming a dense biofilm surrounded by excessive Pel and Psl polysaccharides, which might be induced by FadA. |
| [120] | 2021 Li et al. | In vitro | Fn could co-aggregate with Pa to synergistically invade into pulmonary epithelial cells and transiently resist P. aeruginosa-induced cytotoxic damage to amplify IL-6 and TNF-α associated inflammation in pulmonary epithelial cells simultaneously infected with P. aeruginosa and F. nucleatum. |
| [121] | 2021 Takahashi et al. | In vitro | The culture supernatant of the Fn upregulated the SARS-CoV-2 receptor angiotensin-converting enzyme 2 in A549 alveolar epithelial cells, and induced IL-6 and IL-8 production by A549 alveolar epithelial cells, BEAS-2B bronchial epithelial cells, Detroit 562 pharyngeal epithelial cells, and primary alveolar epithelial cells. |
| [122] | 2020 Rosa et al. | In vivo | The association COPD and periodontitis decreased macrophages, TNF-α and INF-γ in BAL, when compared to the COPD group maintaining emphysema levels by alveolar enlargement reorganization of collagen fibers and also mean linear intercept and mucus. |
| [124] | 2019 Han et al. | In vivo | Although COPD had no effect on periodontitis, 25-OHD3 treatment significantly reduced inflammation by decreasing serum levels of RANKL, TNF-α and IL-1 and increasing that of IL-10, while reducing alveolar bone loss and slightly improving lung function in the periodontitis group or COPD+periodontitis group. |
| [125] | 2018 Miller et al. | In vitro | The presence of Ab increased the abundance of Pg in model dual-species communities,suggesting that both Pg and Ab adapt to each other and have synergistic potential for increased pathogenicity. |
9. Conclusion
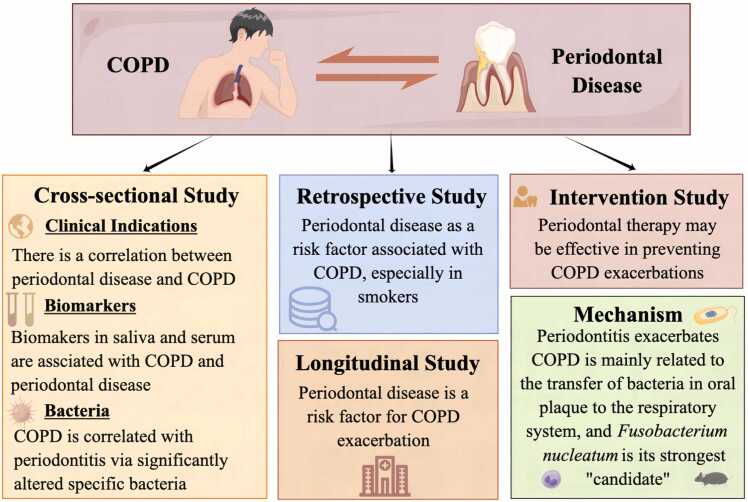
This review summarized the relationship between COPD and periodontal disease (Fig. 1). While the incidence of both diseases is closely related to social factors, such as geographical location and economic development, epidemiological studies across various countries and regions still indicate a positive correlation between the prevalence and severity of periodontal disease and the onset and progression of COPD. Through 16 S rRNA gene sequencing and various laboratory testing methods, researchers have discovered that COPD may be associated with periodontal disease through significant alterations in specific bacteria and biomarkers. Moreover, in vitro and in vivo studies have suggested that periodontal disease exacerbates COPD by transferring Fn in the oral biofilm. Longitudinal research and intervention studies have indicated that periodontal disease is a risk factor for COPD and that periodontal treatment may effectively improve COPD symptoms.
Fig. 1.
The summary of this review. Periodontal disease has an association with COPD.
The association with COVID-19 has recently gained attention as new research [126], [127], [128] field. Similar to the relationship between COPD and periodontal disease, oral infections from periodontitis may enter the respiratory system through the oral-lung pathway, potentially increasing the risk of COVID-19 infection and/or symptoms [129], [130], [131].
In summary, an increasing number of studies have confirmed the association between periodontitis and COPD. However, smoking is a common risk factor for both disease, which make it difficult to find out the direct relationship between these two diseases. Most of the previous studies were cross-sectional or case-control studies and lacked direct evidence from randomized controlled clinical studies. To confirm whether periodontal disease promotes the progression of COPD, and whether periodontal treatment prevents COPD exacerbation, more direct evidence, as well as larger samples and more rigorous epidemiologic study designs, are needed. Moreover, the related mechanisms of influence need to be further investigated.
The association between periodontitis and COPD is complex and multifaceted. Inflammation and the microbiome appear to be the key factors influencing the development of both diseases through shared inflammatory pathways. Understanding these interconnections may provide valuable insights for the prevention and management of periodontitis and COPD as well as for addressing similar pandemics such as COVID-19 more effectively.
Funding
This research was supported by JPSP KAKENHI, Grant Number 20H03863 to S.K.
CRediT authorship contribution statement
Literature collection, P.L and S.K.; writing, P.L., A.L., Yosuke.T., K.N., Y.O., K.T., Yuta.T., T.S., and S.K.; revision, H.N., A.A., T.I., and S.K. All authors have read and agreed to the published the manuscript.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Winning L., Linden G.J. Periodontitis and systemic disease. BDJ Team. 2015;2(10) doi: 10.1007/s40496-017-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polak D., Sanui T., Nishimura F., Shapira L. Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontol 2000. 2020;83(1):46–58. doi: 10.1111/prd.12298. Epub 2020/05/10. [DOI] [PubMed] [Google Scholar]
- 3.Genco R.J., Borgnakke W.S. Diabetes as a potential risk for periodontitis: association studies. Periodontol 2000. 2020;83(1):40–45. doi: 10.1111/prd.12270. Epub 2020/05/10. [DOI] [PubMed] [Google Scholar]
- 4.Orlandi M., Graziani F., D'Aiuto F. Periodontal therapy and cardiovascular risk. Periodontol 2000. 2020;83(1):107–124. doi: 10.1111/prd.12299. Epub 2020/05/10. [DOI] [PubMed] [Google Scholar]
- 5.Schenkein H.A., Papapanou P.N., Genco R., Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020;83(1):90–106. doi: 10.1111/prd.12304. Epub 2020/05/10. [DOI] [PubMed] [Google Scholar]
- 6.Komazaki R., Katagiri S., Takahashi H., Maekawa S., Shiba T., Takeuchi Y., et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-14260-9. Epub 2017/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. Epub 2014/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. Epub 2005/11/22. [DOI] [PubMed] [Google Scholar]
- 9.Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. Epub 2015/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwasaki M., Yoshihara A., Suwama K., Zaitsu T., Suzuki S., Ihira H., et al. A cross-sectional study of the association between periodontitis and physical activity in the Japanese population. J Periodontal Res. 2023;58(2):350–359. doi: 10.1111/jre.13095. Epub 2023/01/16. [DOI] [PubMed] [Google Scholar]
- 11.Salvi G.E., Roccuzzo A., Imber J.C., Stahli A., Klinge B., Lang N.P. Clinical periodontal diagnosis. Periodontol 2000. 2023 doi: 10.1111/prd.12487. Epub 2023/07/15. [DOI] [PubMed] [Google Scholar]
- 12.Tabassum S., Adnan S., Khan F.R. Gingival retraction methods: a systematic review. J Prosthodont. 2017;26(8):637–643. doi: 10.1111/jopr.12522. Epub 2016/07/29. [DOI] [PubMed] [Google Scholar]
- 13.Worthington H.V., Clarkson J.E., Bryan G., Beirne P.V. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev. 2013;(11) doi: 10.1002/14651858.CD004625.pub4. Epub 2013/11/08. [DOI] [PubMed] [Google Scholar]
- 14.Jarhyan P., Hutchinson A., Khaw D., Prabhakaran D., Mohan S. Prevalence of chronic obstructive pulmonary disease and chronic bronchitis in eight countries: a systematic review and meta-analysis. Bull World Health Organ. 2022;100(3):216–230. doi: 10.2471/BLT.21.286870. Epub 2022/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knox-Brown B., Patel J., Potts J., Ahmed R., Aquart-Stewart A., Cherkaski H.H., et al. Small airways obstruction and its risk factors in the Burden of Obstructive Lung Disease (BOLD) study: a multinational cross-sectional study. Lancet Glob Health. 2023;11(1):e69–e82. doi: 10.1016/S2214-109X(22)00456-9. Epub 2022/12/16. [DOI] [PubMed] [Google Scholar]
- 16.Omori H., Higashi N., Nawa T., Fukui T., Kaise T., Suzuki T. Associated factors and comorbidities of airflow limitation in subjects undergoing comprehensive health examination in Japan - survey of chronic obstructive pulmonary disease patients epidemiology in Japan (SCOPE- J) Int J Chron Obstruct Pulmon Dis. 2020;15:3039–3050. doi: 10.2147/COPD.S272588. Epub 2020/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fazleen A., Wilkinson T. Early COPD: current evidence for diagnosis and management. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620942128. Epub 2020/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiropoulou A., Zareifopoulos N., Bellou A., Spiropoulos K., Tsalikis L. Review of the association between periodontitis and chronic obstructive pulmonary disease in smokers. Monaldi Arch Chest Dis. 2019;89(1) doi: 10.4081/monaldi.2019.1018. Epub 2019/04/11. [DOI] [PubMed] [Google Scholar]
- 19.Hobbins S., Chapple I.L., Sapey E., Stockley R.A. Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int J Chron Obstruct Pulmon Dis. 2017;12:1339–1349. doi: 10.2147/COPD.S127802. Epub 2017/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hujoel P.P., Drangsholt M., Spiekerman C., DeRouen T.A. Periodontitis-systemic disease associations in the presence of smoking--causal or coincidental? Periodontol 2000. 2002;30:51–60. doi: 10.1034/j.1600-0757.2002.03005.x. Epub 2002/09/19. [DOI] [PubMed] [Google Scholar]
- 21.Dong J., Li W., Wang Q., Chen J., Zu Y., Zhou X., et al. Relationships between oral microecosystem and respiratory diseases. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.718222. Epub 2022/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai K., Iinuma T., Sato S. Relationship between the oral cavity and respiratory diseases: Aspiration of oral bacteria possibly contributes to the progression of lower airway inflammation. Jpn Dent Sci Rev. 2021;57:224–230. doi: 10.1016/j.jdsr.2021.10.003. Epub 2021/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mammen M.J., Scannapieco F.A., Sethi S. Oral-lung microbiome interactions in lung diseases. Periodontol 2000. 2020;83(1):234–241. doi: 10.1111/prd.12301. Epub 2020/05/10. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang Y., Liu J., Wen S., Xu Y., Zhang Z., Pi Y., et al. Association between chronic obstructive pulmonary disease and periodontitis: The common role of innate immune cells? Cytokine. 2022;158 doi: 10.1016/j.cyto.2022.155982. Epub 2022/08/07. [DOI] [PubMed] [Google Scholar]
- 25.Liu J., Ouyang Y., Zhang Z., Wen S., Pi Y., Chen D., et al. The role of Th17 cells: explanation of relationship between periodontitis and COPD? Inflamm Res. 2022;71(9):1011–1024. doi: 10.1007/s00011-022-01602-1. Epub 2022/07/06. [DOI] [PubMed] [Google Scholar]
- 26.Khijmatgar S., Belur G., Venkataram R., Karobari M.I., Marya A., Shetty V., et al. Oral candidal load and oral health status in chronic obstructive pulmonary disease (COPD) patients: a case-cohort study. Biomed Res Int. 2021;2021 doi: 10.1155/2021/5548746. Epub 20210911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parashar P., Parashar A., Saraswat N., Pani P., Pani N., Joshi S. Relationship between respiratory and periodontal health in adults: a case-control study. J Int Soc Prev Community Dent. 2018;8(6):560–564. doi: 10.4103/jispcd.JISPCD_304_18. Epub 20181129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vadiraj S., Nayak R., Choudhary G.K., Kudyar N., Spoorthi B.R. Periodontal pathogens and respiratory diseases- evaluating their potential association: a clinical and microbiological study. J Conte Dent Pract. 2013;14(4):610–615. doi: 10.5005/jp-journals-10024-1373. Epub 20130701. [DOI] [PubMed] [Google Scholar]
- 29.Sharma N., Shamsuddin H. Association between respiratory disease in hospitalized patients and periodontal disease: a cross-sectional study. J Periodontol. 2011;82(8):1155–1160. doi: 10.1902/jop.2011.100582. Epub 20110110. [DOI] [PubMed] [Google Scholar]
- 30.Moeintaghavi A., Mohammadzadeh Lari S., Shiezadeh F., Mohammadian Z., Tajik S., Nasrabadi N. Relationship between periodontal variables and disease severity in patients with chronic obstructive pulmonary disease. J Adv Periodo Implant Dent. 2018;10(1):1–7. doi: 10.15171/japid.2018.001. Epub 20180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javaheri N., Matin S., Naghizadeh-Baghi A., Bagheri A., Andreasian A., Ghobadi H. Periodontal status, its treatment needs, and its relationship with airflow limitation and quality of life in COPD patients. Eurasia J Med. 2020;52(3):259–264. doi: 10.5152/eurasianjmed.2020.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bomble N., Shetiya S.H., Agarwal D.R. Association of periodontal status with lung function in patients with and without chronic obstructive pulmonary disease visiting a medical hospital in Pune: a comparative study. J Indian Soc Periodo. 2020;24(1):67–71. doi: 10.4103/jisp.jisp_2_19. Epub 20190925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z., Zhang W., Zhang J., Zhou X., Zhang L., Song Y., et al. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol. 2012;39(1):45–52. doi: 10.1111/j.1600-051X.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- 34.Si Y., Fan H., Song Y., Zhou X., Zhang J., Wang Z. Association between periodontitis and chronic obstructive pulmonary disease in a Chinese population. J Periodontol. 2012;83(10):1288–1296. doi: 10.1902/jop.2012.110472. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X., Wang Z., Song Y., Zhang J., Wang C. Periodontal health and quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(1):67–73. doi: 10.1016/j.rmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Kataoka S., Kimura M., Yamaguchi T., Egashira K., Yamamoto Y., Koike Y., et al. A cross-sectional study of relationships between periodontal disease and general health: The Hitachi Oral Healthcare Survey. BMC Oral Health. 2021;21(1) doi: 10.1186/s12903-021-01990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorgensen T. Estrogen use and gallstone disease. Am J Public Health. 1989;79(5):654. doi: 10.2105/ajph.79.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harland J., Furuta M., Takeuchi K., Tanaka S., Yamashita Y. Periodontitis modifies the association between smoking and chronic obstructive pulmonary disease in Japanese men. J Oral Sci. 2018;60(2):226–231. doi: 10.2334/josnusd.17-0225. [DOI] [PubMed] [Google Scholar]
- 39.Terashima T., Chubachi S., Matsuzaki T., Nakajima T., Satoh M., Iwami E., et al. The association between dental health and nutritional status in chronic obstructive pulmonary disease. Chron Respir Dis. 2017;14(4):334–341. doi: 10.1177/1479972316643076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z., Zhou X., Zhang J., Zhang L., Song Y., Hu F.B., et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodo. 2009;36(9):750–755. doi: 10.1111/j.1600-051X.2009.01448.x. [DOI] [PubMed] [Google Scholar]
- 41.Kedlaya M.N., Ramesh A., Hosmane G.B., Bhandary R., Sajna H.R., Thomas B. Comparative evaluation of serum cotinine levels in chronic periodontitis and chronic obstructive pulmonary disease. J Indian Soc Periodo. 2021;25(5):405–410. doi: 10.4103/jisp.jisp_546_20. Epub 20210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rastogi T., Chowdhary Z., Krishna M.K., Mehrotra S., Mohan R. Prevalence of periodontitis in patients with pulmonary disease: A cross-sectional survey in the industrial district of India. J Indian Soc Periodontol. 2019;23(3):269–274. doi: 10.4103/jisp.jisp_435_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raj R., Manu M.K., Prakash P.Y., Singhal D.K., Acharya S. The effect of 6 months or longer duration of chronic obstructive respiratory disease medication on the oral health parameters of adults. Spec Care Dent. 2018;38(3):133–138. doi: 10.1111/scd.12282. Epub 20180330. [DOI] [PubMed] [Google Scholar]
- 44.Bhavsar N.V., Dave B.D., Brahmbhatt N.A., Parekh R. Periodontal status and oral health behavior in hospitalized patients with chronic obstructive pulmonary disease. J Nat Sci Biol Med. 2015;6(1):S93–S97. doi: 10.4103/0976-9668.166097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peter K.P., Mute B.R., Doiphode S.S., Bardapurkar S.J., Borkar M.S., Raje D.V. Association between periodontal disease and chronic obstructive pulmonary disease: a reality or just a dogma? J Periodo. 2013;84(12):1717–1723. doi: 10.1902/jop.2013.120347. Epub 20130123. [DOI] [PubMed] [Google Scholar]
- 46.Russell S.L., Boylan R.J., Kaslick R.S., Scannapieco F.A., Katz R.V. Respiratory pathogen colonization of the dental plaque of institutionalized elders. Spec Care Dent. 1999;19(3):128–134. doi: 10.1111/j.1754-4505.1999.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 47.Baldomero A.K., Siddiqui M., Lo C.Y., Petersen A., Pragman A.A., Connett J.E., et al. The relationship between oral health and COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2019;14:881–892. doi: 10.2147/COPD.S194991. Epub 20190423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leuckfeld I., Obregon-Whittle M.V., Lund M.B., Geiran O., Bjortuft O., Olsen I. Severe chronic obstructive pulmonary disease: association with marginal bone loss in periodontitis. Respir Med. 2008;102(4):488–494. doi: 10.1016/j.rmed.2007.12.001. Epub 20080111. [DOI] [PubMed] [Google Scholar]
- 49.Chrysanthakopoulos N.A., Chrysanthakopoulos P.A. Association between indices of clinically-defined periodontitis and self-reported history of systemic medical conditions. J Investig Clin Dent. 2016;7(1):27–36. doi: 10.1111/jicd.12119. Epub 20140722. [DOI] [PubMed] [Google Scholar]
- 50.Winning L., Patterson C.C., Cullen K.M., Kee F., Linden G.J. Chronic periodontitis and reduced respiratory function. J Clin Periodontol. 2019;46(3):266–275. doi: 10.1111/jcpe.13076. Epub 20190227. [DOI] [PubMed] [Google Scholar]
- 51.Ledić K., Marinković S., Puhar I., Spalj S., Popović-Grle S., Ivić-Kardum M., et al. Periodontal disease increases risk for chronic obstructive pulmonary disease. Coll Antropol. 2013;37(3):937–942. [PubMed] [Google Scholar]
- 52.Lopez-de-Andres A., Vazquez-Vazquez L., Martinez-Huedo M.A., Hernandez-Barrera V., Jimenez-Trujillo I., Tapias-Ledesma M.A., et al. Is COPD associated with periodontal disease? A population-based study in Spain. Int J Chron Obstruct Pulmon Dis. 2018;13:3435–3445. doi: 10.2147/COPD.S174898. Epub 20181018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergstrom J., Cederlund K., Dahlen B., Lantz A.S., Skedinger M., Palmberg L., et al. Dental health in smokers with and without COPD. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059492. Epub 20130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henke C., Budweiser S., Jorres R.A. Lung function and associations with multiple dimensions of dental health: a prospective observational cross-sectional study. BMC Res Notes. 2016;9 doi: 10.1186/s13104-016-2079-2. Epub 20160517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji J., von Schéele I., Bergström J., Billing B., Dahlén B., Lantz A.S., et al. Compartment differences of inflammatory activity in chronic obstructive pulmonary disease. Respir Res. 2014;15(1):104. doi: 10.1186/s12931-014-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oztekin G., Baser U., Kucukcoskun M., Tanrikulu-Kucuk S., Ademoglu E., Isik G., et al. The association between periodontal disease and chronic obstructive pulmonary disease: a case control study. COPD. 2014;11(4):424–430. doi: 10.3109/15412555.2013.858316. Epub 2014/01/01. [DOI] [PubMed] [Google Scholar]
- 57.Yildirim E., Kormi I., Basoglu O.K., Gurgun A., Kaval B., Sorsa T., et al. Periodontal health and serum, saliva matrix metalloproteinases in patients with mild chronic obstructive pulmonary disease. J Periodontal Res. 2013;48(3):269–275. doi: 10.1111/jre.12004. Epub 2012/09/13. [DOI] [PubMed] [Google Scholar]
- 58.Blanco I., Bueno P., Diego I., Perez-Holanda S., Casas-Maldonado F., Esquinas C., et al. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis. 2017;12:561–569. doi: 10.2147/COPD.S125389. Epub 2017/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cadalbert L., Parmar S., Hobbins S., Newby P., Crossley D., Usher A., et al. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. J Clin Periodontol. 2020;47(9):1040–1052. doi: 10.1111/jcpe.13334. Epub 2020/06/23. [DOI] [PubMed] [Google Scholar]
- 60.Yu H., Lin M., Wang X., Wang S., Wang Z. Toll-like receptor 4 polymorphism is associated with increased susceptibility to chronic obstructive pulmonary disease in Han Chinese patients with chronic periodontitis. J Oral Sci. 2016;58(4):555–560. doi: 10.2334/josnusd.16-0187. Epub 2016/12/28. [DOI] [PubMed] [Google Scholar]
- 61.Liu S., Fu Y., Ziebolz D., Li S., Schmalz G., Li F. Transcriptomic analysis reveals pathophysiological relationship between chronic obstructive pulmonary disease (COPD) and periodontitis. BMC Med Genom. 2022;15(1) doi: 10.1186/s12920-022-01278-w. Epub 2022/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rathod S., Shori T., Sarda T.S., Raj A., Jadhav P. Comparative analysis of salivary sialic acid levels in patients with chronic obstructive pulmonary disease and chronic periodontitis patients: a biochemical study. Indian J Dent Res: Publ Indian Soc Dent Res. 2018;29(1):22–25. doi: 10.4103/ijdr.IJDR_106_16. [DOI] [PubMed] [Google Scholar]
- 63.Zhou X., Han J., Song Y., Zhang J., Wang Z. Serum levels of 25-hydroxyvitamin D, oral health and chronic obstructive pulmonary disease. J Clin Periodontol. 2012;39(4):350–356. doi: 10.1111/j.1600-051X.2012.01852.x. Epub 2012/02/03. [DOI] [PubMed] [Google Scholar]
- 64.Jordan A., Sivapalan P., Romer V., Jensen J.U. Time-updated phenotypic guidance of corticosteroids and antibiotics in COPD: rationale, perspective and a proposed method. Biomedicines. 2023;11(5) doi: 10.3390/biomedicines11051395. Epub 2023/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Komerik N., Akkaya A., Yildiz M., Buyukkaplan U.S., Kuru L. Oral health in patients on inhaled corticosteroid treatment. Oral Dis. 2005;11(5):303–308. doi: 10.1111/j.1601-0825.2005.01122.x. Epub 2005/08/27. [DOI] [PubMed] [Google Scholar]
- 66.Man W.H., de Steenhuijsen Piters W.A., Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15(5):259–270. doi: 10.1038/nrmicro.2017.14. Epub 2017/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gleeson K., Eggli D.F., Maxwell S.L. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111(5):1266–1272. doi: 10.1378/chest.111.5.1266. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 68.Huxley E.J., Viroslav J., Gray W.R., Pierce A.K. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4) doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 69.Tan L., Tang X., Pan C., Wang H., Pan Y. Relationship among clinical periodontal, microbiologic parameters and lung function in participants with chronic obstructive pulmonary disease. J Periodontol. 2019;90(2):134–140. doi: 10.1002/JPER.17-0705. Epub 2018/08/08. [DOI] [PubMed] [Google Scholar]
- 70.Zhou X., Wang J., Liu W., Huang X., Song Y., Wang Z., et al. Periodontal status and microbiologic pathogens in patients with chronic obstructive pulmonary disease and periodontitis: a case-control study. Int J Chron Obstruct Pulmon Dis. 2020;15:2071–2079. doi: 10.2147/COPD.S266612. Epub 2020/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leuckfeld I., Paster B.J., Kristoffersen A.K., Olsen I. Diversity of Veillonella spp. from subgingival plaque by polyphasic approach. APMIS. 2010;118(3):230–242. doi: 10.1111/j.1600-0463.2009.02584.x. Epub 2010/02/06. [DOI] [PubMed] [Google Scholar]
- 72.Bassis C.M., Erb-Downward J.R., Dickson R.P., Freeman C.M., Schmidt T.M., Young V.B., et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. 2015;6(2) doi: 10.1128/mBio.00037-15. Epub 2015/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huffnagle G.B., Dickson R.P., Lukacs N.W. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10(2):299–306. doi: 10.1038/mi.2016.108. Epub 2016/12/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris A., Beck J.M., Schloss P.D., Campbell T.B., Crothers K., Curtis J.L., et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187(10):1067–1075. doi: 10.1164/rccm.201210-1913OC. Epub 2013/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moffatt M.F., Cookson W.O. The lung microbiome in health and disease. Clin Med. 2017:525–529. doi: 10.7861/clinmedicine.17-6-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Budden K.F., Shukla S.D., Rehman S.F., Bowerman K.L., Keely S., Hugenholtz P., et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med. 2019;7(10):907–920. doi: 10.1016/S2213-2600(18)30510-1. Epub 2019/04/13. [DOI] [PubMed] [Google Scholar]
- 77.Mammen M.J., Sethi S. COPD and the microbiome. Respirology. 2016;21(4):590–599. doi: 10.1111/resp.12732. [DOI] [PubMed] [Google Scholar]
- 78.Nemoto T., Shiba T., Komatsu K., Watanabe T., Shimogishi M., Shibasaki M., et al. Discrimination of bacterial community structures among healthy, gingivitis, and periodontitis statuses through integrated metatranscriptomic and network analyses. mSystems. 2021;6(6) doi: 10.1128/mSystems.00886-21. Epub 2021/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winning L., Moran G., McClory M., El Karim I., Lundy F.T., Patterson C.C., et al. Subgingival microbial diversity and respiratory decline: a cross-sectional study. J Clin Periodontol. 2023;50(7):921–931. doi: 10.1111/jcpe.13819. Epub 2023/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu X., Chen J., Xu M., Zhu D., Wang X., Chen Y., et al. 16S rDNA analysis of periodontal plaque in chronic obstructive pulmonary disease and periodontitis patients. J Oral Microbiol. 2017;9(1) doi: 10.1080/20002297.2017.1324725. Epub 2017/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu S., Xie G., Chen M., He Y., Yu W., Chen X., et al. Oral microbial dysbiosis in patients with periodontitis and chronic obstructive pulmonary disease. Front Cell Infect Microbiol. 2023;13 doi: 10.3389/fcimb.2023.1121399. Epub 2023/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan L., Wang H., Li C., Pan Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J Periodontal Res. 2014;49(6):760–769. doi: 10.1111/jre.12159. Epub 2014/02/04. [DOI] [PubMed] [Google Scholar]
- 83.Scannapieco F.A., Ho A.W. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol. 2001;72(1):50–56. doi: 10.1902/jop.2001.72.1.50. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 84.Hyman J.J., Reid B.C. Cigarette smoking, periodontal disease: and chronic obstructive pulmonary disease. J Periodontol. 2004;75(1):9–15. doi: 10.1902/jop.2004.75.1.9. Epub 2004/03/18. [DOI] [PubMed] [Google Scholar]
- 85.Katancik J.A., Kritchevsky S., Weyant R.J., Corby P., Bretz W., Crapo R.O., et al. Periodontitis and airway obstruction. J Periodontol. 2005;76(11 Suppl):2161–2167. doi: 10.1902/jop.2005.76.11-S.2161. Epub 2005/11/10. [DOI] [PubMed] [Google Scholar]
- 86.Chen H., Zhang X., Luo J., Dong X., Jiang X. The association between periodontitis and lung function: Results from the National Health and Nutrition Examination Survey 2009 to 2012. J Periodontol. 2022;93(6):901–910. doi: 10.1002/JPER.21-0399. Epub 2021/11/18. [DOI] [PubMed] [Google Scholar]
- 87.Winning L., Polyzois I., Sanmartin Berglund J., Renvert S. Periodontitis and airflow limitation in older Swedish individuals. J Clin Periodontol. 2020;47(6):715–725. doi: 10.1111/jcpe.13287. Epub 2020/04/05. [DOI] [PubMed] [Google Scholar]
- 88.Qian Y., Yuan W., Mei N., Wu J., Xu Q., Lu H., et al. Periodontitis increases the risk of respiratory disease mortality in older patients. Exp Gerontol. 2020;133 doi: 10.1016/j.exger.2020.110878. Epub 2020/02/18. [DOI] [PubMed] [Google Scholar]
- 89.Chung J.H., Hwang H.J., Kim S.H., Kim T.H. Associations Between periodontitis and chronic obstructive pulmonary disease: the 2010 to 2012 Korean national health and nutrition examination survey. J Periodo. 2016;87(8):864–871. doi: 10.1902/jop.2016.150682. Epub 2016/02/26. [DOI] [PubMed] [Google Scholar]
- 90.Lee E., Lee S.W. Prevalence of periodontitis and its association with reduced pulmonary function: results from the Korean National Health and Nutrition Examination Survey. Medicine. 2019;55(9) doi: 10.3390/medicina55090581. Epub 2019/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jung E.S., Lee K.H., Choi Y.Y. Association between oral health status and chronic obstructive pulmonary disease in Korean adults. Int Dent J. 2020;70(3):208–213. doi: 10.1111/idj.12535. Epub 2019/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vollmer A., Vollmer M., Lang G., Straub A., Shavlokhova V., Kubler A., et al. Associations between periodontitis and COPD: an artificial intelligence-based analysis of NHANES III. J Clin Med. 2022;11(23) doi: 10.3390/jcm11237210. Epub 2022/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayes C., Sparrow D., Cohen M., Vokonas P.S., Garcia R.I. The association between alveolar bone loss and pulmonary function: the VA dental longitudinal study. Ann Periodo. 1998;3(1):257–261. doi: 10.1902/annals.1998.3.1.257. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi T., Muro S., Tanabe N., Terada K., Kiyokawa H., Sato S., et al. Relationship between periodontitis-related antibody and frequent exacerbations in chronic obstructive pulmonary disease. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen T.C., Chang P.Y., Lin C.L., Chen C.H., Tu C.Y., Hsia T.C., et al. Risk of periodontal diseases in patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study. Medicine. 2015;94(46) doi: 10.1097/MD.0000000000002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takeuchi K., Matsumoto K., Furuta M., Fukuyama S., Takeshita T., Ogata H., et al. Periodontal status and lung function decline in the community: the Hisayama study. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-31610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takeuchi K., Matsumoto K., Furuta M., Fukuyama S., Takeshita T., Ogata H., et al. Periodontitis is associated with chronic obstructive pulmonary disease. J Dent Res. 2019;98(5):534–540. doi: 10.1177/0022034519833630. [DOI] [PubMed] [Google Scholar]
- 98.Saito M., Shimazaki Y., Yoshii S., Takeyama H. Periodontitis and the incidence of chronic obstructive pulmonary disease: a longitudinal study of an adult Japanese cohort. J Clin Periodo. 2023;50(6):717–726. doi: 10.1111/jcpe.13801. [DOI] [PubMed] [Google Scholar]
- 99.Barros S.P., Suruki R., Loewy Z.G., Beck J.D., Offenbacher S. A cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: modulatory role of systemic biomarkers of inflammation. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0068592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao D., Zhen Z., Pelekos G., Yiu K.H., Jin L. Periodontal disease increases the risk for onset of systemic comorbidities in dental hospital attendees: An 18-year retrospective cohort study. J Periodontol. 2019;90(3):225–233. doi: 10.1002/JPER.18-0224. [DOI] [PubMed] [Google Scholar]
- 101.Silver J.G., Martin A.W., McBride B.C. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. J Clin Periodontol. 1977;4(2):92–99. doi: 10.1111/j.1600-051x.1977.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 102.Lafaurie G.I., Mayorga-Fayad I., Torres M.F., Castillo D.M., Aya M.R., Baron A., et al. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J Clin Periodontol. 2007;34(10):873–879. doi: 10.1111/j.1600-051X.2007.01125.x. [DOI] [PubMed] [Google Scholar]
- 103.Lineberger L.T., De Marco T.J. Evaluation of transient bacteremia following routine periodontal procedures. J Periodontol. 1973;44(12):757–762. doi: 10.1902/jop.1973.44.12.757. [DOI] [PubMed] [Google Scholar]
- 104.Engebretson S., Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Periodontol. 2013;84(4):S153–S169. doi: 10.1902/jop.2013.1340017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simpson T.C., Clarkson J.E., Worthington H.V., MacDonald L., Weldon J.C., Needleman I., et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2022;4(4) doi: 10.1002/14651858.CD004714.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simpson T.C., Weldon J.C., Worthington H.V., Needleman I., Wild S.H., Moles D.R., et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;(11) doi: 10.1002/14651858.CD004714.pub3. Epub 2015/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agado B.E., Crawford B., DeLaRosa J., Bowen D.M., Peterson T., Neill K., et al. Effects of periodontal instrumentation on quality of life and illness in patients with chronic obstructive pulmonary disease: a pilot study. J Dent Hyg. 2012;86(3):204–214. [PubMed] [Google Scholar]
- 108.Kucukcoskun M., Baser U., Oztekin G., Kiyan E., Yalcin F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J Periodontol. 2013;84(7):863–870. doi: 10.1902/jop.2012.120399. [DOI] [PubMed] [Google Scholar]
- 109.Shen T.C., Chang P.Y., Lin C.L., Chen C.H., Tu C.Y., Hsia T.C., et al. Periodontal treatment reduces risk of adverse respiratory events in patients with chronic obstructive pulmonary disease: a propensity-matched cohort study. Medicine. 2016;95(20) doi: 10.1097/MD.0000000000003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sundh J., Tanash H., Arian R., Neves-Guimaraes A., Broberg K., Lindved G., et al. Advanced dental cleaning is associated with reduced risk of COPD exacerbations - a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2021;16:3203–3215. doi: 10.2147/COPD.S327036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou X., Han J., Liu Z., Song Y., Wang Z., Sun Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41(6):564–572. doi: 10.1111/jcpe.12247. [DOI] [PubMed] [Google Scholar]
- 112.Sharma S., Gupta A., Verma A.K., Pathak A., Verma S., Chaudhary S.C., et al. Impact of non-surgical periodontal therapy on pulmonary functions, periodontal health and salivary matrix metalloproteinase-8 of COPD patients with chronic periodontitis: a clinico-biochemical study. Turk Thorac J. 2021;22(4):324–332. doi: 10.5152/TurkThoracJ.2021.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barnes P.J., Burney P.G., Silverman E.K., Celli B.R., Vestbo J., Wedzicha J.A., et al. Chronic obstructive pulmonary disease. Nat Rev Dis Prim. 2015;1 doi: 10.1038/nrdp.2015.76. Epub 20151203. [DOI] [PubMed] [Google Scholar]
- 114.Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- 115.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 116.Hayata M., Watanabe N., Tamura M., Kamio N., Tanaka H., Nodomi K., et al. The periodontopathic bacterium fusobacterium nucleatum induced proinflammatory cytokine production by human respiratory epithelial cell lines and in the lower respiratory organs in mice. Cell Physiol Biochem. 2019;53(1):49–61. doi: 10.33594/000000120. [DOI] [PubMed] [Google Scholar]
- 117.Suzuki R., Kamio N., Sugimoto K., Maruoka S., Gon Y., Kaneko T., et al. Periodontopathic bacterium fusobacterium nucleatum affects matrix metalloproteinase-9 expression in human alveolar epithelial cells and mouse lung. Vivo. 2022;36(2):649–656. doi: 10.21873/invivo.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suzuki R., Kamio N., Kaneko T., Yonehara Y., Imai K. Fusobacterium nucleatum exacerbates chronic obstructive pulmonary disease in elastase-induced emphysematous mice. FEBS Open Bio. 2022;12(3):638–648. doi: 10.1002/2211-5463.13369. Epub 20220130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Q., Tan L., Wang H., Kou Y., Shi X., Zhang S., et al. Fusobacterium nucleatum interaction with pseudomonas aeruginosa induces biofilm-associated antibiotic tolerance via fusobacterium Adhesin A. ACS Infect Dis. 2020;6(7):1686–1696. doi: 10.1021/acsinfecdis.9b00402. Epub 20200504. [DOI] [PubMed] [Google Scholar]
- 120.Li Q., Wang H., Tan L., Zhang S., Lin L., Tang X., et al. Oral pathogen fusobacterium nucleatum coaggregates with pseudomonas aeruginosa to modulate the inflammatory cytotoxicity of pulmonary epithelial cells. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.643913. Epub 20210319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takahashi Y., Watanabe N., Kamio N., Yokoe S., Suzuki R., Sato S., et al. Expression of the SARs-cov-2 receptor ACE2 and proinflammatory cytokines induced by the periodontopathic bacterium. Int J Mol Sci. 2021;22(3) doi: 10.3390/ijms22031352. Epub 20210129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rosa E.P., Murakami-Malaquias-da-Silva F., Palma-Cruz M., de Carvalho Garcia G., Brito A.A., Andreo L., et al. The impact of periodontitis in the course of chronic obstructive pulmonary disease: Pulmonary and systemic effects. Life Sci. 2020;261 doi: 10.1016/j.lfs.2020.118257. Epub 20200818. [DOI] [PubMed] [Google Scholar]
- 123.Bai L., Chen B.Y., Liu Y., Zhang W.C., Duan S.Z. A mouse periodontitis model with humanized oral bacterial community. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.842845. Epub 20220222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han J., Cheng C., Zhu Z., Lin M., Zhang D.X., Wang Z.M., et al. Vitamin D reduces the serum levels of inflammatory cytokines in rat models of periodontitis and chronic obstructive pulmonary disease. J Oral Sci. 2019;61(1):53–60. doi: 10.2334/josnusd.17-0357. [DOI] [PubMed] [Google Scholar]
- 125.Miller D.P., Wang Q., Weinberg A., Lamont R.J. Transcriptome analysis of Porphyromonas gingivalis and Acinetobacter baumannii in polymicrobial communities. Mol Oral Microbiol. 2018;33(5):364–377. doi: 10.1111/omi.12238. Epub 20180803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gupta I., Patel S.A., Kondal D., Goodman M., Mohan S., Ali M.K., et al. Epidemiological pattern of COVID-19 and its association with periodontal health in an urban Indian cohort. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1108465. Epub 2023/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Costa C.A., Vilela A.C.S., Oliveira S.A., Gomes T.D., Andrade A.A.C., Leles C.R., et al. Poor oral health status and adverse COVID-19 outcomes: a preliminary study in hospitalized patients. J Periodo. 2022;93(12):1889–1901. doi: 10.1002/JPER.21-0624. Epub 2022/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gupta S., Sorsa T., Brandt E., Raisanen I.T., Mohindra R., Goyal K. Poor oral health may prolong COVID-19 illness. J Transl Med. 2022;20(1) doi: 10.1186/s12967-022-03310-0. Epub 2022/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baima G., Marruganti C., Sanz M., Aimetti M., Romandini M. Periodontitis and COVID-19: biological mechanisms and meta-analyses of epidemiological evidence. J Dent Res. 2022;101(12):1430–1440. doi: 10.1177/00220345221104725. Epub 2022/07/02. [DOI] [PubMed] [Google Scholar]
- 130.Tamimi F., Altigani S., Sanz M. Periodontitis and coronavirus disease 2019. Periodontol 2000. 2022;89(1):207–214. doi: 10.1111/prd.12434. Epub 2022/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y., Deng H., Pan Y., Jin L., Hu R., Lu Y., et al. Periodontal disease increases the host susceptibility to COVID-19 and its severity: a Mendelian randomization study. J Transl Med. 2021;19(1) doi: 10.1186/s12967-021-03198-2. Epub 2021/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]