Abstract
Iron overload cardiomyopathy (IOC) is a condition in which iron deposition in the heart causes cardiac dysfunction. We described a 21-year-old woman who presented with acute chest pain, dyspnea, and fever. The patient had a history of transfusion-dependent thalassemia (TDT) and secondary hemochromatosis with the latest serum ferritin ranging from 8000 to 15,000. Physical examinations revealed signs of anemia and heart failure. Electrocardiography showed diffuse ST-segment elevation with reciprocal ST-segment depression in aVR and complete atrioventricular block. Cardiac markers were markedly elevated. Echocardiography demonstrated the dilated size, impaired systolic function, global wall hypokinesia, restrictive filling pattern of the left ventricle, and a small amount of pericardial effusion. Coronary angiography showed normal coronary arteries. A cardiac magnetic resonance imaging showed multifocal early and late gadolinium enhancement involving mid-wall and subepicardial areas of biventricular myocardium suggestive of diffuse myocardial injury from an inflammatory process. She was provisionally diagnosed with acute myopericarditis. Ibuprofen and loop diuretic were prescribed; however, cardiogenic shock occurred. Thus, an endomyocardial biopsy was done and revealed diffuse myocardial hemosiderin deposition without evidence of inflammatory cell infiltration. Severe IOC mimicking acute myopericarditis was considered based on an endomyocardial biopsy result. An intravenous iron chelating agent was immediately administered. Unfortunately, cardiogenic shock was refractory resulting in death. This case demonstrated a rare manifestation of IOC, which can masquerade as acute myopericarditis, and emphasized that IOC should be differentially diagnosed, particularly in patients with TDT and hemochromatosis.
Keywords: Cardiac magnetic resonance, Endomyocardial biopsy, Hemochromatosis, Iron overload cardiomyopathy, Myocardial infiltration, Myopericarditis
Introduction
Iron overload cardiomyopathy (IOC) is a form of cardiomyopathy characterized by abnormal iron accumulation in the myocardium, leading to the impairment of cardiac function [1]. The manifestations of IOC, including heart failure and arrhythmia, overlap with myocarditis, causing difficulty in the differential diagnosis between these 2 entities in some situations. Careful cardiac investigation consideration and interpretation are important to make a correct diagnosis, leading to prompt specific management prescriptions that can improve the patient's prognosis.
Case report
A 21-year-old woman presented at the hospital with acute chest pain, dyspnea, and orthopnea for the past 4 days. The patient had experienced a low-grade fever for the preceding 2 weeks. Her past medical history was β-thalassemia/hemoglobin E disease, with regular red blood cell transfusions of 2 units every month. This condition was complicated by secondary hemochromatosis with cardiac, hepatic, and endocrinal involvement (diabetes mellitus). Her serum ferritin levels ranged between 8000 and 15,000 ng/mL over the past year (normal reference, 13-150 ng/mL). Cardiac magnetic resonance imaging (CMR) with a T2* relaxometry, performed 5 years ago, had shown mild cardiac iron deposition with a cardiac T2* of 16 ms (normal reference, > 20 ms). Cardiac size and function were preserved, with a left ventricular ejection fraction (LVEF) of 56%, and no myocardial scar was evident. Severe liver iron deposition with a liver T2* of 0.9 ms (normal reference, > 6.3 ms) and hepatomegaly had been reported. The patient had been undergoing treatment with an oral iron chelating agent deferasirox at a dose of 2,000 mg/day. She had undergone splenectomy 6 months ago due to hypersplenism and had received the AstraZeneca COVID-19 vaccine 2 months prior to this visit. Physical examination revealed a blood pressure of 110/64 mm Hg, a heart rate of 93 beats/min, a respiratory rate of 20 breaths/min, and a body temperature of 36.8°C. The patient appeared pale and jaundiced, with a bronze skin appearance. Cardiovascular examination showed a distended internal jugular vein with a pressure of 12 cm H2O, along with downward and leftward displacement of the cardiac apex. P2 was prominent on precordial auscultation, and a grade II/VI systolic murmur was audible at the left parasternal border. Fine crackles were detected in both lungs, and pitting edema was observed in both legs. Her liver was palpated at a point 4-finger breadth below the right costal margin.
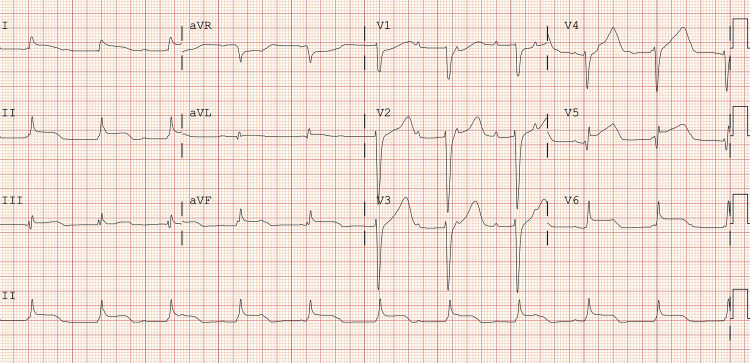
Electrocardiography (ECG) revealed diffuse ST-segment elevation with reciprocal ST-segment depression in aVR and complete atrioventricular block (Fig. 1). Due to the clinical presentation of chest pain and ST-segment elevation on the ECG, emergency coronary angiography was performed, showing a normal appearance of all coronary arteries. Serial cardiac markers with high-sensitivity cardiac troponin I concentrations were 5300 ng/L and 7750 ng/L at 0 and 2 hours after the hospital visit, respectively (normal reference, < 14 ng/L). A complete blood count indicated hypochromic microcytic anemia (hemoglobin of 8.0 g/dL, mean corpuscular volume of 76 fL, red cell distribution width of 22.4%). Liver enzymes were elevated, with an alanine transaminase level of 212 IU/L and an aspartate transaminase level of 58 IU/L. Total and direct bilirubin were 2.81 and 1.63 mg/dL, respectively. Serum ferritin was significantly increased from baseline, with a level of 19,600 ng/mL. Other laboratory tests, including kidney function, nasal swab polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 and 19 subtypes of the respiratory virus, serology for typical causative organisms of myopericarditis, and an autoimmune panel, yielded unremarkable results.
Fig. 1.
Electrocardiography showed diffuse ST-segment elevation with reciprocal ST-segment depression in aVR and complete atrioventricular block.
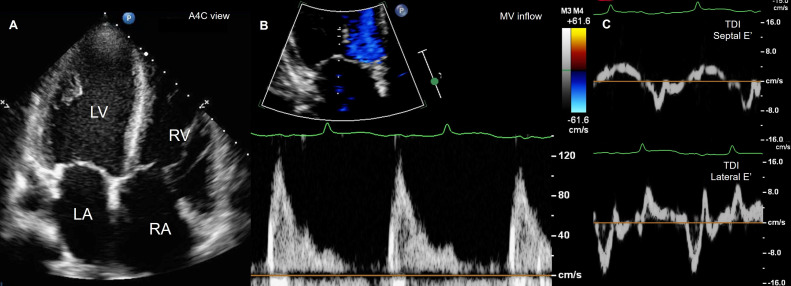
Chest radiography demonstrated an increase in cardiothoracic ratio and pulmonary vascularity with cephalization. Transthoracic echocardiography revealed mild dilatation, impaired systolic function (LVEF 46%), global wall hypokinesia, and restrictive physiology of the left ventricle. Pulmonary hypertension with an estimated mean pulmonary pressure of 33 mm Hg, along with mild dilatation and dysfunction of the right ventricle, was present. A small amount of pericardial effusion was also depicted (Fig. 2). CMR showed multifocal early and late gadolinium enhancement involving mid-wall and subepicardial areas of the left ventricular myocardium, suggestive of diffuse myocardial injury from an inflammatory process. However, the myocardial T2 signal intensity on the T2-weighted images was diffusely low and uninterpretable. T2* relaxometry reported a significant worsening of cardiac iron deposition from mild to severe degree, with T2* of 4.7 ms compared to the previous CMR (Fig. 3). Acute myopericarditis was provisionally diagnosed based on clinical manifestation and investigation results.
Fig. 2.
Transthoracic echocardiography in A4C view revealed mild biventricular dilatation (A), and restrictive physiology of the LV from pulse wave Doppler of MV inflow, E/A = 4.4 (B) and TDI of septal and lateral MV annuli, septal E’= 7.6 cm/s, lateral E’= 13.1 cm/s (C). A4C, apical4-chamber; LA, left atrium; LV, left ventricle; MV, mitral valve; RA, right atrium; RV, right ventricle; TDI, tissue Doppler imaging.
Fig. 3.
CMR with T2-weighted and fat suppression image in short-axis view showed diffusely hypo-signal intensity making it uninterpretable (A). CMR with EGE (B) and LGE (C and D) in short-axis view showed multifocal enhancement involving mid-wall (blue arrow) and subepicardial areas (red arrow) of the left ventricular myocardium suggestive of diffuse myocardial injury from an inflammatory process. CMR, cardiac magnetic resonance imaging; EGE, early gadolinium enhancement; LGE, late gadolinium enhancement.
Oral ibuprofen at a dose of 1800 mg/d and intravenous furosemide at a dose of 80 mg/d were prescribed, resulting in partial improvement of symptoms and a reduction in the degree of atrioventricular block to the first degree. However, worsening heart failure and cardiogenic shock occurred 4 days later. Complete heart block recurred. Subsequent echocardiography revealed deteriorating biventricular function, with an LVEF of 20%. After discussing with the heart team, intravenous methylprednisolone at a dose of 1 mg/kg/d and dobutamine were administered. Endotracheal intubation with positive pressure ventilation, temporary pacemaker placement, and insertion of an intra-aortic balloon pump insertion were performed. An endomyocardial biopsy was carried out, revealing diffuse myocardial hemosiderin deposition without evidence of inflammatory cell infiltration (Fig. 4). Severe IOC, mimicking acute myopericarditis, was considered based on the endomyocardial biopsy result. Intravenous administration of iron chelating agent deferoxamine at a dose of 50 mg/kg/d was initiated. Unfortunately, cardiogenic shock was refractory, and the patient's relatives declined veno-arterial extracorporeal membrane oxygenation support, resulting in the patient's demise.
Fig. 4.
Endomyocardial biopsy revealed diffuse myocardial hemosiderin deposition without evidence of inflammatory cell infiltration.
Discussion
Iron overload cardiomyopathy (IOC), also known as cardiac hemochromatosis, is a form of cardiomyopathy characterized by abnormal iron accumulation in the myocardium, leading to impairment of both ventricular diastolic and systolic function [1]. Iron deposition can occur within the cardiac conducting system and pericardium, resulting in conduction abnormalities (most commonly atrioventricular block) and pericardial constriction [2,3]. IOC arises when the total body iron concentration increases, becomes saturated, and eventually surpasses the capacity of normal transport and storage systems (such as transferrin and the reticuloendothelial system), leading to the intracellular accumulation of nontransferrin-bound iron in cardiac tissues [4]. Furthermore, nontransferrin-bound iron can accumulate systematically and cause damage to other organs, including liver, endocrinal glands, and dermis. Hemochromatosis in patients with thalassemia major, known as secondary hemochromatosis, is caused by frequent red blood cell transfusions, which introduce hemoglobin-bound iron to the system. This, coupled with increased intestinal iron absorption due to the inappropriate suppression of hepcidin resulting from ineffective erythropoiesis, contributes to its development [4]. Nontransferrin-bound iron enters cardiomyocytes in its ferrous form (Fe2+) and is stored as free iron, ferritin, and hemosiderin. Excessive intracellular free iron leads to Fenton reactions, converting Fe2+ into Fe3+, thereby generating an excessive amount of reactive oxygen species (particularly hydroxyl radical) that cannot be adequately counteracted by antioxidants. These free radicals directly damage cellular structures, ultimately impairing overall cardiac functions.
Another significant mechanism of cardiac dysfunction in IOC involves the impaired excitation-contraction coupling. Iron competes with calcium irons and enters cardiomyocytes through L-typed calcium channels. In light of these mechanisms, both restrictive and dilated cardiomyopathy, though not always sequentially, emerge as typical phenotypes of IOC [4,5]. Additionally, in patients with thalassemia major, the incidence of myocarditis is notably high and is considered a common contributor to cardiac dysfunction [6].
Clinical manifestations of IOC and myopericarditis overlap, including heart failure, chest pain, and arrhythmias. Sometimes, distinguishing between these 2 conditions becomes challenging, especially when acute myocarditis is suspected to arise on underlying IOC. However, the onset of myopericarditis is typically acute to subacute, whereas chronic progression is more common in IOC. Cardiac investigations play a pivotal role in differential diagnosis.
Specifically, ST-segment elevation on ECG, significant elevation of cardiac markers, and evidence of inflammation and necrosis based on CMR Lake Louis criteria suggest acute myocarditis [7,8]. Nevertheless, the definitive diagnosis of acute myocarditis relies on the presence of inflammatory cell infiltration and myocardial necrosis in histopathologic specimens from endomyocardial biopsy [7,9]. This is particularly relevant when the myocardium contains extensive iron deposits that render T2-based images (1 of 2 updated Lake Louis criteria required for diagnosing recent myocarditis) uninterpretable due to increased internal magnetic field inhomogeneity caused by intracellular iron [10].
As showcased in this case, the myocardial T2 signal intensity on T2-weighted images was globally low, rendering them noninterpretable. Therefore, early gadolinium enhancement imaging, which was part of the original Lake Louis Criteria but omitted from the updated version, can offer valuable information in such a circumstance (Fig. 3).
The present case serves as an illustrative example of the diagnostic challenge that can arise. Despite an acute onset of chest pain, dyspnea, and fever, along with results from various investigations including ST-segment elevation on the ECG, markedly elevated cardiac markers, and evidence of inflammation on CMR, all of which strongly suggested acute myopericarditis as the most probable diagnosis, the endomyocardial biopsy revealed only hemosiderin deposition without any presence of inflammatory cell infiltration or myocardial necrosis, which contradicted the diagnosis of acute myocarditis. Given this discrepancy, the decision was made to diagnose severe IOC based on the result of the endomyocardial biopsy, which is currently considered the gold standard diagnostic test. However, it is important to note that sampling errors can occur in up to 40% of cases, leading to false-negative biopsy results [11]. Therefore, the possibility of acute myopericarditis coexisting with IOC was considered a viable differential diagnosis.
The prognosis of patients with IOC depends on the severity of cardiac iron load, which can be semi-quantified by CMR-derived T2* relaxation time. Severe cardiac iron deposition, indicated by a T2* value < 10 ms, is associated with the poorest outcome when compared with mild-to-moderate cardiac iron deposition (T2* 10-20 ms), with risks of heart failure and arrhythmias at 1 year being 21%-47% vs 0.2% and 14% vs 4%, respectively [12].
Iron-chelating agents constitute the cornerstone of management for IOC, capable of reducing cardiac iron deposition and improving LVEF, prognosis, and survival [13]. In cases of severe IOC, such as in our presented case, continuous infusion of deferoxamine or a combination of deferoxamine and deferiprone is recommended [14].
Conclusion
This case illustrated a rare manifestation of IOC that can mimic acute myopericarditis, highlighting the importance of considering IOC as a differential diagnosis, particularly in patients with transfusion-dependent thalassemia and hemochromatosis. Careful and thorough cardiac investigations are crucial for arriving at an accurate diagnosis. Upon confirming a diagnosis of severe IOC, the prompt administration of parenteral iron-chelating agents is imperative.
Authors' contributions
PK, NT, and NU drafted the manuscript. PK, AW, NB, CW, and NU were responsible for patient care and data collection. NT, NB, CW, MT, and SS analyzed the data from investigations including blood tests, electrocardiography, echocardiography, coronary angiography, cardiac magnetic resonance, and endomyocardial biopsy. PK, NT, and MT created all the figures. NT, NU, and SS revised the manuscript. All authors provided comments on the report at various stages of development. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethics committee approval was waived.
Consent for publication
Written informed consent was obtained from the patient's next of kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of data and materials
The data for this case report is located at King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Patient consent
Written informed consent was obtained from the patient's next of kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Footnotes
Acknowledgments: The authors acknowledge the support for article processing from the Cardiac Center, King Chulalongkorn Memorial Hospital.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol. 2010;56:1001–1012. doi: 10.1016/j.jacc.2010.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulati V, Harikrishnan P, Palaniswamy C, Aronow WS, Jain D, Frishman WH. Cardiac involvement in hemochromatosis. Cardiol Rev. 2014;22:56–68. doi: 10.1097/CRD.0b013e3182a67805. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz KA, Li Z, Schwartz DE, Cooper TG, Braselton WE. Earliest cardiac toxicity induced by iron overload selectively inhibits electrical conduction. J Appl Physiol (1985) 2002;93:746–751. doi: 10.1152/japplphysiol.01144.2001. [DOI] [PubMed] [Google Scholar]
- 4.Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124:2253–2263. doi: 10.1161/CIRCULATIONAHA.111.050773. [DOI] [PubMed] [Google Scholar]
- 5.Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J Card Fail. 2010;16:888–900. doi: 10.1016/j.cardfail.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Kremastinos DT, Tiniakos G, Theodorakis GN, Katritsis DG, Toutouzas PK. Myocarditis in beta-thalassemia major. A cause of heart failure. Circulation. 1995;91:66–71. doi: 10.1161/01.cir.91.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 9.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 10.Ghugre NR, Enriquez CM, Gonzalez I, Nelson MD, Jr, Coates TD, Wood JC. MRI detects myocardial iron in the human heart. Magn Reson Med. 2006;56:681–686. doi: 10.1002/mrm.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 12.Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miskin H, Yaniv I, Berant M, Hershko C, Tamary H. Reversal of cardiac complications in thalassemia major by long-term intermittent daily intensive iron chelation. Eur J Haematol. 2003;70:398–403. doi: 10.1034/j.1600-0609.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 14.Cappellini MD, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. The management of the cardiac complications of thalassaemia major. Guidelines for the Clinical Managementof Thalassaemia. 2008:83–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this case report is located at King Chulalongkorn Memorial Hospital, Bangkok, Thailand.