Abstract
The divergence of Salmonella enterica and Escherichia coli is estimated to have occurred approximately 140 million years ago. Despite this evolutionary distance, the genomes of these two species still share extensive synteny and homology. However, there are significant differences between the two species in terms of genes putatively acquired via various horizontal transfer events. Here we report on the composition and distribution across the Salmonella genus of a chromosomal region designated SPI-10 in Salmonella enterica serovar Typhi and located adjacent to tRNAleuX. We find that across the Salmonella genus the tRNAleuX region is a hypervariable hot spot for horizontal gene transfer; different isolates from the same S. enterica serovar can exhibit significant variation in this region. Many P4 phage, plasmid, and transposable element-associated genes are found adjacent to tRNAleuX in both Salmonella and E. coli, suggesting that these mobile genetic elements have played a major role in driving the variability of this region.
Salmonella enterica and Escherichia coli are thought to have diverged from a common ancestor approximately 140 million years ago (37, 39). Despite this length of time, the genomes of these two members of the Enterobacteriaceae exhibit significant homology and synteny (35). This conservation of the genome backbone may be a reflection of the similar/overlapping environments occupied by E. coli and S. enterica. Dispersed throughout the genomes of members of S. enterica and other enteric bacteria are a number of horizontally acquired DNA segments (38), some of which contribute to pathogenicity (pathogenicity islands, or PIs). Perhaps the best characterized examples of Salmonella pathogenicity islands (SPIs) are SPI-1 and SPI-2, which encode type III secretion systems (24). PIs are frequently associated with mobile genetic elements, including transposons and bacteriophages, and are often found adjacent to tRNA genes (29, 34). Complete DNA sequencing of the genomes of several S. enterica and E. coli has led to the identification of different combinations of PIs among strains. Although some of the PIs are highly conserved between different S. enterica serovars, others are very divergent (15, 35, 40, 44).
This study investigates the composition of a region adjacent to tRNAleuX, which was termed SPI-10 and is positioned at 93.4 centisomes (genome coordinates 4683690 to 4716539) in the Salmonella enterica serovar Typhi CT18 genome sequence (40). Analysis of the tRNAleuX-associated region in silico and use of microarrays, Southern blotting, and PCR reveal that this is a hot spot for divergence within both Salmonella and E. coli. The many phage, plasmid, and transposable element-related genes or gene fragments found within this region in both S. enterica and E. coli may be a major driving force for the observed hypervariability.
MATERIALS AND METHODS
Strains.
The S. enterica and Salmonella bongori strains used in this study were a combination of both clinical and reference collections (12, 13). Isolates that were cultured for genetic analysis are detailed in Table 1. Those that were analyzed purely in silico are detailed in the “In silico genome analysis” section below. Bacteria were routinely cultured in Luria-Bertani broth or on Luria-Bertani agar overnight at 37°C.
TABLE 1.
Strain information
| Organism | Strain | Sourcea | Analysis by:
|
||||
|---|---|---|---|---|---|---|---|
| Microarrayb | Southern blottingc | PCRd | |||||
| S. enterica serovar Agona | SARB1 | SGSC | Yes | ||||
| S. enterica serovar Anatum | SARB2 | SGSC | Yes | ||||
| S. enterica serovar Binza | S106 | CVL | Yes | ||||
| S. enterica serovar Binza | S111 | CVL | Yes | ||||
| S. enterica serovar Brandenburg | SARB3 | SGSC | Yes | ||||
| S. enterica serovar Cholerasuis | SARB4 | SGSC | Yes | ||||
| S. enterica serovar Decatur | SARB8 | SGSC | Yes | ||||
| S. enterica serovar Derby | S392 | CVL | Yes | ||||
| S. enterica serovar Derby | S394 | CVL | Yes | ||||
| S. enterica serovar Derby | SARB9 | SGSC | Yes | Yes | |||
| S. enterica serovar Derby | SARB10 | SGSC | Yes | ||||
| S. enterica serovar Derby | SARB11 | SGSC | Yes | ||||
| S. enterica serovar Dublin | SARB12 | SGSC | Yes | ||||
| S. enterica serovar Dublin | S193 | CVL | Yes | ||||
| S. enterica serovar Dublin | S16 | CVL | Yes | ||||
| S. enterica serovar Duisburg | SARB15 | SGSC | Yes | ||||
| S. enterica serovar Emek | SARB20 | SGSC | Yes | ||||
| S. enterica serovar Enteritidis | SARB16 | SGSC | Yes | ||||
| S. enterica serovar Enteritidis | S21 | CVL | Yes | ||||
| S. enterica serovar Enteritidis | S97 | CVL | Yes | ||||
| S. enterica serovar Enteritidis | S222 | CVL | Yes | ||||
| S. enterica serovar Gallinarum | SARB21 | SGSC | Yes | ||||
| S. enterica serovar Gallinarum | SG9 | IAH | Yes | ||||
| S. enterica serovar Haifa | SARB22 | SGSC | Yes | ||||
| S. enterica serovar Heidelberg | SARB23 | SGSC | Yes | ||||
| S. enterica serovar Indiana | SARB25 | SGSC | Yes | ||||
| S. enterica serovar Infantis | SARB26 | SGSC | Yes | ||||
| S. enterica serovar Miami | SARB28 | SGSC | Yes | ||||
| S. enterica serovar Montevideo | SARB30 | SGSC | Yes | ||||
| S. enterica serovar Montevideo | S135 | CVL | Yes | ||||
| S. enterica serovar Montevideo | S136 | CVL | Yes | ||||
| S. enterica serovar Muenchen | SARB32 | SGSC | Yes | ||||
| S. enterica serovar Newport | SARB36 | SGSC | Yes | ||||
| S. enterica serovar Newport | S145 | CVL | Yes | ||||
| S. enterica serovar Newport | S146 | CVL | Yes | ||||
| S. enterica serovar Panama | SARB39 | SGSC | Yes | ||||
| S. enterica serovar Paratyphi A | SARB42 | SGSC | Yes | Yes | |||
| S. enterica serovar Paratyphi A | BL6301 | ICC | Yes | ||||
| S. enterica serovar Paratyphi A | BL4781 | ICC | Yes | ||||
| S. enterica serovar Paratyphi A | BL4259 | ICC | Yes | ||||
| S. enterica serovar Paratyphi A | BL5091 | ICC | Yes | ||||
| S. enterica serovar Paratyphi A | BL2846 | ICC | Yes | ||||
| S. enterica serovar Paratyphi B | SARB45 | SGSC | Yes | ||||
| S. enterica serovar Paratyphi C | SARB50 | SGSC | Yes | ||||
| S. enterica serovar Pullorum | SARB51 | SGSC | Yes | ||||
| S. enterica serovar Pullorum | 449/87 | IAH | Yes | ||||
| S. enterica serovar Reading | SARB53 | SGSC | Yes | ||||
| S. enterica serovar Rubislaw | SARB54 | SGSC | Yes | ||||
| S. enterica serovar Saintpaul | SARB56 | SGSC | Yes | Yes | |||
| S. enterica serovar Senftenberg | SARB59 | SGSC | Yes | ||||
| S. enterica serovar Senftenberg | S153 | CVL | Yes | ||||
| S. enterica serovar Senftenberg | S155 | CVL | Yes | ||||
| S. enterica serovar Stanley | SARB60 | SGSC | Yes | ||||
| S. enterica serovar Stanleyville | SARB61 | SGSC | Yes | Yes | |||
| S. enterica serovar Typhi | CT18 | ICC | Yes | Yes | Yes | ||
| S. enterica serovar Typhi | BRD948 Ty2 ΔaroC aroD htrA | ICC | Yes | Yes | |||
| S. enterica serovar Typhi | CVD908 Ty2 ΔaroC aroD | ICC | Yes | ||||
| S. enterica serovar Typhi | SARB63 | SGSC | Yes | Yes | |||
| S. enterica serovar Typhi | SARB64 | SGSC | Yes | Yes | |||
| S. enterica serovar Typhi | KT516 | ICC | Yes | Yes | |||
| S. enterica serovar Typhi | PR39 | ICC | Yes | ||||
| S. enterica serovar Typhi | 9541 | ICC | Yes | ||||
| S. enterica serovar Typhi | 422mar92 | ICC | Yes | ||||
| S. enterica serovar Typhimurium | LT2 | ICC | Yes | Yes | |||
| S. enterica serovar Typhimurium | SL1344 | ICC | Yes | ||||
| Organism | Strain | Sourcea | Analysis by:
|
||||
| Microarrayb | Southern blottingc | PCRd | |||||
| S. enterica serovar Typhimurium | S6332 Copenhagen | CVL | Yes | ||||
| S. enterica serovar Typhimurium | S1055 Copenhagen | CVL | Yes | ||||
| S. enterica serovar Typhimurium | S56 | CVL | Yes | ||||
| S. enterica serovar Typhimurium | S204 | CVL | Yes | ||||
| S. enterica serovar Typhimurium | S23 | CVL | Yes | ||||
| S. enterica serovar Typhimurium | S39 | CVL | Yes | ||||
| S. enterica serovar Typhimurium | 4015 | ICC | Yes | ||||
| S. enterica serovar Typhimurium | 4311 | ICC | Yes | ||||
| S. enterica serovar Typhimurium | mar227 | ICC | Yes | ||||
| S. enterica serovar Typhisuis | SARB70 | SGSC | Yes | ||||
| S. enterica serovar Wien | SARB71 | SGSC | Yes | ||||
| S. bongori | SARC11 | SGSC | Yes | ||||
In silico genome analysis.
The E. coli complete genome sequences analyzed were the nonpathogenic K-12 strain MG1655 (accession number NC_000913) (9) and pathogenic O157:H7 substrain RIMD 0509952 (28) (Sakai outbreak isolate, accession number NC_002695). The Salmonella complete genomes analyzed were S. enterica serovar Typhi CT18 (40) (accession number NC_003198) and Salmonella enterica serovar Typhimurium LT2 (35) (accession number NC_003197). The recently completed, but unannotated, genomes of S. bongori (strain 12419) and Salmonella enterica serovar Enteritidis (strain PT4) (www.sanger.ac.uk/Projects/Salmonella) were compared in detail with the fully sequenced genomes. The partially sequenced genome of Salmonella enterica serovar Paratyphi A (strain ATCC 9150) (www.genome.wustl.edu) was also analyzed, as described below.
Fully sequenced and annotated bacterial genomes were compared pairwise by using MegaBLAST (51) and visualized by using the Artemis Comparison Tool (http://www.sanger.ac.uk/Software/ACT). Complete, but unannotated, genome sequences were compared pairwise with annotated genomes by using the MUMmer DNA-DNA alignment tool (18). Open reading frames were predicted by Glimmer software (17), and the annotation of individual genes was refined by using the sequence alignment tools BLASTN, BLASTX (1), and BLAST 2 sequences (47) and the Conserved Domain Database (33). Artemis (http://www.sanger.ac.uk/Software/Artemis) was used to aid in the annotation of each tRNAleuX island.
All contigs from the incomplete Salmonella serovar Paratyphi A strain ATCC 9150 genome sequence (http://genome.wustl.edu/projects/bacterial/sparatyphiA/)were compared with the fully sequenced Salmonella serovar Typhi CT18 genome (40) and ordered according to the coordinates of the best alignments on the CT18 genome. From this ordered partial genome sequence, four nonoverlapping contigs, which cover the region depicted in Fig. 1 (STY4820-53), were then concatenated, compared with complete genome sequences, and visualized by using the Artemis Comparison Tool. By comparison with Salmonella serovar Typhi CT18, it was found that tRNAleuX mapped between the first two contigs and was missing from the available Salmonella serovar Paratyphi A sequence. PCR and sequencing were carried out to confirm the presence of tRNAleuX at the start of the island in Salmonella serovar Paratyphi A (described below). The other two joins between contigs (within genes resembling Salmonella serovar Typhi CT18 STY4832 and STY4849) were not closed in this way.
FIG. 1.
Comparisons of sequenced E. coli and Salmonella genomes reveal seven different organizations of tRNAleuX-adjacent genes. Predicted intact open reading frames and pseudogenes (marked with a cross) are shown as arrows indicating the direction of transcription. The Salmonella serovar Typhimurium LT2 STY4854-like pseudogene is shown as a cross without an arrow because it has lost its start codon. The boundaries of each island are marked with thick vertical lines. Genes shaded with spots are common to two or more of the islands shown and therefore lie outside of the tRNAleuX-adjacent islands as defined in this study. Genes of related origin or function are shaded similarly, as indicated in the key. The complete fully annotated genome sequences analyzed are those of E. coli nonpathogenic K-12 (strain MG1655 accession number NC_000913) (9) and pathogenic O157:H7 substrain RIMD 0509952 (28) (accession number NC_002695), Salmonella serovar Typhi CT18 (40) (accession number NC_003198), and Salmonella serovar Typhimurium LT2 (35) (accession number NC_003197). Gene numbers assigned by the sequencing projects are indicated for LT2 (35) and CT18 (40). Partially sequenced or fully sequenced, but unannotated, genomes that were also compared are as follows: S. enterica subsp. I serovars Enteritidis (strain PT4) and Paratyphi A (strain ATCC 9150) and S. bongori (stain 12419) (www.sanger.ac.uk/Projects/Salmonella and www.genome.wustl.edu). The genes and pseudogenes shown for Salmonella serovar Paratyphi A are predicted to be present by comparison with Salmonella serovar Typhi CT18. The incomplete Salmonella serovar Paratyphi A genome sequence contains many additional frameshifts and is missing regions between contigs compared with CT18, but as these features remain to be verified, they are not represented here.
Microarray analysis of genomic DNA.
The design and construction of the Salmonella serovar Typhi CT18 microarray used in this study is described elsewhere (48). The array contains 4,097 screened and refined PCR products (200 to 500 bases) from annotated coding sequences from the chromosome of Salmonella serovar Typhi CT18 (40). Genomic DNA was extracted by using the hexadecyltrimethylammonium bromide (Sigma-Aldrich Ltd., Dorset, United Kingdom) method (3). DNA from 40 S. enterica or S. bongori strains (Table 1) plus CT18 itself was sonicated (10 s, level 2, Virsonic sonicator) and labeled with FluoroLink Cy3 or Cy5 dyes by using the Bioprime kit (Invitrogen Ltd., Paisley, United Kingdom). In each case, Salmonella serovar Typhi CT18 DNA was used as the reference strain. Labeled DNA was then purified by using an Autoseq G-50 column (Amersham Biosciences, Chalfont St. Giles, United Kingdom), denatured, and precipitated, and finally, the probes were hybridized to the Salmonella microarray slide overnight at 49°C (http://www.sanger.ac.uk/Projects/Microarrays/arraylab/methods.shtml).
After stringent washing, hybridization results were detected by using a Genepix 4000B scanner (Axon Instruments, Inc., Union City, Calif.) and quantified with Genepix Pro software (Axon Instruments, Inc.). Data analysis was carried out as described by Thomson et al. (48). The final readouts were mean Lowess-normalized Cy3/Cy5 ratio intensities [Ln(Cy3/Cy5)] for up to eight data points (four arrays carried out for each test strain and each gene spotted in duplicate). Data were ordered and labeled according to the Salmonella serovar Typhi CT18 genome gene names (STY0001 to STY4949). The presence/conserved or absence/divergent status of each gene was assigned according to its final Ln(Cy3/Cy5) ratio intensity and was given a standard GeneSpring colored representation (color scale represented in Fig. 2), as follows: absent/divergent genes, Ln(Cy3/Cy5) below 0.3, blue; genes that could not be determined present/conserved or absent/divergent, Ln(Cy3/Cy5) between 0.3 and 0.45; present/conserved genes, Ln(Cy3/Cy5) greater than or equal to 0.45, yellow; missing data, grey.
FIG. 2.
Microarray comparison of Salmonella serovar Typhi CT18 SPI-10 with different salmonellae. The microarray data image was constructed by using GeneSpring software. Functional groups into which the SPI-10 genes can be divided, the direction of their transcription (depicted by arrows for each gene), and the G-C content of this mosaic island (noted in red) are shown at the top. Each row of data is the result of challenging the microarray with 40 different Salmonella isolates, which are labeled along the right hand side and are described in Table 1. Each column represents a specific gene either within (STY4821 to STY4852) or on either side of (STY4820 and STY4853-56) the Salmonella serovar Typhi CT18 tRNAleuX island. The color scheme for the present/conserved or absent/diverged nature of the genes is displayed (bottom). The darkest blue corresponds to those genes that are considered absent/divergent with the highest degree of certainty. The brightest yellow corresponds to genes that are assigned present/conserved with the highest degree of certainty. Those regions that are orange are genes where hybridization with test DNA is higher than that of reference DNA. Grey indicates missing data. It can be seen that data for different strains of the same serovar are generally very similar, with one clear exception: Salmonella serovar Typhi strain KT516 (marked with an asterisk) does not contain an intact CT18-like P4 phage (STY4821 to STY4834).
Southern blotting.
Genomic DNA (2.5 to 5 μg) was restricted with EcoRV (New England Biolabs [United Kingdom] Ltd., Hitchin, United Kingdom) overnight at 37°C and separated on a 1% agarose gel. Using standard methods described previously (45), the gel was treated and transferred to a Hybond-N+ membrane (Amersham Biosciences), which was then UV fixed. Southern blot probes were designed to encompass at least 600 bp of each gene, or genes, to be detected and were based upon either Salmonella serovar Typhimurium LT2 or Salmonella serovar Typhi CT18 genome sequences. Probes were prepared by PCR with primers (see Table S1 in the supplemental material) to detect the following genes: for LT2, STM4493 and STM4496-98; for CT18, aroC (STY2616) and STY4822. PCR was carried out with 10 ng of genomic template DNA, Red Taq (Sigma-Aldrich) in the buffer provided by the manufacturer (containing 1.5 mM Mg2+), 200 μM concentrations of deoxynucleoside triphosphates, and 10 pmol of each primer. PCR conditions were as follows: 94°C for 1 min; 30 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 2 min; and final elongation at 72°C for 2 min. PCR products were purified by using QIA quick PCR purification kits (QIAGEN Ltd., Crawley, United Kingdom). To confirm that the correct PCR products had been amplified, the sizes of the products were checked and restriction patterns (restriction enzymes from New England Biolabs) were assessed: the aroC PCR product was digested with SmaI, the STY4822 PCR product was digested with KpnI, the STM4493 PCR product was digested with BamHI, and the STM4496-98 PCR product was digested with PstI. Random prime labeling of probes (using fluorescein-labeled nucleic acids, random primers, and Klenow polymerase), hybridizations (60°C overnight), and an enhanced chemiluminescence-based detection were carried out by using the Random prime labeling and detection system version II (Amersham Biosciences) according to the manufacturer's instructions.
The stringencies of the Southern hybridizations were assessed by using the CT18-drived aroC probe to blot for aroC in E. coli K-12 (83% identity within the probe region) and Salmonella serovar Typhimurium LT2 (98% identity within the probe region). With 2.5 μg of DNA (the minimum amount used subsequently), a weak, but clearly visible, aroC DNA fragment was seen with K-12 DNA (data not shown). This suggests that, under the blotting conditions used, any gene with at least 83% identity to the probe should be picked up. Also, it should be noted that the aroC probe is the shortest of the probes used in the Southern blotting screen (see Table S1 in the supplemental material), which should make it the least sensitive (45).
PCR screening of tRNAleuX-associated genes in different salmonellae.
PCR comparisons of tRNAleuX-associated genes in different salmonellae were carried out by using either the Salmonella serovar Typhimurium LT2 or Salmonella serovar Typhi CT18 genome sequence for primer design as appropriate. For the PCRs shown in Fig. 3a, Fig. 5i and Fig. 6i to iii, amplification reactions were carried out by using Red Taq DNA polymerase as described above for the preparation of PCR products for Southern blot probes but with 3-min 72°C elongation steps and primers detailed in Table S1 in the supplemental material. For the PCRs shown in Fig. 5ii and Fig. 6iv, the Expand high-fidelity PCR system (Roche Diagnostics Ltd., Lewes, United Kingdom) was used according to the manufacturer's instructions, as it contains a polymerase that is more efficient than Red Taq for amplification of larger DNA fragments, with 8-min elongation steps at 68°C, an annealing temperature of 54°C, and primers that are detailed in Table S1 in the supplemental material.
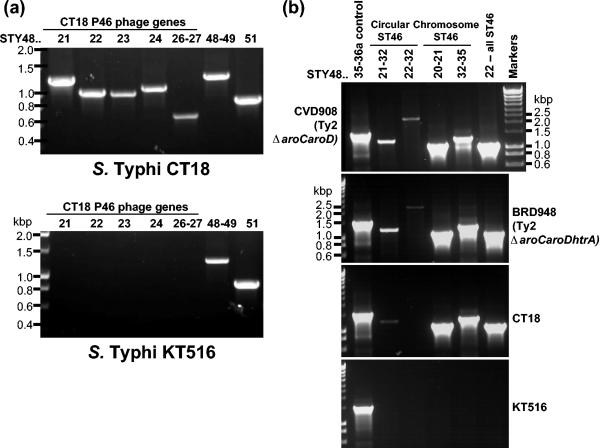
FIG. 3.
(a) PCR analysis confirms the absence of the CT18 P4 phage in Salmonella serovar Typhi KT516. The primers used for these analyses are as follows (see also Table S1 in the supplemental material): lane 1, primer pair 1-3; lane 2, primer pair 4-5; lane 3, primer pair 6-7; lane 4, primer pair 8-9; lane 5, primer pair 10-11; lane 6, primer pair 12-13; lane 7, primer pair 14-15. The CT18 genome numbers for each gene (or genes) amplified and the five PCR products that lie within the CT18 P4 phage (STY4821-24 and STY4826-27) are noted above the gel. PCR analysis of Salmonella serovar Typhi KT516 confirms the observation, made using microarrays (Fig. 2), that this strain does not contain an intact CT18-like P4 phage, whereas transposase STY4848 and the helicase-containing region (STY4849 and STY4851) are present. (b) Circularized ST46 phage DNA was detected by PCR with genomic DNA from Salmonella serovar Typhi strains BRD948, CVD908, or CT18 but not with KT516, which is missing ST46. The CT18 genome numbers for each gene (or genes) amplified are noted above the gel. Whether the primers were designed to amplify chromosomal and/or circular phage DNA products is indicated. The primers used are detailed in Materials and Methods.
FIG. 5.
Examples of PCR analysis of Salmonella serovar Saintpaul SARB56 and Salmonella serovar Stanleyville SARB61 compared with Salmonella serovar Typhimurium LT2. Strain names are noted below each panel. The LT2 genome numbers for each gene (or genes) amplified are noted above each panel. (i) PCR with primers STM4492_for and STM4495_rev (see Table S1 in the supplemental material) reveal that SARB61 and LT2 contain a similarly sized DNA fragment between STM4492 and STM4495. SARB56, on the other hand, contains only a short DNA fragment between STM4492 and STM4495. (ii) PCR with primers STM4490_for and STM4492_rev (see Table S1 in the supplemental material) revealed a similarly sized DNA fragment between the STM4490-like and STM4492-like genes of SARB56 and SARB61, which is larger than the equivalent region in LT2. (iii and iv) Schematic depiction of the conclusions drawn from Southern blotting (Table 2 and Fig. 4), PCR (panels i and ii), and sequencing data comparing SARB56 and SARB61 tRNAleuX islands with that of Salmonella serovar Typhimurium LT2. STM4495 lies adjacent to STM4492 in SARB56, and a novel insertion is present between STM4490 and STM4491 in both SARB56 and SARB61 (containing one predicted gene shown here in black). Genes that were detected by Southern blotting (Table 2) are shown in gray.
FIG. 6.
Examples of PCR comparison of tRNAleuX island genes from LT2 with Salmonella serovar Derby strains SARB9 and SARB10. The LT2 genome numbers for each gene (or genes) amplified are noted above each panel and numbers for genes that were not detected by PCR or where insertions were found to be present compared with LT2 are underlined. The primers used for the PCR analyses shown in panels (i to iii) are as follows (see also Table S1 in the supplemental material): lane 1, primer pair 16-17; lane 2, primer pair 16-19; lane 3, primer pair 20-22; lane 4, primer pair 21-25; lane 5, primer pair 24-26; lane 6, primer pair 24-27; lane 7, primer pair 24-28; lane 8, primer pair 29-30; lane 9, primer pair 29-31. (i) Salmonella serovar Derby SARB9 contains genes similar to LT2 genes STM4490 to STM4499, but STM4488- and STM4489-like genes were not detected. (ii) Salmonella serovar Typhimurium LT2 controls for PCRs shown in panels i and iii. (iii) None of the LT2 tRNAleuX island genes (STM4488 to STM4498) were initially detected in Salmonella serovar Derby SARB10 by PCR. STM4499 (data not shown) and STM4487, which lie on either side of the island, were both present. (iv) PCR with a long-range DNA polymerase with primers STM4487_for and STM4488_rev (see Table S1 in the supplemental material) revealed a larger DNA fragment between STM4487 and STM4488 in SARB10 than in the equivalent region in LT2. (v and vi) Schematic depiction of the gene arrangements in SARB9 and SARB10 supported by PCR data, such as that shown in panels i to iv, Southern blotting (Table 2 and Fig. 4), and sequencing of the SARB10 insertion between tRNAleuX and STM4488 (amplified with primers tRNA_for and STM4488_rev). The sequences that lie between tRNAleuX and STM4490 in SARB9 and between STM4488 and STM4499 in SARB10 remain unknown (shown as question marks). Genes detected by Southern blotting (Table 2) are shown in grey.
A number of PCR products were cloned and sequenced as follows. To confirm the presence of tRNAleuX between STY4820 (similar to STM4487)- and STY4821-like genes in Salmonella serovar Paratyphi A, PCR was carried out with Salmonella serovar Paratyphi A SARB42 template DNA, with Red Taq polymerase (as described above) and primers STM4487_for and STY4821_rev2. Additional novel PCR products were generated from SARB61 and SARB56 genomic DNA with STM4490_for and STM4492_rev primers and from SARB56 with STM4492_for and STM4495_rev primers, all with Red Taq DNA polymerase. Amplification from SARB10 DNA with tRNA_for and STM4488_rev primers was carried out by using the Expand high-fidelity PCR system (conditions described above, but 5-min 68°C elongation steps). For each of these PCRs, the primers are detailed in Table S1 in the supplemental material. Each PCR product, from at least two separate PCRs in each case, was purified by using the QIAGEN PCR purification kit and sequenced both directly and after cloning into pGEM-T Easy (Promega United Kingdom Ltd., Southampton, United Kingdom).
PCR-based detection of circularized ST46 phage.
PCR for detection of circularized ST46 phage in genomic Salmonella serovar Typhi DNA preparations (Fig. 3b) was carried out by using Red Taq DNA polymerase as described above but using 3-min elongation steps. The primers used to amplify the PCR products shown in Fig. 3b were as follows. Circular ST46 was amplified by using either ST46_rev (ATCAATGCCCTGCACTAGCAAC, reverse-strand primer within integrase gene termed STY4821 in CT18) with ST46_for (CAGACCAACGGGATGTTTATGG, forward-strand primer within P4 phage α gene termed STY4832 in CT18) or STY4822_rev2 (CTAGAGCACACCCTGTAATTCTTTG, reverse-strand primer within ST46-specific phage cos site insertion gene termed STY4822 in CT18) with ST46_for. Chromosomal ST46 was amplified using either STY4835_rev (AATGCGGGCAGCAGTGTTTA) with ST46_for or STM4487_for (which recognizes STY4820, listed in Table S1 in the supplemental material) with ST46_rev. Control PCRs amplified either chromosomal genes outside ST46 by using STY4835_for (TAAACACTGCTGCCCGCATT) with STY4836a_rev (CCTGAACCTCTGCTTTGTTA) or all ST46 phage by using STY4822_for with STY4822_rev (listed in Table S1 in the supplemental material). Circular ST46 phage-derived PCR products amplified from BRD948 genomic DNA were gel purified by using a QIAGEN gel purification kit and sequenced to confirm that they were the expected products.
Genome walking for analysis of Salmonella serovar Typhi isolate KT516 tRNAleuX island.
Genome walking was carried out by using the Universal GenomeWalker kit (BD Biosciences, Palo Alto, Calif.). Genomic DNA (1.5 μg) was digested with enzyme DraI, EcoRV, or SspI (New England Biolabs), and DNA was phenol-chloroform purified, precipitated, and resuspended in 20 μl of distilled H2O. Four microliters of each digested DNA solution was ligated to adaptors provided by the manufacturer, the ligase was heat inactivated, and the solution was made up to 80 μl with Tris-EDTA buffer. PCR amplification was carried out by using the Expand high-fidelity PCR system (conditions described above but with 6-min extensions that were modified for the final 20 cycles with 15-s increments per cycle). First-round PCR mixtures contained 1 μl of each ligation mixture as the template and used adaptor primer 1 (AP1, GTAATACGACTCACTATAGGGC) with tRNAfor_outer (CGTTTTCCGCATACCTCTTC) or with STY4835rev_outer (CTTTGCCAGCCGGGAAATAATG) and an annealing temperature of 55°C. Second-round PCR mixtures contained 1 μl of first-round PCR product as a template and used adaptor primer 2 (AP-2, ACTATAGGGCACGCGTGGT) with tRNAfor_inner (AAGTGGCGAAATCGGTAGAC) or with STY4835_rev (see Table S1 in the supplemental material) as appropriate and an annealing temperature of 57°C. PCR products amplified in the second round were gel purified by using the QIAGEN gel purification kit and sequenced.
Novel DNA sequence accession numbers.
The Salmonella serovar Paratyphi A SARB42 tRNAleuX region (STY4820- to STY4821-like genes) PCR product was assigned accession number AY775127. The Salmonella enterica serovar Derby SARB10 insertion between tRNAleuX and the STM4488-like gene was assigned accession number AY786416. The SARB56 novel region within the insertion between STM4490 and STM4491 was assigned accession number AY775129. The SARB61 novel region within the insertion between STM4490 and STM4491 was assigned accession number AY775128. The Salmonella serovar Typhi KT516 genome walking 5′ sequence was assinged accession number AY795072. The Salmonella serovar Typhi KT516 genome walking 3′ sequence was assigned accession number AY795073.
RESULTS AND DISCUSSION
Comparison of tRNAleuX-associated regions of E. coli and Salmonella genomes reveals distinct organizations.
During our efforts to annotate the Salmonella serovar Typhi CT18 genome, we noted that the tRNAleuX-associated region of different S. enterica and E. coli isolates are extremely variable, often encoding completely unrelated DNA sequences and genes. Thus, the tRNAleuX may be one of the most variable regions of the S. enterica and E. coli genomes. Consequently, we decided to analyze this variation within S. enterica in more detail. Initially, we examined the tRNAleuX-associated genes from sequenced Salmonella and E. coli strains by using in silico methods. To support and extend these studies, we utilized S. enterica-based microarrays, Southern blotting, PCR, and DNA sequencing to characterize this region from representative isolates of different S. enterica serovars. Our aim was to try to capture information about the mechanisms driving this diversity and the type of genes acquired by this region in different S. enterica serovars. Since we analyzed a large number of different DNA sequences, we present here the key conclusions of this study but provide an in depth analysis of individual sequences as a supplementary data set. This supplementary set can be used by others to facilitate genetic analysis of particular serovars and to construct broader and more representative S. enterica-based microarrays.
The tRNAleuX-associated region of Salmonella serovar Typhi CT18 is known as SPI-10 and encodes a P4-like phage, the sef/pef fimbrial islet, IS element remnants, and genes of unknown function. Comparisons of the Salmonella serovar Typhi SPI-10 sequence with other sequenced enteric bacteria revealed remarkable heterogeneity (this study and previous partial sequence data) (21). For example, the gene complements at this locus in Salmonella serovar Typhi and Salmonella serovar Typhimurium are entirely different (Fig. 1). The tRNAleuX-associated islands ranged in size from over 44 kbp in E. coli O157:H7 Sakai to just over 11.6 kbp in S. bongori 12418. The predicted characteristics of some of the genes adjacent to tRNAleuX are depicted in Fig. 1 and are described in more detail in the supplemental material (see Tables S2 to S7 in the supplemental material). General themes of the tRNAleuX islands are the appearance of genes related to P4 phages and transposable elements and plasmids, suggesting that mobile elements may drive the hypervariability in this region (Fig. 1). In some cases, the mosaic structures of these islands are also reflected in variations in G-C content. For example, the region encompassing the sef fimbrial genes has a particularly low G-C content (34.14% compared to 52.17% in Salmonella serovar Enteritidis PT4, as previously noted [21], and 34.2% in Salmonella serovar Typhi CT18 compared with 52.09% overall in the genome) (Fig. 2). A region containing three nonphage genes, STY4822-24, inserted into the Salmonella serovar Typhi CT18 P4 phage also has a low G-C content (36.4%) (Fig. 2).
The GntII (subsidiary) system for gluconate metabolism and l-idonic acid catabolism (5) lies immediately 5′ of tRNAleuX in E. coli K-12. A series of small deletions and rearrangements can be detected in different salmonellae, but the region of major divergence begins 3′ of tRNAleuX. Uropathogenic E. coli strain 563 harbors a distinct PI at this locus, the ends of which are clearly defined by repeats of 18 bp (20). In contrast, repeats do not appear to mark the ends of the islands shown in Fig. 1. Orthologs of two genes, yeeN (encoding a conserved hypothetical protein) and yjhP (encoding a putative methyltransferase), are most commonly present at the 3′ end of the tRNAleuX islands. The S. bongori 12419 island ends with two adjacent genes, ORF_5468 and ORF_5469, which are together 84% identical to the full Salmonella serovar Typhi CT18 yjhP-like gene STY4856. In E. coli K-12, the sgc operon, which codes for a potential phosphoenolpyruvate sugar phosphotransferase system, is present upstream of yjhP. This can be considered to be the end of the K-12-specific island, as Salmonella serovar Typhi CT18 also contains orthologs of the sgc system at a different position in the genome (STY1447 to STY1450 and STY1452). Delineation of the borders of the tRNAleuX islands was supported for a number of different Salmonella by microarray analysis, which yielded a positive hybridization for STY4820, just prior to tRNAleuX, for all strains tested (Fig. 2). The 3′ limit of the Salmonella serovar Typhi CT18 serovar-specific PI was less distinct (Fig. 2), but the in silico assignment of STY5852 as the last Salmonella serovar Typhi-specific SPI-10 gene is supported by the microarray data. STY4852 is conserved in all Salmonella serovar Paratyphi A and Salmonella serovar Typhi strains tested but is absent from all other Salmonella strains, whereas STY4853 is detected in many Salmonella strains, with the marked exception of all 10 Salmonella serovar Typhimurium strains tested (Fig. 2). STY4854 is similar in distribution to ST4853, but two Salmonella serovar Typhimurium isolates (39 and 204) show positive STY4854 hybridizations, the majority of Salmonella isolates harbor STY4855, with exceptions being Salmonella serovar Typhimurium isolates 23, 1055, and 56, and all Salmonella isolates tested positive for STY4856 (Fig. 2).
P4 family phages as drivers of diversity in S. enterica.
A P4-related prophage, termed ST46 (48), lies adjacent to tRNAleuX in Salmonella serovar Typhi CT18. P4-like phages are known to target a 20-bp sequence within tRNAleuX, resulting in duplication of the attachment site sequence at either end of the prophage insertion (42). Remnants of one such duplication flank the P4 phage of Salmonella serovar Typhi: 19 bp of the 20-bp tRNAleuX attP P4 phage attachment site are duplicated within the predicted P4 phage-related gene STY4834 at the extreme 3′ end of the prophage element. Salmonella serovar Paratyphi A isolate ATCC 9150 harbors a similar P4-like prophage at the same position (Fig. 1). The only major difference between the Salmonella serovar Typhi and Salmonella serovar Paratyphi A P4 phages are the genes that are inserted into the phage cos site, which is a common spot for integration of nonphage cargo DNA (48). Salmonella serovar Typhi ST46 contains an insertion of three genes with homology to serine/threonine protein kinases (STY4822-23) and a PP2C family-like serine/threonine protein phosphatase (STY4824) (48). The Salmonella serovar Paratyphi A P4-like phage encodes a cos site insertion of three genes that code for a restriction modification system (36). The P4 phages of Salmonella serovar Typhi and Salmonella serovar Paratyphi A both contain an unusual feature, Salmonella serovar Typhi CT18 gene STY4830 (96% identical over all 228 bp in Salmonella serovar Paratyphi A) replaces the normal P4 phage genes ɛ and orf151. These data suggest that Salmonella serovar Paratyphi A and Salmonella serovar Typhi may have acquired their resident phage from a common ancestor whose P4 phage subsequently picked up different cos site insertions.
Salmonella serovar Typhi CT18 microarray data confirmed the presence of a CT18-like P4 phage, STY4821-34, in all but one of the Salmonella serovar Typhi isolates tested and a related phage in all Salmonella serovar Paratyphi A strains. All other S. enterica serovars tested did not contain a closely related P4 phage, although hybridization with one phage gene, STY4826, a homologue of the P4 δ subunit, is common to all but Salmonella enterica serovar Senftenberg and S. bongori (Fig. 2). The Salmonella serovar Typhi-specific nature of STY4822-24 was confirmed by the lack of any hybridization with STY4822-24 by non-serovar Typhi Salmonella isolates (microarray results are shown in Fig. 2; Southern blotting results with STY4822 probe are shown in Table 2 and Fig. 4).
TABLE 2.
Summary of Southern blot data for Salmonella serovar Typhi and Salmonella serovar Typhimurium SPI-10 genes
| S. enterica subsp. I serovar | Strain | Southern blotting bands visible forc:
|
|||
|---|---|---|---|---|---|
| aroC | Salmonella serovar Typhi CT18 ST46 phage gene STY4822 (phage insert) |
Salmonella serovar Typhimurium LT2 tRNAleuX island gene:
|
|||
| STM4493 | STM4496-98 | ||||
| Typhimurium | LT2 | Yes | No | Yes | Yes |
| Typhimurium | SL1344 | Yes | No | Yes | Yes |
| Typhi | CT18 | Yes | Yes | No | No |
| Typhi (Ty2 ΔaroC aroD htrA) | BRD948 | No | Yes | — | — |
| Agona | SARB1 | Yes | No | No | No |
| Anatum | SARB2 | Yes | No | No | No |
| Brandenberg | SARB3 | Yes | No | No | No |
| Choleraesuis | SARB4 | Yes | No | No | No |
| Decatur | SARB8 | Yes | No | No | No |
| Derbyb | SARB9 | Yes | No | Yes | Yes |
| Dublina | SARB12 | Yes | No | No | No |
| Duisburg | SARB15 | Yes | No | No | No |
| Enteritidisa | SARB16 | Yes | No | No | No |
| Emek | SARB20 | Yes | No | No | No |
| Gallinaruma | SARB21 | Yes | No | No | No |
| Haifa | SARB22 | Yes | No | No | No |
| Heidelberg | SARB23 | Yes | No | No | No |
| Indiana | SARB25 | Yes | No | No | No |
| Infantis | SARB26 | Yes | No | No | No |
| Miami | SARB28 | Yes | No | No | No |
| Montevideob | SARB30 | Yes | No | No | No |
| Muenchen | SARB32 | Yes | No | No | No |
| Newportb | SARB36 | Yes | No | No | No |
| Panama | SARB39 | Yes | No | No | No |
| Paratyphi Aa | SARB42 | Yes | No | — | No |
| Paratyphi B | SARB45 | Yes | No | — | No |
| Paratyphi C | SARB50 | Yes | No | — | No |
| Pulloruma | SARB51 | Yes | No | No | No |
| Reading | SARB53 | Yes | No | No | No |
| Rubislaw | SARB54 | Yes | No | No | No |
| Saintpaul | SARB56 | Yes | No | No | Yes |
| Senftenbergb | SARB59 | Yes | No | No | No |
| Stanley | SARB60 | Yes | No | No | No |
| Stanleyville | SARB61 | Yes | No | Yes | Yes |
| Typhi Vi +ve | SARB63 | Yes | Yes | — | — |
| Typhi Vi −ve | SARB64 | Yes | Yes | — | — |
| Typhisuis | SARB70 | Yes | No | No | No |
| Wien | SARB71 | Yes | No | No | No |
FIG. 4.
Examples of Southern blots showing that STY4822 is Salmonella serovar Typhi specific and that the Salmonella serovar Typhimurium SPI-10 genes tested (STM4493 and STM4496-98) are only shared with three other serovars: Salmonella serovar Derby (not shown here), Salmonella serovar Saintpaul, and Salmonella serovar Stanleyville. Data were only included in the final analysis (summarized in Table 2) where a clear aroC control band was visible (with the exception of Salmonella serovar Typhi BRD948, which is an aroC deletion mutant). Strain names and serovars are noted above each panel, and the probes used are indicated at the side. (a) Four Salmonella serovar Typhi strains tested contain STY4822, whereas strains of Salmonella serovar Typhimurium, Salmonella serovar Paratyphi A, Salmonella serovar Paratyphi B, and Salmonella serovar Paratyphi C do not. (b) STY4822 is present in the control Salmonella serovar Typhi CT18, but it is not detected in 11 SARB collection strains tested here. Salmonella serovar Typhimurium LT2 tRNAleuX-adjacent genes, on the other hand, are shared with Salmonella serovar Stanleyville (STM4493 and STM4496-98) and Salmonella serovar Saintpaul (STM4496-98), and are shown in lanes that are boxed.
In addition to the full P4 prophages described above, a number of predicted phage integrases, or fragments of integrases, are also associated with the tRNAleuX region (Fig. 1). For example, Salmonella serovar Typhi and Salmonella serovar Paratyphi A contain identical 159-bp integrase remnants between the end of the P4 phage (STY4834) and the neighboring transposase pseudogene (STY4835). The Salmonella serovar Typhimurium LT2 tRNAleuX island begins with STM4488, which is a highly truncated P4-related integrase (33% identical to P4 int over just 89 amino acids). S. bongori contains an even more truncated integrase gene that is very similar (94% identical) to STM4488 over its 211-bp length.
Salmonella serovar Typhi KT516 is an unusual Salmonella serovar Typhi isolate that does not hybridize with most of the CT18 ST46 phage genes but is positive for the neighboring fimbriae-related and helicase-related CT18 genes (Fig. 2). The absence of the ST46 phage in Salmonella serovar Typhi KT516 was confirmed by PCR (Fig. 3). Thus, although Salmonella serovar Typhi isolates are often described as being highly clonal, variation can be detected in this region even with a small set of sample isolates.
Attempts to amplify across the region from tRNAleuX to STY4835 failed, although the primer sites are intact in KT516 (our unpublished observations). Genome walking from the conserved ends of the KT516 island (tRNAleuX and STY4835) and sequencing of the resulting PCR products has enabled us to begin characterizing the KT516 insertion. A novel P4 integrase pseudogene (76% identical over 139 amino acids to Salmonella serovar Typhi CT18 STY4821 and interrupted by a stop codon) lies next to tRNAleuX. At the other end of the island, next to the STY4835-like gene, we find that STY4834 is conserved but only up to the partial P4 attachment site within this open reading frame. 5′ of this is a novel sequence containing an open reading frame with 42% identity over 147 amino acids to a conserved hypothetical protein in Xylella fastidiosa Ann-1 (accession number NP_297454). Thus, whereas most Salmonella serovar Typhi isolates contain the ST46 prophage, KT516 is carrying novel sequences absent from the other tRNAleuX islands investigated so far.
Detection of circularized ST46 DNA supports a continuing role for P4 phage in genome diversification at the tRNAleuX locus.
The detection of circularized ST46 DNA would suggest that this phage can be mobilized from the lysogenic state and would support a continuing role for P4 phages in genome diversification at the tRNAleuX locus (14). To investigate this possibility, PCR was used to detect circularized ST46 released by Salmonella serovar Typhi BRD948. Putative phage particles were purified from bacterial lysates with chloroform extraction, and these were found to contain detectable circularized phage DNA (data not shown). The phage extracts were not able to cause lysis of target bacteria (E. coli, Salmonella serovar Enteritidis, and Salmonella serovar Typhimurium strains were tested) (data not shown). Salmonella serovar Typhi BRD948 genomic DNA (extracted from bacteria grown to stationary phase) was also tested for the presence of circular ST46 phage DNA by PCR and was found to be clearly positive (Fig. 3b).
Genomic DNA from Salmonella serovar Typhi CVD908 (Ty2 ΔaroC aroD), without the htrA mutation harbored by BRD948 that could potentially affect phage release (htrA is involved in the stress response) (31), was also clearly positive for circular ST46 (Fig. 3b). Importantly, Salmonella serovar Typhi KT516 was negative for both circular and chromosomal ST46 PCR products, consistent with the absence of ST46 in this Salmonella serovar Typhi strain (Fig. 3b). Circular ST46 was also detectable by using Salmonella serovar Typhi CT18 genomic DNA as the template but at a much lower level than with the Ty2-derivative strains. We are currently testing the hypothesis that efficient circularization of ST46 is facilitated by an additional P4 lysogenic phage (ST2_27) known to be present within Ty2 and absent from CT18 (19, 48).
Evidence for genes from other mobile elements in the tRNAleuX-associated region. (i) Plasmid-related genes and IS elements.
SPI-10 of Salmonella serovar Typhi CT18 harbors a truncated pef (plasmid-encoded fimbrial) operon and the full sef (Salmonella serovar Enteritidis fimbriae) operon, forming a pathogenicity islet (16). This Salmonella serovar Typhi islet is associated with six pseudogenes, which are also pseudogenes in Salmonella serovar Typhi strain Ty2 and Salmonella serovar Paratyphi A (Fig. 1) (19, 21, 49), that are likely to inactivate the fimbrial operons. The sef/pef islet is flanked by IS element remnants, which may have facilitated its insertion into the chromosome (16) (Fig. 1). Homologues of the CT18 sef genes STY4836a/sefA-STY4841/sefR were clearly present in all six Salmonella serovar Typhi isolates tested on microarrays (Fig. 2). The majority of these genes, all but sefB, are also detected in Salmonella serovar Paratyphi A, Salmonella enterica serovar Pullorum, Salmonella enterica serovar Gallinarum, Salmonella serovar Enteritidis, and Salmonella enterica serovar Dublin strain 16. This is consistent with our in silico analysis (Fig. 1) and with previous literature describing the sef genes by Southern blotting for sefC and sefD (4) or sefA (16) (Table 2). Hybridization with sefC was observed for all serovars tested, and in some cases, hybridization with sefR was also observed (Fig. 2), suggesting that similar fimbrial genes are present in serovars where the full sef operon is absent. Of the serovars tested, only Salmonella serovar Dublin and Salmonella serovar Enteritidis have been reported to actually elaborate sef fimbriae (16).
The origins of the sef operon are not known, while the pef genes present in the chromosomes of some salmonellae are thought to be of plasmid origin, as full pef fimbrial operons are found on the F-type virulence plasmids of Salmonella serovar Typhimurium (pSLT), Salmonella enterica serovar Choleraesuis (pKDSC50), and Salmonella serovar Enteritidis (pS72) (26). S. bongori SARC11 is not strongly positive with microarray analysis for any of the CT18 plasmid-derived pef genes, consistent with in silico analysis of its tRNAleuX-associated island and the lack of any reported pef-containing plasmid. Of the other salmonellae tested, Salmonella enterica serovar Montevideo, Salmonella serovar Senftenberg, Salmonella serovar Derby, Salmonella enterica serovar Binza, and Salmonella enterica serovar Newport give positive hybridizations with just one of the four CT18 pef genes, STY4846 (Fig. 2), suggesting that the chromosomal pef genes are not present in these serovars. The presence of integrated plasmid-related genes in serovars such as Salmonella serovar Typhi, Salmonella serovar Paratyphi A, and Salmonella serovar Enteritidis strongly suggests that these serotypes had a common ancestor that acquired these genes from a plasmid. Indeed, the Salmonella serovar Enteritidis tRNAleuX region encodes three more putative plasmid-related genes, which lie between IS630 and IS1230 elements at the start of the island (see Table S6 in the supplemental material). IS elements, or their remnants, are present at many points in the tRNAleuX islands of different Salmonella and E. coli strains (Fig. 1) (9) and may have contributed to diversity at tRNAleuX by mediating exchanges between the Salmonella chromosome and horizontally transferred DNA such as plasmids.
(ii) Genes found in conjugative elements are present in the Salmonella serovar Typhimurium tRNAleuX island.
The tRNAleuXisland of Salmonella serovar Typhimurium is distinct in DNA sequence and gene content from SPI-10 of Salmonella serovar Typhi. The island in Salmonella serovar Typhimurium LT2 is 20,842 kbp in length and includes coding sequences designated STM4488 to STM4498 (Fig. 1). The genes in the Salmonella serovar Typhimurium island do not have a clear functional relationship, although a possible link to DNA repair has been suggested (43). This hypothesis is based on the presence of a predicted helicase (STM4489), a putative ATPase involved in DNA repair (STM4496), a predicted type II restriction enzyme methylase subunit (STM4495), and a predicted Mrr restriction endonuclease (STM4490). In addition to these genes, STM4494 is predicted to be an ATPase component of an ABC-type sugar/spermidine/putrescine transport system and STM4491 is a predicted Lon protease. STM4498, STM4497, STM4492, and STM4493 are genes of unknown function.
We have observed a striking homology and synteny between 6 of the 10 predicted open reading frames in Salmonella serovar Typhimurium SPI-10 (STM4491, STM4492, STM4495, STM4496, STM4497, and STM4498) and genes, or fragments of genes, found within conjugative elements harbored by other enteric bacteria (see Table S8 in the supplemental material), with these being an uncharacterized E. coli plasmid p1658/97 (accession number AF550679) and two well-characterized IncJ-related conjugative integrating genomic elements: Vibrio cholerae SXT transposon-like element (accession number AY055428) (7) and Providencia rettgeri conjugative genomic island R391 (the similarity of R391 genes to those of LT2 has been noted previously [10], accession number AY090559). To date, no functional significance has been attributed to these particular genes within SXT or R391 (6). Genes STM4493 and STM4494 are not found in SXT or R391 and may have been acquired by Salmonella serovar Typhimurium separately.
DNA fragments encoding Salmonella serovar Typhimurium LT2 genes STM4496-STM4498, within the integrating conjugative element-related region, and STM4493, which we propose to have been acquired through a separate event, were used to probe Southern blots containing DNA from representatives of different Salmonella enterica subsp. I serovars. The majority of Salmonella strains tested were negative for all of the Salmonella serovar Typhimurium SPI-10 genes. However, STM4496-98 was detected in a small number of serovars (Salmonella serovar Derby SARB9, Salmonella enterica serovar Saintpaul SARB56 and Salmonella enterica serovar Stanleyville SARB61), and STM4493 was detected just in Salmonella serovar Derby SARB9 and Salmonella serovar Stanleyville SARB61 (Fig. 4b and Table 2).
The presence of STM4496-98, but not STM4493, in Salmonella serovar Saintpaul SARB56 backs up the hypothesis that STM4493-94 may have been acquired by Salmonella serovar Typhimurium separately from the surrounding genes. To determine whether both STM4493 and STM4494 were missing from Salmonella serovar Saintpaul SARB56, we carried out PCR across from STM4492- to STM4495-like genes. A much smaller DNA fragment was amplified from SARB56 than from Salmonella serovar Stanleyville SARB61, which was positive for STM4493 by Southern blotting (Table 2), or the Salmonella serovar Typhimurium LT2 control (Fig. 5i). Sequencing of the PCR product confirmed that STM4495 lies right next to STM4492 in Salmonella serovar Saintpaul SARB56 (depicted schematically in Fig.5iii) and that neither STM4493 nor STM4494 are present. This gene arrangement in Salmonella serovar Saintpaul SARB56 is, therefore, similar to that found in the integrating conjugative genomic islands. These data strongly suggest that integration of genes found within conjugative elements into the Salmonella serovar Typhimurium tRNAleuX island occurred prior to insertion of STM4493 and STM4494.
Further variation within Salmonella serovar Derby, Salmonella serovar Saintpaul and Salmonella serovar Stanleyville tRNAleuX islands.
Salmonella serovar Derby, Salmonella serovar Saintpaul and Salmonella serovar Stanleyville were positive for LT2 genes by Southern blotting (Table 2). To detect any additional variation within the tRNAleuX islands in these serovars a detailed comparison with the Salmonella serovar Typhimurium LT2 tRNAleuX-adjacent island was carried out using PCR.
(i) A novel insertion in Salmonella serovar Saintpaul and Salmonella serovar Stanleyville tRNAleuX islands.
Having found variations at the 3′ end of the Salmonella serovar Saintpaul SARB56 tRNAleuX island compared to LT2 (described above), the gene complement at the 5′ tRNAleuX-adjacent end of the Salmonella serovar Saintpaul SARB56 and Salmonella serovar Stanleyville SARB61 islands was investigated. PCR between STM4490- and STM4492-like genes amplified much larger DNA fragments from SARB56 and SARB61 than from the LT2 control (Fig.5ii). Subsequent sequencing revealed a novel DNA sequence between the STM4490- and STM4491-like genes, which is 98% identical between SARB56 and SARB61 over 1,435 bp and contains an open reading frame with similarity (SARB56, 36% identical over 454 amino acids; SARB61, 35% identical over 461 amino acids) to the Methylobacillus flagellatus conserved hypothetical protein ZP_00173826, depicted in Fig. 5iii and iv.
(ii) Intraserovar variation at tRNAleuX in different Salmonella serovar Derby isolates.
Southern blotting suggested that the tRNAleuX island of Salmonella serovar Derby SARB9 may be similar to that found in Salmonella serovar Typhimurium LT2, as it contains both STM4493 and STM4496-98 (Table 2). PCRs were carried out to compare the tRNAleuX islands from two Salmonella serovar Derby isolates (SARB9 and SARB10) with that of Salmonella serovar Typhimurium LT2. Almost all of the Salmonella serovar Typhimurium LT2 tRNAleuX island genes were found to have equivalents in Salmonella serovar Derby SARB9, except that STM4488 and STM4489 at the start of the island were not detected (Fig. 6i). Identical PCR results to those for SARB9 were obtained for Salmonella serovar Derby SARB11 (data not shown). Attempts at long-range PCR from tRNAleuX across to STM4492 for SARB9 were not successful, although both primer sites appeared to be intact (our unpublished observations). With Salmonella serovar Derby isolate SARB10, in contrast to SARB9, none of the Salmonella serovar Typhimurium tRNAleuX island genes could be detected by PCR, except for STM4487/yjgB and STM4499 (both outside of the island), tRNAleuX itself, and the P4 integrase STM4488 (Fig. 6iii). These data indicate that at least two different gene organizations can be found adjacent to tRNAleuX within the Salmonella serovar Derby serovar, both of which may differ from Salmonella serovar Typhimurium LT2.
(iii) Salmonella serovar Derby SARB10 contains a hybrid island related to both Salmonella serovar Typhimurium and Salmonella serovar Enteritidis.
The region encompassing STM4487 to STM4488 in Salmonella serovar Derby SARB10 was amplified, by using a long-range DNA polymerase, and revealed a much larger PCR product than the equivalent region in Salmonella serovar Typhimurium LT2 (Fig. 5iv). Sequencing of the SARB10 insertion, which we narrowed down to a region between tRNAleuX and an STM4488-like gene, revealed that it is very similar to the equivalent section of the Salmonella serovar Enteritidis PT4 tRNAleuX island (compare the Salmonella serovar Enteritidis depicted in Fig. 1 with Salmonella serovar Derby SARB10 in Fig. 6vi). Both Salmonella serovar Derby SARB10 and Salmonella serovar Enteritidis contain a truncated P4-like integrase gene, an IS630-related transposase, and three predicted plasmid-related genes. SARB10 diverges from Salmonella serovar Enteritidis at the end of a partial tRNAleuX P4 phage attachment site duplication (16 bp of the 20-bp attP attachment site are conserved). The SARB10 sequence continues with a pseudogene resembling Salmonella serovar Typhimurium LT2 STM4488 (95% identical over 164 bp but with a frameshift), while Salmonella serovar Enteritidis has instead acquired the IS element-flanked sef/pef islet at this locus. Attempts to PCR amplify the region from STM4488 to STM4499, to detect any genes inserted 3′ of STM4488, were not successful, although the primer sites appear to be present (our unpublished observations).
Conclusions
Most of the SPIs identified in Salmonella serovar Typhi CT18 are largely conserved between different S. enterica subsp. I serovars. In SPI-1 to SPI-5, the only major variation from Salmonella serovar Typhimurium is within SPI-3 where, across a relatively short region (3 kbp), some insertions or deletions have been detected (2). Comparing Salmonella serovar Typhi CT18 with Salmonella serovar Typhimurium LT2, there is a high level of conservation within SPI-1 to SPI-6, except for a large insertion of the tcf fimbrial operon in SPI-6 of Salmonella serovar Typhi (23). SPI-8 and SPI-9 (40) have not yet been thoroughly characterized. Our data suggest that SPI-10 can be placed alongside SPI-7, predicted to be a conjugative transposon (41), as an island in Salmonella serovar Typhi CT18 that is highly variable between different serovars within S. enterica subsp. I.
In some cases, within a single serovar we find significant variation at the tRNAleuX locus from one isolate to another. Even with relatively small numbers of strains, we observed this intraserovar variation both within serovars that (by multilocus enzyme electrophoresis typing) are considered relatively clonal, Salmonella serovar Typhi (46) (Fig. 3), or divergent, Salmonella serovar Derby (8) (Fig. 6). In a microarray-based study by Boyd et al., the tRNAleuX locus was also picked out as one of a small number of regions of major chromosomal variation between Salmonella serovar Typhi isolates (11). Boyd et al. identified three isolates lacking the ST46 phage, one of which was isolated in Indonesia (In15 isolated in 1994), as was the ST46-negative strain analyzed here (KT516 isolated in 1986) (30), while the other two were from different continents (3125, Chile; CDC1707, Liberia) (11). This suggests that the loss of ST46 by Salmonella serovar Typhi isolates has occurred independently in at least three locations worldwide. Differences at the tRNAleuX locus, at least for some serovars, may even prove prevalent enough to be of use in epidemiological studies.
The tRNAleuX island of Salmonella serovar Typhi has been termed SPI-10 (40), even though to our knowledge none of the genes at this locus have been linked to pathogenesis. This island in Salmonella serovar Typhi has instead undergone substantial loss of gene function, through the acquisition of pseudogene mutations, which may contribute to host restriction rather than pathogenesis. The Salmonella serovar Enteritidis tRNAleuX locus has more convincing features of a Salmonella pathogenicity island, as the intact sef operon has been implicated in in vivo pathogenesis with mice (22). There remain a large number of serovars with uncharacterized tRNAleuX loci (Table 2), as they have not been sequenced and contain neither the Salmonella serovar Typhi or Salmonella serovar Typhimurium genes for which we screened nor the previously characterized sef/pef islet (4, 16). It is likely that many more novel gene sets are present in these isolates. It remains to be determined whether these are of importance to the different host restrictions and adaptations observed within S. enterica subsp. I.
The tRNAleuX islands have mosaic structures. For example, within the hybrid island of Salmonella serovar Derby SARB10, we find a partial integrase very similar to Salmonella serovar Typhimurium STM4488 alongside plasmid-related genes and IS elements also seen in Salmonella serovar Enteritidis (Fig. 6iv); the sef/pef islet is present with or without a full P4 phage lysogen in different islands (Fig. 1).
tRNA loci are often the targets for insertion of horizontally transferred DNA (25, 32), and several of the Salmonella pathogenicity islands are located at tRNA genes (7 of the 10 islands identified in Salmonella serovar Typhi are located adjacent to a tRNA) (27, 40, 50). Our in silico and molecular analysis of the tRNAleuX region has revealed such remarkable variation that we believe tRNAleuX in S. enterica to be a locus that is unusually prone to insertion and excision events.
The presence of P4-like phage, many IS elements, and integrase remnants is striking and suggests that this is a common spot for the insertion of transposable elements. The detection of circularized Salmonella serovar Typhi ST46 DNA in strains growing in culture and identification of an Salmonella serovar Typhi strain missing ST46 (Fig. 3) underlines the potential of P4 phages to drive diversity at the tRNAleuX locus. The presence in some serovars of a number of plasmid-derived genes in close association with IS elements is indicative of an IS-mediated acquisition of plasmid DNA. Thus, tRNAleuX may provide a chromosomal locus through which S. enterica can sample different gene sets, a property that could significantly influence the evolution of S. enterica as a species.
Supplementary Material
Acknowledgments
This work was supported by The Wellcome Trust and the BBSRC.
We thank Julian Parkhill and Nicholas Thomson for assistance with in silico genome analysis and advice relating to phage biology.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Amavisit, P., D. Lightfoot, G. F. Browning, and P. F. Markham. 2003. Variation between pathogenic serovars within Salmonella pathogenicity islands. J. Bacteriol. 185:3624-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, and J. A. Smith. 1995. Preparation of genomic DNA from bacteria, p. 2.11-2.22. In K. Struhl (ed.), Short protocols in molecular biology, vol. 3. John Wiley and Sons, Inc., New York, N.Y.
- 4.Baumler, A. J., A. J. Gilde, R. M. Tsolis, A. W. van der Velden, B. M. Ahmer, and F. Heffron. 1997. Contribution of horizontal gene transfer and deletion events to development of distinctive patterns of fimbrial operons during evolution of Salmonella serotypes. J. Bacteriol. 179:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bausch, C., N. Peekhaus, C. Utz, T. Blais, E. Murray, T. Lowary, and T. Conway. 1998. Sequence analysis of the GntII (subsidiary) system for gluconate metabolism reveals a novel pathway for l-idonic acid catabolism in Escherichia coli. J. Bacteriol. 180:3704-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaber, J. W., V. Burrus, B. Hochhut, and M. K. Waldor. 2002. Comparison of SXT and R391, two conjugative integrating elements: definition of a genetic backbone for the mobilization of resistance determinants. Cell. Mol. Life Sci. 59:2065-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaber, J. W., B. Hochhut, and M. K. Waldor. 2002. Genomic and functional analyses of SXT, an integrating antibiotic resistance gene transfer element derived from Vibrio cholerae. J. Bacteriol. 184:4259-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltran, P., J. M. Musser, R. Helmuth, J. J. Farmer III, W. M. Frerichs, I. K. Wachsmuth, K. Ferris, A. C. McWhorter, J. G. Wells, A. Cravioto, et al. 1988. Toward a population genetic analysis of Salmonella: genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc. Natl. Acad. Sci. USA 85:7753-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 10.Boltner, D., C. MacMahon, J. T. Pembroke, P. Strike, and A. M. Osborn. 2002. R391: a conjugative integrating mosaic comprised of phage, plasmid, and transposon elements. J. Bacteriol. 184:5158-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd, E. F., S. Porwollik, F. Blackmer, and M. McClelland. 2003. Differences in gene content among Salmonella enterica serovar Typhi isolates. J. Clin. Microbiol. 41:3823-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd, E. F., F. S. Wang, P. Beltran, S. A. Plock, K. Nelson, and R. K. Selander. 1993. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies I. J. Gen. Microbiol. 139(Pt 6):1125-1132. [DOI] [PubMed] [Google Scholar]
- 13.Boyd, E. F., F. S. Wang, T. S. Whittam, and R. K. Selander. 1996. Molecular genetic relationships of the salmonellae. Appl. Environ. Microbiol. 62:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briani, F., G. Deho, F. Forti, and D. Ghisotti. 2001. The plasmid status of satellite bacteriophage P4. Plasmid 45:1-17. [DOI] [PubMed] [Google Scholar]
- 15.Chan, K., S. Baker, C. C. Kim, C. S. Detweiler, G. Dougan, and S. Falkow. 2003. Genomic comparison of Salmonella enterica serovars and Salmonella bongori by use of an S. enterica serovar Typhimurium DNA microarray. J. Bacteriol. 185:553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collighan, R. J., and M. J. Woodward. 2001. The SEF14 fimbrial antigen of Salmonella enterica serovar Enteritidis is encoded within a pathogenicity islet. Vet. Microbiol. 80:235-245. [DOI] [PubMed] [Google Scholar]
- 17.Delcher, A. L., D. Harmon, S. Kasif, O. White, and S. L. Salzberg. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delcher, A. L., S. Kasif, R. D. Fleischmann, J. Peterson, O. White, and S. L. Salzberg. 1999. Alignment of whole genomes. Nucleic Acids Res. 27:2369-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng, W., S. R. Liou, G. Plunkett III, G. F. Mayhew, D. J. Rose, V. Burland, V. Kodoyianni, D. C. Schwartz, and F. R. Blattner. 2003. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J. Bacteriol. 185:2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobrindt, U., G. Blum-Oehler, G. Nagy, G. Schneider, A. Johann, G. Gottschalk, and J. Hacker. 2002. Genetic structure and distribution of four pathogenicity islands (PAI I536 to PAI IV536) of uropathogenic Escherichia coli strain 536. Infect. Immun. 70:6365-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards, R. A., B. C. Matlock, B. J. Heffernan, and S. R. Maloy. 2001. Genomic analysis and growth-phase-dependent regulation of the SEF14 fimbriae of Salmonella enterica serovar Enteritidis. Microbiology 147:2705-2715. [DOI] [PubMed] [Google Scholar]
- 22.Edwards, R. A., D. M. Schifferli, and S. R. Maloy. 2000. A role for Salmonella fimbriae in intraperitoneal infections. Proc. Natl. Acad. Sci. USA 97:1258-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folkesson, A., A. Advani, S. Sukupolvi, J. D. Pfeifer, S. Normark, and S. Lofdahl. 1999. Multiple insertions of fimbrial operons correlate with the evolution of Salmonella serovars responsible for human disease. Mol. Microbiol. 33:612-622. [DOI] [PubMed] [Google Scholar]
- 24.Galan, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 25.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 26.Haneda, T., N. Okada, N. Nakazawa, T. Kawakami, and H. Danbara. 2001. Complete DNA sequence and comparative analysis of the 50-kilobase virulence plasmid of Salmonella enterica serovar Choleraesuis. Infect. Immun. 69:2612-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen-Wester, I., and M. Hensel. 2002. Genome-based identification of chromosomal regions specific for Salmonella spp. Infect. Immun. 70:2351-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 29.Hou, Y. M. 1999. Transfer RNAs and pathogenicity islands. Trends Biochem. Sci. 24:295-298. [DOI] [PubMed] [Google Scholar]
- 30.Kidgell, C., U. Reichard, J. Wain, B. Linz, M. Torpdahl, G. Dougan, and M. Achtman. 2002. Salmonella typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect. Genet. Evol. 2:39-45. [DOI] [PubMed] [Google Scholar]
- 31.Lowe, D. C., T. C. Savidge, D. Pickard, L. Eckmann, M. F. Kagnoff, G. Dougan, and S. N. Chatfield. 1999. Characterization of candidate live oral Salmonella typhi vaccine strains harboring defined mutations in aroA, aroC, and htrA. Infect. Immun. 67:700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantri, Y., and K. P. Williams. 2004. Islander: a database of integrative islands in prokaryotic genomes, the associated integrases and their DNA site specificities. Nucleic Acids Res. 32:D55-D58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus, S. L., J. H. Brumell, C. G. Pfeifer, and B. B. Finlay. 2000. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2:145-156. [DOI] [PubMed] [Google Scholar]
- 35.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 36.Naderer, M., J. R. Brust, D. Knowle, and R. M. Blumenthal. 2002. Mobility of a restriction-modification system revealed by its genetic contexts in three hosts. J. Bacteriol. 184:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochman, H., S. Elwyn, and N. A. Moran. 1999. Calibrating bacterial evolution. Proc. Natl. Acad. Sci. USA 96:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 39.Ochman, H., and A. C. Wilson. 1987. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J. Mol. Evol. 26:74-86. [DOI] [PubMed] [Google Scholar]
- 40.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 41.Pickard, D., J. Wain, S. Baker, A. Line, S. Chohan, M. Fookes, A. Barron, P. O. Gaora, J. A. Chabalgoity, N. Thanky, C. Scholes, N. Thomson, M. Quail, J. Parkhill, and G. Dougan. 2003. Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. J. Bacteriol. 185:5055-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierson, L. S., III, and M. L. Kahn. 1987. Integration of satellite bacteriophage P4 in Escherichia coli. DNA sequences of the phage and host regions involved in site-specific recombination. J. Mol. Biol. 196:487-496. [DOI] [PubMed] [Google Scholar]
- 43.Porwollik, S., and M. McClelland. 2003. Lateral gene transfer in Salmonella. Microbes Infect. 5:977-989. [DOI] [PubMed] [Google Scholar]
- 44.Porwollik, S., R. M. Wong, and M. McClelland. 2002. Evolutionary genomics of Salmonella: gene acquisitions revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 99:8956-8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Selander, R. K., P. Beltran, N. H. Smith, R. Helmuth, F. A. Rubin, D. J. Kopecko, K. Ferris, B. D. Tall, A. Cravioto, and J. M. Musser. 1990. Evolutionary genetic relationships of clones of Salmonella serovars that cause human typhoid and other enteric fevers. Infect. Immun. 58:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tatusova, T. A., and T. L. Madden. 1999. BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174:247-250. [DOI] [PubMed] [Google Scholar]
- 48.Thomson, N., S. Baker, D. Pickard, J. Wain, D. House, M. Fookes, A. Ivens, N. Hamlin, Z. Bhutta, M. Anjum, M. Woodward, S. Falkow, K. Chan, J. Parkhill, and G. Dougan. 2004. Prophage-like elements in the genome of Salmonella enterica serovar Typhi. Contribution to diversity in S. enterica serovars. J. Mol. Biol. 339:279-300. [DOI] [PubMed] [Google Scholar]
- 49.Townsend, S. M., N. E. Kramer, R. Edwards, S. Baker, N. Hamlin, M. Simmonds, K. Stevens, S. Maloy, J. Parkhill, G. Dougan, and A. J. Baumler. 2001. Salmonella enterica serovar Typhi possesses a unique repertoire of fimbrial gene sequences. Infect. Immun. 69:2894-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wain, J., D. House, D. Pickard, G. Dougan, and G. Frankel. 2001. Acquisition of virulence-associated factors by the enteric pathogens Escherichia coli and Salmonella enterica. Philos. Trans. R. Soc. Lond. B 356:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, Z., S. Schwartz, L. Wagner, and W. Miller. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203-214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.