Abstract
Collagen type IV (COL4) is one of the major components of animals’ and humans’ basement membranes of several tissues, such as skeletal muscles and vascular endothelia. Alterations in COL4 assembly and secretion are associated to muscular disorders in humans and animals among which growth-related abnormalities such as white striping and wooden breast affecting Pectoralis major muscles (PMs) in modern fast-growing (FG) chickens. Considering the high prevalence of these myopathies in FG broilers and that a worsening is observed as the bird slaughter age is increased, the present study was intended to evaluate the distribution and the expression level of COL4 protein and its coding genes in PMs of FG broilers at different stages of muscle development (i.e., 7, 14, 21, 28, 35, and 42 d of age). Medium-growing (MG) chickens have been considered as the control group in consideration of the lower selection pressure on breast muscle growth rate and hypertrophy. Briefly, 5 PM/sampling time/genotype were selected for western blot, immunohistochemistry (IHC), and gene expression analyses. The normalized expression levels of COL4 coding genes showed an overexpression of COL4A2 in FG than MG at d 28, as well as a significant decrease in its expression over their rearing period. Overall, results obtained through the gene expression analysis suggested that selection for the hypertrophic growth of FG broilers may have led to an altered regulation of fibroblast proliferation and COL4 synthesis. Moreover, western blot and IHC analyses suggested an altered secretion and/or degradation of COL4 protein in FG broilers, as evidenced by the fluctuating trend of 2 bands observed in FG over time. In view of the above, the present research supports the evidence about a potential aberrant synthesis and/or degradation of COL4 and corroborates the hypothesis regarding a likely involvement of COL4 in the series of events underlying the growth-related abnormalities in modern FG broilers.
Key words: broiler, genotype, growth-rate, breast meat abnormality, collagen type IV
INTRODUCTION
In the past decade, a group of growth-related abnormalities affecting the Pectoralis major muscles (PMs) of broiler chickens belonging to fast-growing genotypes selected for meat production purposes has abruptly emerged all over the world with impressive incidence levels and severity (Petracci et al., 2019). These spontaneous abnormalities, known as White Striping (WS) and Wooden Breast (WB), often coexist within the same PM, manifest with distinctive phenotypes (Soglia et al., 2019) and exhibit peculiar histological features including the occurrence of inflammatory processes, intense degenerative and occasional regenerative phenomena, lipidosis (especially in WS) and extensive fibrosis (especially in WB) (Soglia et al., 2019). Regarding the relevance of these issues, the findings of a recent survey carried out in Italy on 16,000 breasts highlighted incidence levels exceeding 30 and 60% for WS and WB, respectively (Baldi et al., 2020). In addition, as for their worldwide distribution, the incidence of WB was found to exceed 50 and 60% of the birds in the US and China, respectively (Kuttappan et al., 2013; Xing et al., 2020).
Following their first observation at industrial level, several studies have been initially carried out to evaluate the impact of WS and WB on the main quality traits and technological properties of the forthcoming meat, and later to shed light on the mechanisms eventually underlying their development (Petracci et al., 2019). Despite the recent improvements in understanding the molecular basis of the abovementioned defects, their causative mechanisms are not fully understood (Soglia et al., 2021). For several years, vascular defects leading to hypoxic conditions within the muscles were thought to be the most likely underpinning factors responsible for triggering these myopathies (Sihvo et al., 2017). Other hypotheses suggest mitochondrial dysfunctions as well as altered lipid and glucose metabolisms as phenomena likely predisposing to the development of these conditions (Abasht et al., 2016; Lake and Abasht, 2020). Moreover, Abasht et al. (2021) propose the involvement of endothelial cell dysfunction in the early pathogenic mechanism underpinning WB. Recent studies also pointed out a possible role of endoplasmic reticulum stress (Papah et al., 2018; Bordini et al., 2021, 2022; Greene et al., 2023). Within this context, our previous results built a basis for hypothesizing a key role of collagen type IV (COL4) in the occurrence of endoplasmic reticulum (ER) stress, ultimately responsible for the pathogenesis of these defects. Supporting this hypothesis, alterations in ER evoking the unfolded protein response are among the first ultrastructural and molecular changes observed at the early onset of WB (Papah et al., 2018; Sihvo et al., 2018).
COL4 is one of the major components of the basement membrane (BM) in animals’ and humans’ skeletal muscle fibers and vascular endothelia (Chioran et al., 2017). This network-forming collagen type provides a molecular scaffold along with laminins, proteoglycans, and perlecans. Scaffold assembly is critical for the functionality of COL4 as well as BM itself, since it provides structural support and molecular organization to the extracellular matrix (Bhave et al., 2017). In fact, disruption of the assembly process, intracellularly or extracellularly, can alter its function with potentially severe biologic effects (Bhave et al., 2017). Not surprisingly, defects affecting the COL4-network composing BMs lead to mechanical instability and have systemic consequences, both in humans and animal models (Bhave et al., 2017; Kiss et al., 2019). Notably, mutations in its encoding genes (i.e., COL4A1 and COL4A2) have been previously associated with the development of a collagenopathy (Kuo et al., 2012; Labelle-Dumais et al., 2019), moreover skeletal muscles of mice bearing these mutations exhibit histological features ascribable to myopathic disorders (Guiraud et al., 2017; Labelle-Dumais et al., 2019) along with abnormal angiogenesis (Alavi et al., 2016). Intriguingly, these microscopic features perfectly overlap with those observed in abnormal PMs in modern broilers (Petracci et al., 2019; Soglia et al., 2019) in which evidence of vascular involvement and phlebitis was readily observed before the development of any microscopic lesion (Papah et al., 2017; Sihvo et al., 2017). In addition, recent studies have shown that alterations in intracellular and/or extracellular COL4 accumulation may cause abnormal angiogenesis, due to the direct interaction of the COL4-network of vascular BM with the signaling pathways involved in this process (e.g., integrin signaling) (Jeanne et al., 2015; Trouillet et al., 2017). Finally, COL4 can also exert antiangiogenic effects through the release of bioactive collagen fragments (i.e., arresten and canstatin) generated by the degradation process of its non-collageneous C-terminal fragment (Aikio et al., 2012; Okada and Yamawaki, 2019).
Within this scenario, the present study aimed at evaluating the distribution as well as the expression level of COL4 protein and its coding genes in PMs of modern meat-type chicken hybrids selected both for fast (fast-growing, FG: daily weight gain > 70 g/d) and medium (medium-growing, MG: daily weight gain 40–50 g/d) growth-rate at different stages of muscle development (i.e., 7, 14, 21, 28, 35, and 42 d of age). In detail, considering the high prevalence of growth-related defects in FG broilers and that a worsening of these conditions is observed as the birds grow older, this experimental design will allow to deepen the knowledge regarding the potential implication of COL4 in the onset of these abnormalities. To ascertain the contribution of the rapid growth of modern hybrids, the same analyses have been performed on FG and MG genotypes, with the last considered as the control group in consideration of the lower selection pressure on breast meat yield, as evidenced by their different allometric growth.
MATERIALS AND METHODS
For the present study, a total of 60 PMs belonging to FG (N = 30) and MG (N = 30) broiler chickens were used to investigate the expression level of COL4 coding genes and translated protein, along with its distribution at microscopic level. The experiment was approved by the Ethical Committee of the Italian Ministry of Health (ID: 1194/2021). In detail, FG birds belong to one of the main commercial hybrids worldwide diffused for meat production purposes having an average daily weight gain exceeding 70 g. On the other hand, MG birds (having an average daily weight gain not exceeding 50 g) belong to one of the genotypes approved by ECC. Data concerning the body weight achieved at each sampling time (i.e., 7, 14, 21, 28, 35, and 42 d of age) for both FG and MG chickens are shown in Supplementary Figure S1.
Birds were farmed under homogenous experimental conditions and fed the same commercial diets formulated to meet, and eventually exceed, the nutrient specifications of both genotypes. A detailed description of animal housing, as well as sample collection, classification, preparation, and selection for further analyses, has been previously reported by (Soglia et al., 2022). Briefly, samples were collected by removing the skin and excising the superficial section of the cranial part of the PM. Samples collected for gene and protein quantification were immediately frozen in liquid nitrogen, whereas those undergoing histology and immunohistochemistry analyses were frozen in isopentane cooled using liquid nitrogen. All samples were then stored at -80°C. Among the collected samples at each sampling time (i.e., 7, 14, 21, 28, 35, and 42 d of age), 5 PMs per each genotype (FG and MG) were selected based on their macroscopic traits (e.g., having normal appearance or showing traits ascribable to the WS and/or WB defects) and histological features (i.e., inflammatory cells infiltration, lipid and collagen increased deposition, presence of necrotic fibers and regenerative processes) evaluated by means of hematoxylin and eosin staining. This preliminary histological evaluation was performed to provide an accurate classification and selection of the PMs to be considered for further analyses (Soglia et al., 2022).
Gene Expression Analysis
Total RNA extraction and quantification, as well as the evaluation of its integrity, have been performed following the procedure reported in Soglia et al. (2020). Then, each sample was subjected to genomic DNA (gDNA) removal and 1 μg of total RNA was retrotranscribed to complementary DNA (cDNA) using iScript gDNA Clear cDNA Synthesis Kit (1725035, Bio-Rad Laboratories), as recommended by the manufacturers. Quantification of target genes Collagen type IV alpha 1 (COL4A1), Collagen type IV alpha 2 (COL4A2), and normalizing genes were obtained by conducting quantitative real-time PCR (qRT-PCR) analyses on Rotor-Gene 6000 (Corbett Life Science, Concorde, NSW, Australia), using the standard curve method (Pfaffl et al., 2004). The qRT-PCRs were performed in a total volume of 10 μl using SsoAdvanced Universal SYBR Green Supermix (1725271, Bio-Rad Laboratories). Primer pairs were designed using Primer3Plus web online tool (Untergasser et al., 2012). Supplementary Table S1 reports the complete information concerning primer pairs used in the present study. The ribosomal protein L4 (RPL4), ribosomal protein lateral stalk subunit P0 (RPLP0), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes have been tested to identify the most stable genes to be used to normalize target genes expression (Pfaffl et al., 2004; Zappaterra et al., 2015). As reported by Soglia et al. (2022), the GeNorm algorithm (Vandesompele et al., 2002) highlighted RPL4 and GAPDH as the best couple of normalizing genes for the present research. Per sample, each target and normalizing gene was quantified at least in triplicate to collect no less than 3 replications having coefficients of variation lower than 0.2. Amplification conditions of target and normalizing genes are listed in Supplementary Table S2.
Western Blot
Myofibrillar proteins were extracted from one gram of frozen PM following the procedure described by Soglia et al. (2022). After their extraction and quantification, 10 μg of myofibrillar proteins were loaded in 4% to 15% Mini-PROTEAN TGX Stain-Fee Gels (Bio-Rad Laboratories) and run in a Bio-Rad Mini Protean II electrophoresis apparatus at constant voltage (200 V) for 30 min. Then, gels were activated by UV exposure (Gürtler et al., 2013) and protein fluorescence was acquired to check the separation of the electrophoretic fragments using a ChemiDoc MP Imaging System (Bio-Rad Laboratories) with an Image Lab software (version 5.2.1). Proteins were transferred onto a 0.2 µm nitrocellulose membrane using a Trans-Blot Turbo Transfer System (Bio-Rad Laboratories) and saturated by incubation (45 min, at room temperature while shaking) in 15 mL TBST (20 mM Tris, 150 mM NaCl, 0.1% Tween 20; pH 7.4–7.6) with 5% skimmed milk powder. Membranes were probed (60 min, room temperature while shaking) with a rabbit anticollagen type IV alpha 1 primary antibody (diluted 1:4,000) (LS-C352029, LSBio, Lynnwood, WA) and, after washing, with a secondary anti-rabbit antibody for 60 min (1:15,000) (Merk Millipore, Burlington, MA) treated with HRP-conjugated streptavidin (Merk Millipore) for 20 min. Final detection was performed with enhanced chemiluminescence (Clarity Western ECL Substrate Western Blotting detection kit, Bio-Rad Laboratories), and the images were acquired using the ChemiDoc MP Imaging System (Bio-Rad Laboratories). Densitometry differences were analyzed with Image Lab software, and data were normalized considering the intensity of the fluorescent signal resulting from the proteins loaded in each lane (Valli et al., 2018). Results were expressed as %, considering 100% the intensity of the band assigned to COL4 in the PM belonging to the MG genotype sampled on d 7.
Immunohistochemistry
The distribution of COL4 was assessed by IHC analyses performed implementing the avidin-biotin-peroxidase complex (ABC) method previously described by Soglia et al. (2022), with slight modifications. Briefly, for each PM, serial cross-sections (10 μm thick), cut on a cryostat microtome and mounted on poly-L-lysine coated glass slides (Sigma–Aldrich, St. Louis, MO), were incubated in 5% normal goat serum. Then, the sections were incubated with a rabbit anticollagen type IV alpha 1 diluted 1:400 (LS-C352029, LSBio, Lynnwood, WA) primary antibody and a biotin-conjugated goat anti-rabbit IgG secondary antibody diluted 1:200 (Vector Laboratories, Burlingame, CA) and treated with ABC (Vector elite kit, Vector Laboratories). The immune reactions were visualized through the 3,3′-diaminobenzidine (DAB) chromogen solution supplied by Vector (Vector DAB kit, Vector Laboratories). Finally, the sections were coverslipped with buffered glycerol, pH 8.6.
Statistical Analysis
Data concerning gene expression and protein quantifications were analyzed by using Statistica 10 (StatSoft Inc., 2014). The effect of the genotype (FG vs. MG) on genes (i.e., COL4A1 and COL4A2) and COL4 protein was evaluated within each sampling time (7, 14, 21, 28, 35, and 42 d of age) through the non-parametric Mann-Whitney U test. As regards FG chickens, the evolution over the time of the expression level of COL4 protein and its coding genes has been assessed using the one-way ANOVA option of the GLM procedure, considering the developmental stage (i.e., sampling times) as the main effect. When significant, means were subsequently separated by using the Tukey–HSD test (P ≤ 0.05).
RESULTS
Gene Expression
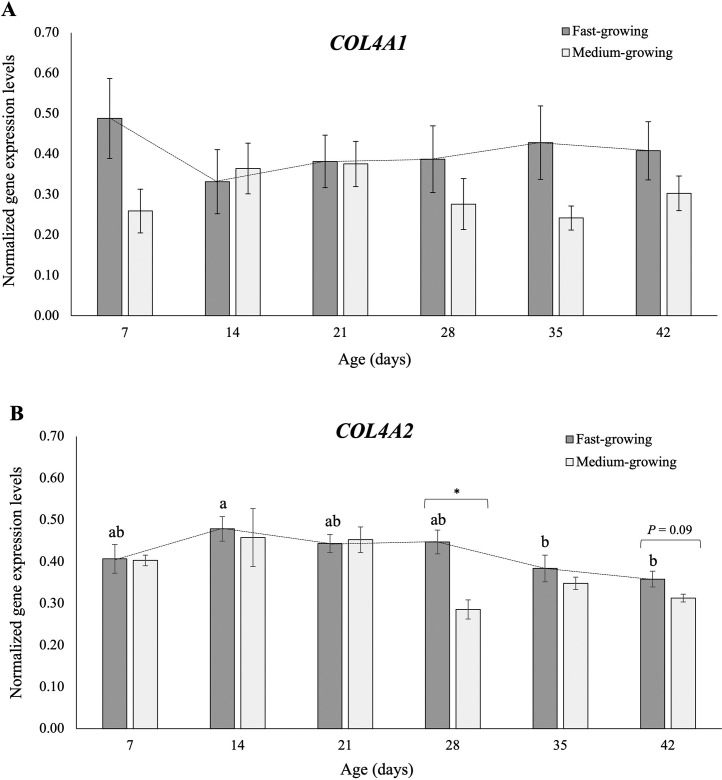
The gene expression study results are reported in Figure 1. In detail, Figures 1A and 1B show the normalized quantifications of COL4A1 and COL4A2, respectively, in both FG and MG chickens, per sampling time (7, 14, 21, 28, 35, and 42 d of age). At each sampling time, the normalized mRNA levels of COL4A1 showed no significant differences between FG and MG. Moreover, no significant differences have been found considering the evolution of COL4A1 normalized expression in FG during the growth period (i.e., from d 7 to d 42). As for the COL4A2 normalized expression, the mRNA level was significantly higher in FG than in MG broilers at d 28 (P < 0.05). Also, a tendency towards significance (P = 0.09) was found at d 42, with a higher level of COL4A2 mRNA found in FG than in MG. As regards other sampling times, no significant differences were found between FG and MG. Considering the effect of developmental stages of PMs on COL4A2 gene expression in FG broilers, the normalized quantifications of this gene have been found significantly different (P < 0.05) as the birds grow older. In particular, COL4A2 expression level significantly decreased in FG from d 14 to d 35 and d 42, as shown in Figure 1B.
Figure 1.
Normalized gene expression values of Collagen type IV alpha 1 chain (COL4A1) and Collagen type IV alpha 2 chain (COL4A2) in Pectoralis major muscles of meat-type chickens belonging to fast-growing (FG) and medium-growing (MG) genotypes at different developmental stages (7, 14, 21, 28, 35, and 42 d of ages). For each panel (A and B), mean values of normalized gene qualifications calculated for each genotype (FG and MG) at each sampling time are shown by the bar graphs. The error bars indicate standard error of mean. The non-parametric Mann–Whitney U was used to test the significance (P ≤ 0.05; *) and trends towards significance (P ≤ 0.10) of differences between FG and MG at each sampling time. Besides, One-way ANOVA was used to assess the evolution of the normalized gene expression levels of COL4A1 and COL4A2 genes in FG over their growth period, which are shown as line graphs. a-b = for FG, mean values followed by different letters significantly differ over the growth period (P ≤ 0.05).
Western Blot
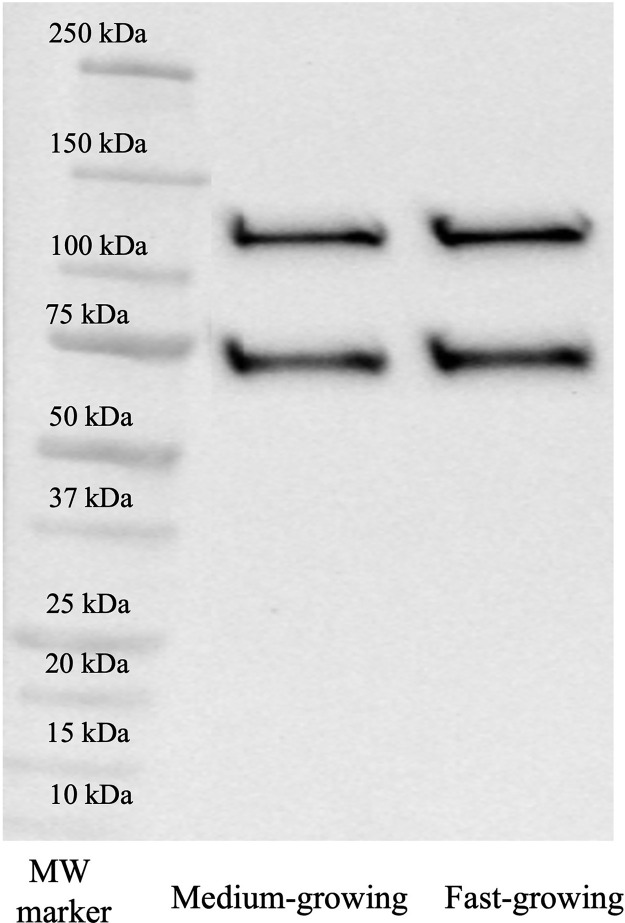
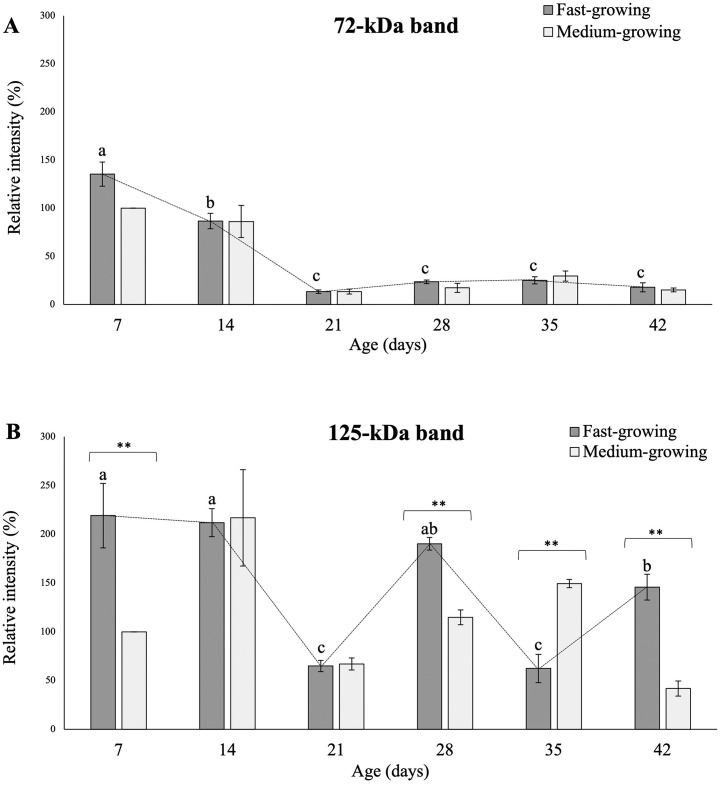
A representative image of the immunoblot obtained for COL4 is reported in Figure 2. Two distinct bands having a molecular weight of 72 and 125 kDa were detected. Figures 3 A and 3B show the outcomes concerning the relative intensity of COL4-related electrophoretic fragments in PMs belonging to FG and MG at different developmental stages (i.e., 7, 14, 21, 28, 35, and 42 d of age).
Figure 2.
Representative image of immunoblot obtained for collagen type IV in Pectoralis major muscles of broiler chickens belonging to fast-growing (FG) and medium-growing (MG) genotypes.
Figure 3.
Relative concentrations of the 2 electrophoretic fragments identified in Pectoralis major muscles of broiler chickens belonging to fast-growing (FG) and medium-growing (MG) genotypes at different ages through the western blot analysis and having a molecular weight of 72 kDa (A) and 125 kDa (B). The results are expressed as %, considering as 100% the intensity of the band respectively assigned to the 72 and 125 kDa fragment in MG broilers at d 7. Values are expressed as mean values (%) ± standard error of mean. Within each sampling time (7, 14, 21, 28, 35, and 42 d), the Mann–Whitney U test was used to assess the differences between FG and MG. * = P ≤ 0.05 and ** = P ≤ 0.01. Moreover, one-way ANOVA was used to assess the evolution of the 72 and 125 kDa electrophoretic fragments (A and B) in FG over their growth period. a-c = for FG, different superscript letters indicate significant differences over the growth period (P ≤ 0.05).
As for the 72 kDa-fragment, no significant differences were found between FG and MG at each sampling time. On the other hand, a significant effect of the genotype was observed for the 125 kDa band whose expression level was found to be higher in FG if compared to MG at 7, 28, and 42 d of age. Conversely, if compared to the level observed in FG, a remarkably higher relative intensity of this electrophoretic fragment was found in MG at d 35.
As for the effect ascribable to the developmental stage, a progressive decline in the relative intensity of the 72 kDa band was observed in FG from d 7 to d 21 whereas no further variations were observed at later stages. On the other hand, a fluctuating trend was observed for the 125 kDa fragment particularly characterized by a significant reduction in its expression level at d 21 and 35 followed by a sharp increase in its content in the subsequent stages (i.e., d 28 and 42).
Immunohistochemistry
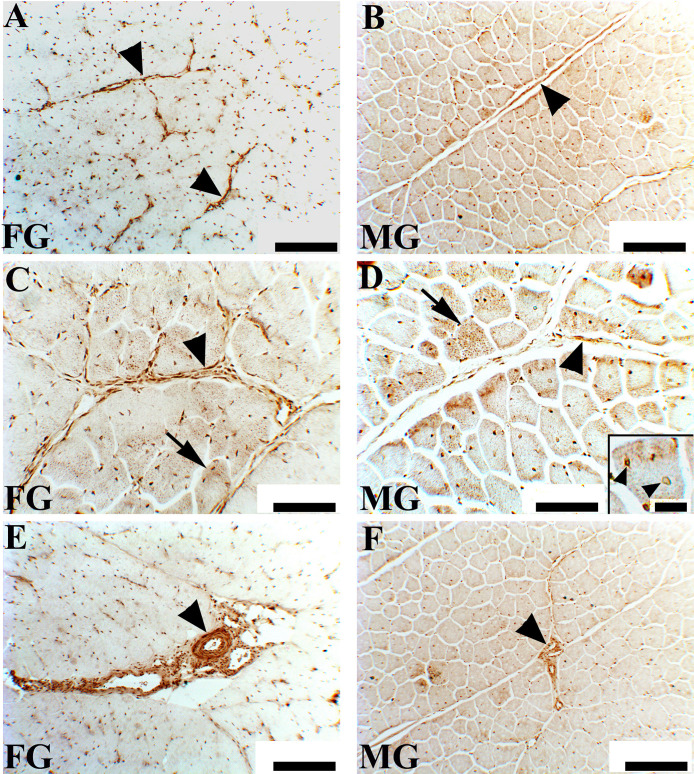
Representative immunoreactivity patterns observed for COL4 in FG and MG broilers are shown in Figure 4. In FG broilers (Figures 4A, 4C, and 4E), intense COL4 immunoreactivity was observed restrictedly to some positions at the peri- and endomysial level. A similar immunolabeling was observed in MG (Figures 4B and 4D, arrowheads). At higher magnification, the perimysial connective tissue of FG appeared to be heavily infiltrated with inflammatory cells and fibroblasts. In addition, some muscle fibers showed intense immunoreactivity in correspondence to the sarcoplasm (Figures 4C and 4D, arrows) as well as to circled structures, which may likely surround nuclei. COL4 immunoreactivity was also observed in the blood vessel wall (Figures 4E and 4F, arrowheads).
Figure 4.
Collagen type IV immunoreactivity (IR) in the Pectoralis major muscle cross-sectional area of fast-growing (FG) (A, C and E) and medium-growing (MG) (B, D and F) broilers chickens. Images (A) to (D) show collagen type IV immunoreactivity (IR) in some tracts in the perimysial and endomysial position (arrowheads). In FG broiler chickens, a loss of the normal architecture of the skeletal muscle was observed: the fibers show a rounded profile (A and C). The MG chicken (B and D) showed a normal polygonal profile of muscle fibers compared to the FG: IR was confined to few/rare areas of the perimysial connective tissue (arrowhead). At higher magnification, the different IR of the perimysial and endomysial connective tissue of the muscle fibers in the FG chickens is even more evident (C and D, arrowheads). In addition, some fibers exhibit intense sarcoplasmic positivity (C and D, arrows). Within the muscle fibers, the intense IR of forms small circular structures (probably around the nuclei) (D, detail arrowheads). Images (E) and (F) show the more intense IR of the blood vessel wall: in FG, abundant IR connective tissue infiltrated by inflammatory and fibroblasts cells surrounded these structures. Scale bars in A, B, E, and F = 200 μm; scale bars in C and D = 100 μm; scale bar in the detail = 50 μm.
DISCUSSION
Studies performed in the past few years have contributed to shed light on biophysical mechanisms underlying the growth-related abnormalities affecting modern FG broilers (Lake and Abasht, 2020; Velleman, 2020; Ayansola et al., 2021; Soglia et al., 2021) and smooth the path for several hypotheses regarding their primary causes. Indeed, other than broad consensus on the alteration of both the muscle architecture and the connective tissue organization of PMs affected by these defects (Velleman et al., 2017), several Authors built their theories about the underlying causes of these conditions on perturbations of capillary to fiber ratio (Petracci et al., 2019; Velleman, 2019; Ayansola et al., 2021), extracellular matrix composition (Velleman, 2020; Bordini et al., 2021), energy metabolism (Abasht et al., 2016; Wang et al., 2023) and mitochondrial functionality (Papah et al., 2017; Hasegawa et al., 2022). As for the extracellular matrix composition, our previous studies suggested a potential involvement of COL4 in triggering the time-series of events ultimately resulting in the development of WS and WB abnormalities (Bordini et al., 2021, 2022). Indeed, the present study has been conceived by the findings obtained through a weighted gene co-expression network analysis (WGCNA) (Bordini et al., 2021) in which COL4A1 and COL4A2 were detected as hub genes significantly associated to the phenotypes related to the occurrence of these defects (i.e., breast weight and width as well as cooking loss). Based on that, the present research focused on the investigation of COL4 expression and distribution in the PMs of FG and MG broilers to further explore its potential role in the onset of the growth-related abnormalities. The outcomes of the preliminary histological evaluations performed as part of this study (Soglia et al., 2022) revealed the presence of an abnormal structural organization of the muscles (i.e., necrotic fibers having rounded profile and internal nuclei, inflammatory cells infiltrations along with proliferations of fat and connective tissue at peri- and endomysial level) in FG from d 28 afterward. Conversely, no microscopic alterations were observed in MG throughout the rearing period (from d 7 to d 42). This evidence seems to support the strong association between the occurrence of WS and WB defects and the impressive growth-rate reached in FG birds through artificial selection (Soglia et al., 2021).
COL4 represents the major component of BM of many tissues (i.e., endothelial, muscle, adipose, and nervous tissues) (Gatseva et al., 2019), and its essential role for BMs stability has been demonstrated by the embryonic lethality of COL4A1/A2 knockout mice (Pöschl et al., 2004). In accordance with COL4 biological importance, COL4A1 and COL4A2 chicken genes were found successfully expressed in PMs of both FG and MG broilers. Concerning COL4 gene expression, it is worth noting that transcription regulation of its coding genes is controlled at several levels. Among them, different studies have demonstrated the post-transcriptional regulatory role of miRNAs (Chen et al., 2020)—small, noncoding RNAs which mediate gene expression by binding complementary sites in the 3’-untranslated region (3’-UTR) of target mRNA (Cushing et al., 2011). In this regard, a recent study carried out to investigate differentially expressed miRNAs in FG broiler slaughtered at commercial age (42 d of age) has identified miR-29 and miR-21 as upregulated in WB cases (Shu et al., 2021). Interestingly, both these miRNAs are known to inhibit COL4A1 and COL4A2 transcription (Takizawa et al., 2012; Takahashi et al., 2012; Chen et al., 2020). The results obtained by Shu et al. (2021) seem to corroborate the outcomes of the present study evidencing a significant reduction in the expression level of COL4A2 observed in FG from d 14 to d 35 and d 42. It is also worth noting that miR-29c, one of the miRNAs having COL4A1 and COL4A2 as target genes, has been shown to promote protein synthesis and skeletal muscle mass accretion (Falcone, 2019; Silva et al., 2019; Alves et al., 2022) by suppressing the expression of genes related to the atrophy process (Silva et al., 2019).
Gene expression of COL4 coding genes is also regulated by Smad family (Abe et al., 2004; Turner et al., 2015; Yu et al., 2022)—a group of proteins involved in fibroblasts differentiation and having roles in mediating fibrosis through TGF-β/Smad interaction (Evans et al., 2003). Notably, Xing et al. (2021) evidenced an increased phosphorylation of Smad2 and Smad3 proteins in WB fillets compared to the normal counterpart, suggesting an activation of Smad signaling pathway. This evidence is in line with the present results showing a higher expression level of COL4A2 gene in FG than MG broilers at d 28 when abnormal collagen deposition likely takes place in WB muscles (Soglia et al., 2022). Similarly, since the COL4A1/A2 genes expression is enhanced in human fibroblasts after TGF-β stimulation (Urushiyama et al., 2015), it could be hypothesized that the higher COL4A2 expression level observed in FG than MG at d 28 may be related to the fibroblast proliferation characterizing PMs affected by WB defect (Papah et al., 2018; Soglia et al., 2022). This hypothesis seems to be supported by our previous study, where FG samples showed a sharp increase, from d 21 and d 28 afterwards, in the synthesis of vimentin (Soglia et al., 2022). Vimentin is a protein involved in fibroblasts proliferation (Velleman, 2019) that has been proposed as a biomarker of regenerative processes in PMs (Soglia et al., 2020); its increased expression from d 21 to d 28 may be, therefore, a direct effect of the fibroblast proliferation taking place at this developmental stage.
In light of the above, it could be hypothesized that genetic selection for the hypertrophic growth of pre-existing fibers pursued in modern FG hybrids may have indirectly led to an overexpression of miRNAs and genes involved in muscle growth, which, in their turn, may be associated to an altered regulation of fibroblast proliferation and COL4 synthesis. In this respect, the immunoreactivity observed by IHC in both FG and MG could be ascribed to the genetic background of the genotypes considered in the present study. Indeed, even though characterized by different growth profiles, not only FG but also MG represents a commercial hybrid selected for meat production purposes. It is also worth mentioning that IHC analyses evidenced an unexpected immunoreactivity for COL4 which was found predominantly at the endothelial level rather than being also uniformly distributed in the BM of the myofibers. That made us wonder whether this pattern was reliable or may be ascribed to issues concerning the primary antibody which was used for both IHC and western blot analyses. However, based on the intense immunoreactivity observed in correspondence to the vessels and respective vascular endothelia (which represent one of the main extracellular spaces in which COL4 is localized) (Meuwissen et al., 2015; Guey and Hervé, 2022), we may assume that the altered positivity observed by IHC may be likely related to an altered synthesis and/or secretion of the protein itself rather than being ascribable to the primary antibody used for immunoblotting. Interestingly, IHC analysis performed on mutant mice exploited as animal model to study the COL4-related disorders (Guiraud et al., 2017; Labelle-Dumais et al., 2019), evidenced a protein distribution comparable with the one observed in the present study. In particular, Guiraud et al. (2017) showed a reduction in the expression of COL4 in the muscle BM of mice bearing a COL4A1 mutation (mapped on coding region and determining a Gly489Val substitution at the amino acid level) that perfectly match with the unpredicted COL4 labeling observed in this study. These Authors hypothesized that the considered COL4A1 mutation may impair the protein assembly and/or its secretion at the basal lamina level surrounding the muscle fibers (Guiraud et al., 2017). They also pointed out an intense labeling of COL4 in correspondence to the vascular endothelia, which basically overlaps the outcomes obtained in the present study. Based on their results, the same Authors suggested an intracellular accumulation of COL4, which further supports the present results highlighting its intense immunoreactivity found in the same position.
As for the Western Blot analysis, the results obtained for the relative intensity of the 125 kDa band seem to further corroborate the hypothesis of an altered COL4 deposition at the BM level. Indeed, when considering the evolution of the protein expression during growth until reaching commercial slaughter age, the fluctuating trend in its content may be presumably ascribed to its altered secretion and/or increased degradation. That, in turn, may contribute to explain the weak positivity at the BM level of myofibers observed by IHC analyses. It should also be noted that the presence of the 72 kDa electrophoretic fragment was not expected whereas, according to the literature, the 125 kDa band might be ascribed to the COL4 alpha 1 chain (Trueb et al., 1982). Considering the high specificity of the primary antibody selected for Western Blot and IHC analyses, we could reasonably exclude the occurrence of any bias in the abovementioned analyses. Notably, dated studies reported the existence of 95 kDa and 60 kDa components resulting from bacterial collagenases digestion of isolated COL4 alpha 1 chain (Trueb et al., 1982; Odermatt et al., 1984). In this respect, the COL4 band having a lower molecular weight (i.e., 72 kDa) detected in the present study may represent a fragment resulting from degradation of the native alpha 1 chain. Furthermore, it might be worth mentioning that an increased degradation of COL4 alpha 1 chain may determine the production of bioactive peptides (e.g., arresten and canstatin) known to have roles in impairing angiogenic process (Aikio et al., 2012). This could be considered particularly interesting in relation to the impaired capillarization characterizing FG broilers (Sihvo et al., 2018), which thus may be worsen by this condition.
A different hypothesis may be pointed out considering that other Authors identified specific bands for COL4 alpha 1 chain having approximative weights of 95 kDa (Angbohang et al., 2021) by using SDS-PAGE analysis of anticollagen type IV antibody under reducing conditions. More specifically, another conjecture may consider the 72 kDa band as the native form of the COL4 alpha 1 chain. In this case, the presence of the 125 kDa band could be likely ascribed to the interaction between the network forming COL4 and the molecules responsible for its assembly, folding, and secretion. Indeed, taking into account the molecular weight of one of the main chaperones involved in COL4 trafficking (i.e., HSP47, 47 kDa), the aggregation of this molecule with COL4 (leading to the development of a fragment having a calculated molecular weight of about 120 kDa) may be hypothesized to be responsible for the heavier band detected in the present study. Interestingly, alterations during the assembly of the alpha chains composing COL4 were demonstrated to imply its accumulation at the endoplasmic reticulum level (Chioran et al., 2017). Supporting this hypothesis, Kuo et al. (2014), who investigated the intracellular accumulation of COL4 in mutant mice, highlighted a substantial co-localization of COL4A1 protein and HSP47 in abnormal mice, suggesting an increased ER retention of this type of collagen in mutant cells. This hypothesis seems to be supported also by our preliminary results showing a similar pattern of expression and bands weight for the HSP47 protein through a western blot analysis (data not shown). Moreover, the abnormal accumulation of COL4 and its chaperone could also explain the characteristic ring-like immunoreactivity observed around the nuclei. In particular, it is plausible that this labeling may be due to the aberrant accumulation of unfolding COL4 and its chaperone in the rough endoplasmic reticulum surrounding the nucleus.
The fluctuating trend of COL4 observed in FG over the time may be explained by considering the potential accumulation of the abovementioned aggregate (i.e., COL4 and its chaperone) followed by its degradation. In fact, in view of the numerous pieces of evidence highlighting an upregulation of metalloproteinases (MMP) specific for COL4 degradation (i.e., MMP-2 and MMP-9) in PMs affected by growth-related abnormalities (Mutryn et al., 2015; Zambonelli et al., 2016; Pampouille et al., 2019; Xing et al., 2021), their contribution in determining COL4 content may be hypothesized. Accordingly, an increased activity of the MMP may also contribute to explain the disrupted BM of the myofibers observed by IHC. In this regard, studies reported in the literature pointed out that the development of hypoxic conditions may trigger an upregulation of MMP activity (Ben-Yosef et al., 2002; Liu et al., 2018). Hence, it might be speculated that the higher susceptibility of the PMs belonging to FG broilers to the occurrence of hypoxia may imply a further activation of MMP which ultimately may worsen the cellular processes leading to the degradation of the myofibers BM. In fact, although an altered distribution of COL4 was observed in both FG and MG chickens, the hypertrophic growth of the former, together with their impaired vascularization resulting in hypoxic conditions, may explain their proneness to develop growth-related defects.
Taken together, the findings achieved in the present study seem to support the potential involvement of COL4 as one of the main primary factors underlying the series of events resulting in the onset of growth-related abnormalities in FG modern broilers. Indeed, these outcomes corroborate the theory suggesting that an altered accumulation of this protein may induce endoplasmic reticulum stress specifically at the endothelial level. On the other hand, the altered distribution of COL4 observed at myofibrillar level, probably related to its increased degradation, may be one of the main causes of the altered extracellular matrix composition characterizing abnormal PMs.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103179.
Appendix. Supplementary materials
References
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abasht B., Papah M.B., Qiu J. Evidence of vascular endothelial dysfunction in wooden breast disorder in chickens: Insights through gene expression analysis, ultra-structural evaluation and supervised machine learning methods. PLoS One. 2021;16 doi: 10.1371/journal.pone.0243983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H., Matsubara T., Iehara N., Nagai K., Takahashi T., Arai H., Kita T., Doi T. Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J. Biol. Chem. 2004;279:14201–14206. doi: 10.1074/jbc.M310427200. [DOI] [PubMed] [Google Scholar]
- Aikio M., Alahuhta I., Nurmenniemi S., Suojanen J., Palovuori R., Teppo S., Sorsa T., López-Otín C., Pihlajaniemi T., Salo T., Heljasvaara R., Nyberg P. Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma. PLoS One. 2012;7:e51044. doi: 10.1371/journal.pone.0051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi M.V., Mao M., Pawlikowski B.T., Kvezereli M., Duncan J.L., Libby R.T., John S.W.M., Gould D.B. Col4a1 mutations cause progressive retinal neovascular defects and retinopathy. Sci. Rep. 2016;6:18602. doi: 10.1038/srep18602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves P.K.N., Cruz A., Silva W.J., Labeit S., Moriscot A.S. miR-29c increases protein synthesis in skeletal muscle independently of AKT/mTOR. Int. J. Mol. Sci. 2022;23:7198. doi: 10.3390/ijms23137198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angbohang A., Huang L., Li Y., Zhao Y., Gong Y., Fu Y., Mao C., Morales J., Luo P., Ehteramyan M., Gao Y., Margariti A., Gu W., Zhang M., Smith A., Shah A.M., Li T., Kong W., Zeng L. X-box binding protein 1-mediated COL4A1s secretion regulates communication between vascular smooth muscle and stem/progenitor cells. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayansola H., Liao C., Dong Y., Yu X., Zhang B., Wang B. Prospect of early vascular tone and satellite cell modulations on white striping muscle myopathy. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Petracci M. Current status of poultry meat abnormalities. Meat Muscle Biol. 2020;4:4. [Google Scholar]
- Ben-Yosef Y., Lahat N., Shapiro S., Bitterman H., Miller A. Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ. Res. 2002;90:784–791. doi: 10.1161/01.res.0000015588.70132.dc. [DOI] [PubMed] [Google Scholar]
- Bhave G., Colon S., Ferrell N. The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. Am. J. Physiol. Renal. Physiol. 2017;313:F596–F602. doi: 10.1152/ajprenal.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini M., Soglia F., Davoli R., Zappaterra M., Petracci M., Meluzzi A. Molecular pathways and key genes associated with breast width and protein content in white striping and wooden breast chicken pectoral muscle. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.936768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini M., Zappaterra M., Soglia F., Petracci M., Davoli R. Weighted gene co-expression network analysis identifies molecular pathways and hub genes involved in broiler white striping and wooden breast myopathies. Sci. Rep. 2021;11:1776. doi: 10.1038/s41598-021-81303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Bai J., Li Y. miR-29 mediates exercise-induced skeletal muscle angiogenesis by targeting VEGFA, COL4A1 and COL4A2 via the PI3K/Akt signaling pathway. Mol. Med. Rep. 2020;22:661–670. doi: 10.3892/mmr.2020.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioran A., Duncan S., Catalano A., Brown T.J., Ringuette M.J. Collagen IV trafficking: the inside-out and beyond story. Dev. Biol. 2017;431:124–133. doi: 10.1016/j.ydbio.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Cushing L., Kuang P.P., Qian J., Shao F., Wu J., Little F., Thannickal V.J., Cardoso W.V., Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 2011;45:287. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R.A., Tian Y.C., Steadman R., Phillips A.O. TGF-β1-mediated fibroblast–myofibroblast terminal differentiation—the role of Smad proteins. Exp. Cell Res. 2003;282:90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- Falcone G. A new role of miR-29c as a potent inducer of skeletal muscle hypertrophy. Acta Physiol. 2019;226:e13320. doi: 10.1111/apha.13320. [DOI] [PubMed] [Google Scholar]
- Gatseva A., Sin Y.Y., Brezzo G., Van Agtmael T. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019;63:297–312. doi: 10.1042/EBC20180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E.S., Maynard C., Mullenix G., Bedford M., Dridi S. Potential role of endoplasmic reticulum stress in broiler woody breast myopathy. Am. J. Physiol. Cell Physiol. 2023;324:C679–C693. doi: 10.1152/ajpcell.00275.2022. [DOI] [PubMed] [Google Scholar]
- Guey S., Hervé D. Main features of COL4A1-COL4A2 related cerebral microangiopathies. Cereb. Circ. Cogn. Behav. 2022;3 doi: 10.1016/j.cccb.2022.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud S., Migeon T., Ferry A., Chen Z., Ouchelouche S., Verpont M.C., Sado Y., Allamand V., Ronco P., Plaisier E. HANAC Col4a1 mutation in mice leads to skeletal muscle alterations due to a primary vascular defect. Am. J. Pathol. 2017;187:505–516. doi: 10.1016/j.ajpath.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler A., Kunz N., Gomolka M., Hornhardt S., Friedl A.A., McDonald K., Kohn J.E., Posch A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013;433:105–111. doi: 10.1016/j.ab.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Hosotani M., Saito M., Nagasawa T., Mori Y., Kawasaki T., Yamada M., Maeda N., Watanabe T., Iwasaki T. Mitochondrial characteristics of chicken breast muscle affected by wooden breast. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022;273 doi: 10.1016/j.cbpa.2022.111296. [DOI] [PubMed] [Google Scholar]
- Jeanne M., Jorgensen J., Gould D.B. Molecular and genetic analysis of collagen type IV mutant mouse models of spontaneous intracerebral hemorrhage identify mechanisms for stroke prevention. Circulation. 2015;131:1555. doi: 10.1161/CIRCULATIONAHA.114.013395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A.A., Somlyai-Popovics N., Kiss M., Boldogkői Z., Csiszár K., Mink M. Type IV collagen is essential for proper function of integrin-mediated adhesion in drosophila muscle fibers. Int. J. Mol. Sci. 2019;20:5124. doi: 10.3390/ijms20205124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo D.S., Labelle-Dumais C., Gould D.B. Col4a1 and col4a2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum. Mol. Genet. 2012;21:R97–R110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo D.S., Labelle-Dumais C., Mao M., Jeanne M., Kauffman W.B., Allen J., Favor J., Gould D.B. Allelic heterogeneity contributes to variability in ocular dysgenesis, myopathy and brain malformations caused by Col4a1 and Col4a2 mutations. Hum. Mol. Genet. 2014;23:1709–1722. doi: 10.1093/hmg/ddt560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., l. Shivaprasad H., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Labelle-Dumais C., Schuitema V., Hayashi G., Hoff K., Gong W., Dao D.Q., Ullian E.M., Oishi P., Margeta M., Gould D.B. COL4A1 mutations cause neuromuscular disease with tissue-specific mechanistic heterogeneity. Am. J. Hum. Genet. 2019;104:847–860. doi: 10.1016/j.ajhg.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J.A., Abasht B. Glucolipotoxicity: a proposed etiology for wooden breast and related myopathies in commercial broiler chickens. Front. Physiol. 2020;11:169. doi: 10.3389/fphys.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang H., Yan L., Du W., Zhang M., Chen H., Zhang L., Li G., Li J., Dong Y., Zhu D. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J. Mol. Cell Cardiol. 2018;121:36–50. doi: 10.1016/j.yjmcc.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Meuwissen M.E.C., Halley D.J.J., Smit L.S., Lequin M.H., Cobben J.M., De Coo R., Van Harssel J., Sallevelt S., Woldringh G., Van Der Knaap M.S., De Vries L.S., Mancini G.M.S. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet. Med. 2015;17:843–853. doi: 10.1038/gim.2014.210. [DOI] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B.F., Lang A.B., Ruttner J.R., Winterhalter K.H., Trüeb B. Monoclonal antibodies to human type IV collagen: useful reagents to demonstrate the heterotrimeric nature of the molecule. Proc. Natl. Acad. Sci. U. S. A. 1984;81:7343–7347. doi: 10.1073/pnas.81.23.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Yamawaki H. A current perspective of canstatin, a fragment of type IV collagen alpha 2 chain. J. Pharmacol. Sci. 2019;139:59–64. doi: 10.1016/j.jphs.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Pampouille E., Hennequet-Antier C., Praud C., Juanchich A., Brionne A., Godet E., Bordeau T., Fagnoul F., Bihan-Duval E.Le, Berri C. Differential expression and co-expression gene network analyses reveal molecular mechanisms and candidate biomarkers involved in breast muscle myopathies in chicken. Sci. Rep. 2019;9:14905. doi: 10.1038/s41598-019-51521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of Wooden Breast Disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Gene expression profiling of the early pathogenesis of wooden breast disease in commercial broiler chickens using RNA-sequencing. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W., Tichopad A., Prgomet C., Neuvians T. Determination of most stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Shu J., Liu Y., Shan Y., Ji G., Ju X., Tu Y., Shi S., Sheng Z., Zhang M., Zou J. Deep sequencing microRNA profiles associated with wooden breast in commercial broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvo H.K., Airas N., Lindén J., Puolanne E. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy. J. Comp. Pathol. 2018;161:1–10. doi: 10.1016/j.jcpa.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Lindén J., Airas N., Immonen K., Valaja J., Puolanne E. Wooden breast myodegeneration of pectoralis major muscle over the growth period in broilers. Vet. Pathol. 2017;54:119–128. doi: 10.1177/0300985816658099. [DOI] [PubMed] [Google Scholar]
- Silva W.J., Graça F.A., Cruz A., Silvestre J.G., Labeit S., Miyabara E.H., Yan C.Y.I., Wang D.Z., Moriscot A.S. miR-29c improves skeletal muscle mass and function throughout myocyte proliferation and differentiation and by repressing atrophy-related genes. Acta Physiol. 2019;226:e13278. doi: 10.1111/apha.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soglia F., Bordini M., Mazzoni M., Zappaterra M., Di Nunzio M., Clavenzani P., Davoli R., Meluzzi A., Sirri F., Petracci M. The evolution of vimentin and desmin in Pectoralis major muscles of broiler chickens supports their essential role in muscle regeneration. Front. Physiol. 2022;13:1839. doi: 10.3389/fphys.2022.970034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soglia F., Mazzoni M., Petracci M. Spotlight on avian pathology: current growth-related breast meat abnormalities in broilers. Avian Pathol. 2019;48:1–3. doi: 10.1080/03079457.2018.1508821. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mazzoni M., Zappaterra M., Di Nunzio M., Babini E., Bordini M., Sirri F., Clavenzani P., Davoli R., Petracci M. Distribution and expression of vimentin and desmin in broiler pectoralis major affected by the growth-related muscular abnormalities. Front. Physiol. 2020;10:1581. doi: 10.3389/fphys.2019.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soglia F., Petracci M., Davoli R., Zappaterra M. A critical review of the mechanisms involved in the occurrence of growth-related abnormalities affecting broiler chicken breast muscles. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StatSoft Inc STATISTICA (Data Analysis Software System), Version 10. Copyright © StatSoft. http://www.statsoft.com/
- Takahashi M., Eda A., Fukushima T., Hohjoh H. Reduction of type IV collagen by upregulated miR-29 in normal elderly mouse and klotho-deficient, senescence-model mouse. PLoS One. 2012;7:e48974. doi: 10.1371/journal.pone.0048974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa T., Mase Y., Ishibashi O., Ishikawa T., Takizawa T., Kiguchi K., Ohba T., Katabuchi H., Takeshita T. MiR-21 is enriched in the RNA-induced silencing complex and targets COL4A1 in human granulosa cell lines. Reprod. Sci. 2012;19:1030–1040. doi: 10.1177/1933719112442245. [DOI] [PubMed] [Google Scholar]
- Trouillet A., Lorach H., Dubus E., El Mathari B., Ivkovic I., Dégardin J., Simonutti M., Paques M., Guillonneau X., Sennlaub F., Sahel J.A., Ronco P., Plaisier E., Picaud S. Col4a1 mutation generates vascular abnormalities correlated with neuronal damage in a mouse model of HANAC syndrome. Neurobiol. Dis. 2017;100:52–61. doi: 10.1016/j.nbd.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Trueb B., Grobli B., Spiess M., Odermatt B.F., Winterhalter K.H. Basement membrane (type IV) collagen is a heteropolymer. J. Biol. Chem. 1982;257:5239–5245. [PubMed] [Google Scholar]
- Turner A.W., Nikpay M., Silva A., Lau P., Martinuk A., Linseman T.A., Soubeyrand S., McPherson R. Functional interaction between COL4A1/COL4A2 and SMAD3 risk loci for coronary artery disease. Atherosclerosis. 2015;242:543–552. doi: 10.1016/j.atherosclerosis.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3–new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushiyama H., Terasaki Y., Nagasaka S., Terasaki M., Kunugi S., Nagase T., Fukuda Y., Shimizu A. Role of α1 and α2 chains of type IV collagen in early fibrotic lesions of idiopathic interstitial pneumonias and migration of lung fibroblasts. Lab. Invest. 2015;95:872–885. doi: 10.1038/labinvest.2015.66. [DOI] [PubMed] [Google Scholar]
- Valli V., Taccari A., Di Nunzio M., Danesi F., Bordoni A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018;107:206–215. doi: 10.1016/j.foodres.2018.02.032. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:0034.1. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman S.G. Recent developments in breast muscle myopathies associated with growth in poultry. Annu. Rev. Anim. Biosci. 2019;7:289–308. doi: 10.1146/annurev-animal-020518-115311. [DOI] [PubMed] [Google Scholar]
- Velleman S.G. Pectoralis major (breast) muscle extracellular matrix fibrillar collagen modifications associated with the wooden breast fibrotic myopathy in broilers. Front. Physiol. 2020;11:461. doi: 10.3389/fphys.2020.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J.R. Fibrillar collagen organization associated with broiler wooden breast fibrotic myopathy. Avian Dis. 2017;61:481–490. doi: 10.1637/11738-080217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wang Z., Brannick E., Abasht B. Integrative transcriptomic and metabolomic analysis reveals alterations in energy metabolism and mitochondrial functionality in broiler chickens with wooden breast. Sci. Rep. 2023;13:4747. doi: 10.1038/s41598-023-31429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T., Zhao X., Zhang L., Li J.L., Zhou G.H., Xu X.L., Gao F. Characteristics and incidence of broiler chicken wooden breast meat under commercial conditions in China. Poult. Sci. 2020;99:620–628. doi: 10.3382/ps/pez560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T., Zhao Z.R., Zhao X., Xu X.L., Zhang L., Gao F. Enhanced transforming growth factor-beta signaling and fibrosis in the pectoralis major muscle of broiler chickens affected by wooden breast myopathy. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.Y., Sun Q., Zhang Y.M., Zou L., Zhao Y.Y. TGF-β/Smad signaling pathway in tubulointerstitial fibrosis. Front. Pharmacol. 2022;13:866. doi: 10.3389/fphar.2022.860588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambonelli P., Zappaterra M., Soglia F., Petracci M., Sirri F., Cavani C., Davoli R. Detection of differentially expressed genes in broiler pectoralis major muscle affected by white striping – wooden breast myopathies. Poult. Sci. 2016;95:2771–2785. doi: 10.3382/ps/pew268. [DOI] [PubMed] [Google Scholar]
- Zappaterra M., Braglia S., Bigi M., Zambonelli P., Davoli R. Comparison of expression levels of fourteen genes involved in the lipid and energy metabolism in two pig breeds. Livest. Sci. 2015;181:156–162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.