Summary
Background
Guideline-directed medical therapy (GDMT) is the cornerstone in the treatment of patients with heart failure and reduced ejection fraction (HFrEF) and novel substances such as sacubitril/valsartan (S/V) and sodium-glucose co-transporter-2 inhibitors (SGLT2i) have demonstrated marked clinical benefits. We investigated their implementation into real-world HF care in Germany before, during, and after the COVID-19 pandemic period.
Methods
The IQVIA LRx data set is based on ∼80% of 73 million people covered by the German statutory health insurance. Prescriptions of S/V were used as a proxy for HFrEF. Time trends were analysed between Q1/2016 and Q2/2023 for prescriptions for S/V alone and in combination therapy with SGLT2i.
Findings
The number of patients treated with S/V increased from 5260 in Q1/2016 to 351,262 in Q2/2023. The share of patients with combination therapy grew from 0.6% (29 of 5260) to 14.2% (31,128 of 219,762) in Q2/2021, and then showed a steep surge up to 54.8% (192,429 of 351,262) in Q2/2023, coinciding with the release of the European Society of Cardiology (ESC) guidelines for HF in Q3/2021. Women and patients aged >80 years were treated less often with combined therapy than men and younger patients. With the start of the COVID-19 pandemic, the number of patients with new S/V prescriptions dropped by 17.5% within one quarter, i.e., from 26,855 in Q1/2020 to 22,145 in Q2/2020, and returned to pre-pandemic levels only in Q1/2021.
Interpretation
The COVID-19 pandemic was associated with a 12-month deceleration of S/V uptake in Germany. Following the release of the ESC HF guidelines, the combined prescription of S/V and SGLT2i was readily adopted. Further efforts are needed to fully implement GDMT and strengthen the resilience of healthcare systems during public health crises.
Funding
Supported by Novartis Pharma GmbH, Nuremberg, Germany.
Keywords: Heart failure, COVID-19, Sacubitril-valsartan, Sodium-glucose co-transporter-2 inhibitors, Guideline-directed medical therapy, Evidence-based practice, Real-world
Research in context.
Evidence before this study
Guideline-directed medical therapy (GDMT) is the cornerstone of a successful treatment strategy in patients with heart failure and reduced ejection fraction (HFrEF). Besides established pharmacotherapy, novel substances such as sacubitril/valsartan (S/V) and sodium-glucose co-transporter-2 inhibitors (SGLT2i) have demonstrated marked clinical benefits. The prompt initiation of GDMT after a diagnosis of HFrEF is considered safe and efficacious, whereas the deferred initiation is associated with worse prognosis. We searched PubMed for peer-reviewed literature from database inception to 05/31/2023 on the impact of the COVID-19 pandemic on the implementation of novel GDMT for HFrEF. The following search string was used: ((COVID-19) OR (SARS-CoV-2) OR (coronavirus)) AND ((prescription∗) OR (prescription drugs) OR (drug utilization) OR (implementation)) AND ((sacubitril-valsartan) OR (sacubitril valsartan sodium hydrate) OR (LCZ696) OR (sodium-glucose transporter 2 inhibitors) OR (SGLT-2 inhibitors) OR (gliflozin∗)). We found two studies, which had investigated the change of novel GDMT in patients with HFrEF during the COVID-19 pandemic. The first study was a brief report on the prevalent prescription filling patterns of GDMT throughout the year 2020 in the United States. It reported that the prescription fills of S/V and SGLT2i increased during the COVID-19 pandemic. The second study compared observed and forecasted new S/V prescriptions between March and December 2020 in Italy. The study reported that the initiation of new HF patients on S/V decreased by nearly 40% in this period. However, both studies concentrated only on the first months of the pandemic, precluding conclusions for longer-term and recovery effects. Furthermore, prevalent and incident prescriptions were not jointly examined, no subgroup analyses were performed, and the impact of the new HF guidelines of the European Society of Cardiology (published in August 2021) on prescribing behaviour was not addressed.
Added value of this study
Our study describes the diffusion of novel GDMT into the real-world HF care in Germany before, during, and after the COVID-19 pandemic. The number of prevalent patients with S/V prescriptions continuously increased between Q1/2016 and Q2/2023. After the release of the ESC HF guidelines in August 2021, prescribing behaviours markedly changed from isolated S/V therapy to combined therapy with S/V and SGLT2i. Even though the implementation of novel GDMT increased overall, women and patients >80 years seemed to be disadvantaged. Most importantly, the COVID-19 pandemic notably compromised the initiation of disease modifying drug therapy resulting in a 12-month period of delayed treatment initiation of S/V.
Implications of all the available evidence
Further efforts are needed to fully implement GDMT in HFrEF including S/V and SGLT2i. Health policy makers need to take action to ensure the access to care for patients with chronic illness and thereby to strengthen the resilience of healthcare systems for future public health crises.
Introduction
Heart failure (HF) affects more than 64 million patients worldwide imposing a major burden on health systems and societies.1,2 In Germany, the prevalence of HF is slightly higher than in other Western European countries, with approximately 4% in the general population.2,3 The number of HF hospitalizations has increased substantially over time with HF remaining the most common cause of hospitalization and in-hospital death.4 Guideline-directed medical therapy (GDMT) is the cornerstone of HF therapy.5 Recent clinical trials in patients with HF and a reduced ejection fraction (HFrEF) have demonstrated the benefit of novel pharmacotherapies (sacubitril/valsartan [S/V] and sodium-glucose co-transporter-2 inhibitors (SGLT2i),6, 7, 8 besides traditional substance classes such as beta-blockers (BB), mineralocorticoid receptor antagonists (MRA) and angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARB). S/V and SGLT2i were shown to reduce HF hospitalizations,6, 7, 8 improve health-related quality of life,6,9,10 and reduce progression of chronic kidney disease (CKD).11, 12, 13 Furthermore, the stepwise addition of substance classes followed by multiple up-titration visits is time-consuming, and deferral of GDMT initiation associates with worse outcomes.14 Hence, the recent recommendation in the European Society of Cardiology (ESC) HF guidelines centers on early commencement of quadruple therapy (S/V, SGLT2i, BB, and MRA) accompanied by accelerated up-titration to improve outcomes in patients with HFrEF (class IC recommendation).15,16 Recently, the STRONG-HF trial supported this concept as it demonstrated the benefit and safety of an intensified, early up-titration of GDMT.17
However, the implementation of GDMT for patients with HFrEF into daily medical practice faces major barriers.18 The EVOLUTION HF trial showed that, compared to other GDMT, the time needed to initiate S/V and/or SGLT2i was particularly long.19 This indicates that the implementation of novel GDMT may be medically, economically, and logistically challenging, but also impeded by inertia. Apart from these well-known physician-, patient-, and system-related factors,18,20 further external factors constitute challenges for the “real world of guideline implementation”. This includes public health crises, e.g. the recent COVID-19 pandemic, which mandated numerous individual protective and general lock-down measures, and thereby massively affected health care utilization.21
We investigated the transfer of recommendations on novel GDMT into clinical practice in Germany over the period from January 2016 to June 2023. We aimed to quantify the potential influence of the new HF guidelines and the impact of the COVID-19 pandemic on prescription rates in real-world HF care.
Methods
Study design and database
We here report a descriptive, retrospective analysis of patients from the IQVIA longitudinal prescription (LRx) database and the IQVIA Analytic Platform on both patient and physician level including all adult patients with a prescription of S/V following its approval by the European Medicines Agency (EMA) in November 2015.22 The observation period was from the first quarter of the year 2016 (Q1/2016) to the end of Q2/2023. Due to the unavailability of clinical characteristics and diagnoses in the LRx database, use of S/V served as a proxy for HFrEF. This assumption is founded on the restricted indication of S/V for the treatment of symptomatic patients with HFrEF.23
The IQVIA LRx database is an anonymized prescription data set based on community pharmacy data comprising about 80% of the prescriptions in the German statutory health insurance (SHI) population (∼73 million people). Extrapolation of LRx patient counts to national pharmacy claims (SHI and private health insurance) using established methods (i.e. the ratio of observed claimed packs in LRx vs. total national claims in the IQVIA PharmaScope database) allows inferring conclusions about total national prescriptions. Apart from longitudinal information about the prescribed treatment course over time for an individual patient (e.g. product name, substance, dose), the database also provides basic patient characteristics including age and sex. While age was directly available in the database, sex was derived from the patient's first name. If such derivation was not possible because of ambiguity, no information on sex was available. In sex-specific analyses, such cases were therefore excluded.
The IQVIA Analytic Platform covers anonymized data on a representative share of all sick-fund prescriptions in Germany and is based on a trust centre that includes anonymized aggregated data from representative panels of about 50% of the German primary care physicians (PCP; either general practitioners or general internists) and cardiologists, respectively. It enables analyses of prescribing behaviours among the totality of PCP offices (n∼36,950) and cardiologist offices (n∼1800), in Germany.
To assess the potential impact of the COVID-19 pandemic and the release of the ESC HF guidelines on novel GDMT prescriptions, the following time points were defined: 22nd of March 2020 for the implementation of nation-wide public restrictions (in accordance with Enners et al.24), and 27th of August 2021 for the official publication of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure.5 All data were analysed using Microsoft Excel.
Patient-level analysis
For each quarter, the number of patients receiving prescriptions of S/V (and concomitant SGLT2i) prescriptions were calculated. Thereby, “prevalent prescriptions” were defined as all filed prescriptions of a respective quarter, and “incident prescriptions” were defined as prescriptions that were newly started in a respective quarter. For prevalent patients with combined therapy, the therapy sequence was analysed, i.e. whether the treatment was started (i) with S/V alone followed by later addition of SGLT2i, (ii) with S/V and SGLT2i at the same time, or (iii) with SGLT2i alone followed by later addition of S/V. Incident treatment with S/V was defined as a new S/V prescription without any preceding S/V prescription within the last 12 months, either (a) S/V alone without concomitant SGLT2i, (b) the simultaneous initiation of S/V and SGLT2i, or (c) the addition of S/V to pre-existing SGLT2i. Co-prescription of SGLT2i was assessed for dapagliflozin, empagliflozin, or ertugliflozin. The following subgroups were compared: sex (female vs. male) and age (>80 years vs. ≤80 years).
Physician-level analysis
The aim of the physician-level analysis was to assess the breadth and depth of the diffusion of novel GDMT into ambulatory HF care. The prevalent share of offices with at least one patient on S/V prescription (i.e. “breadth”) and the number of patients on drug per prescribing office (i.e. “depth”) were calculated for each quarter. We compared S/V prescription rates (as share of offices with ≥1 patient on medication and number of patients on medication per office) between PCPs and cardiologists.
Role of the funding source
Novartis Pharma GmbH, Nuremberg/Germany, supported the process of data set extraction via the IQVIA institute and contributed intellectual content to the final version of the manuscript. Novartis Pharma GmbH was not involved in the conceptualization of the study design, data analysis or data interpretation.
Results
Prevalent patients with S/V therapy
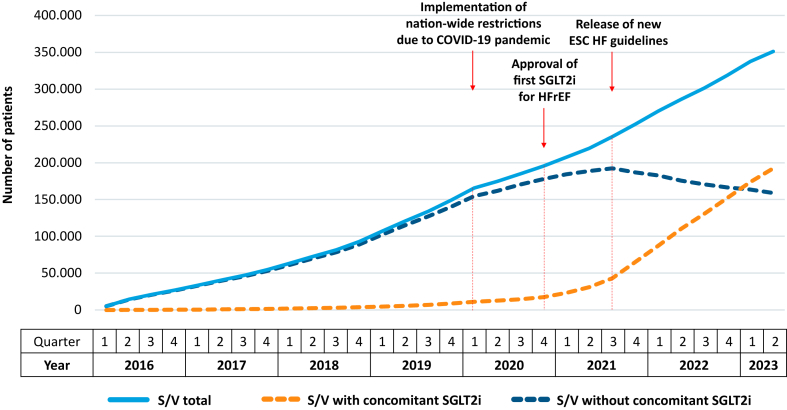
The number of prevalent patients treated with S/V continuously increased from 5260 in Q1/2016 to 351,262 in Q2/2023 (Fig. 1). However, coinciding with the start of the COVID-19 pandemic, the quarterly growth decreased by about 50% from 16,507 in Q1/2020 to 8804 in Q2/2020. As depicted in Table 1, the number of patients treated with S/V alone declined slowly (99.4% of total S/V prescriptions in Q1/2016 vs. 45.2% in Q2/2023), whereas the number of patients jointly treated with S/V and SGLT2i increased from 29 (0.6%) to 192,429 (54.8%). This trend was markedly accelerated after the release of the ESC HF guidelines, with the quarterly growth for combined therapy almost doubling from 11,929 in Q3/2021 to 22,033 in Q4/2021.
Fig. 1.
Temporal trend of prevalent patients with S/V prescriptions. COVID-19 = coronavirus disease 2019, ESC = European Society of Cardiology, HF = heart failure, HFrEF = heart failure with reduced ejection fraction, SGLT2i = sodium-glucose co-transporter-2 inhibitor, S/V = sacubitril/valsartan.
Table 1.
Prevalent patients with S/V prescriptions.
| Drug(s) | 2016 |
2017 |
2018 |
2019 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| S/V total (% of S/V total) | 5260 (100%) | 14,521 (100%) | 21,181 (100%) | 27,142 (100%) | 33,722 (100%) | 40,489 (100%) | 47,034 (100%) | 54,703 (100%) | 63,632 (100%) | 72,704 (100%) | 81,644 (100%) | 92,798 (100%) | 107,069 (100%) | 121,044 (100%) | 134,080 (100%) | 149,169 (100%) |
| S/V alone (% of S/V total) | 5231 (99.4%) | 14,366 (98.9%) | 20,905 (98.7%) | 26,747 (98.5%) | 33,164 (98.3%) | 39,621 (97.9%) | 45,811 (97.4%) | 53,148 (97.2%) | 61,586 (96.8%) | 70,129 (96.5%) | 78,501 (96.2%) | 88,926 (95.8%) | 102,343 (95.6%) | 115,240 (95.2%) | 127,190 (94.9%) | 140,347 (94.1%) |
| S/V + SGLT2i (% of S/V total) | 29 (0.6%) | 156 (1.1%) | 276 (1.3%) | 395 (1.5%) | 558 (1.7%) | 868 (2.1%) | 1223 (2.6%) | 1556 (2.8%) | 2047 (3.2%) | 2576 (3.5%) | 3143 (3.8%) | 3872 (4.2%) | 4726 (4.4%) | 5804 (4.8%) | 6890 (5.1%) | 8822 (5.9%) |
| Drug(s) | 2020 |
2021 |
2022 |
2023 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | |
| S/V total (% of S/V total) | 165,676 (100%) | 174,480 (100%) | 184,947 (100%) | 195,261 (100%) | 207,290 (100%) | 219,762 (100%) | 235,367 (100%) | 252,061 (100%) | 270,326 (100%) | 286,160 (100%) | 301,287 (100%) | 318,595 (100%) | 337,463 (100%) | 351,262 (100%) |
| S/V alone (% of S/V total) | 154,620 (93.3%) | 161,763 (92.7%) | 170,448 (92.2%) | 177,760 (91.0%) | 184,194 (88.9%) | 188,633 (85.8%) | 192,310 (81.7%) | 186,971 (74.2%) | 182,817 (67.6%) | 175,685 (61.4%) | 170,785 (56.7%) | 166,561 (52.3%) | 163,538 (48.5%) | 158,833 (45.2%) |
| S/V + SGLT2i (% of S/V total) | 11,056 (6.7%) | 12,717 (7.3%) | 14,500 (7.8%) | 17,501 (9.0%) | 23,096 (11.1%) | 31,128 (14.2%) | 43,057 (18.3%) | 65,090 (25.8%) | 87,509 (32.4%) | 110,476 (38.6%) | 130,502 (43.3%) | 152,035 (47.7%) | 173,925 (51.5%) | 192,429 (54.8%) |
S/V = sacubitril/valsartan, SGLT2i = sodium–glucose co-transporter 2 inhibitor.
In Q1/2016, 1517 women (28.8%) and 3375 men (64.2%) were treated with S/V. In 368 patients (7.0%), no information on sex was available. In Q2/2023, the respective shares were 29.4%, 63.3%, and 7.3%, respectively. After exclusion of patients with unknown sex, the cleared proportion of women treated with S/V remained relatively unchanged over time at around 31% (Table 2). In the subgroup of simultaneous treatment with S/V and SGLT2i, the proportion of women was always lower (5.6% in Q1/2016 and 28.1% in Q2/2023) when compared to the whole cohort.
Table 2.
Share of women amongst prevalent patients with S/V prescriptions.
| Drug(s) | 2016 |
2017 |
2018 |
2019 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| S/V total (% of S/V total) | 1517 (31.0%) | 4299 (31.9%) | 6054 (30.7%) | 7570 (30.1%) | 9185 (29.4%) | 11,184 (29.8%) | 12,995 (29.9%) | 14,919 (29.5%) | 17,455 (29.7%) | 20,008 (29.7%) | 22,602 (29.9%) | 25,660 (29.8%) | 29,856 (30.0%) | 34,177 (30.3%) | 37,796 (30.3%) | 42,217 (30.4%) |
| S/V alone (% of S/V alone) | 1516 (31.2%) | 4268 (32.1%) | 5999 (30.8%) | 7501 (30.3%) | 9100 (29.7%) | 11,049 (30.1%) | 12,790 (30.2%) | 14,654 (29.9%) | 17,091 (30.0%) | 19,558 (30.1%) | 22,053 (30.3%) | 25,022 (30.3%) | 29,034 (30.6%) | 33,166 (30.9%) | 36,600 (30.9%) | 40,618 (31.1%) |
| S/V + SGLT2i (% of S/V + SGLT2i) | 2 (5.6%) | 31 (20.2%) | 55 (20.8%) | 69 (18.2%) | 85 (16.1%) | 136 (16.8%) | 204 (17.9%) | 265 (18.2%) | 364 (19.1%) | 450 (18.6%) | 549 (18.5%) | 637 (17.5%) | 822 (18.4%) | 1011 (18.5%) | 1196 (18.4%) | 1598 (19.2%) |
| Drug(s) | 2020 |
2021 |
2022 |
2023 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | |
| S/V total (% of S/V total) | 46,978 (30.4%) | 49,357 (30.4%) | 52,397 (30.4%) | 55,364 (30.4%) | 59,086 (30.6%) | 62,758 (30.7%) | 67,135 (30.7%) | 72,105 (30.7%) | 77,777 (30.9%) | 83,199 (31.3%) | 87,564 (31.3%) | 92,697 (31.3%) | 98,878 (31.6%) | 103,246 (31.7%) |
| S/V alone (% of S/V alone) | 45,029 (31.3%) | 47,130 (31.3%) | 49,912 (31.4%) | 52,265 (31.6%) | 54,712 (31.9%) | 56,478 (32.2%) | 58,109 (32.5%) | 57,557 (33.1%) | 57,378 (33.7%) | 56,350 (34.5%) | 55,284 (34.8%) | 54,484 (35.2%) | 54,263 (35.7%) | 53,101 (36.0%) |
| S/V + SGLT2i (% of S/V + SGLT2i) | 1949 (18.7%) | 2227 (18.6%) | 2486 (18.2%) | 3099 (18.9%) | 4374 (20.2%) | 6280 (21.6%) | 9026 (22.5%) | 14,549 (24.0%) | 20,398 (25.1%) | 26,849 (26.1%) | 32,280 (26.6%) | 38,213 (27.1%) | 44,615 (27.7%) | 50,145 (28.1%) |
S/V = sacubitril/valsartan, SGLT2i = sodium–glucose co-transporter 2 inhibitor.
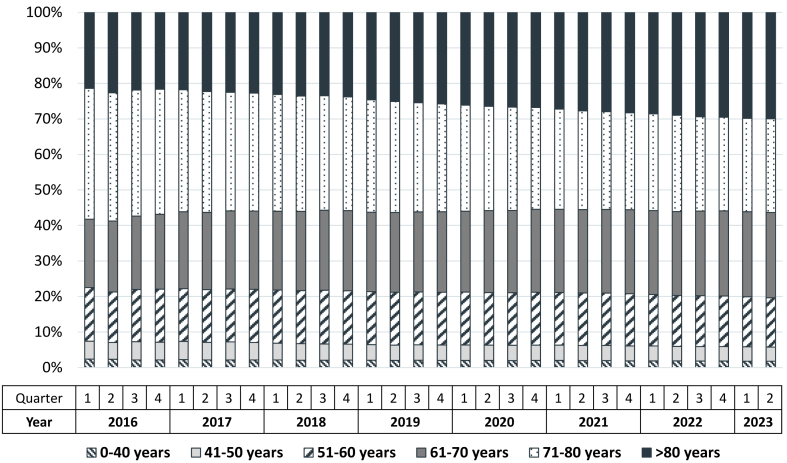
Between Q1/2016 and Q2/2023, the share of patients older than 80 years increased from 21.4% % to 29.9% (Fig. 2 and Table 3). At the same time, the shares of patients younger than 60 years and those in the age category of 71–80 years decreased. Similar to female sex, patients older than 80 years were consistently less prevalent in the subgroup of combined treatment with S/V and SGLT2i (5.3% in Q1/2026 and 24.8% in Q2/2023) when compared to the whole cohort (Table 3).
Fig. 2.
Distribution of prevalent patients with S/V prescriptions according to age over time.
Table 3.
Share of elderly (>80 years) amongst prevalent patients with S/V prescriptions.
| Drug(s) | 2016 |
2017 |
2018 |
2019 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| S/V total (% of S/V total) | 1125 (21.4%) | 3286 (22.6%) | 4627 (21.8%) | 5874 (21.6%) | 7341 (21.8%) | 9022 (22.3%) | 10,584 (22.5%) | 12,446 (22.8%) | 14,695 (23.1%) | 17,121 (23.5%) | 19,166 (23.5%) | 22,088 (23.8%) | 26,357 (24.6%) | 30,361 (25.1%) | 34,078 (25.4%) | 38,448 (25.8%) |
| S/V alone (% of S/V alone) | 1124 (21.5%) | 3282 (22.8%) | 4610 (22.1%) | 5858 (21.9%) | 7308 (22.0%) | 8970 (22.6%) | 10,503 (22.9%) | 12,334 (23.2%) | 14,544 (23.6%) | 16,941 (24.2%) | 18,947 (24.1%) | 21,823 (24.5%) | 26,024 (25.4%) | 29,919 (26.0%) | 33,520 (26.4%) | 37,700 (26.9%) |
| S/V + SGLT2i (% of S/V + SGLT2i) | 2 (5.3%) | 5 (2.9%) | 17 (6.1%) | 17 (4.2%) | 34 (6.0%) | 52 (6.0%) | 81 (6.6%) | 111 (7.2%) | 151 (7.4%) | 180 (7.0%) | 220 (7.0%) | 265 (6.9%) | 332 (7.0%) | 442 (7.6%) | 558 (8.1%) | 749 (8.5%) |
| Drug(s) | 2020 |
2021 |
2022 |
2023 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | |
| S/V total (% of S/V total) | 43,299 (26.1%) | 46,133 (26.4%) | 49,357 (26.7%) | 52,291 (26.8%) | 56,404 (27.2%) | 60,895 (27.7%) | 65,819 (28.0%) | 71,234 (28.3%) | 77,182 (28.6%) | 82,916 (29.0%) | 88,496 (29.4%) | 93,972 (29.5%) | 100,597 (29.8%) | 105,196 (29.9%) |
| S/V alone (% of S/V alone) | 42,337 (27.4%) | 44,962 (27.8%) | 47,941 (28.1%) | 50,368 (28.3%) | 53,636 (29.1%) | 56,768 (30.1%) | 59,294 (30.8%) | 59,539 (31.8%) | 60,144 (32.9%) | 59,455 (33.8%) | 59,303 (34.7%) | 58,671 (35.2%) | 58,597 (35.8%) | 57,441 (36.2%) |
| S/V + SGLT2i (% of S/V + SGLT2i) | 962 (8.7%) | 1171 (9.2%) | 1415 (9.8%) | 1923 (11.0%) | 2768 (12.0%) | 4127 (13.3%) | 6525 (15.2%) | 11,695 (18.0%) | 17,037 (19.5%) | 23,461 (21.2%) | 29,193 (22.4%) | 35,301 (23.2%) | 42,000 (24.1%) | 47,755 (24.8%) |
S/V = sacubitril/valsartan, SGLT2i = sodium–glucose co-transporter 2 inhibitor.
Therapy sequence for prevalent patients with combined therapy (S/V + SGTL2i)
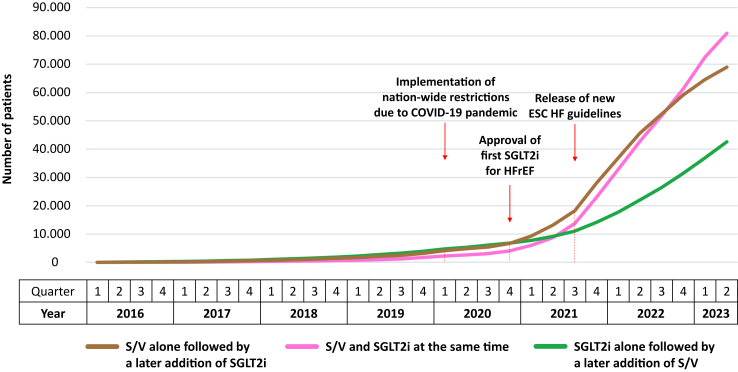
As shown in Fig. 3, there was a continuous surge for all three subgroups of patients receiving simultaneous treatment with S/V and SGLT2i since the year 2019. In Q1/2019, treatment initiation with SGLT2i alone followed by a later addition of S/V was most frequent (n = 2265), but was surpassed by the other two therapy sequences in Q3/2021. In Q2/2023, jointly initiating S/V with SGLT2i constituted the most frequent approach (n = 80,926). Overall, joint initiation of S/V with SGLT2i increased by a factor of 103 from Q1/2019 until Q2/2023 (growth factor 41 for S/V alone, and 19 for the addition of S/V to pre-existing SGLT2i, respectively).
Fig. 3.
Temporal trend of therapy sequence of prevalent patients with combined prescriptions of S/V and SGLT2i. COVID-19 = coronavirus disease 2019, ESC = European Society of Cardiology, HF = heart failure, HFrEF = heart failure with reduced ejection fraction, SGLT2i = sodium-glucose co-transporter-2 inhibitor, S/V = sacubitril/valsartan.
Incident patients with S/V therapy
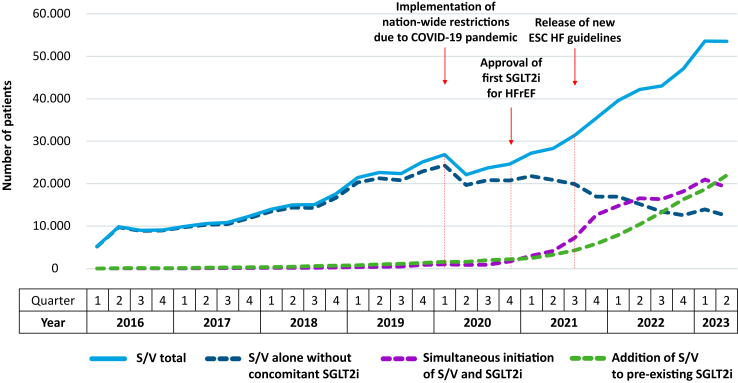
As depicted in Fig. 4, the number of patients with new S/V prescriptions increased from Q1/2016 (n = 5238) to Q2/2023 (n = 53,534). However, there was a drop of 17.5% between Q1/2020 (n = 26,855) and Q2/2020 (n = 22,145), at the time when the nation-wide public restrictions due to the COVID-19 pandemic had to be implemented in Germany. The absolute number of new S/V prescriptions did not return to the pre-pandemic level before Q1/2021 (n = 27,197).
Fig. 4.
Temporal trend of incident patients with S/V prescriptions. COVID-19 = coronavirus disease 2019, ESC = European Society of Cardiology, HF = heart failure, HFrEF = heart failure with reduced ejection fraction, SGLT2i = sodium-glucose co-transporter-2 inhibitor, S/V = sacubitril/valsartan.
Before the approval of the first SGLT2i for HFrEF in Q4/2020, the increase of new S/V prescriptions was driven by prescribing S/V without concomitant SGLT2i. Thereafter, the simultaneous initiation of S/V and SGLT2i or the addition of S/V to pre-existing SGLT2i became the favoured approach. This trend was further accelerated after Q3/2021, i.e. concurring with the release of the new ESC HF guidelines.
Physician-level analysis
For PCPs, the proportion of offices with at least one patient receiving S/V continuously increased from 3.3% in Q1/2016 and exceeded 80% since Q3/2021 (Table 4). For the combination of S/V and SGLT2i, the share of PCP offices more than tripled after Q2/2021, from 20.7% to 65.2% in Q2/2023. For cardiologists, the proportion of offices with at least one patient receiving S/V peaked at 91.5% in Q3/2021 followed by a slight decline to 90.0% in Q2/2023. However, the share of cardiologist offices prescribing the combined therapy more than doubled from 34.4% in Q2/2021 to 74.2% in Q2/2023.
Table 4.
Prescriptions of S/V overall and S/V with SGLT2i according to prescribing specialty.
| Physician | Drug(s) | Variable | 2016 |
2017 |
2018 |
2019 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| Primary care physician | S/V | Share of officesa (%) | 3.3 | 13.1 | 20.3 | 26.0 | 29.8 | 34.7 | 38.6 | 40.6 | 45.2 | 48.2 | 51.5 | 54.0 | 57.4 | 61.6 | 65.0 | 67.3 |
| Number of patients per office (n) | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.8 | 2.0 | 2.0 | 2.2 | 2.3 | 2.4 | 2.6 | 2.9 | 3.1 | 3.3 | ||
| S/V + SGLT2i | Share of officesa (%) | 0.0 | 0.1 | 0.1 | 0.4 | 0.5 | 0.6 | 0.9 | 1.1 | 1.3 | 1.6 | 2.1 | 2.5 | 3.1 | 3.7 | 4.5 | 6.1 | |
| Number of patients per office (n) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Cardiologist | S/V | Share of officesa (%) | 29.2 | 54.6 | 65.3 | 69.9 | 69.5 | 71.2 | 75.5 | 77.7 | 79.2 | 80.6 | 79.6 | 81.0 | 79.0 | 79.3 | 80.5 | 84.0 |
| Number of patients per office (n) | 2.4 | 4.0 | 4.8 | 5.6 | 6.1 | 6.7 | 7.1 | 7.8 | 9.1 | 9.8 | 10.1 | 11.4 | 11.3 | 12.4 | 13.1 | 14.0 | ||
| S/V + SGLT2i | Share of officesa (%) | n/a | 0.9 | 1.4 | 2.3 | 0.9 | 1.7 | 0.9 | 2.6 | 2.8 | 4.0 | 4.1 | 5.9 | 5.7 | 7.1 | 7.4 | 11.6 | |
| Number of patients per office (n) | n/a | 1.0 | 1.0 | 1.0 | 1.0 | 1.3 | 1.5 | 1.2 | 1.0 | 1.1 | 1.1 | 1.1 | 1.2 | 1.1 | 1.2 | 1.3 | ||
| Physician | Drug(s) | Variable | 2020 |
2021 |
2022 |
2023 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | |||
| Primary care physician | S/V | Share of officesa (%) | 71.0 | 72.1 | 74.5 | 75.6 | 76.4 | 78.3 | 80.2 | 81.3 | 81.0 | 81.0 | 80.9 | 80.6 | 84.4 | 84.1 |
| Number of patients per office (n) | 3.5 | 3.6 | 3.8 | 4.0 | 4.1 | 4.3 | 4.6 | 4.9 | 5.1 | 5.5 | 5.9 | 6.2 | 6.4 | 6.8 | ||
| S/V + SGLT2i | Share of officesa (%) | 8.3 | 8.5 | 9.8 | 12.1 | 15.7 | 20.7 | 29.4 | 39.3 | 45.0 | 50.8 | 54.5 | 57.2 | 63.1 | 65.2 | |
| Number of patients per office (n) | 1.1 | 1.1 | 1.1 | 1.1 | 1.2 | 1.2 | 1.3 | 1.5 | 1.7 | 1.9 | 2.0 | 2.2 | 2.4 | 2.5 | ||
| Cardiologist | S/V | Share of officesa (%) | 84.8 | 85.9 | 86.2 | 86.7 | 89.8 | 89.8 | 91.5 | 91.5 | 90.9 | 89.9 | 89.0 | 88.1 | 91.0 | 90.0 |
| Number of patients per office (n) | 14.9 | 14.4 | 15.1 | 16.1 | 16.0 | 16.4 | 16.7 | 18.2 | 18.3 | 19.0 | 19.2 | 20.0 | 19.6 | 19.6 | ||
| S/V + SGLT2i | Share of officesa (%) | 14.8 | 14.1 | 17.9 | 22.8 | 27.4 | 34.4 | 49.7 | 61.3 | 64.2 | 68.3 | 70.2 | 71.0 | 74.4 | 74.2 | |
| Number of patients per office (n) | 1.3 | 1.3 | 1.3 | 1.5 | 1.8 | 2.1 | 2.6 | 3.4 | 4.2 | 4.5 | 4.7 | 5.3 | 5.5 | 5.7 | ||
Primary care physician = general practitioner or general internist, S/V = sacubitril/valsartan, SGLT2i = sodium–glucose co-transporter 2 inhibitor, n/a = not available.
with at least one patient on drug.
Discussion
The current study analysed temporal prescribing trends for novel HF GDMT in Germany before, during and after the COVID-19 pandemic associating the 2021 updated HF clinical guidelines with prescribing behaviors. The speed by which new evidence for GDMT may translate into everyday practice has been difficult to assess in the past. We found a steady increase of S/V prescriptions between January 2016 and June 2023, accompanied by a gradual shift after mid-2021 from isolated S/V therapy to combined therapy with S/V and SGLT2i, coinciding with the release of the ESC HF guidelines in Q3 2021. As a result of the COVID-19 pandemic-related restrictions, new S/V prescriptions dropped by 17.5% within a quarter, and the delayed treatment initiation remained compromised for 12 months.
Impact of the 2021 ESC HF guidelines on the prescribing behaviour for novel GDMT
The temporal association in 2021 suggests that the updated ESC recommendations for early and prompt initiation of all four pillars for patients with HFrEF5,15 quickly affected prescribing behaviours in terms of a surge in combined prescriptions of S/V and SGLT2i. In fact, simultaneous prescription of both novel GDMT evolved from the least often to the most frequent modality of therapy initiation. Likewise, the share of PCP and cardiologist offices prescribing the combination of S/V and SGLT2i both markedly increased after Q3/2021. When compared to PCPs, cardiologists seemed to be more prone to prescribe S/V overall and the combined therapy, respectively. This corroborates previous studies reporting differences in the prescribing behaviour of GDMT between general practitioners and cardiologists.25,26 These discrepancies become even more relevant since, in Germany, within the first year after hospitalisation for HF, 99.6% of the patients are seen by their general practitioner, but only 36.6% by their cardiologist.27 Therefore, future research needs to investigate the determinants of prescribing behaviour including physicians' preferences and costs of drugs.
Our study corroborates recent findings by Pierce et al. from the United States (US) on the relatively rapid early adoption of SGLT2i when compared to the early adoption of S/V in patients with HFrEF.28 Since the EMA approvals of dapagliflozin29 and empagliflozin30 for HFrEF in November 2020 and in May 2021, respectively, the observed shift towards combined prescription in our study (i.e. uptake of SGLT2i) might have been promoted by various factors including its pre-existing approval for type 2 diabetes (T2D), and for CKD in August 2021 (dapagliflozin). Furthermore, dosing characteristics of SGLT2i (administration once per day, no up-titration) and the minimal effect on blood pressure might facilitate its application. Nevertheless, the uptake of S/V is also rising steadily over time. In contrast to SGLT2i, S/V demonstrated survival benefits in comparison to an active comparator6 and was shown to promote reverse cardiac remodeling.31,32 Moreover, Pierce et al. pointed out that the overall use of SGLT2i is still low, with only 1 in 5 patients hospitalized for HFrEF being discharged with SGLT2i, irrespective of T2D or CKD.28
Even though clinical guidelines have become an increasingly better adopted cornerstone of clinical care,18,33 major gaps remain between evidence from pivotal randomized outcome trials and uptake of GDMT in daily practice.34 Implementation interventions have been shown to improve uptake of GDMT in HF,35, 36, 37 but they share great heterogeneity and were not always accompanied by improvements in clinical outcomes.36 Ultimately, considerable efforts are still needed to understand how evidence-based clinical knowledge can more effectively be translated to and used in real-world settings.
Despite improvements over time, less women and patients aged >80 years appeared to benefit from S/V and SGLT2i as a combined regimen. This is in line with previous studies reporting sex- and age-based differences of GDMT for patients with HFrEF.38, 39, 40, 41, 42 Recently, another study analysing claims data of up to 180,386 Medicare beneficiaries with HFrEF between 2016 and 2019 from the US showed that patients aged ≥85 years were less likely to initiate S/V.43 Among many factors, uncertainty regarding the effectiveness and safety might contribute to hesitant adoption of novel GDMT in elderly patients, even though studies had demonstrated the efficacy, effectiveness, and safety of both S/V and SGLT2i across multiple subgroups including elderly patients.39,41,42,44,45
The importance of novel GDMT was underscored in the recently published 2023 Focused Update of the 2021 ESC HF guidelines.46 Notably, this update placed special emphasis on SGLT2i, which now have been given a class IA recommendation across the ejection fraction spectrum, i.e. including not only HFrEF but also HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF).
Impact of the COVID-19 pandemic on the initiation of novel GDMT
We observed a temporal association between the beginning of the nation-wide public restrictions related to the COVID-19 pandemic in March 2020 and a decline of new S/V prescriptions by 17.5% following the respective first quarter of the year 2020. Previous studies reported on the changes of drug and healthcare utilization during the COVID-19 pandemic due to countermeasures such as stay-at-home orders or lockdown policies.21,24,47,48 A systematic review including 81 studies from 20 countries demonstrated overall pandemic-related reductions in global healthcare utilization by 37% and for cardiovascular diseases by 29%.21 In Germany, physician-patient consultations and the corresponding treatment cases markedly decreased in the course of the pandemic: when compared to the pre-pandemic year, treatment cases in the last week of March 2020 declined by 40% for cardiologists (>50,000 cases) and by 39% for general practitioners (>500,000 cases).49 It seems plausible that—among other factors – limited access to care might have contributed to the decreased prescription rates, including S/V. In fact, our findings are consistent with previous studies from Europe (e.g. Italy) reporting underprescription of cardiovascular drugs such as S/V and direct oral anticoagulants during COVID-19 lockdown periods.50,51 In the study of Rosano et al., the decline of new S/V prescriptions was even more pronounced, i.e. close to 40%, when comparing observed and forecasted incident S/V prescriptions between March and December 2020 in Italy.50 With regards to the number of prevalent patients with S/V prescriptions, a study by Vaduganathan et al. from the US showed that prescription fills for GDMT in HF were maintained during the COVID-19 pandemic,52 even though patient-level data were not available. In fact, prescription fills of brand-name therapies (i.e., S/V and SGLT2i) even steeply increased from March to December 2020. For both generic and brand-name therapies, prescription fill patterns from mail-order pharmacies increased substantially over expected trends beginning in March 2020.52 However, this does not explain the steep increase in brand-name fills.
Overall, it is noteworthy that during the COVID-19 pandemic the number of prevalent patients with S/V prescriptions continuously increased, while the number of incident patients with new S/V prescriptions declined. By comparing our results with previous findings, nation-specific differences need to be considered. Most importantly, the characteristics and severity of pandemic-related countermeasures and public restrictions including access to physicians and pharmacies might have varied between countries. Furthermore, there are notable discrepancies of access to medication between countries regarding for example out-of-pocket costs, co-payments, in-hospital dispensation, accessibility to chain, independent or mail-order pharmacies, among others. Besides pharmacotherapy in HF, the influence of the COVID-19 pandemic on prescribing behaviour was also observed for antibiotic therapy.24,53,54 Further research is warranted to unravel the effects of pandemic-related alterations in prescribing behaviours (e.g. antimicrobial resistance for antibiotics or excess mortality for patients with HF).
Besides adding further evidence to the underprescription of HF-specific GDMT due to the COVID-19 pandemic, our results extend previous findings by providing an estimate for the pandemic-related timely delay of the initiation of disease modifying pharmacotherapy. New S/V prescription rates did not reach pre-pandemic level before Q1/2021 resulting in a 12-month period of retarded initiation of disease modifying drug therapy. Hence, it can be hypothesized that the prompt initiation of potentially life-saving medication might have been postponed or even suspended, which might have affected outcomes, especially in patients with incident HF during this period. The increased mortality risk of deferring GDMT in patients with HFrEF has been reported previously. Zaman et al. investigated the survival benefit for three different classes of GDMT (i.e., BB, ACEi, and MRA) and showed that non-adherence with starting all three classes was associated with a 12.2% increased risk of death within one year.14 Tromp et al. estimated that optimal GDMT with all pillars of quadruple therapy would result in a survival benefit of 5 years for an exemplary patient aged 70 years in comparison with no pharmacotherapy treatment.55
Apart from the underprescription of GDMT, several other unfavourable effects of the COVID-19 pandemic on HF care have been reported,56 including the marked decline (e.g., 31% in Germany, 49% in the US) in HF hospitalization during the COVID-19 pandemic,47,57,58 but also in-hospital quality of care (e.g., increased in-hospital mortality and increased length of hospitalization).58,59 Even though the full amount of the long-term negative effects of the COVID-19 pandemic remain yet unknown, our results add further evidence to the multidimensionality of this public health crisis. Our findings raise intriguing questions regarding the resilience of the German healthcare system by highlighting its shortcomings in terms of access to life-saving medication for patients with chronic diseases during public health emergencies. This also sheds light on the adverse unintended consequences of strict lock-down policies, and structural weaknesses in our healthcare system. For the future, advancing its pandemic preparedness will be essential.60 As pointed out by the latest Health Policy Studies of the Organisation for Economic Co-operation and Development (OECD), health policy makers need to take adequate actions towards preventing or mitigating disruptions of care continuity for patients with chronic illness throughout future public health crises and ultimately strengthening the resilience of healthcare systems.61 As such, digital pathways (e.g. increased use of telehealth services) might help to ensure continuity and quality of care.
Limitations
The limitations of the current report need to be acknowledged. First, the descriptive nature of our study does not allow to infer causation or quantify the significance of observed trends. For example, there are several reasons why patients might have missed care opportunities during the COVID-19 pandemic including lockdown-related limited access to care or personal fear of becoming infected in ambulatory care practices. Second, other guideline recommendations (e.g. for T2D) might have mediated the altered prescribing behaviour observed after Q3/2021. Yet, the new German Clinical Practice Guidelines (NVL) Diabetes Type 2 were released only in May 2023 stating for the very first time that for patients with established cardiovascular disease a combination of metformin and a SGLT2 inhibitor or a GLP-1-RA shall be considered. Hence, a significant influence on prescribing behaviour of SGLT2 inhibitors for patients with T2D in Germany during the course of our study is unlikely. Furthermore, the very low penetrance of SGLT2i before Q3/2021 and the strong surge afterwards is suggestive of an influence related to the new ESC HF guidelines. Third, information on the indication of SGLT2i prescription (i.e. for T2D, for CKD, or for HF) is not electronically saved in Germany. However, for patients receiving SGLT2i before Q3/2021, it may be reasonably inferred that initiation was predominantly attributed to T2D. Fourth, as a consequence of choosing S/V as a proxy for HFrEF, we were unable to evaluate prescriptions of SGLT2i in patients not receiving S/V.
Conclusions
This health claims analysis indicates that the implementation of novel GDMT in patients with HFrEF is increasingly adopted in real-world HF care. Adoption appeared enhanced by the new ESC HF guidelines, but temporarily retarded by the COVID-19 pandemic. Women and patients aged 80 years and above are less likely to receive GDMT. Further efforts are needed to fully implement GDMT into routine HF care in Germany, including both S/V and SGLT2i, and to strengthen the resilience of healthcare systems during public health crises.
Contributors
FK, RW, MB, MS, UR, and SS conceptualized this study. UR was involved in data acquisition. FK and UR accessed and verified the data. FK, RW, MB, MS, and SS were involved in data analysis. FK, RW, SvH, AA, MB, MS, and SS were involved in data interpretation. FK wrote the original draft of the paper and produced the manuscript figures and tables. All authors contributed intellectual content to the manuscript, approved its final version and agreed to be accountable for all aspects of the work.
Data sharing statement
The data are available from the corresponding author on reasonable request. More information about the IQVIA longitudinal prescription (LRx) database and the IQVIA Analytic Platform is provided on the IQVIA website (https://www.iqvia.com/).
Declaration of interests
FK reports personal fees from Novartis and a grant from the German Research Council (Deutsche Forschungsgemeinschaft, DFG; Project No. 413657723).
UR is an employee of Novartis Pharma GmbH, Germany.
RW reported receiving personal fees from AstraZeneca, Bayer, Daiichi Sankyo, Novartis, Pfizer, Pharmacosmos, Servier and Vifor; grants and personal fees from Boehringer Ingelheim, CVRx, and Medtronic; grants from Bundesministerium für Bildung und Forschung, the European Union, and Deutsche Forschungsgemeinschaft.
SvH has been a paid consultant for and/or received honoraria payments from AstraZeneca, Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Pharmacosmos, Respicardia, Roche, Servier, Sorin, and Vifor. S.v.H reports research support from Amgen, Boehringer Ingelheim, IMI, and the German Center for Cardiovascular Research (DZHK).
AA reports consulting fees and speaker's honoraria from Boston Scientific and Bayer Vital GmbH.
MB is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Abbott, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, ReCor, Servier and Vifor.
MS has received speaker honoraria and/or consulting fees from BMS, CSL Vifor, Daiichi Sankyo, MSD, Novartis, Pfizer, and Sanofi.
SS has received honoraria as speaker or member of advisory boards by AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, NovoNordisk, Pfizer, Servier, Vifor; he reports research support from Alnylam, Akcea, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Lilly, MSD, Novartis, NovoNordisk, Pfizer, Servier.
Acknowledgements
Novartis Pharma GmbH, Nuremberg/Germany, supported the process of data set extraction via the IQVIA institute.
References
- 1.Groenewegen A., Rutten F.H., Mosterd A., Hoes A.W. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savarese G., Becher P.M., Lund L.H., Seferovic P., Rosano G.M.C., Coats A.J.S. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2022;118(17):3272–3287. doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- 3.Stork S., Handrock R., Jacob J., et al. Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol. 2017;106(11):913–922. doi: 10.1007/s00392-017-1137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dörr M., Riemer U., Christ M., et al. Hospitalizations for heart failure: still major differences between East and West Germany 30 years after reunification. ESC Heart Fail. 2021;8(4):2546–2555. doi: 10.1002/ehf2.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 6.McMurray J.J.V., Packer M., Desai A.S., et al. Angiotensin–Neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 7.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 8.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 9.Kosiborod M.N., Jhund P.S., Docherty K.F., et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141(2):90–99. doi: 10.1161/CIRCULATIONAHA.119.044138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler J., Anker S.D., Filippatos G., et al. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021;42(13):1203–1212. doi: 10.1093/eurheartj/ehaa1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damman K., Gori M., Claggett B., et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Jhund P.S., Solomon S.D., Docherty K.F., et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction. Circulation. 2021;143(4):298–309. doi: 10.1161/CIRCULATIONAHA.120.050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zannad F., Ferreira J.P., Pocock S.J., et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function. Circulation. 2021;143(4):310–321. doi: 10.1161/CIRCULATIONAHA.120.051685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaman S., Zaman S.S., Scholtes T., et al. The mortality risk of deferring optimal medical therapy in heart failure: a systematic comparison against norms for surgical consent and patient information leaflets. Eur J Heart Fail. 2017;19(11):1401–1409. doi: 10.1002/ejhf.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021;42(6):681–683. doi: 10.1093/eurheartj/ehaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdin A., Bauersachs J., Soltani S., Eden M., Frey N., Böhm M. A practical approach to the guideline-directed pharmacological treatment of heart failure with reduced ejection fraction. ESC Heart Fail. 2022;10(1):24–31. doi: 10.1002/ehf2.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mebazaa A., Davison B., Chioncel O., et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938–1952. doi: 10.1016/S0140-6736(22)02076-1. [DOI] [PubMed] [Google Scholar]
- 18.Seferović P.M., Polovina M., Adlbrecht C., et al. Navigating between Scylla and Charybdis: challenges and strategies for implementing guideline-directed medical therapy in heart failure with reduced ejection fraction. Eur J Heart Fail. 2021;23(12):1999–2007. doi: 10.1002/ejhf.2378. [DOI] [PubMed] [Google Scholar]
- 19.Savarese G., Kishi T., Vardeny O., et al. Heart failure drug treatment—inertia, titration, and discontinuation: a multinational observational study (EVOLUTION HF) JACC Heart Fail. 2023;11(1):1–14. doi: 10.1016/j.jchf.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Rosano G.M.C., Moura B., Metra M., et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23(6):872–881. doi: 10.1002/ejhf.2206. [DOI] [PubMed] [Google Scholar]
- 21.Moynihan R., Sanders S., Michaleff Z.A., et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11(3) doi: 10.1136/bmjopen-2020-045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency . Entresto; 2023. European public assessment report.https://www.ema.europa.eu/en/medicines/human/EPAR/entresto [Google Scholar]
- 23.European Medicines Agency . Entresto; 2015. Summary of product characteristics.https://www.ema.europa.eu/documents/product-information/entresto-epar-product-information_en.pdf [Google Scholar]
- 24.Enners S., Gradl G., Kieble M., Böhm M., Laufs U., Schulz M. Utilization of drugs with reports on potential efficacy or harm on COVID-19 before, during, and after the first pandemic wave. Pharmacoepidemiol Drug Saf. 2021;30(11):1493–1503. doi: 10.1002/pds.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutten F.H., Grobbee D.E., Hoes A.W. Differences between general practitioners and cardiologists in diagnosis and management of heart failure: a survey in every-day practice. Eur J Heart Fail. 2003;5(3):337–344. doi: 10.1016/s1388-9842(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 26.Remme W.J., McMurray J.J., Hobbs F.D., et al. Awareness and perception of heart failure among European cardiologists, internists, geriatricians, and primary care physicians. Eur Heart J. 2008;29(14):1739–1752. doi: 10.1093/eurheartj/ehn196. [DOI] [PubMed] [Google Scholar]
- 27.Störk S., Peters-Klimm F., Bleek J., Ninic R., Klöss A. Springer Berlin Heidelberg; 2021. Sektorübergreifende Versorgung bei Herzinsuffizienz; pp. 109–130. [Google Scholar]
- 28.Pierce J.B., Vaduganathan M., Fonarow G.C., et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US. JAMA Cardiol. 2023;8:652. doi: 10.1001/jamacardio.2023.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Medicines Agency . Forxiga; 2023. European public assessment report.https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga [Google Scholar]
- 30.European Medicines Agency . Jardiance; 2023. European public assessment report.https://www.ema.europa.eu/en/medicines/human/EPAR/jardiance [Google Scholar]
- 31.Martens P., Beliën H., Dupont M., Vandervoort P., Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther. 2018;36(4) doi: 10.1111/1755-5922.12435. [DOI] [PubMed] [Google Scholar]
- 32.Januzzi J.L., Camacho A., Piña I.L., et al. Reverse cardiac remodeling and outcome after initiation of sacubitril/valsartan. Circ Heart Fail. 2020;13(6) doi: 10.1161/CIRCHEARTFAILURE.119.006946. [DOI] [PubMed] [Google Scholar]
- 33.Woolf S.H., Grol R., Hutchinson A., Eccles M., Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530. doi: 10.1136/bmj.318.7182.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brownson R.C., Eyler A.A., Harris J.K., Moore J.B., Tabak R.G. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102–111. doi: 10.1097/PHH.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Güder G., Störk S., Gelbrich G., et al. Nurse-coordinated collaborative disease management improves the quality of guideline-recommended heart failure therapy, patient-reported outcomes, and left ventricular remodelling. Eur J Heart Fail. 2015;17(4):442–452. doi: 10.1002/ejhf.252. [DOI] [PubMed] [Google Scholar]
- 36.Shanbhag D., Graham I.D., Harlos K., et al. Effectiveness of implementation interventions in improving physician adherence to guideline recommendations in heart failure: a systematic review. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roccaforte R., Demers C., Baldassarre F., Teo K.K., Yusuf S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. Eur J Heart Fail. 2005;7(7):1133–1144. doi: 10.1016/j.ejheart.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal A., Peters S.A.E., Chandramouli C., Lam C.S.P., Figtree G.A., Arnott C. Guideline-directed medical therapy in females with heart failure with reduced ejection fraction. Curr Heart Fail Rep. 2021;18(5):284–289. doi: 10.1007/s11897-021-00524-z. [DOI] [PubMed] [Google Scholar]
- 39.Murphy S.P., Ward J.H., Piña I.L., et al. Age differences in effects of sacubitril/valsartan on cardiac remodeling, biomarkers, and health status. JACC Heart Fail. 2022;10(12):976–988. doi: 10.1016/j.jchf.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Seo W.-W., Park J.J., Park H.A., et al. Guideline-directed medical therapy in elderly patients with heart failure with reduced ejection fraction: a cohort study. BMJ Open. 2020;10(2) doi: 10.1136/bmjopen-2019-030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene S.J., Choi S., Lippmann S.J., et al. Clinical effectiveness of sacubitril/valsartan among patients hospitalized for heart failure with reduced ejection fraction. J Am Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.121.021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez F.A., Serenelli M., Nicolau J.C., et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age. Circulation. 2020;141(2):100–111. doi: 10.1161/CIRCULATIONAHA.119.044133. [DOI] [PubMed] [Google Scholar]
- 43.Lee Y.C., Lin J.K., Ko D., et al. Frailty and uptake of angiotensin receptor neprilysin inhibitor for heart failure with reduced ejection fraction. J Am Geriatr Soc. 2023;71(10):3110–3121. doi: 10.1111/jgs.18481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filippatos G., Anker S.D., Butler J., et al. Effects of empagliflozin on cardiovascular and renal outcomes in heart failure with reduced ejection fraction according to age: a secondary analysis of EMPEROR-Reduced. Eur J Heart Fail. 2022;24(12):2297–2304. doi: 10.1002/ejhf.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jhund P.S., Fu M., Bayram E., et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36(38):2576–2584. doi: 10.1093/eurheartj/ehv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonagh T.A., Metra M., Adamo M., et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2023 doi: 10.1002/ejhf.3024. [DOI] [PubMed] [Google Scholar]
- 47.Baum A., Schwartz M.D. Admissions to veterans affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96–99. doi: 10.1001/jama.2020.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taxiarchi V.P., Senior M., Ashcroft D.M., et al. Changes to healthcare utilisation and symptoms for common mental health problems over the first 21 months of the COVID-19 pandemic: parallel analyses of electronic health records and survey data in England. Lancet Reg Health Eur. 2023;32 doi: 10.1016/j.lanepe.2023.100697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangiapane S., Zhu L., Czihal T., von Stillfried D. 2020. Veränderung der vertragsärztlichen Leistungsinanspruchnahme während der COVID-Krise–Tabellarischer Trendreport für das 1. Halbjahr 2020 des Zentralinstituts (Zi) für die kassenärztliche Versorgung in Deutschland. [Google Scholar]
- 50.Rosano G.M.C., Celant S., Olimpieri P.P., et al. Impact of the COVID-19 pandemic on prescription of sacubitril/valsartan in Italy. Eur J Heart Fail. 2022;24(5):855–860. doi: 10.1002/ejhf.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onder G., Olimpieri P.P., Celant S., et al. Under-prescription of direct oral anticoagulants for treatment of non-valvular atrial fibrillation and venous thromboembolism in the COVID-19 lockdown period. Eur J Prev Cardiol. 2022;29(4):e149–e152. doi: 10.1093/eurjpc/zwab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaduganathan M., Li D., Van Meijgaard J., Warraich H.J. Prescription filling patterns of evidence-based medical therapies for heart failure during the COVID-19 pandemic in the United States. J Card Fail. 2021;27(11):1280–1284. doi: 10.1016/j.cardfail.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaduganathan M., Van Meijgaard J., Mehra M.R., Joseph J., O'Donnell C.J., Warraich H.J. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA. 2020;323(24):2524. doi: 10.1001/jama.2020.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong X., Pate A., Yang Y.-T., et al. Impact of COVID-19 on broad-spectrum antibiotic prescribing for common infections in primary care in England: a time-series analyses using OpenSAFELY and effects of predictors including deprivation. Lancet Reg Health Eur. 2023;30 doi: 10.1016/j.lanepe.2023.100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tromp J., Ouwerkerk W., van Veldhuisen D.J., et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73–84. doi: 10.1016/j.jchf.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Cowie M.R., Mourilhe-Rocha R., Chang H.Y., et al. The impact of the COVID-19 pandemic on heart failure management: global experience of the OPTIMIZE Heart Failure Care network. Int J Cardiol. 2022;363:240–246. doi: 10.1016/j.ijcard.2022.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhatt A.S., Moscone A., McElrath E.E., et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.König S., Hohenstein S., Meier-Hellmann A., Kuhlen R., Hindricks G., Bollmann A. In-hospital care in acute heart failure during the COVID-19 pandemic: insights from the German-wide Helios hospital network. Eur J Heart Fail. 2020;22(12):2190–2201. doi: 10.1002/ejhf.2044. [DOI] [PubMed] [Google Scholar]
- 59.Keshvani N., Mehta A., Alger H.M., et al. Heart failure quality of care and in-hospital outcomes during the COVID-19 pandemic: findings from the Get with the Guidelines-Heart Failure registry. Eur J Heart Fail. 2022;24(6):1117–1128. doi: 10.1002/ejhf.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization (WHO) 2022. Imagining the future of pandemics and epidemics: a 2022 perspective.https://apps.who.int/iris/rest/bitstreams/1457114/retrieve [Google Scholar]
- 61.Organisation for Economic Co-operation and Development (OECD) 2023. Ready for the next crisis? Investing in health system resilience.https://www.oecd-ilibrary.org/sites/1e53cf80-en/index.html?itemId=/content/publication/1e53cf80-en [Google Scholar]