Abstract
Comparison of the O antigens of Shigella boydii types 10 and 6 by chemical analysis and nuclear magnetic resonance spectroscopy showed that their structures are similar, with the only difference being the presence or absence of d-ribofuranose, which is the immunodominant sugar in S. boydii type 10. In S. boydii type 6, a residue previously reported as α-d-GlcpA, was shown to be β-d-GlcpA as in S. boydii type 10. S. boydii types 10 and 6 are reported not to cross-react serologically, and the role of d-ribofuranose in the specificity of S. boydii was confirmed by making a mutant of type 10 that lacked d-ribofuranose. However, S. boydii type 11, which has a d-ribofuranose but with different linkage does show cross-reaction with type 10. The O-antigen gene loci of S. boydii types 10 and 6 were shown to be virtually identical except that orf8 (wbaM), which was confirmed as the ribofuranosyltransferase gene, is interrupted by IS629 in type 6. Therefore, it is proposed that the O-antigen gene cluster of S. boydii type 6 was derived from type 10 by an IS element insertion.
Shigellae are well-known human pathogens (20), and four species are formally recognized: Shigella boydii, S. dysenteriae, S. flexneri, and S. sonnei. There are 46 Shigella O serotypes but only 33 distinct O-antigen forms, as the rest are variants that arose by phage modification (1, 2, 10, 12) and all but 1 are in reality forms of Escherichia coli (14).
The O antigen, which consists of many repeats of an O unit, is a part of the lipopolysaccharide (LPS) present in the outer membrane of gram-negative bacteria. It is one of the most variable cell constituents and a major determinant of immunospecificity.
The O-antigen gene clusters are normally located between galF and gnd in E. coli. There are three types of genes commonly involved in the biosynthesis of the O antigen: genes for nucleotide sugar synthesis, genes encoding sugar transferases, and O-unit-processing genes wzx (encoding O-unit flippase) and wzy (encoding O-unit polymerase). The diverse forms of O antigen are almost entirely due to genetic variation in the O-antigen gene cluster. It was suggested that the expansion of O-antigen diversity was achieved by either obtaining new clusters or modifying existing O-antigen gene clusters (15).
In this study, the structure and gene cluster of S. boydii type 10 O antigen were studied and compared with those of type 6. The structure of the type 6 O unit reported earlier (5) was also reexamined and revised. The aim was to discover the details of the immunogenic and evolutionary relationship between types 10 and 6. Finally, genes specific to types 10 and 6 were also identified and a PCR-based method for distinguishing the two types was established.
Elucidation of S. boydii type 10 O-antigen structure.
S. boydii type 10 strain G1009 and type 6 strain G1007 were obtained from the Institute of Epidemiology and Microbiology, Chinese Academy of Preventive Medicine, Beijing, People's Republic of China. Bacteria were grown to late log phase under constant aeration in Luria-Bertani broth at 37°C and pH 7.0 as previously described (16). The LPS (5.7%) was isolated from dried cells by the phenol-water method (24) and purified by precipitation of nucleic acids and proteins with CCl3CO2H (26). The LPS was degraded with aqueous 2% acetic acid (100°C, 1 h) or with aqueous 12% ammonia (37°C, 16 h) to give polysaccharide I (PSI) and PSII, which were isolated with yields of 50 and 70%, respectively, by gel chromatography on Sephadex G-50 (S).
Sugar analysis by gas-liquid chromatography of the alditol acetates (18) and acetylated methyl and (S)-2-octyl (11) glycosides showed that PSI contains d-ribose, d-galactose, and d-mannose in a ratio of ∼1:2:6, as well as d-galactosamine and d-glucuronic acid (d-GlcA). Methylation analysis of PSII (9), including carboxyl reduction of the methylated polysaccharide (LiBH4 in aqueous 70% 2-propanol, 20°C, 16 h) demonstrated terminal ribofuranose (Ribf), 3,4-disubstituted Gal, 2- and 6-substituted Man, 3-substituted GalNAc, and 4-substituted GlcA.
1H and 13C nuclear magnetic resonance (NMR) spectra of PSII showed a hexasaccharide O unit (six anomer signals at δH 4.73 to 5.14, δC 96.1 to 109.0) containing one GalNAc residue (signals for GalNAc C2 at δC 52.4 and an N-acetyl group at δH 2.01, δC 23.8). The NMR spectra of PSI differed in the splitting of some signals owing to a partial loss of ribose during mild acid degradation of the LPS.
The 1H and 13C NMR spectra of PSII were assigned by two-dimensional 1H, 1H and 1H, 13C shift-correlated spectroscopy (6) (for chemical shifts, see Table S1 in the supplemental material [http://202.113.31.179/35/download/boydii10.html]). On the basis of 3JH,H coupling constants and 13C NMR chemical shifts compared to published data (3), it was found that all hexose residues occur in the pyranose form, with Gal and both Man residues (ManI and ManII) α linked and GalNAc and GlcA β linked. Rib is present in the β-furanose form. Interresidue ManI H1/ManII H2, ManII H1/GalNAc H3, GalNAc H1/Gal H3, Rib H1/GlcA H4, and GlcA H1/Gal H4 correlations in the two-dimensional rotating-frame nuclear Overhauser effect spectrum of PSII revealed the monosaccharide sequence in the O unit. Therefore, the O antigen of S. boydii type 10 has the structure shown in Fig. 1.
FIG. 1.
Structures of the O antigens of S. boydii types 10 (this work) and 6 (revised in this work). Shown are chemical repeating units, which may be any cyclic permutation of the biological repeating units (O units) that are assembled and then polymerized in the biosynthesis of the O antigen.
Revision of the S. boydii type 6 O-antigen structure.
The structure of the S. boydii type 10 O antigen is remarkably similar to that reported for the O antigen of type 6 (5), differing only in the presence of an additional sugar residue, viz. Ribf, and in the configuration of GlcA (β in type 10 versus α in type 6). Since the anomeric configurations in type 6 were determined by chromium trioxide oxidation, a method now considered to have poor reliability, we reinvestigated the type 6 O-antigen polysaccharide by the same NMR spectroscopy approach as described above for type 10 (for 1H and 13C NMR chemical shifts of the type 6 polysaccharide, see Table S1 in the supplemental material.). As a result, the configuration of GlcA in type 6 was revised from α to β, whereas the rest of the polysaccharide structure was confirmed. Therefore, the O antigen of type 6 has the structure shown in Fig. 1, which differs from that of type 10 only in the absence of Ribf.
It is interesting that the type 6 and 10 O antigens were reported not to cross-react serologically (7), indicating that the ribosyl moiety is immunodominant. The O antigen of S. boydii 11 also has a β-d-Ribf residue that is 1,3 linked to l-Rha (13). S. boydii type10, with a different ribosyl linkage, does cross-react with type 11, with 8- and 16-fold reductions in the endpoints for the type 10 and 11 antisera, respectively (7). As most of the type 10 immunogenicity must lie with the Ribf residue, it appears that most of the specificity lies in the linkages.
Nucleotide sequence analysis of the S. boydii type 10 O-antigen gene cluster.
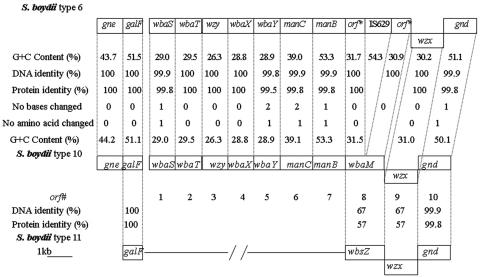
The O-antigen gene cluster of type 10 strain G1009 was amplified by PCR and sequenced, and the sequence data were analyzed as previously described (8). A sequence of 13,402 bases, including galF (positions 1 to 765) and gnd (positions 11979 to 13402), was obtained. Nine open reading frames excluding galF and gnd were identified, all transcribed from galF to gnd, except for orf9, which was transcribed in the opposite direction (Fig. 2). All are present in the type 6 sequence, except that orf8 of type 6 is inactivated by an IS insertion. The levels of DNA identity for these genes ranged from 99.8 to 100%. This high level of identity and the similarity of the structures of types 10 and 6 indicate that the genes have the same functions in both and so were given the same names (Table S2 in the supplemental material).
FIG. 2.
Comparison of the O-antigen gene clusters of S. boydii types 10, 6, and 11. Gene names are given for S. boydii type 10. All of the genes are transcribed in the galF-to-gnd direction except for the wzx genes of the three S. boydii types.
The proposed functions for the genes other than orf8 are as for type 6 (22), but at that time we were not aware that synthesis of the GalNAc residue requires the presence of a gne gene for a UDP-GlcNAc-4-epimerase and so did not notice its absence. In E. coli, the gne gene can be with other O-antigen genes between galF and gnd or immediately upstream of galF (21). Given the absence of a gne gene between galF and gnd, the region upstream of galF was amplified with primers WL-4225 and WL-4226 (Table S3 in the supplemental material) and sequenced for both types 6 and 10 and a gne gene found as shown in Fig. 2.
Ribofuranose is the only sugar not present in type 6, and orf8, which is disrupted in type 6, appears to be the ribosyltransferase gene and has been named wbaM. The product of wbaM belongs to pfam00156 (E value = 3.2 × e−4), a family that includes a range of diverse phosphoribosyltransferases. WbaM also showed 67% identity with WbsZ of type 11, which is a putative ribosyltransferase (19). wbaM is 100% identical to the corresponding parts of the type 6 gene, which is interrupted by IS629 (positions 9724 to 11036) (Fig. 2). Disruption of wbaM by insertion of IS629 in type 6 strongly supports the proposal that wbaM is the ribosyltransferase gene responsible for transferring Ribf to GlcA via a 1,4 linkage in the O antigen of type 10.
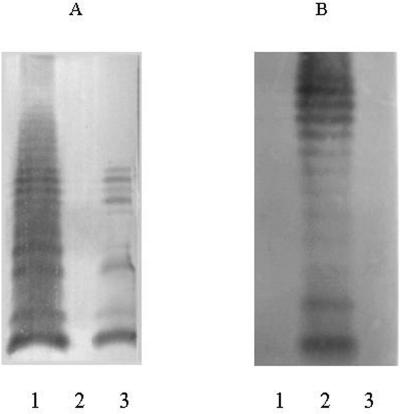
To confirm the function of WbaM, the wbaM gene of type 10 was replaced with a chloramphenicol acetyltransferase (cat) gene (4, 25) with primers WL-2990 and WL-2991 (Table S3 in the supplemental material). The mutant strain had an obvious immunoreaction with type 6-specific rabbit antiserum and had no immunoreaction with type 10-specific rabbit antiserum (Chengdu Institute of Biological Products). LPS was prepared from types 6 and 10 and the wbaM-deficient mutant, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and silver stained as previously described (23). Western immunoblotting was also carried out as described by Sambrook et al. (17). Filters were probed with type 6- or 10-specific rabbit antiserum used at a dilution of 1:1,000, followed by goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) used at a dilution of 1:2,000. The mutant had a positive reaction with type 6-specific antiserum and a negative reaction with type 10-specific antiserum (Fig. 3). These data indicate that disruption of wbaM changes the type 10 O antigen to the type 6 O antigen and confirm that wbaM is the ribosyltransferase gene responsible for transferring Ribf to GlcA in the O antigen of type 10.
FIG. 3.
Western immunoblot analysis. LPS samples from the wbaM-deficient mutant strain of S. boydii type 10 and wild-type strains of S. boydii types 10 and 6 were probed with S. boydii type 6 (A)- and 10 (B)-specific antisera. Lanes: 1, G1005 (S. boydii type 6 strain); 2, G1009 (S. boydii type 10 strain); 3, H1108 (wbaM-deficient mutant strain of S. boydii type 10).
Transferase genes for other linkages, the O-antigen-processing genes wzx and wzy, proposed for type 6 (22), were also present in type 10 and are expected to have the same functions. To confirm the designation of orf3 as wzy, a mutant strain of type 10 was constructed by replacing orf3 with a cat gene (4, 25) with primers WL-2992 and WL-2993 (Table S3 in the supplemental material). This mutant produced only the lipid A-core part of the LPS and a single O unit, while the wild-type strain produced normal LPS. The semirough-LPS phenotype was complemented by plasmid pLW1116 containing orf3 to give the smooth phenotype as observed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown).
S. boydii type 6 is a clone that recently emerged from type 10.
The O-antigen gene clusters of types 10 and 6 differ by only eight nucleotides or seven amino acids, if IS629 is excluded (Fig. 2), indicating that they diverged recently. The two halves of wbaM, interrupted by IS629 in type 6 (22), were 100% identical to the corresponding parts of orf8 in type 10. This also indicates that the IS was inserted only recently. This is in agreement with the observation that types 6 and 10 are very similar in their housekeeping genes, with the type 6 and 10 strains differing by two single-base substitutions in the 7,160 bp sequenced (14). On the basis of those facts, we propose that the O-antigen gene cluster of type 6 was formed by interrupting the ribosyltransferase gene of the O-antigen gene cluster of type 10 with IS629.
As already described, the transcriptional direction of the wzx gene of type 10 was the opposite of that of the other genes in the O-antigen gene cluster. This atypical feature was observed in types 6 and 11. The wzx genes of types 10 and 6 have 67% identity with wzx of type 11 (Fig. 2), which was shown to be functional (19). We proposed previously that part of the O-antigen gene cluster of type 6 was derived from that of type 11 (19), but this was before the structure and the gene cluster of type 10 O antigen were investigated. We now propose that the gene cluster of type 6 was derived directly from that of type 10, and it is type 10 that is most closely related to type 11. Now that we know the function of wbaM, we can see that in the divergence of the region common to types 10 and 11, the ribofuranose transferase has changed not only the acceptor sugar, which is galactose or glucose, respectively, but also the linkage, which is either 1 to 4 or 1 to 3, respectively. The divergence of this region must have taken some time as the transferases and wzx genes both have only 57% identity at the amino acid level. There is no similarity in the remaining parts of the type 10 and 11 gene cluster, and there was presumably a recombination event at the boundary of the shared and unshared segments.
Identification of S. boydii type 10- and 6-specific genes.
In general, sugar transferase genes and O-antigen-processing genes are specific to O antigens. Two pairs of primers for both wzx and wzy (Table S4 in the supplemental material) were used to screen against DNA pools representing all 186 O serotypes of E. coli and Shigella strains by PCR (8). All of the primer pairs used produced a band of the predicted size from the pools containing types 10 and 6, respectively, while no other pools produced these bands. This showed that the wzx and wzy genes were specific to types 10 and 6. To distinguish between types 10 and 6, two pairs of primers were designed on the basis of wbaM (Table S4 in the supplemental material). PCR products of 648 and 814 bases were obtained from the pool containing type 10, but each primer pair gave the nearly 2-kb PCR products expected from the pool containing type 6. These PCR products were not produced from other pools with either primer pair. Thus, the primers based on wbaM were highly specific to types 6 and 10 and size can be used to distinguish between types 10 and 6 by PCR.
Concluding remarks.
The structure of the S. boydii type 10 O antigen was determined, and that of type 6 was revised, showing that they differed only by the presence or absence of a d-ribofuranose residue. Absence of serological cross-reaction between the two suggested that ribofuranose is the immunodominant sugar in type 10. The O-antigen gene clusters of types 10 and 6 were almost identical except that wbaM in type 6, shown to encode the ribofuranosyltransferase, was interrupted by IS629. We propose that the O-antigen gene cluster of S. boydii type 6 was derived from S. boydii type 10 by an IS element (IS629) insertion and that the latter has a more distant relationship with S. boydii type 11.
Nucleotide sequence accession number.
The DNA sequence of the S. boydii type 10 O-antigen gene cluster has been deposited in GenBank under accession number AY693427.
Acknowledgments
S.N., L.F., and J.Y. contributed equally to this work.
We thank all of the individuals and institutes for kindly supplying strains and plasmids.
This work was supported by grant RF NSh-1557.2003.3, a grant from the Russiian Foundation for Basic Research (03-04-39020) to S.N.S, the 863 Program (2002AA2Z2051), the Chinese National Science Fund for Distinguished Young Scholars (30125001), the Cooperation Research Fund for Nankai and Tianjin Universities from the Chinese Ministry of Education, the NSFC General Program (30270029, 30370339, 30370023), and funds from the Science and Technology Committee of Tianjin City (013181711) to L.W.
REFERENCES
- 1.Allison, G. E., and N. K. Verma. 2000. Serotype-converting bacteriophages and O antigen modification in Shigella flexneri. Trends Microbiol. 8:17-23. [DOI] [PubMed] [Google Scholar]
- 2.Bagdian, G., O. Luderitz, and A. M. Staub. 1966. Immunochemical studies on Salmonella. XI. Chemical modification correlated with conversion of group B by bacteriophage 27. Ann. N. Y. Acad. Sci. 133:405-424. [DOI] [PubMed] [Google Scholar]
- 3.Bock, K., and C. Pedersen. 1983. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv. Carbohydr. Chem. Biochem. 41:27-66. [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmitriev, B. A., L. V. Backinowsky, V. L. L'vov, Y. A. Knirel, N. K. Kochetkov, and N. A. Khomenko. 1975. The structure of the chemical repeating-unit of the O-specific polysaccharide chain of Shigella boydii 6 lipopolysaccharide. Carbohydr. Res. 41:329-338. [DOI] [PubMed] [Google Scholar]
- 6.Duus, J. Ø., C. H.; Gotfredsen, and K. Bock. 2000. Carbohydrate structural determination by NMR spectroscopy: modern methods and limitations. Chem. Rev. 100:4589-4614. [DOI] [PubMed] [Google Scholar]
- 7.Ewing, W. H. 1986. Edwards and Ewing's identification of the Enterobacteriaceae, 4th ed. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 8.Feng, L., S. N. Senchenkova, J. Yang, A. S. Shashkov, J. Tao, H. Guo, G. Zhao, Y. A. Knirel, P. Reeves, and L. Wang. 2004. Structural and genetic characterization of the Shigella boydii type 13 O antigen. J. Bacteriol. 182:383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakomori, S. I. 1964. A rapid permethylation of glycolipids and polysaccharides catalyzed by methylsulfinyl carbanion in dimethylsulfoxide. J. Biochem. 55:205-208. [PubMed] [Google Scholar]
- 10.Huan, T. P., D. A. Bastin, B. L. Whittle, A. A. Lindberg, and N. Verma. 1997. Molecular characterization of the genes involved in O-antigen modification, attachment, integration and excision in Shigella flexneri bacteriophage SfV. Gene 195:217-227. [DOI] [PubMed] [Google Scholar]
- 11.Leontein, K., and J. Lönngren. 1993. Determination of the absolute configuration of sugars by gas-liquid chromatography of their acetylated 2-octyl glycosides. Methods Carbohydr. Chem. 9:87-89. [Google Scholar]
- 12.Lindberg, A. A., C. G. Helleqvist, G. Bagdian-Motta, and P. H. Mäkela. 1978. Lipopolysaccharide modification accompanying antigenic conversion by phage P27. J. Gen. Microbiol. 107:279-287. [Google Scholar]
- 13.L'vov, V. L., A. P. Iakovlev, A. S. Shashkov, and B. A. Dmitriev. 1991. Antigenic polysaccharides of Shigella bacteria. Structure of the polysaccharide chain of the lipopolysaccharide from Shigella boydii type 11. Bioorg. Khim. 17:111-120. [PubMed] [Google Scholar]
- 14.Pupo, G. M., R. Lan, and P. R. Reeves. 2000. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc. Natl. Acad. Sci. USA 97:10567-10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves, P. R., and L. Wang. 2002. Genomic organization of LPS-specific loci. Curr. Top. Microbiol. Immunol. 264:109-135. [PubMed] [Google Scholar]
- 16.Robbins, P. W., and T. Uchida. 1962. Studies on the chemical basis of the phage conversion of O antigens in the E-group Salmonella. Biochemistry 1:323-335. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Sawardeker, J. S., J. H. Sloneker, and A. Jeanes. 1965. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 37:1602-1603. [Google Scholar]
- 19.Tao, J., L. Feng, H. Guo, Y. Li, and L. Wang. 2004. The O antigen gene cluster of Shigella boydii O11 and functional identification of its wzy gene. FEMS Microbiol. Lett. 234:125-132. [DOI] [PubMed] [Google Scholar]
- 20.Wachsmuth, K., and G. K. Morris. 1989. Shigella, p. 447-462. In M. P. Doyle (ed.), Foodborne bacterial pathogens. Marcel Dekker, Inc., New York, N.Y.
- 21.Wang, L., S. Huskic, A. Cisterne, D. Rothemund, and P. R. Reeves. 2002. The O-antigen gene cluster of Escherichia coli O55:H7 and identification of a new UDP-GlcNAc C4 epimerase gene. J. Bacteriol. 184:2620-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, L., W. Qu, and P. R. Reeves. 2001. Sequence analysis of four Shigella boydii O antigen loci: implication for Escherichia coli and Shigella relationships. Infect. Immun. 69:6923-6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, L., and P. R. Reeves. 1994. Involvement of the galactosyl-1-phosphate transferase encoded by the Salmonella enterica rfbP gene in O antigen subunit processing. J. Bacteriol. 176:4348-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 25.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 91:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zych, K., F. V. Toukach, N. P. Arbatsky, K. Kolodziejska, S. N. Senchenkova, A. S. Shashkov, Y. A. Knirel, and Z. Sidorczyk. 2001. Structure of the O-specific polysaccharide of Proteus mirabilis D52 and typing of this strain to Proteus serogroup O33. Eur. J. Biochem. 268:4346-4351. [DOI] [PubMed] [Google Scholar]