Summary
Background
Identifying patients at high residual risk of atherosclerotic cardiovascular disease (ASCVD) despite statin-treatment is of paramount clinical importance. We aim to investigate if non-high-density lipoprotein cholesterol (non-HDL-C) identifies residual risk of ASCVD and death in statin-treated patients with ischemic heart disease and low-density lipoprotein cholesterol (LDL-C) ≤ 1.8 mmol/L.
Methods
Leveraging Danish regional and national registries, we identified statin-treated patients with ischemic heart disease who underwent coronary angiography (CAG) and attained LDL-C ≤ 1.8 mmol/L within a year post-CAG. Outcomes were myocardial infarction (MI), ASCVD (MI or ischemic stroke), and all-cause death occurring from one year after CAG to end of follow-up. Cox regression analyses obtained adjusted hazard ratios (HR).
Findings
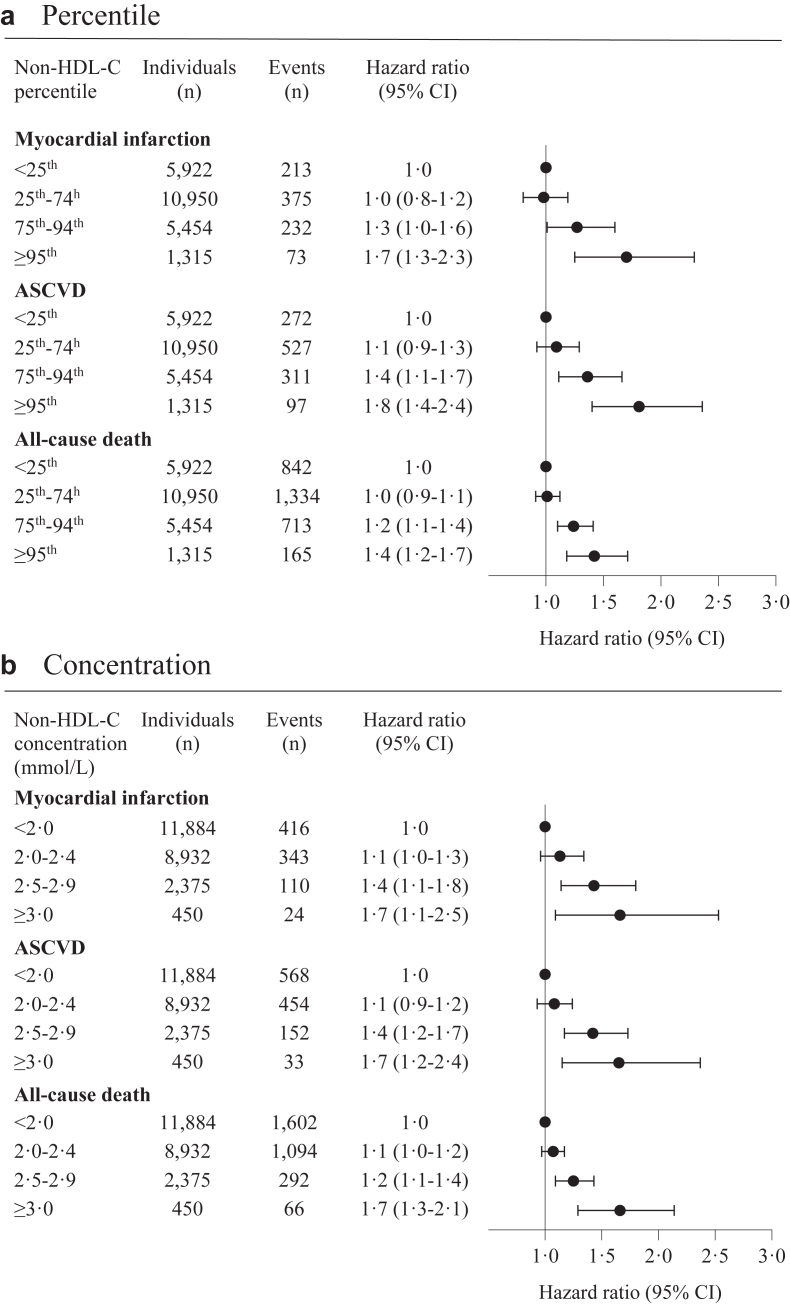
Between January 1, 2011, and December 31, 2020, we included 23,641 statin-treated patients with ischemic heart disease and LDL-C ≤ 1.8 mmol/L. During median follow-up of 4.1 years (IQR 2.4–6.1), 893 (3.8%) patients developed MI, 1207 (5.1%) ASCVD, and 3054 (12.9%) patients died. For ASCVD the adjusted HRs (95% confidence interval) for non-HDL-C < 25th percentile (<1.7 mmol/L) versus 25th–74th (1.7–2.1 mmol/L), 75th–94th (2.2–2.6 mmol/L), and ≥95th (≥2.7 mmol/L) percentile were 1.1 (0.9–1.3), 1.4 (1.1–1.7), and 1.8 (1.4–2.4), and for all-cause death 1.0 (0.9–1.1), 1.2 (1.1–1.4), and 1.4 (1.2–1.7), respectively.
Interpretation
In a contemporary secondary prevention cohort of patients with well-managed LDL-C, non-HDL-C emerges as an easily accessible marker to detect patients facing high residual risk of ASCVD and death. These findings are important for preventive strategies extending beyond LDL-C targets.
Funding
Research grant from the Novo Nordisk Foundation.
Keywords: Non-HDL cholesterol, Residual risk, Cardiovascular events
Research in context.
Evidence before this study
Lowering of LDL-C is the mainstay in secondary prevention of atherosclerotic cardiovascular disease; however, large clinical trials reveal that residual risk persist in patients with well-controlled LDL-C. Although there is evidence of causality between LDL-C concentration and risk of ASCVD, LDL-C does not include the total atherogenic cholesterol burden and the predictive ability of LDL-C in statin-treated patients is limited. On the contrary, guidelines acknowledge that non-HDL-C is a measure of all atherogenic cholesterol and residual cholesterol-related risk is more accurately measured by non-HDL-C. High-sensitive C-reactive protein (hsCRP) has recently been examined in statin-treated patients at high risk of ASCVD and was found to be a predictor of residual risk in patients with LDL-C < 70 mg/dL. Historical studies have shown that hsCRP and remnant cholesterol (which is included in non-HDL-C) are highly correlated and dual elevated hsCRP and remnant cholesterol were associated with the worst prognosis of atherosclerotic cardiovascular disease in a recent general population study.
To assess the uniqueness of our study hypothesis, we searched Pubmed until August 8, 2023, with search terms including “non-HDL cholesterol/remnant cholesterol/remnant lipoprotein/triglyceride-rich lipoprotein”, “statin”, “cardiovascular disease/coronary artery disease/myocardial infarction”, and “residual risk”. Two observational studies with limited statistical power (n = 1000) of patients with established ASCVD and LDL-C ≤ 1.8 mmol/L (<70 mg/dL) showed a positive association between triglyceride-rich-lipoprotein/remnant-like lipoprotein and future cardiovascular disease. Thus, no studies of sufficient statistical power have hitherto specifically examined the impact of non-HDL-C on residual cardiovascular risk in statin-treated patients with documented coronary artery disease and LDL-C ≤ 1.8 mmol/L.
Added value of this study
The present study is the most comprehensive analysis of the association between non-HDL-C and ASCVD in patients with established ischemic heart disease and well-controlled LDL-C. This study reveals that calculation of non-HDL-C is a simple and robust measure to identify patients who have a high residual risk of a future myocardial infarction, ischemic stroke, and death in a cohort of patients with already established ischemic heart disease and well-controlled LDL-C. We advocate that the estimates have a high generalisability to patients with ischemic heart disease worldwide as they are derived from a powerful cohort of more than 23,000 well-defined statin-treated patients with ischemic heart disease and LDL-C ≤ 1.8 mmol/L. Furthermore, robust results in assessed subgroups vouch for a widely generalizable association between non-HDL-C and the specified outcomes.
Implications of all the available evidence
A direct clinical implication is to incorporate the calculation of non-HDL-C in the standard lipid profile worldwide. This simple, inexpensive measure with no inconvenience for the patients and no extra laboratory requirements or additional assays can identify patients with already well-controlled LDL-C who have high residual risk. Based on all available evidence, non-HDL-C can now be considered a better predictor of ASCVD than LDL-C in both primary and secondary preventive therapy of patients receiving statins—down to a level of LDL-C below 1.8 mmol/L. Future research should focus on i) whether hsCRP and non-HDL-C identifies the same or two different high-risk patient populations, and ii) whether non-HDL-C should be the optimal cholesterol target when LDL-C is low.
Introduction
Approaches to identify residual cardiovascular risk in patients with or at high risk of atherosclerotic cardiovascular disease (ASCVD) beyond low-density lipoprotein cholesterol (LDL-C) gain traction. Subclinical inflammation, as measured by high-sensitive C-reactive protein (hsCRP), is a stronger predictor of ASCVD compared with LDL-C in statin-treated patients.1,2 However, hsCRP is an exclusive biomarker not available in a standard laboratory panel.
International guidelines of ASCVD prevention unanimously agree upon LDL-C reduction to target levels whereas non-high-density lipoprotein cholesterol (non-HDL-C) recommendations diverge; from part of risk stratification algorithms in primary prevention3, 4, 5 to treatment strategies and goals.6 Non-HDL-C is a united measure of the total atherogenic cholesterol summarising cholesterol in LDL and triglyceride-rich lipoprotein (TRL) particles, i.e., apolipoprotein B (apoB) particles.7 Although LDL-C values are close to non-HDL-C values in many patients, some patients have significantly higher non-HDL-C levels than LDL-C levels (i.e., higher contribution of cholesterol from TRLs),8 which may particularly be true when LDL-C is treated to low levels, like in secondary prevention. As the cholesterol content in TRL (called remnant cholesterol) is positively correlated with hsCRP,9,10 non-HDL-C may act as an inexpensive and easily accessible measure of the attributable risk from both LDL-C, TRL, and hsCRP.
Previous studies have shown that non-HDL-C, apoB, and remnant cholesterol compared with LDL-C better reflect residual cardiovascular risk in the statin-treated general population and in secondary prevention cohorts not treated to LDL-C target levels.11, 12, 13, 14 Evidence is lacking in patients with already achieved secondary preventive cholesterol targets.
We aimed to test the hypothesis that non-HDL-C is associated with residual risk of myocardial infarction (MI), ASCVD, and death in statin-treated patients with ischemic heart disease and well-controlled LDL-C ≤ 1.8 mmol/L.
Methods
Study design
This cohort study was performed using data from regional and national health registries in Denmark. Linkage between registries at an individual level with no loss to follow is possible due to the unique sex-defined 10-digit Central Personal Registry number assigned to each Danish resident at birth or immigration. The Danish National Health Service provides tax-funded medical care for all Danish residents.
We gathered the study population from the Western Denmark Heart Registry covering both rural and urban areas of more than 3 million inhabitants (approximately 55% of the total Danish population).15 Since 1999 all coronary angiographies (CAGs) has been recorded in this clinical database of prospectively collected information by the treating physician at time of examination.
This study was approved by the Danish Data Protection Agency (record no. 1-16-02-193-18). According to Danish regulations, observational registry-based studies do not require approval from ethics committee and informed consent from participants were exempted (record no: 1-45-70-24-22).
Participants
The study population included all adults referred to first time CAG between January 1, 2011 and December 31, 2020, with indication ischemic heart disease, i.e., ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, unstable angina pectoris, and stable angina pectoris. We chose the first CAG as index procedure if the patient underwent multiple examinations during inclusion.
Patients were required to have cholesterol levels measured within one year after CAG. In case of more than one lipid panel within this period, the latest panel including total cholesterol, high-density lipoprotein cholesterol (HDL-C), and LDL-C was chosen as index measure. We chose the latest panel to allow for up-titration in cholesterol-lowering therapy and time to achieve treatment to target levels of LDL-C. We excluded patients without statin use and patients with LDL-C > 1.8 mmol/L. Data on blood sample measurements were collected from The Laboratory Information System, which holds information of blood samples in Western Denmark including analyses codes, measurement units, dates of test collection, and results.
Statin use was defined as at least one redeemed prescription of any statin between 30 and 365 days prior to index cholesterol measurement. The 30-day quarantine period of statin use was to ensure reasonable time for the drug to obtain steady state. Data of medical therapy were obtained from the Danish National Prescription Registry, which holds information on all prescriptions reimbursed by the Danish National Health Service (Supplementary Fig. S1 and Table S1).
Procedures
Total cholesterol, HDL-C, and triglycerides were measured using standard hospital assays. We calculated baseline non-HDL-C as total cholesterol—HDL-C. LDL-C was calculated by the laboratory using the Friedewald formula (total cholesterol—HDL-C—triglycerides/2.2) as recommended in guidelines.16 If triglycerides were >4.0 mmol/L, direct enzymatic techniques determined LDL-C. We had no knowledge of fasting state. We calculated remnant cholesterol as non-HDL-C—LDL-C.
Non-HDL-C groups were defined according to percentiles (<25th, 25th–74th, 75th–94th, ≥95th) and concentration (<2.0, 2.0–2.4, 2.5–2.9, ≥3.0 mmol/L), respectively.
Study outcomes were MI, ASCVD, and all-cause death. ASCVD was defined as an occurrence of either MI or ischemic stroke by retrieving hospital discharge diagnoses from the Danish National Patient Register; a nationwide registry established in 1977, which holds records on 99.4% of all discharges from Danish hospitals using diagnoses coded according to the International Classification of Diseases codes, 10th revision (Supplementary Table S1). In validation studies both MI and ischemic stroke have shown performance with positive predictive values up to 97%.17,18 Information on all-cause death was obtained from the Civil Registration System and main causes of death from the Danish Register of Causes of Death.
Clinical baseline characteristics (smoking, body mass index (BMI), and family history of coronary heart disease) were obtained at time of CAG from the Western Denmark Heart Registry. Comorbidity (diabetes, hypertension, previous MI, and previous ischemic stroke) were obtained from both Western Denmark Heart Registry and the Danish National Patient Register. For diabetes, insulin and non-insulin medication use within one year prior to CAG was also considered (Supplementary Table S1). Acute coronary syndrome covered index CAG due to ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and unstable angina pectoris. Obstructive coronary artery disease (CAD) was defined as ≥50% stenosis in ≥1 coronary vessel, fractional flow reserve ≤0.80, or instant wave-free ratio ≤0.89 in at least one lesion. No obstructive CAD was defined as no vessel disease or diffuse vessel disease.
Statistical analyses
Start of follow-up began one year after CAG and continued until an outcome occurred, death, emigration (n = 46), or end of follow-up (November 1, 2022).
The distribution of non-HDL-C was presented in Kernel density plots. The relationship between non-HDL-C, LDL-C, remnant cholesterol, and triglycerides is visualised by scatter plots and enumerated by Spearman’s correlation coefficients. Non-HDL-C was measured in SI units (mmol/L; multiply by 38.67 to convert to mg/dL).
Baseline characteristics were presented as number and proportion for categorical variables and as median with interquartile range (IQR) for continuous variables.
Absolute event rates per 1000 person-years were calculated for the outcomes according to non-HDL-C percentile and concentration.
The relative effect estimates were obtained using the Cox proportional hazards regression model. Time since start of follow-up was underlying time scale. The assumption of proportional hazards was examined graphically using log-log plots and was satisfied for every outcome. Crude and adjusted hazard ratios (HR) were presented with 95% confidence intervals. Adjustment included the following covariates: age (continuous), sex, smoking (categorical), hypertension, and LDL-C (continuous). In sensitivity analyses we provided analyses without adjustment for LDL-C, and with additional adjustment for diabetes, high-intensity statin, and BMI. If number of events were below ten, adjustment was not performed. Also, in sensitivity analyses, we computed Fine–Gray subdistribution HRs to allow for competing risk of death. For comparison we calculated HRs for conventional risk factors (e.g., diabetes, BMI, and smoking).
The association of non-HDL-C and the outcomes was analysed in four manners: 1) non-HDL-C as a continuous variable yielding an estimate of the HR per 1 mmol/L increase in non-HDL-C, 2) restricted cubic spline model with 5 knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentile,19 3) non-HDL-C divided in groups according to percentile, and 4) non-HDL-C divided in clinically chosen groups according to concentration.
For comparison we examined the predictive capability of LDL-C, remnant cholesterol, and triglycerides according to percentile levels (<25th, 25th–74th, 75th–94th, ≥95th). Also, for LDL-C we used a restricted cubic spline analysis.
Missing values for covariates in the adjustment model (9% for smoking) were handled by multiple imputation using chained equations generating ten imputations. Missing values were assumed to be missing at random and imputation was based upon the variables listed in Supplementary Table S2. Distribution of baseline characteristics in patients with complete and incomplete information of smoking and complete case analysis are listed in Supplementary Table S2 and S3.
We performed stratified analyses by sex, age, indication for CAG as acute coronary syndrome or stable angina, and in presence of obstructive CAD. For each specified subcohort, the distribution of non-HDL-C was re-evaluated according to percentiles. Multiple imputation was done separately for each cohort.
Several sensitivity analyses were performed. The cohort was divided according to achievement of LDL-C ≤1.4 mmol/L and >1.4 mmol/L at index measure. Further we evaluated whether time of index measure during one year after CAG (divided into quartiles: 0–90, 91–180, 181–270, and 271–364 days) biased the association between non-HDL-C and the outcomes. Baseline characteristics are provided for each quartile (Supplementary Table S4). We evaluated if advanced start of follow-up at date of index measure changed the association between non-HDL-C and the outcomes. Last, we calculated mean non-HDL-C and LDL-C by year of CAG and examined the association between non-HDL-C and the outcomes stratified according to year of CAG. Statistical analyses were performed using Stata/MP v. 17.0 (StataCorp LP, College Station, TX) and graphical illustrations using Graph Pad.
Role of the funding source
The study was funded by a research grant from the Novo Nordisk Foundation (grant number NNF22OC0074083). The funder of the study had no role in study design, data collection, data analyses, data interpretation, or writing of the report.
Results
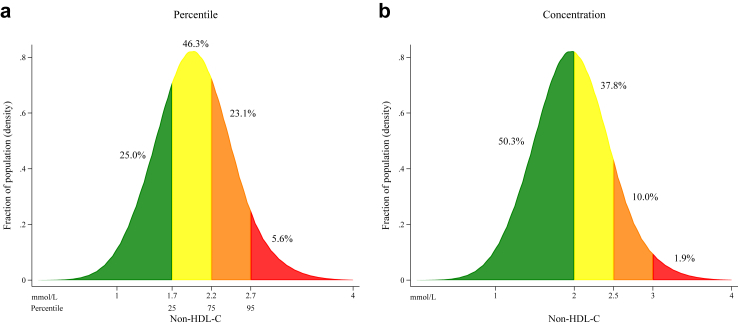
Based on a population of 105,804 patients undergoing CAG in Western Denmark between January 1, 2011, and December 31, 2020, we identified a total of 23,641 patients with ischemic heart disease and LDL-C ≤ 1.8 mmol/L within one year after CAG (Supplementary Fig. S2). In total, 5922 (25.0%) had non-HDL-C <25th percentile (1.7 mmol/L), 10,950 (46.3%) between 25th–74th percentile (1.7–2.1 mmol/L), 5454 (23.1%) between 75th–94th percentile (2.2–2.6 mmol/L), and 1315 (5.6%) ≥95th percentile (≥2.7 mmol/L) (Fig. 1). There was a positive correlation between non-HDL-C and LDL-C, and non-HDL-C and remnant cholesterol (Supplementary Fig. S3a and b) whereas LDL-C was not correlated with triglycerides (Supplementary Fig. S3c). Patients with non-HDL-C ≥95th percentile were younger, more were active smokers, and had more comorbidity compared with non-HDL-C percentile <25th (Table 1). Moreover, patients with high non-HDL-C were less likely to receive high-intensity statin treatment (non-HDL-C ≥95th percentile 862 patients (66%) versus 4857 patients (82%) in <25th percentile group). The overall cohort was predominantly comprised of patients with obstructive CAD (n = 18,578 (79%)) of whom 10,335 (56%) were classified as single vessel disease. High-intensity statin-treated patients were younger and more had obstructive CAD while the prevalence of diabetes were lower when compared to low-intensity statin-treated patients (Supplementary Table S5). Patients with obstructive CAD and STEMI had lower median LDL-C and non-HDL-C compared with no obstructive CAD. Diabetic patients had lower LDL-C but equal non-HDL-C compared to non-diabetic patients. And last, with higher BMI non-HDL-C increased whereas LDL-C remained unchanged. Overall, baseline characteristics were minimally associated with achieved LDL-C and non-HDL-C.
Fig. 1.
Distribution of non-high-density lipoprotein cholesterol levels in the study cohort. Legend: The distribution of non-high-density lipoprotein cholesterol (non-HDL-C) in 23,641 statin-treated patients with ischemic heart disease examined by coronary angiography and low-density lipoprotein cholesterol (LDL-C) ≤ 1.8 mmol/L from the Western Denmark Heart Registry. Fractions of the overall study cohort are coloured according to percentiles (<25th, 25th–74th, 75th–94th, and ≥95th) (Fig. 1a) and clinically chosen concentration groups (<2.0, 2.0–2.4, 2.5–2.9, ≥3.0 mmol/L) (Fig. 1b). Patients with non-HDL-C > 4.0 mmol/L (n = 15) are omitted for illustrative purposes.
Table 1.
Baseline characteristics of 23,641 statin-treated patients with ischemic heart disease and LDL-C ≤ 1.8 mmol/L.
| Non-HDL-C percentile |
||||
|---|---|---|---|---|
| <25th | 25th–74th | 75th–94th | ≥95th | |
| Number | 5922 (25.0) | 10,950 (46.3) | 5454 (23.1) | 1315 (5.6) |
| Non-HDL-C, mmol/L | 1.5 (1.3–1.6) | 2.0 (1.8–2.1) | 2.4 (2.3–2.5) | 2.9 (2.8–3.1) |
| Non-HDL-C, mg/dL | 58 (50.3–61.9) | 77.3 (69.6–81.2) | 92.8 (88.9–96.7) | 112.1 (108.3–119.9) |
| Age, years | 67 (59–74) | 67 (58–74) | 66 (57–73) | 64 (55–71) |
| Women | 1458 (24.6) | 3015 (27.5) | 1669 (30.6) | 415 (31.6) |
| Men | 4464 (75.4) | 7935 (72.5) | 3785 (69.4) | 900 (68.4) |
| Smokinga | ||||
| Never | 1808 (30.5) | 3216 (29.4) | 1429 (26.2) | 283 (21.5) |
| Former | 2146 (36.2) | 4011 (36.6) | 1994 (36.6) | 511 (38.9) |
| Active | 1474 (24.9) | 2730 (24.9) | 1477 (27.1) | 404 (30.7) |
| BMI, kg/m2a | 26 (24–29) | 27 (24–30) | 28 (25–31) | 29 (26–33) |
| Diabetes | 1247 (21.1) | 2006 (18.3) | 1286 (23.6) | 449 (34.1) |
| Hypertension | 3335 (56.3) | 6423 (58.7) | 3526 (64.6) | 919 (69.9) |
| Family history of CHDa | 2066 (34.9) | 3926 (35.9) | 2174 (39.9) | 539 (41.0) |
| Previous myocardial infarctionb | 163 (2.8) | 342 (3.1) | 216 (4.0) | 76 (5.8) |
| Previous ischemic strokeb | 126 (2.1) | 243 (2.2) | 172 (3.2) | 43 (3.3) |
| Total cholesterol, mmol/L | 2.8 (2.5–3.1) | 3.2 (3.0–3.5) | 3.5 (3.4–3.8) | 4.0 (3.7–4.2) |
| LDL-C, mmol/L | 1.1 (0.9–1.2) | 1.5 (1.3–1.6) | 1.6 (1.5–1.8) | 1.6 (1.5–1.8) |
| HDL-C, mmol/L | 1.3 (1.1–1.6) | 1.3 (1.0–1.6) | 1.2 (1.0–1.4) | 1.0 (0.9–1.2) |
| Triglyceride, mmol/La | 0.9 (0.7–1.1) | 1.1 (0.8–1.4) | 1.7 (1.3–2.1) | 2.9 (2.5–3.5) |
| Indication for CAG | ||||
| STEMI | 1758 (29.5) | 2829 (25.8) | 1238 (22.7) | 297 (22.6) |
| NSTEMI | 1659 (28.0) | 2804 (25.6) | 1360 (24.9) | 313 (23.8) |
| Unstable angina pectoris | 340 (5.7) | 724 (6.6) | 377 (6.9) | 109 (8.3) |
| Stable angina pectoris | 2165 (36.6) | 4593 (41.9) | 2479 (45.5) | 596 (45.3) |
| CAD | ||||
| Obstructive CAD | 4814 (81.3) | 8634 (78.8) | 4141 (75.9) | 989 (75.2) |
| No obstructive CAD | 1108 (18.7) | 2316 (21.2) | 1313 (24.1) | 326 (24.8) |
| Revascularizationc | ||||
| PCI | 3874 (65.4) | 6761 (61.7) | 3195 (58.6) | 757 (57.6) |
| CABG | 587 (9.9) | 1229 (11.2) | 643 (11.8) | 132 (10.0) |
| Medication | ||||
| High-intensity statind | 4857 (82.0) | 8292 (75.7) | 3778 (69.3) | 862 (65.6) |
| Aspirine | 5597 (94.5) | 10,135 (92.6) | 5030 (92.2) | 1186 (90.2) |
Values are median (interquartile range) or numbers (%).
BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CAG, coronary angiography; CHD, coronary heart disease; HDL-C, high-density lipoprotein cholesterol, LDL-C, low-density lipoprotein cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Missing values: smoking (9.1%), BMI (3.9%), family history of CAD (10.3%), triglyceride (0.2%).
More than 30 days before CAG.
Within 90 days after CAG.
Atorvastatin 40–80 mg. Rosuvastatin 20–40 mg.
Prescription redeemed within one year before CAG and 30 days after CAG.
During median 4.1 (IQR 2.4–6.1) years of follow-up, 893 (3.8%) patients had an MI, 1207 (5.1%) ASCVD, and 3054 (12.9%) died. The event rates per 1000 person-years in the overall cohort were 8.6 (7.5–9.8), 11.9 (11.3–12.6), and 29.3 (28.3–30.4) for MI, ASCVD, and all-cause death, respectively. Main causes of death were due to cancer (29%) and cardiovascular diseases (28%) (Supplementary Table S6).
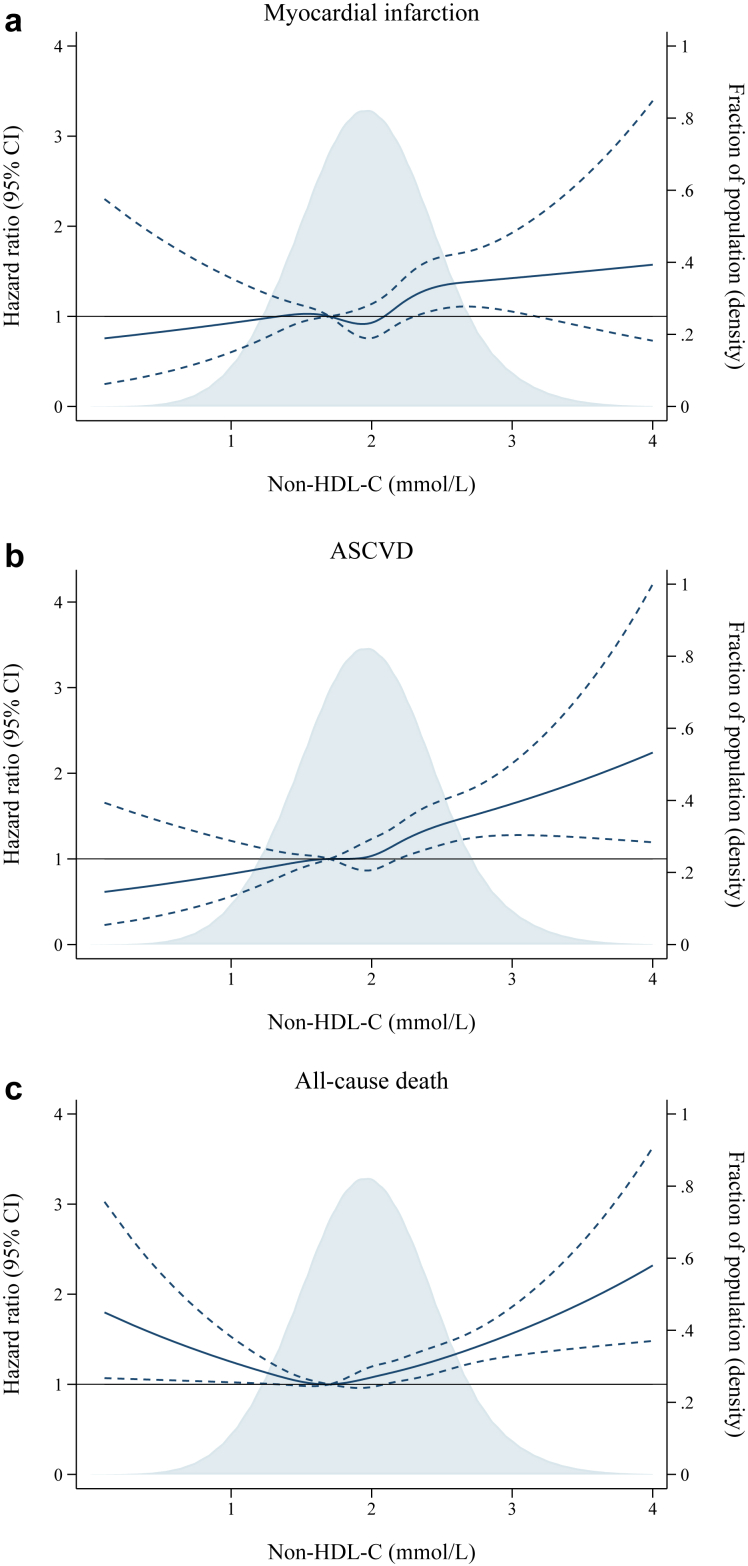
In the overall cohort, there was a curve-linear association between higher non-HDL-C and higher risk for MI and ASCVD (Fig. 2a and b). Per 1 mmol/L higher non-HDL-C, the adjusted HR was 1.3 (1.1–1.6) for MI and 1.4 (1.2–1.6) for ASCVD (Supplementary Table S7). For all-cause death, a J-curved association was found with the lowest risk of all-cause death at non-HDL-C 1.7 mmol/L, and increased risk both below and above this point (Fig. 2c).
Fig. 2.
Multivariable adjusted hazard ratios for myocardial infarction, atherosclerotic cardiovascular disease, and all-cause death according to non-high-density lipoprotein cholesterol level. Legend: Restricted cubic spline analysis showing the multivariable adjusted hazard ratio (solid line) and 95% confidence interval (stippled lines) for myocardial infarction (Fig. 2a), atherosclerotic cardiovascular disease (Fig. 2b), and all-cause death (Fig. 2c) according to non-HDL-C concentration in statin-treated patients with ischemic heart disease and low-density lipoprotein cholesterol ≤1.8 mmol/L from the Western Denmark Heart Registry. Five knots were chosen at the 5th, 27.5th, 50th, 72.5th, and 95th percentile. Reference level was chosen at the 25th percentile (non-HDL-C 1.7 mmol/L). Hazard ratios were adjusted for age, sex, smoking, hypertension, and low-density lipoprotein cholesterol. Patients with non-HDL-C > 4 mmol/L (n = 15), were omitted to avoid extremely wide confidence intervals. Kernel density plots (shaded area) illustrate the distribution of non-HDL-C. Abbreviation: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval, non-HDL-C; non-high-density lipoprotein cholesterol.
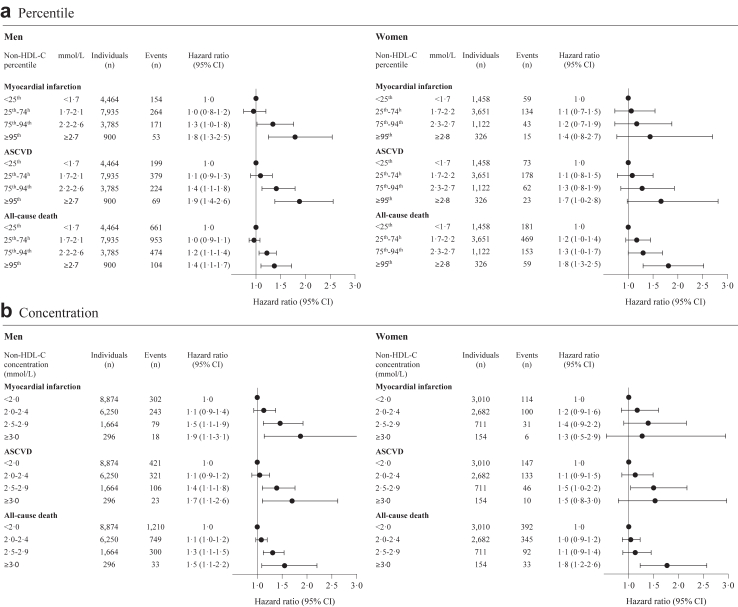
The adjusted HR according to non-HDL-C percentile groups and clinically chosen concentration groups are shown in Fig. 3. For MI, the multivariable adjusted HRs for non-HDL-C <25th percentile versus 25th–74th, 75th–94th, and ≥95th percentile were 1.0 (0.8–1.2), 1.3 (1.0–1.6) and 1.7 (1.3–2.3), respectively. For ASCVD, the corresponding adjusted HRs were 1.1 (0.9–1.3), 1.4 (1.1–1.7), and 1.8 (1.4–2.4), and for all-cause death 1.0 (0.9–1.1), 1.2 (1.1–1.4), and 1.4 (1.2–1.7), respectively (Fig. 3a). Similar results were seen for the clinically chosen concentration groups of non-HDL-C (Fig. 3b). In men and women separately, and in patients below and above 65 years of age there was a similar association between non-HDL-C and risk of MI and ASCVD (Fig. 4, Supplementary Fig. S4). In patients below 65 years of age the association between non-HDL-C and all-cause death was less pronounced (Supplementary Fig. S4). In non-diabetic patients, we found a similar association of non-HDL-C with ASCVD and all-cause death as in the overall cohort (Supplementary Fig. S5). For comparison, the predictive strength of conventional risk factors (e.g., diabetes and smoking) on the cardiovascular outcomes were weaker compared to that of non-HDL-C and non-existing for BMI (Supplementary Table S8).
Fig. 3.
Association between non-high-density lipoprotein cholesterol and myocardial infarction, atherosclerotic cardiovascular disease, and all-cause death in the study cohort. Legend: Multivariable adjusted hazard ratios for myocardial infarction, atherosclerotic cardiovascular disease, and all-cause death according to non-HDL-C percentile (Fig. 3a) and concentration group (Fig. 3b). Hazard ratio adjusted for age, sex, smoking, hypertension, and low-density lipoprotein cholesterol. Abbreviation: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; non-HDL-C, non-high-density lipoprotein cholesterol.
Fig. 4.
Men and women. Legend: Multivariable adjusted hazard ratios for myocardial infarction, atherosclerotic cardiovascular disease, and all-cause death according to non-HDL-C percentile (Fig. 4a) and concentration group (Fig. 4b) in men and women separately. Hazard ratio adjusted for age, smoking, hypertension, and low-density lipoprotein cholesterol. Abbreviation: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; non-HDL-C, non-high-density lipoprotein cholesterol.
In total, 13,808 (58%) patients presented with acute coronary syndrome and 9833 (42%) with stable angina and non-HDL-C level was associated with higher risk of MI, ASCVD, and death in both subgroups (Supplementary Fig. S6). The event rates per 1000 person-years for MI and ASCVD, but not death, were higher among patients with acute coronary syndrome compared to patients with stable angina: 10.8 (8.7–11.9) versus 6.1 (5.4–6.9) for MI, 14.2 (13.2–15.2) versus 9.0 (8.2–10.0) for ASCVD, and 30.4 (29.0–31.8) versus 27.9 (26.4–29.5) for death. In patients with obstructive CAD the results resembled the overall cohort (Supplementary Fig. S7).
Absolute event rates per 1000 person-years stratified by sex, age below/above 65 years, acute coronary syndrome, and stable angina are shown in Supplementary Fig. S8. In general, there was a stepwise increase in absolute event rates for MI and ASCVD across non-HDL-C percentile groups. Event rates of death were not uniform across patient subgroups. A U-shaped relation was found with both low and high levels of non-HDL-C showing highest rates of death for patients above 65 years of age.
Crude HRs and HRs in adjustment models excluding LDL-C gave similar results. When including diabetes, high-intensity statin, and BMI to the main adjustment model estimates did not change substantially. Neither when taking competing risk of death into account (Supplementary Table S9 and S10). Complete case analyses revealed similar results (Supplementary Table S2).
Higher LDL-C percentile was only minimally predictive of an increased risk of MI and ASCVD, whereas extremely low LDL-C was associated with an increased risk for all three outcomes (Supplementary Figs. S9 and S10a). On the contrary, higher remnant cholesterol and triglycerides percentile showed an incremental association of MI, ASCVD, and death (Supplementary Figs. S10b and S11).
In sensitivity analyses when stratifying patients according to LDL-C ≤ 1.4 mmol/L and >1.4 mmol/L the association between non-HDL-C and ASCVD was similar in patients with LDL-C > 1.4 mmol/L but attenuated in patients with LDL ≤ 1.4 mmol/L (Supplementary Table S11). Remnant cholesterol did not differ in the two groups (Supplementary Table S12).
Median time from CAG to redeemed statin prescription was 6 days (IQR 3–38 days), and median time from CAG to index cholesterol measure was 8.3 months (IQR 5.5–10.4 months). Irrespective of non-HDL-C percentile, median number of lipid profile measurements before start of follow-up was 3 (IQR 2–4). Time of index measure did not influence the association of non-HDL-C and the outcomes (Supplementary Table S13), nor did advanced start of follow-up (Supplementary Table S14), or year of CAG (Supplementary Fig. S12 and Table S15).
Discussion
This large and contemporary cohort of 23,641 consecutive patients with ischemic heart disease undergoing CAG for evaluation of CAD provides important and novel insights into the association of non-HDL-C and residual risk of MI, ASCVD, and all-cause death in statin-treated patients who had already achieved well-controlled LDL-C levels. The present results show that higher non-HDL-C is strongly associated with higher risk of MI, ASCVD, and all-cause death in presence of well-controlled LDL-C levels. These findings were consistent across multiple subgroup analyses and the predictive power of non-HDL-C was similar or higher than for conventional risk factors such as diabetes, BMI, and smoking. Taken together, these data are valuable for clinical practice and future guidelines as they demonstrate that assessment of non-HDL-C in patients who are believed to be optimally treated, identifies patients who remain at high residual risk of MI, ASCVD, and all-cause death.
Now, with this study, two markers (hsCRP and non-HDL-C) can identify patients at high residual risk of ASCVD and death when LDL-C target is achieved. The present study supports knowledge that LDL-C has limited capability in terms of predicting residual risk in patients with LDL-C ≤ 1.8 mmol/L. Not to confuse with stating that further LDL-C reduction will not reduce residual risk, but the predictive power of LDL-C is challenged. This study settles that the predictive capability is inherent within remnant cholesterol (i.e., the cholesterol content in TRL particles) being a part of non-HDL-C. As already presented, a recent meta-analysis based on three randomised trials of high-risk patients with raised triglyceride levels (PROMINENT, REDUCE-IT, STRENGTH) found that hsCRP was a stronger predictor of residual risk than LDL-C in statin-treated patients suggesting that this marker could be used to guide additional preventive therapies.1 Interestingly, results from a general population study show that dual elevated hsCRP and remnant cholesterol confers the highest risk of ASCVD.9 How this specific association confer risk in statin-treated patients is unknown.
However, hsCRP is an exclusive biomarker not readily accessible for clinicians to take advantage of. Viewed in this context, our study provides important information by showing that the simpler, more widely available and easy to adopt calculation of non-HDL-C, is of great value for assessment of residual risk in statin-treated patients with ischemic heart disease. This is of major clinical relevance given that LDL-C can now be controlled in most patients due to development of additional non-statin pharmacological treatments such ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. These treatments, together with statins, also substantially lowers non-HDL-C, hence residual risk due to elevated non-HDL-C is modifiable.14,20 A finding of special clinical interest is that only 66% of patients with high non-HDL-C were treated with high-intensity statins as compared with 82% among those with the lowest non-HDL-C percentile. Although beyond the scope of the paper, this suggests an opportunity for optimisation of statin treatment, and potentially add-on lipid-lowering therapies to lower residual risk through non-HDL-C reduction, even when LDL-C is treated to target levels. Furthermore, since the highest non-HDL-C percentile also holds proportionally more patients with obesity, diabetes, and hypertension it is essential to optimise therapies that have demonstrated cardiovascular benefits in these patient populations. These include, but are not necessarily limited to, selective sodium glucose transporter (SGLT)-2 inhibitors, glucagon like peptide (GLP)-1 analogues and carefully titrated antihypertensive treatment.21,22
In addition to non-HDL-C, there is an increasing interest in residual risk associated with the number of circulating atherogenic lipoproteins measured as apoB. Given that these two measures provide information (cholesterol content versus number of atherogenic particles) on the same lipoproteins, they are highly correlated.23 Indeed, in a previous study, only few statin-treated patients were found to have discordant values of non-HDL-C versus apoB, and discordance was not associated with risk for future MI.11 Further, a study based on statin-treated patients from the FOURIER and IMPROVE-IT trials found that the association of non-HDL-C and apoB with future MI was similar with adjusted HRs of 1.16 (1.11–1.22) and 1.19 (1.14–1.25) per 1 standard deviation increase, respectively.24 However, when adjusted for each other, risk seems to track most with apoB. Taken together, this suggest that apoB may be marginally more important for risk than non-HDL-C (i.e., lowering of particle number is important for risk reduction) but, as these markers are highly correlated, they provide similar predictive value in most patients. ApoB is not part of the standard lipid profile in Denmark and thus not available based on national health registries. However, non-HDL-C calculated from the standard lipid profile within clinical practice, will provide sufficient information of residual risk in patients in a cost-effective manner.7
Clinical practice has generally focused on the use of LDL-C for assessment of cholesterol-mediated risk for future ASCVD events. Given that LDL-C constitute the majority of non-HDL-C, these two cholesterol measures are highly correlated, that is, when LDL-C is low then non-HDL-C is often also low, and vice versa. Thus, when simply comparing the individual associations of LDL-C and non-HDL-C with ASCVD events in an overall population, the adjusted HRs per 1 mmol/L increase in LDL-C and non-HDL-C are similar.13 Thus, to demonstrate the incremental value of assessing non-HDL-C beyond LDL-C, recent studies have used discordance analyses. The advantage of such analyses is that the results are not diluted by concordant data in which risk predictions cannot differ. Previous studies among patients not treated with statin therapy have shown that elevated non-HDL-C better reflects risk than LDL-C.25, 26, 27 Only few studies have specifically assessed the incremental value of non-HDL-C or TRL over LDL-C among statin-treated patients.11,28 Patients with LDL-C target achievement was an absolute minority in these studies. With the present study, we show that remnant cholesterol and triglycerides, as collinear with non-HDL-C, are carriers of the predictive capability of ASCVD down to a level of LDL-C below 1.8 mmol/L. We advocate for using non-HDL-C, in contrast to remnant cholesterol, since calculated non-HDL-C is freed from uncertainties of Friedewald’s formula at low LDL-C and high triglyceride levels, which are extremely relevant in this specific cohort in terms of residual risk. Although triglycerides are a predictive measure as well, this finding completely relies of the inherited relationship between triglycerides and remnant cholesterol due to Friedewald’s formula. Consequently, when adjusting for non-HDL-C, the association between triglycerides and MI, ASCVD, or all-cause death disappears. This underscores one of the key benefits of utilizing non-HDL-C for risk prediction, as it encompasses the predictive value of triglycerides (when assessing non-HDL-C there is no additional predictive value by also looking at triglycerides). This aligns with the recommendations provided in dyslipidaemia guidelines, including those from the European Society of Cardiology.16
Regarding all-cause death, we believe the J-curved association observed in the crude HR was confounded by older age among patients with non-HDL-C < 25th percentile. After multivariable adjustment, we saw an incremental association of non-HDL-C and HR for all-cause death. Still, in the spline analysis low levels of non-HDL-C was predictive of a higher HR for all-cause death. As LDL-C composes the majority non-HDL-C, we argue that the association between non-HDL-C and all-cause death, mirrors the known association with LDL-C and all-cause death in which no causal association is proven but low LDL-C being a proxy for, e.g., malnutrition due to cancer.29
In addition to the limitations introduced by the study design, which we attempted to address in sensitivity analyses, several points are to be mentioned. First, we cannot determine if there is a causative effect from non-HDL-C particles itself or if non-HDL-C is a marker of residual risk caused by other factors. However, we have provided numerous sensitivity analyses in which we found that the observed association was robust. Also in terms of identifying patients with high residual risk this point is not fundamental. To reduce the risk of confounding we chose to adjust the models for known clinical covariates that are usually adjusted for in clinical risk stratification. We chose deliberately not to adjust for BMI and diabetes in the main analysis, since BMI and diabetes is a mediator through strong correlation with triglyceride and TRL.30 In sensitivity analyses we adjusted for diabetes, high-intensity statin, and BMI which did not change the conclusions, nor when excluding patients with diabetes. Using national registry data based on current clinical practice in Denmark, unfortunately hsCRP was not available although highly desired for research purposes. Since we only included patients with LDL-C ≤ 1.8 mmol/L, we find it reasonable to assume that a redeemed statin prescription, in this cohort, does in fact mean that patients were taking statins. Use of ezetimibe was not prescribed regularly in Denmark during the study period. Requiring prescription of statins introduce the possibility of confounding by indication i.e., if patients with the most severe angiographic findings are those treated to lowest LDL-C, then patients with lowest LDL-C could be the ones with highest risk due to other factors than LDL-C and non-HDL-C. However, when comparing baseline characteristics of patients receiving high- and low-intensity statins we did not find support of confounding by indication. The risk of introducing bias due to start of follow-up one year after CAG is negligible as supported by sensitivity analyses showing that the association between non-HDL-C and the outcomes was neither dependent of time of index measure nor advanced start of follow-up. Furthermore, the overall analysis was independent of year of CAG.
Our study does not determine a clinical cut-off value of non-HDL-C concentration that should be considered a new target for therapy, but it illustrates the spectrum of non-HDL-C and how residual risk differs accordingly. Non-HDL-C as a predictor of cardiovascular events in patients with LDL-C ≤ 1.4 mmol/L was attenuated. This may be due to low power as of a reduced number of events in this well-treated patient group or, simply that the predictive capability of non-HDL-C is challenged at low levels, just like the case for LDL-C. Non-HDL-C has now been included in the primary prevention risk prediction model SCORE2.5 With the accumulating evidence inclusion of non-HDL-C in global secondary prevention risk prediction is promising.
In conclusion, in patients with ischemic heart disease and well-managed LDL-C, non-HDL-C was a predictor of residual risk of MI, ASCVD, and all-cause death. These results are valuable for clinical practice as they demonstrate the importance of assessing non-HDL-C when LDL-C is well-controlled to identify patients who face a high risk of MI, ASCVD, and death, in whom intensification of preventive management may be warranted.
Contributors
MKH, MBM and MM designed the study. MKH analysed the data and wrote the first draft of the manuscript. KKWO was responsible for data management of collected data from national and regional registries and supervised data analyses. PGT supervised data analyses and verified the results reported. MBM and MM supervised the data process and critically reviewed the manuscript. All authors had full access to all data in the study and accept responsibility to submit for publication.
Data sharing statement
Open access to data is not allowed by the Danish Data Protection Agency. However, given a reasonable request, additional analyses can be done after contacting the corresponding author.
Declaration of interests
MKH has received a research grant from the Novo Nordisk Foundation (grant number NNF22OC0074083). MBM has received lecture fees from Novo Nordisk, AstraZeneca, Amarin, Sanofi, and Amgen. KKWO is supported by a research grant from the Danish Cardiovascular Academy funded by the Danish Heart Association and Novo Nordisk. MM is supported by a grant from the Novo Nordisk Foundation (grant number NNF22OC0074083), has received institutional research grants from Novo Nordisk and Bayer, and has received lecture and/or advisory board fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Novo Nordisk. PGT has no conflicts of interest.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100774.
Appendix A. Supplementary data
References
- 1.Ridker P.M., Bhatt D.L., Pradhan A.D., Glynn R.J., MacFadyen J.G., Nissen S.E. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401:1293–1301. doi: 10.1016/S0140-6736(23)00215-5. [DOI] [PubMed] [Google Scholar]
- 2.Guedeney P., Claessen B.E., Kalkman D.N., et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2019;73:2401–2409. doi: 10.1016/j.jacc.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 3.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC) Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehac458. [DOI] [PubMed] [Google Scholar]
- 5.SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42:2439–2454. doi: 10.1093/eurheartj/ehab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson G.J., Thanassoulis G., Anderson T.J., et al. 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Nordestgaard B.G., Langlois M.R., Langsted A., et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM. Atherosclerosis. 2020;294:46–61. doi: 10.1016/j.atherosclerosis.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Nordestgaard B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 9.Doi T., Langsted A., Nordestgaard B.G. Dual elevated remnant cholesterol and C-reactive protein in myocardial infarction, atherosclerotic cardiovascular disease, and mortality. Atherosclerosis. 2023;379 doi: 10.1016/j.atherosclerosis.2023.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Varbo A., Benn M., Tybjærg-Hansen A., Nordestgaard B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 11.Johannesen C.D.L., Mortensen M.B., Langsted A., Nordestgaard B.G. Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J Am Coll Cardiol. 2021;77:1439–1450. doi: 10.1016/j.jacc.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Johannesen C.D.L., Mortensen M.B., Langsted A., Nordestgaard B.G. ApoB and non-HDL cholesterol versus LDL cholesterol for ischemic stroke risk. Ann Neurol. 2022;92:379–389. doi: 10.1002/ana.26425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boekholdt S.M., Arsenault B.J., Mora S., et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307:1302–1309. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 14.Vallejo-Vaz A.J., Fayyad R., Boekholdt S.M., et al. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation. 2018;138:770–781. doi: 10.1161/CIRCULATIONAHA.117.032318. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M., Maeng M., Madsen M., Sørensen H.T., Jensen L.O., Jakobsen C.-J. The Western Denmark heart registry: its influence on cardiovascular patient care. J Am Coll Cardiol. 2018;71:1259–1272. doi: 10.1016/j.jacc.2017.10.110. [DOI] [PubMed] [Google Scholar]
- 16.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 17.Krarup L.-H., Boysen G., Janjua H., Prescott E., Truelsen T. Validity of stroke diagnoses in a national register of patients. Neuroepidemiology. 2007;28:150–154. doi: 10.1159/000102143. [DOI] [PubMed] [Google Scholar]
- 18.Sundbøll J., Adelborg K., Munch T., et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell F.E.J. 2nd ed. Springer International Publishing; 2015. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. [Google Scholar]
- 20.Robinson J.G., Wang S., Smith B.J., Jacobson T.A. Meta-analysis of the relationship between non–high-density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol. 2009;53:316–322. doi: 10.1016/j.jacc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Marx N., Federici M., Schütt K., et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043–4140. doi: 10.1093/eurheartj/ehad192. [DOI] [PubMed] [Google Scholar]
- 22.Mancia G., Rosei E.A., Azizi M., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 23.Welsh C., Celis-Morales C.A., Brown R., et al. Comparison of conventional lipoprotein tests and apolipoproteins in the prediction of cardiovascular disease. Circulation. 2019;140:542–554. doi: 10.1161/CIRCULATIONAHA.119.041149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marston N.A., Giugliano R.P., Melloni G.E.M., et al. Association of apolipoprotein B-containing lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis: distinguishing between particle concentration, type, and content. JAMA Cardiol. 2022;7:250–256. doi: 10.1001/jamacardio.2021.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora S., Buring J.E., Ridker P.M. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina M.J., D’Agostino R.B., Zdrojewski T., et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol. 2015;22:1321–1327. doi: 10.1177/2047487315569411. [DOI] [PubMed] [Google Scholar]
- 27.Sniderman A.D., St-Pierre A.C., Cantin B., Dagenais G.R., Després J.-P., Lamarche B. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. Am J Cardiol. 2003;91:1173–1177. doi: 10.1016/s0002-9149(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y.-X., Zhang H.-W., Jin J.-L., et al. Prognostic utility of triglyceride-rich lipoprotein-related markers in patients with coronary artery disease. J Lipid Res. 2020;61:1254–1262. doi: 10.1194/jlr.RA120000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johannesen C.D.L., Langsted A., Mortensen M.B., Nordestgaard B.G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. 2020;371 doi: 10.1136/bmj.m4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varbo A., Freiberg J.J., Nordestgaard B.G. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the Copenhagen general population study. Clin Chem. 2018;64:219–230. doi: 10.1373/clinchem.2017.279463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.