Abstract
In previous experiments we were able to separate, using a nondestructive separation technique, culturable and nonculturable bacteria, from a Luria-Bertani (LB) medium culture of Escherichia coli incubated for 48 h. We observed in the nonculturable bacterial population an increase in oxidative damage and up-induction of most defenses against reactive oxygen species (ROS), along with a decrease in cytoplasmic superoxide dismutases. In this study, using the same separation technique, we separated into two subpopulations a 10-h LB medium culture containing only culturable bacteria. For the first time, we succeeded in associating physical separation with physiological differences. Although the levels of defense against ROS (RpoS, RpoH, OxyR, and SoxRS regulons) and oxidative damage (carbonyl contents) were apparently the same, we found that bacteria in one subpopulation were more sensitive to LB medium starvation and to various stresses, such as phosphate buffer starvation, heat shock, and hydrogen peroxide exposure. Based on these results, we suggest that these physiological differences reflect uncharacterized bacterial modifications which do not directly involve defenses against ROS.
Biological aging could be defined as the gradual decline in the capacity of an organism to resist stress, damage, and disease. In 1956, Denham Harman (10) postulated that this ubiquitous progressive decay in the functional capacity of aging eukaryotes is a consequence of the accumulation of oxidative damage caused by reactive oxygen species (ROS) (12); this was called the free radical theory (10). A small percentage of oxygen is chemically reduced by addition of single electrons, and the products are sequentially converted into ROS, including the superoxide anion, hydrogen peroxide, and the hydroxyl radical (8). ROS have been shown to cause molecular damage relatively indiscriminately to proteins, lipids, and nucleic acids (3, 9).
In cells of prokaryotes, such as Escherichia coli, entering a nonproliferating state (stationary phase) due to nutrient depletion, the bacteria gradually lose the ability to divide and reproduce (21). Similar to eukaryotes, the life span of a starved bacterium appears to be limited by the cell's ability to combat ROS. Indeed, Dukan and Nyström demonstrated the existence of an accumulation of oxidized proteins during starvation of an E. coli population (6). Moreover, the life span of growth-arrested wild-type E. coli can be increased >100% by omitting oxygen during stasis (7). This process has been referred to as conditional senescence elicited by growth arrest (17). Given that one of the criteria for defining senescence is an increase in the mortality rate over time (12), it appears that prokaryotes such as E. coli also senesce (20). More recently, using an ultracentrifugation separation technique, we (4) isolated a nonculturable subpopulation from a Luria-Bertani (LB) medium culture of E. coli incubated for 48 h. We suggested that the main reason for the loss of culturability observed after 48 h was a decrease in cytoplasmic superoxide dismutase activity (SodA, SodB). In nonculturable bacteria, a decrease in SodA and SodB activity seemed to be associated with an increase in oxidative damage and up-induction of stress regulons. Thus, indicator genes controlled by RpoS, SoxRS, RpoH, RpoE, and CpxR were up-induced in a nonculturable fraction. Nonculturable cells exhibited greater oxidation damage than culturable cells. Hence, these results suggested that in E. coli cells the loss of reproductive ability and an increase in oxidative damage were associated (4). Taken together, all these results suggested that ROS are involved in bacterial loss of culturability during the stationary phase. However, and even if the scientific community agrees with the ROS theory, the first events leading to this bacterial loss of culturability were unclear.
In this study, we tried to gain further insight into the events leading to the loss of the reproductive ability of E. coli during the stationary phase. We were able to separate a 10-h LB medium culture of E. coli into two subpopulations containing only culturable cells. While these subpopulations appeared to be identical in terms of stress defense and oxidative damage, we observed that one subpopulation (higher density), which contained more than 75% of the cells, was more sensitive to LB medium starvation and to various stresses, such as phosphate buffer starvation, heat shock, and hydrogen peroxide exposure.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type E. coli MG1655 was grown at 37°C with aeration in LB broth with constant rotation at 200 rpm. Growth was monitored by measuring the turbidity with a Beckman photometer. The culture conditions were fixed as follows. Overnight cultures on LB medium were first diluted 100-fold into 10 ml of fresh LB medium. Four hours later, an early-stationary-phase culture (optical density at 600 nm, 4) was diluted 10-fold into 10 ml of fresh LB medium. Viable cell counts were determined at various times by spreading aliquots of the cultures on LB agar plates. The total cell concentration, as determined with a flow cytometer or by microscopic analysis, was always equivalent to the culturable cell concentration. Where indicated below, conditioned media were used. Overnight cultures on LB medium were first diluted 100-fold into 10 ml of fresh LB medium. Ten hours later, cultures were centrifuged (4°C, 20 min), and the conditioned medium, designated LB10H, was collected.
Radioselectan equilibrium density gradient.
Bacteria were washed in cold phosphate buffer (pH 7, 0.05 M) (or cold water without loss of culturability) and concentrated in cold 26.45% radioselectan (sodium and meglumine amidotrizoate; final volume, 10 ml; final cell concentration, 5 × 1010 cells/ml). Gradients were prepared as described previously (4) by using 10 ml of 37% radioselectan in each polycarbonate centrifuge tube (25 by 89 mm) layered with 1 ml of a bacterial suspension (5 × 1010 cells) without a loss of resolution. Gradients were spun at 55,000 rpm in a Ti70 rotor by using a Beckman tabletop ultracentrifuge at 4°C for 3 h. After collection, the cells in the sample were pelleted, rinsed, and resuspended in sterile MilliQ water for carbonyl analysis or in phosphate buffer (pH 7, 0.05 M) for measurement of viability. This separation was performed more than 20 times for 10-h cultures.
Total cell and culturable cell concentrations for each sample were determined by using a flow cytometer and by plating on LB agar, respectively. For each cell preparation and separation step, no differences between the total and culturable cell concentrations were found. Indeed, we observed that 100% of the cells were culturable.
Microscopic observation of cells and nucleoids.
To observe the shape and size of cells and nucleoids by microscopy, cells were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), which binds specifically to DNA, by using the method developed by Hiraga et al. (11).
Cell assays and viability measurements.
The two subpopulations were subjected to different stresses, including starvation (0.05 M phosphate buffer [pH 7] at 37°C), heat shock at 60°C, and exposure to 8.8 mM H2O2 (the reaction was stopped with 10 U of catalase from bovine liver per ml). Culturable bacteria were assayed by plating samples from suspensions onto LB agar plates after serial dilution in cold phosphate buffer (0.05 M, pH 7, 4°C). Colonies were counted after 48 h of incubation at 37°C. The results were expressed as percentages (challenged culturable cells/nonchallenged culturable cells). These experiments were repeated 6 times for starvation and 12 times for the other stresses to obtain representative results.
Chemicals and reagents.
Anti-DnaK monoclonal mouse antibodies were purchased from StressGen Biotechnologies (Victoria, Canada), and anti-RpoS monoclonal mouse antibodies were purchased from Neoclone (Washington, D.C.). Anti-SodA and anti-SodB polyclonal rabbit antibodies were gifts from D. Touati. Anti-mouse and anti-rabbit immunoglobulin G-peroxidase conjugates were obtained from Sigma. The chemiluminescence blotting substrate (ECL+) and Hybond-P (polyvinylidene difluoride membrane) were obtained from Amersham and were used according to the manufacturer's instructions. Protein assay reagents were purchased from Bio-Rad. X-Omat AR-5 was purchased from Eastman Kodak. Radioselectan (76%) was purchased from Schering.
Carbonylation assays.
As described previously (6), crude protein extracts were obtained by using a French pressure cell; the proteins were reacted with the carbonyl reagent 2,4-dinitrophenylhydrazine and dot blotted onto polyvinylidene difluoride membranes, and oxidatively modified proteins were detected with anti-2,4-dinitrophenyl hydrazone antibodies (OxyBlot kit; Intergen).
RESULTS
During our first study (4), which was performed with a 39% radioselectan gradient and only culturable cells, we observed only one band after ultracentrifugation. In the present study, we decreased the radioselectan gradient concentration to 37%, and as described below, we were able to separate cell subpopulations during entrance into the stationary phase. Moreover, we verified that our first results obtained after 48 h (4) were reproducible with a 37% radioselectan gradient (data not shown).
Fractionation of E. coli cells by radioselectan solution centrifugation.
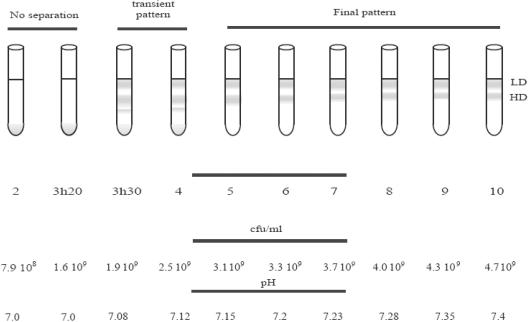
The previous experiments suggested that there is a possible chronology of events that lead bacteria to a nonculturable state (4). In order to determine the first event, we tried to separate cells before the appearance of the first nonculturable bacteria. Then we examined the separation pattern at various times when cells were entering the stationary phase in LB medium (100% of the cells were culturable). With 37% radioselectan, for up to 3 h 20 min of incubation, we could not detect any differences in the bacterial population; all of the cells settled and formed one pellet (Fig. 1). After 3.5 h of incubation, we observed four distinct bands. Finally, after 5 h of incubation we observed only two distinct bands. As the total cell numbers and the CFU counts were the same, each band contained only culturable bacteria. The low-density (LD) band remained at the same position and was the same thickness (5 mm) for all samples after 3.5 h of incubation, while the cell content decreased. Using a flow cytometer, we determined that for the culture incubated for 10 h the bottom band contained almost 75% of all cells (data not shown). The thickness of the high-density (HD) band decreased from 2.2 cm at 5 h to 1.3 cm after 7 h of incubation and then stayed the same. Cells from both bands were stained with DAPI, and the cell morphology was examined; even if we observed changes in cell shape associated with entrance into the stationary phase, no characteristic difference was observed between the two subpopulations (data not shown). We decided to focus on the 10-h culture.
FIG. 1.
Radioselectan solution centrifugation of E. coli cells at various stages of the transition from exponential growth to the stationary phase. E. coli was grown in LB medium. Cultures were harvested at different times and immediately subjected to centrifugation. The number of culturable cells and the pH of the medium were measured for each time. In the final tube the bacterial population was separated into two bands, the LD bacteria and the HD bacteria. Representative results are shown. The experiments were repeated three times.
Subpopulations of bacteria have the same levels of oxidative damage and defense against ROS.
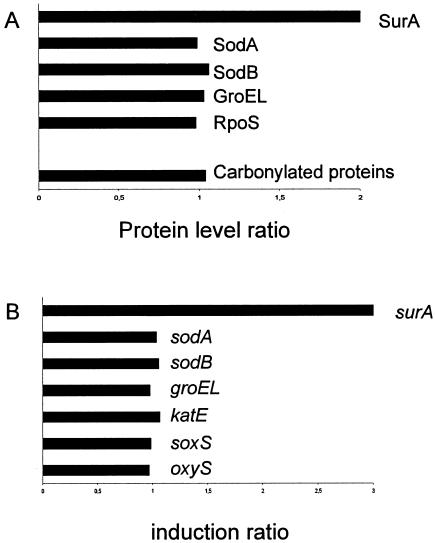
We wondered whether the physical heterogeneity was associated with any biochemical modification. We observed previously after 48 h of culture of a nonculturable subpopulation a decrease in the SodA and SodB proteins associated with induction of various stress regulons (4). Here, the amounts of the SodA, SodB, GroEL (for the rpoH regulon), and RpoS proteins or the carbonyl contents (specific marker for protein oxidation) were identical for subpopulations separated from a 10-h E. coli culture (Fig. 2A). These results suggested that the two subpopulations had the same background of stress proteins to resist oxidative stress.
FIG. 2.
Protein and carbonyl contents (A) and differential levels of selected transcripts (B) in high- and low-density cells from an LB medium culture of E. coli incubated for 10 h. The bacteria, grown in LB medium, were separated by ultracentrifugation in a radioselectan solution. Comparisons were performed by dividing the transcript levels and protein levels (as measured by Western blotting with specific antibodies against selected proteins) in HD bacteria with the levels in LD bacteria. The standard deviation was always less than 15%. The experiments were repeated three times.
Transcriptome preanalysis.
Previous results were confirmed at the transcriptional level. Using transcriptome preanalysis, we did not observe any difference at the RNA level between the two subpopulations for the various regulons mentioned above (Fig. 2B). However, some genes were found to be expressed differently in the two subpopulations. The only gene which was known to be involved in E. coli survival in the stationary phase was surA, which was overexpressed threefold in the high-density cells (Fig. 2B). surA encodes a periplasmic peptidyl-prolyl isomerase involved in the maturation of outer membrane porins and in stationary-phase survival (14, 15). SurA antibody analysis confirmed this up-induction at the protein level (Fig. 2A). Even if surA might be a member of the RpoE regulon, no difference was observed for other genes regulated by RpoE (data not shown). Altogether, these results suggest that at 10 h all the cells had the same oxidative stress resistance background and the same perception (RNAs) of these stresses even though there were some differences, such as differences in surA.
A subpopulation of bacteria became more sensitive to various stresses.
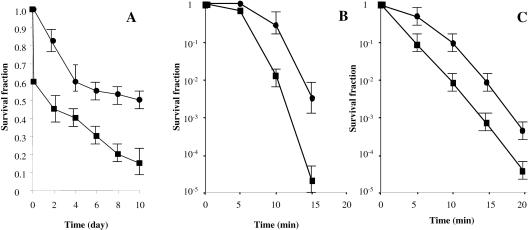
E. coli responds to starvation by developing increased resistance to a variety of environmental stresses, such as oxidation or heat shock (13, 19). We tested whether the two subpopulations became more resistant to various stresses, including (i) phosphate starvation, (ii) heat shock, and (iii) hydrogen peroxide. Figure 3 shows that the HD cells were much more sensitive to all stresses than the LD cells. This result suggested that even if the defenses against ROS are apparently the same in the two subpopulations, the E. coli culture is physiologically heterogeneous.
FIG. 3.
Survival rates after phosphate buffer starvation, hydrogen peroxide exposure, and heat shock exposure of the two subpopulations isolated from an LB medium culture of E. coli incubated for 10 h. Bacteria were separated by ultracentrifugation in the radioselectan solution. (A) Ten milliliters of phosphate buffer was initially inoculated with 107 bacteria/ml. The experiments were repeated six times. (B and C) Ten milliliters of phosphate buffer was initially inoculated with 108 bacteria/ml and exposed to 60°C (B) or 8.8 mM H2O2 (C). The experiments were repeated 12 times. Samples were taken at intervals. Culturable bacteria were assayed by plating samples from suspensions onto LB agar plates after serial dilution in cold phosphate buffer. Colonies were counted after 48 h of incubation at 37°C. Symbols: ▪, HD bacteria; •, LD bacteria.
One bacterial subpopulation is more sensitive to LB medium starvation.
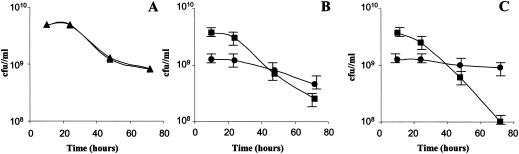
Finally, we monitored the behavior of the HD and LD subpopulations during LB medium starvation. We first separated tetracycline-resistant parental E. coli cultures (subpopulations HDT and LDT) and kanamycin-resistant parental E. coli cultures (subpopulations HDK and LDK) after 10 h of growth. We mixed (final cell concentration, 5 × 109 bacteria/ml) 25% LDT cells and 75% HDK cells in the LB medium which supported growth during this 10 h (LB10H conditioned medium). The cultures were incubated at 37°C and 200 rpm for several days, and the culturable bacteria were examined. As shown in Fig. 4A, radioselectan cell separation had no effect on the behavior of the culture. Moreover, as observed for phosphate buffer starvation, we observed that HDK (or HDT) cells incubated alone on LB10H were more sensitive than LDK (or LDT) cells (Fig. 4B). As shown in Fig. 4C, in the presence of HDK cells, LDT cells were more resistant to LB medium starvation and HDK cells were more sensitive. Taken together, these results suggested that LDT cells accounted for the majority of the future culturable bacteria detected during LB medium starvation and that HDK cells accounted for the majority of the future nonculturable bacteria. Moreover, HDK cells might have served as a nutrient source during starvation.
FIG. 4.
Subpopulation behavior during LB medium starvation. Tetracycline-resistant (MG1655 zhj-3076::Tn10 or zah-281::Tn10) and kanamycin-resistant (MG1655 rha::Tn5 or purD81::Tn5) cells were separated by ultracentrifugation in the radioselectan solution. After separation, the tetracycline-resistant culture was divided into HDT and LDT subpopulations, and the kanamycin-resistant culture was divided into HDK and LDK subpopulations. (A) Evolution of MG1655 culturability during the stationary phase without (▴) or with (▵) the ultracentrifugation step. The open and solid symbols overlap. (B) Evolution of culturability of HDT or HDK cells alone (▪) or LDT or LDK cells alone incubated in LB10H. (C) Evolution of culturability of a mixed population (25% LDT cells and 75% HDK cells) in LB10H (final cell concentration, 5 × 109 bacteria/ml). The experiments were repeated three times.
DISCUSSION
In recent studies on bacterial physiology during starvation-induced stasis, workers turned to the free radical hypothesis proposed for eukaryote aging to explain the progressive decline in the culturability of growth-arrested bacterial cells (17). Accordingly, a number of regulatory networks are functionally integrated in starved cells of bacteria in order to reduce oxidation of target macromolecules and to enhance the cell's ability to withstand environmental insults. However, despite the fact that starved wild-type E. coli cells have an enhanced capacity to manage oxidative stress, they gradually lose the ability to reproduce. Different studies have suggested that bacteria become nonculturable due to cellular deterioration via oxidation and that the cells are moribund (2, 4, 5). In this study, using a nondestructive separation technique, we observed for the first time that most of the future nonculturable cells come from one of two subpopulations. However, the two subpopulations appeared to have the same basal-level defenses against ROS.
Indeed, using this technique, we were able to detect some heterogeneity in the bacterial population before it entered stasis. We linked the physical separation of an LB medium culture of E. coli incubated for 10 h into two subpopulations with different buoyant densities to physiological differences. Even if all the bacteria throughout the experiments had the same colony-forming capacity and had apparently identical levels of oxidative damage and defense against ROS, one subpopulation was more prone to become nonculturable after various stresses (phosphate buffer starvation, heat shock, hydrogen peroxide). Moreover, the reconstruction experiment with LB10H indicated that the HD cells accounted for the majority of the future nonculturable cells during starvation. At least two parameters are important for bacterial protection against stresses, the basal level of defense and the ability to respond to stress. If this is taken into account, one possible explanation of all the results might be that HD cells were not able to respond to stresses as well as LD cells responded. To our knowledge, this is the first time that it has been possible to demonstrate that all cells from the same culture are not identical in the face of loss of culturability. Indeed, it is commonly thought that self-replication of bacteria, such as E. coli, is symmetrical, and the cytoplasm seems to be dispersed randomly and nonconservatively during division. Damaged and nonfunctional molecules are distributed equally in both daughter cells, and old macromolecules are rapidly diluted as long as the environment supports growth and proliferation (18). Although the two subpopulations separated at 10 h evolved in the same environment, they differed in the rate of loss of culturability. Based on these results, we suggest that the physiological differences reflect uncharacterized modifications which do not directly involve defense against ROS.
We suggest that the presence of HD cells at 10 h might be a bacterial population strategy for long-term survival in the stationary phase. The HD cells, which have a higher die-off rate, could provide nutrients for other cells (16). Indeed, a possible explanation for the increased resistance of the LDT cells in the presence of HDK cells and the loss of resistance of the HDK cells in the presence of LDT cells might be competition for nutrients provided by HDK cells. Nutrient assimilation seemed to be greater in the LDT cells, which could also have facilitated the response of LDT cells to stresses. The strategy might consist of diffusion of small molecules which could ensure the survival or the growth of growth advantage in stationary phase cells (22), which represented part of the population. Indeed, the SurA up-induction observed in the HD cells might reflect disorder in maintaining the outer membrane integrity (15). SurA appears to play a pivotal role in the biogenesis of trimeric outer membrane porins (OmpA, OmpC, and OmpF) that are involved in the diffusion of selected small molecules through this barrier (1). Further investigation is necessary to identify the main reason behind bacterial disorders; transcriptome analysis should provide more information about the chain of events that lead to the loss of bacterial culturability.
Acknowledgments
We thank Susanne Behrens, University of California, San Francisco, for providing SurA antibodies. We also thank V. Méjean, M. Chippaux, and P. Moreau, Laboratoire de Chimie Bactérienne, Marseille, France, for carefully reading the manuscript. We thank D. Touati and F. Taddei for valuable discussions.
This work was supported by ATI and ACI Jeunes Chercheurs. C.C. was a recipient of a fellowship from Ministère de l'Education Nationale.
REFERENCES
- 1.Behrens, S., R. Maier, H. de Cock, F. X. Schmid, and C. A. Gross. 2001. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 20:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogosian, G., and E. Bourneuf. 2001. A matter of bacterial life and death. EMBO J. 2:770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies, K., S. Lin, and R. Pacifici. 1987. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J. Biol. Chem. 262:9914-9920. [PubMed] [Google Scholar]
- 4.Desnues, B., C. Cuny, G. Grégori, S. Dukan, H. Aguilaniu, and T. Nyström. 2003. Differential oxidative damage and expression of stress defence regulons in culturable and non-culturable Escherichia coli cells. EMBO Rep. 4:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dukan, S., S. Belkin, and D. Touati. 1999. Reactive oxygen species are partially involved in the bactericidal action of hypochlorous acid. Arch. Biochem. Biophys. 367:311-316. [DOI] [PubMed] [Google Scholar]
- 6.Dukan, S., and T. Nyström. 1998. Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev. 12:3431-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dukan, S., and T. Nyström. 1999. Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J. Biol. Chem. 274:26027-26032. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich, I. 1978. The biology of oxygen radicals. Science 201:875-880. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell, B., and J. Gutteridge. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harman, D. 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 2:298-300. [DOI] [PubMed] [Google Scholar]
- 11.Hiraga, S., H. Niki, T. Ogura, C. Ichinose, H. Mori, B. Ezaki, and A. Jaffe. 1989. Chromosomal partitioning in Escherichia coli chromosomal replication: novel mutants producing anucleate cells. J. Bacteriol. 171:1496-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, F., D. Sinclair, and L. Guarente. 1999. Molecular biology of aging. Cell 96:291-302. [DOI] [PubMed] [Google Scholar]
- 13.Kolter, R., D. Siegele, and A. Tormo. 1993. The stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 47:855-874. [DOI] [PubMed] [Google Scholar]
- 14.Lazar, S. W., M. Almirón, A. Tormo, and R. Kolter. 1998. Role of the Escherichia coli SurA protein in stationary-phase survival. J. Bacteriol. 180:5704-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazar, S. W., and R. Kolter. 1996. SurA assists the folding of Escherichia coli outer membrane proteins. J. Bacteriol. 178:1770-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64:503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyström, T. 1999. Starvation, cessation of growth and bacterial aging. Curr. Opin. Microbiol. 2:214-219. [DOI] [PubMed] [Google Scholar]
- 18.Nyström, T. 2002. Translational fidelity, protein oxidation, and senescence: lessons from bacteria. Ageing Res. Rev. 1:693-703. [DOI] [PubMed] [Google Scholar]
- 19.Siegele, D., and R. Kolter. 1992. Life after log. J. Bacteriol. 174:345-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vulic, M., and R. Kolter. 2002. Alcohol-induced delay of viability loss in stationary-phase cultures of Escherichia coli. J. Bacteriol. 184:2898-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu, H.-S., N. Roberts, F. Singleton, R. Attwell, D. Grimes, and R. Colwell. 1982. Survival and viability of non-culturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microbiol. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 22.Zambrano, M., and R. Kolter. 1996. GASPing for life in stationary phase. Cell 86:181-184. [DOI] [PubMed] [Google Scholar]