Key Points
Question
What are the outcomes of neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo among patients with early triple-negative breast cancer enrolled in East/Southeast Asia (Asia) in the KEYNOTE-522 study?
Findings
In this secondary analysis of a phase 3, double-blind randomized clinical trial, among the 216 patients enrolled in Asia, there was a bigger difference in pathologic complete response with pembrolizumab vs placebo (58.7% vs 40.0%) and better event-free survival (91.2% vs 77.2% at 36 months) compared with the overall study population; safety was as anticipated.
Meaning
Outcomes in the KEYNOTE-522 study for patients enrolled in Asia were consistent with those in the overall study population; these findings support the use of this therapy regimen as standard of care for patients in Asia.
This secondary analysis of the KEYNOTE-522 randomized clinical trial evaluates efficacy and safety outcomes of neoadjuvant and adjuvant pembrolizumab for patients enrolled in East and Southeast Asia.
Abstract
Importance
In the phase 3 KEYNOTE-522 study, addition of pembrolizumab to neoadjuvant chemotherapy followed by adjuvant pembrolizumab significantly increased pathologic complete response (pCR) and event-free survival (EFS) vs neoadjuvant chemotherapy in patients with early triple-negative breast cancer.
Objective
To evaluate efficacy and safety outcomes for patients enrolled in East/Southeast Asia (Asia) in KEYNOTE-522.
Design, Setting, and Participants
KEYNOTE-522, a multicenter, double-blind, randomized clinical trial, enrolled 1174 patients between March 7, 2017, and September 13, 2018. For interim EFS and overall survival (OS) analyses (data cutoff, March 23, 2021), median follow-up was 39.8 months (range, 30.4-46.9 months) for pembrolizumab plus chemotherapy and 40.8 months (range, 30.1-46.9 months) for placebo plus chemotherapy. Data cutoff for pCR analysis was September 24, 2018. This secondary analysis included adults enrolled in Asia with newly diagnosed, previously untreated, nonmetastatic triple-negative breast cancer (tumor stage T1c and nodal stage N1-2 or tumor stage T2-4 and nodal stage N0-2) and Eastern Cooperative Oncology Group performance status of 0 to 1, regardless of programmed cell death ligand 1 (PD-L1) status.
Intervention
Patients were randomized 2:1 to 4 cycles of pembrolizumab (200 mg every 3 weeks) or placebo plus carboplatin and paclitaxel and another 4 cycles of pembrolizumab or placebo plus doxorubicin or epirubicin and cyclophosphamide before surgery. After definitive surgery, patients received pembrolizumab or placebo every 3 weeks for 9 cycles or until recurrence or unacceptable toxic effects.
Main Outcomes and Measures
The main outcome was pCR (no evidence of primary tumor after neoadjuvant therapy or carcinoma in situ after neoadjuvant therapy and no regional lymph node involvement after neoadjuvant therapy) at the time of definitive surgery and EFS.
Results
A total of 216 of 1174 randomized patients (all female; median [range] age, 46.0 [24.0-71.0] years) were from Korea, Japan, Taiwan, and Singapore (136 in the pembrolizumab plus chemotherapy group and 80 in the placebo plus chemotherapy group). Of these patients, 104 (76.5%) in the pembrolizumab plus chemotherapy group and 60 (75.0%) in the placebo plus chemotherapy group had a tumor PD-L1 combined positive score of 1 or greater. Pathologic complete response was 58.7% (95% CI, 46.7%-69.9%) with pembrolizumab plus chemotherapy and 40.0% (95% CI, 26.4%-54.8%) with placebo plus chemotherapy; benefit was observed regardless of PD-L1 status. Thirteen patients (9.6%) in the pembrolizumab plus chemotherapy group and 20 patients (25.0%) in the placebo plus chemotherapy group had EFS events (hazard ratio, 0.35; 95% CI, 0.17-0.71). The 36-month EFS rate was 91.2% (95% CI, 85.0%-94.9%) with pembrolizumab plus chemotherapy and 77.2% (95% CI, 66.3%-85.0%) with placebo plus chemotherapy. Grade 3 to 4 treatment-related adverse events occurred in 109 patients (80.1%) receiving pembrolizumab plus chemotherapy and 64 patients (81.0%) receiving placebo plus chemotherapy.
Conclusions and Relevance
In this subgroup analysis of patients enrolled in Asia in KEYNOTE-522, neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab led to clinically meaningful improvements in pCR and EFS vs neoadjuvant chemotherapy alone. These findings support the use of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab as a standard-of-care therapy for patients in Asian countries with early triple-negative breast cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT03036488
Introduction
Triple-negative breast cancer (TNBC) has a poor prognosis and is recognized as the most difficult-to-treat breast cancer subtype.1,2 Neoadjuvant chemotherapy is standard-of-care treatment for early TNBC.3,4,5 The short-term goal of such therapy is to achieve a pathologic complete response (pCR) because of its association with significant improvements in long-term clinical outcomes. A meta-analysis of 25 studies in early TNBC demonstrated an 81% lower risk of death and a 76% lower risk of progression, recurrence, or death among patients who attain a pCR after neoadjuvant chemotherapy.6 However, median pCR rates were only 35.0% (range, 16.7%-67.0%) among the cohort studies and 41.0% (range, 26.7%-62.0%) among the clinical trials included in the meta-analysis.6 These findings underscore the need for new treatment approaches for patients with early TNBC to reduce disease recurrence and prolong survival.
In the phase 3 KEYNOTE-522 study (Study of Pembrolizumab [MK-3475] Plus Chemotherapy vs Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs Placebo as Adjuvant Therapy in Participants With Triple Negative Breast Cancer), addition of the anti–programmed cell death protein 1 monoclonal antibody pembrolizumab to neoadjuvant chemotherapy followed by adjuvant pembrolizumab significantly increased pCR and event-free survival (EFS) vs neoadjuvant chemotherapy alone in patients with early TNBC. In the overall study population, the pCR rate was 64.8% (95% CI, 59.9%-69.5%) in the pembrolizumab and neoadjuvant chemotherapy group vs 51.2% (95% CI, 44.1%-58.3%) in the neoadjuvant chemotherapy alone group (P < .001 for between-group difference),7 and EFS at 36 months was 84.5% (95% CI, 81.7%-86.9%) in the pembrolizumab and neoadjuvant chemotherapy group vs 76.8% (95% CI, 72.2%-80.7%) in the neoadjuvant chemotherapy alone group (hazard ratio [HR], 0.63; 95% CI, 0.48-0.82; P < .001).8 Benefits were observed regardless of tumor programmed cell death ligand 1 (PD-L1) expression.7,8 On the basis of these results, neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab after surgery for the treatment of patients with high-risk, early-stage TNBC has received regulatory approval in several countries.9,10,11
The incidence of breast cancer has decreased over the past 2 decades in the US and most of Europe but has increased in many countries in Asia, with mean annual increases of 0.37% in Singapore, 1.28% in Japan, 2.65% in Korea, and 2.76% in China.12 Although there has been evidence of decreasing breast cancer mortality in the US and Europe, mortality rates have remained stable or have increased across many countries in Asia.12,13 In 2020 alone, it was estimated that more than 1 million women were diagnosed with breast cancer in Asian countries and nearly 350 000 women died of breast cancer, accounting for 45.4% of global breast cancer cases and 50.5% of global breast cancer deaths.14 Additionally, evidence suggests that the genomic profile and tumor microenvironment can differ between Asian and non-Asian patients with breast cancer,15,16,17,18 which could, in turn, influence responses to treatment. Considering these potential differences and the high burden of breast cancer in the Asia population,19 we conducted a subgroup analysis to evaluate efficacy and safety outcomes for patients enrolled in countries in East and Southeast Asia (hereafter referred to as Asia) in KEYNOTE-522.
Methods
Study Design and Patients
KEYNOTE-522 is an ongoing phase 3, double-blind, placebo-controlled, international randomized clinical trial that enrolled 1174 patients between March 7, 2017, and September 13, 2018. Detailed methods were previously published7,8 and are available in the protocol (Supplement 1). Demographic information, including race and ethnicity, was previously reported for the entire study population in the primary publication.7,8 The inclusion criterion for the current subgroup analysis was geographically specific and thus not broken down by race or ethnicity. Briefly, eligible patients were 18 years or older with centrally confirmed, newly diagnosed, previously untreated, nonmetastatic TNBC (tumor stage T1c, nodal stage N1-2 or tumor stage T2-4, nodal stage N0-2 per the American Joint Committee on Cancer20) as determined by investigator radiologic or clinical assessment. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 and a tissue sample available for PD-L1 assessment. Patients were eligible for the study regardless of PD-L1 expression. An external, independent data monitoring committee oversaw the study, periodically assessed safety, and assessed efficacy at prespecified interim analyses. The study protocol and its amendments were approved by institutional review boards or independent ethics committees at each study site. The study was conducted in accordance with the standards of Good Clinical Practice. All patients provided written informed consent before enrollment. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Randomization and Study Treatment
Patients were stratified before randomization based on nodal status (positive or negative), tumor size (T1-T2 or T3-T4), and schedule of carboplatin administration (weekly or every 3 weeks). Randomization (block size of 6) was performed using a central interactive voice-response system with an integrated Web-response system from March 23, 2017, to September 23, 2018, with a data cutoff date for interim EFS and overall survival (OS) of March 24, 2021. Patients, investigators, the sponsor, and other study site staff were blinded to treatment assignment. A total of 1174 patients were randomized 2:1 to receive pembrolizumab or placebo. In the first neoadjuvant phase (cycles 1-4), patients received intravenous pembrolizumab (200 mg every 3 weeks) or placebo in combination with carboplatin (area under the concentration time curve, 5 mg/mL/min every 3 weeks or 1.5 mg/mL/min weekly) plus paclitaxel (80 mg/m2 weekly). In the second neoadjuvant phase (cycles 5-8), patients continued treatment with pembrolizumab or placebo in combination with doxorubicin or epirubicin (60 mg/m2 or 90 mg/m2, respectively, every 3 weeks) and cyclophosphamide (600 mg/m2 every 3 weeks). After surgery, patients received adjuvant therapy with intravenous pembrolizumab (200 mg every 3 weeks) or placebo for 9 cycles. Patients who completed or discontinued the first neoadjuvant treatment could start the second neoadjuvant treatment or undergo surgery; patients who completed or discontinued the second neoadjuvant treatment could proceed to surgery. Adjuvant therapy with capecitabine was not permitted. Patients discontinued treatment if they had disease progression, recurrence, or unacceptable adverse events (AEs).
End Points
The study’s primary end points were pCR (no evidence of primary tumor after neoadjuvant therapy or carcinoma in situ after neoadjuvant therapy [ypT0/Tis] and no regional lymph node involvement after neoadjuvant therapy [ypN0]) at the time of definitive surgery and EFS. Secondary end points included pCR (ypT0/Tis ypN0) and EFS in patients who had tumors that were PD-L1 positive, pCR (ypT0 ypN0) and pCR (ypT0/Tis) in all patients and in patients who had tumors that were PD-L1 positive, OS, and safety.
Assessments
After completion of neoadjuvant therapy, pCR was assessed according to the definitions of the pathologic stages ypT0/Tis ypN0, ypT0 ypN0, and ypT0/Tis, as determined by a local pathologist who was blinded to treatment group assignment. Follow-up for disease status and survival occurred every 3 months for the first 2 years, every 6 months from years 3 to 5, and yearly thereafter. Event-free survival was assessed by the investigator and defined as time from randomization to first occurrence of disease progression that precluded definitive surgery, local or distant recurrence, a second primary cancer, or death from any cause. Overall survival was defined as time from randomization to death from any cause.
Expression of PD-L1 in tumor samples was determined using immunohistochemical analysis (PD-L1 IHC 22C3 pharmDx, Agilent Technologies). The combined positive score (CPS) was defined as the number of PD-L1–positive cells (tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells multiplied by 100. Tumors with a CPS of 1 or greater were considered PD-L1 positive.
Adverse events were monitored throughout the study and for 30 days after discontinuation of treatment (90 days for serious AEs). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Immune-mediated AEs were based on a prespecified list of Medical Dictionary for Regulatory Activities terms.
Statistical Analysis
The study was powered to test hypotheses in the global population; no α was assigned to analyses in the subgroup of patients enrolled in Asia; therefore, the results reported herein are descriptive only. Efficacy was assessed in the intention-to-treat population (all randomized patients); safety was assessed in all randomized and treated patients based on the treatment received. For pCR, the estimated treatment difference was based on the unstratified Miettinen and Nurminen method; patients without pCR data for any reason or who received neoadjuvant chemotherapy not specified in the protocol were counted as non-pCR. For EFS and OS, HRs and associated 95% CIs were estimated based on an unstratified Cox proportional hazards regression model with the Efron method of tie handling and treatment as a covariate. Analyses were conducted using SAS version 9.4 (SAS Institute).
Results
Patients
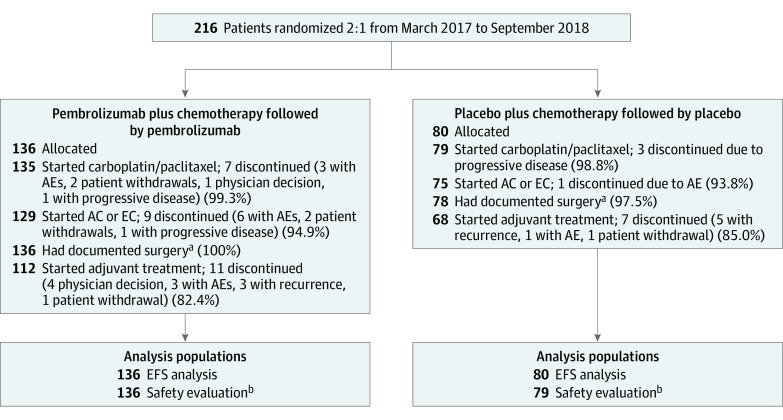
Of 1174 patients randomized in KEYNOTE-522 between March 23, 2017, and September 24, 2018, the subgroup of patients enrolled in Asia included 216 patients (18.4%; 136 receiving pembrolizumab plus chemotherapy and 80 receiving placebo plus chemotherapy) in Korea (n = 86), Japan (n = 76), Taiwan (n = 50), and Singapore (n = 4). The list of countries that enrolled patients in the global KEYNOTE-522 study is provided in eTable 1 in Supplement 2. Patient disposition and analysis populations are shown in Figure 1. Patient demographics and baseline disease characteristics were generally similar between the treatment groups (Table 1). All patients were female, and the median age was 46.0 years (range, 24.0-71.0 years). Of the 216 patients, 189 (87.5%) had an ECOG performance status score of 0. A total of 164 patients (75.9%) had tumors that were PD-L1 positive (ie, PD-L1 CPS ≥1), including 104 (76.5%) in the pembrolizumab plus chemotherapy group and 60 (75.0%) in the placebo plus chemotherapy group; 175 patients (81.0%) had a baseline tumor size of T1 or T2. At the data cutoff date for the interim EFS and OS analyses (March 23, 2021), median time from randomization to data cutoff was 39.8 months (range, 30.4-46.9 months) in the pembrolizumab plus chemotherapy group and 40.8 months (range, 30.1-46.9 months) in the placebo plus chemotherapy group.
Figure 1. Patient Disposition and Analysis Populations: Subgroup of Patients Enrolled in Asia.
AC indicates doxorubicin-cyclophosphamide; AE, adverse event; EC, epirubicin-cyclophosphamide; and EFS, event-free survival.
aPatients did not have to complete all neoadjuvant therapy to undergo surgery.
bIncludes all patients who received at least 1 dose of study treatment or underwent surgery.
Table 1. Patient Demographics and Baseline Characteristics: Subgroup of Patients Enrolled in Asiaa.
| Characteristic | Pembrolizumab plus chemotherapy (n = 136) | Placebo plus chemotherapy (n = 80) |
|---|---|---|
| Sex | ||
| Female | 136 (100.0) | 80 (100.0) |
| Male | 0 | 0 |
| Age, median (range), y | 46.0 (26.0-71.0) | 47.0 (24.0-71.0) |
| Aged <65 y | 129 (94.9) | 71 (88.8) |
| Menopausal status | ||
| Premenopausal | 85 (62.5) | 43 (53.8) |
| Postmenopausal | 51 (37.5) | 37 (46.3) |
| ECOG PS | ||
| 0 | 118 (86.8) | 71 (88.8) |
| 1 | 18 (13.2) | 9 (11.3) |
| ERBB2 (previously HER2/neu) status | ||
| 0-1+ by IHC | 119 (87.5) | 68 (85.0) |
| 2+ by IHC (but FISH negative) | 16 (11.8) | 12 (15.0) |
| Tumor PD-L1 CPS | ||
| <1 | 32 (23.5) | 19 (23.8) |
| ≥1 | 104 (76.5) | 60 (75.0) |
| ≥10 | 56 (41.2) | 32 (40.0) |
| Carboplatin schedule | ||
| Weekly | 91 (66.9) | 47 (58.8) |
| Every 3 wk | 45 (33.1) | 33 (41.3) |
| Tumor size | ||
| T1/T2 | 109 (80.1) | 66 (82.5) |
| T3/T4 | 27 (19.9) | 14 (17.5) |
| Nodal involvement | ||
| Positive | 64 (47.1) | 41 (51.3) |
| Negative | 72 (52.9) | 39 (48.8) |
| Enrollment location | ||
| Korea | 56 (41.2) | 30 (37.5) |
| Japan | 45 (33.1) | 31 (38.8) |
| Taiwan | 33 (24.3) | 17 (21.3) |
| Singapore | 2 (1.5) | 2 (2.5) |
Abbreviations: CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; PD-L1, programmed cell death ligand 1.
Data are presented as number (percentage) of participants unless otherwise indicated. Data cutoff date was March 23, 2021.
Efficacy
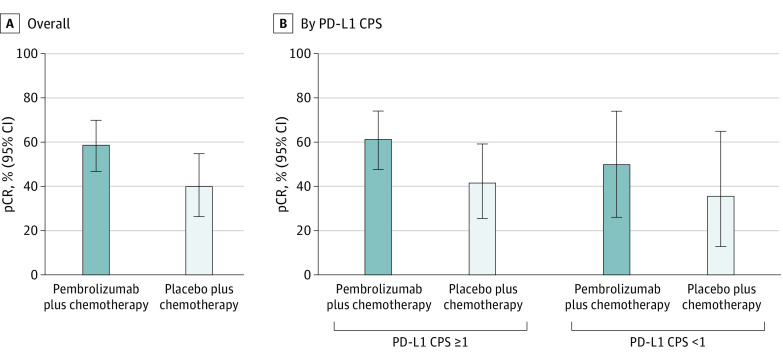
The data cutoff date for the pCR analysis (interim analysis 1) was September 24, 2018. Pathologic complete response was achieved in 44 of 75 patients (58.7%; 95% CI, 46.7%-69.9%) in the pembrolizumab plus chemotherapy group and 20 of 50 patients (40.0%; 95% CI, 26.4%-54.8%) in the placebo plus chemotherapy group, for a treatment difference of 18.7% (95% CI, 0.7%-35.4%) (Figure 2A). Results by PD-L1 expression are shown in Figure 2B. In patients with PD-L1 CPS of 1 or higher, pCR was attained in 35 of 57 patients (61.4%; 95% CI, 47.6%-74.0%) in the pembrolizumab plus chemotherapy group and 15 of 36 patients (41.7%; 95% CI, 25.5%-59.2%) in the placebo plus chemotherapy group, for a treatment difference of 19.7% (95% CI, −1.2% to 39.0%). In patients with PD-L1 CPS less than 1, pCR was achieved in 9 of 18 patients (50.0%) in the pembrolizumab plus chemotherapy group and 5 of 14 patients (35.7%) in the placebo plus chemotherapy group, for a treatment difference of 14.3% (95% CI, −20.4% to 45.3%).
Figure 2. Pathologic Complete Response (pCR) in the Overall Subgroup of Patients Enrolled in Asia and by Programmed Cell Death Ligand 1 (PD-L1) Combined Positive Score (CPS).
Pathologic complete response was defined as no evidence of primary tumor after neoadjuvant therapy or carcinoma in situ after neoadjuvant therapy and no regional lymph node involvement after neoadjuvant therapy. Data cutoff date was September 24, 2018.
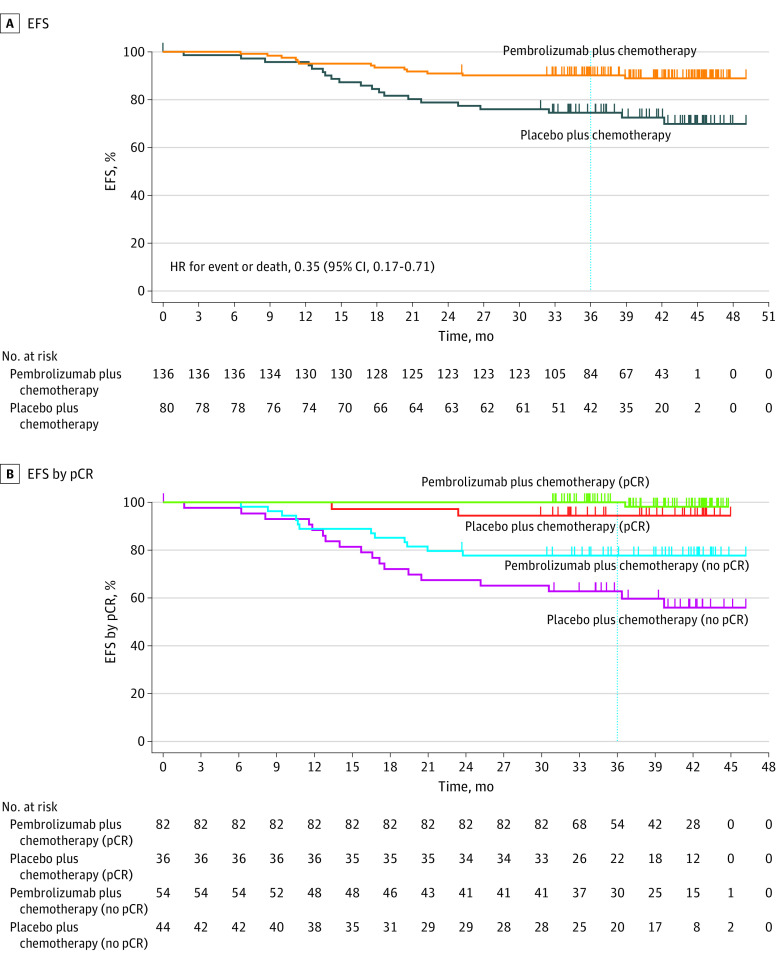
The data cutoff date for the EFS and OS analyses (interim analysis 4) was March 23, 2021. A total of 13 of 136 patients (9.6%) in the pembrolizumab plus chemotherapy group and 20 of 80 patients (25.0%) in the placebo plus chemotherapy group experienced an EFS event (HR, 0.35; 95% CI, 0.17-0.71) (eTable 2 in Supplement 2; Figure 3A). The most common first EFS event in both treatment groups was distant recurrence, which occurred in 8 patients (5.9%) in the pembrolizumab plus chemotherapy group and 15 patients (18.8%) in the placebo plus chemotherapy group (eTable 2 in Supplement 2). Median EFS was not reached in either treatment group. At 36 months, the EFS rate was 91.2% (95% CI, 85.0%-94.9%) in the pembrolizumab plus chemotherapy group and 77.2% (95% CI, 66.3%-85.0%) in the placebo plus chemotherapy group (Figure 3A).
Figure 3. Event-Free Survival (EFS) in the Overall Subgroup of Patients Enrolled in Asia and by Pathologic Complete Response (pCR).
Pathologic complete response was defined as no evidence of primary tumor after neoadjuvant therapy or carcinoma in situ after neoadjuvant therapy and no regional lymph node involvement after neoadjuvant therapy. Data cutoff date was March 23, 2021.
Event-free survival according to the outcome (yes or no) of pCR (ypT0/Tis ypN0) was a prespecified, nonrandomized, exploratory analysis. In patients with a pCR, 1 of 82 patients (1.2%) experienced an EFS event and the 36-month EFS rate was 100% (95% CI, 100%-100%) in the pembrolizumab plus chemotherapy group, and 2 of 36 patients (5.6%) experienced an EFS event and the 36-month EFS rate was 94.4% (95% CI, 79.6%-98.6%) in the placebo plus chemotherapy group (Figure 3B). In patients without a pCR, 12 of 54 patients (22.2%) in the pembrolizumab plus chemotherapy group had an EFS event, and the 36-month EFS rate was 77.7% (95% CI, 64.1%-86.7%); 18 of 44 patients (40.9%) in the placebo plus chemotherapy group had an EFS event, and the 36-month EFS rate was 62.8% (95% CI, 46.6%-75.3%).
Six of 136 patients (4.4%) died in the pembrolizumab plus chemotherapy group, and 14 of 80 patients (17.5%) died in the placebo plus chemotherapy group (HR, 0.24; 95% CI, 0.09-0.62) (eFigure in Supplement 2). The 36-month OS rate was 95.6% (95% CI, 90.4%-98.0%) in the pembrolizumab plus chemotherapy group and 81.6% (95% CI, 70.9%-88.7%) in the placebo plus chemotherapy group.
Safety
The data cutoff date for the safety analysis (interim analysis 4) was March 23, 2021. In the combined neoadjuvant and adjuvant phases, treatment-related AEs of any grade were reported in 135 of 136 patients (99.3%) in the pembrolizumab plus chemotherapy group and 79 of 79 patients (100%) in the placebo plus chemotherapy group. The most common treatment-related AEs in patients who received pembrolizumab were alopecia, nausea, and anemia (Table 2). Grade 3 or 4 treatment-related AEs were experienced by 109 patients (80.1%) in the pembrolizumab plus chemotherapy group and 64 patients (81.0%) in the placebo plus chemotherapy group. There were no treatment-related AEs leading to death. Treatment-related AEs leading to discontinuation of any study treatment occurred in 19 patients (14.0%) in the pembrolizumab plus chemotherapy group and 7 patients (8.9%) in the placebo plus chemotherapy group. In the adjuvant phase, treatment-related AEs of any grade occurred in 40 of 112 patients (35.7%) in the pembrolizumab plus chemotherapy group and 22 of 68 patients (32.4%) in the placebo plus chemotherapy group. Grade 3 or 4 treatment-related AEs occurred in 6 patients (5.4%) in the pembrolizumab plus chemotherapy group and 1 patient (1.5%) in the placebo plus chemotherapy group, and treatment-related AEs of any grade that led to discontinuation of study treatment occurred in 1 patient (0.9%) in the pembrolizumab plus chemotherapy group and 0 patients in the placebo plus chemotherapy group.
Table 2. Summary of AEs in Combined and Adjuvant Phases: Subgroup of Patients Enrolled in Asiaa.
| AE | Pembrolizumab plus chemotherapyb | Placebo plus chemotherapyc | ||
|---|---|---|---|---|
| Any grade | Grade 3 or 4d | Any grade | Grade 3 or 4d | |
| Combined phases | ||||
| Treatment-related AEs | 135 (99.3) | 109 (80.1) | 79 (100.0) | 64 (81.0) |
| Led to discontinuation of any study drug | 19 (14.0) | 12 (8.8) | 7 (8.9) | 5 (6.3) |
| Most common AEs (≥20% in either treatment group) | ||||
| Alopecia | 111 (81.6) | 0 | 59 (74.7) | 0 |
| Nausea | 93 (68.4) | 2 (1.5) | 49 (62.0) | 0 |
| Anemia | 76 (55.9) | 30 (22.1) | 40 (50.6) | 10 (12.7) |
| Peripheral sensory neuropathy | 75 (55.1) | 3 (2.2) | 39 (49.4) | 3 (3.8) |
| Neutrophil count decreased | 70 (51.5) | 61 (44.9) | 42 (53.2) | 34 (43.0) |
| Rash | 53 (39.0) | 3 (2.2) | 14 (17.7) | 1 (1.3) |
| Constipation | 52 (38.2) | 0 | 27 (34.2) | 0 |
| Pyrexia | 46 (33.8) | 3 (2.2) | 15 (19.0) | 0 |
| ALT level increased | 44 (32.4) | 9 (6.6) | 24 (30.4) | 3 (3.8) |
| White blood cell count decreased | 38 (27.9) | 24 (17.6) | 21 (26.6) | 11 (13.9) |
| Neutropenia | 38 (27.9) | 37 (27.2) | 24 (30.4) | 24 (30.4) |
| Vomiting | 37 (27.2) | 0 | 17 (21.5) | 1 (1.3) |
| Fatigue | 35 (25.7) | 0 | 14 (17.7) | 1 (1.3) |
| Myalgia | 35 (25.7) | 0 | 23 (29.1) | 0 |
| Pruritus | 34 (25.0) | 0 | 12 (15.2) | 0 |
| Decreased appetite | 33 (24.3) | 3 (2.2) | 14 (17.7) | 1 (1.3) |
| Stomatitis | 33 (24.3) | 0 | 16 (20.3) | 0 |
| AST level increased | 30 (22.1) | 3 (2.2) | 16 (20.3) | 1 (1.3) |
| Diarrhea | 29 (21.3) | 0 | 10 (12.7) | 0 |
| Febrile neutropenia | 29 (21.3) | 29 (21.3) | 12 (15.2) | 12 (15.2) |
| Immune-mediated AEs and infusion reactions | 50 (36.8) | 17 (12.5) | 17 (21.5) | 1 (1.3) |
| Led to discontinuation of any study drug | 9 (6.6) | 7 (5.1) | 1 (1.3) | 0 |
| Hypothyroidism | 19 (14.0) | 0 | 5 (6.3) | 0 |
| Infusion reactions | 17 (12.5) | 3 (2.2) | 7 (8.9) | 0 |
| Hyperthyroidism | 8 (5.9) | 1 (0.7) | 1 (1.3) | 0 |
| Severe skin reactions | 8 (5.9) | 6 (4.4) | 3 (3.8) | 1 (1.3) |
| Pneumonitis | 6 (4.4) | 2 (1.5) | 2 (2.5) | 0 |
| Thyroiditis | 4 (2.9) | 0 | 1 (1.3) | 0 |
| Vasculitis | 4 (2.9) | 0 | 0 | 0 |
| Adrenal insufficiency | 2 (1.5) | 1 (0.7) | 0 | 0 |
| Colitis | 2 (1.5) | 0 | 0 | 0 |
| Pancreatitis | 2 (1.5) | 2 (1.5) | 0 | 0 |
| Type 1 diabetes | 2 (1.5) | 2 (1.5) | 0 | 0 |
| Myocarditis | 1 (0.7) | 1 (0.7) | 0 | 0 |
| Hypophysitis | 0 | 0 | 1 (1.3) | 0 |
| Adjuvant phase | ||||
| Treatment-related AEs | 40 (35.7) | 6 (5.4) | 22 (32.4) | 1 (1.5) |
| Led to discontinuation of study drug | 1 (0.9) | 1 (0.9) | 0 | 0 |
| Most common (≥2% in either treatment group) | ||||
| Pruritus | 5 (4.5) | 0 | 2 (2.9) | 0 |
| Rash | 5 (4.5) | 1 (0.9) | 1 (1.5) | 0 |
| ALT level increased | 3 (2.7) | 0 | 1 (1.5) | 0 |
| Arthralgia | 3 (2.7) | 0 | 2 (2.9) | 0 |
| Fatigue | 3 (2.7) | 0 | 0 | 0 |
| Hypothyroidism | 1 (0.9) | 0 | 3 (4.4) | 0 |
| Neutrophil count decreased | 1 (0.9) | 0 | 2 (2.9) | 0 |
| Ejection fraction decreased | 0 | 0 | 2 (2.9) | 1 (1.5) |
| Headache | 0 | 0 | 3 (4.4) | 0 |
| Rash (maculopapular) | 0 | 0 | 4 (5.9) | 0 |
| White blood cell count decreased | 0 | 0 | 2 (2.9) | 0 |
| Immune-mediated AEs and infusion reactions | 8 (7.1) | 4 (3.6) | 4 (5.9) | 0 |
| Led to discontinuation of any study drug | 1 (0.9) | 1 (0.9) | 0 | 0 |
| Pneumonitis | 3 (2.7) | 1 (0.9) | 0 | 0 |
| Severe skin reactions | 2 (1.8) | 1 (0.9) | 0 | 0 |
| Hypothyroidism | 1 (0.9) | 0 | 3 (4.4) | 0 |
| Infusion reactions | 1 (0.9) | 0 | 0 | 0 |
| Pancreatitis | 1 (0.9) | 1 (0.9) | 0 | 0 |
| Type 1 diabetes | 1 (0.9) | 1 (0.9) | 0 | 0 |
| Hyperthyroidism | 0 | 0 | 1 (1.5) | 0 |
| Thyroiditis | 0 | 0 | 1 (1.5) | 0 |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Data are presented as number (percentage) of participants unless otherwise indicated. Data cutoff date was March 23, 2021.
Includes 136 patients in the combined phase and 112 patients in the adjuvant phase.
Includes 79 patients in the combined phase and 68 patients in the adjuvant phase.
There were no treatment-related AEs leading to death.
Immune-mediated AEs and infusion reactions in the combined neoadjuvant and adjuvant phases were reported in 50 of 136 patients (36.8%) in the pembrolizumab plus chemotherapy group and 17 of 79 patients (21.5%) in the placebo plus chemotherapy group; the most common events in patients who received pembrolizumab were hypothyroidism, infusion reactions, hyperthyroidism, and severe skin reactions (Table 2). Grade 3 or 4 immune-mediated AEs and infusion reactions were experienced by 17 patients (12.5%) in the pembrolizumab plus chemotherapy group and 1 patient (1.3%) in the placebo plus chemotherapy group. Immune-mediated AEs and infusion reactions leading to discontinuation of any study treatment occurred in 9 patients (6.6%) in the pembrolizumab plus chemotherapy group and 1 patient (1.3%) in the placebo plus chemotherapy group. In the adjuvant phase, immune-mediated AEs and infusion reactions of any grade occurred in 8 patients (7.1%) in the pembrolizumab plus chemotherapy group and 4 patients (5.9%) in the placebo plus chemotherapy group. Grade 3 or 4 immune-mediated AEs and infusion reactions occurred in 4 patients (3.6%) in the pembrolizumab plus chemotherapy group and 0 patients in the placebo plus chemotherapy group, and immune-mediated AEs and infusion reactions of any grade that led to discontinuation of study treatment occurred in 1 patient (0.9%) in the pembrolizumab plus chemotherapy group and 0 patients in the placebo plus chemotherapy group.
Discussion
KEYNOTE-522 is the first prospective, phase 3 randomized clinical study of pembrolizumab in patients with early TNBC in the neoadjuvant and adjuvant settings. Results of this subgroup analysis of patients enrolled in countries in Asia showed that treatment with neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab led to clinically meaningful improvements in pCR and EFS compared with neoadjuvant chemotherapy alone. Adverse events were consistent with the known safety profiles of each agent.
Importantly, efficacy outcomes in the subgroup of patients enrolled in Asia were generally consistent with those of the overall study population. Although both the overall population and the subgroup had higher pCR in the pembrolizumab plus chemotherapy group than in the placebo plus chemotherapy group, pCR was numerically lower among patients enrolled in Asia (58.7% vs 40.0%) than the overall study population (64.8% vs 51.2%).7 However, the between-group difference in pCR in favor of pembrolizumab plus chemotherapy was numerically larger in patients enrolled in Asia relative to the overall study population (18.7% vs 13.6%).7 The prespecified, nonrandomized, exploratory analysis of EFS was conducted according to the outcome (yes or no) of pCR (ypT0/Tis ypN0). The proportion of patients in the pembrolizumab plus chemotherapy group with an improvement in EFS at 36 months vs placebo plus chemotherapy was numerically higher in the subgroup of patients enrolled in Asia (91.2% for pembrolizumab plus chemotherapy and 77.2% for placebo plus chemotherapy) than in the overall study population (84.5% for pembrolizumab plus chemotherapy and 76.8% for placebo plus chemotherapy).8 At the time of this analysis, data on OS were immature, and further follow-up is ongoing. Thus far, favorable trends in OS have been observed in the pembrolizumab plus chemotherapy group in both the overall study population8 and in the subgroup of patients enrolled in Asia.
The types and severity of treatment-related AEs were similar in both the overall study population8 and in the subgroup of patients enrolled in Asia during the combined neoadjuvant and adjuvant phases. In both populations, the most common treatment-related AEs of any grade in the pembrolizumab plus chemotherapy group were nausea, alopecia, and anemia, and the most frequent treatment-related AEs of grade 3 or greater were neutropenia and decreased neutrophil count.8 In both treatment groups, the rate of discontinuation due to treatment-related AEs was somewhat lower in the subgroup of patients enrolled in Asia (placebo, 8.9%; pembrolizumab, 14.0%) compared with the overall study population (placebo, 14.1%; pembrolizumab, 27.7%)8 during the combined neoadjuvant and adjuvant phases. Additionally, the incidence of pneumonitis was not substantially elevated in the subgroup of patients enrolled in Asia compared with the overall study population. This finding suggests that tolerability was at least as good in the subgroup of patients enrolled in Asia compared with the overall study population.
Other studies have evaluated the addition of PD-L1 inhibitors to neoadjuvant chemotherapy in patients with early TNBC. In the phase 3 IMpassion031 study (Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer), 88 of 333 patients (26%) were Asian.21 In that subgroup, pCR was achieved in 57% of patients in the atezolizumab plus chemotherapy group (n = 47) and 34% of patients in the placebo plus chemotherapy group (n = 41), for a treatment difference of 23% (95% CI, 3%-44%). The EFS and OS data were immature at the time of analysis.21 The pCR results from IMpassion031 are consistent with the analysis described herein. Results from other studies evaluating the addition of a PD-L1 inhibitor to neoadjuvant chemotherapy in patients with early TNBC include the phase 2 I-SPY2 (Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer) (durvalumab, olaparib, and chemotherapy),22 GeparNuevo (Addition of PD-L1 Antibody MEDI4736 to a Taxane-Anthracycline Chemotherapy in Triple Negative Breast Cancer) (durvalumab priming then durvalumab and chemotherapy),23 and the phase 3 NeoTRIPaPDL1 study (Neoadjuvant Therapy in TRIPle Negative Breast Cancer With antiPDL1) (atezolizumab and chemotherapy).24 However, in these latter studies, fewer patients received combination therapy, and results were not available in patients from Asian countries.
Analysis of biomarkers in future studies could potentially provide insight into our findings. The current results complement those seen with pembrolizumab in the phase 3 KEYNOTE-355 study, which enrolled patients with previously untreated, locally recurrent and inoperable, or metastatic TNBC. In this setting, among the 160 patients enrolled in countries in Asia, treatment with pembrolizumab plus chemotherapy led to clinically meaningful improvement in OS and PFS compared with placebo plus chemotherapy overall and in patients with PD-L1 CPS of 1 or greater tumors.25
Limitations
This study has certain limitations. This secondary analysis was exploratory, and no formal statistical testing was performed. Furthermore, this analysis included the subgroup of patients enrolled in certain Asian countries (Korea, Japan, Taiwan, and Singapore) that were defined by geographic region of enrollment. As such, generalizability of the current results beyond these countries should be approached with caution.
Conclusions
In this secondary analysis of patients enrolled in KEYNOTE-522 in East and Southeast Asia, neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab led to clinically meaningful improvements vs neoadjuvant chemotherapy alone. These results support the use of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab as a standard-of-care therapy in patients in countries in Asia with early TNBC, consistent with the global population.
Trial Protocol and Statistical Analysis Plan
eTable 1. List of Countries that Enrolled Patients in the Global KEYNOTE-522 Study
eTable 2. First EFS Events by Category: Subgroup of Patients Enrolled in Asia
eFigure. Overall Survival: Subgroup of Patients Enrolled in Asia
Data Sharing Statement
References
- 1.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619-626. doi: 10.1158/1055-9965.EPI-17-0627 [DOI] [PubMed] [Google Scholar]
- 2.Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176-198. doi: 10.1158/2159-8290.CD-18-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Kyriakides S, Ohno S, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220. doi: 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 4.Park YH, Senkus-Konefka E, Im SA, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with early breast cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31(4):451-469. doi: 10.1016/j.annonc.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 5.Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485-1505. doi: 10.1200/JCO.20.03399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang M, O’Shaughnessy J, Zhao J, et al. Association of pathologic complete response with long-term survival outcomes in triple-negative breast cancer: a meta-analysis. Cancer Res. 2020;80(24):5427-5434. doi: 10.1158/0008-5472.CAN-20-1792 [DOI] [PubMed] [Google Scholar]
- 7.Schmid P, Cortes J, Pusztai L, et al. ; KEYNOTE-522 Investigators . Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 8.Schmid P, Cortes J, Dent R, et al. ; KEYNOTE-522 Investigators . Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556-567. doi: 10.1056/NEJMoa2112651 [DOI] [PubMed] [Google Scholar]
- 9.KEYTRUDA® (pembrolizumab). Full Prescribing Information, Merck Sharp & Dohme LLC; 2022. [Google Scholar]
- 10.European Commission approves KEYTRUDA® (pembrolizumab) plus chemotherapy as neoadjuvant treatment, then continued as adjuvant monotherapy after surgery for locally advanced or early-stage triple-negative breast cancer at high risk of recurrence. May 24, 2022. Accessed October 19, 2022. https://www.merck.com/news/european-commission-approves-keytruda-pembrolizumab-plus-chemotherapy-as-neoadjuvant-treatment-then-continued-as-adjuvant-monotherapy-after-surgery-for-locally-advanced-or-early-stage-triple/
- 11.Merck’s KEYTRUDA® (pembrolizumab) receives four new approvals in Japan, including in high-risk earlystage triple-negative breast cancer (TNBC). September 27, 2022. Accessed October 27, 2022. https://www.merck.com/news/mercks-keytruda-pembrolizumab-receives-four-new-approvals-in-japan-including-in-high-risk-early-stage-triple-negative-breast-cancer-tnbc/
- 12.Luo C, Li N, Lu B, et al. Global and regional trends in incidence and mortality of female breast cancer and associated factors at national level in 2000 to 2019. Chin Med J (Engl). 2022;135(1):42-51. doi: 10.1097/CM9.0000000000001814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1495-1506. doi: 10.1158/1055-9965.EPI-15-0535 [DOI] [PubMed] [Google Scholar]
- 14.Lei S, Zheng R, Zhang S, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). 2021;41(11):1183-1194. doi: 10.1002/cac2.12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo TK, Lee WS, Kim J, et al. Mutational analysis of triple-negative breast cancer using targeted kinome sequencing. J Breast Cancer. 2022;25(3):164-177. doi: 10.4048/jbc.2022.25.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH, Lu YS, Cheng AL, et al. Disparity in tumor immune microenvironment of breast cancer and prognostic impact: Asian versus Western populations. Oncologist. 2020;25(1):e16-e23. doi: 10.1634/theoncologist.2019-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niyomnaitham S, Parinyanitikul N, Roothumnong E, et al. Tumor mutational profile of triple negative breast cancer patients in Thailand revealed distinctive genetic alteration in chromatin remodeling gene. PeerJ. 2019;7:e6501. doi: 10.7717/peerj.6501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan Z, Ding Y, Kim J, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9(1):1725. doi: 10.1038/s41467-018-04129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yap YS, Lu YS, Tamura K, et al. Insights Into breast cancer in the East vs the West: a review. JAMA Oncol. 2019;5(10):1489-1496. doi: 10.1001/jamaoncol.2019.0620 [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 21.Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090-1100. doi: 10.1016/S0140-6736(20)31953-X [DOI] [PubMed] [Google Scholar]
- 22.Pusztai L, Yau C, Wolf DM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell. 2021;39(7):989-998.e5. doi: 10.1016/j.ccell.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279-1288. doi: 10.1093/annonc/mdz158 [DOI] [PubMed] [Google Scholar]
- 24.Gianni L, Huang CS, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33(5):534-543. doi: 10.1016/j.annonc.2022.02.004 [DOI] [PubMed] [Google Scholar]
- 25.Takano T, Cortes J, Cescon DW, et al. KEYNOTE-355 Asian subset: pembrolizumab + chemotherapy vs placebo + chemotherapy for triple-negative breast cancer. Presented at: Japanese Society of Medical Oncology (JSMO); February 17–19, 2022; Kyoto, Japan. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. List of Countries that Enrolled Patients in the Global KEYNOTE-522 Study
eTable 2. First EFS Events by Category: Subgroup of Patients Enrolled in Asia
eFigure. Overall Survival: Subgroup of Patients Enrolled in Asia
Data Sharing Statement