Abstract
Pasteurella multocida is the causative agent of a range of diseases with economic importance in production animals. Many systems have been employed to identify virulence factors of P. multocida, including in vivo expression technology (IVET), signature-tagged mutagenesis, and whole-genome expression profiling. In a previous study in which IVET was used with P. multocida, nrfE was identified as a gene that is preferentially expressed in vivo. In Escherichia coli, nrfE is part of the formate-dependent nitrite reductase system involved in utilizing available nitrite as an electron accepter during growth under anaerobic conditions. In this study, we constructed an isogenic P. multocida strain that was unable to reduce nitrite under either aerobic or anaerobic conditions, thereby demonstrating that P. multocida nrfE is essential for nitrite reduction. However, the nrfE mutant was still virulent in mice. Real-time reverse transcription-PCR analysis indicated that nrfE was regulated independently of nrfABCD by an independent promoter that is likely to be upregulated in vivo.
Pasteurella multocida is a gram-negative bacterial pathogen that is responsible for a number of diseases that are prevalent worldwide, including bovine hemorrhagic septicemia, avian fowl cholera, porcine atrophic rhinitis, and lapine snuffles. The worldwide economic cost of these diseases in production animals is significant, but despite considerable research, safe and effective vaccines against pasteurellosis are still lacking. The molecular mechanisms of P. multocida pathogenesis are still largely unknown, and only a few virulence factors have been identified. These factors include toxins (8), capsule (5, 9), iron acquisition proteins (4, 13, 25), and hemagglutinins (25). Therefore, it is likely that many virulence factors remain uncharacterized. Identification of novel virulence factors could be used to identify new candidate vaccine antigens or targets for antimicrobial compounds.
Numerous methods have been utilized to identify genes expressed during Pasteurella infections, including in vivo expression technology (IVET) (21), signature-tagged mutagenesis (13, 16), and whole-genome expression profiling (6). In the P. multocida IVET study a number of genes that are upregulated in vivo in mice were identified (21). One of these genes, nrfE, was selected for further characterization.
The formate-dependent nitrite reductase (Nrf) system is present in a number of enteric bacteria, including Escherichia coli and Salmonella spp., and in the species closely related to P. multocida, Haemophilus influenzae and Actinobacillus actinomycetemcomitans (28). The Nrf system in E. coli is encoded by a seven-gene operon (nrfABCDEFG) and uses nitrite as an alternate electron acceptor for oxygen during anaerobic growth. nrfA encodes a 50-kDa cytochrome that utilizes nitrite as an electron acceptor, while nrfBCD encode proteins that are essential for electron transfer to the catalytic subunit, NrfA (22). nrfEFG have been proposed to encode proteins that form a heme lyase required for attachment of a heme group to the site of catalysis of NrfA (11, 14, 22). nrfE has been shown to be essential for formate-dependent nitrite reduction in E. coli (11) and has been identified in P. multocida as a gene that is upregulated in vivo during infection (21). For this reason it was of interest to determine what role nrfE plays in the metabolism of P. multocida during growth in vitro and during infection of the host.
In this study, the function of the nrfE gene of P. multocida was characterized by using a strain in which nrfE was inactivated; additionally, an analysis of transcriptional regulation of the P. multocida nrf operon was conducted.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are shown in Table 1. P. multocida and E. coli were grown with aeration at 37°C in brain heart infusion broth (BHI) and 2YT (Oxoid, Hampshire, England), respectively. Kanamycin (50 μg/ml) and tetracycline (2.5 μg/ml for P. multocida and 10 μg/ml for E. coli) were added to solid and liquid media when they were required.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli DH5α | F−endA1 hsdR17(rk− mk+) thi-1 λ−recA1 gyrA96 relA1 Φ80dlacZΔM15 | Bethesda Research Laboratories, Rockville, Md. |
| P. multocida strains | ||
| X-73 | Serotype A:1 wild-type strain | 18 |
| AL362 | X-73 nrfE::tet(M) mutant | This study |
| AL464 | E. coli DH5α harboring pAL263 | This study |
| AL465 | AL362 harboring pAL99 | This study |
| AL466 | AL362 harboring pAL263 | This study |
| Plasmids | ||
| pAL99 | 40-bp EcoRI fragment containing P. multocida tpiA promoter region cloned into pPBA1100 EcoRI site | 17 |
| pAL209 | 2.1-kb insert containing nrfE cloned into pWSK129, Kanr | This study |
| pAL211 | pWSK129 containing the nrfE::tet(M) cassette, Kanr Tetr | This study |
| pAL263 | 2-kb insert containing full nrfE gene in pAL99 (nrfE expression driven by tpiA promoter) | This study |
| pBA1100 | P. multocida-E. coli shuttle vector, pUC18 derivative | 19 |
| pVB101 | tet(M) gene from Tn916 cloned into pBR322, Tetr Ampr | Vickers Burdett, Duke University, Durham, N.C. |
| pWSK129 | Low-copy-number E. coli vector, Kanr | 29 |
Modified CDM for nitrite reduction studies.
A modified version of the chemically defined medium (CDM) described by Jablonski et al. (24) was used for nitrite reduction studies. Unless specifically indicated otherwise, the concentrations of solutions were the concentrations described by Jablonski et al. (24). Briefly, the CDM was prepared as a 5× stock devoid of l-arginine, l-serine, l-glutamic acid, l-phenylalanine, l-leucine, l-isoleucine, l-aspartic acid, l-tyrosine, MgSO4, and glucose but supplemented with 50 g of Casamino Acids (Sigma) per liter. The 5× stock was filter sterilized and diluted to a 1× working concentration by using one of three different filter-sterilized 2.5× base solutions. The first 2.5× base solution (normal CDM) contained l-aspartic acid, l-tyrosine, and MgSO4, while the second base solution was identical except that it was supplemented to give 1× CDM working concentrations of 0.4% glycerol and 40 mM sodium fumarate (glycerol/fumarate CDM). The third solution was the same as the solution used for glycerol/fumarate CDM except that it was supplemented to give final concentrations of 2 mM sodium nitrite and 1 mM sodium nitrate (nitrite/nitrate CDM).
Recombinant DNA techniques.
Genomic DNA was purified by using the cetyltrimethylammonium bromide method (1). Plasmid DNA was purified either by the alkaline lysis method (2) or by using anion-exchange columns (QIAGEN, Hilden, Germany). PCR amplification of DNA was carried out with Taq polymerase by using the reaction conditions specified by the manufacturer (Roche Molecular Biochemicals, Basel, Switzerland). DNA was introduced into E. coli by the chemical transformation method of Hanahan (15) and into P. multocida by the electroporation method of Jablonski et al. (23). DNA sequencing was carried out by using BigDye Ready Reaction DyeDeoxy terminator cycle sequencing kits (Perkin-Elmer, Foster City, Calif.), and the reaction mixtures were analyzed with a 373A DNA sequencing system. Oligonucleotides used in this study are shown in Table 2. Prior to sequencing or cloning, PCR fragments were purified either by polyethylene glycol precipitation or by passage through a QIAGEN PCR purification kit. DNA dam methylation was conducted as specified by the manufacturer (Roche).
TABLE 2.
Oligonucleotides used in this study
| Oligonu- cleotide | Sequence (5′-3′)a | Positionb |
|---|---|---|
| 683 | CAATTTCAATCAATGCTCCCAC | NA |
| 1914 | GCACATGTCGACTGGATTGGGGTAG | 23284-23308 |
| 1915 | GGTAAATCTAGACATAAGCTTGGTTTT | 25218-25190 |
| 2106 | GCCCTTTCCGATAAATTGCAA | 1670646-1670626 |
| 2107 | ATCGCGGCTAATGGTGCTT | 1670549-1670567 |
| 2123 | AATGGCATGTTGGTCTTGTAAAAG-3′ | 20017-20040 |
| 2124 | CCACCACTTGCCCATGTTG | 20112-20094 |
| 2277 | GCCGGTAGTGTTGATGGA | 23172-23189 |
| 2278 | AGCAAGGCGCCTAGTGCC | 25411-25394 |
| 2283 | TTGATAGAGCTCTTCAAATTT | 23510-23530 |
| 2284 | CATACTCATGAGCTCACCTGT | 25494-25474 |
| 2363 | CCATCGCCGTTTATTTGTTCTT | 22553-22574 |
| 2364 | TGCCGTACAACGTATCACATGA | 22677-22656 |
| 2398 | GATTTTTGGCTTTACTGATCGCTT | 23560-23583 |
| 2399 | CGCAGTACCAATCATATACGGC | 23660-23639 |
| 2403 | GGACAAATGACGCTAGCATAAATG | 23449-23472 |
| 2404 | ACATCGCAAAGGCTTTATGCTAC | 23367-23389 |
| 2405 | AGCGTCATTTGTCCTGCATAGA | 23462-23441 |
| 2407 | AAGCCAAAAAACCAAGTTCTGG | 23571-23550 |
| 2442 | CACTATGATCCCAGAACTTGGTTTT | 23540-23564 |
Underlined bases indicate engineered restriction sites.
Position in the Pm70 genome (accession number NC_002663). NA, not applicable.
Construction of an nrfE::tet(M) mutant by allelic exchange.
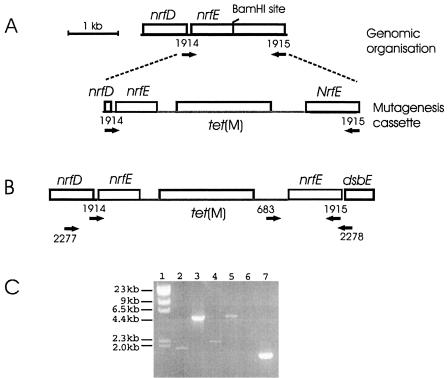
A mutagenesis construct containing the P. multocida nrfE gene disrupted by tet(M) was made (Fig. 1). Oligonucleotides 1914 and 1915 (Table 2) were used to amplify a 2.1-kb DNA fragment by PCR from P. multocida X-73 genomic DNA, and this fragment was then ligated into pWSK129 to generate pAL209 (Table 1). A 3.2-kb BamHI-digested fragment of pVB101 (Table 1) containing tet(M) was cloned into the unique BamHI site in pAL209 to form pAL211. The pAL211 sequence was verified by DNA sequencing, and the plasmid was dam methylated prior to electroporation into P. multocida X-73 (dam methylase; New England Biolabs). Allelic recombinants were selected on BHI agar with tetracycline (2.5 μg/ml). One putative mutant was identified, and the nrfE::tet(M) genotype was confirmed by PCR (Fig. 1C) and Southern hybridization by using an internal nrfE probe generated by PCR performed with the 1914 and 1915 oligonucleotides (data not shown). The nrfE::tet(M) mutant was designated AL362.
FIG. 1.
Schematic diagram of the P. multocida nrfE mutagenesis construct and confirmation of the nrfE mutant by PCR. (A) A single 1.8-kb fragment containing nrfE was amplified by PCR by using primers 1914 and 1915 (indicated by the arrows labeled 1914 and 1915). This fragment was digested with BamHI to obtain two 900-bp fragments that were then ligated to either end of tet(M) to produce the mutagenesis cassette used for allelic exchange. (B) Schematic diagram of the genome organization around nrfE after insertion of the tet(M) cassette. The labeled arrows indicate primers used for PCR. (C) The genotype of AL362 was investigated by PCR. Genomic DNA from AL362 (lanes 3, 5, and 7) was compared to genomic DNA from wild-type strain X-73 (lanes 2, 4, and 6). Lanes 2 and 3, amplification with 1914 and 1915; lanes 4 and 5, amplification with 2277 and 2278; lanes 6 and 7, amplification with tet(M) primer 683 together with genomic primer 2278. Lane 1 contained λ DNA digested with HindIII.
RNA isolation.
Bacteria were harvested from four replicate cultures at an A600 of 0.5 (4 × 109 CFU/ml), added to 0.1 volume of ice-cold killing buffer (0.05 M Tris-HCl [pH 7.5], 15 mg of sodium azide per ml, 0.6 mg of chloramphenicol per ml), and pelleted by centrifugation. RNA was isolated from the bacteria by using Trizol (Gibco/BRL) as described by the manufacturer. Purified RNA was treated with DNase (20 U for 20 min at 37°C), and the RNA was further purified by using RNeasy columns (QIAGEN).
RT and real-time RT-PCR.
Primers for real-time reverse transcription (RT)-PCR were designed with the Primer Express software (ABI) (Table 2). Reverse transcription reactions were performed at 42°C for 2 h, and the reaction mixtures contained 10 μg of total RNA, 15 μg of random hexamers, 5 U of Superscript II reverse transcriptase (Gibco/BRL), and each deoxynucleoside triphosphate at a concentration of 200 μM. Synthesized cDNA samples were diluted 80-fold prior to real-time RT-PCR, which was carried out by using an ABI PRISM model 7700 sequence detector with product accumulation quantified by incorporation of the fluorescent dye SYBR Green. Triplicate real-time RT-PCRs were performed by using 2.4 μl of cDNA with the SYBR Green PCR mixture (ABI) and each gene-specific primer at a concentration of 50 nM in a 20-μl (final volume) reaction mixture. Gene-specific standard curves were constructed from known concentrations of P. multocida X-73 genomic DNA and were used to determine relative template concentrations in each reaction mixture. gyrB was used as a normalizer for all reactions performed with primers 2106 and 2107 (Table 2). All RT-PCRs amplified a single product, as determined by melting curve analysis.
Nitrite reduction assay.
The nitrite reduction assay was based on the method used for E. coli by Hussain et al. (22). For aerobic studies, 1 ml of normal CDM was inoculated with P. multocida and grown at 37°C overnight. Two hundred microliters of this preparation was used to inoculate 1 ml of nitrate/nitrite CDM in triplicate cultures, which were then incubated at 37°C overnight. The overnight cultures were diluted 1/10 in fresh glycerol/fumarate CDM and incubated for 24 h or until an optical density at 600 nm of >0.5 was reached. Aliquots (50 μl) of each test culture were mixed with 0.5 ml of 1% (wt/vol) sulfanilamide in 1 M HCl and 0.5 ml of 0.02% N-1-naphthylethylenediamine dihydrochloride. Formation of an intense pink color after 30 s indicated the presence of nitrite, and the absorbance of each sample was measured at 530 nm.
Disk diffusion SNAP susceptibility assay.
An agar suspension of each P. multocida test strain was made by adding 1 ml of a suspension containing 108 CFU of bacteria per ml to 3 ml of nitrite/nitrate CDM containing 3% (wt/vol) Noble agar (Oxoid) and pouring the preparation onto plates. Whatman paper disks (diameter, 4 mm) were then soaked in 15 μl of a 250 mM solution of S-nitroso-N-acetyl-penicillamine (SNAP) (dissolved in methanol) and placed in the center of each plate. Disks soaked in methanol were used as controls, and the plates were incubated overnight under either aerobic or anaerobic growth conditions.
Mouse virulence assay.
Individual strains of P. multocida X-73 were grown overnight in BHI, diluted 1:100 in 5 ml of fresh BHI, and incubated with shaking at 37°C for 4 to 6 h. The absorbance at 600 nm of each of the cultures was determined, after which the cultures were diluted in sterile phosphate-buffered saline (pH 7.2) to obtain the required number of CFU. Groups of five 6-week-old female BALB/c mice were inoculated intraperitoneally with 100 μl of cells from appropriately diluted cell suspensions. The mice were monitored closely for the onset of symptoms and euthanized when they were moribund. Cell suspensions were plated onto BHI agar and counted after growth for 16 h at 37°C to determine the number of CFU.
Competition growth assays in mice.
Competition growth assays were conducted as described previously (16). Briefly, 106 CFU of wild-type and mutant strains was injected into mice, and blood was recovered after 6 h. For the in vitro assay, a 100-fold dilution of the mixed bacterial preparation was grown for 6 h at 37°C, diluted appropriately, and plated onto nutrient agar. Bacteria were plated onto BHI agar and BHI agar containing tetracycline, and the relative competitive index was determined by dividing the percentage of tetracycline-resistant colonies (AL362) obtained in vivo by the percentage of tetracycline-resistant colonies obtained in vitro. Significance was determined by calculating a P value from an approximate z test for the difference between two proportions.
Sequence analysis and statistical programs.
Sequences were aligned by using the lalign algorithm (20). Statistical analyses were conducted by using the InStat program (Graphpad Software Inc.)
RESULTS
Genetic organization of the nrf locus of P. multocida.
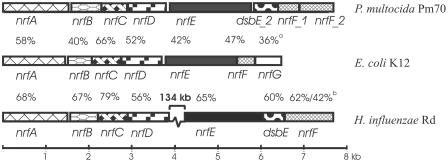
The organization of the nrf locus in P. multocida strain Pm70 (25) was compared to the organization of the corresponding loci in E. coli K-12 (3) and H. influenzae Rd (12) (Fig. 2). The P . multocida Pm70 nrf locus comprised eight open reading frames, nrfABCDE, dsbE_2, nrfF_1, and nrfF_2. The E. coli nrf locus organization was very similar to the P. multocida nrf locus organization, except that there was no dsb homolog. Although P. multocida had two genes annotated as nrfF (nrfF_1 and nrfF_2), nrfF_2 exhibited higher identity to the E. coli nrfG gene than to the nrfF gene, indicating that nrfF_2 is most likely an ortholog of nrfG. However, the organization of the nrf genes in H. influenzae was quite different; nrfABCD was organized as one locus, and nrfEF and dsbE were organized as a second locus 134 kb downstream from nrfF. H. influenzae contained a single dsbE homologue and a single nrfF gene but no nrfG. However, the nrfF gene of H. influenzae showed partial homology to nrfF_1 (first 150 bp) and partial homology to nrfF_2 (bp 150 to 270), indicating that the NrfF protein in H. influenzae may have two domains that perform the function of nrfF and nrfG. The P. multocida nrfABCDEF genes showed the highest levels of identity to the nrf genes of H. influenzae. This was not unexpected as H. influenzae and P. multocida are closely related and the presence and similar positions of the dsbE gene indicate that they may have originally had a similar nrf locus arrangement.
FIG. 2.
Genomic organization of the formate-dependent nitrite reduction (nrf) locus in selected gram-negative bacteria. Genomic organizations shown are for the following strains: P. multocida Pm70 (GenBank accession number NC_002663), H. influenzae Rd (GenBank accession number NC_000907), and E. coli K-12 (GenBank accession number NC_000913). Open reading frames are indicated by labeled boxes. Cross-hatched boxes indicate predicted orthologs, and the percentage above each box indicates the level of protein sequence identity to the P. multocida Pm70 protein. The superscript a indicates homology to nrfF_2, and the superscript b indicates homology to nrfF_1 and nrfF_2.
Construction and complementation of the nrfE mutant strain AL362.
A P. multocida nrfE::tet(M) mutant designated AL362 was constructed by allelic exchange by using a tet(M)-disrupted copy of nrfE (Fig. 1) (see Materials and Methods). A plasmid containing an uninterrupted copy of nrfE was constructed to complement AL362 by cloning nrfE into the vector pAL99, which contained the P. multocida tpiA promoter upstream of the cloning site. The complete nrfE coding fragment was amplified by PCR from P. multocida X-73 genomic DNA by using primers 2283 and 2284 (Table 2), digested with SacI, and cloned into the SacI site of pAL99 to generate pAL263 (Table 1). The nrfE insert was verified by DNA sequencing to be identical to wild-type nrfE and was in the same orientation as the tpiA promoter in pAL99. Plasmid pAL263 was transformed into strain AL362, yielding the complemented strain AL466. pAL99 was also transformed into AL362 as a vector control to construct strain AL465.
Nitrite reduction studies of the P. multocida nrfE gene.
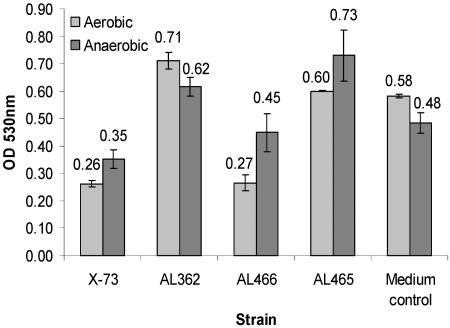
The ability of the P. multocida nrfE mutant to reduce nitrite was assessed by using a modified nitrite reduction assay (22). When grown both aerobically and anaerobically, wild-type strain X-73 was found to reduce nitrite (Nrf+ phenotype), whereas the mutant strain AL362 was unable to do so (Nrf− phenotype) (Fig. 3). The nrfE-complemented strain AL466 was able to reduce nitrite at wild-type levels, indicating restoration of the Nrf+ phenotype (Fig. 3). Thus, the P. multocida nrfE gene is essential for nitrite reduction under both aerobic and anaerobic conditions. A higher level of nitrite was present in the AL362 strain cultures (and the anaerobic AL465 culture) than in the original CDM medium, indicating that during growth additional nitrite was produced. This may have been due to the actions of nitrate reductases (e.g., the product of the nap operon [periplasmic nitrate reductase]), which have been shown to be present in P. multocida strain Pm70 (25).
FIG. 3.
Nitrite reduction by P. multocida strains grown either aerobically or anaerobically. Strains were grown in nitrate/nitrite CDM, and culture supernatants were tested for the presence of nitrite after 18 h (aerobic) or 72 h (anaerobic). The values are the means ± standard deviations for triplicate cultures. When grown both aerobically and anaerobically, wild-type strain X-73 reduced nitrite, whereas mutant strain AL362 was unable to reduce nitrite. Additionally, under both growth conditions, the nrfE-complemented strain AL466 was able to reduce nitrite at a level that was not significantly different from the level of nitrite reduction of wild-type strain X-73 (P > 0.05) but was significantly different from the levels of nitrite reduction of AL362 and AL465 (P < 0.05). The levels of nitrite in the vector control AL465 culture and the uninoculated medium control were significantly different from the levels for all of the other strains (P < 0.001) but not from each other in the aerobically grown cultures. In the anaerobic cultures, the medium control contained smaller amounts of nitrite than the amounts observed during the aerobic nitrite assays and was not significantly different from the wild-type X-73, AL362, and AL466 cultures (P > 0.05), but it was significantly different from AL465 cultures (P < 0.01). OD 530nm, optical density at 530 nm.
Nitric oxide reduction tests.
nrfE mutant strains of E. coli have been shown in disk diffusion assays to be sensitive to nitric oxide (NO) released by SNAP, whereas the wild-type strains were resistant (27). Disk diffusion assays were used to compare the sensitivities of strains AL362, AL465, and AL466 and wild-type strain X-73 to SNAP under both aerobic and anaerobic conditions. However, none of the P. multocida strains tested showed growth inhibition by SNAP (data not shown), indicating that inactivation of nrfE in P. multocida did not affect reduction of nitric oxide.
Virulence in mice.
To determine the virulence of the nrfE mutant AL362, 2 × 102 CFU of P. multocida X-73 and 10-fold dilutions of AL362 (range, 2 × 102 to 2 × 105 CFU) were injected intraperitoneally into mice (groups of five mice). There were no survivors in any of the test groups, indicating that nrfE is not required for P. multocida virulence in mice.
To quantitatively assess the growth rate of the nrfE mutant in vivo, competitive growth assays were used to compare the relative levels of survival of AL362 and X-73 in vitro and in vivo in mice. By using this method, mutants were identified as attenuated if the ratio of mutant to wild-type bacteria recovered after in vivo growth was significantly less than the ratio of mutant to wild-type bacteria recovered after in vitro growth. Mutant AL362 was found not to be significantly attenuated for growth in vivo compared to X-73 in any of the mice tested (P > 0.05), which is consistent with the challenge results described above.
Transcriptional analysis of nrfE.
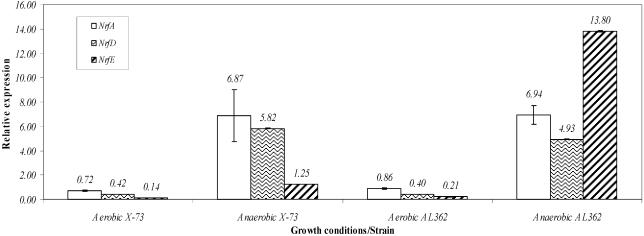
Transcriptional regulation of the Nrf operon under various growth conditions was investigated by using quantitative real-time RT-PCR. Cultures of X-73 and AL362 were grown either aerobically or anaerobically in the same medium used for the nitrite reduction experiments (nitrate/nitrite CDM). Real-time RT-PCR was carried out by using primers that amplified internal regions within nrfA (region A), nrfD (region D), and nrfE (region E) from X-73 and AL362 cDNA (Fig. 4 and 5). gyrB was used as a normalizer for all reactions, as described previously (6), in order to determine relative expression from triplicate data sets. The expression of nrfA, nrfD, and nrfE was significantly higher (at least ninefold higher; P < 0.001, as determined by the Tukey-Kramer multiple-comparison test) during anaerobic growth than during aerobic growth in both X-73 and AL362 (Fig. 5). Thus, nrfA, nrfD, and nrfE were expressed at low levels during growth under aerobic conditions but were significantly upregulated during growth under anaerobic conditions. However, the nrfE expression in mutant AL362 increased 66-fold when this strain was grown anaerobically compared to when it was grown aerobically, which was significantly different (P < 0.001) from the increase in nrfE expression observed in X-73 (Fig. 5). Furthermore, during anaerobic growth the expression of nrfE was significantly different from the expression of nrfA and nrfD (P < 0.001) in both X-73 and AL362, whereas during aerobic growth the expression of nrfA, the expression of nrfD, and the expression of nrfE were not significantly different (P > 0.05) (Fig. 5). In addition, there were no statistically significant differences between the expression of nrfA and the expression of nrfD when either X-73 or AL362 was grown anaerobically. Taken together, these data indicated that nrfA, nrfD, and nrfE were all upregulated during anaerobic growth in both strains, but nrfE was expressed at much higher levels in strain AL362 (with nrfE inactivated), probably from an uncharacterized promoter between nrfD and nrfE. The level of expression of nrfE in X-73 grown anaerobically was also significantly lower than the levels of expression of nrfA and nrfD, suggesting that a transcriptional terminator or transcriptional attenuation sequence was present in this region. In E. coli, nrfA is under the control of FNR (10). A putative FNR recognition sequence (5′-TTGATCAAGCGCAA-3′) was identified 128 bp upstream of nrfA in the Pm70 genome. However, no potential FNR recognition site was located upstream of nrfE, indicating that, unlike nrfA, the hypothesized nrfE promoter is not likely to be regulated by FNR.
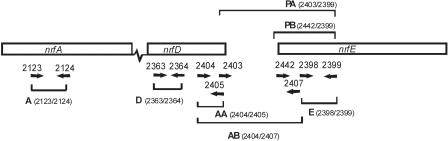
FIG. 4.
Schematic diagram of the positions of primers used in real-time RT-PCRs to assess the levels of transcripts at different points between nrfA and nrfE. The PCR sets were designated A, D, E, AA, AB, PA, and PB. The arrows and the numbers 2123, 2124, 2363, 2364, 2404, 2405, 2403, 2442, 2407, 2398, and 2399 indicate primers 2123, 2124, 2363, 2364, 2404, 2405, 2403, 2442, 2407, 2398, and 2399, respectively.
FIG. 5.
Relative levels of nrfA, nrfD, and nrfE expression as determined by real-time RT-PCR (normalized with gyrB) during anaerobic growth of P. multocida X-73 and AL362. Cultures of X-73 and AL362 were grown simultaneously in conditions optimal for nitrite reduction. The values are means ± standard deviations for relative expression determined from a minimum of three reactions.
The transcriptional regulation of nrfE in both wild-type strain X-73 and nrfE mutant AL362 grown anaerobically was investigated further by using real-time RT-PCR and a series of primer sets (Table 3 and Fig. 4 and 6). The relative level of transcripts was significantly less (P < 0.001) for primer set A than for primer set D (Table 3) in both X-73 and in AL362, indicating either that there was transcriptional attenuation within nrfD or there was instability at the 3′ end of the transcript. The relative level of transcripts dropped even further from primer set AA to primer set AB (Table 3), indicating that there was further transcript attenuation or degradation in the intergenic region between nrfD and nrfE that was independent of expression of nrfE. Importantly, there was a greater increase in the relative level of transcripts between primer sets PA and PB (Table 3) (P < 0.001) in AL362 than in X-73. These data indicated that there was a promoter between the binding sites of primers 2403 and 2442 (Fig. 6) which was regulated either directly or indirectly by the level of active NrfE. Thus, nrfE was expressed at low levels in X-73 but at much higher levels in AL362, in which no active NrfE was produced. Furthermore, these data indicated that a low level of transcription of nrfE probably occurred from the nrfA promoter, but most nrfE expression was a result of the nrfE promoter and this promoter was itself regulated by the expression levels of nrfE. Northern blot analysis was used in an attempt to confirm this proposal; however, no transcripts were detected from nrfA, nrfD, or nrfE (data not shown). A positive control (tpiA) simultaneously detected a transcript of the predicted size, suggesting that the inability to detect transcripts from the nrf locus was most likely due to low abundance and/or instability and/or the large size of the mRNA transcripts.
TABLE 3.
Transcriptional regulation of expression of the nrf operon of P. multocida
| P. multocida strain | Relative expression with the following primer setsa:
|
||||||
|---|---|---|---|---|---|---|---|
| A | D | AA | AB | PA | PB | E | |
| X-73 | 6.9 ± 2.14 | 5.8 ± 0.04 | 0.9 ± 0.01 | 0.3 ± 0.01 | 0.2 ± 0.01 | 1.2 ± 0.01 | 1.4 ± 0.01 |
| AL362 | 6.9 ± 0.77 | 4.9 ± 0.03 | 0.8 ± 0.01 | 0.3 ± 0.004 | 0.3 ± 0.003 | 10.8 ± 0.11 | 13.3 ± 0.14 |
The values are means ± standard deviations for relative expression determined from triplicate reactions.
FIG. 6.
Locations of real-time PCR primers within nrfD and nrfE from Pm70 (GenBank accession number NC_002663). Primer sites and directions are indicated by arrows below the sequence. Translational start and stop codons are indicated by boldface type.
DISCUSSION
The P. multocida formate-dependent nitrite reduction (nrf) locus has not been characterized previously, nor has its role in virulence been assessed. The organization of the nrf operon in P. multocida is somewhat different from the organization of this operon in members of the family Enterobacteriaceae (Fig. 2), with the presence of two annotated nrfF homologues, no nrfG, and an additional gene, dsbE_2, although nrfF_2 is most likely an ortholog of nrfG. In E. coli nrfF and nrfG (together with nrfE) have been proposed to be essential for nitrite reduction (11), although their role in P. multocida is still uncertain. The closely related bacterium H. influenzae Rd displayed an organization similar to that of P. multocida, although it had a single nrfF homologue and nrfE, nrfF, and dsbE were clustered 134 kb from nrfABCD. The predicted H. influenzae nrfF gene contains two domains with similarity to nrfF_1 and nrfF_2 in P. multocida and nrfF and nrfG in E. coli, indicating that its product may perform the function of both proteins. If the nrf system in H. influenzae is active, it is clear that the nrfE, nrfF, and dsbE genes must be transcribed from their own promoter due to their isolation from the rest of the nrf locus. As shown in this study, the P. multocida nrfE gene is regulated independently of the nrfABCD genes. It is therefore probable that the P. multocida nrfF_1, nrfF_2, and dsbE_2 genes are also regulated separately from the P. multocida nrfABCD genes. The functions of DsbE_2 in P. multocida and DsbE in H. influenzae are unknown, although active DsbA, DsbD, and DsbB have been shown to be essential for Nrf activity and maturation of c-type cytochromes in E. coli (26). As there is a second dsbE gene (dsbE_1) in the P. multocida Pm70 genome, it is possible that DsbE_2 may play a specialized role in maturation of nrfA (c552) in P. multocida, although at this stage we have no direct evidence for this.
In this work, nrfE was shown to be critical for nitrite reduction, which correlates with previous findings for E. coli (11). The nrfE mutant AL362 was unable to reduce nitrite either aerobically or anaerobically, whereas the wild-type X-73 strain was able to reduce nitrite under both conditions. In E. coli, formate-dependent nitrite reduction has been shown to be repressed under aerobic growth conditions and to be upregulated anaerobically (10). Unlike E. coli, P. multocida lacks any of the aerobic nitrate reductases (such as nir), so it is likely that the nrf locus has a dual function during both aerobic and anaerobic growth.
The reduction of nitric oxide by E. coli was investigated recently by Poock et al. (27), who found that strains deficient in Nrf activity were not able to reduce the nitric oxide released from SNAP. This property was investigated in the strain of P. multocida in which nrfE was inactivated (AL362), as the reduction of NO in the host might be an important virulence factor. However, AL362 was identical to wild-type strain X-73 in terms of resistance to the NO released by SNAP. Therefore, it is probable that P. multocida has alternative pathways for detoxifying NO that are not present in the E. coli strains.
The inactivation of nrfE in P. multocida did not result in attenuation in mice, as determined by either direct challenge experiments or competitive growth assays. These results indicated that although nrfE is essential for nitrite reduction in P. multocida, it is not essential for virulence despite being upregulated during a mouse infection (21). P. multocida has a number of predicted alternative electron acceptor systems (e.g., Nap, Dms, and Frd), and it is likely that when one of these systems is unavailable (such as Nrf), the other systems are utilized and hence P. multocida survival in vivo is not adversely affected.
Expression of the nrf operon in E. coli has been demonstrated to be regulated by FNR in response to anaerobic conditions and to be activated by the NarL or NarP proteins in response to nitrate or nitrite (7). We showed that the P. multocida nrf locus is also upregulated under anaerobic conditions, and a putative FNR recognition sequence was identified upstream of the P. multocida nrfA gene. However, there was also a low level of expression of the nrf locus during aerobic growth. Although nitrite reduction occurs under both aerobic and anaerobic conditions, high levels of nitrite reduction are not required during aerobic growth due to the availability of aerobic electron acceptors, and hence, nrfE is expressed only at very low levels. Correspondingly, it is likely that the upregulation of nrfE under anaerobic conditions is due to use of nitrite as an alternate electron acceptor by P. multocida when oxygen is unavailable.
Expression of the entire nrf operon in E. coli has been proposed to be driven by a single promoter upstream of nrfA (7). In this study, when X-73 was grown anaerobically, nrfE was expressed at lower levels than nrfA and nrfD (Fig. 5). However, when mutant AL362 was grown under anaerobic conditions, nrfE was expressed twofold more than nrfA and nrfD. Despite the differences in nrfE expression between X-73 and AL362 when the organisms were grown anaerobically, the levels of transcription of nrfA and nrfD were not significantly different for the two strains. These results indicate that expression of nrfE is not under the control of the nrfA promoter (pnrfA) and are consistent with the presence of an independent promoter between nrfD and nrfE and attenuation of the nrfA transcript in the same region. This is consistent with our previous work in which we identified nrfE as a gene that is downstream of an in vivo active promoter (21). To address this issue, we used real-time RT-PCR to show the presence of a potential nrfE promoter (pnrfE) within the nrfD-nrfE intergenic region. As mentioned previously, nrfE has been proposed to form part of a heme lyase that is responsible for attaching a heme group to the active site of NrfA (c552) (14). As we found that nrfE was expressed at significantly higher levels in AL362 than in X-73, it is likely that nrfE is upregulated because the NrfE protein is nonfunctional. This upregulation is probably due to an as-yet-uncharacterized regulatory feedback loop.
The data presented here indicate that P. multocida X-73 has a functional Nrf system that is active both aerobically and anaerobically. This work also demonstrated that in P. multocida nrfE is essential for Nrf activity but is not required for virulence in mice. nrfE has also been shown to be upregulated independent of the pnrfA promoter by an unknown promoter that is active preferentially in vivo.
Acknowledgments
Components of this work were supported by grants from the Australian Research Council.
We gratefully acknowledge the excellent technical assistance of Ian McPherson and Vicki Vallance.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
- 2.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett, 3rd, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 4.Bosch, M., E. Garrido, M. Llagostera, A. M. Perez de Rozas, I. Badiola, and J. Barbe. 2002. Pasteurella multocida exbB, exbD and tonB genes are physically linked but independently transcribed. FEMS Microbiol. Lett. 210:201-218. [DOI] [PubMed] [Google Scholar]
- 5.Boyce, J. D., and B. Adler. 2000. The capsule is a virulence determinant in the pathogenesis of Pasteurella multocida M1404 (B:2). Infect. Immun. 68:3463-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce, J. D., I. Wilkie, M. Harper, M. L. Paustian, V. Kapur, and B. Adler. 2002. Genomic scale analysis of Pasteurella multocida gene expression during growth within the natural chicken host. Infect. Immun. 70:6871-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browning, D. F., C. M. Beatty, A. J. Wolfe, J. A. Cole, and S. J. Busby. 2002. Independent regulation of the divergent Escherichia coli nrfA and acsP1 promoters by a nucleoprotein assembly at a shared regulatory region. Mol. Microbiol. 43:687-701. [DOI] [PubMed] [Google Scholar]
- 8.Chanter, N. 1990. Molecular aspects of the virulence of Pasteurella multocida. Can. J. Vet. Res. 54(Suppl.):S45-S47. [PubMed] [Google Scholar]
- 9.Chung, J. Y., I. Wilkie, J. D. Boyce, K. M. Townsend, A. J. Frost, M. Ghoddusi, and B. Adler. 2001. Role of capsule in the pathogenesis of fowl cholera caused by Pasteurella multocida serogroup A. Infect. Immun. 69:2487-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darwin, A., H. Hussain, L. Griffiths, J. Grove, Y. Sambongi, S. Busby, and J. Cole. 1993. Regulation and sequence of the structural gene for cytochrome c552 from Escherichia coli: not a hexahaem but a 50 kDa tetrahaem nitrite reductase. Mol. Microbiol. 9:1255-1265. [DOI] [PubMed] [Google Scholar]
- 11.Eaves, D. J., J. Grove, W. Staudenmann, P. James, R. K. Poole, S. A. White, I. Griffiths, and J. A. Cole. 1998. Involvement of products of the nrfEFG genes in the covalent attachment of haem c to a novel cysteine-lysine motif in the cytochrome c552 nitrite reductase from Escherichia coli. Mol. Microbiol. 28:205-216. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, K. McKenney, G. Sutton, W. FitzHugh, C. Feilds, J. D. Gocayne, J. Scott, R. Shirley, L. Liu, A. Glodek, J. M. Kelley, J. F. Weidman, C. A. Phillips, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, D. T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchman, J. L. Furhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. McDonald, K. V. Small, C. M. Fraser, H. O. Smith, and J. C. Venter. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 13.Fuller, T. E., M. J. Kennedy, and D. E. Lowery. 2000. Identification of Pasteurella multocida virulence genes in a septicemic mouse model using signature-tagged mutagenesis. Microb. Pathog. 29:25-38. [DOI] [PubMed] [Google Scholar]
- 14.Grove, J., S. Busby, and J. Cole. 1996. The role of the genes nrf EFG and ccmFH in cytochrome c biosynthesis in Escherichia coli. Mol. Gen. Genet. 252:332-341. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-135. In D. M. Glover (ed.), DNA cloning: a practical approach, vol. 1. IRL Press, Washington, D.C.
- 16.Harper, M., J. D. Boyce, I. W. Wilkie, and B. Adler. 2003. Signature-tagged mutagenesis of Pasteurella multocida identifies mutants displaying differential virulence characteristics in mice and chickens. Infect. Immun. 71:5440-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper, M., A. D. Cox, F. St. Michael, I. W. Wilkie, J. D. Boyce, and B. Adler. 2004. A heptosyltransferase mutant of Pasteurella multocida produces a truncated lipopolysaccharide structure and is attenuated in virulence. Infect. Immun. 72:3436-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heddleston, K. L., and P. A. Rebers. 1972. Fowl cholera. Cross immunity induced in turkeys with formalin-killed in-vivo-propagated Pasteurella multocida. Avian Dis. 16:578-586. [PubMed] [Google Scholar]
- 19.Homchampa, P., R. A. Strugnell, and B. Adler. 1997. Cross protective immunity conferred by a marker-free aroA mutant of Pasteurella multocida. Vaccine 15:203-208. [DOI] [PubMed] [Google Scholar]
- 20.Huang, X., and W. Miller. 1991. A time efficient, linear space local similarity algorithm. Adv. Appl. Math. 12:337-357. [Google Scholar]
- 21.Hunt, M. L., D. J. Boucher, J. D. Boyce, and B. Adler. 2001. In vivo-expressed genes of Pasteurella multocida. Infect. Immun. 69:3004-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain, H., J. Grove, L. Griffiths, S. Busby, and J. Cole. 1994. A seven-gene operon essential for formate-dependent nitrite reduction to ammonia by enteric bacteria. Mol. Microbiol. 12:153-163. [DOI] [PubMed] [Google Scholar]
- 23.Jablonski, L., N. Sriranganathan, S. M. Boyle, and G. R. Carter. 1992. Conditions for transformation of Pasteurella multocida by electroporation. Microb. Pathog. 12:63-68. [DOI] [PubMed] [Google Scholar]
- 24.Jablonski, P. E., M. Jaworski, and C. J. Hovde. 1996. A minimal medium for growth of Pasteurella multocida. FEMS Microbiol. Lett. 140:165-169. [DOI] [PubMed] [Google Scholar]
- 25.May, B. J., Q. Zhang, L. L. Li, M. L. Paustian, T. S. Whittam, and V. Kapur. 2001. Complete genomic sequence of Pasteurella multocida, Pm70. Proc. Natl. Acad. Sci. USA 98:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metheringham, R., K. L. Tyson, H. Crooke, D. Missiakas, S. Raina, and J. A. Cole. 1996. Effects of mutations in genes for proteins involved in disulphide bond formation in the periplasm on the activities of anaerobically induced electron transfer chains in Escherichia coli K12. Mol. Gen. Genet. 253:95-102. [DOI] [PubMed] [Google Scholar]
- 27.Poock, S. R., E. R. Leach, J. W. Moir, J. A. Cole, and D. J. Richardson. 2002. Respiratory detoxification of nitric oxide by the cytochrome c nitrite reductase of Escherichia coli. J. Biol. Chem. 277:23664-23669. [DOI] [PubMed] [Google Scholar]
- 28.Potter, L., H. Angove, D. Richardson, and J. Cole. 2001. Nitrate reduction in the periplasm of gram-negative bacteria. Adv. Microb. Physiol. 45:51-112. [DOI] [PubMed] [Google Scholar]
- 29.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]