Abstract
In 2021, the H5N1 virus lineage 2.3.4.4b spread to the Americas, causing high mortality in wild and domestic avian populations. South American countries along the Pacific migratory route have reported wild bird deaths due to A/H5Nx virus since October 2022. However, limited genomic data resulted in no cases reported in Brazil until May 2023.
Brazil reported its first case of highly pathogenic avian influenza virus (HPAI A/H5N1) in May 2023. The virus was detected in Cabot's tern specimen in Marataízes, Espírito Santo. Cases were also found in backyard poultry and other wild birds, but no human or commercial poultry cases occurred. HPAI poses risks to the poultry industry, food security, and public health.
Researchers used next-gen sequencing and phylogenetic analysis to study the Brazilian sample. It confirmed its affiliation with the 2.3.4.4b clade and proximity to sequences from Chile and Peru.
This sheds light on the spread and evolution of HPAI A/H5N1 in the Americas, emphasizing continuous monitoring to mitigate risks for both avian and human populations. Understanding the virus's genetics and transmission allows implementing effective control measures to protect public health and the poultry industry.
Keywords: Avian influenza virus, Brazilian first report, Complete genetic characterization
1. Introduction
Since 1996, H5N1 viruses have been spreading globally, including the recent introduction of the 2.3.4.4b lineage in the Americas in 2021, affecting both wild and domestic avian populations with a high mortality rate (Bevins et al., 2022).
Reports of mortality in wild birds in several South American countries along the Pacific migratory route have been attributed to the A/H5Nx virus since October 2022 (Gamarra-Toledo et al., 2023). Nevertheless, genomic data on the viruses entering South America are still scarce, and until date, no cases had been reported in Brazilian territory.
The complete sequencing of the first case of infection in a wild bird with the highly pathogenic avian influenza virus (HPAI A/H5N1), which occurred in May 2023 in Brazil, is herein reported. In this first case, A/H5N1 was detected in two specimens of migratory seabirds found in the city of Marataízes, state of Espírito Santo, at Latitude −21.0366 S and Longitude −40.81760167 W, both belonging to the species known as Cabot's tern (Thalasseus acuflavidus).
Cases in backyard poultry and other species of wild birds were subsequently detected after this initial outbreak. Nonetheless, there have been no reported cases in commercial poultry or human cases in Brazilian territory so far.
HPAI represents a risk to the poultry industry, food security, and public health. Biosafety measures, quarantine, culling of infected birds, disinfection, and vaccination are generally strategies used to control the disease (WOAH, 2022). Transmission occurs primarily through direct contact with respiratory secretions and faces of infected birds, as well as through contaminated objects. Furthermore, migratory birds are susceptible to infection and can even spread the virus during their migratory routes (Imai et al., 2013).
HPAI is a viral disease that affects birds and, in rare cases, can be transmitted to humans. Avian Influenza Virus (AIV) can cross the species barrier and infect humans, causing severe illness. By identifying genetic recombination events, especially involving both avian and human-adapted lineages, the probability of zoonotic transmission and potential pandemic threats can be assessed (Blagodatski et al., 2021).
Genetic recombination plays a significant role in the evolution of influenza viruses. It can result in the emergence of lineages with altered antigenic properties, leading to the evasion of pre-existing immunity in both birds and humans. Identifying recombination events helps monitor the virus evolution and understand the potential risks associated with new strains (Petrova & Russell, 2018).
2. Materials and methods
A sample of the central nervous system of a dead specimen of Cabot's tern was collected by the Official Veterinary Service of the state of Espírito Santo and sent to the Federal Laboratory of Agropecuary Defense in São Paulo (LFDA-SP), the national reference laboratory for avian influenza diagnosis.
The sample was tested for A/H5 by two RT-qPCR techniques (VetMAX Gold AIV, Thermo Fisher; Spackman et al., 2002), and fragment sequencing (Sanger et al., 1977) was performed of the HA gene, segment 4 to confirm for presence of a polybasic motif at the cleavage site compatible with high pathogenicity viruses. Additionally, the sample was tested by RT-PCR for neuraminidase subtyping (Fereidouni et al., 2009).
All tests were performed in accordance with the general requirements for the competence of testing and calibration laboratories of ISO/IEC 17025/2017 (International Organization for Standardization / International Electrotechnical Commission) and Chapter 3.3.4. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of WOAH (World Organisation for Animal Health).
Subsequently, the inactivated sample was sent to the Federal Laboratory of Agropecuary Defense in Minas Gerais (LFDA-MG) for next-generation complete genome sequencing on a solid phase using the Nextera XT DNA Library Prep Kit (Illumina Inc.) on a MiSeqTM platform by Illumina Inc. The library preparation process involved several steps and was adapted from the protocol described by Gauthier et al. (2021).
Raw data generated from the sequencing was analyzed in Varsmetagen (https://varsomics.com/varsmetagen/), an online platform for bioinformatic analysis and interpretation of NGS data for infectious diseases. Varsmetagen implements a viromic pipeline with the following steps: (1) quality control of raw sequences with metrics and removal of low-quality sequences (5 bp and 9 bp removed from 3′ and 5′ respectively, mean quality score lower than 20 and sequences shorter than 50 bp); (2) mapping of the obtained sequences to the closest host genome (Gallus gallus, assembly: GCF_016699485.2) using BWA with default parameters to remove contaminants; (3) taxonomic identification using Kraken2 (Wood et al., 2019) with a custom 33-kmer length database composed of NCBI RefSeq complete genomes for Viruses, Bacteria, and Human plus complete viral genomes from Genbank as downloaded in Oct-2021; (4) assembly with Spades 3.13 to recover the viral genome (Antipov et al., 2020); (5) search for distant homologous sequences using Hidden Markov Models (HMM) of viral proteins implemented in the eegNOG database (Huerta-Cepas et al., 2019); and (6) complementary analyses involving confirmation and genome coverage metrics.
The sequence for the hemagglutinin (HA) segment 4 was used to infer the phylogenetic relationships. H5Nx sequences were obtained from the GenBank and GISAID (Global Initiative on Sharing All Influenza Data) databases. Additionally, the 100 most closely related sequences in the BLAST (Basic Local Alignment Search Tool) (Zhang et al., 2000 and Morgulis et al., 2008) analysis to the sequences obtained in Brazil were added for the segments encoding HA. Low coverage sequences (<90 % of the total length) were excluded from the analysis. The Flusurver genotyping tool (available at https://flusurver.bii.a-star.edu.sg/), enabled by GISAID data (Elbe & Buckland-Merrett, 2017) was used to confirm the classification clade.
Multiple sequence alignment was performed using the "MUSCLE" (Multiple Sequence Comparison by Log-Expectation) software (Edgar, 2004), associated with the "MEGA" (Molecular Evolutionary Genetics Analysis) software (Kumar et al., 2018).
Phylogenetic inference was performed by maximum likelihood using the RAxML (Randomized Axelerated Maximum Likelihood) (Stamatakis, 2014).
Phylogenetic trees were constructed using the "MEGA" software (Kumar et al., 2018), and a bootstrap of 1000 iterations was used to assess the robustness of the clusters formed in the phylogenetic tree, allowing for the confidence in the support of each node in the topology by repeating the phylogenetic analysis on pseudo-replicas of the original alignment (Felsenstein, 1985). The outgroup for rooting the tree was performed using the reference sequence for HA from the first outbreak in China (AF148678.1/A/goose/Guangdong/1/96/H5N1).
3. Results and discussion
The sample was initially confirmed as positive for A/H5 by two RT-qPCR techniques and fragment sequencing was performed. The partial sequencing of the HA gene, segment 4, confirmed the presence of a polybasic motif at the cleavage site, PLREKRKKR/GLF, compatible with high pathogenicity viruses. Additionally, sample positive for influenza A/H5 underwent RT-PCR for neuraminidase subtyping resulting in N1 positive.
The quality metrics of complete genetic sequencing were adequate to obtain the genome with read length de 150 pb forward e 150 pb reverse, library insert size 350 pb, Phred quality score of 92,44 % and base pair adequate composition percentage of each of the four nucleotides.
The complete sequences obtained by NGS of the eight segments of A/H5N1 were deposited in GenBank under the indicated codes OR269884 (segment 1 - PB2), OR269885 (segment 2 - PB1), OR269886 (segment 3 - PA), OR269887 (segment 4 - HA), OR269888 (segment 5 - NP), OR269889 (segment 6 - NA), OR269890 (segment 7 - M2), and OR269891 (segment 8 - NEP) and was confirmed to belong to the 2.3.4.4b clade using genotyping tool in sequences of segment 4 - HA and segment 6 - NA.
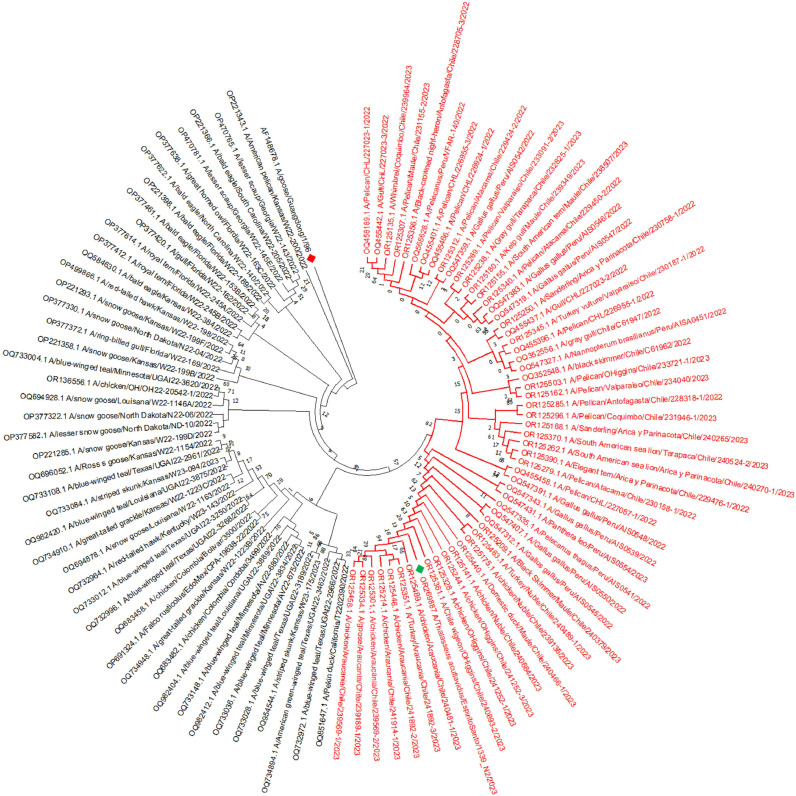
The A(H5N1) viral sequence obtained from Thalasseus acuflavidus in Brazil grouped together with all the sequences of A/H5N1 viruses detected in Chile and Peru in 2022 and 2023, forming a single cluster with 82 % bootstrap support value, confirming its closest phylogenetic proximity to these sequences from South American countries facing the Pacific Ocean (Fig. 1).
Fig. 1.
Nucleotide based phylogenetic relatedness of A/H5. Maximum likelihood and trees and Hasegawa-Kishino-Yano model (Hasegawa et al., 1985) of complete nucleotide sequences of segment 4 hemagglutinin were generated with MEGA X software version 11.0 (Kumar et al., 2018). Legend: OR269887 A/Thalasseus acuflavidus/EspiritoSanto/1339_N2/2023, AF148678 A/goose/Guangdong/1/1996 (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)

The level of similarity between Brazilian and Chilean/Peruvian strains ranged from 99 % (p-distance, MEGA). The differences are nucleotide substitutions, mostly transitions, occurring throughout the entire Brazilian sequence.
Therefore, the migratory route of these wild birds across the Pacific Flyway, including Cabot's tern, (Alkie et al., 2022; PAHO/WHO, 2023) would explain the topology of the phylogenetic tree, following the geographical dispersal of these wild migratory birds.
4. Conclusions
Passive and active surveillance in domestic and wild birds must be continuous in Brazil and throughout South America. HPAI A/H5Nx viruses in Brazil have only been detected so far in backyard and wild birds. Additional sequencing of A/H5Nx and non-A/H5 positive selections should be continued to identify possible reassortment events with locally circulating AIV strains.
The identification of possible rearrangement events in Avian Influenza Virus strains is crucial for monitoring viral evolution, assessing the risks of zoonotic transmission, developing effective vaccines and implementing control strategies by Governments, health and veterinary organizations, as well as the poultry industry, must work together to monitor and respond quickly to any suspected or confirmed cases in order to prevent the spread of the disease and protect both animal and human health.
Ethical statement for solid state ionics
Hereby, I Anselmo Vasconcelos Rivetti Júnior consciously assure that for the manuscript First Report and Genetic Characterization of the Highly Pathogenic Avian Influenza A(H5N1) Virus in Cabot's tern (Thalasseus acuflavidus), Brazil the following is fulfilled:
-
1)
This material is the authors' own original work, which has not been previously published elsewhere.
-
2)
The paper is not currently being considered for publication elsewhere.
-
3)
The paper reflects the authors' own research and analysis in a truthful and complete manner.
-
4)
The paper properly credits the meaningful contributions of co-authors and co-researchers.
-
5)
The results are appropriately placed in the context of prior and existing research.
-
6)
All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
-
7)
All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
The violation of the Ethical Statement rules may result in severe consequences.
To verify originality, your article may be checked by the originality detection software iThenticate. See also http://www.elsevier.com/editors/plagdetect.
I agree with the above statements and declare that this submission follows the policies of Solid State Ionics as outlined in the Guide for Authors and in the Ethical Statement.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alkie T.N., Lopes S., Hisanaga T., Xu W., Suderman M., Koziuk J., et al. A threat from both sides: Multiple introductions of genetically distinct H5 HPAI viruses into Canada via both East Asia-Australasia/Pacific and Atlantic flyways. Virus evolution. 2022;8(2) doi: 10.1093/ve/veac077. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antipov D., Raiko M., Lapidus A., Pevzner P.A. Metaviralspades: Assembly of viruses from metagenomic data. Bioinformatics (Oxford, England) 2020;36(14):4126–4129. doi: 10.1093/bioinformatics/btaa490. [DOI] [PubMed] [Google Scholar]
- Bevins S.N., Shriner S.A., Cumbee J.C., Jr., Dilione K.E., Douglass K.E., Ellis J.W.…Lenoch J.B. Intercontinental movement of highly pathogenic avian influenza A (H5N1) clade 2.3. 4.4 virus to the United States. Emerging Infectious Diseases. 2022;28(5):1006. doi: 10.3201/eid2805.220318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagodatski A., Trutneva K., Glazova O., Mityaeva O., Shevkova L., Kegeles E., Onyanov N., Fede K., Maznina A., Khavina E., Yeo S., Park H., Volchkov P. Avian influenza in wild birds and poultry: Dissemination pathways, monitoring methods, and virus ecology. Pathogens (Basel, Switzerland) 2021;10(5):630. doi: 10.3390/2Fpathogens10050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Global Challenges (Hoboken, NJ) 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the Comparative Method. The American Naturalist. 1985;125(1):1–15. doi: 10.1086/703055. http://www.jstor.org/stable/2461605 [DOI] [PubMed] [Google Scholar]
- Fereidouni S.R., Starick E., Grund C., Globig A., Mettenleiter T.C., Beer M., Harder T. Rapid molecular subtyping by reverse transcription polymerase chain reaction of the neuraminidase gene of avian influenza A viruses. Vet Microbiol. 2009;135(3–4):253–260. doi: 10.1016/j.vetmic.2008.09.077. 30. [DOI] [PubMed] [Google Scholar]
- Gamarra-Toledo V., Plaza P.I., Gutiérrez R., Luyo P., Hernani L., Angulo F., Lambertucciet S.A. Avian flu threatens Neotropical birds. Science (New York, N.Y.) 2023;379(6629):246. doi: 10.1126/science.adg2271. -246. [DOI] [PubMed] [Google Scholar]
- Gauthier N.P.G., Nelson C., Bonsall M.B., Locher K., Charles M., MacDonald C., Krajden M., Chorlton S.D., Manges A.R. Nanopore metagenomic sequencing for detection and characterization of SARS-CoV-2 in clinical samples. PloS One. 2021;16(11) doi: 10.1371/journal.pone.0259712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Dating the human-ape split by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution. 1985;22:60–174. doi: 10.1007/bf02101694. [DOI] [PubMed] [Google Scholar]
- Huerta-Cepas J., Szklarczyk D., Heller D., Hernández-Plaza A., Forslund S.K., Cook H., Mende D.R., Letunic I., Rattei T., Jensen L.J., von Mering C., Borket P. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Research. 2019;47 doi: 10.1093/nar/gky1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Herfst S., Sorrell E.M., Schrauwen E.J.A., Linster M., De Graaf M., Fouchier R.A.M, Kawaoka Y. Transmission of influenza A/H5N1 viruses in mammals. Virus Research. 2013;178:15–20. doi: 10.1016/j.virusres.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgulis A., Coulouris G., Raytselis Y., Madden T.L., Agarwala R., Schaffer A.A. Database indexing for production MegaBLAST searches. Bioinformatics (Oxford, England) 2008;15:1757–1764. doi: 10.1093/bioinformatics/btn322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAHO/WHO . PAHO/WHO; Washington, DC, USA: 2023. Epidemiological update outbreaks of avian influenza caused by influenza A(H5N1) in the region of the Americas. [Google Scholar]
- Petrova V.N., Russell C.A. The evolution of seasonal influenza viruses. Nature Reviews Microbiology. 2018;16:47–60. doi: 10.1038/nrmicro.2017.118. pages. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A.R. DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences. 1977;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E., Senne D.A., Myers T.J., Bulaga L.L., Garber L.P., Perdue M.L., Lohman K., Daum L.T., Suarez D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology. 2002;40(9):3256–3260. doi: 10.1128/jcm.40.9.3256-3260.2002. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England) 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D.E., Lu J., Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biology. 2019;20:257. doi: 10.1186/2Fs13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Organisation for Animal Health - WOAH (2022). Terrestrial Animal Health Code. Chapter 10.4. Article 10.4.1. Infection With High Pathogenicity Avian Influenza Viruses. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_avian_influenza_viruses.htm.
- Zhang Z., Schwartz S., Wagner L., Miller W. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]