Summary
We report a technique to generate a murine model of lung metastases by selectively injecting tumor cells into the right heart ventricle under ultrasound guidance. First, we describe cell preparation and reference animal preparation as previously described. We then detail the technique using a previously described 3D-printed instrument stabilization device. Finally, we describe tumor growth surveillance using bioluminescent imaging.
For complete details on the use and execution of this protocol, please refer to Labora et al.1
Subject areas: Cancer, Model Organisms
Graphical abstract

Highlights
-
•
Ultrasound-guided seeding of tumor cells in the lungs
-
•
Alternative route of cell delivery to the lungs
-
•
Use of a prototype device to enhance injection stability
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
We report a technique to generate a murine model of lung metastases by selectively injecting tumor cells into the right heart ventricle under ultrasound guidance. First, we describe cell preparation and reference animal preparation as previously described. We then detail the technique using a previously described 3D-printed instrument stabilization device. Finally, we describe tumor growth surveillance using bioluminescent imaging.
Before you begin
This protocol describes steps for generating a lung metastatic model of melanoma or pancreatic cancer with luciferase-expressing B16F10 (B16F10-luc) cells and luciferase-expressing KP4662-G12C gp100 (KP4662-G12C gp100-luc) respectively in C57BL/6 mice. Bioluminescent imaging (BLI) is used to monitor tumor burden. Approval from the appropriate animal research review board should be obtained prior to conducting any experiments involving live vertebrates.
Institutional permissions
All reported animal studies were approved by the University of California, Los Angeles, Institutional Animal Care and Use Committee (IACUC), ARC-2012-089.
Cell culture preparation
Timing: 5–7 days
-
1.
Thaw the tumor cells of interest and culture cells in DMEM supplemented with 10% FBS.
-
2.
Maintain cells in culture at 37°C in 5% CO2.
-
3.
Prepare for injection when the preferred confluency is achieved.
Note: We recommend not exceeding 80% confluency prior to cell injection. Over confluency of cells can compromise cell viability.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| D-luciferin, potassium salt | BioVision Technologies | 7903 |
| Fluriso (isoflurane, USP) | VetOne | NDC13985-528-60 |
| PBS 1× | Corning | 21-031-CM |
| 0.25% Trypsin-EDTA 1× | Gibco | 25200–056 |
| DMEM | Corning | 10017CM |
| Fetal bovine serum (FBS) | Omega Scientific | FB-01 |
| Experimental models: Cell lines | ||
| Murine: B16F10-luciferase (B16F10-luc), P3-P10 | ATCC | CRL-6475 |
| Murine: KP4662G12Cgp100-luc, P3-P10 | Radu Laboratory | |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6, aged 6–8 weeks, female | The Jackson Laboratory | JAX:000664 |
| Software and algorithms | ||
| Living Image v.4.5 | PerkinElmer | 128110 |
| Other | ||
| Vevo 2100 imaging system | VisualSonics | https://www.biotech.cornell.edu/sites/default/files/2020-06/Vevo%202100%20Operator%20Manual.pdf |
| Vevo imaging station | VisualSonics | https://www.biotech.cornell.edu/sites/default/files/2020-06/Vevo%202100%20Operator%20Manual.pdf |
| MicroScan transducer | VisualSonics | https://www.biotech.cornell.edu/sites/default/files/2020-06/Vevo%202100%20Operator%20Manual.pdf |
| 3D-printed prototype device | N/A | N/A |
| IsoTec 5 isoflurane vaporizer | Datex Ohmeda | N/A |
| Ultrasound gel | Medline | MDS092005 |
| U-100 insulin syringes (30G) | EasyTouch | 830565 |
| Sterile alcohol prep pads | Fisherbrand | 22-363-750 |
| Toothed forceps | N/A | N/A |
| Electric shaver | N/A | N/A |
| Gel cream hair remover | Veet | 3116876 |
| Cotton-tipped applicators | Fisherbrand | Ref. 22363157 |
| Kimwipes delicate task wipes | Kimtech | Code: 34155 |
| Micropore surgical tape | 3M | 1530–0 |
| Microcentrifuge tube, 1.5 mL | Thermo Scientific | 3448 |
| Stainless steel laboratory spatula | N/A | N/A |
| Artificial tears lubricant ophthalmic ointment | Henry Schein | NDC 11695-6832-1 |
| Thermo-Peep heated pad | K&H Pet Products | https://khpet.com/collections/small-animal-products/products/thermo-peep-heated-pad |
| Absorbent blue pad | Denville | 1158J48 |
Materials and equipment
Note: The Vevo Imaging System refers to the ultrasound machine used in establishing this protocol. The MicroScan Transducer is an individually purchased compatible ultrasound probe. The Vevo Imaging Station encompasses the animal physiological monitoring and maneuvering system. It contains a temperature-controlled heated platform, an integrated rail base to maneuver the animal in x, y, and z axes, and the transducer mounting system to secure the ultrasound probe. While Visual Sonics no longer sells this model, the Vevo F2 and Vevo F2 LT are appropriate alternatives. The link in the Key Resources Table refers to a comprehensive user manual for the Vevo 2100 Imaging System, which also includes the Vevo Imaging Station and MicroScan Transducer.
Note: the 3D-printed needle stabilization device that our group has previously published is a critical material for this protocol.1 Please refer to this cited publication for device schematic and dimensions that can be used for 3D printing as well as assembly instructions.
Step-by-step method details
Animal preparation: Fur removal
Timing: 5–10 min per mouse, 24 h prior to injection
This step prepares the animal for ultrasound-guided tumor cell injection by exposing skin required for efficient signal transduction by the ultrasound probe through ultrasound gel.
Note: The steps for fur removal are identical to those previously described except for site of fur removal.1 Instead of removing fur from the anterior abdomen, we remove fur from the anterior thorax.
-
1.Prepare depilatory station as previously described:1
-
a.Turn on the heated platform and set to 37°C.
-
b.Ensure that there is sufficient isoflurane and oxygen to carry out the procedure.
-
c.Place an absorbent blue pad on the heated platform and assemble tools:
-
i.Electric shaver.
-
ii.Unscented depilatory cream.
-
iii.PBS.
-
iv.Metal spatula.
-
v.Kimwipes.
-
vi.Cotton-tipped applicators.
-
i.
-
a.
-
2.Induce anesthesia as previously described:1
-
a.Transfer one mouse at a time to the anesthesia chamber and induce general anesthesia as per local animal review committee protocol.
-
b.Apply ophthalmic ointment to both eyes for anesthetic events lasting longer than 5 min.
-
c.Transfer anesthetized mouse to the preheated platform draped with an absorbent pad and continue anesthesia administration via nose cone.
-
a.
-
3.Remove fur:1
-
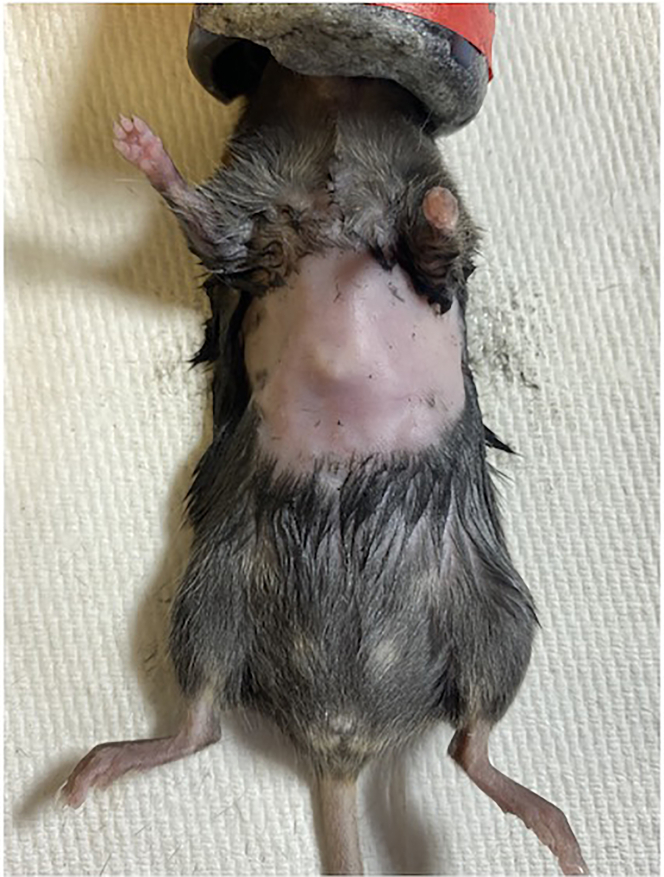
a.Using an electric shaver, remove fur from the anterior thorax from the suprasternal fossa to the costal margin (Figure 1).
-
b.Complete fur removal with depilatory cream as previously described.
-
c.Dispense a dime-sized amount of unscented depilatory cream and spread evenly over the shaved area using a cotton-tipped applicator.
-
d.Wait for approximately 2 min.
-
e.Using a metal spatula, gently remove cream and fur until skin is completely fur-free.
-
a.
-
4.
Transfer the mouse back to the cage on a heated platform and monitor for recovery from anesthesia.1
-
5.
Repeat above steps until all mice have undergone hair removal and have recovered from anesthesia.1
Figure 1.
Suggested extent of hair removal prior to ultrasound imaging
Ultrasound imaging and injection station preparation
Timing: 30 min
This step prepares the imaging and injection station for use and confirms operational status of all experimental equipment as previously described.1
Note: The steps for imaging and injection station preparation are identical to those previously described except for the insulin syringe.1 In this protocol, a 30-gauge insulin syringe is used. The following steps are abbreviated from the originally published protocol.1
-
6.
Ensure that tubing connections and anesthetic delivery cone are oriented on the right side of the Vevo Imaging Station such that when a mouse is placed in the supine position the right side of the mouse will face the proceduralist.
-
7.
Review supplies for induction and maintenance of anesthesia and ensure sufficient isoflurane and oxygen to perform the procedure.
-
8.
Turn on the physiological controller from the Vevo Imaging Station and set the platform temperature to 37°C.
-
9.
Warm ultrasound gel.
-
10.
Assemble required instruments and materials as previously described.1
-
11.
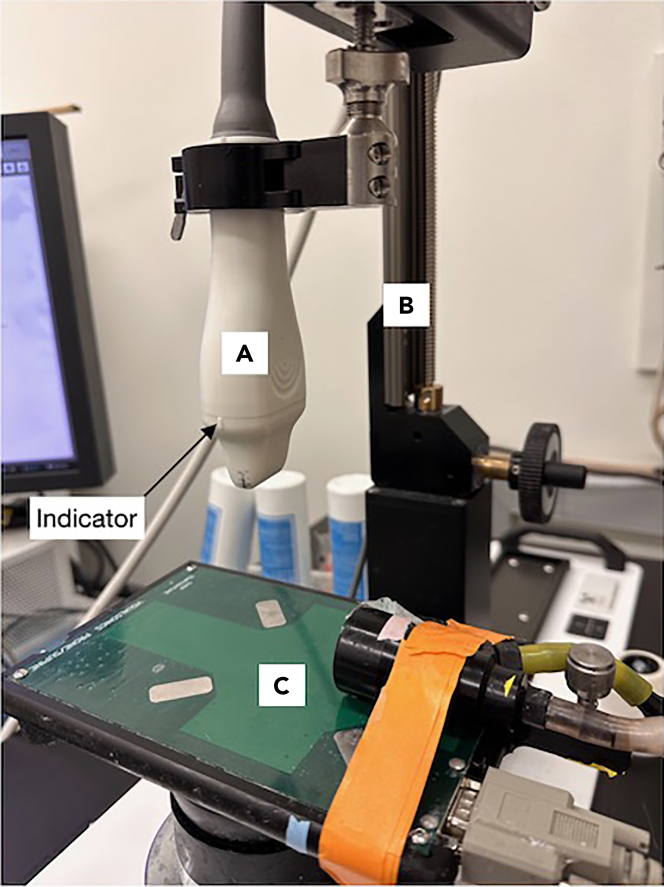
Select and mount the MicroScan Transducer hereafter referred to as “ultrasound probe” (Figure 2).
-
12.
Orient the ultrasound probe indicator toward the mouse’s right side (facing the injector).
-
13.
Secure the needle stabilization device to the ultrasound probe with the syringe-holding side facing the injector (Figure 3)
-
14.
Position the imaging station and ultrasound screen to maximize the injector’s ergonomics and accessibility to the injection site.
Figure 2.
Appropriately mounted MicroScan Transducer (ultrasound probe) within the Vevo Imaging Station
(A) MicroScan Transducer (ultrasound probe).
(B) Transducer mounting system.
(C) Animal physiological monitoring and maneuvering system.
Figure 3.
Appropriately mounted and positioned needle stabilization device
(A) Mounted ultrasound probe.
(B) Needle stabilization device mounted in correct orientation on ultrasound probe. Note that ultrasound probe indicator snuggly fits into notch within needle stabilization device.
Cell preparation for injection
Timing: 1 h
This step prepares tumor cells for injection with the desired cell density and volumes.
-
15.
Detach cells adhered to the tissue culture plate suspend in cell culture media according to the preferred cell detachment protocol.
Note: The B16F10-luc cells and KP4662-G12C gp100-luc cells used in this protocol were detached from a TC-treated 10 cm plate by washing the cells once with 2 mL PBS following incubation with 2 mL 0.25% trypsin. B16F10-luc cells were incubated for 2–3 min while KP4662-G12C gp100-luc cells were incubated for 5–7 min. The trypsin reaction was halted with 5 mL of cell culture media.
-
16.
Centrifuge cell suspension at 450 × g for 5 min at 4°C. Remove supernatant.
-
17.
Resuspend cells in at least 5 mL chilled PBS to wash the cells.
-
18.
Repeat centrifugation and PBS wash (steps 16 and 17) twice more for a total of 3 washes.
Note: Repeated washing of cells with PBS removes the FBS present in culture media prior to injection.
-
19.
After the 3rd wash resuspend cells in 5 mL PBS and pass through a sterile 40 μm filter into a new, sterile conical vial.
-
20.
Count cells and record viability.
-
21.
Perform final centrifugation of cells at 450 × g for 5 min at 4°C.
Note: We recommend that the viability of cells used in this protocol to be at least 90%. We have not tested this protocol with cells less viable than this threshold. We theorize that using less viable cells can adversely affect the reproducible establishment of lung metastases.
-
22.
Resuspend cells in volume of chilled PBS to reach desired cell concentration.
Note: Recommended injection volume is 100 μL per mouse as previously described.2 Volume of prepared cells should be sufficient for all mice in study plus 5 additional mice.
-
23.
Perform an additional cell count on this final cell suspension to record viability and concentration of viable cells at the time of injection.
-
24.
Keep cell suspension on ice until needed for injection.
Note: When initially establishing this protocol it is recommended to first optimize the number of tumor cells injected. This must be done for each cell line to be investigated. Optimal cell number is determined as the number of cells that consistently establish BLI signal in the anterior thoracic region within the desired time frame (for example 3–5 days). The B16F10-luc cells used in this protocol were injected at a concentration of 1.25–10 × 106 cells/mL, total volume of 100 μL per mouse as previously described.1 The KP4662-G12C-gp100-luc cells used in this protocol were injected at a concentration of 5–10 × 106 cells/mL, total volume of 100 μL per mouse. The ranges provided here are broad to reflect all the concentrations trialed while working with these cell lines. Titrations should reflect the experimental model and desired experimental design.
Ultrasound-guided injection
Timing: 10–15 min per mouse
This step injects prepared tumor cells into the right ventricle using a short-axis or cross-sectional view of the heart.
-
25.Anesthetize mice.
-
a.Anesthetize one mouse at a time in the anesthesia induction chamber as per local animal review committee protocol.Note: It is recommended to minimize the time under isoflurane due to the reduction in sympathetic tone which reduces cardiac output in mice.3Note: We used 5% isoflurane in oxygen (5 L per min) for anesthesia induction and 2.5% isoflurane in oxygen (5 L per min) for anesthesia maintenance as previously described.1
-
b.Apply ophthalmic ointment to both eyes for anesthetic events lasting longer than 5 min.
-
c.Transfer mouse to pre-heated animal imaging platform and maintain anesthesia via nose cone.
-
a.
-
26.
Secure mouse to platform by using approximately 1 inch of paper tape for each paw, such that the mouse is supine with face inside the nose cone and each limb fully extended (Figure 4).
Note: The electrocardiogram (ECG) pads on the Imaging Station will not be utilized during this procedure, so it is not required to have each paw make contact with the pads. However, they can be used as landmarks to aid in mouse positioning.1
Note:Figure 4 and subsequent Figure 5 depict the nose cone and mouse oriented on the left side, such that the left side of the mouse faces the injector. These images were taken when utilizing the potential solution described in troubleshootingproblem 1: difficulty obtaining a short axis cardiac window without shadowing artifact. To first establish a short axis cardiac window the mouse is oriented as described in step 25 (Figure 5).
-
27.
Sanitize the ultrasound probe with an alcohol wipe or 70% ethanol spray.1
-
28.
Wipe the previously shaved area of the mouse with an alcohol swab.1
-
29.
Tilt the imaging platform so that the mouse is head down, reminiscent of reverse Trendelenburg position (Figure 5).
Note: Positioning the mouse this way facilitates a cross-sectional view of the heart while minimizing the shadowing artifact from the ribs and sternum. We prefer a cross-sectional view in which the ultrasound probe is perpendicular to the ventricles. This allows simultaneous visualization of both ventricles. The most difficult aspect of this technique is obtaining a clear window without shadowing that would obscure the needle (see troubleshootingproblem 1: difficulty obtaining a short axis cardiac window without shadowing artifact).
-
30.
Apply a generous layer of pre-warmed ultrasound gel to the mouse.1
-
31.
Lower the ultrasound probe using the height control until it meets the ultrasound gel layer. Watch the ultrasound screen for the ultrasound image to come into view.1
-
32.
Using the fine forward and backward control knobs, scan the mouse and use anatomic landmarks to obtain an ideal window (Figure 6).
Note: Appropriate anatomical landmarks include: left ventricle (LV), right ventricle (RV), intraventricular septum (IVS), tricuspid/mitral/aortic valves, and tracing of the aorta back to the left atrium.
Note: Depending on the anatomy of an individual mouse, it may be necessary to adjust the angle of the platform to obtain the desired window. Obtaining an ideal injection window is the most challenging aspect of the procedure. Difficulty in obtaining a window can lengthen procedure time and total time under anesthesia. An experienced sonographer can obtain an injection window in less than two minutes, but a challenging window can take up to five minutes. If a window is not obtained within five minutes, it is recommended to abort the procedure and allow the mouse to recover from anesthesia. A second attempt can be made after the remaining mice in the cage have been injected.
-
33.
While the injector identifies the relevant landmarks, an assistant should remove the prepared cells from ice, thoroughly resuspend settled cells by vigorous tapping or gentle vortex, and aspirate 100 μL through the needle of 30-gauge insulin syringe.
Note: The assistant may need to aspirate a small amount of additional volume to aid in degassing of needle.
CRITICAL: It is essential to degas the needle thoroughly as even small amounts of air can prove lethal for the mouse.
-
34.
Adjust the gain as needed to clearly distinguish the ventricular cavity from the ventricular walls. As the ventricular cavities are filled with blood, they should appear black.
-
35.
Adjust the depth to fill 75% of the screen with the target anatomy to maximize image quality.
-
36.
Load the device with the syringe by snapping it into place (Figure 7).
Note: The needle stabilization device restricts lateral movement, maintaining the needle in plane with forward advancement.1 This procedure can be done without the assistance of the device but requires greater dexterity and has a steeper learning curve.
-
37.
Adjust the angle of entry so that the needle can be visualized within the gel cap at the intended site of entry.
-
38.
Using toothed forceps, grasp the skin lateral to the intended site of needle entry and gently pull the skin taut.1
-
39.
Gently advance the needle using the dominant hand.1
-
40.
Advance the needle through the skin and intercostal muscles.
-
41.
Release the skin and drop the forceps as they are no longer needed.
-
42.
Assess needle trajectory to ensure that no further angle adjustment is needed to enter the right ventricle.
Note: The right ventricle is much smaller and should be inferior to the left ventricle. A steeper angle may be required to avoid unnecessarily piercing the left ventricle.
-
43.
Advance the needle until it enters the right ventricle cavity. The needle should be seen between the walls of the ventricle during ventricular contraction (Figure 8).
Note: It is important for the needle to be in-plane so that the needle tip is visualized. Failure to do so can result in the needle entering the interventricular septum and lead to injection into the muscle wall rather than the ventricular cavity.
-
44.
Aspirate to confirm blood return.
-
45.
Inject cells at a relatively slow and constant rate.
Note: While the injector focuses on position of the needle during injection, an assistant can confirm blood return and dispensing rate.
CRITICAL: A rapid bolus into the ventricular cavity can cause cardiovascular collapse if cell suspension is rapidly administered.
-
46.
Gently remove the needle along the track of the needle stabilization device and discard it.
-
47.
Raise the ultrasound probe, remove the tape securing the mouse to the platform and recover the mouse by hand, applying pressure to the injection site with the left thumb.
Note: Pressure should be firm, but not so firm as to restrict diaphragmatic excursion during respiration as mouse regains consciousness.
-
48.
Transfer the mouse to a cage on a heated platform once the mouse has recovered enough from anesthesia to start ambulating.
Figure 4.
Mouse appropriately secured to imaging platform with paper tape
Figure 5.
Suggested positioning of the platform for cardiac imaging
Figure 6.
Short axis cardiac window with labeled anatomic landmarks
Figure 7.
Image of loaded syringe stabilization device
Figure 8.
Ultrasound image of needle positioned within the right ventricle
Bioluminescent imaging
Timing: 5–10 min per cage
Duration: Every 3 days until experimental endpoint is reached.
This step measures tumor cell engraftment and growth via bioluminescent imaging.
Note: Imaging frequency of once every 3 days is necessary when initially characterizing the growth kinetics of each cell line and number of cells injected, as time to initial signal can vary.
-
49.
Anesthetize mice as per local animal review committee protocol. Anesthesia is maintained during imaging via nose cone per local animal review committee protocol.
Optional: To minimize time under anesthesia, intraperitoneal injections of D-Luciferin can be performed without anesthesia.
-
50.
Perform 50 μL intraperitoneal injections of D-Luciferin (50 mg/kg).
-
51.
Acquire bioluminescent images at 5 min post-injection for the mice injected with B16F10-luc cells and 15 min post-injection for the mice injected with KP4662-G12C gp100-luc cells using the IVIS Lumina III imaging system.
Note: Waiting period for image acquisition post-luciferin injection must be optimized for each cell line due to differing expression levels of luciferase.
Note: For this protocol, we performed a four-image series with an F/Stop of 1.2 at 1, 5, 10, and 20 s as previously described.1 All images displayed in this protocol were from the fourth image (F/Stop 1 at 20 s) using a color intensity scale ranging from 1.00 × 105 to 1.00 × 108.
-
52.
Transfer mice to a cage on heated platform and monitor for recovery from anesthesia.1
Expected outcomes
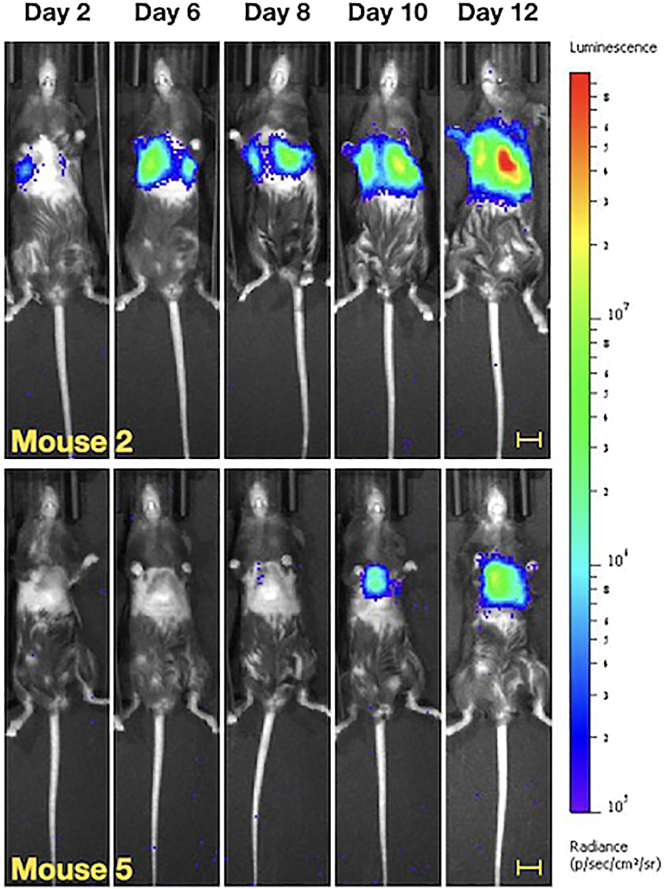
In the B16F10-luc model, 100% of mice developed BLI signal in the lungs bilaterally by 12 days post-injection (Figure 9). Bioluminescent imaging (BLI) signal correlated with visible, hyperpigmented lesions in the lungs at necropsy (Figure 10). In the KP4662-G12C gp100-luc model, 100% of mice developed BLI signal in the bilateral lungs by 14 days post-injection (Figure 11). At necropsy, cystic, off-white macrometastatic lesions were observed in the lungs (Figure 12). Some mice may develop initial BLI signal as early as 3–6 days post-injection. Sometimes, initial signal may be limited to one lung or the other. Subsequent imaging will demonstrate bilateral signal. This is distinct from the boot-shaped left-sided cardiac signal from inadvertent injection into the ventricular wall muscle (Figure 13). If injections are performed under ultrasound-guidance using in plane technique, intraventricular injection should occur infrequently (less than 1%). This technique was developed to improve engraftment rates of our cell line of interest, the KP4662-G12C gp100-luc line. While we do not yet know how route of cell delivery affects the biology of metastatic development, we were able to greatly improve engraftment rates and obtain more uniform signal using this technique compared with tail vein injections (Figure 14). As discussed in the limitations section however, we do not suggest that this technique is superior to standard tail vein injection. Rather, it is an alternative strategy to developing lung metastases with a unique set advantages and disadvantages.
Figure 9.
Representative BLI imaging of C57BL/6 mice injected with 1 × 106 B16F10-luc cells from 2 to 12 days post-injection
Bars denote 1 cm.
Figure 10.
Representative BLI imaging of C57BL/6 mice injected with 1 × 106 B16F10-luc cells per mouse at experimental endpoint (12 days post-injection) alongside harvested lungs demonstrating pigmented metastatic lesions
Bars denote 1 cm.
Figure 11.
Representative BLI imaging of C57BL/6 mice injected with 1 × 106 CGF3R-L3-luc cells per mouse from 3 to 14 days post-injection alongside harvested lungs demonstrating cystic metastatic lesions at experimental endpoint (day 14)
Bars denote 1 cm. White outline highlights examples of cystic metastatic lesions within lung tissue.
Figure 12.
Representative BLI imaging of C57BL/6 mice injected with 1 × 106 CGF3R-L3-luc cells per mouse at experimental endpoint (14 days post-injection) alongside harvested lungs demonstrating cystic metastatic lesions
Bars denote 1 cm. White outline highlights examples of cystic metastatic lesions within lung tissue.
Figure 13.
Example of boot shaped BLI signal resulting from intracardiac injection along with necropsy photo
This mouse was injected with 1 × 106 B16F10-luc cells per mouse. Arrows showing mediastinal tumor burden. Bar denotes 1 cm.
Figure 14.
Representative BLI imaging of C57BL/6 mice injected with 1 × 106 B16F10-luc cells from 2 to 12 days post injection
Mice were injected using either the standard tail vein technique (left) or ultrasound-guided right ventricle (US-RV) technique (right). Bars denote 1 cm.
Limitations
A major limitation of this technique is the amount of time it takes compared to the standard tail vein technique. While this technique results in very homogenous signal across mice and eliminates the problem of tail engraftment, it does require more time. An experienced tail vein injector requires between 30 s to 1 min per mouse. An experienced injector, aided by a stabilization device, can perform this procedure within two to 5 min. The injection itself, defined as advancing the needle through the subcutaneous tissue and into the ventricle, requires less than 30 s. The most time-consuming aspects of the procedure are obtaining an injection window, especially for novice sonographers, and the manual recovery of the mouse. To minimize time between injections, a second person focused on post-procedure recovery is advisable. A major benefit of using ultrasound guidance in conjunction with the needle stabilization device is that only one needle stick is required, reducing injection attempts and trauma. With proper technique, this minimally invasive approach should not be more traumatic than a subcutaneous injection, and may be less stressful to mice as it occurs under anesthesia. Due to the investment required in terms of equipment and training, this protocol is best suited to a group already using ultrasound for in vivo work. Using our needle-stabilization device however, our group was able to train personnel from another lab with no prior ultrasound experience to reliably perform this technique with fewer than five 1-h training sessions.
It also must be emphasized that success of homogeneous bilateral lung engraftment of cells is due in part to the cell line’s tropism for the lung. Lung metastases were unable to be reliably established with our KP4662-G12C gp100-luc using the standard tail vein approach.
While the two cell lines reported in this protocol establish themselves well in the lung microenvironment, it can be reasoned that other models may result in a longer time to initial bioluminescent signal or greater variability in growth kinetics. Some of these obstacles may be overcome with increasing the number of cells injected. If tumor burden variability is greater than the tolerances accepted, cells from tumors displaying the quickest growth kinetics can be cultured ex vivo and injected into a second cohort of mice using this protocol. This process can be repeated several times to develop a cell line possessing increased tropism for the lung compared to the original.
An additional limitation of this protocol is the use of bioluminescent imaging to monitor tumor engraftment and growth, as it requires the cell line to express a foreign protein (firefly luciferase). Immunogenicity to such reporter proteins and potential changes to the tumor microenvironment have been previously described and were not explored in the development of this protocol.3,4 Alternative methods of quantifying reporterless tumor burden in the lungs, such as qRT-PCR, would need to be performed at experimental endpoint.5 This approach is limited to experiments in which the outcome of interest is survival as metastatic engraftment can only be confirmed post-mortem.
Troubleshooting
Problem 1: Difficulty obtaining a short axis cardiac window without shadowing artifact
After identifying anatomical landmarks, it can sometimes be difficult to obtain a safe injection window (Steps 25 to 28). This is most often due to shadowing artifact from the ribs or sternum.
Potential solution
In steps 25 to 28, we offer suggestions for initial ultrasound probe positioning. However, individual mice have slightly different positions of the heart and slight adjustments of the platform and/or probe angle may be needed to obtain an ideal window. Ultrasound windows can be optimized by adding more gel, increasing probe pressure, or changing the position of the mouse. Adjusting the position of the mouse by changing the platform angle and/or probe angle is often more effective than simply increasing probe pressure. Rib shadowing is less obstructive because the ribs are much thinner than the sternum. Fine adjustments are typically sufficient to obtain a clear injection window. When the desired cross-sectional view is directly below the sternum, it may be necessary to apply probe pressure at an angle. To accomplish this, the heated platform is tilted to bring the right side of the mouse down (Figure 15). Gravity helps bring the heart closer to the ultrasound probe and pull the heart lateral to the sternum. Then, probe pressure is slightly increased so that the heart slides to the right side of the sternum. More important than the amount of pressure is the direction of force. Application of excess force may shift the cardiac structures, but also impair venous return to the heart and lead to cardiac arrest. If the heart rate begins to slow, this is a sign that excessive pressure has been applied. Heart rate is observed by visualization of ventricular systole/diastole on the ultrasound screen.
Figure 15.
Example of possible positioning strategies to obtain an ideal injection window
Problem 2: Poor post-procedure recovery
Poor post-procedure recovery describes unanticipated mortality following a technically successful, atraumatic (one pass with the needle) injection (Steps 40 and 41).
Potential solution
Difficulty with recovery was observed when we first established this procedure and was attributed to several possible factors. The primary factor was length of time under anesthesia. To minimize time under anesthesia, we only anesthetize one mouse at a time. Furthermore, we keep procedure time under 7 min (up to 5 min for obtaining an injection window and up to 2 min for intraventricular injection). The lengthiest part of the procedure is obtaining the injection window. If there is great difficulty in obtaining an injection window despite applying the maneuvers described in troubleshooting problem 1: difficulty in obtaining a short axis cardiac window without shadowing artifact, we return the mouse to its cage and proceed with the next mouse. After injecting the remaining mice, we make a second attempt to obtain a suitable injection window. Often, an optimal window is obtained without the need for additional troubleshooting. A second factor is ultrasound probe pressure. Excessive probe pressure can cause cardiac compromise by restricting both cardiac inflow and outflow. Probe pressure is applied judiciously and strategically, after first attempting to adjust the platform and/or probe angle. Another possible cause is failure to apply direct pressure to the puncture wound leading to hematoma formation and possible pericardial tamponade. The mice should be held using two hands for warmth with the nondominant thumb applying direct pressure to the site of needle entry.
Problem 3: Extracardiac injection
The right ventricle cavity is much smaller than the left ventricle. If care is not taken to advance the needle into the cavity using in plane technique, it is possible to lose visualization of the needle tip (Step 38). When this occurs, the needle tip is most commonly in the ventricular wall. Injection into the ventricular muscle will result in a characteristic, boot shaped BLI signal in the absence of bilateral lung signal (Figure 13). It is much more difficult to mistakenly inject cells into the left ventricle as it is easy to distinguish the ventricles in short axis view (the left ventricle has much thicker walls and a much larger cavity). Inadvertent injection into the left ventricle will result in BLI signal throughout the body as opposed to signal restricted to the thoracic region (Figure 16).
Figure 16.
Representative image demonstrating the different patterns of metastatic engraftment seen with left ventricular vs. right ventricular injection
C57BL/6 mice were injected with 1 × 106 B16F10-luc cells per mouse. BLI imaging was performed at 8 days post-injection. Bars denote 1 cm.
Potential solution
To avoid mis-injection, it is important to obtain an optimal injection window as previously described. It is also critical to use in plane technique to permit continuous visualization of the full length of the needle. Do not advance the needle farther into the cavity than necessary. It is also critical to confirm blood return upon aspiration prior to cell injection.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Timothy Donahue, MD (tdonahue@mednet.ucla.edu).
Materials availability
Timothy Donahue, MD (tdonahue@mednet.ucla.edu) or Caius Radu, MD (cradu@mednet.ucla.edu).
Data and code availability
Timothy Donahue, MD (tdonahue@mednet.ucla.edu) or Caius Radu, MD (cradu@mednet.ucla.edu).
Acknowledgments
This research was supported in part by a grant from the Jonsson Comprehensive Cancer Center, a grant from the H & H Lee Surgical Research Scholars Program, the National Institutes of Health (NIH) National Cancer Institute (NCI) Research Supplements to Promote Diversity in Health-Related Research (R01CA250529), and an NIH grant R01CA260678.
Author contributions
Conceptualization, A.L.; methodology, A.L. and A.C.; validation, A.L. and A.C.; investigation, A.L., A.C., H.L., and E.T.; writing – original draft, A.L. and A.C.; writing – review and editing, A.L., A.C., H.L., and E.T.; supervision, C.R. and T.D.; funding, C.R. and T.D.
Declaration of interests
The 3D-printed prototype device has been submitted for a provisional patent (U.S. Provisional Application No. 63/386,961) listing A.L. as the inventor.
Contributor Information
Amanda Labora, Email: alabora@mednet.ucla.edu.
Timothy Donahue, Email: tdonahue@mednet.ucla.edu.
References
- 1.Labora A., Lee H., Chan C., Tabornal E., Le T., Rashid K., Abt E., Yamao T., Mandl H., Creech A., Premji A., et al. Generation of liver metastases in a mouse model using ultrasound-guided intravenous injection. STAR Protocols. 2023;4 doi: 10.1016/j.xpro.2023.102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou H., Zhao D. Ultrasound imaging-guided intracardiac injection to develop a mouse model of breast cancer brain metastases followed by longitudinal MRI. J. Vis. Exp. 2014;85:51146. doi: 10.3791/51146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L., Bommireddy R., Munoz L.E., Guin R.N., Wei C., Ruggieri A., Menon A.P., Li X., Shanmugam M., Owonikoko T.K., et al. Expression of tdTomato and luciferase in a murine lung cancer alters the growth and immune microenvironment of the tumor. PLoS One. 2021;16 doi: 10.1371/journal.pone.0254125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultheiß C., Binder M. Overcoming unintended immunogenicity in immunocompetent mouse models of metastasis: the case of GFP. Sig Transduct Target Ther. 2022;7:68. doi: 10.1038/s41392-022-00929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahn M.L., Dean C.A., Jo D.B., Coyle K.M., Marcato P. Human-specific GAPDH qRT-PCR is an accurate and sensitive method of xenograft metastasis quantification. Mol. Ther. Methods Clin. Dev. 2021;20:398–408. doi: 10.1016/j.omtm.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Timothy Donahue, MD (tdonahue@mednet.ucla.edu) or Caius Radu, MD (cradu@mednet.ucla.edu).