Abstract
Objective
To investigate the therapeutic potential of dimethyl fumarate (DMF) in improving erectile function of bilateral cavernous nerve injury (BCNI) rats, along with elucidating its underlying mechanisms.
Methods
A BCNI rat model was established by clamping bilateral cavernous nerve (CN). DMF was given by gavage at low (20 mg/kg/day) and high (40 mg/kg/day) dosages for a duration of 4 weeks. Erectile function was assessed by electrical stimulation of CN. Penis and CN tissues were collected for subsequent analysis. Additionally, PC-12 cell line was used to verify the mechanism of DMF in vitro. Nfe2l2 or Ho-1 gene knockdown PC-12 cell lines were constructed by lentiviral transfection, respectively. A damaged cell model was induced using H2O2. And then molecular biological methods were employed to analyze cellular molecules and proteins.
Results
DMF administration for 4 weeks led to improvements in erectile function, reduced fibrosis of penis corpus cavernosum in BCNI rats. The morphology of CN was improved and the number of nerve fibers increased. Furthermore, the levels of nNOS, NO, and cGMP were increased, while Ca2+ was decreased in penis corpus cavernosum. Notably, the levels of ROS, 3-NT and NLRP3 inflammasomes production were reduced, alongside increased expression of Nrf2 and HO-1 proteins in the dorsal penile nerve (DPN) and CN. In vitro, DMF increased cell viability, reduced ROS level, promoted SOD, diminished 3-NT, MDA and DNA damage markers, and inhibited the activation of NLRP3 inflammasomes in H2O2 induced PC-12 cells. Nfe2l2 knockdown and Ho-1 knockdown significantly attenuated the protective effect of DMF, respectively. Furthermore, inhibition of ROS production by N-acetylcysteine led to a reduction in NLRP3 inflammasome activation in H2O2 induced PC-12 cells.
Conclusions
DMF improved erectile function of BCNI rats by protecting nerves through inhibiting oxidative stress and the activation of NLRP3 inflammasome-mediated pyroptosis via activation of Nrf2/HO-1 pathway.
Keywords: Dimethyl fumarate, Erectile dysfunction, Cavernous nerve injury, Oxidative stress, Pyroptosis, Nrf2/HO-1
Graphical abstract
Highlights
-
•
Dimethyl fumarate enhanced cavernous nerve regeneration and improved erectile function.
-
•
Activated Nrf2/HO-1 signaling plays a key role in oxidative stress and intraneural NLRP3 inflammasome-mediated pyroptosis.
-
•
Oxidative stress and pyroptosis in injured nerve were inhibited by dimethyl fumarate.
Non-standard abbreviations and acronyms
- DMF
Dimethyl fumarate
- BCNI
Bilateral cavernous nerve injury
- CN
Cavernous nerve
- DPN
Dorsal penile nerve
- NED
Neurogenic erectile dysfunction
- RP
Radical prostatectomy
- PDE5i
Phosphodiesterase type 5 inhibitors
- ROS
Reactive oxygen species
- MPGs
Major pelvic ganglions
- ICP
Intracavernous pressure
- MAP
Mean arterial pressure
- DHE
Dihydroethidium
- PI
Propidium
- NAC
N-acetyl-l-cysteine
1. Introduction
Neurogenic erectile dysfunction (NED) is the persistent inability to initiate and sustain the penile erection for satisfactory sexual intercourse due to neurogenic disorder [1]. The main etiology of NED is cavernous nerve (CN) injury caused by radical prostatectomy (RP) for prostate cancer [2]. Several studies have exhibited the prevalence rate of erectile dysfunction (ED) after RP is up to 85 % [3]. Long-term denervation leads to the morphological changes in the penile corpus cavernosum, including increased collagen synthesis, decreased content of corpora cavernosa smooth muscle and the down-regulated neuronal nitric oxide synthase (nNOS)/nitric oxide (NO) pathway [[4], [5], [6]]. These changes result in a poor response to phosphodiesterase type 5 inhibitors (PDE5i), the first line treatment for ED [7,8]. Thus, it is necessary to investigate the pathogenetic mechanism of NED and identify novel therapeutic targets.
Oxidative stress refers to an imbalanced state between the level of antioxidants and the generation of reactive oxygen species (ROS) within a biological system. This physiological state, characterized by an excess of ROS, can result in detrimental consequences such as cellular death and the impairment of tissue functionality [9]. Oxidative stress has been shown to inhibit the nerve regeneration after the cavernous nerve injury [10]. Wang et al. demonstrated the levels of oxidative stress of serum and corpus cavernosum were elevated and contributed to the development of ED in CN injured rat model [11]. Several studies have demonstrated that ROS production might trigger NLRP3 inflammasome-mediated pyroptosis, which participated in the initiation and progression of diverse pathological conditions, including but not limited to cardiovascular diseases, spinal cord injury and chronic degenerative diseases [[12], [13], [14], [15], [16], [17]]. Pyroptosis is a form of inflammatory programmed cell death, which is classified as innate immune response against both exogenous and endogenous stimuli [18]. The activation of NLRP3 inflammasomes leads to the subsequent activation of Caspase-1, which, in turn, triggers the cleavage of Gasdermin-D (GSDMD) into its N-terminal domain (GSDMD-N) and facilitates the activation of pro-inflammatory factors, namely interleukin-18 (IL-18) and interleukin-1β (IL-1β). Ultimately, GSDMD-N assembles into cytotoxic pores on the plasma membrane, instigating the execution of pyroptosis [18,19].
Nuclear factor erythroid-derived 2-like 2 (Nrf2) represents a pivotal participant in the intracellular antioxidant response process, rendering it one of the most crucial cytoprotective factors. Within the cytoplasm, Kelch-like ECH-associated protein 1 (Keap1) assumes the role of a negative regulator by binding to Nrf2 and promoting its degradation through ubiquitination. Upon exposure to oxidative stress, Keap1 is modified and Nrf2 is released from Keap1. Consequently, Nrf2 translocates to the nucleus, where it orchestrates the activation of a constellation of cytoprotective genes via binding to the antioxidant response element (ARE) [18,20,21]. Among these genes, heme oxygenase-1 (HO-1) emerges as a principal target protein of Nrf2 and it is the rate-limiting enzyme that degrades heme into free iron, carbon monoxide, and biliverdin [22,23]. Notably, HO-1 and its metabolites perform a critical role in the maintenance of cellular redox homeostasis [24]. Therefore, Nrf2/HO-1 pathway exerts vital effect on reducing oxidative stress and promoting cell survival.
Dimethyl fumarate (DMF) is an effective drug approved by the USA Food and Drug Administration (FDA) for the management of relapsing-remitting multiple sclerosis. Notably, both DMF and its metabolite, monomethyl fumarate, demonstrate the ability to degrade Keap1, thereby facilitating the accumulation of Nrf2 and exert the role of reducing oxidative stress. Multiple sclerosis is a chronic autoimmune disorder affecting the central nervous system. The underlying pathophysiology involves significant neurodegenerative changes and demyelination of nerve fibers. Oxidative stress and neuroinflammation are key contributors to the development and progression of the disease. DMF has been recognized for its neuroprotective and immunomodulatory properties, primarily through the activation of Nrf2 and its downstream antioxidant pathway. Additionally, DMF exhibits therapeutic potential by inhibiting NF-κB signaling pathway and activating the HCAR2-AMPK-Sirt1 signaling pathway via interacting with the Nrf2 signaling pathway [[25], [26], [27]]. The neuroprotective effects of DMF have also been observed in the context of peripheral nerve injury. Studies have demonstrated that oxidative stress played a significant role in the development of neuropathic pain, which could activate pain pathways through various mechanisms. By activating Nrf2 and its downstream antioxidant pathways, DMF exhibits the potential to alleviate neuropathic pain [[28], [29], [30]]. Moreover, DMF demonstrates the ability to mitigate Wallerian degeneration of injured nerves and promote nerve repair and regeneration in sciatic nerve injury murine model, achieved through its neuroprotective and immunomodulatory actions [31,32]. Nevertheless, to date, there is a dearth of literature documenting the utilization of DMF as a therapeutic intervention for NED. Additionally, the efficacy of DMF in ameliorating erectile dysfunction in NED patients by mitigating the extent of nerve impairment through the activation of the Nrf2/HO-1 antioxidant pathway, remains unexplored and warrants further investigation.
In summary, our hypothesis posits that oxidative stress and the activation of NLRP3 inflammasome-mediated pyroptosis may play a contributory role in the development and progression of NED. Furthermore, we propose that the administration of DMF can ameliorate erectile dysfunction in NED patients by mitigating CN injury and facilitating nerve repair via activating Nrf2/HO-1 antioxidant pathway. The primary objective of this study is to elucidate the specific mechanism by which DMF treats NED in bilateral cavernous nerve injury (BCNI) rat model. The findings of this study provide promising therapeutic targets and a theoretical foundation for the treatment of NED patients.
2. Materials and methods
2.1. Animal and experimental design
All animal experiments were approved by the Animal Care and Use Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Approval No. TJH-202110012). Thirty-two 8-week-old specific pathogen free male Sprague Dawley rats were housed in our animal facility with a temperature of 22 ± 2 °C and a 12:12 h dark-light cycle. Food and water were available ad libitum. They were divided into one Sham group and three BCNI groups randomly, 8 rats respectively, after one week acclimatization. Two groups of BCNI rats were treated with DMF (HY-17363, MedChemexpress) by oral gavage with the dosages of 20 mg/kg/d and 40 mg/kg/d, as the low dosage group (BCNI+DMF-LD) and high dosage group (BCNI+DMF-HD) [33], while the other groups were treated with vehicle (0.08 % methylcellulose in saline) postoperatively.
2.2. Establishment of BCNI rat model
All rats were anesthetized with pentobarbital sodium at a dosage of 40 mg/kg. Subsequently, a 2–3 cm long midline abdominal incision was performed to expose the prostate. After identification of major pelvic ganglions (MPGs) and CNs on the surface of posterolateral prostate, no further surgical operations were performed on the rats allocated to the Sham group. In the rats of BCNI groups, the bilateral CNs were crushed at 5 mm distal to bilateral MPGs for 2 min with an ophthalmic needle holder with the same force.
2.3. Evaluation of erectile function
Following four weeks of treatment with either DMF or a vehicle solution subsequent to the surgical procedure, erectile function was evaluated by electrostimulation to CNs according to the previously described methodology [34]. Briefly, MPGs and CNs were exposed with the abovementioned method. To measure intracavernous pressure (ICP), a 25-gauge needle filled with 200U/ml heparin solution was inserted into the left penile crura. Simultaneously, mean arterial pressure (MAP) was monitored using a heparinized PE-50 tube inserted into the left carotid artery. Electrical stimulation of the CNs was executed via a bipolar hook electrode positioned between the MPG and the site of injury in the CNs. The stimulus parameters were set as follows: voltage 5.0V, frequency 15Hz, pulse width 1.2 msec and a duration of 1 min. The tubes measuring ICP and MAP and the electrode were connected to the pressure transducers (BL-420F, Techman, Chengdu, China) to record the measurement of ICP and MAP. Erectile function was evaluated by the ratios between max ICP and MAP and total ICP (represented by the area under the curve, AUC). Following the assessment of erectile function, both penile tissue and CNs were meticulously harvested for subsequent analysis.
2.4. Masson trichrome staining
Penile tissue specimens, which had been fixed in a 4 % paraformaldehyde solution, were subjected to paraffin embedding and subsequently sectioned into 5 μm-thick slices. Masson trichrome staining was performed as the standard protocol described. The ratio of smooth muscle and collagen in corpus cavernosum was evaluated with Image-Pro Plus software (Version 6.0).
2.5. Immunohistochemistry (IHC)
Penis and CN tissues were fixed, embedded and cut into sections as mentioned above. As described in previous study [35], sections were incubated with anti-α-SMA (1:200, rabbit, Cat#GB111364, Servicebio), anti-3-Nitrotyrosine (3-NT, 1:50, mouse, Cat#ab61392, abcam), anti-NLRP3 (1:50, rabbit, Cat#IMG-6668A, NOVUS Biologicals) and anti-Caspase-1 (1:200, rabbit, Cat#22915-AP, Proteintech) overnight at 4 °C. Then the sections were washed by PBS and incubated by relevant secondary antibodies for 1 h at room temperature. Five microscopic images were randomly taken.
2.6. Immunofluorescence (IF)
The paraffin-embedded sections of penis and CN tissues and PC-12 cell slides were used for IF to explore the expression of target proteins. As described in previous study [35], the primary antibodies used for incubating sections were anti-neuronal nitric oxide synthase (nNOS, 1:100, mouse, Cat#610308, BD Pharmingen), anti-ATF3 (1:100, rabbit, Cat#A13469, Abclonal), anti-neurofilament 200 (NF-200, 1:200, rabbit, Cat#18934-1-AP, Proteintech), anti-Nrf2 (1:200, rabbit, Cat#16396-1-AP, Proteintech), anti–HO–1 (1:100, rabbit, Cat#ab68477, abcam), anti-GSDMD (1:500, rabbit, Cat#P30823, abmart). Then appropriate secondary antibodies were selected for further incubating. Five microscopic images were randomly taken and the semiquantitative analysis of relative fluorescence intensity was determined by Image-Pro Plus software (Version 6.0).
2.7. Transmission electron microscopy (TEM)
For TEM analysis, fresh penis was cut into slices with the thickness no more than 1 mm. The slices were fixed in the 0.1 M PBS containing 2.5 % glutaraldehyde for 4h. Then the slices were soaked in 0.1 M PBS containing 1 % osmium tetroxide for 2h at room temperature. The slices were dehydrated and treated with propylene oxide. Thereafter, the slices were cut into ultrathin sections (60–80 nm) and stained with uranyl acetate and lead citrate. Finally, the ultrastructural features of the tissue were captured through transmission electron microscope (Hitachi HT7700, Tokyo, Japan).
2.8. Measurement of NO, cyclic guanosine monophosphate (cGMP) and Ca2+ levels
The fresh penis corpus cavernosum was used to detect the concentration of NO, cGMP and Ca2+. Total Nitric Oxide Assay Kit (S0024, Beyotime) was applied to detect the level of NO and Rat cGMP Enzyme Linked Immunosorbent Assay (ELISA) Kit (JM-01434R2, JINGMEI) was used to measure the concentration of cGMP. The content of Ca2+ was determined by Calcium Colorimetric Assay Kit (S1063S, Beyotime). They were performed according to the manufacturer's protocols. The levels of NO, cGMP and Ca2+ were normalized to the protein concentration.
2.9. Cell culture and cell viability assay
The PC-12 cell line was derived from a pheochromocytoma in male rats, and the highly differentiated PC-12 cell line has a remarkable neuronal phenotype [[36], [37], [38]]. The highly differentiated PC-12 cell line was purchased by Procell. PC-12 cells were sustained in RPMI 1640 (PYG0066, Boster) containing 10 % fetal bovine serum (FCS500, Excell) and 1 % penicillin/streptomycin at 37 °C with 5 % CO2.
To assess the cytotoxicity of DMF and hydrogen peroxide (H2O2, AR1108, Boster) on PC-12 cells, various concentrations and durations of exposure to DMF and H2O2 were employed. 104 cells per well were inoculated and cultured in 96-well plates for 24h. After the treatment of DMF and H2O2, cell viability was assessed using the Cell Counting Kit-8 (CCK-8, AR1160, Boster) performed referring to the manufacturer's protocol. The absorbance of each well was measured by a microplate reader (Multiskan FC, Thermo Fisher Scientific, USA) at 450 nm.
2.10. Construction of lentivirus and infection of target PC-12 cell lines
Nrf2 small hairpin RNA (Nrf2 shRNA) and HO-1 small hairpin RNA (HO-1 shRNA) were designed to knockdown Nfe2l2 and Ho-1 of PC-12 cell line, respectively. In parallel, the negative control shRNA targeted sequences with no homology to any known rat genes was constructed. The specific sequences of the shRNAs are provided in Supplementary Table 1. The lentiviral vectors were generated and the competent cells containing target plasmids were constructed by Genomeditech. The plasmids were extracted by FastPure EndoFree Plasmid Mini Kit (DC203-01, Vazyme). The HEK-293T packaging cell line was cultured and transfected with target plasmids and lentiviral packaging plasmids to generate lentiviruses. Following 48-h incubation period, the supernatant containing virus was harvested and purified. Prior to lentiviral infection, the PC-12 cell line was cultivated in 12-well plates until reaching a confluence of approximately 70 %. Subsequently, the PC-12 cells were treated with the lentiviral supernatant for a duration of 24 h, followed by the replacement of the culture medium with fresh RPMI complete medium for an additional 48-h incubation period. The PC-12 cell line was harvested and screened with 2 μg/ml puromycin to obtain stable Nfe2l2 and Ho-1 knockdown cell lines, respectively. The knockdown efficiency of Nfe2l2 and Ho-1 was evaluated by western blot analysis and quantitative reverse transcription polymerase chain reaction (qRT-PCR).
2.11. qRT-PCR analysis
Total RNA of PC-12 cells was extracted with TRIzol reagent (G3013, Severicebio) and cDNA was synthesized with Hifair® Ⅲ Reverse Transcriptase (11111ES92, YEASEN). qRT-PCR was conducted to quantify the transcripts using Hieff® qPCR SYBR Green Master Mix (11202ES03, YEASEN) and QuantStudio 6 Flex system (Appliedbiosystems, Thermofisher, USA). The relative levels of target RNA were calculated using the 2−ΔΔCt method. The specific primers used in qRT-PCR are shown in Table 1.
Table 1.
Sequences of primers for qRT-PCR.
| Primers | Sequences |
|---|---|
| β-actin | F 5′-ATCATTGCTCCTCCTGAGCG-3′ |
| R 5′-GAAAGGGTGTAAAACGCAGCTC-3′ | |
| Nrf2 | F 5′-CCAAGCATATCACAACCATT-3′ |
| R 5′-GACCACTGTATAGGATCATAGG-3′ | |
| HO-1 | F 5′-CTTTCAGAAGGGTCAGGTGTC-3′ |
| R 5′-TGCTTGTTTCGCTCTATCTCC-3′ |
2.12. Cell experimental design
PC-12 cells were categorized into five distinct experimental groups as follows: I. Control group: PC-12 cells were only treated with 0.1 % dimethyl sulfoxide (DMSO); II. H2O2 group: PC-12 cells were treated with H2O2 and 0.1 % DMSO; III. DMF group: PC-12 cells were treated with H2O2 and DMF (DMF was dissolved in 0.1 % DMSO); IV. sh-Nrf2 group: Nfe2l2 knockdown PC-12 cells were treated with H2O2 and DMF (DMF was dissolved in 0.1 % DMSO); V. sh–HO–1 group: Ho-1 knockdown PC-12 cells were treated with H2O2 and DMF (DMF was dissolved in 0.1 % DMSO).
2.13. Western blot
PC-12 cells were employed for the subsequent protein content analyses and lysed by RIPA lysate (R0010, Solarbio) containing protease inhibitor cocktails (HY-K0010, MedChemexpress). Then the solution was centrifugated after ultrasonic processing. Nuclear-Cytosol Extraction Kit (P1200-50, Applygen) was used to isolate nuclear and cytosolic fractions. The protein concentration was measured by BCA assay (AR0146, Boster). 30 μg protein samples were run on 10 % sodium dodecyl sulfate-polyacrylamide gel and then transferred to the PVDF membrane. The membrane was blocked in 5 % bovine serum albumin dissolved in Tris-buffered saline-Tween solution for 1 h and then incubated with primary antibodies overnight at 4 °C. The primary antibodies included anti-Nrf2 (1:1000, rabbit, Cat#ab92946, abcam), anti–HO–1 (1:1000, mouse, Cat#66743-1-Ig, Proteintech), anti-NLRP3 (1:500, rabbit, Cat#IMG-6668A, NOVUS Biologicals), anti-Caspase-1 (1:1000, rabbit, Cat#22915-AP, Proteintech), anti-GSDMD (1:1000, rabbit, Cat#P30823, abmart), anti-Cleaved-Caspase-1 (1:1000, rabbit, Cat#AF4005, Affinity), anti-β-actin (1:1000, rabbit, Cat#20536-1-AP; Proteintech) and anti-Histone3 (1:1000, rabbit, Cat#17168-1-AP, Proteintech). Following incubation with primary antibodies, appropriate secondary antibodies were applied and incubated for 1 h at room temperature. The relative intensity of protein bands was assessed using ImageJ software ((https://imagej.nih.gov/ij/docs/index.html) with the corresponding β-actin and Histone3 as the control bands.
2.14. Detection of ROS
ROS in the dorsal penile nerve (DPN) and CN was detected by Dihydroethidium (DHE) probe (D7008, Sigma-Aldrich). Fresh-frozen penis and CN tissues were sectioned and subsequently incubated with the DHE probe solution for 30 min at 37 °C in a light-protected environment according to the protocol. Subsequently, nuclear staining was carried out using 4,6-diamidino-2-phenylindole (DAPI, G1012, Servicebio).
Total intracellular ROS in PC-12 cells was investigated with H2DCFH-DA probe (BL714A, Biosharp). The PC-12 cell slides were incubated with 10 μM H2DCFH-DA probe solution for 30 min at 37 °C in darkness according to the manufacturer's protocol.
Five microscopic images were randomly taken and the semiquantitative analysis of positive area was determined by Image-Pro Plus (Version 6.0).
2.15. Measurement of intracellular malondialdehyde (MDA) and superoxide dismutase (SOD) levels
The contents of MDA and SOD were used to reflect the levels of oxidative stress and the antioxidant activity in PC-12 cells, respectively. The levels of MDA and SOD were measured with MDA Content Assay Kit (BC0025, Solarbio) and Total Superoxide Dismutase Assay Kit with WST-8 (S0101S, Beyotime) according to the manufacturer's protocols, respectively. Protein concentrations were used to normalize the levels of MDA and SOD.
2.16. Propidium (PI)/Hoechst33342 staining
PC-12 cells were stained with Hoechst 33342/PI Double Stain Kit (CA1120, Solarbio) referring to the manufacturer's protocol. Briefly, the cells were washed with PBS and stained with 0.5 % PI and 0.5 % Hoechst33342 dissolved in cell staining buffer at 4 °C for 30 min in darkness. Afterwards, the cells were washed with PBS again and photographed using a fluorescence microscope (Olympus). The rate of PI-positive cells was calculated by ImageJ software.
2.17. Measurement of lactate dehydrogenase (LDH) release
The LDH level of cell supernatant was measured with LDH Cytotoxicity Assay Kit (C0016, Beyotime) according to the protocol. The absorbance of each well was detected by microplate reader at 490 nm.
2.18. Measurement of IL-1β and IL-18 concentrations
The IL-1β and IL-18 concentrations of cell supernatant were detected by ELISA method. Rat IL-1β ELISA kit (ERC007.96, NeoBioscience) and Rat IL-18 ELISA kit (ERC010.96, NeoBioscience) were used according to the instructions. The absorbance of each well was measured by microplate reader at 450 nm.
2.19. Statistical analysis
All results were presented as mean ± standard deviation and analyzed with GraphPad Prism software (version 8.0.1). One-way ANOVA with Dunnett's multiple comparisons was performed to determine the significance among the groups in vivo and in vitro. P < 0.05 was considered as statistically significance.
3. Results
3.1. Effect of DMF on erectile function of BCNI rats
To assess the erectile function of BCNI rats, the ratio of Max ICP to MAP and the total ICP were computed (Fig. 1). In comparison to Sham group, erectile function was significantly impaired in BCNI rats (P < 0.05). Subsequent to the administration of varying dosages of DMF, a marked improvement in erectile function was observed in BCNI rats (P < 0.05). These results provide compelling evidence for the successful establishment of the BCNI rat model and underscore the preventive potential of DMF treatment against erectile dysfunction in BCNI rats.
Fig. 1.
Effect of DMF on erectile function of BCNI rats (n = 8). (A) Representative ICP and AP with the electrical stimulation of cavernous nerve. The erectile function was evaluated by (B) max ICP/MAP and (C) total ICP (represented by area under the curve). #P < 0.05 compared to Sham group. *P < 0.05 compared to BCNI group. BCNI: Bilateral cavernous nerve injury; DMF: Dimethyl fumarate; ICP: intracavernous pressure; AP: Arterial pressure; MAP: Mean arterial pressure.
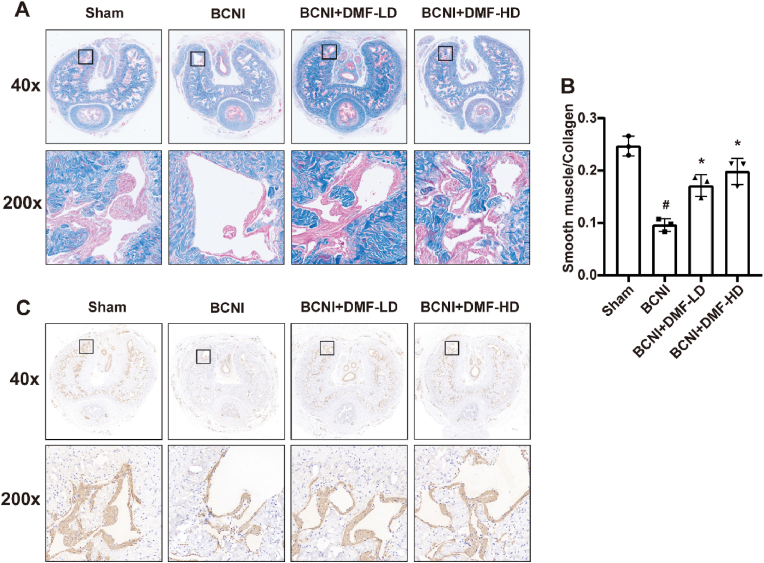
3.2. Effect of DMF on penile fibrosis of BCNI rats
The denervation of penis corpus cavernosum would contribute to the development of penile fibrosis. Following the successful establishment of BCNI rat model, Masson trichrome staining indicated the ratio between smooth muscle and collagen decreased in penis corpus cavernosum (P < 0.05, Fig. 2A–B). In BCNI+DMF-LD and BCNI+DMF-HD groups, the smooth muscle contents both increased (P < 0.05). IHC analysis of α-SMA, a marker of smooth muscle, also confirmed that DMF treatment could ameliorate the decrease in smooth muscle content caused by the injury of CNs (Fig. 2C). The above results demonstrated that DMF had a positive effect on the penile fibrosis caused by denervation.
Fig. 2.
Effect of DMF on penile fibrosis of BCNI rats. (A) Representative images of Masson trichrome staining. Smooth muscle and collagen were stained red and blue, respectively. (B) Semi-quantitative data of the ratio of smooth muscle and collagen in corpus cavernosum. (C) Representative images of immunohistochemical staining for α-SMA in corpus cavernosum. All experiments were performed in triplicate (n = 3). #P < 0.05 compared to Sham group. *P < 0.05 compared to BCNI group. BCNI: Bilateral cavernous nerve injury. DMF: Dimethyl fumarate.
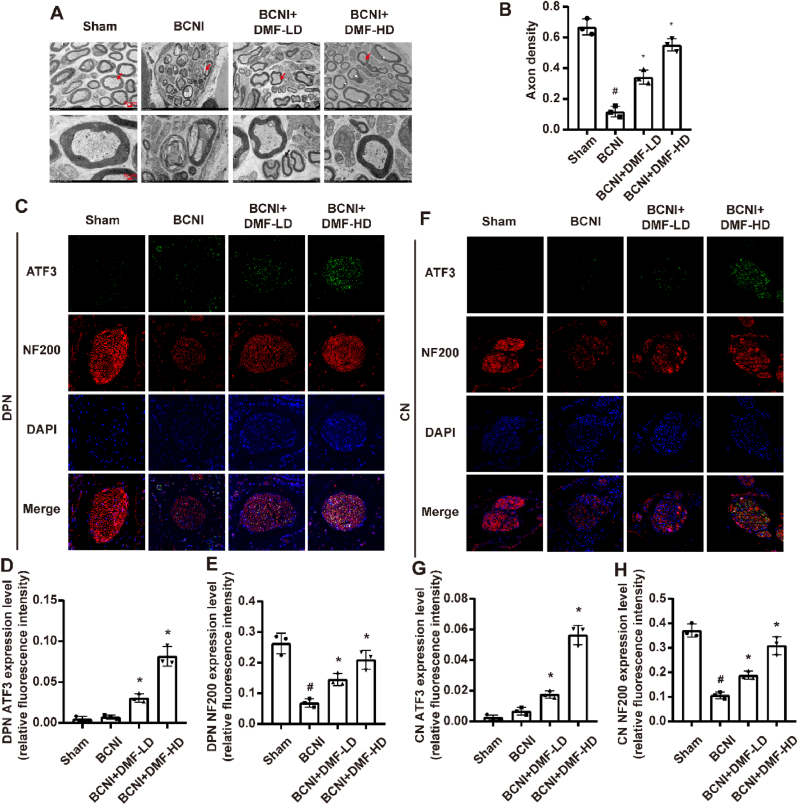
3.3. Effect of DMF on nerve repair of BCNI rats
TEM was employed to investigate the morphologic alterations in the myelinated fibers of CN (Fig. 3A). In BCNI rats, myelin debris and a reduction in intact axons were evident when compared to the Sham group. However, following treatment with a lower dosage of DMF, some regenerating axons were emerged, characterized by smaller dimensions and thinner myelin sheaths (indicated by red arrow) in CN. In BCNI+DMF-HD group, the regenerated axons gradually reached the level of maturity before CN injury. For quantitative analyses, we recorded axon density as the myelinated axon area in the total sectioned nerve area. Axon density decreased after BCNI and was increased by the treatment of both low and high dosage DMF (P < 0.05, Fig. 3B).
Fig. 3.
Effect of DMF on nerve regeneration of BCNI rats. (A) Ultrastructural changes of cavernous nerve. (B) Quantitative analysis of the axon density (myelinated axon area/total sectioned nerve area) of CN. (C, F) Representative images of immunofluorescent staining for ATF3 (green) and NF200 (red) in DPN and CN, respectively. (D, E, G, H) Semi-quantitative data of ATF3 and NF200 expression as relative fluorescence intensity in DPN and CN, respectively. There were three replicates in these experiments (n = 3). Original magnification is × 400. #P < 0.05 compared to Sham group. *P < 0.05 compared to BCNI group. BCNI: Bilateral cavernous nerve injury. DMF: Dimethyl fumarate. DPN: Dorsal penile nerve. CN: Cavernous nerve.
Studies have shown that part of nerve fibers in DPN originated from CN and nerve fibers were decreased in DPN after the CN was injured [39,40]. Activating transcription factor 3 (ATF3) is a transcription factor activated after peripheral axonal injury and enhances the injured axon to regenerate [41,42]. Neurofilament 200 (NF200) serves as a crucial component of neuronal cytoskeleton and is utilized as a marker protein for nerve fibers [43]. Double IF staining for ATF3 and NF200 revealed that there were no significant alterations in ATF3 content, while fewer NF200-positive nerve fibers were identified (P < 0.05) in rat DPN and CN tissue of BCNI group compared to Sham group. After treatment of DMF, significant increase of them was observed (P < 0.05, Fig. 3C–H). These findings demonstrate the potential of DMF to enhance ATF3 expression and promote the restoration of NF200-positive nerve fibers in the injured nerve tissues.
3.4. Effect of DMF on nNOS and NO/cGMP pathway in penis corpus cavernosum of BCNI rats
IF staining for nNOS showed that significant low expression of nNOS was induced by BCNI in penis corpus cavernosum (P < 0.05). nNOS expression levels were significantly restored in BCNI+DMF-LD and BCNI+DMF-HD groups (P < 0.05, Fig. 4A–B).
Fig. 4.
Effect of DMF on nNOS and NO/cGMP pathway in penis corpus cavernosum of BCNI rats. (A) Representative images of immunofluorescent staining for nNOS in penis corpus cavernosum. (B) Semi-quantitative data of nNOS expression as relative fluorescence intensity in penis corpus cavernosum. There were three replicates in this experiment (n = 3). (C, D, E) NO content, cGMP content and Ca2+ concentration in penis corpus cavernosum (n = 8), respectively. Original magnification is × 400. #P < 0.05 compared to Sham group. *P < 0.05 compared to BCNI group. BCNI: Bilateral cavernous nerve injury; DMF: Dimethyl fumarate.
Furthermore, the contents of NO and cGMP exhibited a marked decline subsequent to BCNI in penis corpus cavernosum (P < 0.05) and the contents were significantly enhanced by the treatment of DMF (P < 0.05, Fig. 4C–D). In addition, there was a significant increase of Ca2+ in penis corpus cavernosum of BCNI rats and this change was rescued by DMF treatment (P < 0.05, Fig. 4E).
3.5. Effect of DMF on oxidative stress in DPN and CN of BCNI rats
DHE probe was stained to detect the ROS production in DPN and CN. The result showed that the content of ROS was significantly elevated after BCNI (P < 0.05). The accumulation of ROS was reduced by the treatment of DMF (P < 0.05, Fig. 5A–D). Additionally, 3-NT was used as an indicator of oxidative stress which was detected by IHC. The content of 3-NT was upregulated coordinately in BCNI group and was reduced following DMF intervention (Fig. 5E).
Fig. 5.
Effect of DMF on oxidative stress in DPN and CN of BCNI rats. (A, C) Representative images of DHE staining for ROS in DPN and CN, respectively. (B, D) Semi-quantitative data of ROS level as relative fluorescence intensity in DPN and CN, respectively. (E) Representative images of immunohistochemical staining for 3-NT in DPN and CN, respectively. There were three replicates in these experiments (n = 3). Original magnification is × 200 for (A), × 400 for (C) and × 400 for (E). #P < 0.05 compared to Sham group. *P < 0.05 compared to BCNI group. BCNI: Bilateral cavernous nerve injury. DMF: Dimethyl fumarate. DPN: Dorsal penile nerve. CN: Cavernous nerve. DHE: Dihydroethidium. ROS: Reactive oxygen species. 3-NT: 3-Nitrotyrosine.
3.6. Effect of DMF on NLRP3 inflammasome-mediated pyroptosis in DPN and CN of BCNI rats
To investigate whether NLRP3 inflammasome-mediated pyroptosis was activated in BCNI rats and the therapeutic effect of DMF on pyroptosis, the protein expression of GSDMD, NLRP3 and Caspase-1 was evaluated by IF and IHC staining. Within the DPN and CN of BCNI rats, there was a notably elevated GSDMD content when compared to normal rats (P < 0.05). Remarkably, this elevation was significantly mitigated subsequent to DMF treatment (P < 0.05, Fig. 6A–D). The IHC results of NLRP3 and Caspase-1 were consistent with GSDMD. The content of them was elevated by BCNI and was reduced through administration of DMF (Fig. 6E–F).
Fig. 6.
Effect of DMF on NLRP3 inflammasome-mediated pyroptosis in DPN and CN of BCNI rats. (A, C) Representative images of immunofluorescent staining for GSDMD in DPN and CN, respectively. (B, D) Semi-quantitative data of GSDMD expression as relative fluorescence intensity in DPN and CN, respectively. (E) Representative images of immunohistochemical staining for NLRP3 in DPN and CN, respectively. (F) Representative images of immunohistochemical staining for Caspase-1 in DPN and CN, respectively. There were three replicates in these experiments (n = 3). Original magnification is × 400. #P < 0.05 compared to Sham group. *P < 0.05 compared to BCNI group. BCNI: Bilateral cavernous nerve injury. DMF: Dimethyl fumarate. DPN: Dorsal penile nerve. CN: Cavernous nerve.
3.7. Effect of DMF on Nrf2/HO-1 pathway in DPN and CN of BCNI rats
DMF is an effective activator of Nrf2 antioxidant pathway, and HO-1 is a downstream protein of Nrf2. HO-1 and its metabolites have robust antioxidant ability. Based on this knowledge, we formulated the hypothesis that DMF exerted its inhibitory effects on oxidative stress and NLRP3 inflammasome-mediated pyroptosis primarily through the activation of the Nrf2/HO-1 pathway. The results of IF indicated the protein expression of Nrf2 and HO-1 had no significant change in BCNI rats. After the intervention of DMF, the protein levels of Nrf2 and HO-1 were both increased (P < 0.05, Fig. 7A–H).
Fig. 7.
Effect of DMF on Nrf2/HO-1 pathway in DPN and CN of BCNI rats. (A, B) Representative images of immunofluorescent staining for Nrf2 and HO-1 in DPN. (C, D) Semi-quantitative data of Nrf2 and HO-1 expression as relative fluorescence intensity in DPN. (E, F) Representative images of immunofluorescent staining for Nrf2 and HO-1 in CN. (G, H) Semi-quantitative data of Nrf2 and HO-1 expression as relative fluorescence intensity in CN. There were three replicates in these experiments (n = 3). Original magnification is × 400. *P < 0.05 compared to BCNI group. BCNI: Bilateral cavernous nerve injury. DMF: Dimethyl fumarate. DPN: Dorsal penile nerve. CN: Cavernous nerve.
In conclusion, DMF demonstrated the ability to enhance erectile function of BCNI rats by protecting CN from oxidative stress and NLRP3 inflammasome-mediated pyroptosis via activating Nrf2/HO-1 pathway. To further support this conclusion, we subsequently verified these findings through in vitro experimentation.
3.8. Effect of DMF on cell viability of H2O2 induced PC-12 cells
To evaluate the cytotoxicity of DMF and H2O2, PC-12 cells were treated with different concentrations of DMF and H2O2 for different time intervals. Cell viability was measured by CCK-8 assay. The results of DMF and H2O2 treatment showed that concentration of DMF up to 20 μmol/L and concentration of H2O2 up to 40 μmol/L had significant effect on viability of PC-12 cells for 24h (P < 0.05, Fig. 8 A, C). The viability of PC-12 cells treated with 10 μmol/L DMF had no significant change within 48h (Fig. 8B). However, treatment with 40 μmol/L H2O2 for 12h significantly decreased the viability of PC-12 cells (P < 0.05, Fig. 8D). Therefore, cells were incubated with 10 μmol/L DMF for 2h before exposure to 40 μmol/L H2O2. After 6h of H2O2 treatment, expression of Nrf2 and HO-1 was detected. Other parameters were all measured after 12h exposure to H2O2. The result of CCK-8 assay showed that DMF improved the viability of H2O2 induced PC-12 cells (P < 0.05, Fig. 8E).
Fig. 8.
Effect of DMF on cell viability of H2O2 induced PC-12 cells. (A) CCK-8 detected the viability of PC-12 cells treated with DMF (0, 2, 5, 10, 20 μmol/L) for 24h. *P < 0.05 compared to 0 μmol/L DMF. (B) CCK-8 detected the viability of PC-12 cells treated with 10 μmol/L DMF for 0, 6, 12, 24, 48h. *P < 0.05 compared to 0 h. (C) CCK-8 detected the viability of PC-12 cells treated with H2O2 (0, 20, 40, 60, 80,100 μmol/L) for 24h. *P < 0.05 compared to 0 μmol/L H2O2. (D) CCK-8 detected the viability of PC-12 cells treated with 40 μmol/L H2O2 for 0, 6, 12, 24, 48h. *P < 0.05 compared to 0 h. (E) CCK-8 detected the viability of PC-12 cells after pretreatment with DMF for 2h and incubation with 40 μmol/L H2O2 for 12h. There were four replicates in these experiments (n = 4). #P < 0.05 compared to Control group. *P < 0.05 compared to H2O2 group. DMF: Dimethyl fumarate. CCK-8: Cell counting assay-8.
3.9. Construction of Nfe2l2 and Ho-1 knockdown PC-12 cell lines
After the infection of lentivirus and screened by puromycin, the knockdown efficiency of Nfe2l2 and Ho-1 was evaluated by Western blot and qRT-PCR. Western blot analysis showed that Nrf2 shRNA-2 and Nrf2 shRNA-3 had an effective inhibition of Nrf2 protein expression, and all HO-1 sh-RNAs could inhibit HO-1 protein expression (P < 0.05, Fig. 9A–D). The results of qRT-PCR demonstrated that all Nrf2 shRNAs and HO-1 shRNAs decreased the levels of Nfe2l2 and Ho-1 mRNAs, respectively (P < 0.05, Fig. 9E–F). Nrf2 shRNA-3 and HO-1 shRNA-3 had the highest knockdown efficiency of Nfe2l2 and Ho-1. Therefore, PC-12 cell lines which were infected by the lentivirus with Nrf2 shRNA-3 and HO-1 shRNA-3 were selected for the further research.
Fig. 9.
Construction of Nfe2l2 and Ho-1 knockdown PC-12 cell lines. (A) Representative western blot bands for Nrf2 in PC-12 cells. (B) Semi-quantitative analysis of Nrf2. (C) Representative western blot bands for HO-1 in PC-12 cells. (D) Semi-quantitative analysis of HO-1. (E) mRNA level of Nfe2l2. (F) mRNA level of Ho-1. There were three replicates in these experiments (n = 3). *P < 0.05 compared to Negative Control.
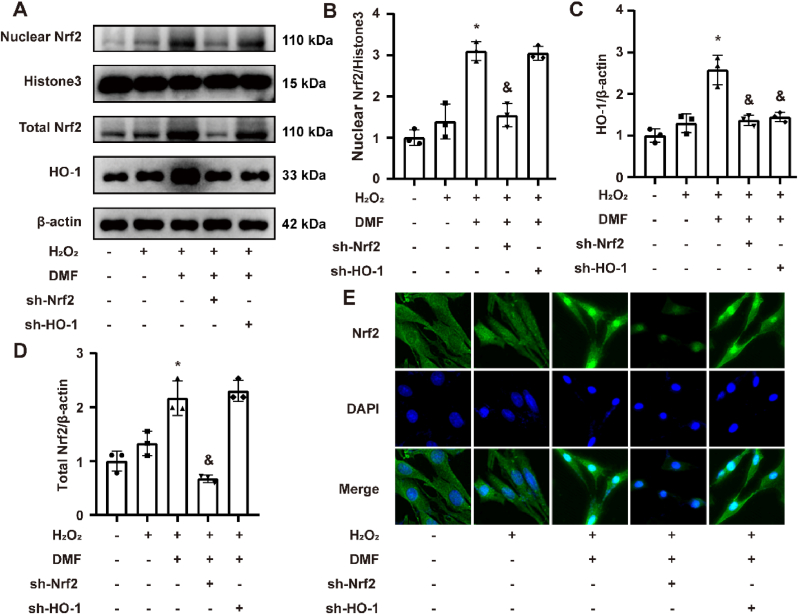
3.10. Effect of DMF on Nrf2/HO-1 pathway in H2O2 induced PC-12 cells
The cell experimental groups were established in accordance with the abovementioned method. Western blot results revealed H2O2 had no significant effect on total Nrf2 protein content, nuclear Nrf2 content and total HO-1 content in PC-12 cells. DMF pretreatment increased the content of total Nrf2, nuclear Nrf2 and total HO-1 (P < 0.05). After Nfe2l2 knockdown, the content of total Nrf2, nuclear Nrf2 and total HO-1 was inhibited compared to DMF group (P < 0.05). The content of total Nrf2, nuclear Nrf2 had no significant change and the content of HO-1 was decreased in sh–HO–1 group compared to DMF group (P < 0.05, Fig. 10A–D). The results of cell IF staining were similar to those of Western blot. The content of total Nrf2, nuclear Nrf2 had no obvious change in H2O2 group. After DMF treatment, the content of total Nrf2, nuclear Nrf2 was elevated obviously especially nuclear Nrf2 and they were not affected by knockdown of Ho-1. After knockdown of Nfe2l2, the content of total Nrf2, nuclear Nrf2 could not be elevated by treatment of DMF (Fig. 10E).
Fig. 10.
Effect of DMF on Nrf2/HO-1 pathway in H2O2 induced PC-12 cells. (A) Representative western blot bands for total Nrf2, HO-1 and nuclear Nrf2 in PC-12 cells. (B, C, D) Semi-quantitative analysis of nuclear Nrf2, total Nrf2 and HO-1. (E) Representative images of immunofluorescent staining for Nrf2 in PC-12 cells. There were three replicates in these experiments (n = 3). Original magnification is × 400. *P < 0.05 compared to H2O2 group. &P < 0.05 compared to DMF group. DMF: Dimethyl fumarate.
3.11. Effect of DMF on oxidative stress in H2O2 induced PC-12 cells
ROS levels were assessed through H2DCFH-DA staining, with the results revealing that DMF effectively suppressed ROS production induced by H2O2 in PC-12 cells (P < 0.05). ROS levels were elevated in both sh-Nrf2 and sh–HO–1 groups compared to DMF group (P < 0.05, Fig. 11A–B). Both 53BP1 and γ-H2AX play pivotal roles in the regulation of DNA damage and repair processes, rendering them valuable markers for DNA damage assessment [44]. IF staining results for 3-NT, 53BP1 and γ-H2AX demonstrated that they were all increased in H2O2 group and DMF intervention reduced the content of these molecules (P < 0.05). After knockdown of Nfe2l2 and Ho-1, the inhibitive effects of DMF were reversed and the content of these molecules increased again (P < 0.05, Fig. 11C–H). MDA serves as a product of lipid peroxidation and is recognized as a marker of oxidative stress, whereas SOD is an efficacious antioxidant substance. MDA and SOD assay kits were used to measure the content of MDA and the activity of SOD in PC-12 cells. Results indicated the MDA level was increased (P < 0.05), and SOD activity had no significant change by H2O2 administration. After DMF pretreatment, MDA level was remarkably decreased and SOD activity were significantly elevated. Nfe2l2 and Ho-1 knockdown could inhibit the beneficial protective effect of DMF, respectively (P < 0.05, Fig. 11I–J).
Fig. 11.
Effect of DMF on oxidative stress in H2O2 induced PC-12 cells. (A) Representative images of H2DCFH-DA staining for ROS in PC-12 cells. (B) Semi-quantitative data of ROS level as relative fluorescence intensity in PC-12 cells. (C, E, G) Representative images of immunofluorescent staining for 3-NT, 53BP1 and γ-H2AX in PC-12 cells, respectively. (D, F, H) Semi-quantitative data of 3-NT, 53BP1 and γ-H2AX in PC-12 cells, respectively. (I, J) SOD activity, MDA level in PC-12 cells, respectively. There were three replicates in these experiments (n = 3). Original magnification is × 200 for (A) and × 400 for (C, E, G). #P < 0.05 compared to Control group. *P < 0.05 compared to H2O2 group. &P < 0.05 compared to DMF group. DMF: Dimethyl fumarate. ROS: Reactive oxygen species. 3-NT: 3-Nitrotyrosine. SOD: Superoxide dismutase. MDA: Malondialdehyde.
3.12. Effect of DMF on NLRP3 inflammasome-mediated pyroptosis in H2O2 induced PC-12 cells
The integrality of plasma membrane was evaluated by PI staining. The result showed that DMF pretreatment eliminated the increased number of PI positive cells induced by H2O2 intervention (P < 0.05). The numbers of PI positive cells were both elevated in sh-Nrf2 and sh–HO–1 groups compared to DMF group (P < 0.05, Fig. 12A–B). Moreover, DMF was indicated to reduce the H2O2-induced LDH release and Nfe2l2 and Ho-1 knockdown reversed the inhibitory effect of DMF (P < 0.05, Fig. 12C). Subsequently, Western blot showed DMF pretreatment reduced the elevated content of NLRP3, GSDMD-T, GSDMD-N, pre-Caspase-1 and cle-Caspase-1 caused by H2O2 induced PC-12 cells(P < 0.05). These proteins were all increased after Nfe2l2 and Ho-1 knockdown (P < 0.05, Fig. 12D–I). ELISA analysis was performed to detect the concentrations of IL-1β and IL-18 in cell supernatant. It demonstrated DMF pretreatment significantly ameliorated the increased levels of IL-1β and IL-18 induced by H2O2 (P < 0.05). Nfe2l2 and Ho-1 knockdown reduced the inhibitory effect of DMF (P < 0.05, Fig. 12J–K).
Fig. 12.
Effect of DMF on NLRP3 inflammasome-mediated pyroptosis in H2O2 induced PC-12 cells. (A) PI (red)/Hoechst33342(blue) double fluorescent staining for PC-12 cells. (B) The rate of PI positive PC-12 cells. (C) LDH release in cell supernatant. (D) Representative western blot bands for NLRP3, GSDMD-T, GSDMD-N, pro-Caspase-1, cle-Caspase-1 in PC-12 cells. (E, F, G, H, I) Semi-quantitative analysis of NLRP3, GSDMD-T, GSDMD-N, pro-Caspase-1, cle-Caspase-1, respectively. (J, K) IL-1β and IL-18 concentrations in cell supernatant, respectively. There were three replicates in these experiments (n = 3). Original magnification is × 200. #P < 0.05 compared to Control group. *P < 0.05 compared to H2O2 group. &P < 0.05 compared to DMF group. DMF: Dimethyl fumarate. PI: Propidium. LDH: Lactate dehydrogenase.
3.13. Effect of ROS inhibitor on NLRP3 inflammasome-mediated pyroptosis in H2O2 induced PC-12 cells
N-acetyl-l-cysteine (NAC) is an effective antioxidant which scavenges ROS directly and can be converted into metabolites capable of stimulating glutathione synthesis [45]. 5 mmol/L NAC was pretreated for 2 h before H2O2 intervention in PC-12 cells. H2DCFH-DA staining indicated that NAC significantly inhibited the accumulation of ROS (P<0.05, Fig. 13A and B). Furthermore, Western blot analysis demonstrated NAC decreased the contents of NLRP3, GSDMD-T, GSDMD-N, pre-Caspase-1, cle-Caspase-1 increased by H2O2 intervention (P<0.05, Fig. 13C–H). In conclusion, DMF exerted its inhibitory effect on NLRP3 inflammasome-mediated pyroptosis by reducing ROS production.
Fig. 13.
Effect of ROS inhibitor on NLRP3 inflammasome-mediated pyroptosis in H2O2 induced PC-12 cells. (A) Representative images of H2DCFH-DA staining for ROS in PC-12 cells. (B) Semi-quantitative data of ROS level as relative fluorescence intensity in PC-12 cells. (C) Representative western blot bands for NLRP3, GSDMD-T, GSDMD-N, pro-Caspase-1, cle-Caspase-1 in PC-12 cells. (D, E, F, G, H) Semi-quantitative analysis of NLRP3, GSDMD-T, GSDMD-N, pro-Caspase-1, cle-Caspase-1, respectively. There were three replicates in these experiments (n = 3). Original magnification is × 200. #P < 0.05 compared to Control group. *P < 0.05 compared to H2O2 group. ROS: Reactive oxygen species. NAC: N-acetyl-l-cysteine.
4. Discussion
In our study, we established the BCNI rat model by clamping bilateral CN. After treating BCNI rats with DMF for 4 weeks, erectile function was severely impaired in BCNI rats and DMF could improve erectile function of BCNI rats. Following CN injury, we observed structural damage of the nerves, decreased nerve fibers, reduced content of nNOS in the corpus cavernosum, inhibition of the NO/cGMP pathway, and an elevated level of corpus cavernosum fibrosis. However, DMF treatment ameliorated these changes significantly. To explore the potential mechanisms underlying the effects of DMF, we investigated the levels of oxidative stress and activation of the NLRP3 inflammasome-mediated pyroptosis in the DPN and CN. Our findings confirmed the involvement of oxidative stress and NLRP3 inflammasome-mediated pyroptosis in the development of CN dysfunction following injury. Moreover, DMF treatment effectively promoted Nrf2 and HO-1 protein expression. DMF exerted its protective effects on the injured nerves by reducing oxidative stress and inhibiting NLRP3 inflammasome-mediated pyroptosis. Based on this observation, we hypothesized that DMF exerted therapeutic effects on NED by inhibiting oxidative stress and NLRP3 inflammasome-mediated pyroptosis in injured nerves through the activation of the Nrf2/HO-1 pathway. To validate this hypothesis, we conducted in vitro experimentations using the PC-12 cell line, Nfe2l2 and Ho-1 knockdown PC-12 cell lines. The results demonstrated that DMF increased the content of Nrf2 and facilitated its nuclear translocation, leading to an increase in HO-1 expression. Furthermore, H2O2 intervention induced oxidative stress and activated NLRP3 inflammasome-mediated pyroptosis, which were effectively inhibited by DMF pretreatment. However, the protective effects of DMF were reversed by knockdown of Nfe2l2 and Ho-1, respectively. In summary, we demonstrated that DMF inhibited oxidative stress and NLRP3 inflammasome-mediated pyroptosis in injured CN through activation of the Nrf2/HO-1 pathway. These findings contribute to our understanding of DMF as a potential therapeutic option for NED and shed light on the underlying mechanisms involved (Fig. 14).
Fig. 14.
The summary of therapeutic effect of DMF on ED via activating Nrf2/HO-1 pathway in BCNI rats. Oxidative stress and NLRP3 inflammasome-mediated pyroptosis are involved in the pathological process of neurological dysfunction in BCNI rats. DMF can improve erectile function by reducing the degree of CN damage and promoting nerve repair through inhibiting the oxidative stress and activation of NLRP3 inflammasome-mediated pyroptosis via activating Nrf2/HO-1 antioxidant pathway in BCNI rats. DMF: Dimethyl fumarate. 3-NT: 3-Nitrotyrosine. SOD: Superoxide dismutase. MDA: Malondialdehyde.
Prostate cancer is a prevalent malignancy that poses a significant threat to male lives and profoundly impacts their quality of life [46]. RP is considered the optimal treatment for primary prostate cancer [39]. However, due to the possibility of CN injury during RP, NED is the main complication caused by CN injury. Epidemiologic studies have shown that the incidence of ED after RP can be as high as 85 % [3]. Although the implementation of nerve-sparing techniques and minimal invasive surgery has reduced the occurrence of NED, complete avoidance of this complication remains challenging. Approximately 50 % of patients experience ED within 18 months following bilateral nerve-sparing RP [47]. In addition, other pelvic surgeries represented by radical resection of rectal carcinoma may also damage the CN intraoperatively, leading to the occurrence of postoperative NED, which greatly affects the quality of life of patients.
During normal penile erection, sexual stimulation triggers nerve impulses that propagate from the CN to the penis, stimulating the release of NO catalyzed by nNOS. NO activates guanylate cyclase, which converts guanosine triphosphate (GTP) into cGMP. Subsequently, cGMP activates downstream pathways, leading to a decrease in cytoplasmic Ca2+ concentration and relaxation of smooth muscle cells of corpus cavernosum. PDE5i are the first-line therapeutic medications for ED as they catalyze the deactivation of cGMP into 5′-GMP [48,49]. However, PDE5i therapy often falls short in effectively treating NED. Studies have shown that PDE5i such as vardenafil and tadalafil had limited therapeutic effects on ED after RP [50,51]. This is likely due to the long-term denervation that leads to structural changes in the penile corpus cavernosum [7]. Consequently, the protection and regeneration of nerves have become crucial approaches for the treatment of NED.
Studies have demonstrated that the accumulation of ROS could hinder nerve regeneration after CN injury and contribute to increased levels of oxidative stress in the serum and corpus cavernosum, leading to damage of tissues and exacerbation of ED [10,11]. ROS refers to a group of strong reactive oxidizing substances with unpaired electrons generated by endogenous and exogenous stimuli. Excessive ROS inflict continuous damage on lipids, proteins, and DNA, resulting in irreversible oxidative damage [52]. NLRP3 inflammasome can be activated by diverse stimuli, such as extracellular ATP, cholesterol crystal and potassium efflux. Notably, these stimuli converge on a common activation mechanism associated with ROS, suggesting that ROS may also be triggered through the activation of the NLRP3 inflammasome and its downstream pyroptosis pathway [53]. Actually, it has been experimentally proven that ROS could stimulate the NLRP3 inflammasome, exacerbating subsequent inflammatory cascades [54]. Activated NLRP3 inflammasome cleaves pro-Caspase-1 to cle-Caspase-1, which promotes the activation of IL-18 and IL-1β and drives cleavage of GSDMD to GSDMD-N. Ultimately, GSDMD-N inserts into the plasma membrane, forming cytotoxic pores and inducing pyroptosis. Therefore, inhibiting local oxidative stress and reducing ROS accumulation in the CN may represent a potential therapeutic strategy for the treatment of NED.
DMF has been established as an effective activator of Nrf2 pathway, enhancing the content of Nrf2 by degrading Keap1, a protein that binds to Nrf2 and promotes its ubiquitination [21]. Upon activation, Nrf2 translocates into the nucleus and binds to antioxidant response element regions, facilitating the transcription of genes involved in antioxidant defense [20]. One prominent antioxidant protein activated by Nrf2 is HO-1 [22]. Prior research has indicated that DMF exerted neuroprotective effects and promoted nerve regeneration through its antioxidant properties via activation of Nrf2/HO-1 pathway [[28], [29], [30], [31], [32]]. Our study demonstrated that DMF effectively suppressed oxidative stress and the activation of NLRP3 inflammasome-mediated pyroptosis via Nrf2/HO-1 pathway in vivo and in vitro. To confirm the role of ROS reduction in DMF's inhibitory effect on NLRP3 inflammasome activation, we employed NAC as a positive control. Our findings provided evidence that the inhibitory effect of DMF on NLRP3 inflammasome activation was dependent on the reduction of ROS production. Furthermore, our study revealed that DMF treatment increased the protein expression of ATF3, a marker for axon repair, and NF200, a marker for nerve fibers. This finding further supported the notion that DMF protected and promoted the repair of injured CN, ultimately leading to improved erectile function in the BCNI rat model.
There are also some limitations in this study. We performed rescue experimentations in vitro to validate that DMF inhibits oxidative stress and NLRP3 inflammasome-mediated neural death through Nrf2/HO-1 pathway. Transgenic mice are necessary for the validation of this pathway before future use of DMF in the clinic. It should be noted that activation of the Nrf2/HO-1 pathway in aged rat and human cavernous tissue for a short period of time can improve endothelial function [55]. Hence, it is uncertain whether DMF promotes penile erection by ameliorating endothelial cell function, which necessitates further investigation. Finally, it is important to note that impaired erectile function after BCNI is mainly caused by an imbalance between adrenergic and nitrergic nerves [56,57]. The current study solely investigated the impact of DMF on nitrergic nerves. Though the neuroprotective effects of DMF appear to be similar across specific neurons, there remains a requirement to fully determine the impacts of DMF on adrenergic neurons in the forthcoming researches.
5. Conclusion
This study demonstrated the therapeutic effect of DMF on NED through the inhibition of oxidative stress and the activation of NLRP3 inflammasome-mediated pyroptosis in injured nerves via the activation of Nrf2/HO-1 pathway. These findings offer novel insights into the protective mechanism of DMF against NED, thus providing a valuable theoretical foundation for future clinical applications.
Funding information
This work was supported by the grant from the National Natural Science Foundation of China (No. 81901472).
Declaration of competing interest
All authors have made substantial contributions to the study and have read and approved the final manuscript. The authors have no conflict of interest to disclose.
Acknowledgement
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102938.
Contributor Information
Jihong Liu, Email: jhliu@tjh.tjmu.edu.cn.
Yajun Ruan, Email: ruanyajun@hust.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Lue T.F. Neurogenic erectile dysfunction. Clin. Auton. Res. : Official J. Clin. Autonomic Res. Soc. Oct 2001;11(5):285–294. doi: 10.1007/bf02332973. [DOI] [PubMed] [Google Scholar]
- 2.Wang R. Penile rehabilitation after radical prostatectomy: where do we stand and where are we going? J. Sex. Med. Jul 2007;4(4 Pt 2):1085–1097. doi: 10.1111/j.1743-6109.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 3.Emanu J.C., Avildsen I.K., Nelson C.J. Erectile dysfunction after radical prostatectomy: prevalence, medical treatments, and psychosocial interventions. Curr. Opin. Support. Palliat. Care. Mar 2016;10(1):102–107. doi: 10.1097/spc.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weyne E., Castiglione F., Van der Aa F., Bivalacqua T.J., Albersen M. Landmarks in erectile function recovery after radical prostatectomy. Nat. Rev. Urol. May 2015;12(5):289–297. doi: 10.1038/nrurol.2015.72. [DOI] [PubMed] [Google Scholar]
- 5.Hu W.L., Hu L.Q., Song J., et al. Fibrosis of corpus cavernosum in animals following cavernous nerve ablation. Asian J. Androl. Jun 2004;6(2):111–116. [PubMed] [Google Scholar]
- 6.Leungwattanakij S., Bivalacqua T.J., Usta M.F., et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J. Androl. Mar-Apr 2003;24(2):239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 7.Fode M., Ohl D.A., Ralph D., Sønksen J. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. Nov 2013;112(7):998–1008. doi: 10.1111/bju.12228. [DOI] [PubMed] [Google Scholar]
- 8.Mulhall J.P. Penile rehabilitation following radical prostatectomy. Curr. Opin. Urol. Nov 2008;18(6):613–620. doi: 10.1097/MOU.0b013e3283136462. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J., Fang S., Yuan Y., et al. Green tea polyphenols protect spinal cord neurons against hydrogen peroxide-induced oxidative stress. Neural Regenerat. Res. Jul 15 2014;9(14):1379–1385. doi: 10.4103/1673-5374.137591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z.K., Yu H.L., Liu B., Wang H., Luo Q., Ding X.G. Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury. Neural Regenerat. Res. Aug 2016;11(8):1312–1321. doi: 10.4103/1673-5374.189197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H., Ding X.G., Li S.W., et al. Role of oxidative stress in surgical cavernous nerve injury in a rat model. J. Neurosci. Res. Jun 2015;93(6):922–929. doi: 10.1002/jnr.23545. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Z., He Y., Ming H., Lei S., Leng Y., Xia Z.Y. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J. Diabetes Res. 2019;2019 doi: 10.1155/2019/8151836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X., Zhang H., Qi W., et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. Feb 7 2018;9(2):171. doi: 10.1038/s41419-017-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen S., He F., Cheng C., Xu B., Sheng J. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. Jan 2021;133 doi: 10.1016/j.biopha.2020.110990. [DOI] [PubMed] [Google Scholar]
- 15.Al Mamun A., Wu Y., Monalisa I., et al. Role of pyroptosis in spinal cord injury and its therapeutic implications. J. Adv. Res. Feb 2021;28:97–109. doi: 10.1016/j.jare.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Negro R., Wu H. TRPM2, linking oxidative stress and Ca(2+) permeation to NLRP3 inflammasome activation. Curr. Opin. Immunol. Feb 2020;62:131–135. doi: 10.1016/j.coi.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abais J.M., Xia M., Zhang Y., Boini K.M., Li P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxidants Redox Signal. May 1 2015;22(13):1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu F., Lan Z., Xin Z., et al. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell. Physiol. Apr 2020;235(4):3207–3221. doi: 10.1002/jcp.29268. [DOI] [PubMed] [Google Scholar]
- 19.Shen R., Yin P., Yao H., et al. Punicalin ameliorates cell pyroptosis induced by LPS/ATP through suppression of ROS/NLRP3 pathway. J. Inflamm. Res. 2021;14:711–718. doi: 10.2147/jir.S299163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi A., Kang M.I., Watai Y., et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell Biol. Jan 2006;26(1):221–229. doi: 10.1128/mcb.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsuishi Y., Motohashi H., Yamamoto M. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front. Oncol. 2012;2:200. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Na H.K., Surh Y.J. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic. Biol. Med. Feb 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- 23.Ryter S.W., Choi A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. : J. Lab. Clin. Med. Jan 2016;167(1):7–34. doi: 10.1016/j.trsl.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q.F., Zhu Y.S., Jiang H., Xu H., Sun Y. Heme oxygenase-1 mediates the anti-inflammatory effect of isoflurane preconditioning in LPS-stimulated macrophages. Acta Pharmacol. Sin. Feb 2009;30(2):228–234. doi: 10.1038/aps.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R., Rezk A., Ghadiri M., et al. Dimethyl fumarate treatment mediates an anti-inflammatory shift in B cell subsets of patients with multiple sclerosis. J. Immunol. (Baltimore, Md : 1950. Jan 15 2017;198(2):691–698. doi: 10.4049/jimmunol.1601649. [DOI] [PubMed] [Google Scholar]
- 26.Majkutewicz I. Dimethyl fumarate: a review of preclinical efficacy in models of neurodegenerative diseases. Eur. J. Pharmacol. Jul 5 2022;926 doi: 10.1016/j.ejphar.2022.175025. [DOI] [PubMed] [Google Scholar]
- 27.Montes Diaz G., Hupperts R., Fraussen J., Somers V. Dimethyl fumarate treatment in multiple sclerosis: recent advances in clinical and immunological studies. Autoimmun. Rev. Dec 2018;17(12):1240–1250. doi: 10.1016/j.autrev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D., Little J.W., Doyle T., Neumann W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. Sep 1 2011;51(5):951–966. doi: 10.1016/j.freeradbiomed.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grace P.M., Gaudet A.D., Staikopoulos V., et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. Dec 2016;39(12):862–879. doi: 10.1016/j.tins.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Ma J., Lacagnina M.J., et al. Oral dimethyl fumarate reduces peripheral neuropathic pain in rodents via NFE2L2 antioxidant signaling. Anesthesiology. Feb 2020;132(2):343–356. doi: 10.1097/aln.0000000000003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szepanowski F., Donaldson D.M., Hartung H.P., et al. Dimethyl fumarate accelerates peripheral nerve regeneration via activation of the anti-inflammatory and cytoprotective Nrf2/HO-1 signaling pathway. Acta Neuropathol. Mar 2017;133(3):489–491. doi: 10.1007/s00401-017-1676-z. [DOI] [PubMed] [Google Scholar]
- 32.Linker R.A., Lee D.H., Ryan S., et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain : J. Neurol. Mar 2011;134(Pt 3):678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 33.Pitarokoili K., Ambrosius B., Meyer D., Schrewe L., Gold R. Dimethyl fumarate ameliorates lewis rat experimental autoimmune neuritis and mediates axonal protection. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0143416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Zhou B., Yu Z., et al. Baicalein alleviates erectile dysfunction associated with streptozotocin-induced type I diabetes by ameliorating endothelial nitric oxide synthase dysfunction, inhibiting oxidative stress and fibrosis. J. Sex. Med. Aug 2020;17(8):1434–1447. doi: 10.1016/j.jsxm.2020.04.390. [DOI] [PubMed] [Google Scholar]
- 35.Cui K., Ruan Y., Wang T., et al. FTY720 supplementation partially improves erectile dysfunction in rats with streptozotocin-induced type 1 diabetes through inhibition of endothelial dysfunction and corporal fibrosis. J. Sex. Med. Mar 2017;14(3):323–335. doi: 10.1016/j.jsxm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Oprea D., Sanz C.G., Barsan M.M., Enache T.A. PC-12 cell line as a neuronal cell model for biosensing applications. Biosensors. Jul 8 2022;(7):12. doi: 10.3390/bios12070500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye K., He A., Wu M., et al. In vitro study of decellularized rat tissues for nerve regeneration. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.986377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannaccini M., Calatayud M.P., Poggetti A., et al. Magnetic nanoparticles for efficient delivery of growth factors: stimulation of peripheral nerve regeneration. Adv. Healthcare Mater. Apr 2017;6(7) doi: 10.1002/adhm.201601429. [DOI] [PubMed] [Google Scholar]
- 39.Yucel S., Baskin L.S. Identification of communicating branches among the dorsal, perineal and cavernous nerves of the penis. J. Urol. Jul 2003;170(1):153–158. doi: 10.1097/01.ju.0000072061.84121.7d. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y.L., Chao T.T., Wu Y.N., et al. nNOS-positive minor-branches of the dorsal penile nerves is associated with erectile function in the bilateral cavernous injury model of rats. Sci. Rep. Jan 17 2018;8(1):929. doi: 10.1038/s41598-017-18988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gey M., Wanner R., Schilling C., Pedro M.T., Sinske D., Knöll B. Atf3 mutant mice show reduced axon regeneration and impaired regeneration-associated gene induction after peripheral nerve injury. Open Biol. Aug 2016;6(8) doi: 10.1098/rsob.160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seijffers R., Mills C.D., Woolf C.J. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J. Neurosci. : Official J. Soc. Neurosci. Jul 25 2007;27(30):7911–7920. doi: 10.1523/jneurosci.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin J., Shi J., Min X., et al. The GDF11 promotes nerve regeneration after sciatic nerve injury in adult rats by promoting axon growth and inhibiting neuronal apoptosis. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma A., Alswillah T., Singh K., et al. USP14 regulates DNA damage repair by targeting RNF168-dependent ubiquitination. Autophagy. 2018;14(11):1976–1990. doi: 10.1080/15548627.2018.1496877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martacic J., Filipovic M.K., Borozan S., et al. N-acetyl-L-cysteine protects dental tissue stem cells against oxidative stress in vitro. Clin. Oral Invest. Nov 2018;22(8):2897–2903. doi: 10.1007/s00784-018-2377-2. [DOI] [PubMed] [Google Scholar]
- 46.Schatten H. Brief overview of prostate cancer statistics, grading, diagnosis and treatment strategies. Adv. Exp. Med. Biol. 2018;1095:1–14. doi: 10.1007/978-3-319-95693-0_1. [DOI] [PubMed] [Google Scholar]
- 47.Tal R., Valenzuela R., Aviv N., et al. Persistent erectile dysfunction following radical prostatectomy: the association between nerve-sparing status and the prevalence and chronology of venous leak. J. Sex. Med. Oct 2009;6(10):2813–2819. doi: 10.1111/j.1743-6109.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 48.Küthe A., Wiedenroth A., Mägert H.J., et al. Expression of different phosphodiesterase genes in human cavernous smooth muscle. J. Urol. Jan 2001;165(1):280–283. doi: 10.1097/00005392-200101000-00079. [DOI] [PubMed] [Google Scholar]
- 49.Lima T.F.N., Bitran J., Frech F.S., Ramasamy R. Prevalence of post-prostatectomy erectile dysfunction and a review of the recommended therapeutic modalities. Int. J. Impot. Res. May 2021;33(4):401–409. doi: 10.1038/s41443-020-00374-8. [DOI] [PubMed] [Google Scholar]
- 50.Montorsi F., Brock G., Lee J., et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur. Urol. Oct 2008;54(4):924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 51.Aydogdu O., Gokce M.I., Burgu B., Baltacı S., Yaman O. Tadalafil rehabilitation therapy preserves penile size after bilateral nerve sparing radical retropubic prostatectomy. Int. Braz J. Urol. : Official J. Brazilian Soc. Urol. May-Jun 2011;37(3):336–344. doi: 10.1590/s1677-55382011000300007. ; discussion 344-6. [DOI] [PubMed] [Google Scholar]
- 52.Trachootham D., Lu W., Ogasawara M.A., Nilsa R.D., Huang P. Redox regulation of cell survival. Antioxidants Redox Signal. Aug 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tschopp J., Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. Mar 2010;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 54.Shirasuna K., Takano H., Seno K., et al. Palmitic acid induces interleukin-1β secretion via NLRP3 inflammasomes and inflammatory responses through ROS production in human placental cells. J. Reprod. Immunol. Aug 2016;116:104–112. doi: 10.1016/j.jri.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Angulo J., El Assar M., Sevilleja-Ortiz A., et al. Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention. Redox Biol. Sep 2019;26 doi: 10.1016/j.redox.2019.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martínez-Salamanca J.I., La Fuente J.M., Martínez-Salamanca E., et al. α(1A)-Adrenergic receptor antagonism improves erectile and cavernosal responses in rats with cavernous nerve injury and enhances neurogenic responses in human corpus cavernosum from patients with erectile dysfunction secondary to radical prostatectomy. J. Sex. Med. Dec 2016;13(12):1844–1857. doi: 10.1016/j.jsxm.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Martínez-Salamanca J.I., La Fuente J.M., Fernández A., et al. Nitrergic function is lost but endothelial function is preserved in the corpus cavernosum and penile resistance arteries of men after radical prostatectomy. J. Sex. Med. Mar 2015;12(3):590–599. doi: 10.1111/jsm.12801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.