Key Points
Question
Does tafamidis, 80 mg, affect cardiac function in patients with transthyretin amyloid cardiomyopathy?
Findings
In this post hoc analysis of the randomized Tafamidis in Transthyretin Cardiomyopathy Clinical Trial including 441 patients, over 30 months, there was less pronounced worsening of left ventricular (LV) stroke volume, LV global longitudinal strain, and LV filling pressure (estimated using septal and lateral E/e′ ratio) in patients treated with tafamidis, 80 mg, vs placebo.
Meaning
Compared with placebo, tafamidis, 80 mg, attenuated the decline of LV systolic and diastolic function in patients with transthyretin amyloid cardiomyopathy.
This post hoc analysis of the randomized Tafamidis in Transthyretin Cardiomyopathy Clinical Trial examines the effect of tafamidis vs placebo on 30-month changes in cardiac function in patients with transthyretin amyloid cardiomyopathy.
Abstract
Importance
Tafamidis has been shown to improve survival in patients with transthyretin amyloid cardiomyopathy (ATTR-CM) compared with placebo. However, its effect on cardiac function has not been fully characterized.
Objective
To examine the effect of tafamidis on cardiac function in patients with ATTR-CM.
Design, Setting, and Participants
This was an exploratory, post hoc analysis of the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT), a multicenter, international, double-blind, placebo-controlled phase 3 randomized clinical trial conducted from December 2013 to February 2018. The ATTR-ACT included 48 sites in 13 counties and enrolled patients aged 18 to 90 years with ATTR-CM. Data were analyzed from July 2018 to September 2023.
Intervention
Patients were randomized to tafamidis meglumine, 80 mg or 20 mg, or placebo for 30 months.
Main Outcomes and Measures
Patients were categorized based on left ventricular (LV) ejection fraction at enrollment as having heart failure with preserved ejection fraction (≥50%), mildly reduced ejection fraction (41% to 49%), or reduced ejection fraction (≤40%). Changes from baseline to month 30 in LV ejection fraction, LV stroke volume, LV global longitudinal strain, and the ratio of early mitral inflow velocity to septal and lateral early diastolic mitral annular velocity (E/e′) were compared in patients receiving tafamidis, 80 mg, vs placebo.
Results
A total of 441 patients were randomized in ATTR-ACT, and 436 patients had available echocardiographic data. Of 436 included patients, 393 (90.1%) were male, and the mean (SD) age was 74 (7) years. A total of 220 (50.5%), 119 (27.3%), and 97 (22.2%) had heart failure with preserved, mildly reduced, and reduced LV ejection fraction, respectively. Over 30 months, there was less pronounced worsening in 4 of the echocardiographic measures in patients receiving tafamidis, 80 mg (n = 176), vs placebo (n = 177) (least squares mean difference: LV stroke volume, 7.02 mL; 95% CI, 2.55-11.49; P = .002; LV global longitudinal strain, −1.02%; 95% CI, −1.73 to −0.31; P = .005; septal E/e′, −3.11; 95% CI, −5.50 to −0.72; P = .01; lateral E/e′, −2.35; 95% CI, −4.01 to −0.69; P = .006).
Conclusions and Relevance
Compared with placebo, tafamidis, 80 mg, attenuated the decline of LV systolic and diastolic function over 30 months in patients with ATTR-CM. Approximately half of patients had mildly reduced or reduced LV ejection fraction at enrollment, suggesting that ATTR-CM should be considered as a possible diagnosis in patients with heart failure regardless of underlying LV ejection fraction.
Trial Registration
ClinicalTrials.gov Identifier: NCT01994889
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a progressive, fatal disease caused by the deposition of transthyretin (TTR) amyloid fibrils in the myocardium, leading to cardiomyopathy and symptoms of heart failure.1,2 The 2 forms of the disease are hereditary (ATTRv-CM), in which a pathogenic TTR variant is present, and wild type (ATTRwt-CM), in which no variant is identified.1 Prognosis is poor in patients with untreated ATTR-CM, with median survival estimates of 2 to 6 years after diagnosis, depending on factors such as genotype and stage of disease.3
In ATTR-CM, the buildup of amyloid deposits in the myocardium leads to increased ventricular wall thickness and diastolic dysfunction.2 ATTR-CM has been observed in relatively high proportions (ie, 6% to 13%) of older patients with heart failure with preserved ejection fraction (HFpEF) and increased left ventricular (LV) wall thickness and may be a frequently overlooked cause of HFpEF.4,5 However, LV ejection fraction (LVEF) tends to decrease in advanced stages of the disease.6,7 Echocardiographic measures of both systolic and diastolic function, including LVEF, longitudinal strain, LV stroke volume (SV), and early mitral inflow velocity to septal/early diastolic mitral annular velocity (E/e′; a marker of LV filling pressures), have been shown to be independent prognostic factors for mortality in patients with ATTR-CM.8,9
Tafamidis is a TTR kinetic stabilizer that inhibits tetramer dissociation, the rate-limiting step in TTR amyloidogenesis.10 In the randomized Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT), tafamidis was shown to significantly reduce mortality and cardiovascular-related hospitalizations and to reduce decline in functional capacity and quality of life compared with placebo.11 Despite a recognized clinical benefit of tafamidis, its effect on cardiac function as measured by echocardiographic parameters has not been fully characterized. Three recent reports from single-center studies of tafamidis reported delays in cardiac structural and functional deterioration in patients with ATTR-CM treated with tafamidis compared with treatment-naive patients, thereby suggesting that tafamidis may attenuate the decline of cardiac function.12,13,14
The primary aim of this post hoc analysis of the ATTR-ACT was to assess the effect of tafamidis, 80 mg, the approved dose for ATTR-CM,15 vs placebo on echocardiographic measures of cardiac function that have previously been identified as prognostic factors for mortality in ATTR-CM.8,9 By focusing on these prognostic echocardiographic measures, we aimed to better understand the underlying basis of improved survival with tafamidis.
Methods
Trial Design and Patients
A full description of the design and methodology of ATTR-ACT has been published,11,16 and the trial protocol and statistical analysis plan can be found in Supplement 1. Briefly, ATTR-ACT was an international, multicenter, double-blind, placebo-controlled phase 3 randomized clinical trial of tafamidis for ATTR-CM. Eligible patients were aged 18 to 90 years with biopsy-confirmed ATTRv-CM or ATTRwt-CM, a medical history of heart failure, and end-diastolic interventricular septal wall thickness of 12 mm or greater. Patients were randomized 2:1:2 to receive once-daily oral tafamidis meglumine, 80 mg, tafamidis meglumine, 20 mg, or matching placebo for 30 months.
ATTR-ACT was approved by the independent review board or ethics committee at each site and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All participants provided written informed consent. ATTR-ACT followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Clinical and Echocardiographic Evaluations
Patient demographic characteristics, TTR genotype, New York Heart Association (NYHA) class, and additional clinical characteristics were collected at baseline. Race was collected by self-report at the time of enrollment based on categories provided by the investigator from a case report form that was standardized across sites; race categories included American Indian or Alaska Native, Asian, Black, multiple races, Native Hawaiian or Other Pacific Islander, White, and other or unknown race.
All patients underwent 2-dimensional (2-D) echocardiography with Doppler at baseline and at months 6, 18, and 30, following a standardized, prespecified protocol. Focused apical 4-chamber, 3-chamber, and 2-chamber (A4C, A3C, and A2C) views of the left ventricle were obtained at high frame rates (≥30 frames per second) for speckle-tracking strain analysis measurement of LV global longitudinal strain (GLS). Images were reviewed and analyzed at the MedPace Imaging Core Labs (Cincinnati, Ohio), where they underwent an initial quality control review to assess completeness and adequacy of the data before analysis by an independent, blinded cardiologist and sonographer. TOMTEC medical imaging software (TOMTEC Imaging Systems) was used for review of all images and for making all measurements. All echocardiographic measurements were made according to published guidelines.17,18
LVEF was estimated using the LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV), measured by the biplane method (modified Simpson rule) when 2 acceptable quality orthogonal views (A4C and A2C) were available or by the single-plane method when only an acceptable quality A4C view was available. LVEF was calculated using the formula (LVEDV – LVESV) / LVEDV × 100. HFpEF, heart failure with mildly reduced ejection fraction (HFmrEF), and heart failure with reduced ejection fraction (HFrEF) were defined as LVEF of 50% or greater, 41% to 49%, and 40% or less, respectively, based on current clinical practice guidelines.19
LVSV was calculated from the LV outflow tract (LVOT) diameter and velocity time integral (VTI) values using the formula π(LVOT diameter / 2)2 × LVOT VTI. The LVOT diameter is a linear, 2-D measurement obtained in the parasternal long axis. The electronic caliper is placed at the insertion point of the noncoronary cusp of the aortic valve and the right coronary cusp of the aortic valve. The LVOT VTI was obtained by placing the pulsed-wave Doppler proximal to the aortic valve in the apical 5-chamber view and tracing the LVOT flow profile.
LV GLS, which measures subendocardial longitudinal fiber systolic function, was calculated as the percentage change in longitudinal deformation of the LV myocardium between end-diastole and end-systole in the apical views and evaluated using 2-D speckle-tracking echocardiographic analysis. In each apical view (A4C, A3C, and A2C), longitudinal systolic strain was measured in 6 segments (basal, mid, and apical), and the mean of the measurements was calculated. LV GLS was subsequently determined from the mean peak value of longitudinal systolic strain from the A4C, A3C, and A2C views.
LV circumferential strain is a measure of the shortening deformation of the left ventricle between end-diastole and end-systole in the parasternal short axis (PSAX) view. Basal, mid, and apical global circumferential strain were calculated as the mean peak value of 2-D circumferential speckle tracking–derived strain of the LV basal, mid, and apical segments, respectively. LV radial strain is a measure of the thickening deformation of the LV between end-diastole and end-systole in the PSAX view. Basal, mid, and apical radial strain were calculated as the mean peak value of 2-D speckle tracking–derived strain of the LV basal, mid, and apical segments, respectively. Regional (segmental) strain values were extracted from the TOMTEC software in the A4C view (longitudinal strain) and PSAX view (circumferential and radial strain) to allow comparisons between tafamidis and placebo.
Septal and lateral E/e′ were calculated using early mitral inflow (E) velocity (measured at the tips of the mitral leaflets in the A4C view using pulsed wave Doppler) and septal and lateral early mitral annular (e′) tissue Doppler imaging velocities. The left atrial diameter was measured at ventricular end-systole in the parasternal long axis (for anteriorposterior) and A4C (for mediolateral and superior-inferior) views.
Statistical Analysis
All analyses were post hoc and were therefore considered exploratory. Baseline demographic, clinical, and echocardiographic characteristics were summarized in all patients in ATTR-ACT according to LVEF category. Differences between LVEF groups were assessed for statistical significance. P values were calculated using 1-way analysis of variance to compare means for continuous variables, the Kruskal-Wallis test to compare medians for continuous variables, the χ2 test to compare proportions with cell counts of 5 or greater, and Fisher exact test to compare proportions with cell counts less than 5.
The main analysis compared changes in LVEF, LVSV, LV GLS, and septal and lateral E/e′ from baseline to months 6, 18, and 30 in the tafamidis, 80 mg, and placebo groups. Changes in regional measures of LV longitudinal, circumferential, and radial strain and left atrial diameter from baseline to month 30 were also compared between treatment groups. Only patients treated with tafamidis, 80 mg, were assessed in comparison with placebo in these longitudinal analyses because it is the approved dose for ATTR-CM.15 Change from baseline in the selected echocardiographic measures was evaluated at each postbaseline time point using a mixed-effect model repeated-measures analysis of covariance with an unstructured covariance matrix: center and patients within center were random effects; treatment, visit, TTR genotype (variant and wild-type), and visit by treatment interaction were fixed effects; and baseline echocardiographic variables were covariates. The potential impact of baseline LVEF on tafamidis efficacy was explored by adding LVEF at baseline, with a 2-way interaction with treatment, to models for all-cause mortality, cardiovascular-related hospitalizations, and the 4 echocardiographic measures (LVSV, LV GLS, lateral E/e′, and septal E/e′). Two-tailed P values < .05 were considered significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Baseline Characteristics by LVEF Category in All Patients in ATTR-ACT
ATTR-ACT enrolled 441 patients, of whom 176 received tafamidis, 80 mg, 88 received tafamidis, 20 mg, and 177 received placebo. Of 436 included patients, 393 (90.1%) were male; 18 (4.1%) were Asian, 63 (14.4%) were Black, 352 (80.7%) were White, and 3 (0.7%) were other races; and the mean (SD) age was 74 (7) years. A total of 220 patients (50.5%) had HFpEF, 119 (27.3%) had HFmrEF, and 97 (22.2%) had HFrEF at enrollment (Table 1). Five patients with missing LVEF data at enrollment were excluded from this part of the analysis.
Table 1. Baseline Demographic Characteristics and Clinical, Laboratory, and Echocardiographic Measures Stratified by Left Ventricular Ejection Fraction (LVEF) Category.
| Characteristic | No. (%) | P valuea | ||
|---|---|---|---|---|
| HFpEF (n = 220) | HFmrEF (n = 119) | HFrEF (n = 97) | ||
| Age, y | ||||
| Mean (SD) | 74.7 (7.1) | 73.4 (6.6) | 74.5 (7.3) | .25 |
| Median (IQR) | 75 (71-80) | 74 (70-78) | 75 (70-79) | |
| Sex | ||||
| Female | 23 (10.5) | 9 (7.6) | 11 (11.3) | .60 |
| Male | 197 (89.5) | 110 (92.4) | 86 (88.7) | |
| Race | ||||
| Asian | 11 (5.0) | 3 (2.5) | 4 (4.1) | .01 |
| Black | 21 (9.5) | 18 (15.1) | 24 (24.7) | |
| White | 186 (84.5) | 98 (82.4) | 68 (70.1) | |
| Other raceb | 2 (0.9) | 0 | 1 (1.0) | |
| TTR genotype | ||||
| Wild-type | 176 (80.0) | 90 (75.6) | 64 (66.0) | .03 |
| Variant | 44 (20.0) | 29 (24.4) | 33 (34.0) | |
| NYHA class | ||||
| I/II | 162 (73.6) | 86 (72.3) | 49 (50.5) | <.001 |
| III | 58 (26.4) | 33 (27.7) | 48 (49.5) | |
| NT-proBNP, median (IQR), pg/mL | 2535.1 (1529.0-4157.3) | 3161.0 (2075.0-4694.1) | 4093.0 (2614.0-6133.0) | <.001 |
| KCCQ overall summary score, mean (SD) | 70.1 (20.1) | 67.5 (19.4) | 58.4 (24.2) | <.001 |
| 6MWT distance, mean (SD), m | 364.2 (118.8) | 369.7 (109.1) | 300.5 (134.0) | <.001 |
| Comorbidities | ||||
| Hypertension | 111 (50.5) | 64 (53.8) | 52 (53.6) | >.99 |
| Atrial fibrillation | 107 (48.6) | 65 (54.6) | 54 (55.7) | .99 |
| Coronary artery disease | 48 (21.8) | 25 (21.0) | 18 (18.6) | >.99 |
| Chronic kidney disease | 35 (15.9) | 18 (15.1) | 32 (33.0) | .94 |
| Diabetes | 18 (8.2) | 7 (5.9) | 8 (8.2) | >.99 |
| Echocardiographic measures | ||||
| LVEF, mean (SD), % | 56.5 (4.5) | 45.5 (2.6) | 33.9 (5.0) | <.001 |
| LV mass | ||||
| Total, No. | 217 | 115 | 97 | NA |
| Mean (SD), g | 283.1 (84.5) | 304.9 (94.2) | 312.1 (89.7) | .01 |
| LV end-diastolic interventricular septal wall thickness | ||||
| Total, No. | 218 | 116 | 97 | NA |
| Mean (SD), mm | 16.3 (3.6) | 16.6 (3.8) | 16.8 (3.8) | .47 |
| LV posterior wall thickness | ||||
| Total, No. | 217 | 115 | 97 | NA |
| Mean (SD), mm | 16.7 (3.8) | 17.0 (4.1) | 16.9 (4.3) | .82 |
| LV stroke volume | ||||
| Total, No. | 211 | 116 | 94 | NA |
| Mean (SD), mL | 49.8 (17.2) | 44.1 (15.2) | 37.4 (12.4) | <.001 |
| LV global longitudinal strainc | ||||
| Total, No. | 217 | 119 | 97 | NA |
| Mean (SD), % | −10.6 (3.5) | −8.9 (3.1) | −7.1 (2.5) | <.001 |
| LV mid global circumferential strain | ||||
| Total, No. | 215 | 116 | 93 | NA |
| Mean (SD), % | −19.1 (9.8) | −15.2 (6.9) | −12.4 (7.5) | <.001 |
| Septal E/e′ | ||||
| Total, No. | 212 | 117 | 92 | NA |
| Mean (SD) | 22.9 (8.7) | 24.0 (11.2) | 26.6 (11.9) | .02 |
| Lateral E/e′ | ||||
| Total, No. | 215 | 117 | 94 | NA |
| Mean (SD) | 17.2 (8.1) | 16.2 (6.6) | 17.3 (8.2) | .43 |
Abbreviations: 6MWT, 6-minute walk test; E/e′, mitral inflow E wave/early diastolic mitral annular velocity; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; NA, not applicable; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; TTR, transthyretin.
P values are from 1-way analysis of variance for age, KCCQ overall summary score, 6MWT distance, comorbidities, and echocardiographic measures; from the χ2 test for sex, TTR genotype, and NYHA class; from Fisher exact test for race; and from the Kruskal-Wallis test for NT-proBNP values.
Race data were collected at enrollment via self-report by the patients and categorized as Asian, Black, White, and other race, which included American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, multiple races, and other or unknown race.
Reference values for LV global longitudinal strain: normal (≤−18%), borderline abnormal (−18% to −16%), and abnormal (≥−16%). LV global longitudinal strain is expressed as a negative number; the closer the absolute value to zero, the worse the LV global longitudinal strain.
Median (IQR) age in patients with HFpEF, HFmrEF, and HFrEF were 75 (71-80), 74 (70-78), and 75 (70-79) years, respectively (Table 1). The proportion of patients with self-reported Black race, a variant TTR genotype, and NYHA class III heart failure was lowest in the HFpEF category and highest in the HFrEF category (Table 1). Patients with HFpEF had lower N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentration and higher (better) Kansas City Cardiomyopathy Questionnaire overall summary scores than patients with HFmrEF and HFrEF (Table 1). They also had lower LV mass, higher LVSV, lower septal E/e′, and better (ie, more negative) LV GLS and mid global circumferential strain values (Table 1). Six-minute walk test distance was similar in patients with HFpEF and HFmrEF and lowest in patients with HFrEF (Table 1).
Changes in Echocardiographic Measures Over Time by Treatment Group
Changes in echocardiographic measures were assessed in the 176 patients in the tafamidis, 80 mg, group and the 177 patients in the placebo group (Table 2).20 Mean LVEF, LVSV, LV GLS, and septal and lateral E/e′ were similar between the treatment groups at baseline (Table 2). Worsening of each echocardiographic measure was observed after 30 months, with greater decline in patients receiving placebo than in those receiving tafamidis, 80 mg (Figure 1). The least squares mean differences among those receiving tafamidis, 80 mg, vs placebo in change from baseline to month 30 were 2.09% (95% CI, −0.62 to 4.79; P = .13) for LVEF, 7.02 mL (95% CI, 2.55-11.49; P = .002) for LVSV, −1.02% (95% CI, −1.73 to −0.31; P = .005) for LV GLS, −3.11 (95% CI, −5.50 to −0.72; P = .01) for septal E/e′, and −2.35 (95% CI, −4.01 to −0.69; P = .006) for lateral E/e′ (Figure 1). Decline in LVEF was apparent from month 6 onwards with placebo, whereas there was minimal decline with tafamidis, 80 mg, until month 30 (Figure 2A). Decline in LVSV and LV GLS was apparent from month 6 onwards in all patients (Figure 2B and C). Increasing E/e′ was apparent from month 6 through month 30 with placebo; however, these only minimally changed at each time point after month 6 with tafamidis, 80 mg (Figure 2D and E).
Table 2. Baseline Demographic Characteristics and Clinical, Laboratory, and Echocardiographic Measures Stratified by Treatment Groupc.
| Characteristic | No. (%) | |
|---|---|---|
| Tafamidis, 80 mg (n = 176) | Placebo (n = 177) | |
| Age, y | ||
| Mean (SD) | 75.2 (7.2) | 74.1 (6.7) |
| Median (IQR) | 76.0 (71-81) | 74.0 (71-79) |
| Sex | ||
| Female | 18 (10.2) | 20 (11.3) |
| Male | 158 (89.8) | 157 (88.7) |
| Race | ||
| Asian | 11 (6.3) | 5 (2.8) |
| Black | 26 (14.8) | 26 (14.7) |
| White | 136 (77.3) | 146 (82.5) |
| Other racea | 3 (1.7) | 0 |
| TTR genotype | ||
| Wild-type | 134 (76.1) | 134 (75.7) |
| Variant | 42 (23.9) | 43 (24.3) |
| NYHA class | ||
| I/II | 121 (68.8) | 114 (64.4) |
| III | 55 (31.3) | 63 (35.6) |
| NT-proBNP, median (IQR), pg/mL | 3122.0 (1826.0-4948.5) | 3161.0 (1864.4-4825.0) |
| KCCQ overall summary score, mean (SD) | 67.1 (21.3) | 65.9 (21.7) |
| 6MWT distance, mean (SD), m | 344.8 (120.3) | 353.3 (126.0) |
| Comorbidities | ||
| Hypertension | 90 (51.1) | 84 (47.5) |
| Atrial fibrillation | 93 (52.8) | 89 (50.3) |
| Coronary artery disease | 35 (19.9) | 40 (22.6) |
| Chronic kidney disease | 31 (17.6) | 41 (23.2) |
| Diabetes | 14 (8.0) | 13 (7.3) |
| Echocardiographic variables | ||
| LVEF | ||
| Total, No. | 173 | 175 |
| Mean (SD), % | 48.0 (10.5) | 48.6 (9.5) |
| LV mass | ||
| Total, No. | 169 | 175 |
| Mean (SD), g | 296.4 (92.3) | 287.4 (85.1) |
| LV end-diastolic interventricular septal wall thickness | ||
| Total, No. | 171 | 175 |
| Mean (SD), mm | 16.7 (3.9) | 16.2 (3.5) |
| LV posterior wall thickness | ||
| Total, No. | 169 | 175 |
| Mean (SD), mm | 16.7 (3.8) | 16.7 (4.1) |
| LV stroke volume | ||
| Total, No. | 170 | 168 |
| Mean (SD), mL | 45.5 (16.9) | 45.1 (16.9) |
| LV global longitudinal strainb | ||
| Total, No. | 173 | 173 |
| Mean (SD), % | –9.3 (3.7) | –9.4 (3.6) |
| Septal E/e′ | ||
| Total, No. | 169 | 169 |
| Mean (SD) | 24.8 (11.0) | 24.2 (10.4) |
| Lateral E/e′ | ||
| Total, No. | 171 | 172 |
| Mean (SD) | 17.1 (7.7) | 17.2 (7.8) |
| LVEF category, No./total No. (%) | ||
| HFpEF | 87/173 (50.3) | 88/175 (50.3) |
| HFmrEF | 44/173 (25.4) | 50/175 (28.6) |
| HFrEF | 42/173 (24.3) | 37/175 (21.1) |
Abbreviations: 6MWT, 6-minute walk test; E/e′, mitral inflow E wave/early diastolic mitral annular velocity; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; TTR, transthyretin.
Race data were collected at enrollment via self-report by the patients and categorized as Asian, Black, White, and other race, which included American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, multiple races, and other or unknown race.
Reference values for LV global longitudinal strain: normal (≤−18%), borderline abnormal (−18% to −16%), and abnormal (≥−16%). LV global longitudinal strain is expressed as a negative number; the closer the absolute value to zero, the worse the LV global longitudinal strain.
Some data were previously presented in Damy et al.20
Figure 1. Least Squares (LS) Mean Changes From Baseline to Month 30 With Tafamidis, 80 mg, vs Placebo.
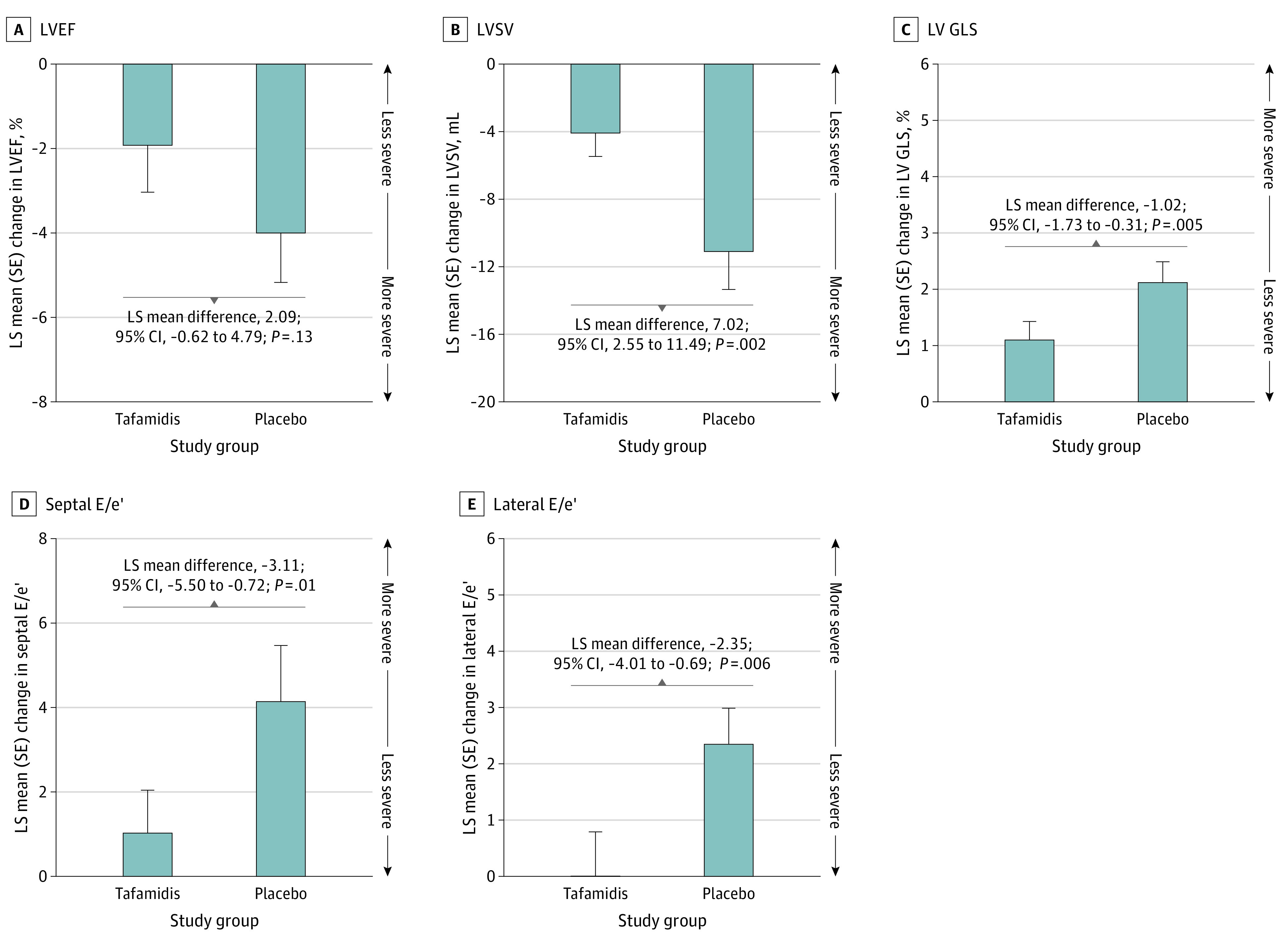
Data are shown for left ventricular ejection fraction (LVEF), left ventricular stroke volume (LVSV), left ventricular (LV) global longitudinal strain (GLS), early mitral inflow velocity to septal/early diastolic mitral annular velocity (E/e′), and lateral E/e′. LS mean differences represent tafamidis, 80 mg, minus placebo. LV GLS is expressed as a negative number; therefore, a change in the positive direction indicates worsening of this measure.
Figure 2. Least Squares (LS) Mean Changes From Baseline Over Time With Tafamidis, 80 mg, vs Placebo.
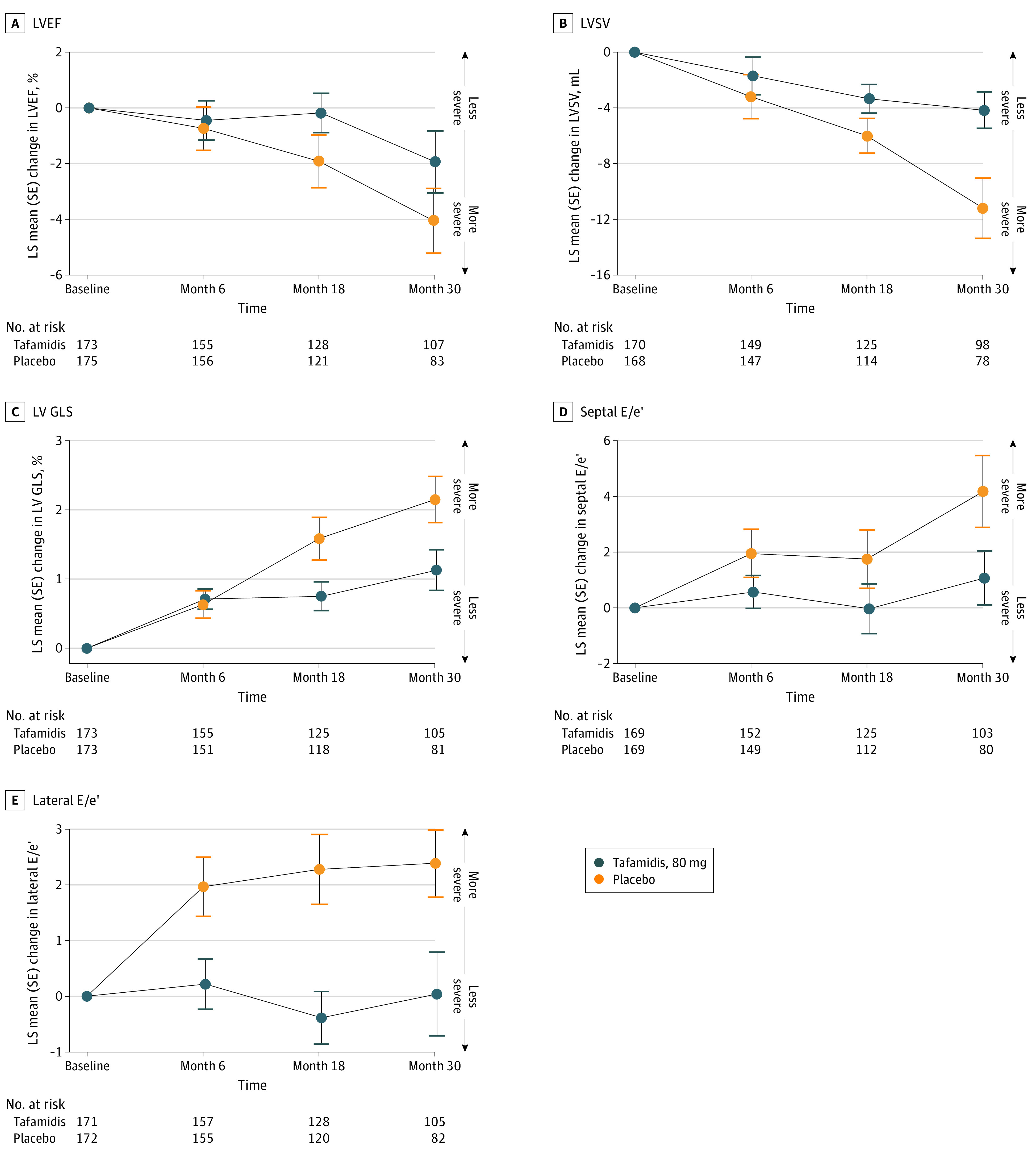
Data are shown for left ventricular ejection fraction (LVEF), left ventricular stroke volume (LVSV), left ventricular (LV) global longitudinal strain (GLS), early mitral inflow velocity to septal/early diastolic mitral annular velocity (E/e′), and lateral E/e′. LS mean differences represent tafamidis, 80 mg, minus placebo. LV GLS is expressed as a negative number; therefore, a change in the positive direction indicates worsening of this measure.
Analyses of changes in regional measures of strain showed less decline with tafamidis, 80 mg, vs placebo in midseptal (least squares mean difference, −1.84; 95% CI, −2.79 to −0.89; P < .001) and basal-lateral (−1.36; 95% CI, −2.55 to −0.16; P = .03) LV longitudinal strain, mid (−2.92; 95% CI, −4.77 to −1.07; P = .002) and apical (−3.07; 95% CI, −5.56 to −0.59; P = .02) LV circumferential strain, and mid (3.91; 95% CI, 0.97 to 6.84; P = .009) and apical (4.27; 95% CI, 0.17 to 8.38; P = .04) LV radial strain (eTable 1 in Supplement 2). There were no differences in change from baseline to month 30 in left atrial diameter with tafamidis, 80 mg, vs placebo (eTable 2 in Supplement 2).
Effect of Baseline LVEF on Treatment
There was no significant interaction between the treatment effect with tafamidis, 80 mg, and baseline LVEF for all-cause mortality (P for interaction = .40) and cardiovascular-related hospitalizations (P for interaction = .35) or for change from baseline in LVSV (P for interaction = .23), LV GLS (P for interaction = .66), septal E/e′ (P for interaction = .66), or lateral E/e′ (P for interaction = .84).
Discussion
This post hoc analysis from ATTR-ACT showed that in patients with ATTR-CM, treatment with tafamidis, 80 mg, attenuated the decline in cardiac function over 30 months compared with placebo. Differences between tafamidis, 80 mg, and placebo were observed both for LV systolic parameters (LVSV, LV GLS) and for LV diastolic indices (E/e′). Changes in LVEF followed a similar pattern of attenuated decline with tafamidis, 80 mg, but the difference between tafamidis and placebo did not reach statistical significance. These 5 echocardiographic parameters hold particular significance, as they have been identified as independent prognostic factors for mortality in patients with ATTR-CM,8,9 thereby suggesting that the effect of tafamidis on cardiac function in patients with ATTR-CM may underlie improved survival.11 Our findings provide additional insight into the clinical benefit of tafamidis for patients with ATTR-CM and emphasize the importance of early intervention.
Prior results from 3 single-center studies also reported attenuated decline in cardiac function in patients with ATTR-CM when treated with tafamidis.12,13,14 The first of these was a retrospective study that used echocardiography and found less deterioration in LV GLS, myocardial work index, and efficiency after 12 months in patients with ATTR-CM treated with tafamidis free acid, 61 mg (equivalent to tafamidis meglumine, 80 mg; n = 23), compared with untreated patients (n = 22).14 A single-center, open-label study that also used echocardiography found that patients with ATTR-CM treated with tafamidis free acid, 61 mg (n = 62), showed stable measurements in LV GLS, right ventricular GLS, and left atrial reservoir strain after a median 8.5 months follow-up, whereas treatment-naive patients (n = 54) had clear signs of disease progression in these measures after a median follow-up of 10.5 months.13 Lastly, another single-center, open-label study used cardiac magnetic resonance imaging and found that patients treated with tafamidis free acid, 61 mg (n = 35), had stable measurements in LVEF, LV mass index, and extracellular volume after a median follow-up of 9 months compared with treatment-naive patients (n = 19), who showed clear disease progression as assessed by these measures after a median follow-up of 12 months.12 In the current study, the beneficial effect of tafamidis was observed only in measures of LV function, as there was no difference between treatment groups in the change in left atrial diameter. However, left atrial diameter was the only left atrial echocardiographic parameter assessed in ATTR-ACT.
We also assessed strain parameters according to myocardial region and found that, at baseline, LV longitudinal strain followed the apical sparing pattern typically observed in patients with ATTR-CM.21,22 Attenuated decline in LV longitudinal strain was observed with tafamidis, 80 mg, vs placebo in mid and basal regions, whereas attenuated decline in LV circumferential and radial strain was observed in mid and apical regions. A similar analysis from the phase 3 APOLLO study of the RNA interference therapeutic patisiran in patients with ATTRv amyloidosis and symptomatic polyneuropathy showed that in the cardiac subpopulation (patients with baseline LV wall thickness ≥13 mm and no history of aortic valve disease or hypertension), patisiran vs placebo prevented the deterioration of LV GLS over 18 months.23 This effect was primarily driven by differences in the basal region, whereas we found attenuated decline in longitudinal strain in both the basal and mid regions (the APOLLO analysis did not examine circumferential or radial strain). However, comparisons between APOLLO and ATTR-ACT are complicated by differences in patient populations; ATTR-ACT enrolled patients with ATTRv and ATTRwt amyloidosis and a diagnosis of cardiomyopathy, whereas APOLLO enrolled patients with ATTRv amyloidosis and a diagnosis of polyneuropathy. Baseline clinical, laboratory, and echocardiographic characteristics were markedly different among patients enrolled in APOLLO vs ATTR-ACT,24 and patients with ATTR-CM (ATTR-ACT patients) have much more advanced disease compared with patients with transthyretin amyloid polyneuropathy (enrolled in the APOLLO trial).
Approximately half of the patients in ATTR-ACT had HFmrEF or HFrEF at enrollment. Those with self-reported Black race and those with ATTRv amyloidosis were more likely to present with HFmrEF or HFrEF than HFpEF, likely reflecting the high proportion of Black Americans (3% to 4%) with the V122I variant, which is associated with more severe LV systolic dysfunction than ATTRwt amyloidosis.8,25 Consistent with prior reports,7 patients with HFrEF or HFmrEF vs HFpEF at enrollment had signs of more advanced disease, including a greater proportion in NYHA class III and higher median NT-proBNP concentration, both of which are associated with poorer outcomes in ATTR-CM.26,27 Nevertheless, baseline LVEF was not found to impact the efficacy benefit of tafamidis in terms of mortality, cardiovascular-related hospitalizations, or changes in echocardiographic measures (LVSV, LV GLS, and lateral and septal E/e′) in our analysis. Of note, patients in all 3 LVEF categories, including those with HFpEF, already showed evidence of severely reduced LV GLS at enrollment, highlighting that LV GLS is a more sensitive measure of systolic function than LVEF28,29 and that other measures in addition to LVEF should be considered when characterizing the cardiac function of patients with ATTR-CM.
Strengths and Limitations
Strengths of our analysis of ATTR-ACT echocardiographic data include the relatively large size of the study population, the blinded comparison of tafamidis with placebo, the 30-month follow-up duration, and the use of a core laboratory to standardize the assessment of echocardiographic data. Study limitations are that these analyses were post hoc and not predefined, and the number of patients with ATTRv amyloidosis was relatively small. Furthermore, the longitudinal analyses included only patients receiving tafamidis, 80 mg, and not those receiving tafamidis, 20 mg. The rationale for excluding the latter group was that tafamidis, 80 mg, is the approved dose for ATTR-CM. However, this design decision precludes the generalization of the results to the tafamidis, 20 mg, dose. Our study is also limited by the lack of more detailed indices of left atrial size and function beyond the left atrial diameter measurement available in ATTR-ACT. Given the limitations of the left atrial diameter, we cannot exclude the possibility that tafamidis, 80 mg, results in changes in left atrial volume and function compared with placebo in the setting of ATTR-CM.
Conclusions
Echocardiographic data from patients with ATTR-CM in ATTR-ACT showed that, compared with placebo, tafamidis attenuated the decline in LV systolic and diastolic function typically seen in untreated patients with ATTR-CM. The high proportion of patients presenting with reduced LVEF at enrollment in ATTR-ACT suggests that ATTR-CM should be considered as a possible diagnosis in all patients with heart failure, regardless of LVEF. These clinical insights support the need for early diagnosis and intervention in patients with ATTR-CM to prevent progressive decline in cardiac function.
Trial Protocol
eTable 1. Change From Baseline to Month 30 in Echocardiographic Segmental Strain Measures: Tafamidis, 80 mg, vs Placebo
eTable 2. Change From Baseline to Month 30 in Left Atrial Diameter: Tafamidis, 80 mg, vs Placebo
Data Sharing Statement
References
- 1.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872-2891. doi: 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. doi: 10.1016/j.jchf.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 3.Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16-26. doi: 10.1161/CIRCULATIONAHA.118.038169 [DOI] [PubMed] [Google Scholar]
- 4.González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585-2594. doi: 10.1093/eurheartj/ehv338 [DOI] [PubMed] [Google Scholar]
- 5.AbouEzzeddine OF, Davies DR, Scott CG, et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6(11):1267-1274. doi: 10.1001/jamacardio.2021.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castaño A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163-178. doi: 10.1007/s10741-014-9462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106(10):528-540. doi: 10.1016/j.acvd.2013.06.051 [DOI] [PubMed] [Google Scholar]
- 8.Chacko L, Martone R, Bandera F, et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J. 2020;41(14):1439-1447. doi: 10.1093/eurheartj/ehz905 [DOI] [PubMed] [Google Scholar]
- 9.Bukhari S, Bashir Z, Shpilsky D, Eisele YS, Soman P. Abstract 16145: reduced ejection fraction at diagnosis is an independent predictor of mortality in transthyretin amyloid cardiomyopathy. Circulation. 2020;142(suppl 3):A16145-A16145. doi: 10.1161/circ.142.suppl_3.16145 [DOI] [Google Scholar]
- 10.Bulawa CE, Connelly S, Devit M, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629-9634. doi: 10.1073/pnas.1121005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer MS, Schwartz JH, Gundapaneni B, et al. ; ATTR-ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 12.Rettl R, Mann C, Duca F, et al. Tafamidis treatment delays structural and functional changes of the left ventricle in patients with transthyretin amyloid cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2022;23(6):767-780. doi: 10.1093/ehjci/jeab226 [DOI] [PubMed] [Google Scholar]
- 13.Rettl R, Duca F, Binder C, et al. Impact of tafamidis on myocardial strain in transthyretin amyloid cardiomyopathy. Amyloid. 2023;30(1):127-137. doi: 10.1080/13506129.2022.2131385 [DOI] [PubMed] [Google Scholar]
- 14.Giblin GT, Cuddy SAM, González-López E, et al. Effect of tafamidis on global longitudinal strain and myocardial work in transthyretin cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2022;23(8):1029-1039. doi: 10.1093/ehjci/jeac049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfizer . Vyndaqel (tafamidis meglumine) and Vyndamax (tafamidis) prescribing information. Accessed December 4, 2022. https://www.fda.gov/media/126283/download
- 16.Maurer MS, Elliott P, Merlini G, et al. ; ATTR-ACT Study Investigators . Design and rationale of the phase 3 ATTR-ACT Clinical Trial (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Circ Heart Fail. 2017;10(6):e003815. doi: 10.1161/CIRCHEARTFAILURE.116.003815 [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233-270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 19.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;S1071-9164(21)00050-6. doi: 10.1002/ejhf.211533663906 [DOI] [Google Scholar]
- 20.Damy T, Garcia-Pavia P, Hanna M, et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2021;23(2):277-285. doi: 10.1002/ejhf.2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840-1849. doi: 10.1161/CIRCULATIONAHA.113.006242 [DOI] [PubMed] [Google Scholar]
- 22.Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442-1448. doi: 10.1136/heartjnl-2012-302353 [DOI] [PubMed] [Google Scholar]
- 23.Minamisawa M, Claggett B, Adams D, et al. Association of patisiran, an RNA interference therapeutic, with regional left ventricular myocardial strain in hereditary transthyretin amyloidosis: the APOLLO study. JAMA Cardiol. 2019;4(5):466-472. doi: 10.1001/jamacardio.2019.0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah SJ. Targeted therapeutics for transthyretin cardiac amyloidosis. Circulation. 2019;139(4):444-447. doi: 10.1161/CIRCULATIONAHA.118.037593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22(3):171-174. doi: 10.3109/13506129.2015.1051219 [DOI] [PubMed] [Google Scholar]
- 26.Nativi-Nicolau J, Judge DP, Hoffman JE, et al. Natural history and progression of transthyretin amyloid cardiomyopathy: insights from ATTR-ACT. ESC Heart Fail. 2021;8(5):3875-3884. doi: 10.1002/ehf2.13541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grogan M, Scott CG, Kyle RA, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014-1020. doi: 10.1016/j.jacc.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 28.Abou R, van der Bijl P, Bax JJ, Delgado V. Global longitudinal strain: clinical use and prognostic implications in contemporary practice. Heart. 2020;106(18):1438-1444. doi: 10.1136/heartjnl-2019-316215 [DOI] [PubMed] [Google Scholar]
- 29.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37(15):1196-1207. doi: 10.1093/eurheartj/ehv529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Change From Baseline to Month 30 in Echocardiographic Segmental Strain Measures: Tafamidis, 80 mg, vs Placebo
eTable 2. Change From Baseline to Month 30 in Left Atrial Diameter: Tafamidis, 80 mg, vs Placebo
Data Sharing Statement


