Abstract
Brain gray matter (GM) reductions have been reported after breast cancer chemotherapy, typically in small and/or cross-sectional cohorts, most commonly using voxel-based morphometry (VBM). There has been little examination of approaches such as deformation-based morphometry (DBM), machine-learning-based brain aging metrics, or the relationship of clinical and demographic risk factors to GM reduction. This international data pooling study begins to address these questions. Participants included breast cancer patients treated with (CT+, n=183) and without (CT-, n=155) chemotherapy and noncancer controls (NC, n=145), scanned pre- and post-chemotherapy or comparable intervals. VBM and DBM examined GM volume. Estimated brain aging was compared to chronological aging. Correlation analyses examined associations between VBM, DBM, and brain age, and between neuroimaging outcomes, baseline age, and time since chemotherapy completion. CT+ showed longitudinal GM volume reductions, primarily in frontal regions, with a broader spatial extent on DBM than VBM. CT- showed smaller clusters of GM reduction using both methods. Predicted brain aging was significantly greater in CT+ than NC, and older baseline age correlated with greater brain aging. Time since chemotherapy negatively correlated with brain aging and annual GM loss. This large-scale data pooling analysis confirmed findings of frontal lobe GM reduction after breast cancer chemotherapy. Milder changes were evident in patients not receiving chemotherapy. CT+ also demonstrated premature brain aging relative to NC, particularly at older age, but showed evidence for at least partial GM recovery over time. When validated in future studies, such knowledge could assist in weighing the risks and benefits of treatment strategies.
Keywords: brain aging, breast cancer, chemotherapy, gray matter, magnetic resonance imaging (MRI)
Introduction
Cognitive problems frequently occur after chemotherapy for non-CNS cancers, at least partially attributable to neurotoxicity of chemotherapeutic regimens (Ahles & Root, 2018; Janelsins, Kesler, Ahles, & Morrow, 2014; Lange, Joly, et al., 2019). Neuroimaging studies have reported gray matter (GM) reductions after chemotherapy in many brain regions, predominantly in cortex, particularly in the frontal lobes (Amidi et al., 2017; Amidi & Wu, 2019; Apple et al., 2017; Bergouignan et al., 2011; Chen et al., 2018; Conroy et al., 2013; Correa et al., 2013; Correa et al., 2017; de Ruiter et al., 2012; Henneghan et al., 2020; Hosseini, Koovakkattu, & Kesler, 2012; Inagaki et al., 2007; Jenkins et al., 2016; Kaiser, Bledowski, & Dietrich, 2014; Kesler et al., 2013; Koppelmans et al., 2012; Lepage et al., 2014; Li & Caeyenberghs, 2018; McDonald, Conroy, Ahles, West, & Saykin, 2010; McDonald, Conroy, Smith, West, & Saykin, 2013; McDonald & Saykin, 2013; Mentzelopoulos et al., 2021; Niu et al., 2021; Simó et al., 2015; Sousa, Almeida, Bessa, & Pereira, 2020; Stouten-Kemperman, de Ruiter, Caan, et al., 2015; Stouten-Kemperman, de Ruiter, Koppelmans, et al., 2015; Yoshikawa et al., 2005). This is consistent with the importance of the prefrontal cortex for multiple aspects of cognitive functioning. However, most of these studies have been cross-sectional, lacking baseline assessments prior to systemic treatment. Longitudinal studies have baseline data, but typically have small sample sizes. Furthermore, studies often lack a disease-specific control group of chemotherapy-naïve patients, making it harder to attribute findings to chemotherapy exposure versus more general effects of the cancer disease process.
GM reductions after chemotherapy may resemble those observed with normal aging. This is consistent with evidence from clinical and preclinical studies that chemotherapy can have neurotoxic effects that mimic those underlying cellular aging (Henneghan et al., 2020; Koppelmans et al., 2012; Seigers, Schagen, Van Tellingen, & Dietrich, 2013). Machine-learning algorithms are particularly suited to capture the subtle and spatially heterogenous morphological brain changes characteristic of brain aging. This provides a metric that estimates an individual’s brain age based on neuroimaging data. For instance, an estimated brain age of 70 in a 60-year-old suggests 10 years of premature brain aging. This approach has demonstrated premature brain aging in disorders characterized by cognitive dysfunction (e.g., Alzheimer’s disease, multiple sclerosis) (Boyle et al., 2021; Cole, 2018, 2020; Cole & Franke, 2017; Franke & Gaser, 2019; Franke, Gaser, Manor, & Novak, 2013; Gaser, Franke, Klöppel, Koutsouleris, & Sauer, 2013; Henneghan et al., 2020). One study to date has applied a brain aging algorithm approach to cancer patients. Breast cancer patients were evaluated pre-chemotherapy and on average one month and one year post-chemotherapy, and compared with noncancer controls. One month after completion of chemotherapy, breast cancer patients showed two years of premature brain aging compared to controls (Henneghan et al., 2020).
There is little knowledge on the trajectory of GM reductions after chemotherapy. Some research has shown lower GM compared to controls up to 20 years after treatment (Koppelmans et al., 2012). In contrast, several studies have reported partial recovery from GM reductions in the months to years after treatment (Conroy et al., 2013; Inagaki et al., 2007; Lepage et al., 2014; McDonald et al., 2010). One commonly used method is voxel-based morphometry (VBM), an automated technique for examination of GM density/volume on a voxel-by-voxel basis across the entire brain (Ashburner & Friston, 2000, 2001; Good et al., 2001). A related technique, deformation-based morphometry (DBM) (Ashburner et al., 1998), does not include a segmentation step and is less susceptible to variations in image quality (Schwarz & Kašpárek, 2011). Only one prior study has used DBM to examine brain volumetric changes after cancer and treatment (Blommaert et al., 2019). Several risk factors have also been reported for cognitive problems after chemotherapy, including older age (Ahles et al., 2010; Lange, Heutte, et al., 2019; Schilder et al., 2010); however, no prior work has described risk factors for GM reductions after chemotherapy.
In this study, an initiative of the International Cognition and Cancer Task Force (ICCTF) Neuroimaging Working Group, data from six centers in the USA and Europe were combined to investigate whether pre- to post-chemotherapy treatment neuroimaging changes in breast cancer patients could be detected across an aggregated dataset using volume change metrics (VBM, DBM) and brain aging analyses. In addition to including patients who received chemotherapy (CT+), we included chemotherapy-naïve patients (CT-) and noncancer controls (NC). We expected GM reductions after chemotherapy that would be most prominent in cortical brain regions, and premature brain aging compared to chemotherapy-naïve patients and noncancer controls. We further evaluated whether these MRI indices of chemotherapy neurotoxicity were associated with time since treatment and whether older age was a risk factor.
Methods
Contributions
Invitations to participate were extended to attendees of ICCTF conferences via personal communications. Inclusion criteria were longitudinal high-resolution T1-weighted brain MRI data acquired at 3T in breast cancer patients who received chemotherapy (CT+) with one measurement prior to chemotherapy (baseline) and at least one measurement after chemotherapy (follow-up). Additionally, longitudinal data from breast cancer patients who did not receive chemotherapy (CT-), and noncancer controls (NC) were included as available. Information was gathered about available cognitive and behavioral data, but there was too much heterogeneity of measures among the cohorts for meaningful integration of those data. Approval of local Institutional Review Boards to participate or a waiver to provide anonymized data was required before data aggregation. Terms of access agreements were executed for each participating institution.
Data were contributed by four institutions from the USA (City of Hope, Indiana University, University of Michigan, and University of Vermont) and two from Europe (KU Leuven, Belgium, and The Netherlands Cancer Institute, the Netherlands). Some results from individual datasets were published previously (Blommaert et al., 2019; Chen et al., 2018; McDonald et al., 2013). Descriptions of participants, scanners, and acquisition parameters by institution are summarized in Supplementary Table 1. Anonymized data were securely uploaded to a high-performance computing server of Indiana University for analysis.
Volume change metrics
Locally developed MATLAB scripts (The MathWorks, Inc., Natick, MA), SPM12, and CAT12 were used to implement modulated VBM and DBM methods (see Supplementary Material) (Ashburner & Friston, 2000, 2001; Ashburner et al., 1998; Good et al., 2001). Statistical comparisons within and between groups and over time, including interaction analyses, were conducted using a full factorial model in SPM12, with factors of Group (three independent levels: CT+, CT-, NC) and Time (two non-independent levels: baseline and follow-up). Data acquisition site, age, and interscan interval were included as covariates for DBM and VBM. Intracranial volume (ICV) was additionally included for VBM. Overall significance was set at family-wise error (FWE) correction P<0.05 to address multiple comparisons. Clusters were then considered significant at cluster-level Puncorrected<0.05. Mean values for clusters showing significant reduction in GM volume from baseline to follow-up in the cancer groups were extracted using MATLAB for post-hoc correlation analyses. To evaluate overall GM loss and to account for the large interscan interval range we calculated annual GM loss by subtracting total GM volume (calculated in CAT12) at follow-up from total GM volume at baseline, divided by the interscan interval in years.
Brain age analysis
brainageR software (available on https://github.com/james-cole/brainageR/releases/tag/1.0, also see Cole et al. (Cole et al., 2017)) was used for generating brain-predicted age values at both timepoints. This involved using preprocessed brain volume images and comparing them to a machine learning model of brain aging trained on MRIs from healthy individuals (see Supplementary Material). Spearman’s correlations of chronological age with brain age at baseline were used to assess the accuracy of brain age predictions. Between-group differences in brain aging ratio (brain age at follow-up minus brain age at baseline)/(age at follow-up minus age at baseline) were assessed with ANOVA, followed by paired comparisons, and considered significant at P<0.05.
Correlation analyses
Spearman correlations evaluated associations between volume change metrics (annual GM loss, clusters showing GM volume reduction on VBM or DBM), and brain aging ratio, and between time since chemotherapy and age at baseline and volume change metrics (annual GM loss, significant VBM and DBM clusters) and brain aging ratio. To reduce the chance of type I error, P<0.01 was considered statistically significant for all correlation analyses.
Results
Patient characteristics
At baseline participants were 50 years old on average and mean time between baseline and follow-up measurement (interscan interval) was 7.6 months. No significant differences were found for these variables between treatment groups. Annual GM loss was numerically larger for CT+ participants (Table 1). Duration of follow-up since chemotherapy was available for 181 out of 183 patients and was on average 3.8 months (range 0–15.0, SD 3.0). Chemotherapeutic regimens varied and consisted of alkylating agents and taxanes, with or without anthracyclines or antimetabolite agents (Supplementary Table 1).
Table 1.
Participant Characteristics
| CT+ (n=183) | CT- (n=155) | NC (n=145) | P | |
|---|---|---|---|---|
| Age | 49.9 (10.1) | 51.5 (8.9) | 49.7 (10.8) | 0.24 |
| Range 28–82 | Range 30–75 | Range 20–78 | ||
| Breast cancer stage | ||||
| 0 | 0 (0%) | 39 (25.2%) | ||
| I | 52 (28.4) | 98 (6.2%) | ||
| II | 86 (47.0%) | 16 (10.3%) | ||
| III | 28 (15.3%) | 0 (0%) | ||
| Missing | 17 (9.3%) | 2 (1.3%) | ||
| Interscan interval (months) | 7.5 (3.4) | 7.8 (3.3) | 7.6 (3.6) | 0.73 |
| Range 2.4–23.1 | Range 2.1–18.7 | Range 3.0–26.7 | ||
| Annual gray matter loss (ml/year) | -9.2 (32.2) | -4.4 (23.1) | -5.5 (26.2) | 0.24 |
Values are mean (SD); CT+=Breast cancer patients treated with chemotherapy; CT-=Breast cancer patients treated without chemotherapy; NC=Healthy controls with no cancer diagnosis. Significance of group differences was assessed with ANOVA.
VBM and DBM
The CT+ group (Table 2, Figure 1) showed volume reduction in left dorsolateral prefrontal cortex (DLPFC) and cerebellar GM from baseline to follow-up using VBM. DBM also showed volume reduction in left DLPFC, as well as additional reductions in right DLPFC and left anterior prefrontal cortex. The CT- group (Table 2, Figure 2) showed small clusters of volume reduction in right middle temporal and anterior prefrontal, left dorsal posterior cingulate, and left cerebellar GM from baseline to follow-up using VBM. Right anterior prefrontal cortex reduction was also evident using DBM. There were no regions where either cancer group showed significant volume increases, and no significant changes were seen in the NC group. Between-group interaction analyses were largely nonsignificant, though in one interaction NC showed relative reductions over time in left orbitofrontal cortex and right putamen compared to CT+ (Table 2), due to a slight (nonsignificant) decline in NC and a slight (nonsignificant) increase in CT+.
Table 2.
VBM and DBM Results
| MNI peak (x,y,z) | Cluster level | k | Z | Brain region | |
|---|---|---|---|---|---|
|
| |||||
| P FWE-corrected | Puncorrected | ||||
| VBM CT+ Baseline > Follow-up | |||||
| −28 45 44 | 0.002 | 0.030 | 46 | 6.19 | Left dorsolateral prefrontal cortex (BA9) |
| −26 −80 −44 | <0.001 | 0.004 | 90 | 5.04 | Left cerebellum |
|
| |||||
| VBM CT- Baseline > Follow-up | |||||
|
| |||||
| 60 2 −32 | 0.001 | 0.013 | 63 | 5.56 | Right middle temporal gyrus (BA21) |
| 40 52 −15 | 0.002 | 0.039 | 41 | 5.30 | Right anterior prefrontal cortex (BA10) |
| −2 −38 51 | 0.002 | 0.032 | 45 | 5.17 | Left dorsal posterior cingulate cortex (BA 31) |
| −36 −68 −21 | 0.001 | 0.015 | 61 | 5.07 | Left cerebellum |
|
| |||||
| DBM CT+ Baseline > Follow-up | |||||
|
| |||||
| −27 51 27 | <0.001 | <0.001 | 1565 | 6.31 | Left anterior prefrontal cortex (BA10) |
| 27 42 28 | <0.001 | 0.003 | 367 | 5.50 | Right dorsolateral prefrontal cortex (BA9) |
| −48 34 15 | 0.002 | 0.042 | 149 | 5.14 | Left dorsolateral prefrontal cortex (BA46) |
|
| |||||
| DBM CT- Baseline > Follow-up | |||||
|
| |||||
| 44 50 −15 | 0.002 | 0.047 | 141 | 5.10 | Right anterior prefrontal cortex (BA10) |
|
| |||||
| DBM NC reduction from Baseline to Follow-up Relative to CT+ | |||||
|
| |||||
| −15 42 −18 | 0.001 | 0.020 | 202 | 5.54 | Left orbitofrontal cortex (BA11) |
| 24 12 −9 | 0.001 | 0.015 | 224 | 5.47 | Right putamen |
MNI=Montreal Neurological Institute coordinates; FWE=Family-wise error; k=cluster extent; VBM=Voxel-based morphometry; DBM=Deformation-based morphometry; BA=Brodmann Area; CT+=Breast cancer patients treated with chemotherapy; CT-=Breast cancer patients treated without chemotherapy; NC=Healthy controls with no cancer diagnosis
Figure 1.
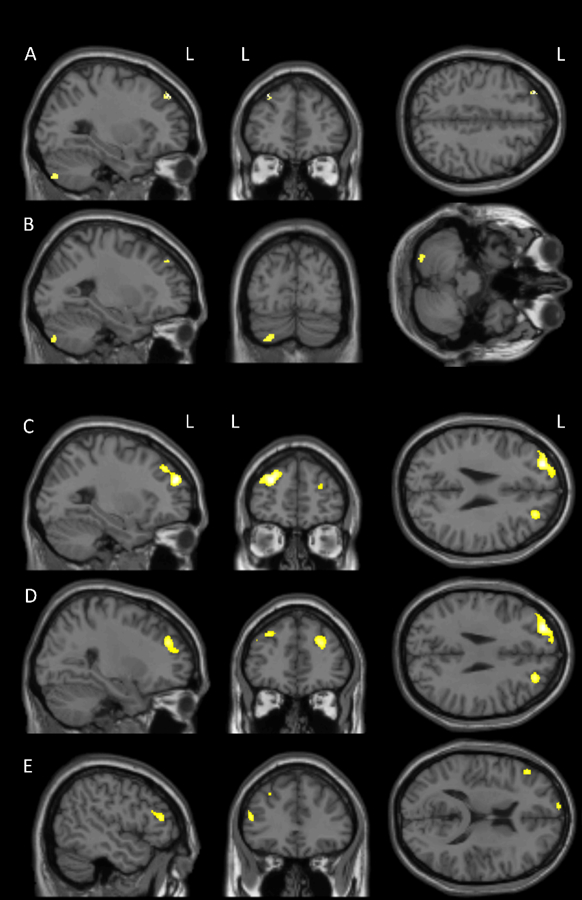
Gray matter volume reduction from baseline to follow-up in breast cancer patients treated with chemotherapy (CT+ group) as seen on voxel-based morphometry (VBM) in A) left dorsolateral prefrontal cortex and B) left cerebellum, and on deformation-based morphometry (DBM) in C) left anterior prefrontal cortex, D) right dorsolateral prefrontal cortex, and E) left dorsolateral prefrontal cortex (overall FWE-corrected p<0.05, cluster-level puncorrected<0.05).
Figure 2.
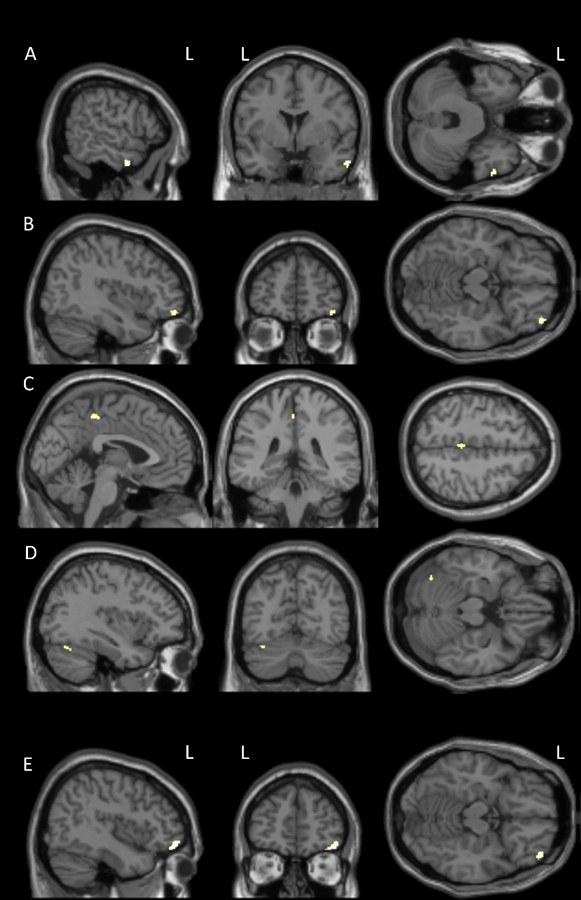
Gray matter volume reduction from baseline to follow-up in breast cancer patients not treated with chemotherapy (CT- group) as seen on voxel-based morphometry (VBM) in A) right middle temporal gyrus, B) right anterior prefrontal cortex, C) left dorsal posterior cingulate cortex, and D) left cerebellum, and on deformation-based morphometry (DBM) in E) right anterior prefrontal cortex (overall FWE-corrected p<0.05, cluster-level puncorrected<0.05).
Brain age analyses
Chronological age and brain age estimated at baseline showed correlations (r) of 0.77 (CT+), 0.79 (CT-) and 0.82 (NC, all Ps<0.001, Supplementary Figure 1). Brain aging ratio was significantly different between groups (P=0.035). Post-hoc tests showed a significant difference between the CT+ and NC groups (P=0.01). Mean brain aging ratio was highest for the CT+ group (1.7), followed by the CT- (1.0) and NC (0.4) groups (Figure 3). The interscan interval for chemotherapy-treated patients was on average 7.5 months. As the brain age ratio was 1.7, this indicates longitudinal brain aging of 7.5 months*1.7=12.9 months, resulting in premature brain aging of (12.9–7.5)=5.4 months.
Figure 3.
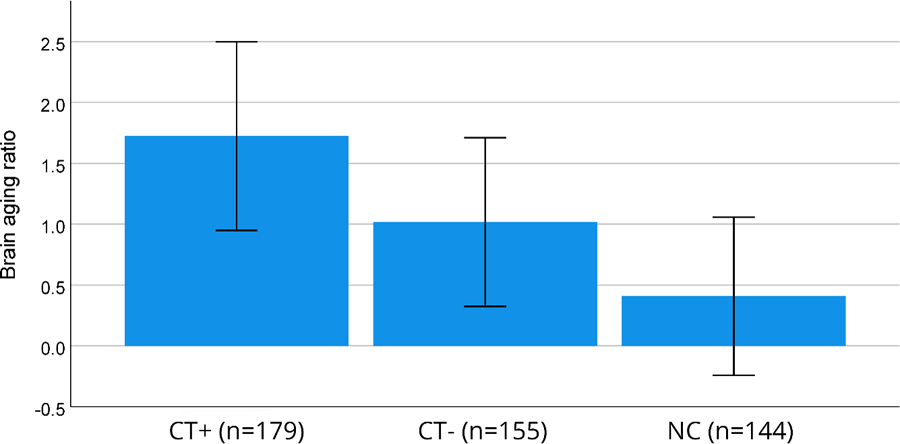
Brain aging ratio per group, defined as longitudinal brain aging/chronological aging. Error bars show SEM.
Associations between volume change metrics and brain age analyses
Annual GM loss was negatively correlated with brain aging ratio (i.e., a decline in GM was associated with an increase in brain aging ratio; CT+: r=−0.44, P<0.001, CT-: r=−0.34, P<0.001, NC: r=−0.20, P=0.014), indicating greater GM loss with greater brain aging. No significant correlations were found between brain aging ratio and VBM or DBM clusters showing significant change over time in the CT+ group. In the CT- group, there were significant negative correlations between brain aging ratio and VBM volume changes in the left dorsal posterior cingulate (r=−0.26, P=0.001), right middle temporal (r=−0.24, P=0.003), and right anterior prefrontal (r=−0.22, P=0.006) regions, indicating greater GM loss with greater brain aging.
Associations between time since treatment, age at baseline, and neuroimaging outcomes
Time since chemotherapy was positively correlated with annual GM loss (r=0.21, P=0.004) and negatively correlated with brain aging ratio (r=−0.20, P=0.007, Figure 4, upper panel), indicating that patients with longer time since treatment showed less GM loss and lower brain aging ratios. There were no significant correlations between time since treatment and VBM or DBM changes. Age at baseline was negatively correlated with annual GM loss in the CT+ group (r=−0.35, P<0.001), indicating greater GM loss with older age at baseline. This was not seen in CT- or NC (r=−0.04 and r=−0.06, respectively, Ps>0.45). Age at baseline was positively correlated with brain aging ratio in the CT+ group (r=0.27, P<0.001, Figure 4, lower panel), indicating greater brain aging with older age. This was not seen in CT- or NC (r=0.12 and r=−0.05, respectively, Ps>0.16). The only significant correlation between age at baseline and VBM or DBM clusters showing change over time was a positive correlation in the CT- group (r=0.24, P=0.003) for the left cerebellar VBM cluster, suggesting older patients showed less volume loss in this region over time.
Figure 4.
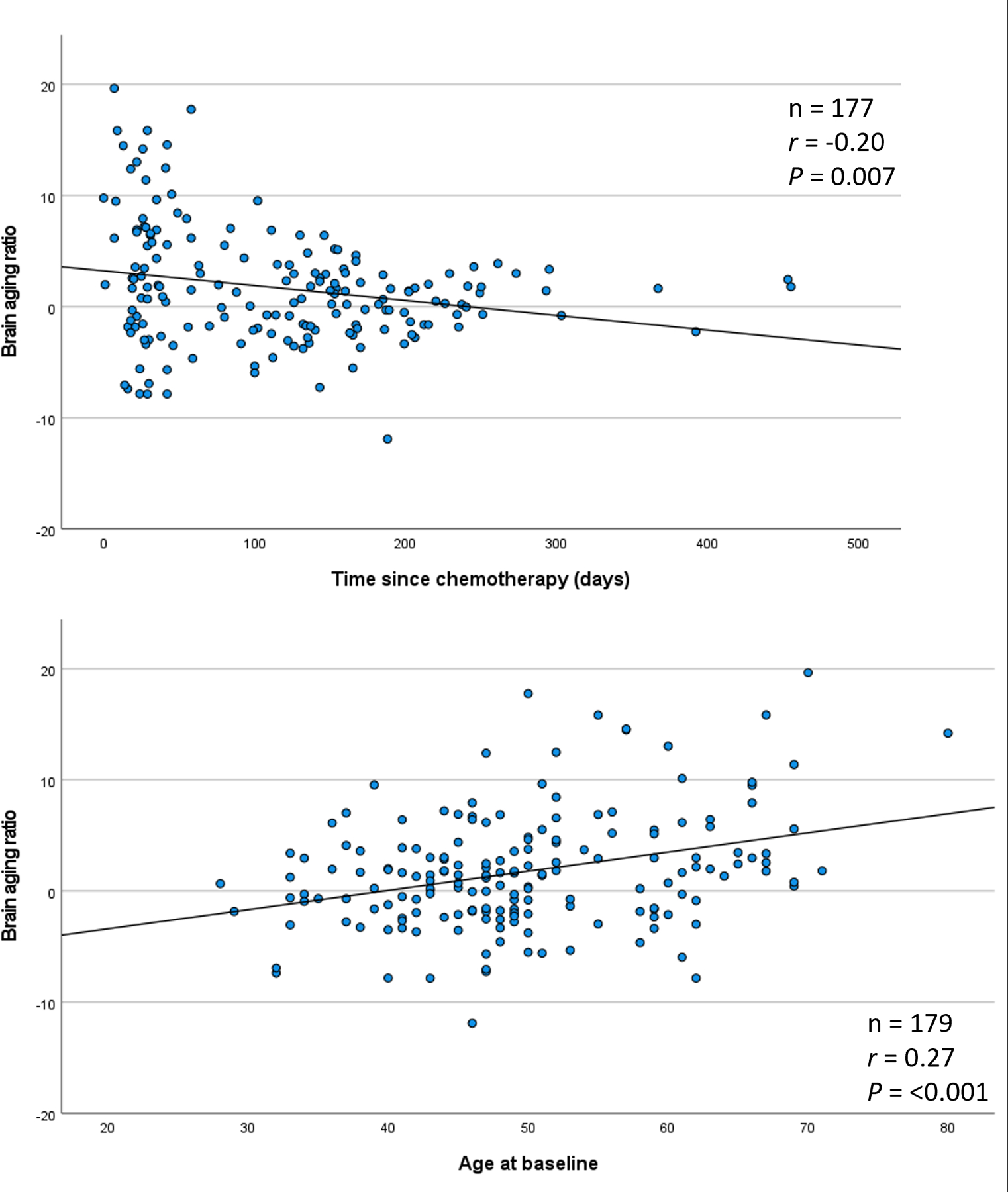
Upper panel: correlation of time since chemotherapy with brain aging ratio. Lower panel: Correlation of age at baseline with brain aging ratio in patients treated with chemotherapy.
Discussion
This study represents the first implementation of a large multicenter neuroimage pooling strategy assessing alterations in cerebral structure after breast cancer chemotherapy. Within one year after chemotherapy completion patients showed reduced GM volume and premature brain aging. Volume change (VBM and DBM) analyses showed bilateral GM reductions after chemotherapy, predominantly in prefrontal brain regions. The involvement of the prefrontal cortex is consistent with its prominent role in cognitive functioning, and the cognitive problems commonly observed in cancer patients post-chemotherapy (Ahles & Root, 2018; Janelsins et al., 2014; Lange, Joly, et al., 2019). Our findings are also consistent with prior studies that were typically small and/or cross-sectional (Amidi et al., 2017; Amidi & Wu, 2019; Apple et al., 2017; Bergouignan et al., 2011; Chen et al., 2018; Conroy et al., 2013; Correa et al., 2013; Correa et al., 2017; de Ruiter et al., 2012; Henneghan et al., 2020; Hosseini et al., 2012; Inagaki et al., 2007; Jenkins et al., 2016; Kaiser et al., 2014; Kesler et al., 2013; Koppelmans et al., 2012; Lepage et al., 2014; Li & Caeyenberghs, 2018; McDonald et al., 2010; McDonald et al., 2013; McDonald & Saykin, 2013; Mentzelopoulos et al., 2021; Niu et al., 2021; Simó et al., 2015; Sousa et al., 2020; Stouten-Kemperman, de Ruiter, Caan, et al., 2015; Stouten-Kemperman, de Ruiter, Koppelmans, et al., 2015; Yoshikawa et al., 2005), which also reported GM reductions in chemotherapy-treated patients. The apparent vulnerability of the prefrontal cortex to neurotoxicity of chemotherapy is consistent with the results of a recent meta-analysis (Niu et al., 2021), and is generally in agreement with the notion that this region of the brain is particularly sensitive to neurodegeneration (McDonald & Saykin, 2013). Our results also suggest that DBM and VBM may provide complementary information, as both similarities and differences were seen in the results from the two methods (Schwarz & Kašpárek, 2011). Patients not receiving chemotherapy also showed some GM reductions over time, though these were less prominent (i.e., smaller cluster sizes) than those seen in chemotherapy-treated patients. These findings may indicate changes related to the cancer disease process and/or to other systemic treatments (Ahles & Root, 2018; Janelsins et al., 2014; Lange, Joly, et al., 2019). We did not find any within-group volume changes in noncancer controls, though in one interaction NC showed reduced left orbitofrontal and right putamen volume over time relative to the CT+ group, a finding of unclear significance.
Acceleration of the biological aging process by cancer therapies is an influential conceptual framework (Ahles & Root, 2018; Carroll et al., 2019; Guida et al., 2019; Hurria, Jones, & Muss, 2016). Within this framework, premature brain aging may explain how systemic cancer therapies cause cognitive dysfunction. Our machine-learning-based brain age analyses showed premature brain aging in chemotherapy-treated patients. Brain age increased 1.7 times faster than chronological aging (i.e., time between MRI scans), translating into an average of 5.4 months of premature brain aging 4 months after chemotherapy. This significantly differed from noncancer controls, and was not seen in breast cancer patients who did not receive chemotherapy. Kesler and coworkers also investigated chemotherapy-associated premature brain aging in breast cancer patients with a machine-learning-based approach (Henneghan et al., 2020). Similar to our findings, they found increased premature brain aging one month post-chemotherapy compared to noncancer controls. This difference disappeared one year after chemotherapy. Several differences between that study and ours should be noted, particularly the larger sample size in the current study, the inclusion of a disease-specific control group, the large variability between measurements (whereas timepoints in the Kesler et al. study were more centered around one month and one year post-treatment), and the use of a different brain age algorithm.
To evaluate the validity of a machine-learning-based brain age measure as a global measure for premature brain aging, we determined associations with annual GM loss (averaged across brain GM). In all three groups, annual GM loss showed a highly significant association with the brain aging ratio, indicating that greater GM loss is associated with greater premature brain aging. This confirms the validity of brain aging ratio as a general as opposed to brain region-specific marker of chemotherapy-induced premature brain aging. Of note, annual GM loss was not significantly higher in chemotherapy-treated patients than those who did not receive chemotherapy or noncancer controls. This suggests that the brain aging ratio may be a more sensitive measure for chemotherapy-associated neurotoxicity than GM volume. Voxel-wise analyses and brain age estimations likely provide complementary methods to measure neurodegenerative consequences of cancer treatment. Whereas voxel-based methods provide a well-established approach to assess regional changes in brain tissue suggestive of neurotoxicity, brain age estimations may provide a global assessment of brain health that is more sensitive than measurements of overall tissue reduction. Since the algorithm is trained on multiple scanners, it might be more stable across different imaging protocols and scanners.
The large variation in time since chemotherapy completion allowed us to investigate time since treatment effects, although still within a relatively short timeframe. Time since chemotherapy was positively associated with annual GM loss and brain aging ratio, both indicating that the extent of premature brain aging decreased over time in the timeframe covered in the current study (up to 455 days after chemotherapy). We therefore found no support for the accelerated aging hypothesis, which proposes that age-associated declines after cancer follow a steeper slope than those without a cancer history (Ahles, Root, & Ryan, 2012). In contrast, our findings seem to be more compatible with partial recovery after chemotherapy-induced brain aging. This is in agreement with studies in breast cancer patients that found partial recovery from chemotherapy associated GM reductions; two studies found partial recovery one year versus one month post-chemotherapy (Lepage et al., 2014; McDonald et al., 2010), whereas one study found GM reduction one year, but not three years after chemotherapy (Inagaki et al., 2007). Another VBM study similarly showed a positive correlation between GM density and time since chemotherapy (Conroy et al., 2013). These findings suggest that some recovery from chemotherapy-associated GM reduction occurs.
An important topic in the study of cancer and cognition is whether some patients are at risk for cognitive decline after receiving chemotherapy. Several studies point to older age as a risk factor (Ahles et al., 2010; Lange, Heutte, et al., 2019; Schilder et al., 2010). We observed that age at chemotherapy treatment was significantly associated with both annual GM loss as well as brain aging ratio; the older a patient was before receiving chemotherapy, the more GM loss and the higher the brain aging ratio after chemotherapy. Importantly, these associations were not observed in the other two groups. This may point to an underlying mechanism for older age as a risk factor for chemotherapy-associated cognitive problems.
There are limitations to the current study, most inherent to a retrospective data pooling project. Clinical and treatment variables (e.g., disease characteristics, chemotherapy, radiotherapy, and endocrine treatment regimens/doses) were not gathered consistently across sites, and so could not be included in analyses. Similarly, we could not include cognitive or behavioral data, as there was insufficient overlap between measures used in the different independent cohorts. We therefore could not assess whether decreases in GM or degree of premature brain aging were associated with cognitive or behavioral change. The maximum time since chemotherapy completion was 15 months, but 75% of the MRI data were acquired within 6 months after chemotherapy, limiting interpretation of results over 6 months post-chemotherapy. Although all MRIs were T1-weighted high-resolution scans acquired on 3T scanners, MRI protocols and scanners varied, which could contribute to differences in image inhomogeneity and contrast distribution. Importantly, the brain age algorithm was also trained on MRI scans acquired with different protocols from different vendors. While brain age is a promising biomarker, in the currently applied algorithm it is unclear how variables are being combined to make predictions. There was also variability in the applied chemotherapeutic regimens, although most patients received alkylating agents and taxanes, with or without anthracyclines or antimetabolite agents. Overall, sample heterogeneity may have contributed to the lack of significant interaction effects. Strengths of the present study include the large sample size, the wide age range of participants, the wide range in time intervals between measurements, the longitudinal nature of the data, and the inclusion of both disease-specific and noncancer control groups.
Conclusions
We found that breast cancer chemotherapy is associated with frontal and cerebellar GM decline and premature brain aging within one year after treatment. Our findings suggest that some recovery occurs over time, and that older age is a risk factor for chemotherapy-associated premature brain aging. When validated in future studies with longer follow-ups, ideally with harmonized scan protocols, such knowledge could be used for tailored patient education, early cognitive interventions, and in weighing the risks and benefits of treatment strategies.
Supplementary Material
Supplementary Figure 1. Scatter plot of age and brain age values at baseline measurement.
CT+=Breast cancer patients treated with chemotherapy; CT-=Breast cancer patients treated without chemotherapy; NC=Healthy controls with no cancer diagnosis.
Acknowledgements
The authors wish to posthumously acknowledge the contributions of John D. West, MS to this research.
Funding Sources
Sources of support for gathering and sharing the data analyzed for this paper include National Institutes of Health grants R03 AG045090, R01 NR010939, and R01 CA101318, Breast Cancer Research Foundation grant BCRF-9 2008–2016, Dutch Cancer Society grant 2009–4284, Research Foundation Flanders (FWO) grant G.048010N, and Stichting tegen Kanker.
Footnotes
This study was presented in part at the Internation Cognition and Cancer Task Force (ICCTF) conference in Denver, CO in 2020.
Conflict of Interest
Sources of support for gathering and sharing the data analyzed for this paper include National National Institutes of Health grants R03 AG045090, R01 NR010939, and R01 CA101318, Breast Cancer Research Foundation grant BCRF-9 2008–2016, Dutch Cancer Society grant 2009–4284, Research Foundation Flanders (FWO) grant G.048010N, and Stichting tegen Kanker. AJS has received support from Springer-Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior). All other authors declared no conflicts of interest that are relevant to the content of this article.
Compliance with Ethical Standards
Written informed consent was obtained from all participants as per protocols approved by the respective Institutional Review Board at each participating institution (City of Hope, Indiana University, KU Leuven, The Netherlands Cancer Institute, University of Michigan, and University of Vermont).
Data Availability Statement
Inter-institutional terms of access agreements enacted for the purposes of this data pooling study do not permit resharing of the study data. Those interested on obtaining data could contact the individual participating institutions, and the corresponding author can facilitate reasonable such requests.
References
- Ahles TA, & Root JC (2018). Cognitive Effects of Cancer and Cancer Treatments. Annu Rev Clin Psychol, 14, 425–451. doi: 10.1146/annurev-clinpsy-050817-084903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Root JC, & Ryan EL (2012). Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol, 30(30), 3675–3686. doi: 10.1200/jco.2012.43.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, … Kaufman PA (2010). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol, 28(29), 4434–4440. doi: 10.1200/jco.2009.27.0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amidi A, Agerbæk M, Wu LM, Pedersen AD, Mehlsen M, Clausen CR, … Zachariae R (2017). Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav, 11(3), 769–783. doi: 10.1007/s11682-016-9552-3 [DOI] [PubMed] [Google Scholar]
- Amidi A, & Wu LM (2019). Structural brain alterations following adult non-CNS cancers: a systematic review of the neuroimaging literature. Acta Oncol, 58(5), 522–536. doi: 10.1080/0284186x.2018.1563716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple AC, Ryals AJ, Alpert KI, Wagner LI, Shih PA, Dokucu M, … Wang L (2017). Subtle hippocampal deformities in breast cancer survivors with reduced episodic memory and self-reported cognitive concerns. Neuroimage Clin, 14, 685–691. doi: 10.1016/j.nicl.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2000). Voxel-based morphometry--the methods. Neuroimage, 11(6 Pt 1), 805–821. doi: 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2001). Why voxel-based morphometry should be used. Neuroimage, 14(6), 1238–1243. doi: 10.1006/nimg.2001.0961 [DOI] [PubMed] [Google Scholar]
- Ashburner J, Hutton C, Frackowiak R, Johnsrude I, Price C, & Friston K (1998). Identifying global anatomical differences: deformation-based morphometry. Hum Brain Mapp, 6(5–6), 348–357. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergouignan L, Lefranc JP, Chupin M, Morel N, Spano JP, & Fossati P (2011). Breast cancer affects both the hippocampus volume and the episodic autobiographical memory retrieval. PLoS One, 6(10), e25349. doi: 10.1371/journal.pone.0025349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blommaert J, Schroyen G, Vandenbulcke M, Radwan A, Smeets A, Peeters R, … Deprez S (2019). Age-dependent brain volume and neuropsychological changes after chemotherapy in breast cancer patients. Hum Brain Mapp, 40(17), 4994–5010. doi: 10.1002/hbm.24753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R, Jollans L, Rueda-Delgado LM, Rizzo R, Yener GG, McMorrow JP, … Whelan R (2021). Brain-predicted age difference score is related to specific cognitive functions: a multi-site replication analysis. Brain Imaging Behav, 15(1), 327–345. doi: 10.1007/s11682-020-00260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Van Dyk K, Bower JE, Scuric Z, Petersen L, Schiestl R, … Ganz PA (2019). Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer, 125(2), 298–306. doi: 10.1002/cncr.31777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Jin T, Patel SK, Ye N, Sun CL, Ma H, … Hurria A (2018). Gray matter density reduction associated with adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat, 172(2), 363–370. doi: 10.1007/s10549-018-4911-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH (2018). Neuroimaging studies illustrate the commonalities between ageing and brain diseases. Bioessays, 40(7), e1700221. doi: 10.1002/bies.201700221 [DOI] [PubMed] [Google Scholar]
- Cole JH (2020). Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol Aging, 92, 34–42. doi: 10.1016/j.neurobiolaging.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, & Franke K (2017). Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends Neurosci, 40(12), 681–690. doi: 10.1016/j.tins.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Cole JH, Underwood J, Caan MW, De Francesco D, van Zoest RA, Leech R, … Sharp DJ (2017). Increased brain-predicted aging in treated HIV disease. Neurology, 88(14), 1349–1357. doi: 10.1212/wnl.0000000000003790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, … Saykin AJ (2013). Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat, 137(2), 493–502. doi: 10.1007/s10549-012-2385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Root JC, Baser R, Moore D, Peck KK, Lis E, … Relkin N (2013). A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav, 7(4), 478–490. doi: 10.1007/s11682-013-9221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Root JC, Kryza-Lacombe M, Mehta M, Karimi S, Hensley ML, & Relkin N (2017). Brain structure and function in patients with ovarian cancer treated with first-line chemotherapy: a pilot study. Brain Imaging Behav, 11(6), 1652–1663. doi: 10.1007/s11682-016-9608-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, … Schagen SB (2012). Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp, 33(12), 2971–2983. doi: 10.1002/hbm.21422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, & Gaser C (2019). Ten years of BrainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front Neurol, 10, 789. doi: 10.3389/fneur.2019.00789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Gaser C, Manor B, & Novak V (2013). Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci, 5, 90. doi: 10.3389/fnagi.2013.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Franke K, Klöppel S, Koutsouleris N, & Sauer H (2013). BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS One, 8(6), e67346. doi: 10.1371/journal.pone.0067346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage, 14(1 Pt 1), 21–36. doi: 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Guida JL, Ahles TA, Belsky D, Campisi J, Cohen HJ, DeGregori J, … Hurria A (2019). Measuring aging and identifying aging phenotypes in cancer survivors. J Natl Cancer Inst, 111(12), 1245–1254. doi: 10.1093/jnci/djz136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneghan A, Rao V, Harrison RA, Karuturi M, Blayney DW, Palesh O, & Kesler SR (2020). Cortical brain age from pre-treatment to post-chemotherapy in patients with breast cancer. Neurotox Res, 37(4), 788–799. doi: 10.1007/s12640-019-00158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Koovakkattu D, & Kesler SR (2012). Altered small-world properties of gray matter networks in breast cancer. BMC Neurol, 12, 28. doi: 10.1186/1471-2377-12-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurria A, Jones L, & Muss HB (2016). Cancer treatment as an accelerated aging process: assessment, biomarkers, and interventions. Am Soc Clin Oncol Educ Book, 35, e516–522. doi: 10.1200/edbk_156160 [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, … Uchitomi Y (2007). Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer, 109(1), 146–156. doi: 10.1002/cncr.22368 [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, & Morrow GR (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry, 26(1), 102–113. doi: 10.3109/09540261.2013.864260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V, Thwaites R, Cercignani M, Sacre S, Harrison N, Whiteley-Jones H, … Harder H (2016). A feasibility study exploring the role of pre-operative assessment when examining the mechanism of ‘chemo-brain’ in breast cancer patients. Springerplus, 5, 390. doi: 10.1186/s40064-016-2030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Bledowski C, & Dietrich J (2014). Neural correlates of chemotherapy-related cognitive impairment. Cortex, 54, 33–50. doi: 10.1016/j.cortex.2014.01.010 [DOI] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, & Dhabhar FS (2013). Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun, 30 Suppl(0), S109–116. doi: 10.1016/j.bbi.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, … Schagen SB (2012). Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat, 132(3), 1099–1106. doi: 10.1007/s10549-011-1888-1 [DOI] [PubMed] [Google Scholar]
- Lange M, Heutte N, Noal S, Rigal O, Kurtz JE, Lévy C, … Joly F (2019). Cognitive changes after adjuvant treatment in older adults with early-stage breast cancer. Oncologist, 24(1), 62–68. doi: 10.1634/theoncologist.2017-0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, … Castel H (2019). Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol, 30(12), 1925–1940. doi: 10.1093/annonc/mdz410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage C, Smith AM, Moreau J, Barlow-Krelina E, Wallis N, Collins B, … Scherling C (2014). A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus, 3, 444. doi: 10.1186/2193-1801-3-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, & Caeyenberghs K (2018). Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: a systematic review. Neurosci Biobehav Rev, 92, 304–317. doi: 10.1016/j.neubiorev.2018.05.019 [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, & Saykin AJ (2010). Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat, 123(3), 819–828. doi: 10.1007/s10549-010-1088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Smith DJ, West JD, & Saykin AJ (2013). Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun, 30 Suppl(0), S117–125. doi: 10.1016/j.bbi.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, & Saykin AJ (2013). Alterations in brain structure related to breast cancer and its treatment: chemotherapy and other considerations. Brain Imaging Behav, 7(4), 374–387. doi: 10.1007/s11682-013-9256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzelopoulos A, Gkiatis K, Karanasiou I, Karavasilis E, Papathanasiou M, Efstathopoulos E, … Matsopoulos GK (2021). Chemotherapy-induced brain effects in small-cell lung cancer patients: a multimodal MRI study. Brain Topogr, 34(2), 167–181. doi: 10.1007/s10548-020-00811-3 [DOI] [PubMed] [Google Scholar]
- Niu R, Du M, Ren J, Qing H, Wang X, Xu G, … Zhou P (2021). Chemotherapy-induced grey matter abnormalities in cancer survivors: a voxel-wise neuroimaging meta-analysis. Brain Imaging Behav, 15(4), 2215–2227. doi: 10.1007/s11682-020-00402-7 [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, … Schagen SB (2010). Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol, 28(8), 1294–1300. doi: 10.1200/jco.2008.21.3553 [DOI] [PubMed] [Google Scholar]
- Schwarz D, & Kašpárek T (2011). Comparison of two methods for automatic brain morphometry analysis. Radioengineering, 20(4), 996–1001. [Google Scholar]
- Seigers R, Schagen SB, Van Tellingen O, & Dietrich J (2013). Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav, 7(4), 453–459. doi: 10.1007/s11682-013-9250-3 [DOI] [PubMed] [Google Scholar]
- Simó M, Root JC, Vaquero L, Ripollés P, Jové J, Ahles T, … Rodríguez-Fornells A (2015). Cognitive and brain structural changes in a lung cancer population. J Thorac Oncol, 10(1), 38–45. doi: 10.1097/jto.0000000000000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa H, Almeida S, Bessa J, & Pereira MG (2020). The developmental trajectory of cancer-related cognitive impairment in breast cancer patients: a systematic review of longitudinal neuroimaging studies. Neuropsychol Rev, 30(3), 287–309. doi: 10.1007/s11065-020-09441-9 [DOI] [PubMed] [Google Scholar]
- Stouten-Kemperman MM, de Ruiter MB, Caan MW, Boogerd W, Kerst MJ, Reneman L, & Schagen SB (2015). Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum Brain Mapp, 36(11), 4638–4647. doi: 10.1002/hbm.22942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, & Schagen SB (2015). Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging Behav, 9(2), 275–284. doi: 10.1007/s11682-014-9305-0 [DOI] [PubMed] [Google Scholar]
- Yoshikawa E, Matsuoka Y, Inagaki M, Nakano T, Akechi T, Kobayakawa M, … Uchitomi Y (2005). No adverse effects of adjuvant chemotherapy on hippocampal volume in Japanese breast cancer survivors. Breast Cancer Res Treat, 92(1), 81–84. doi: 10.1007/s10549-005-1412-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Scatter plot of age and brain age values at baseline measurement.
CT+=Breast cancer patients treated with chemotherapy; CT-=Breast cancer patients treated without chemotherapy; NC=Healthy controls with no cancer diagnosis.
Data Availability Statement
Inter-institutional terms of access agreements enacted for the purposes of this data pooling study do not permit resharing of the study data. Those interested on obtaining data could contact the individual participating institutions, and the corresponding author can facilitate reasonable such requests.


