Abstract
Multiple promoters drive the expression of the principal cell division gene, ftsZ, in bacterial systems. Primer extension analysis of total RNA from Mycobacterium tuberculosis and a Mycobacterium smegmatis transformant containing 1.117 kb of the upstream region of M. tuberculosis ftsZ and promoter fusion studies identified six ftsZ transcripts and their promoters in the ftsQ open reading frame and ftsQ-ftsZ intergenic region. The presence of multiple promoters reflects the requirement to maintain a high basal level of, or to differentially regulate, FtsZ expression during different growth conditions of the pathogen in vivo.
Bacterial cell division is a tightly regulated process that involves spatial and temporal control of the replication and segregation of the chromosome (karyokinesis) and cytoplasmic division of the cell (cytokinesis) (12, 27, 37). The principal cell division protein FtsZ initiates septation through polymerization to form a ring-like structure at the leading edge of the invaginating septum to guide cytokinesis (1, 5, 27, 37). Although the function of FtsZ seems to be similar in all bacterial cells, transcriptional regulation of the gene differs remarkably, apparently in compliance with the physiological demands and growth conditions of different bacterial genera and species. In Escherichia coli, six promoters distributed in the upstream ftsQ, ftsA, and ddlB coding sequences drive one-third of the total ftsQAZ transcripts. The remaining two-thirds of ftsZ transcription is driven by promoters that are placed beyond 6 kb upstream of ddlB gene (10, 18, 40). Some of these promoters are upregulated by protein factors, such as SdiA, which regulates the ftsQ2p promoter (46), and the response regulator RcsB, which regulates the ftsA1p promoter (8, 22). Like for E. coli, the presence of multiple promoters for ftsZ has been reported for Bartonella bacilliformis and Bartonella henselae (17), Shewanella violacea (31), Neisseria gonorrhoeae (20), and Thermoplasma acidophilum (48). However, in the differentiating bacterium Caulobacter crescentus, monocistronic ftsZ is driven by a single promoter under the control of the global cell cycle regulator CtrA (32). In Bacillus subtilis, where the organization of the genes in the dcw cluster is similar to that in E. coli, among the three promoters that drive ftsA-ftsZ cotranscription, two are active during vegetative growth (SigA dependent) and one is active during sporulation (SigH dependent) (24). Moreover, the response regulator YycF of the YycG/YycF two-component system binds directly to the nonessential P1 promoter upstream of ftsAZ and activates transcription of the gene (21, 30). In Streptomyces, among the three ftsZ promoters present in the ftsQ-ftsZ intergenic region, one promoter is constitutively active, the second one is active during vegetative growth, and the third one is active during sporulation (11, 19, 33, 41). A minor sporulation-specific ftsZ transcript was detected from the ftsQ open reading frame (ORF) in Streptomyces griseus (11, 33) and in Streptomyces coelicolor (19, 39). In Corynebacterium glutamicum ATCC 13869 (Brevibacterium lactofermentum), there is a less abundant short transcript originating from the ftsQ-ftsZ intergenic region and a more abundant transcript starting inside ftsQ (29, 42).
Mycobacterium tuberculosis, which is a member of the lower Actinomycetes group and is similar to Corynebacterium and Streptomyces, is known to shut down its proliferation inside activated macrophages (34, 35). Similarly, proliferation of the pathogen is arrested at a uniform stage of the cell cycle when the cell enters the state of dormancy and is resumed when the cell comes out of dormancy (36, 47). Thus, the regulation of cell division is a key process that is obligatory for the pathogen for the successful establishment of infection as well as for survival and proliferation inside host cells. Although the mechanism of the control of septation in this pathogen is not known, a critical intracellular concentration of FtsZ protein, the level of which decreases in stationary phase, was found to be required for productive septum formation (13). This finding suggests a regulatory mechanism that controls the critical level of FtsZ protein inside the cells. We have initiated studies on the transcriptional regulation of the ftsZ gene of M. tuberculosis, and recently we have reported the identification of three specific regions upstream of the ftsZ ORF, which elicited ftsZ-specific transcription (43). These regions were the ftsQ-ftsZ intergenic region (172 bp) and the 5′ 467-bp and 3′ 217-bp regions of the ftsQ ORF, with maximal activity coming from within the ftsQ ORF. In continuation of these observations, here we report the identification of multiple transcripts and their start sites at the nucleotide level, and the corresponding putative promoters, by primer extension analysis from these regions on the RNA from mid-log-phase M. tuberculosis cells and on Mycobacterium smegmatis transformant carrying the M. tuberculosis ftsZ upstream region.
For this study, M. tuberculosis H37Ra cells, the isogenic attenuated mutant strain of pathogenic M. tuberculosis H37Rv, and an M. smegmatis transformant carrying the pMN406-Q1K1 construct containing the ftsQ ORF-ftsQ-ftsZ intergenic region were grown to mid-log phase (an optical density of approximately 0.8 at 600 nm) in Middlebrook 7H9 (Difco) liquid medium supplemented with albumin-dextrose-catalase (ADC) enrichment and hygromycin selection (50 μg/ml) wherever applicable. The isolation of RNA, removal of genomic DNA contamination from the samples, and quantitation of total RNA from M. tuberculosis cells and M. smegmatis transformants were all carried out as previously described (43). For primer extension analysis, 5′ end-labeled primers (Table 1) of 1.5 × 106 cpm were hybridized to 2 μg of total RNA for 10 min at 65°C followed by another 10 min of hybridization at a suitable annealing temperature (Table 1). Primer was extended by using Moloney murine leukemia virus RNase H− reverse transcriptase enzyme (MBI Fermentas) at either 42 or 45°C for 1 h in the presence of either 1 or 5 mM deoxynucleoside triphosphates. All of the reactions were repeated with two different dilutions of total RNA preparations from at least three independent mid-log-phase cultures. Control primer extension reactions were performed on E. coli tRNA (1 μg) (Sigma) to rule out extended products due to nonspecific annealing. Similarly, in order to rule out primer-extended product being a falloff product due to GC-rich sequences, primer extension reactions were performed on single-stranded sense strand DNA (200 ng) of the corresponding regions that was amplified from the PCR product of the 1.202-kbp region (Q1-ZPE segment) from the ATG of ftsQ to the annealing site of the ZPE primer inside the ftsZ ORF by using only the forward Q1 primer (57) as described previously (16). The primer extension products were analyzed in 6 or 8% denaturing polyacrylamide gel with 7 M urea, along with a corresponding sequencing reaction, using a Thermosequenase cycle sequencing kit (USB) on the PCR product template Q1-ZPE that was amplified from genomic DNA of M. tuberculosis H37Ra.
TABLE 1.
Primers used in primer extension
| Primer | Sequence | Annealing temperature (°C) |
|---|---|---|
| ZPE | 5′ CGATCATTCGGTTGACGGCGTTGAC 3′ | 55 |
| K1 | 5′ CGGGATCCCGTTCGGCTTCCTCCCTGGTGGGG 3′ | 61 |
| Igf | 5′ CGCGGATCCATGGCTACGACGGTTGCTAACCGTGCAGG 3′ | 60 |
| K2r | 5′ GCTAACCGTGCAGGCGCGCCGATAC 3′ | 60-58-55 |
| P2r | 5′ CGGGATCCCGCCGCCTTGGTCGTCGGATCG 3′ | 58 |
| P1P2r | 5′ GGGTACTGCCGCTGCACCCGCGCAC 3′ | 62 |
| Revgfp2PE | 5′ CGGTGAACAGCTCCTCGCCCTTCGAC 3′ | 58 |
Identification of the origin of ftsZ transcripts from the ftsQ-ftsZ intergenic region.
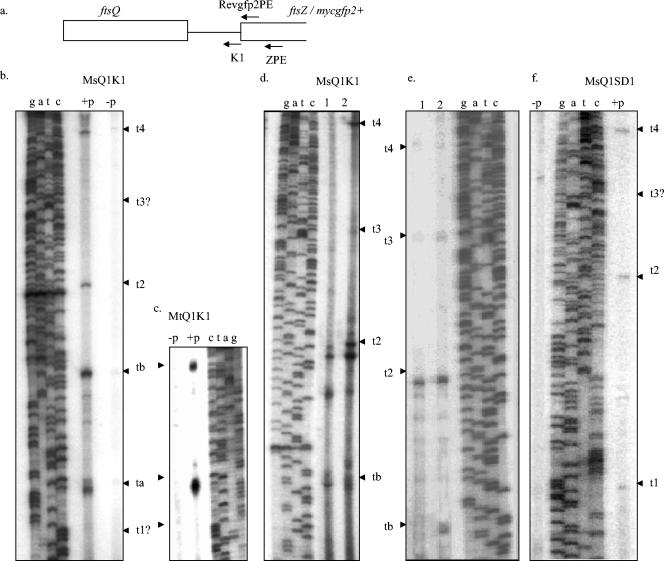
Primer extension analysis of RNA from M. tuberculosis cells with ZPE and K1 primers, which anneal to the M. tuberculosis ftsZ gene and the ftsQ-ftsZ intergenic region, respectively (Table 1), identified two transcripts, t1 and t2, originating from the intergenic region. However, extension using the Revgfp2PE primer, which anneals to the mycgfp2+ gene in the pMN406-Q1K1 construct, on RNA from M. smegmatis transformants harboring multicopy reporter plasmid pMN406-Q1K1 (43) containing the 1.117-kb M. tuberculosis ftsZ promoter region (ftsQ ORF and ftsQ-ftsZ intergenic region) identified t2 and, instead of t1, two additional transcripts, ta and tb, which were not found in M. tuberculosis. The control experiments using a mixture of E. coli tRNA and single-stranded sense strand DNA did not show any corresponding bands (Fig. 1b, lane cz), indicating that the primer extension reactions did not involve either nonspecific extension or secondary structure-related falloff of the enzyme. The control experiments for the primer extension of RNA from M. smegmatis transformant were carried out using RNA from M. smegmatis transformants containing promoterless vector control (pMN406-Δpimyc) (43). Primer extension analyses using RNA from M. smegmatis transformants containing the individual 172-bp ftsQ-ftsZ intergenic region and the 3′ 217-bp and 5′ 467-bp regions of the ftsQ ORF in the reporter plasmid (43) were not carried out in order not to miss any transcripts that originate near the junctions of these cloned regions.
FIG. 1.
Primer extension analysis of RNA from M. tuberculosis. (a) Schematic locations of ZPE and K1 primers. (b to d) Phosphorimaging profile of primer extension analysis using ZPE on M. tuberculosis RNA. Lane cz, control extension with ZPE on E. coli tRNA (1 μg) mixed with single-stranded sense strand DNA (200 ng) of the 1.117-kb promoter region. (e and f) Phosphorimaging profile of primer extension using K1 on M. tuberculosis (Mt) RNA.
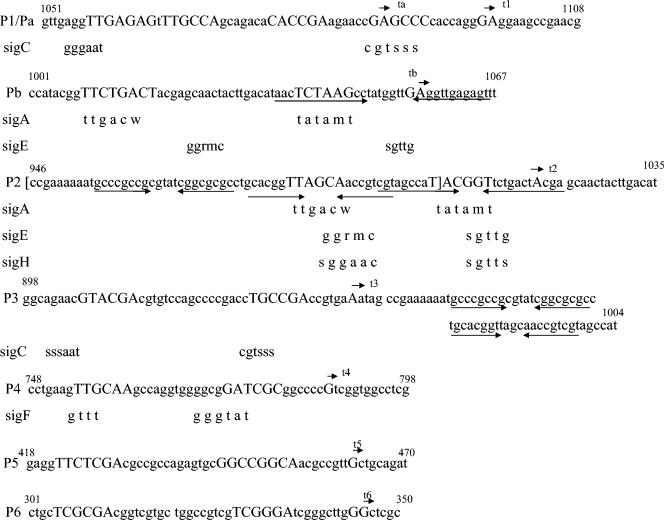
The transcript t1, which was identified in M. tuberculosis, starts at the A or G 12 or 13 bp upstream of the ATG of ftsZ and just includes the predicted ribosome binding site (RBS) GGAGGAAG of the cognate mRNA (Fig. 1b). The potential RBS where the transcription start lies can form stem-loop structures in several conformations with sequences just downstream of ATG of the ftsZ gene. The presence of such stem-loop structures and a strong RBS at the 5′ end of the RNA is known to stabilize the cognate mRNA molecule (3, 4, 26). The putative promoter P1, which drives the expression of the t1 transcript, has CGAGCCCC as the −10 sequence and TTGCCA as the −35 sequence (Table 3) (see Fig. 5). Although the −35 sequence shows a σA-like promoter consensus, the −10 sequence does not. Therefore, P1 could be classified under type C promoters (23). If the −10 region (CGAGCC) is considered with a −35-like (GAGAGT) sequence, with a 24-nucleotide gap between them, it shows σC consensus (38, 45) (see Fig. 5). However, in mycobacteria the gap between −10 and −35 sequences of various promoters reported so far are found to vary between 12 and 22 nucleotides only and not 24 nucleotides (23).
TABLE 3.
Putative promoter sequences of M. tuberculosis ftsZ
| Putative promoter | −35 | −10 | +1 | Distance of +1 from ATG of ftsZ | Type |
|---|---|---|---|---|---|
| P1 | GAGAGTTTGCCA | CGAGCCCC | G/A | 13 or 12 | C |
| P2 | TTAGCA | TACGGT | A | 100 | A |
| P3 | GTACGA | TGCCGA | A | 176 | C |
| P4 | GTTGCAA | GATCGC | G | 331 | C |
| P5 | TTCTCGA | GGCCGGCA | G | 655 | C |
| P6 | TCGCGA | TCGGGA | GG | 773 or 772 | C |
FIG. 5.
Promoter sequences of M. tuberculosis ftsZ and comparisons with different sigma factor consensuses. The number of nucleotides are based on the M. tuberculosis Q1-ZPE region, with ftsQ ATG as 1. s denotes g or c; w denotes a or t; r denotes a or g; and m denotes a or c. The top arrow is the +1 site. Capital letters indicate −10 and −35 sequences. Convergent arrows indicate palindrome sequences. These promoter sequences were cloned by using primers shown in Table 2. The portion of the P2 sequence in square brackets is P2Δ.
In order to verify whether the same transcript is produced in the M. smegmatis transformant, extension was carried out on RNA from the transformant by using primer Revgfp2PE, the 3′ end of which anneals against G of the ATG codon of mycgfp2+ in the promoter probe vector (43) (Fig. 2a and Table 1). Instead of the t1 transcript, the primer extension reaction identified the transcript ta, which starts at the G that is 24 bp upstream of the ATG of ftsZ (Fig. 2b; see Fig. 5). Similar to P1, the corresponding Pa promoter for the ta transcript possessed TTGAGA as the −35 sequence of σA consensus, but there was no clear −10 region similar to the −10 consensus of σA (see Fig. 5). Therefore, Pa could also be considered a type C promoter (23). It may be noted that both P1 and Pa promoters overlap with each other, suggesting that the transcription initiation complex can engage only one of them at any time point if both are functional in the same organism. Such promoters, having conserved the −35 region but without −10 consensus, are known to be targets of regulation by extracellular function sigma factors (15). The M. smegmatis genome lacks a σC homologue but possesses sequences for eight additional extracellular function sigma factors which are absent in M. tuberculosis (38). Therefore, it is likely that the overlapping P1 and Pa promoters might be targets of regulation by different sigma factors in M. tuberculosis and M. smegmatis. Another possibility for the presence of t1 and ta transcripts is that the products originated from differential processing of a single pre-mRNA due to altered secondary structure in these two mycobacterial species, owing to the GC-rich mycgfp2+ sequence, instead of the ftsZ ORF, downstream of the promoter region in the transformant. One distant possibility is sequence polymorphism, similar to a previous report (6), between the ftsZ promoter region of the attenuated H37Ra strain, from which RNA was prepared for primer extension, and of the virulent H37Rv strain, the DNA of which was used to generate the insert in the pMN406-Q1K1 construct (43). We ruled out this possibility, as the sequence of the Pfu polymerase (MBI Fermentas)-amplified product from the ftsQ ORF-ftsQ-ftsZ intergenic-ftsZ ORF region (Q1-ZPE) from H37Ra genomic DNA with the ZPE, K2r, P2r, and P1P2r primers (Table 1) was identical to the reported sequence of the ftsQ-ftsZ locus of M. tuberculosis H37Rv (9).
FIG. 2.
Primer extension analyses of RNA from mycobacterial transformants. (a) Schematic locations of ZPE, K1, and Revgfp2PE primers. (b) Phosphorimaging profile of primer extension analysis using Revgfp2PE on M. smegmatis Q1K1. (c) Phosphorimaging profile of primer extension analysis with Revgfp2PE of RNA from M. tuberculosis Q1K1. (d) Phosphorimaging profile of primer extension analysis of RNA of M. smegmatis Q1K1 with K1 primer. Extension products from 500 ng (lane 1) and 2 μg (lane 2) of RNA are shown. (e) Phosphorimaging profile of primer extension analysis with K1 primer of RNA from M. smegmatis Q1K1 (lane 2) and M. smegmatis Q1SD1 (lane 1). (f) Phosphorimaging profile of primer extension analysis with ZPE primer of RNA from M. smegmatis Q1SD1. MsQ1K1 +p, M. smegmatis transformed with pMN406-Q1K1; MsQ1K1 −p, M. smegmatis transformed with promoterless vector control pMN406-Δpimyc; and MsQ1SD1 +p, M. smegmatis transformed with pMN406-Q1SD1. t1 to t4, ta, and tb are extension products representing transcripts.
Extension with the same primer, Revgfp2PE, of RNA from M. smegmatis transformants detected yet another transcript, tb (Fig. 2b; see Fig. 5). The product starts at the A or G at 62 or 63 bp, respectively, upstream of the ATG of the ftsZ gene. The corresponding promoter region, Pb (−35 TTCTGA and −10 TCTAAG with a 21- to 22-bp spacer region) shows a high degree of consensus to σA promoters (see Fig. 5). In addition to the presence of a clear σA promoter consensus, there could also be a probable σE or σH consensus with −35-like GCAAC and −10-like GGTTG, but with a 24-bp spacer, just immediately upstream of the +1 site (see Fig. 5). However, the tb transcript was absent from extension using the ZPE primer on the RNA from M. tuberculosis mid-log-phase cells (Fig. 1b). The absence of tb in mid-log-phase M. tuberculosis cells could be due to the possibility that the Pb promoter might be under regulated activity, as there is a strong hairpin loop present downstream of the −10 sequence of Pb involving the start site of the tb transcript. Such secondary structure downstream of the −10 sequence might be suggestive of a repression mechanism, as repressors are shown to maximally bind to palindrome sequences that are present downstream of −30 of promoter regions and up to +20 of the ORF (25). It is also possible that the activity of Pb is below detection limits during mid-log phase in M. tuberculosis. The presence of a large amount of transcribed RNA from the multicopy plasmid pMN406-Q1K1 in M. smegmatis transformant might have enabled its detection in the transformant. Another possibility is that antibiotic selection of M. smegmatis transformant might have induced stress response-specific sigma factors, such as σE or σH, for which consensus exists upstream of tb, which in turn might have activated the Pb promoter. Extension reactions using the primer K1, which binds upstream of ZPE and Revgfp2PE, the 3′ end of which anneals to 24 bp upstream of the ATG of ftsZ (Fig. 1a and 2a and Table 1) or mycgfp2+ in pMNQ1K1 (Fig. 2a), confirmed the observation that tb is present in M. smegmatis transformant only and not in M. tuberculosis cells (Fig. 1f and 2d).
Primer extension with the ZPE, K1, and Revgfp2PE primers identified a fourth transcript, t2, from the RNA of both M. tuberculosis cells and the M. smegmatis transformant (Fig. 1b, d, and e and 2b, d, e, and f). The +1 site starts at the A 100 bp upstream of the ATG of the ftsZ gene (Table 3). The corresponding promoter, P2, shows a canonical σA-type consensus having −35 TTAGCA and −10 TACGGT with a spacer region of 13 bp. Alternately, there could be σE- or σH-type consensus having −35-like GGAAC and −10-like GGTTC with a 14-bp spacer (see Fig. 5). Incidentally, the promoter region contains palindromic sequences upstream of as well as between the −35 and −10 regions (see Fig. 5). Such promoters are subject to regulation and could be probable transcription factor binding sites, as in the case of the tipA-p promoter of Streptomyces (44). It may be noted that the −10 region of P2 and the −35 region of Pb overlap (see Fig. 5), indicating the possibility of differential engagement of the two promoters by RNA polymerase. Another possibility is of promoter occlusion (2) of Pb by the higher level of activity of P2 in M. tuberculosis.
Transcripts ta and tb are not specific to ftsZ.
In order to verify whether ta and tb represent differentially expressed true transcripts, RNA was prepared from M. tuberculosis integrant carrying pMN406-Q1K1 derivative (unpublished data) at the L5 lambda attachment site. Primer extension analysis using the Revgfp2PE and K1 primers identified the same ta and tb transcripts, instead of t1, from the RNA of M. tuberculosis transformants too (Fig. 2c). The presence of these transcripts in M. tuberculosis transformants rules out the possibility, suggested earlier, of repression or downregulated expression from the Pa and Pb promoters in M. tuberculosis. However, antibiotic-induced sigma factor-mediated activation of these promoters cannot be ruled out. In order to verify this possibility, the 1,843-bp region starting from the ATG of M. tuberculosis ftsQ up to the 726-bp region of M. tuberculosis ftsZ was PCR amplified from M. tuberculosis genomic DNA by using Q1 and SD1 primers (Table 2) and KOD XL DNA polymerase (Novagen) and cloned into the pMN406-Δpimyc vector (43) at the SspI and NheI sites, replacing the mycgfp2+ gene, to obtain pMN406-Q1SD1. In this construct, M. tuberculosis ftsZ was directly under the regulation of its own native 1.117-kb promoter region. Extension of the ZPE primer on the RNA from M. smegmatis transformant containing pMN406-Q1SD1 identified t1 and t2 transcripts, originating from the intergenic region, but not ta or tb transcripts (Fig. 2f). This observation was again confirmed by the extension of K1 primer on the same RNA, wherein as expected only t2 and not tb could be detected, as K1 anneals at the P1/Pa region (Fig. 2e, lane 1). This result ruled out the possibility of firing of Pa and Pb promoters by antibiotic-induced sigma factors. The fact that ta and tb transcripts are absent in the negative control (pMN406-Δpimyc) and that two independent primers, K1 and Revgfp2PE, have identified them in M. smegmatis and M. tuberculosis transformants only when mycgfp2+ is present downstream of the 1.117-kb M. tuberculosis ftsZ promoter region (Fig. 2b and c) and not when the ftsZ ORF is present (Fig. 2f and 1b) proves that the two transcripts are the processed ends due to a modified conformation of the untranslated M. tuberculosis ftsZ leader sequence in the presence of the GC-rich mycgfp2+ sequence downstream of the promoter region. Since the two transcripts do not pertain to ftsZ transcription, as evident from the primer extension result with RNA from M. smegmatis transformant (pMN406-Q1SD1) (Fig. 2e and f), the probable reason for their mycgfp2+-specific origin was not investigated.
TABLE 2.
Primers used in the cloning of promoter sequences
| Primer | Sequence | Region or promoter cloned |
|---|---|---|
| T1f | 5′ GCTCTAGAGTTGAGGTTGAGAGTTTGCCAGCAGACACAC 3′ | P1 |
| K1 | 5′ CGGGATCCCGTTCGGCTTCCTCCCTGGTGGGG 3′ | P1 |
| Tbf | 5′ GCTCTAGACCATACGGTTCTGACTACGAGCAACTAC 3′ | Pb |
| Tbr | 5′ CGGGATCCAAACTCTCAACCTCAACCATAGGCTTAGAG 3′ | Pb |
| K2 | 5′ GCTCTAGACCGAAAAAATGCCCGCCGCGTATC 3′ | P2 |
| T2r | 5′ GCGGATCCATGTCAAGTAGTTGCTCGTAGTCAGAACCGTATGGC 3′ | P2 |
| Igf | 5′ GCGGATCCTAAATGGCTACGACGGTTGCTAACCGTGCAGG 3′ | P2Δ, P3 |
| T3f | 5′ GCTCTAGAGGCAGAACGTACGACGTGTCCAGCCCC 3′ | P3 |
| T4 | 5′ CTAGACCTGAAGTTGCAAGCCAGGTGGGGCGGATCGCGGCCCCGTCG 3′ | P4 |
| T4c | 5′ GATCCGACGGGGCCGCGATCCGCCCCACCTGGCTTGCAACTTCAGGT 3′ | P4 |
| T5f | 5′ GCTCTAGAGAGGTTCTCGACGCCGCCAG 3′ | P5 |
| T5r | 5′ CGGGATCCCGATCTGCAGCAACGGCGTTGCCG 3′ | P5 |
| Q1 | 5′ GCGGGATCCGATATCATGACGGAACACAACGAGGACCCACAGATCGAGCGC 3′ | Q1SD1 |
| SD1 | 5′ GATATCGCTAGCGATGGCGATCTCGGCCGCTTTGAGCGACCGGCCTTC 3′ | Q1SD1 |
Identification of the origin of ftsZ transcripts from the ftsQ ORF.
Primer extension analysis on the RNA from M. tuberculosis cells and M. smegmatis transformants containing pMN406-Q1K1 with the primers ZPE, K1, Revgfp2PE, K2r, Igf, P2r, and P1P2r (Table 1) identified four transcripts: t3 and t4, originating in the 3′ 217-bp region of the ftsQ ORF, and t5 and t6, originating in the 5′ 467-bp region of the ftsQ ORF (Fig. 3 and 4). The extension of ZPE, K1, and Revgfp2PE primers identified transcript t3 as originating inside the ftsQ ORF at the A, which is 4 nucleotides upstream of the end of the stop codon of ftsQ and 176 bp upstream of the ATG of ftsZ (Fig. 1b and c and 2b, d, e, and f; Table 3). Similarly, t4 was found to originate approximately 320 bp upstream of the ATG of ftsZ and about 145 bp upstream of the stop codon of ftsQ. In order to precisely map t3 and t4 transcripts, K2r and Igf primers were used for primer-extension on total RNA from M. tuberculosis and M. smegmatis transformant containing pMN406-Q1K1 (Table 1 and Fig. 3a).
FIG. 3.
Primer extension analysis in the 3′ portion of the ftsQ ORF. (a) Schematic locations of Igf and K2r primers. (b to e) Phosphorimaging profile of primer extension using K2r (b and c) and Igf (d and e) primers. t3 and t4 are extension products representing transcripts.
FIG. 4.
Primer extension analysis in the 5′ portion of the ftsQ ORF. (a to c) Phosphorimaging profile of primer extension using P2r and P1P2r primers. (a) Extension with P2r of RNA from M. smegmatis transformed with pMN406-Q1K1. +p1 and +p2 are two different reactions from two different samples. (b and c) Extension with P1P2r primer of RNA from M. smegmatis Q1K1 (MsQ1K1) and M. tuberculosis (Mt). Primer extension products from 2 μg (lane 1) and 500 ng (lane 2) of RNA are shown. (d) Schematic locations of P2r and P1P2r primers and +1 sites of ftsZ transcripts (t1 to t6).
Primer K2r, the 3′ end of which lies in the ftsQ-ftsZ intergenic region 153 bp upstream of the ATG of ftsZ, was annealed to RNA with a gradual reduction of temperature (Table 1 and Fig. 3a) owing to the high secondary structure in the region. The 3′ end of the primer Igf anneals 142 bp upstream of ATG (Table 1 and Fig. 3a). The extension of both of the primers detected t3 transcript (Fig. 3c and e and 5), confirming the earlier observation. The corresponding promoter, P3, lacks consensus to σA-, σC-, or σH-type promoters and could be grouped as a C type promoter (Table 3 and Fig. 5). Since P3 lacks consensus with any of the known sigma factor-specific promoters and t3 starts in an AU-rich region downstream of which there is a probable strong stem-loop structure, t3 could be a RNase-processed product, as found in the ftsQAZ transcript of E. coli (7). The extension of K2r and Igf mapped t4, originating at the G 331 bp upstream of the ATG of ftsZ and 158 nucleotides upstream of the end of the stop codon of ftsQ, confirming the earlier observation with ZPE, K1, and Revgfp2PE primers (Fig. 3b and d and Table 3). The corresponding promoter, P4, has a GC-rich −10 region (GATCGC) and a weak −35 consensus (TTGCAA) for σA, indicating that it could probably be a type C promoter (Table 3 and Fig. 5). The alternate possibility is a weak σF consensus promoter (GTTG-13 bp-GCGGAT) (Fig. 5).
In order to verify the observation of the absence of promoter activity in the 261-bp PstI fragment of the ftsQ ORF (43), extension was performed with the P2r primer (Table 1 and Fig. 4d), the 3′ end of which anneals at 416 bp upstream of the ATG of ftsZ and 244 bp upstream of the stop codon of ftsQ. P2r extension did not generate any product from M. tuberculosis RNA or from RNA of M. smegmatis transformant from the 261-bp region of the ftsQ ORF (43). Instead, it gave a faint extension product, t5, on RNA from the M. smegmatis transformant containing pMN406-Q1K1, but not from M. tuberculosis, above 650 bp upstream of the ATG of ftsZ that lies in the 467-bp 5′ region of the ftsQ ORF (Fig. 4a). In order to precisely map transcript t5, extension was carried out using the P1P2r primer (Table 1 and Fig. 4d), the 3′ end of which anneals to 597 bp upstream of the ATG of ftsZ. The product t5 was found to be present only in M. smegmatis transformant, starting at the G, 655 bp upstream of the ATG of the ftsZ gene and 482 nucleotides upstream of the stop codon of ftsQ and lies in the 467-bp 5′ portion of ftsQ (Fig. 4a and b). The corresponding promoter, P5, having only a weak consensus to the −35 region (TTCTCG) of σA, lacks consensus to any of the known sigma factor-specific promoters. Therefore, it could be a type C promoter (Table 3 and Fig. 5). The reason for the absence of t5 transcript in M. tuberculosis could be a low level of P5 activity or differential regulation at the mid-log phase of growth. Besides t5, one more transcript, t6, which starts at the G 772 or 773 nucleotides upstream of the ATG of ftsZ and 599 or 600 nucleotides upstream of the stop codon of ftsQ and lies in the 5′ 467-bp portion of ftsQ, could be detected by P1P2r primer extension of RNA from M. tuberculosis and M. smegmatis transformant containing pMN406-Q1K1 (Fig. 4c). The corresponding P6 promoter did not show consensus to any of the known sigma factor-specific promoters (Table 3 and Fig. 5).
Activity of the mapped putative promoter sequences in vivo.
Although our previous study (43) showed promoter activity from the individual 5′ 467-bp (initiating t5 and t6 transcripts) and 3′ 217-bp (initiating t3 and t4 transcripts) regions of M. tuberculosis ftsQ as well as the ftsQ-ftsZ intergenic region (initiating t1 and t2 transcripts), the presence of multiple origins of transcripts from each cloned region and also the presence of potential stem-loop structures in some of the promoter regions raise the possibility that some of the 5′ ends identified could be due to RNA processing. Due to the lack of RNase mutant strains for mycobacteria, the transcripts with secondary structure near their origin could not be tested to determine whether they were RNase-processed products. Since the majority of the mycobacterial promoters are not active in E. coli (23), E. coli RNase mutant strains also could not be used for this purpose. Instead, the activity of the putative promoters was determined in the reporter fusion study using the promoter probe vector pMN406-Δpimyc (43). Since P6 is the most distal promoter detected, there is no promoter sequence beyond P6 in the M. smegmatis transformant containing pMN406-Q1K1. Therefore, t6 transcript cannot be a processed product. Hence, except P6, other putative promoter sequences, namely, P1, P2, P3, P4, and P5, encompassing exclusively the −10, −35, and +1 start sites of the respective transcripts (Fig. 5), were PCR amplified with primers (Table 2) and cloned in pMN406-Δpimyc vector, and the sequences were confirmed by using Revgfp2PE primer (Table 1) and analyzed for their activity in M. smegmatis transformants. The respective sequences of the P1, P2, P3, P4, and P5 promoters that were cloned are indicated in Fig. 5. Since P2 has secondary structure regions overlapping with the −10 and −35 sequences, in order to confirm the promoter activity of P2, the −10 sequence was deleted by cloning a 59-bp PCR-amplified truncated P2 (P2Δ) (Fig. 5). As an internal negative control, a 67-bp Pb sequence was PCR amplified and cloned. Except truncated P2Δ and the negative control Pb, all of the specific promoter sequences, namely, P1 to P5, drove the expression of mycgfp2+ (Fig. 6). These observations confirmed that the regions, predicted based on 5′ end mapping of ftsZ transcripts, are true promoters and the transcripts t1 to t5 (and t6) are true transcripts and not RNase-processed products. These observations indicate that the palindrome sequences in these promoter regions are not RNase processing sites but might be involved in promoter regulation. Careful mutational analyses of the palindrome structures are needed to fully understand the transcriptional regulation of the M. tuberculosis ftsZ gene.
FIG. 6.
Fluorescence microscopic observation of M. smegmatis transformants containing various promoter fusion constructs. (a) Dark-field fluorescence microscopy of the cells containing the constructs indicated in the figure. (b) Figures of phase-contrast microscopy of the corresponding fluorescent M. smegmatis cells. Cells are photographed and shown at ×25,000 magnification. pMN406-Q1K1 (43) was taken as the positive control, whereas pMN406-Δpimyc (43) was used as the negative control. Brightness and contrast have been modified with Adobe Photoshop 6.0.
In summary, we have identified and mapped six transcripts (t1 to t6) and their putative promoters (P1 to P6) for the ftsZ gene of M. tuberculosis. Among them, P1 and P2 are in the ftsQ-ftsZ intergenic region, while P3 and P4 are in the 3′ 217-bp region and P5 and P6 are in the 5′ 467-bp region of the ftsQ ORF. The identification of these transcripts, their respective putative promoters, and their distribution are in concurrence with our previous study (43), which demonstrated promoter activity in the ftsQ-ftsZ intergenic region and 3′ 217-bp and 5′ 467-bp regions of the ftsQ ORF. The absence of any transcript from the 261-bp P1P2 region of ftsQ also correlates with the absence of promoter activity noted for this region in our previous study (43). The quantitation of the relative intensities of the extension products of t1, t2, t3, and t4 transcripts (Fig. 1b and 2f) showed that both in M. tuberculosis cells and in M. smegmatis transformants, the promoter strength of the 3′ 217-bp region of the ftsQ ORF is 1.3 to 1.8 times higher than that of the 172-bp M. tuberculosis ftsQ-ftsZ intergenic region. These values are comparable to the values (1.4 to 1.8) obtained in the semiquantitative reverse transcription-PCR analyses of the M. tuberculosis ftsZ transcripts originating from the respective regions in M. tuberculosis (43) and in M. smegmatis transformant containing pMN406-Q1K1 (Fig. 6 of reference 43). The promoters in the ftsQ ORF are GC rich and lack consensus to promoters that are recognized by the plethora of characterized sigma factors present in M. tuberculosis. Their high GC content also shows concurrence with their activity in M. smegmatis but lack of activity in E. coli (43). The presence of multiple promoters for ftsZ might facilitate differential regulation of gene expression in response to various environmental signals corresponding to different stages of the life cycle of the pathogen. Alternately, considering the slow rate of transcription in mycobacteria (28), multiple promoters might enable the maintenance of the high intracellular concentration of FtsZ required for cell division in M. tuberculosis and M. smegmatis (13, 14).
Acknowledgments
This work was supported by a research grant from the Council of Scientific & Industrial Research, Government of India.
We express sincere thanks to H. S. Rajeswari for technical assistance, R. Srinivasan for discussions, and P. D. V. Krishna for the technical support in phosphorimaging. We acknowledge the DBT-Supported Phosphorimaging Facility, Division of Biological Sciences, Indian Institute of Science (IISc), and the infrastructure support from the ICMR-Funded Centre for Advanced Study in Molecular Medical Microbiology at the Department of Microbiology and Cell Biology, IISc, from the FIST Programme, Department of Science and Technology, Government of India, and from the DSA Programme, University Grants Commission, Government of India. S.R. acknowledges a Senior Research Fellowship from the Council of Scientific & Industrial Research, Government of India.
REFERENCES
- 1.Addinall, S. G., and B. Holland. 2002. The tubulin ancestor, FtsZ, draughtsman, designer and driving force for bacterial cytokinesis. J. Mol. Biol. 318:219-236. [DOI] [PubMed] [Google Scholar]
- 2.Adhya, S., and M. Gottesman. 1982. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell 29:939-944. [DOI] [PubMed] [Google Scholar]
- 3.Agaisse, H., and D. Lereclus. 1996. STAB-SD: a Shine-Dalgarno sequence in the 5′ untranslated region is a determinant of mRNA stability. Mol. Microbiol. 20:633-643. [DOI] [PubMed] [Google Scholar]
- 4.Belasco, J. G., G. Nilsson, A. von Gabain, and S. N. Cohen. 1986. The stability of E. coli gene transcripts is dependent on determinants localized to specific mRNA segments. Cell 46:245-251. [DOI] [PubMed] [Google Scholar]
- 5.Bi, E. F., and J. Lutkenhaus. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161-164. [DOI] [PubMed] [Google Scholar]
- 6.Brosch, R., W. J. Philipp, E. Stavropoulos, M. J. Colston, S. T. Cole, and S. V. Gordon. 1999. Genomic analysis reveals variation between Mycobacterium tuberculosis H37Rv and the attenuated M. tuberculosis H37Ra strain. Infect. Immun. 67:5768-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cam, K., G. Rome, H. M. Krisch, and J. P. Bouché. 1996. RNase E processing of essential cell division genes mRNA in Escherichia coli. Nucleic Acids Res. 24:3065-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carballes, F., C. Bertrand, J. P. Bouche, and K. Cam. 1999. Regulation of Escherichia coli cell division genes ftsA and ftsZ by the two-component system rcsC-rcsB. Mol. Microbiol. 34:442-450. [DOI] [PubMed] [Google Scholar]
- 9.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Dewar, S. J., and R. Dorazi. 2000. Control of division gene expression in Escherichia coli. FEMS Microbiol. Lett. 187:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Dharmatilake, A. J., and K. E. Kendrick. 1994. Expression of the division-controlling gene ftsZ during growth and sporulation of the filamentous bacterium Streptomyces griseus. Gene 147:21-28. [DOI] [PubMed] [Google Scholar]
- 12.Donachie, W. D. 2001. Co-ordinate regulation of the Escherichia coli cell cycle or the cloud of unknowing. Mol. Microbiol. 40:779-785. [DOI] [PubMed] [Google Scholar]
- 13.Dziadek, J., M. V. Madiraju, S. A. Rutherford, M. A. Atkinson, and M. Rajagopalan. 2002. Physiological consequences associated with overproduction of Mycobacterium tuberculosis FtsZ in mycobacterial hosts. Microbiology 148:961-971. [DOI] [PubMed] [Google Scholar]
- 14.Dziadek, J., S. A. Rutherford, M. V. Madiraju, M. A. Atkinson, and M. Rajagopalan. 2003. Conditional expression of Mycobacterium smegmatis ftsZ, an essential cell division gene. Microbiology 149:1593-1603. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes, N. D., Q. L. Wu, D. Kong, X. Puyang, S. Garg, and R. N. Husson. 1999. A mycobacterial extracytoplasmic sigma factor involved in survival following heat shock and oxidative stress. J. Bacteriol. 181:4266-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finckh, U., P. A. Lingenfelter, and D. Myerson. 1991. Producing single stranded DNA probes with the Taq DNA polymerase: a high yield protocol. BioTechniques 10:35-39. (Erratum, 12:382, 1992.) [PubMed] [Google Scholar]
- 17.Fiskus, W., I. Padmalayam, T. Kelly, C. Guibao, and B. R. Baumstark. 2003. Identification and characterization of the DdlB, FtsQ and FtsA genes upstream of FtsZ in Bartonella bacilliformis and Bartonella henselae. DNA Cell Biol. 22:743-752. [DOI] [PubMed] [Google Scholar]
- 18.Flardh, K., P. Palacios, and M. Vicente. 1998. Cell division genes ftsQAZ in Escherichia coli require distant cis-acting signals upstream of ddlB for full expression. Mol. Microbiol. 30:305-315. [DOI] [PubMed] [Google Scholar]
- 19.Flärdh, K., E. Leibovitz, M. J. Buttner, and K. F. Chater. 2000. Generation of a non-sporulating strain of Streptomyces coelicolor A3(2) by the manipulation of a developmentally controlled ftsZ promoter. Mol. Microbiol. 38:737-749. [DOI] [PubMed] [Google Scholar]
- 20.Francis, F., S. Ramirez-Arcos, H. Salimnia, C. Victor, and J. R. Dillon. 2000. Organization and transcription of the division cell wall (dcw) cluster in Neisseria gonorrhoeae. Gene 251:141-151. [DOI] [PubMed] [Google Scholar]
- 21.Fukuchi, K., Y. Kasahara, K. Asai, K. Kobayashi, S. Moriya, and N. Ogasawara. 2000. The essential two-component regulatory system encoded by yycF and yycG modulates expression of the ftsAZ operon in Bacillus subtilis. Microbiology 146:1573-1583. [DOI] [PubMed] [Google Scholar]
- 22.Gervais, F. G., P. Phoenix, and G. R. Drapeau. 1992. The rcsB gene, a positive regulator of colanic acid biosynthesis in Escherichia coli, is also an activator of ftsZ expression. J. Bacteriol. 174:3964-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez, M., and I. Smith. 2000. Determinants of mycobacterial gene expression, p. 111-129. In G. F. Hatfull and W. R. Jacobs, Jr. (ed.), Molecular genetics of mycobacteria. ASM Press, Washington, D.C.
- 24.Gonzy-Treboul, G., C. Karmazyn-Campelli, and P. Stragier. 1992. Developmental regulation of transcription of the Bacillus subtilis ftsAZ operon. J. Mol. Biol. 224:967-979. [DOI] [PubMed] [Google Scholar]
- 25.Gralla, J. D., and J. C. Collado-Vides. 1996. Organization and function of transcription regulatory elements, p. 1232-1245. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 26.Hansen, M. J., L. H. Chen, M. L. Fejzo, and J. G. Belasco. 1994. The ompA 5′ untranslated region impedes a major pathway for mRNA degradation in Escherichia coli. Mol. Microbiol. 12:707-716. [DOI] [PubMed] [Google Scholar]
- 27.Harry, E. J. 2001. Bacterial cell division: regulating Z-ring formation. Mol. Microbiol. 40:795-803. [DOI] [PubMed] [Google Scholar]
- 28.Harshey, R. M., and T. Ramakrishnan. 1976. Purification and properties of DNA-dependent RNA polymerase from Mycobacterium tuberculosis H37Rv. Biochim. Biophys. Acta 432:49-59. [DOI] [PubMed] [Google Scholar]
- 29.Honrubia, M. P., F. J. Fernandez, and J. A. Gil. 1998. Identification, characterization, and chromosomal organization of the ftsZ gene from Brevibacterium lactofermentum. Mol. Gen. Genet. 259:97-104. [DOI] [PubMed] [Google Scholar]
- 30.Howell, A., S. Dubrac, K. K. Andersen, D. Noone, J. Fert, T. Msadek, and K. Devine. 2003. Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol. Microbiol. 49:1639-1655. [DOI] [PubMed] [Google Scholar]
- 31.Ishii, A., K. Nakasone, T. Sato, M. Wachi, M. Sugai, K. Nagai, and C. Kato. 2002. Isolation and characterization of the dcw cluster from the piezophilic deep-sea bacterium Shewanella violacea. J. Biochem. (Tokyo) 132:183-188. [DOI] [PubMed] [Google Scholar]
- 32.Kelly, A. J., M. J. Sackett, N. Din, E. Quardokus, and Y. V. Brun. 1998. Cell cycle-dependent transcriptional and proteolytic regulation of FtsZ in Caulobacter. Genes Dev. 12:880-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwak, J., A. J. Dharmatilake, H. Jiang, and K. E. Kendrick. 2001. Identification and differential regulation of ftsZ transcription during septation of Streptomyces griseus. J. Bacteriol. 183:5092-5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagrange, P. H. 1984. Cell-mediated immunity and delayed-type hypersensitivity, p. 681-720. In G. P. Kubica and L. G.Wayne (ed.), The mycobacteria: a sourcebook, part B. Marcel Dekker, Inc., New York, N.Y.
- 35.Lefford, M. J. 1984. Diseases in mice and rats, p. 947-977. In G. P. Kubica and L. G. Wayne (ed.), The mycobacteria: a sourcebook, part B. Marcel Dekker, Inc., New York, N.Y.
- 36.Lim, A., M. Eleuterio, B. Hutter, B. Murugasu-Oei, and T. Dick. 1999. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J. Bacteriol. 181:2252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutkenhaus, J., and S. G. Addinall. 1997. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 38.Manganelli, R., R. Provvedi, S. Rodrigue, J. Beaucher, L. Gaudreau, and I. Smith. 2004. Sigma factors and global gene regulation in Mycobacterium tuberculosis. J. Bacteriol. 186:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCormick, J. R., and R. Losick. 1996. Cell division gene ftsQ is required for efficient sporulation but not growth and viability in Streptomyces coelicolor A3(2). J. Bacteriol. 178:5295-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mengin-Lecreulx, D., J. Ayala, A. Bouhss, J. van Heijenoort, C. Parquet, and H. Hara. 1998. Contribution of the Pmra promoter to expression of genes in the Escherichia coli mra cluster of cell envelope biosynthesis and cell division genes. J. Bacteriol. 180:4406-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikulik, K., E. Zhulanova, M. Kratky, O. Kofronova, and O. Benada. 2000. Isolation and characterization of dcw cluster from Streptomyces collinus producing kirromycin. Biochem. Biophys. Res. Commun. 268:282-288. [DOI] [PubMed] [Google Scholar]
- 42.Ramos, A., M. P. Honrubia, D. Vega, J. A. Ayala, A. Bouhss, D. Mengin-Lecreulx, and J. A. Gil. 2004. Characterization and chromosomal organization of the murD-murC-ftsQ region of Corynebacterium glutamicum ATCC 13869. Res. Microbiol. 155:174-184. [DOI] [PubMed] [Google Scholar]
- 43.Roy, S., M. A. Mir, S. P. Anand, M. Niederweis, and P. Ajitkumar. 2004. Identification and semi-quantitative analysis of Mycobacterium tuberculosis H37Rv ftsZ gene-specific promoter activity-containing regions. Res. Microbiol. 155:817-826. [DOI] [PubMed] [Google Scholar]
- 44.Strohl, W. R. 1992. Compilation and analysis of DNA sequence associated with apparent Streptomyces promoters. Nucleic Acids Res. 20:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun, R., P. J. Converse, C. Ko, S. Tyagi, N. E. Morrison, and W. R. Bishai. 2004. Mycobacterium tuberculosis ECF sigma factor sigC is required for lethality in mice and for the conditional expression of a defined gene set. Mol. Microbiol. 52:25-38. [DOI] [PubMed] [Google Scholar]
- 46.Wang, X. D., P. A. de Boer, and L. I. Rothfield. 1991. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 10:3363-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaoi, T., P. Laksanalamai, A. Jiemjit, H. K. Kagawa, T. Alton, and J. D. Trent. 2000. Cloning and characterization of ftsZ and pyrF from the archaeon Thermoplasma acidophilum. Biochem. Biophys. Res. Commun. 275:936-945. [DOI] [PubMed] [Google Scholar]